Abstract
Pseudoaneurysms of the mitral-aortic intervalvular fibrosa are rare complications that can develop after mitral or aortic valve surgery, endocarditis, or Takayasu arteritis. The optimal timing of surgery to avoid potentially life-threatening complications of pseudoaneurysms has not been established, and watchful waiting has been adopted in specific situations. We describe the case of a 50-year-old man in whom a pseudoaneurysm of the mitral-aortic intervalvular fibrosa developed after aortic root replacement with a homograft. After 13 years of watchful waiting, reoperation was deemed necessary because the pseudoaneurysm had grown to 48 mm and the aortic regurgitation caused by the degenerated homograft had become severe. This case highlights the need for increased awareness of mitral-aortic intervalvular fibrosa pseudoaneurysms and their management.
Keywords: Aneurysm, false/complications/etiology/surgery; cardiac surgical procedures/methods; follow-up studies; heart valve prosthesis implantation/adverse effects; reoperation; treatment outcome; watchful waiting
The mitral-aortic intervalvular fibrosa (MAIVF) is a fibrous structure that connects the anterior mitral leaflet to the posterior portion of the aortic annulus between the left and right fibrous trigones. The histologic characteristics of this region make it particularly vulnerable to trauma from previous mitral or aortic valve surgery, endocarditis, or Takayasu arteritis.1–4 These events can lead to formation of a pseudoaneurysm that may remain stable for years, or enlarge progressively, eventually compressing nearby structures. Moreover, rupture of this type of pseudoaneurysm can be catastrophic. We describe the case of a patient whose pseudoaneurysm of the MAIVF needed surgical treatment years after aortic root replacement with a homograft, and we discuss the rationale for our clinical decisions.
Case Report
In July 2016, a 50-year-old man presented at our hospital with severe aortic regurgitation caused by a degenerated aortic homograft and a progressively enlarging pseudoaneurysm of the MAIVF, which had developed after aortic root replacement 13 years earlier. In that operation, an aortic valve damaged by Staphylococcus aureus endocarditis was replaced, and a homograft was inserted in the subcoronary position. In June 2003, 6 weeks later, a new episode of S. aureus infective endocarditis led to periannular abscess formation, so the previous homograft was removed and a new one was inserted as a free-standing root replacement. The patient was asymptomatic afterwards. In July 2004, routine echocardiograms showed a 26-mm pseudoaneurysm of the MAIVF. During the next few years, the size of the cavity did not change substantially, so the treatment team decided upon watchful waiting. However, after 7 years of follow-up, serial echocardiograms and cardiovascular magnetic resonance images showed gradual enlargement of the pseudoaneurysm, and progressive deterioration of the homograft was causing aortic regurgitation. In the 13th year of follow-up, the pseudoaneurysm had grown to 48 mm and bulged into the left atrial cavity (Fig. 1), and aortic regurgitation had become severe. Computed tomographic angiograms showed no coronary artery disease; however, the pseudoaneurysm substantially displaced the proximal left anterior descending and left circumflex coronary arteries (Fig. 2). Therefore, in July 2016, the patient was scheduled for a 3rd operation.
Fig. 1.
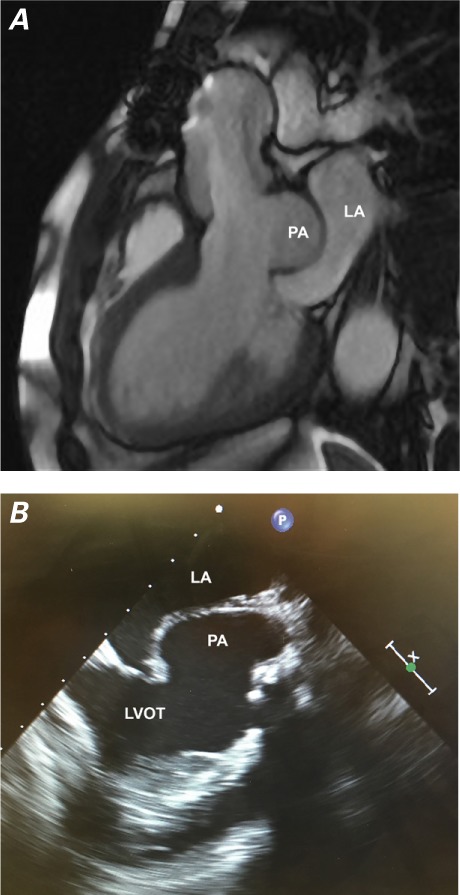
A) Cardiovascular magnetic resonance image and B) transesophageal echocardiogram show the pseudoaneurysm (PA) bulging into the left atrium (LA).
