Abstract
AIM:
The aim of this study was to compare and evaluate the penetration depth of irrigant after using manual, passive ultrasonic, and diode laser–assisted irrigant activation technique.
Materials and Methods:
Ninety extracted single-rooted human mandibular premolars were selected (N = 90). Teeth were decoronated and working length was standardized for 12 mm. Root canals were shaped using ProTaper Universal F3. Saline was used as an irrigant in between every successful instrumentation. After completion of chemomechanical preparation, root canals were filled with Rhodamine-B-labeled sodium hypochlorite (3%). Teeth samples were divided into three experimental groups. In Group 1 (n = 30), manual irrigant activation was performed for 30 seconds with master cone gutta-percha (F3) in gentle up and down motion. In Group 2 (n = 30), ultrasonic irrigant activation was performed using passive ultrasonic endo tips for 30 seconds. In Group 3 (n = 30), laser activation was performed using diode laser for 30 seconds with 200-µm tips. Transverse sections were made at 2, 5, and 8 mm from the root apex and were observed under confocal laser scanning microscopy. Maximum penetration depth of irrigant was recorded and statistically analyzed.
Result:
In the all three groups, coronal third (sections at 8 mm from root apex) showed the highest penetration depth of irrigant. Laser activation (Group 3) showed the highest penetration depth in all the three sections when compared with manual and passive ultrasonic irrigant activation. One-way analysis of variance and post hoc test showed there were significantly high differences among all the three groups and also at all three levels (P < 0.05).
Conclusion:
Diode laser–assisted irrigant activation technique had better penetration depth in all the three aspects of root dentin.
KEYWORDS: Confocal laser scanning microscopy, diode laser, passive ultrasonic irrigation, sodium hypochlorite, tubular penetration
INTRODUCTION
Success of root canal treatment depends on diagnosis, establishing an appropriate treatment plan, understanding the canal anatomy and its application, debridement, disinfection of root dentin, and adequate obturation of the root canal system.[1] Shaping and cleaning of the root canal includes removal of vital and necrotic tissues along with reduction in microorganisms and their by-products.[2]
Major challenge in shaping the root canal system is its complex anatomical structure that includes lateral canals, accessory canals, canal curvatures, fins, cul-de-sacs, and isthmus.[3] In addition to these complexities in the root canal, there are evidences of microorganisms in the dentinal tubules of infected root dentin. So the penetration depth of endodontic irrigants into the dentinal tubules significantly determines the outcome of root canal treatment.[4]
Various endodontic irrigants are used effectively to disinfect these root canal complexities and dentinal tubules in root dentin. Among the endodontic irrigants, sodium hypochlorite can penetrate into the dentinal tubules up to 300 µm.[5] The penetration of sodium hypochlorite into the root dentin depends on its time, concentration, temperature, and activation technique.[6,7]
Endodontic irrigants can be activated by manual irrigation activation and irrigant activation using various devices such as sonic, ultrasonic, apical negative pressure irrigant system,[7] and plastic rotary files.[8] There are evidence in literature that ultrasonic agitation can increase the penetration depth of irrigant even in the apical third of root canal.[9] Dental lasers have unique properties such as photochemical, photoacoustic, and photothermal effects.[10] Various properties of lasers such as smear layer removal, disinfection of root canal, and postoperative pain management have been studied in literature.[11] But very limited research has been performed on diode laser as irrigant activation. Thus, the aim of this study was to compare and evaluate the penetration depth of irrigant after using manual, passive ultrasonic, and diode laser–assisted irrigant activation technique.
MATERIALS AND METHODS
Ninety single-rooted extracted human mandibular premolars were collected (N = 90) after the approval of the intuitional ethics committee of Sri Ramachandra Institute Higher Education and Research, Chennai, Tamil Nadu, India. Teeth with root caries, restoration, resorbed roots, severely angulated roots, and calcified root canals were excluded from the study. Roots were cleaned of tissue with an ultrasonic scaler and stored in distilled water. Each tooth was examined radiographically to confirm that the canals were not calcified. The teeth were then decoronated with a diamond disk to produce a standardized root length of 12 mm to facilitate instrumentation.[12] An ISO size 10 K-file was inserted until visible at the apical foramen. Working length was determined by reducing 1 mm from this length. After access cavity preparation, canals were shaped using ProTaper Universe (Dentsply Maillefer, Ballaigues, Switzerland) up to size F3. For smear layer removal, 1 ml of 17% ethylenediaminetetraacetic acid (EDTA) solution was used finally for 1 minute, and the canals were rinsed with saline. Then, the canals were dried using absorbent points. After chemomechanical preparation, canals were filled with 3% sodium hypochlorite (Prime Dental, Bhiwandi, Maharastra, India), which was mixed with 0.1 wt% fluorescent Rhodamine B isothiocyanate (Sigma-Aldrich, Bangalore, Karnataka, India). The mixture was delivered using conventional syringe.
