Abstract
Background:
Methyl methacrylate monomer of denture base resins was modified with several monomers to achieve better physico-mechanical properties without compromising the biocompatibility. However, there are no consensuses on the best strategy to achieve best modified monomer.
Purpose:
To identify and evaluate the differences in the properties between conventional and modified monomers and to verify the influence of several variables on the properties of denture base acrylic resin.
Materials and Methods:
This study was executed by following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. In-vitro studies that investigated the properties of conventional and modified monomers were selected. Searches were carried out in the Ebscohost, PubMed, Semantic scholar and J-stage databases. The search commenced from the year 1995 and the last search was done till November 2018. A comparison was performed between modified and unmodified monomers. The analyses were carried out using fixed-effect models.
Results:
The meta-analysis results showed high heterogeneity in all aspects, and higher flexural strength for monomers modified with 20% methacrylic acid.
Conclusion:
Although the articles included in this meta-analysis showed high heterogeneity and high risk of bias, the in-vitro literature seems to suggest that use of modified monomers could improve the properties of denture base resins. Other variants of monomer modifications and their tested parameters were discussed in this systematic review as well. Dimensional accuracy is an unexplored variable to be evaluated extensively in the future researches.
KEYWORDS: Monomer, Methyl-methacrylate, properties, biocompatibility
INTRODUCTION
The field of prosthetic dentistry is null without dental materials. Both are inextricably connected to each other since they have evolved. Prosthodontists are the ones who encounter with multitudinous materials. A good dentist can aptly identify and use any given material whereas a good prosthodontist is an authoritative chemist who has engulfed the science of reactions and manipulations of the materials. An eternal friendship between a prosthodontist and denture base acrylic resins exists.
Poly(methyl methacrylate) (PMMA) is the most commonly used denture base material for the past 60 years. Although numerous denture base polymers have been invented and introduced into dentistry to overcome issues related with PMMA resin, it has remained the material of choice for the fabrication of denture bases. It is the combination of various desirable properties of PMMA resin that accounts for the universal consumption though its properties are not ideal in every aspect.[1]
The limitations of heat-cured PMMA resin with respect to strength, peculiarly under impact and fatigue conditions, were investigated by several researchers.[2] The use of alternative materials with considerable improvement in strength would resist the denture fracture though it might be minimized by proper denture design and by following appropriate fabrication techniques.[3,4]
Also, the prosthesis installation within the oral cavity changes the bio-environment and paves the way for the deposition of biofilms on the prosthetic device.[5] The porous and irregular surfaces of acrylic resins favor microbial adhesion, accumulation, and colonization, which are determining agents in the majority of oral problems such as candidiasis, sore/burning mouth, and glossodynia.[6,7] PMMA resin with antimicrobial properties would be ideal to improve the patients’ quality of life and to impede discomfort with cost-effectiveness. Handful of studies have aimed at reducing or eliminating bacterial and fungal contamination by addition of antimicrobial agents[8,9] without compromising or rather deteriorating the mechanical properties of dental materials.
In the recent years, several modifications have been experimented in the denture base PMMA resins to improvise the physico-mechanical and biological properties. These modifications can be broadly classified into polymer and monomer modifications. The advances in polymer technology have produced novel polymers such as high-impact polymers and fiber-reinforced polymers with improved impact strength and fatigue resistance. The polymer modifications are achieved either by incorporating additives such as rubber,[10] styrene-butadiene,[11] Al2O3,[12] and β-AgVO3[13] or by adding inorganic fillers such as metallic oxides,[14] fluorinated glass fillers,[15] hydroxyapatite whiskers,[16] zirconia,[17] and organic fibers[18] of carbon/graphite, aramid, ultra-high modulus polyethylene, nylon, and jute. Reviews of studies regarding the polymeric modifications are available in the dental literature.[18,19,20,21] However, literature reviews on monomer modifications are not available yet.
The purpose of this study was to (1) systematically review the published researches pertaining to the modifications of denture base acrylic resin monomer, and (2) identify tests used for physico-mechanical and biological properties analysis.
MATERIALS AND METHODS
PICO model
Patient intervention comparison outcome model includes studies pertaining to denture base acrylic resins. Intervention was the quantitative modification of the monomer. Comparators were the conventional monomers without modifications. Outcomes were the effect of modified monomers on the properties of denture base acrylic resins.
Search strategy
The electronic search was independently performed by two reviewers. The databases used for search were EBSCOhost (http://web.a.ebscohost.com/ehost/publication), PubMed (www.ncbi.nlm. nih.gov. /entrez/query.fcgi), Semantic Scholar (https://www.semanticscholar.org/search), and J-STAGE (https://www.jstage.jst.go.jp). The present systematic review included research titles published from the year 1995 to 2018 in which comparison between unmodified and modified monomers was executed. On the basis of MeSH terms (PubMed), Boolean phrases (EBSCOhost), and other free-term keywords such as denture bases, monomers, polymethyl methacrylate, methacrylates, methylmethacrylate, methacrylic acid, denture base resins, denture base monomers, denture base modified monomers, modified denture bases, denture base co-monomer, monomer modification, anti-microbial monomer, anti-fungal monomer, denture base novel monomer, and denture base properties, the studies were assessed for the relevance. In case of any disagreement regarding the inclusion of a study, a third reviewer was consulted to achieve a consensus. Full texts of all relevant research studies that satisfied the inclusion criteria were downloaded. No blinding was carried out regarding the authors, journals, date of publication, or results.
Inclusion and exclusion criteria
Three main aspects were considered when reports were identified for inclusion: substratum, material, and testing methods. Eligible substrata consisted of modifications made in the monomer as liquid substitute and volume percentage of the monomer substitute. The exclusion criteria included studies pertaining to the monomers of light-cure composite resins, monomers of dental adhesives, pits and fissure sealants, and monomers of tissue conditioners and soft lining materials.
Data extraction
A data extraction criterion was defined and assessed by two authors. The data were extracted from the full-text articles selected independently. The authors classified the information on authors/year, monomer, its availability, monomer added to proprietary monomer (MMA) in percent, denture base materials, polymer/monomer (P/L) ratio, curing regimen, property studied, results, conclusions, and risk of bias.
Assessment of risk of bias
Risk of bias was evaluated according to the articles’ elucidation of the following parameters: randomization of samples/specimens, use of control group, use of materials according to the manufacturer’s instructions, preparation of samples according to ISO/ADA/ANSI standards with dimensions, sample preparation by the single operator, description of sample-size calculation, and blinding of the operator of the testing machine. Articles with one-to-three reported items were classified to possess high risk of bias, four or five reported items as medium risk of bias, and six or seven reported items as low risk of bias.[22,23,24]
Data synthesis
Considering the heterogeneity of the studies with respect to research design, methodology executed, sample sizes, storage, and polymerization method, only studies that met particular criteria were included in the meta-analysis. For flexural strength, the inclusion criteria were the following: moderate or high risk of bias; compliance with ISO standards for specimen dimension, and P/L ratio; reported means and standard deviations for the groups. The wt/vol% of substituted monomer (MAA) into the proprietary monomer (MMA) was kept constant (20%) to avoid heterogeneity. When the studies were similar in terms of these substrata, heterogeneity was further evaluated through the Q, I2, and τ2 tests.