LVOT = left ventricular outflow tract
Fig. 2.
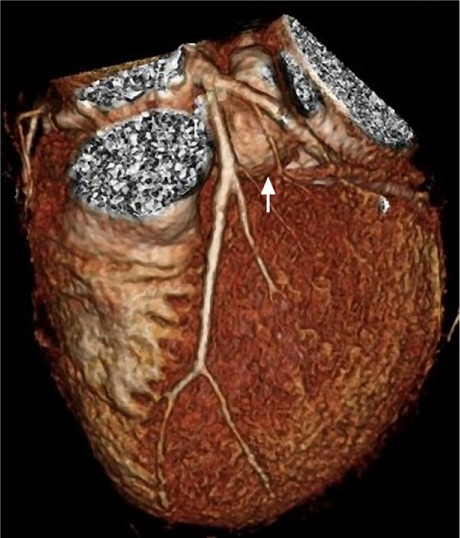
Three-dimensional computed tomogram shows the pseudoaneurysm (arrow) between the proximal left anterior descending and left circumflex coronary arteries.
We administered heparin, cannulated the femoral vein and artery, and established cardiopulmonary bypass before sternotomy. The patient was cooled to a temperature of 32 °C. A vent was inserted into the right superior pulmonary vein. The previously implanted homograft appeared to be severely calcified. After clamping the distal ascending aorta and infusing blood cardioplegic solution retrograde, we made a transverse aortotomy just superior to the distal suture line of the homograft. When we excised the deteriorated cusps of the homograft, the pseudoaneurysm was easily accessible (Fig. 3). No active endocarditis was evident.
Fig. 3.
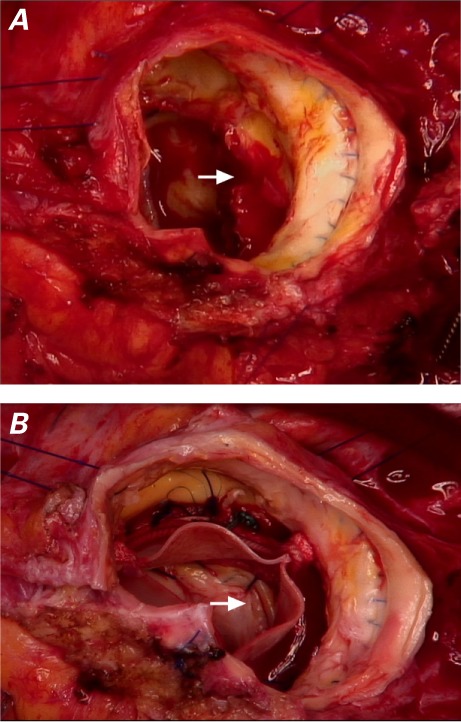
Intraoperative photographs show A) the opening of the pseudoaneurysm, seen through the homograft after cusp removal (arrow); and B) the closed pseudoaneurysm (arrow) after prosthesis implantation.
The opening of the pseudoaneurysm was between the anterior mitral leaflet and the portion of the aortic annulus corresponding to the commissure between the left coronary and noncoronary cusps of the aortic valve. We inserted a 27-mm Carpentier-Edwards Perimount aortic prosthesis (Edwards Lifesciences Corporation) inside the previously implanted homograft, and we excluded the pseudoaneurysm from the circulation by using interrupted 3-0 Ethibond Excel® sutures (Ethicon, a Johnson & Johnson company). Every pledgeted suture was passed through the mitral annulus, then through the aortic annulus of the homograft, and finally through the sewing ring of the aortic prosthesis to anchor it (Figs. 3 and 4). We then placed a Dacron prosthesis (Terumo Aortic) between the homograft and the ascending aorta, to achieve tension-free repair.