The samples were randomly divided into three groups according to irrigation activation protocol. In Group 1 (n = 30), manual irrigant activation was performed with master cone gutta-percha (F3) in gentle up and down motion for 30 s for each sample. In Group 2 (n = 30), passive ultrasonic irrigant activation was performed using stainless-steel ISO size 25 ultrasonic endo tip inside the canal for 30 seconds at a power setting of 3, with a piezoelectric ultrasonic unit (Woodpecker, Guilin, Guangxi, China).[13] The tip of the instrument was kept 1 mm short of the root apex. In Group 3 (n = 30), diode laser–assisted irrigant activation was performed using 940-nm diode laser (Epic; Biolase, Irvine, California, USA) with the power of 1.5 W in continuous mode. The size of the tip was 200 µm (E2-14; Biolase) and it was moved from apical to coronal in helical motion three times with each cycle for 5 seconds, with short interval between every cycle. After irrigant activation, transverse sections were made using slow-speed diamond disk at 2 mm (apical), 5 mm (middle), and 8 mm (coronal) from root apex.
These sections were examined using a confocal laser scanning microscope (CLSM). The image was divided into four equal parts and the penetration depth was measured at each part. The average value was recorded as the penetration depth of irrigant. These specimens were evaluated by a single operator, who was blinded to the groups.[14]
Statistical analysis was performed using one-way analysis of variance (ANOVA) and post hoc test to compare the coronal, middle, and apical values. The significance level was set at P < 0.05.
RESULT
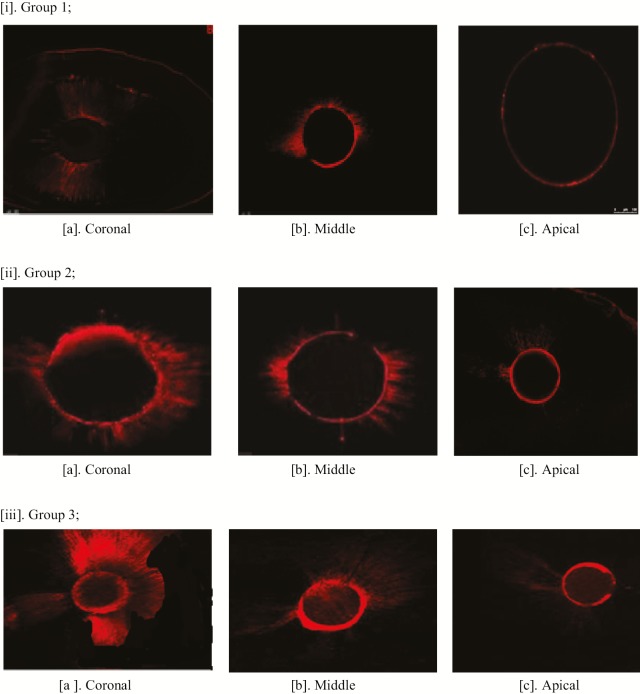
The penetration depth of the groups are reported in Table 1 and Figure 1. At the coronal (at 8 mm), middle (at 5 mm), and apical (at 2 mm), laser activation group showed the highest penetration depth. Lesser penetration of irrigant was observed in apical third (at 2 mm) when compared with middle third (at 5 mm) and coronal third (at 8 mm) in all the three groups. Figure 2 shows the CLSM images of penetration depth of irrigants.
Table 1.