The fixed-effect and random-effects models were considered appropriate for the meta-analysis of any quantitative data. However, considering the substrata, fixed-effect model was executed. In this review, it was possible to group four studies that evaluated the modified monomer (MAA). Other forms of evaluations such as publication bias and subgroup analysis were precluded because of insufficient number of included studies. All analyses were executed with statistical software (Comprehensive Meta-Analysis; version 3, Biostatistics).
RESULTS
Search and selection
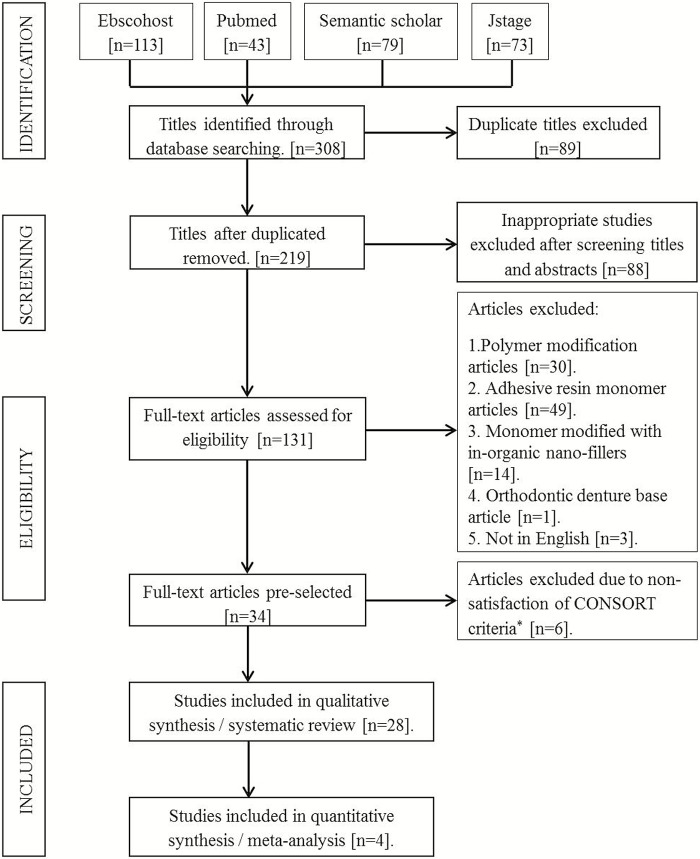
A total of 308 titles were obtained through the electronic search. Once duplicates, studies not relevant to the research question, and articles not in English were excluded; only 54 full-text articles were left. Thirty-four studies where the methodology for modification was not elucidated and monomer modifications were performed by incorporation of inorganic nanoparticle powder in monomer were further excluded. Finally, 20 studies were considered suitable for the present systematic review [Figure 1].[25] The exclusion criteria according to CONSORT* included incomplete description of materials, methods or details of the experimental procedure, not following standardized testing protocols, absence of sample size calculation, incomplete or inappropriate statistical analysis, no conflict of interests statement, limited interpretation, and comparison of results with other available dental literature.
Figure 1.
PRISMA flow chart
Risk of bias
From the 28 researches evaluating the properties of denture base acrylic resin with modified monomers, 17 researches presented a moderate risk of bias and only 11 presented high risk of bias. The most common risks of bias in the studies evaluating modified monomers were the sample randomization, sample preparation by a single operator, calculation for sample size, and blinding the operator of the testing machine. The studies evaluating modified monomers scored exclusively poor with respect to the sample randomization, sample preparation by a single operator, sample size calculations, and blinding protocols.
Meta-analysis
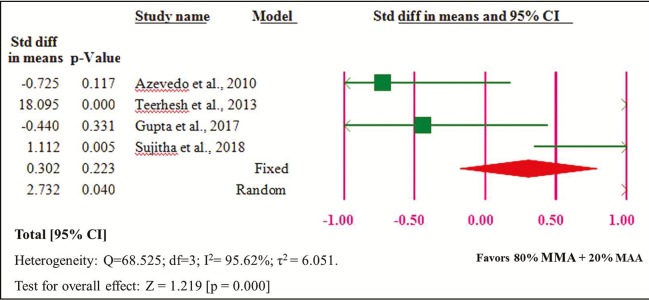
The 80% MMA + 20% MAA group showed significantly higher flexural strength than the 100% MMA group [Figure 2]. The heterogeneity of the four studies included in the meta-analysis was high (Q = 68.525, heterogeneity P = 0.000, I2 = 95.62%, τ2 = 6.051).
Figure 2.
Forest plot of comparison of 100% MMA and 80% MMA + 20% MAA
Descriptive analysis
From the included researches in the systematic review, the modification of MMA was done basically by certain monomers or solutions that can be categorized as fluoromonomers[26,27,28,29,30] (n = 5), phosphate monomers[31,32,33] (n = 3), methacrylic acid monomer[34,35,36,37,38,39] (n = 6), itaconate monomers[40,41,42] (n = 3), nitromonomers[5,43,44,45,46] (n = 5), and nonspecific monomers or solutions (n = 6). Nonspecific monomers include Norbonyl/phenyl methacrylate (NBMA/PHMA),[47] polyhedral oligosilsesquioxane (POSS),[48] methacrylated dendrimer (DD1),[49] 1,3-bis(3-methacryloxypropyl)-1,1,3,3 tetramethyl disiloxane (BMPMS),[50] silver nitrate (AgNO3) solution,[51] and N-acetyl cysteine (NAC).[52]
The monomers used to substitute with MMA (wt/vol%) were either experimentally synthesized[33,42,44,45,47,49,51] or purchased.[5,26,27,28,29,30,32,34,35,36,37,38,39,40,41,43,46,48,50,52] For the specimen preparation, the polymer beads were synthesized by bulk polymerization,[29,47,49,50] suspension polymerization,[26,27,28,33,34] or from the commercially available pre-polymers. The commercially available pre-polymers used were Lucitone 550,[30,35,43,45] Lucitone 199,[31,32] Paladent 20,[48] Trevalon,[36] Vertex,[37,44] DPI,[38,39] Ondacryl,[5] Ivoclar Vivadent,[51] Biocryl,[40,41,42] QC-20, Acron MC,[46] and Uniffasttrad.[52] Researches included in this review, based upon the polymerization process, used heat-cured PMMA resin,[29,30,31,32,35,36,37,38,39,40,41,42,43,44,45,46,47,48,50,51] autopolymerizing resin,[26,27,28,33,34,49,52] and microwave irradiation.[5,46]
From Table 1, it can be understood that a number of curing regimens were followed and executed for both heat-cured and microwave-irradiated specimen preparations. The activator used in autopolymerizing resin were AIBN,[26,27,28] N,N-dimethyl-p-toluidine,[33] and N,N-dimethyl aniline.[34] The substitution of the monomers to MMA in wt/vol% is critical. For perfluoroalkylethyl acrylate monomers, polymer beads were not formed beyond 25 wt%.[26,27,28] Also, the wt/vol% of substituted monomer affects the dough-forming time, which was recorded and mentioned in two[30,46] of the selected researches. In all the studies, the control group (0%) was used to compare with the monomer-substituted groups. A vivid wt/vol% of substituted monomers into the proprietary monomer was demonstrated. The most commonly executed wt/vol% of substituted monomer were 10%[29,30,31,32,33,34,35,37,39,40,41,42,44,46,47,50] and 20%.[29,30,31,32,33,34,35,36,38,39,46,47]
Table 1.