Fig. 4.
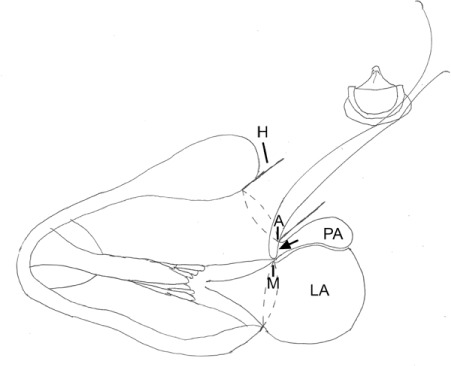
Diagram shows closure of the 8-mm opening of the pseudoaneurysm (arrow). To exclude the 48-mm pseudoaneurysm (PA), sutures were passed through the mitral annulus (M), homograft annulus (A), and bioprosthesis.
H = homograft; LA = left atrium
The patient recovered uneventfully and was discharged from the hospital 7 days after the procedure. A transthoracic echocardiogram one year later showed no pseudoaneurysm (Fig. 5).
Fig. 5.
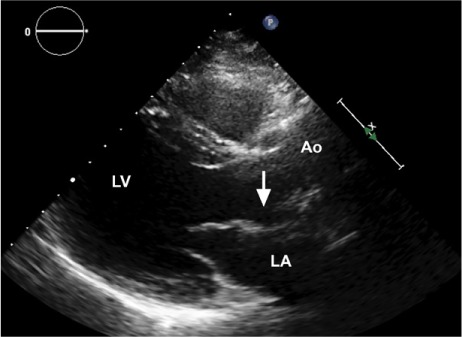
Transthoracic echocardiogram one year postoperatively shows the former site of the pseudoaneurysm (arrow).
Ao = aorta; LA = left atrium; LV = left ventricle
Discussion
The MAIVF, a fibrous structure that connects the mitral and aortic valves, is important to their anatomic and functional integrity. In most people, the MAIVF is shaped like a triangle, with its base positioned at the level of the anterior-medial mitral annulus, between the lateral and medial trigones.5 The MAIVF is susceptible to the formation of pseudoaneurysms, which can result from trauma and tears caused by suture traction during aortic or mitral valve surgery.1,2 In addition, pseudoaneurysms can develop consequent to infective endocarditis.3 Because the MAIVF is relatively avascular and histologically equivalent to the mitral valve leaflet structure,6 it offers little resistance to infections that can lead to formation of abscesses or pseudoaneurysms. Pseudoaneurysms associated with Takayasu arteritis have also been described.4
An MAIVF pseudoaneurysm can cause life-threatening conditions, such as rupture into the left atrium leading to left ventricular outflow tract-to-left atrial communication. Alternatively, rupture into the pericardial sac can cause cardiac tamponade. However, a pseudoaneurysm may remain relatively stable for a long time, appearing as a pulsating cavity with systolic expansion. If it grows, it may compress the pulmonary artery or the left coronary artery, causing angina. Embolization of a thrombus formed in a pseudoaneurysm of the MAIVF may cause stroke.7–9
Approaches to this pathologic condition depend on the presentation. Aggressive surgical treatment is not always necessary.2 In the absence of endocarditis, hemodynamic impairment, or periprosthetic leaks, conservative treatment of a stable pseudoaneurysm is reasonable.2 Gin and colleagues10 described a case of pseudoaneurysm that was managed conservatively for 9 years after its diagnosis.
Our patient had a history of aortic root replacement because of endocarditis. Although his pseudoaneurysm grew, he was asymptomatic until the previously implanted homograft deteriorated, causing severe aortic regurgitation and prompting additional surgery. Repeat surgery can be challenging when it involves replacing the aortic root in the presence of a degenerated and calcified homograft and closing an extensive pseudoaneurysm that displaces the left coronary artery. Accordingly, we initiated cardiopulmonary bypass through the femoral artery and vein before performing the sternotomy. We simplified the surgical procedure to avoid en bloc removal of the calcified homograft. The pseudoaneurysm was excluded through the homograft, and the new prosthesis was implanted inside the homograft with use of the same sutures.