Summary of studies included in the systematic review
| Studies | Monomer and its property | Availability | Modified MMA in vol/wt % | Material, trade name and curing regimen | P/L ratio | Evaluated properties* | Results and conclusions |
|---|---|---|---|---|---|---|---|
| Hayashi et al.[26] (2003) | Perfluoro octylethyl acrylate (C8F) Hydrophobic monomer | Monomer: Purchased Polymer: Synthesized by suspension polymerization | 0 and 25 wt% | Heat-cured in presence of 0.045 g of α-α′-azobis isobutyronitrile (AIBN) at 70ºC for 6 hours and then at 100ºC for 0.8 hour | 2:1 | CA Tg KH TS MA | Increase in the CA and Tg. No significant difference in KH. Decrease in TS. C8F reduced the MA |
| Kubota et al.[27] (2005) | Perfluoro (butylethyl [C4F], hexylethyl [C6F], octylethyl [C8F], decylethyl [C10F]) acrylates Hydrophobic monomers | Monomer: Purchased. Polymer: Synthesized by suspension polymerization | 0 and 25 wt% | Heat-cured in presence of 0.015 wt% of AIBN at 70ºC for 6 hours and then at 100ºC for 0.8 hours | 2:1 | Tg KH TS | Increase in the carbon chain length increased Tg and decreased KH and TS. However, this research concluded that the fluoromonomer-added denture base resins are acceptable for clinical use with regard to mechanical properties |
| Kobayashi et al.[28] (2006) | C4F, C6F, C8F and C10F Hydrophobic monomers | Monomers: Purchased. Polymer: Synthesized by suspension polymerization | 0 and 25 wt% | Heat-cured in presence of 1.5 g of α-α′-azobis isobutyronitrile [AIBN] at 70ºC for 6 hours and then at 100ºC for 0.8 hours | 2:1 | CA MA | Increase in the carbon chain length increased the CA and decreased MA |
| Kurata et al.[29] (2007) | A. Fluoro-monomers: 1. α-Trifluoro methacrylic acid t-butyl [MAF-TBE]. 2. α-Trifluoro methacrylic acid 2-methyl-2-adamantyl (MAF-MAE). 3. 2-Trifluoro methyl norbornene-2-carboxylic acid t-butyl (MAF-TBN) B. Styrene monomers: 1. p-t-Butoxy styrene (PTBS). 2. p-Acetoxy styrene (PACS). 3. p-Ethoxy ethoxystyrene (PEES) |
Purchased | 0, 10, 20, 30 mol% | Bulk polymerization. Heat-cured at 51ºC for 24 hours in a water bath in the presence of 50 mg of BPO followed by 100ºC for 1 hour in a dry oven | N/A | CS FS WS | CS and FS decreased with increase in mol% of substituted monomers whereas MAF-TBN and PEES showed least values. Fluoro- and styrene monomers decreased the WS with increase in mol%. In all the tests, MAF-TBN at 30 mol% showed rubber-like consistency indicating poor polymerization characteristics |
| Cunha et al.[30] (2009) | Fluoralkyl methacrylate (FMA) Hydrophobic monomer | Purchased | 0, 10, 20 vol% | Lucitone 550 Heat-cured at 73°C for 90 minutes and 100°C for 30 minutes | 21 g/ 10 mL | FS YM Ra | Ra was unaffected. Addition of FMA decreased FS and YM regardless of the tested concentration. However, this decrease may be clinically acceptable |
| Dhir et al.[31] (2007) | Phosphate-containing monomer | Not mentioned | 0, 10 and 20 vol% | Lucitone 199 Heat-cured at 73°C for 90 minutes and 100°C for 30 minutes | 3:1 | FS WS solubility ColS and staining | Increase in the phosphate monomer concentration decreased the FS and increased WS and solubility. No difference was found with respect to ColS and staining |
| Puri et al.[32] (2008) | Ethylene glycol methacrylate phosphate (EGMP) | Purchased | 0, 10, 15, 20 vol% | Lucitone 199 Heat-cured at 74°C for 9 hours | 3:1 | IS FT CA | Addition of EGMP did not adversely affect IS and FT. However, CA decreased with increase in vol% |
| Raj et al.[33] (2011) | Methallyl phosphate (MAP) Antimicrobial monomer | Monomer: Synthesized Polymer: Synthesized by suspension/ bead polymerization | 0, 5, 10, 15, 20, 25% | Autopolymerized with 1.2 g of BPO and 0.75 mL of dimethyl para toluidine | N/A | Adhesion assay | MAP inhibited the adhesion and colonization of Candida albicans |
| Park et al.[34] (2003) | Methacrylic acid (MAA). Antimicrobial monomer | Purchased | 0, 5, 10, 20% | Autopolymerized with 1 wt % of BPO and 0.5 vol % of N,N- dimethyl aniline in water at 55 ± 1°C under air pressure of 300 kPa for 15 minutes | 3:1 | MA CA | MA and CA decreased with increasing concentrations of MAA |
| Azevedo et al.[35] (2010) | MAA Antimicrobial monomer | Purchased | 0, 10, 20, 50 vol % | Lucitone 550 Heat-cured at 73°C for 90 minutes and 100°C for 30 minutes | 21 g/ 10 mL | VHRaFS | At 50 vol%, VH decreased. A decreasing Ra was found in all concentrations and improved FS |
| Jain et al.[36] (2013) | MAA Antimicrobial monomer | Purchased | 0, 12, 16, 20 % | Trevalon Heat-cured according to manufacturer’s instruction | 3:1 | FS | FS decreased with increasing concentrations of MAA |
| Al-Ali et al.[37] (2015) | MAA, ethyl acrylate (EA) and butyl methacrylate (BMA) | Purchased. | 0, 5, 10, 15% | Vertex Heat-cured from room temperature to 100°C and maintained for 30 minutes | 22 g/ 10 mL | CP | All the monomers successfully co-polymerized with MMA at all concentrations |
| Gupta et al.[38] (2017) | MAA Antimicrobial monomer | Purchased | 0, 15, 20, 25% | DPI Heat-cured at 74°C for 8 hours and then at 100°C for 30 minutes | 2.5:1 | FS MA | Addition of MAA did not affect FS. However, with increase in concentration and number of days, decreased the CFU |
| Sujitha et al.[39] (2018) | MAA Antimicrobial monomer | Purchased | 0, 10, 20 vol% | DPI Heat-cured according to manufacturer’s instruction | 3:1 | FS, Ra | Decreased FS and improved Ra with increasing concentrations of MAA |
| Spasojević et al.[40] (2012) | Dimethyl itaconate (DMI) and Di-n-butyl itaconate (DBI) | DMI: Purchased DBI: Synthesized | 0, 2.5, 5.0, 7.5, 10 wt%. | Biocryl Heat-cured at 100°C for 30 minutes in the water bath | 2:1 | RM WS DC | RM decreased with increased concentrations of itaconates. WS decreased with increased concentrations of itaconates. DC increased for DBI at 7.5 and 10% |
| Spasojević et al.[41] (2015) | DMI and DBI | DMI: Purchased DBI: Synthesized | 0, 2.5, 5.0, 7.5, 10 wt% | Biocryl Heat-cured at 100°C for 30 minutes in the water bath | 2:1 | CP Tg TS SH IS | CP was confirmed with PMMA. Tg, TS and IS decreased with increased concentrations of itaconates. SH was unaffected with DMI whereas increased with increased concentration of DBI |
| Spasojević et al.[42] (2015) | Di-tetra hydrofurfuryl itaconate (DTHFI) | Synthesized | 0, 2.5, 5.0, 7.5, 10 wt% | Biocryl Heat-cured at 100°C for 30 minutes in the water bath | 2:1 | RM, CP, WS, Tg, TS, SH, IS | RM decreased with increased concentrations of itaconates. CP was confirmed with PMMA. WS decreased with increased concentrations of itaconates. Tg, TS and SH decreased with increased concentrations of itaconates. IS was unaffected. |
| Paleari et al.[43] (2011) | 2-tert-butyl aminoethyl methacrylate (TBAEMA) Antimicrobial monomer | Purchased | 0.00, 0.50, 1.00, 1.50, 1.75, 2.00% | Lucitone 550 Heat-cured at 73°C for 90 minutes and 100°C for 30 minutes | 21 g/10mL | FS | Increasing TBAEMA concentrations reduced the FS |
| Rodriguez et al.[5] (2013) | TBAEMA Antimicrobial monomer | Purchased | 0, 1, 2, 3, 4% | Onda-cryl Microwave curing. Cured for 3 minutes at 320 W + 4 minutes at 0 W + 3 minutes at 720 W | 14 g/ 7 mL | N2 ratio CP Tg FS | Amine group indicates antimicrobial activity. TBAEMA co-polymerizes with MMA. Both Tg and FS decreased with increased concentration of TBAEMA |
| Mirizadeh et al.[44] (2018) | N,N-dimethyl aminoethyl methacrylate- octyl bromide (DMAEMA-OB). Quaternized ammonium antimicrobial monomer (QAM) | Synthesized | 0, 8, 10, 12 wt% | Vertex Heat-cured at 70ºC for 1 hour and at 100ºC for 2 hours (dry heat) | 50 g/ 25 mL | CFU (direct contact test) FS WS | QAM was significantly effective against gram- positive and gram-negative bacteria and fungi. FS decreased with increasing concentrations of QAM. WS increased with increasing concentrations of QAM |
| Regis et al.[45] (2011) | Methacryloyl oxyundecyl pyridinium bromide (MUPB) Antimicrobial monomer | Synthesized | 0.0, 0.3, 0.6 wt% | Lucitone 550 Heat-cured at 73°C for 90 minutes and 100°C for 30 minutes | 21g/ 10mL | VH FS Ra ColS | Reinforcing MUPB with MMA did not alter the VH. FS significantly decreased at 0.6% concentration. Ra ColS were not affected at 0.3 or 0.6% |
| Aydogan Ayaz and Durkan .[46] (2013) | Acrylamide monomer (AA) | Purchased | 0, 5, 10, 15, 20%. | QC 20 Heat-cured in a thermally controlled autoclave device at 60°C for 30 minutes, and further cured at 130°C for 20 minutes. Acron MC: Microwave irradiation at 500 W for 3 minutes | N/A | FS FM CP | FS and FM increased with increased concentrations of AA. Successful copolymerization of AA with PMMA |
| Umemoto and Kurata.[47] (1997) | Norbonyl methacrylate (NBMA) and phenyl methacrylate (PHMA). Both are hydrophobic monomers | Synthesized | 0, 10, 20, 30, 40 wt% | Bulk polymerization Heat-cured at 55ºC for 24 hours in water bath in the presence of 0.5 wt% of benzoyl peroxide (BPO) followed by 100ºC for 1 hour in a dry oven | N/A | CS WS FS DMTA PS | Addition of NBMA and PHMA to MMA decreased WS and improved mechanical properties. Showed less PS than PMMA |
| Kim et al.[48] (2007) | Polyhedral oligosil sesquioxane (POSS) | Purchased | 0 and 1.44% | Paladent 20 Curing regimen not specified | N/A | In vitro cytotoxicity tests: 1. MTT assay 2. Agar overlay test 3. Mutagenesis assay | In all assays, addition of POSS with MMA showed less cytotoxicity than the control |
| Kawaguchi et al.[49] (2011) | Methacrylated dendrimer (DD1) compared with ethylene glycol di methacrylate (EGDMA) Cross-linker | Monomer: Synthesized Polymer: Synthesized | 1.1, 2.3, 4.6, 6.9, 9.1 vol% of both monomers | Bulk polymerization 2% BPO was added to powder and mixed with monomer and polymerized in distilled water at 55°C under pressure of 0.4 MPa for 20 minutes | 10 g/ 7 mL | FSFMVH | Addition of DD1 improved only FM. FS and VH remained unaffected. The effect of vol % was significant only on FS |
| Aoyagi et al.[50] (2012) | 1,3-bis(3-methacryloxy propyl)-1,1,3,3- tetramethyl disiloxane (BMPMS) | Purchased | 0, 10, 30, 50, 70 mol% | Bulk polymerization Heat-cured at 53ºC for 5 days in a water bath in presence of 0.5 mass% of BPO followed by 100ºC for 1 hour in dry oven | N/A | WS WSol | WS and W Sol decreased with increasing concentrations of BMPMS |
| Al-Husayni et al.[51] (2014) | Silver nitrate (AgNO3) | Synthesized | 9.375, 15, 30, 60, 120, 150, 300, 600, 900 ppm | Ivoclar vivadent Heat-cured. Regimen not specified | 2.25 g/LmL | IS FS TS | 1. IS was high at 60 ppm. In the rest of concentrations, IS was not affected. 2. FS and TS decreased with increase in ppm. 3. Discoloration was observed at 300 ppm |
| Jiao et al.[52] (2015) | N-acetyl cysteine (NAC) | Purchased | 0, 0.15, 0.3, 0.6, 0.9 wt% | Unifast trad. Autopolymerized resin | 1 g per 0.5 mL | MTT assay DegC FS VH Ra | Higher cell viability with NAC. DegC reduced except at 0.15% of NAC. FS decreased with increasing concentrations of NAC. VH was unaffected at 0.15% beyond which it decreased. Ra was affected at a concentration beyond 0.3% |
*CS = compressive strength, WS = water sorption, FS = flexural strength, DMTA = dynamic mechanical thermal analysis, PS = polymerization shrinkage, CA = contact angle, Tg = glass transition temperature, KH = knoop hardness, TS = tensile strength, MA = microbial adhesion, MTT = tetrazolium, ColS = color stability, FT = fracture toughness, YM = Young’s modulus, Ra = surface roughness, VH = Vicker hardness, ZI = zone of inhibition, TB= = Trypan blue, FM = flexure modulus, WSol = water solubility, CP = co-polymerization, CFU = colony-forming unit, RM= residual monomer, DC = diffusion coefficient, SH = shoreD hardness; DegC = degree of conversion
Vivid description about the testing methodologies of the properties in each of the studies was provided. All the aspects of the properties of the denture base resins were studied. However, the polymerization shrinkage, which is the commonest troubleshoot, has been studied only in one study.[47] Every single study included here followed the standardized testing protocols, inclusive of specimen dimensions, stipulated for denture base resins.