Case-by-case analysis is necessary to select the optimal approach toward pseudoaneurysms of the MAIVF in high-risk patients who need reoperation. Our patient was managed conservatively for 13 years before surgery was deemed necessary. In addition, our surgical strategy enabled us to reduce the duration of the procedure, which would have been longer had we excluded the pseudoaneurysm with use of a patch and completely excised the homograft.
Acknowledgment
We thank Deborah Morris-Rosendahl, PhD (Head, Clinical Genetics and Genomics Service, Royal Brompton & Harefield NHS Foundation Trust, London), for her editing of the manuscript.
References
- 1.Spampinato RA, Borger MA, Strotdrees E, Mohr FW. Pseudoaneurysm of the mitral-aortic intervalvular fibrosa as a complication after minimally invasive mitral valve repair. Interact Cardiovasc Thorac Surg. 2013;16(3):396–8. doi: 10.1093/icvts/ivs502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grimaldi A, Ho SY, Pozzoli A, Sora N, Taramasso M, Benussi S et al. Pseudoaneurysm of mitral-aortic intervalvular fibrosa. Interact Cardiovasc Thorac Surg. 2011;13(2):142–7. doi: 10.1510/icvts.2011.269258. [DOI] [PubMed] [Google Scholar]
- 3.Yokoyama Y, Tamaki S, Kato N, Yokote J, Mutsuga M. Pseudoaneurysm from the mitral-aortic intervalvular fibrosa following endocarditis. Jpn J Thorac Cardiovasc Surg. 2003;51(8):374–7. doi: 10.1007/BF02719470. [DOI] [PubMed] [Google Scholar]
- 4.Tufekcioglu O, Ozlu MF, Cay S, Tuna F, Basar N, Gurel OM, Ozcan F. Pseudoaneurysm of the mitral-aortic intervalvular fibrosa in a patient with Takayasu's arteritis. Can J Cardiol. 2008;24(9):718. doi: 10.1016/s0828-282x(08)70675-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavalcanti JS, Vieira de Melo NC, Simoes de Vasconcelos R. Morphological study of the human mitral-aortic intervalvular fibrosa. Braz J Morphol Sci. 2005;22(1):37–40. Available from: https://pdfs.semanticscholar.org/56c9/6a9e88bd2eefba2d8653a4f86cc0b16c06ab.pdf. [Google Scholar]
- 6.Misfeld M, Sievers HH. Heart valve macro- and microstructure. Philos Trans R Soc Lond B Biol Sci. 2007;362(1484):1421–36. doi: 10.1098/rstb.2007.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koch R, Kapoor A, Spencer KT. Stroke in patient with an intervalvular fibrosa pseudoaneurysm and aortic pseudoaneurysm. J Am Soc Echocardiogr. 2003;16(8):894–6. doi: 10.1067/S0894-7317(03)00408-5. [DOI] [PubMed] [Google Scholar]
- 8.Ferreira R, Ferreira N, Melica B, Gonzaga A, Antunes M. Pseudoaneurism with systolic compressive effect on the left coronary artery: a rare complication after infective endocarditis. Eur Heart J Cardiovasc Imaging. 2015;16(10):1100. doi: 10.1093/ehjci/jev176. [DOI] [PubMed] [Google Scholar]
- 9.Apostolidou E, Beale C, Poppas A, Stockwell P. Pseudoaneurysm of the mitral-aortic intervalvular fibrosa: a case series with literature review. CASE (Phila) 2017;1(6):221–6. doi: 10.1016/j.case.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gin A, Hong H, Rosenblatt A, Black M, Ristow B, Popper R. Pseudoaneurysms of the mitral-aortic intervalvular fibrosa: survival without reoperation. Am Heart J. 2011;161(1):130.e1–5. doi: 10.1016/j.ahj.2010.09.013. [DOI] [PubMed] [Google Scholar]