DISCUSSION
Fluoromonomers have an inherent property of “water-shedding” because of the fluorine ions; thus they possess hydrophobicity and have been exploited in the field of prosthetic dentistry. This is a novel approach by substituting fluoromonomers in MMA and reducing the water sorption,[29] less acute contact angle,[26,28] and thus decreased microbial adhesion especially Candidal adhesion as far as denture stomatitis is concerned.[26,28] These monomers were also incorporated in the proprietary monomers of denture soft/resilient liners[53,54] to reduce the water sorption and solubility in oral environment and to curb the Candidal adhesion. Nevertheless, the effect of fluoromonomers on glass transition temperature[26,27] (Tg), surface hardness,[26,27] surface roughness[30] (Ra), tensile,[26,27] compressive,[29] and flexural strength[29,30] were also investigated. Though this addition causes some deterioration to the mechanical properties, it can be acceptable for clinical use. Extensive biocompatibility assay has to be executed with this monomer.
Addition of phosphate monomers to MMA caused increased water sorption, solubility,[31] and more acute contact angles.[32] From these results, it is inferred that these monomers are hydrophilic in nature. Performance of these monomers with respect to color stability and staining was acceptable. Though there is slight decrease in the mechanical properties such as flexural strength, impact strength, and fracture toughness, there were no devastating effects stating its limitations in clinical usage. However, it is also said to be an antifungal monomer by inhibiting the adhesion and colonization of Candida albicans.[33] Moreover, there is a huge lacuna with respect to the biocompatibility assays for these groups of monomers.
Methacrylic acid (MAA) is primarily an antimicrobial monomer substituted in MMA. MAA decreased the microbial adhesion[34,38] and also had more acute contact angle.[34] The hydrophilicity of this monomer has to be extensively studied with respect to water sorption and solubility. Substitution of MAA improved the surface roughness.[35,39] Chemical characterization and copolymerization was demonstrated when added to MMA.[37] MAA caused reduction in surface hardness.[35] With respect to flexural strength, antonymous conclusions were demonstrated stating both increased[35,38] and decreased[36,39] flexural strength (FS) upon MAA substitution. This monomer is also open for the future impact strength and biocompatibility tests.
Evidence of copolymerization was demonstrated when itaconate monomers were added to MMA. Residual monomer content was significantly reduced with the substitution of itaconate monomers in MMA. This is due to the increase in degree of conversion of the monomers to polymeric units.[40,42] Water sorption increased with increasing concentrations of itaconate monomers.[40,42] Glass transition temperature and tensile strength decreased with itaconates. Dimethyl itaconate compromised the impact strength[41] and di-tetrahydro furfuryl itaconate reduced the surface hardness.[42] Light has to be thrown with respect to flexural strength and biocompatibility assays with these monomers.
Nitromonomer is a new term introduced here for the convenience of explanation about some nitrogen-containing monomers in the form of either amino/ammonium/pyridinium or amides. These monomers can be also tagged as antimicrobial monomers. High N2 ratio and copolymerization were demonstrated when 2-tert-butyl aminoethyl methacrylate (TBAEMA) was added to MMA.[5] Reduced flexural strength, Tg, and increased water sorption at raised concentrations were also observed.[5,44] Quaternized ammonium monomer (DMAEMA-OB) was effective against gram-positive and gram-negative bacteria and fungi.[44] Another quaternized ammonium anti-microbial monomer (QAM), 2-(methacryloyloxy) ethyl trimethyl ammonium chloride (MADQUAT), was proved to be an efficient antifungal monomer possessing both fungistatic and -cidal actions.[55] However, its copolymerization with MMA, physico-mechanical properties, and biocompatibility are yet unknown.
Numerous novel QAMs were synthesized and were experimented with dental restorative composite and adhesive resins. Dimethylaminododecyl methacrylate (DMADDM) and dimethylammoniumethyl dimethacrylate (DMAEDM) were found to possess “time-kill” antibacterial properties. Both monomers had significantly lesser cytotoxicity than the bisphenol A-glycidyl methacrylate (Bis-GMA).[56] DMADDM was proved to be an antimicrobial denture base monomer against the formation of multispecies biofilm on the denture surfaces. It changed the structure of multi-biofilm and inhibited the biofilm metabolic activities, hyphal development, and virulence-related genes’ expression of C. albicans. It also reduced the Candidal receptors on the host cells, thus reducing cell damage as well as being biocompatible at a concentration of 1.65–3.3%.[57] Methacryloxyethyl cetyl dimethyl ammonium chloride (DMAE-CB) can be used as an antibacterial monomer by incorporating into dental restorative materials.[58] Unpolymerized 2-methacryloxyethyl dodecyl methyl ammonium bromide (MAE-DB) and 2-methacryloxyethyl hexadecyl methyl ammonium bromide (MAE-HB) cross-linking monomers showed a strong bactericidal activity against eight different strains of oral bacteria. They had significantly lesser cytotoxicity than that of Bis-GMA.[59] These monomers have a unique property of “contact-kill.” Any microorganism that comes in contact with the denture base containing QAM gets killed. QAM intrinsically possessing positive charge has high affinity toward negatively charged cell membrane; it disrupts the lipid bilayer, leads to leakage of intracellular accessories, and eventually results in microbial death. They can be also designated as cationic monomers. These QAMs, however, have a wide scope of research in developing newer denture base resins.
Two pyridinium-based nitromonomers were used in dentistry. Methacryloyl oxyundecyl pyridinium bromide (MUPB) added to MMA reduced the flexural strength, whereas surface roughness, hardness, and color stability were unaffected.[45] Methacryloyloxy-dodecyl-pyridinium-bromide (MDPB) is being extensively used in the field of research pertained to restorative resins as bactericidal monomer against oral bacteria. The cytotoxicity was similar to those of other monomers used in dental materials.[60] Acrylamide monomer in MMA increased the flexural strength and modulus by copolymerizing with PMMA.[46]
NBMA and PHMA are hydrophobic monomers that reduced water sorption and improved the mechanical properties. NBMA with MMA showed less polymerization shrinkage, and this may be attributed to the fact that norbonyl compounds polymerize through ring-opening metathesis polymerization (ROMP).[47] POSS with MMA at all experimental concentrations yielded lesser cytotoxicity than the PMMA.[48] DD1 with MMA did not affect the flexural strength and surface hardness.[49] Water sorption and solubility decreased significantly with the addition of BMPMS with MMA, and thus, can be considered as hydrophobic monomer.[50] Substituting AgNO3 solution with MMA increased impact strength at 60 ppm concentration. Discoloration was observed at 300 ppm. This is because of the oxidative reaction of Ag with the atmosphere. With increase in the concentration, the flexural and tensile strength decreased.[51] NAC is a cellular friendly product, and hence, showed higher cell viability when added to MMA. Surface roughness and hardness were affected at a concentration beyond 0.3% and 0.15%, respectively. Flexural strength decreased with increasing concentrations of NAC.[52]
In this systematic review, the selected studies evaluated only the experimental monomers and commercially available products using these monomers are yet to be launched in the market.
LIMITATIONS
Cautious interpretations of the results of the present review are mandatory because in vitro laboratory experiments have intrinsic limitations while trying to simulate oral conditions such as masticatory loads, masticatory cycles, temperature fluctuations, microbial flora, and salivary flow rate. Properly designed randomized controlled trials with long follow-up duration are essential to deduce at a conclusion as to whether monomer modifications will result in improved clinical success rates when compared with conventional PMMAs. None of the studies included have a complete research about a monomer, especially with respect to dimensional accuracy of denture base.
CONCLUSION
Monomer modification improved the physico-mechanical properties and biocompatibility of the denture base resins.
Though some monomers compromised specific properties, their performance in the clinical scenario is well accepted.
Evaluation of dimensional stability/accuracy should be given high priority because it leads to several problems such as pain, tissue irritation, hyperplastic tissues, and occlusal errors.
Numerous explicit researches to be undertaken to develop an ideal denture base resin because commercially available products do not suffice the geriatric population.
Numerous antimicrobial monomers used with restorative resins shall be experimented with denture base resins to reduce microbial adhesion.
Combination of two or more monomers of different inherent properties may be attempted and patented for commercial manufacture.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.al-Mulla MA, Murphy WM, Huggett R, Brooks SC. Effect of water and artificial saliva on mechanical properties of some denture-base materials. Dent Mater. 1989;5:399–402. doi: 10.1016/0109-5641(89)90108-5. [DOI] [PubMed] [Google Scholar]
- 2.Anderson GC, Schulte JK, Arnold TG. Dimensional stability of injection and conventional processing of denture base acrylic resin. J Prosthet Dent. 1988;60:394–8. doi: 10.1016/0022-3913(88)90292-2. [DOI] [PubMed] [Google Scholar]
- 3.Anthony DH, Peyton FA. Evaluating dimensional accuracy of denture bases with a modified comparator. J Prosthet Dent. 1959;9:683–92. [Google Scholar]
- 4.Anthony DH, Peyton FA. Dimensional accuracy of various denture-base materials. J Prosthet Dent. 1962;12:67–81. [Google Scholar]
- 5.Rodriguez LS, Paleari AG, Giro G, de Oliveira Junior NM, Pero AC, Compagnoni MA. Chemical characterization and flexural strength of a denture base acrylic resin with monomer 2-tert-butylaminoethyl methacrylate. J Prosthodont. 2013;22:292–7. doi: 10.1111/j.1532-849X.2012.00942.x. [DOI] [PubMed] [Google Scholar]
- 6.Valentini F, Luz MS, Boscato N, Pereira-Cenci T. Biofilm formation on denture liners in a randomised controlled in situ trial. J Dent. 2013;41:420–7. doi: 10.1016/j.jdent.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Wady AF, Machado AL, Zucolotto V, Zamperini CA, Berni E, Vergani CE. Evaluation of Candida albicans adhesion and biofilm formation on a denture base acrylic resin containing silver nanoparticles. J Appl Microbiol. 2012;112:1163–72. doi: 10.1111/j.1365-2672.2012.05293.x. [DOI] [PubMed] [Google Scholar]
- 8.Fan C, Chu L, Rawls HR, Norling BK, Cardenas HL, Whang K. Development of an antimicrobial resin—A pilot study. Dent Mater. 2011;27:322–8. doi: 10.1016/j.dental.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Gjorgievska E, Apostolska S, Dimkov A, Nicholson JW, Kaftandzieva A. Incorporation of antimicrobial agents can be used to enhance the antibacterial effect of endodontic sealers. Dent Mater. 2013;29:e29–34. doi: 10.1016/j.dental.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Jagger DC, Harrison A, Jandt KD. The reinforcement of dentures. J Oral Rehabil. 1999;26:185–94. doi: 10.1046/j.1365-2842.1999.00375.x. [DOI] [PubMed] [Google Scholar]
- 11.Rodford R. The development of high impact strength denture-base materials. J Dent. 1986;14:214–7. doi: 10.1016/0300-5712(86)90004-7. [DOI] [PubMed] [Google Scholar]
- 12.Yadav NS, Elkawash H. Flexural strength of denture base resin reinforced with aluminium oxide and processed by different processing techniques. J Adv Dent Res. 2011;2:33–6. [Google Scholar]
- 13.de Castro DT, Valente ML, Agnelli JA, Lovato da Silva CH, Watanabe E, Siqueira RL, et al. In vitro study of the antibacterial properties and impact strength of dental acrylic resins modified with a nanomaterial. J Prosthet Dent. 2016;115:238–46. doi: 10.1016/j.prosdent.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Nuñez-Anita RE, Acosta-Torres LS, Vilar-Pineda J, Martínez-Espinosa JC, de la Fuente-Hernández J, Castaño VM. Toxicology of antimicrobial nanoparticles for prosthetic devices. Int J Nanomedicine. 2014;9:3999–4006. doi: 10.2147/IJN.S63064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Bakri IA, Harty D, Al-Omari WM, Swain MV, Chrzanowski W, Ellakwa A. Surface characteristics and microbial adherence ability of modified polymethylmethacrylate by fluoridated glass fillers. Aust Dent J. 2014;59:482–9. doi: 10.1111/adj.12218. [DOI] [PubMed] [Google Scholar]
- 16.Pan Y, Liu F, Xu D, Jiang X, Yu H, Zhu M. Novel acrylic resin denture base with enhanced mechanical properties by incorporation of PMMA-modified hydroxyapatite. Mater Int. 2013;23:89–93. [Google Scholar]
- 17.Ayad NM, Badawi MF, Fatah AA. Effect of reinforcement of high-impact acrylic resin with zirconia on some physical and mechanical properties. Rev Clín Pesq Odontol. 2008;4:145–51. [Google Scholar]
- 18.Vallittu PK. A review of fiber-reinforced denture base resins. J Prosthodont. 1996;5:270–6. doi: 10.1111/j.1532-849x.1996.tb00511.x. [DOI] [PubMed] [Google Scholar]
- 19.Alla RK, Sajjan S, Alluri VR, Ginjupalli K, Upadhya N. Influence of fiber reinforcement on the properties of denture base resins. J Biomater Nanobiotechnol. 2013;4:91–7. doi: 10.4236/jbnb.2013.41012. [Google Scholar]
- 20.Vallittu PK. A review of methods used to reinforce polymethyl methacrylate resin. J Prosthodont. 1995;4:183–7. doi: 10.1111/j.1532-849x.1995.tb00338.x. [DOI] [PubMed] [Google Scholar]
- 21.Gad MM, Fouda SM, Al-Harbi FA, Näpänkangas R, Raustia A. PMMA denture base material enhancement: A review of fiber, filler, and nanofiller addition. Int J Nanomedicine. 2017;12:3801–12. doi: 10.2147/IJN.S130722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maia LC, Antonio AG. Systematic reviews in dental research: A guideline. J Clin Pediatr Dent. 2012;37:117–24. doi: 10.17796/jcpd.37.2.h606137vj3826v61. [DOI] [PubMed] [Google Scholar]
- 23.Astudillo-Rubio D, Delgado-Gaete A, Bellot-Arcís C, Montiel-Company JM, Pascual-Moscardó A, Almerich-Silla JM. Mechanical properties of provisional dental materials: A systematic review and meta-analysis. PLos One. 2018;13:e0193162. doi: 10.1371/journal.pone.0196264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarkis-Onofre R, Skupien JA, Cenci MS, Moraes RR, Pereira-Cenci T. The role of resin cement on bond strength of glass-fiber posts luted into root canals: a systematic review and meta-analysis of in vitro studies. Oper Dent. 2014;39:E31–44. doi: 10.2341/13-070-LIT. [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA statement. J Clin Epidemiol. 2009;62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi R, Kubota T, Mega J. Application of fluoroalkyl acrylate monomer for a denture base material. Int J Oral-Med Sci. 2003;1:124–9. [Google Scholar]
- 27.Kubota T, Kobayashi M, Hayashi R, Ono A, Mega J. Influence of carbon chain length of fluorinated alkyl acrylate on mechanical properties of denture base resin. Int J Oral-Med Sci. 2005;4:92–6. [Google Scholar]
- 28.Kobayashi M, Kubota T, Mega J. Application of fluorinated alkyl acrylate to denture base resin. Influence of carbon chain length of fluorinated alkyl acrylate on bacterial adherence. Int J Oral-Med Sci. 2006;4:136–41. [Google Scholar]
- 29.Kurata S, Morishita K, Shimoyama K, Umemoto K. Basic study on the application of novel functional monomers to a denture base resin. Dent Mater J. 2008;27:273–7. doi: 10.4012/dmj.27.273. [DOI] [PubMed] [Google Scholar]
- 30.Cunha TR, Regis RR, Bonatti MR, de Souza RF. Influence of incorporation of fluoroalkyl methacrylates on roughness and flexural strength of a denture base acrylic resin. J Appl Oral Sci. 2009;17:103–7. doi: 10.1590/S1678-77572009000200006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhir G, Berzins DW, Dhuru VB, Periathamby AR, Dentino A. Physical properties of denture base resins potentially resistant to Candida adhesion. J Prosthodont. 2007;16:465–72. doi: 10.1111/j.1532-849X.2007.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puri G, Berzins DW, Dhuru VB, Raj PA, Rambhia SK, Dhir G, et al. Effect of phosphate group addition on the properties of denture base resins. J Prosthet Dent. 2008;100:302–8. doi: 10.1016/S0022-3913(08)60210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raj PA, Dentino AR. New phosphated poly(methyl methacrylate) polymers for the prevention of denture-induced microbial infection: An in vitro study. Clin Cosmet Investig Dent. 2011;3:25–32. doi: 10.2147/CCIDEN.S16860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park SE, Periathamby AR, Loza JC. Effect of surface-charged poly(methyl methacrylate) on the adhesion of Candida albicans. J Prosthodont. 2003;12:249–54. doi: 10.1016/s1059-941x(03)00107-4. [DOI] [PubMed] [Google Scholar]
- 35.Azevedo AM, Regis RR, Chaves CAL, Souza RF, Fernandes RM. Physical properties of a denture base acrylic resins after incorporation of anionic charges. Rev Odontol Bras Central. 2010;19:290–4. [Google Scholar]
- 36.Jain T, Yadav NS, Pandita A, Feroz SM, Kartika UK, Singh PP. A comparative evaluation of flexural strength of commercially available acrylic and modified polymethylmethacrylate: An in vitro study. J Contemp Dent Pract. 2013;14:80–3. doi: 10.5005/jp-journals-10024-1275. [DOI] [PubMed] [Google Scholar]
- 37.Al-Ali AAS, Kassab-Bashi TY. Fourier transform infra red (FTIR) spectroscopy of new copolymers of acrylic resin denture base materials. Int J Enhanced Res Sci Tech Eng. 2015;4:172–80. [Google Scholar]
- 38.Gupta L, Aparna IN, Bhat S, Ginjupalli K. Effect of comonomer of methacrylic acid on flexural strength and adhesion of Staphylococcus aureus to heat polymerized poly (methyl methacrylate) resin: An in vitro study. J Indian Prosthodont Soc. 2017;17:149–55. doi: 10.4103/jips.jips_257_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sujitha K, Bharathi M, Lakshminarayana S, Shareef A, Lavanya B, SivKumar V. Physical properties of heat cure denture base resin after incorporation of methacrylic acid. Contemp Clin Dent. 2018;9:251–5. doi: 10.4103/ccd.ccd_172_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spasojević P, Stamenković D, Pjanović R, Bošković-Vragolović N, Dolić J, Grujić S, et al. Diffusion and solubility of commercial poly(methyl methacrylate) denture base material modified with dimethyl itaconate and di-n-butyl itaconate during water absorption/desorption cycles. Polym Int. 2012:61. doi: 10.1002/pi.4202. [Google Scholar]
- 41.Spasojević P, Zrilić M, Panic V, Stamenkovic D, Seslija S, Velickovic S. The mechanical properties of a poly(methyl methacrylate) denture base material modified with dimethyl itaconate and di-n-butyl itaconate. Int J Polym Sci. 2015:1–9. doi: 10.1155/2015/561012. [Google Scholar]
- 42.Spasojević P, Panić V, Šešlija S, Nikolić V, Popović IG, Velicković S. Poly(methyl methacrylate) denture base materials modified with ditetrahydro furfuryl itaconate: Significant applicative properties. J Serb Chem Soc. 2015;80:1177–92. [Google Scholar]
- 43.Paleari AG, Marra J, Pero AC, Rodriguez LS, Ruvolo-Filho A, Compagnoni MA. Effect of incorporation of 2-tert-butylaminoethyl methacrylate on flexural strength of a denture base acrylic resin. J Appl Oral Sci. 2011;19:195–9. doi: 10.1590/S1678-77572011000300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mirizadeh A, Atai M, Ebrahimi S. Fabrication of denture base materials with antimicrobial properties. J Prosthet Dent. 2018;119:292–8. doi: 10.1016/j.prosdent.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 45.Regis RR, Zanini AP, Della Vecchia MP, Silva-Lovato CH, Oliveira Paranhos HF, de Souza RF. Physical properties of an acrylic resin after incorporation of an antimicrobial monomer. J Prosthodont. 2011;20:372–9. doi: 10.1111/j.1532-849X.2011.00719.x. [DOI] [PubMed] [Google Scholar]
- 46.Aydogan Ayaz E, Durkan R. Influence of acrylamide monomer addition to the acrylic denture-base resins on mechanical and physical properties. Int J Oral Sci. 2013;5:229–35. doi: 10.1038/ijos.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Umemoto K, Kurata S. Basic study of a new denture base resin applying hydrophobic methacrylate monomer. Dent Mater J. 1997;16:21–30. doi: 10.4012/dmj.16.21. [DOI] [PubMed] [Google Scholar]
- 48.Kim SK, Heo SJ, Koak JY, Lee JH, Lee YM, Chung DJ, et al. A biocompatibility study of a reinforced acrylic-based hybrid denture composite resin with polyhedraloligosilsesquioxane. J Oral Rehabil. 2007;34:389–95. doi: 10.1111/j.1365-2842.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 49.Kawaguchi T, Lassila LV, Vallittu PK, Takahashi Y. Mechanical properties of denture base resin cross-linked with methacrylated dendrimer. Dent Mater. 2011;27:755–61. doi: 10.1016/j.dental.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 50.Aoyagi Y, Umemoto K, Kurata S. Chemical properties of 1,3-bis(3-methacryloxypropyl)-1,1,3,3-tetramethyldisiloxane-methyl methacrylate copolymer. Dent Mater J. 2012;31:215–8. doi: 10.4012/dmj.2009-121. [DOI] [PubMed] [Google Scholar]
- 51.Al-Husayni OKA, Hatoor NAF. Effect of silver nitrate incorporation into heat polymerized acrylic resin on some mechanical properties. J Bagh College Dentistry. 2014;26:78–85. [Google Scholar]
- 52.Jiao Y, Ma S, Li J, Shan L, Yang Y, Li M, et al. The influences of N-acetyl cysteine (NAC) on the cytotoxicity and mechanical properties of poly-methylmethacrylate (PMMA)-based dental resin. PeerJj. 2015;3:e868. doi: 10.7717/peerj.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hayakawa I, Akiba N, Keh E, Kasuga Y. Physical properties of a new denture lining material containing a fluoroalkyl methacrylate polymer. J Prosthet Dent. 2006;96:53–8. doi: 10.1016/j.prosdent.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 54.Yoshida K, Kurogi T, Torisu T, Watanabe I, Murata H. Effects of 2,2,2-trifluoroethyl methacrylate on properties of autopolymerized hard direct denture reline resins. Dent Mater J. 2013;32:744–52. doi: 10.4012/dmj.2013-103. [DOI] [PubMed] [Google Scholar]
- 55.Stopiglia CD, Collares FM, Ogliari FA, Piva E, Fortes CB, Samuel SM, et al. Antimicrobial activity of [2-(methacryloyloxy)ethyl]trimethylammonium chloride against Candida spp. Rev Iberoam Micol. 2012;29:20–3. doi: 10.1016/j.riam.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 56.Li F, Weir MD, Fouad AF, Xu HH. Time-kill behaviour against eight bacterial species and cytotoxicity of antibacterial monomers. J Dent. 2013;41:881–91. doi: 10.1016/j.jdent.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang K, Ren B, Zhou X, Xu HH, Chen Y, Han Q, et al. Effect of antimicrobial denture base resin on multi-species biofilm formation. Int J Mol Sci. 2016;17:1033. doi: 10.3390/ijms17071033. doi:10.3390/ijms1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiao YH, Chen JH, Fang M, Xing XD, Wang H, Wang YJ, et al. Antibacterial effects of three experimental quaternary ammonium salt (QAS) monomers on bacteria associated with oral infections. J Oral Sci. 2008;50:323–7. doi: 10.2334/josnusd.50.323. [DOI] [PubMed] [Google Scholar]
- 59.Huang L, Xiao YH, Xing XD, Li F, Ma S, Qi LL, et al. Antibacterial activity and cytotoxicity of two novel cross-linking antibacterial monomers on oral pathogens. Arch Oral Biol. 2011;56:367–73. doi: 10.1016/j.archoralbio.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 60.Imazato S, Ebi N, Tarumi H, Russell RR, Kaneko T, Ebisu S. Bactericidal activity and cytotoxicity of antibacterial monomer MDPB. Biomaterials. 1999;20:899–903. doi: 10.1016/s0142-9612(98)00247-6. [DOI] [PubMed] [Google Scholar]