Comparison of penetration depth (μm) into root dentinal tubules
| Group | n | Mean | Standard Deviation | Standard Error | |
|---|---|---|---|---|---|
| Coronal (at 8 mm) | 1 | 30 | 155.677667 | 20.7208257 | 3.7830879 |
| 2 | 30 | 374.054667 | 33.1011586 | 6.0434171 | |
| 3 | 30 | 427.673667 | 28.6567671 | 5.2319859 | |
| Total | 90 | 319.135333 | 121.4840162 | 12.8055397 | |
| Middle (at 5 mm) | 1 | 30 | 91.208000 | 13.5227482 | 2.4689048 |
| 2 | 30 | 168.783000 | 21.1523032 | 3.8618645 | |
| 3 | 30 | 208.584333 | 19.1338175 | 3.4933411 | |
| Total | 90 | 156.191778 | 52.2190228 | 5.5043683 | |
| Apical (at 2 mm) | 1 | 30 | 30.490667 | 6.5074646 | 1.1880951 |
| 2 | 30 | 53.830667 | 10.2919681 | 1.8790477 | |
| 3 | 30 | 85.201667 | 6.9714542 | 1.2728076 | |
| Total | 90 | 56.507667 | 23.9219708 | 2.5215971 |
Figure 1.
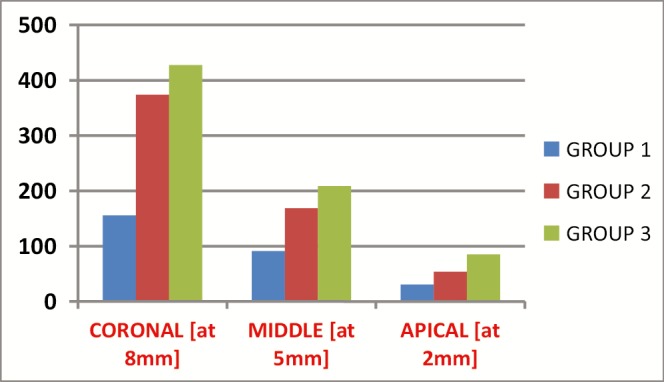
Graphical representation of comparison of penetration depth (µm) into root dentinal tubules
Figure 2.
Confocal laser scanning microscopy images of penetration depth
One-way ANOVA and post hoc test showed there were significantly high differences among all the three groups and also at all three levels (P < 0.05).
DISCUSSION
One of the major objective of the root canal treatment is disinfection of root canal system. There are evidences of microbial colonies even after thorough chemomechanical debridement. Microorganisms invade not only the main root canal system but also the dentinal tubules.[15] These microorganisms can produce endotoxin that can penetrate up to 500 µm into the root dentin. Zou et al.[5] stated that sodium hypochlorite can penetrate into the dentinal tubules when it is activated.
Gu et al.[16] reviewed the contemporary irrigant agitation technique and devices. They stated that manual activation of the root canal irrigant can be performed using hand file, gutta-percha, and microendobrushes, and mechanical activation can be performed using sonic and ultrasonic irrigant activation system.[16] In this study, we used master cone gutta-percha as manual irrigant activation in Group 1.
Passive ultrasonic can be performed with a small file or ultrasonic endo tip (ISO size 10–25), oscillating in the root canal using a piezoelectric ultrasonic device.[17] This produces significant acoustic microstreaming, which can adequately activate the endodontic irrigants and also remove organic content in the root canal system.[18] Hence, we used passive ultrasonic irrigant activation in Group 2.
Dental lasers have been widely used in root canal treatment for disinfection of root canal system.[19] Various lasers, such as CO2, Nd:YAG, Er:YAG, Er:YSGG, argon, and diode laser, have been used for disinfection.[20] Photothermal property of diode laser in the root canal can increase the temperature of irrigant at any concentration.[21] Increase in temperature of sodium hypochlorite can reduce its viscosity and improve its ability to penetrate into the root dentin.[22] In this study, we used diode laser–assisted irrigant activation in Group 3.
In a previous study, Rhodamine B dye was mixed with sodium hypochlorite and fluorescein dye was mixed with resin sealer, and the penetration depth was studied under CLSM.[12] CLSM is comparatively accurate and enables simpler sample preparation.[23] CLSM produces fewer artifacts compared to all other microscopy methodologies.[24] Hence, CLSM was used in our methodology for this study.
In our study, based on the given methodology, diode laser–assisted irrigant activation clearly showed the highest amount of penetration depth into the root dentin. This supports the previous study conducted using diode laser and comparing various irrigant combinations.[25] The reason behind the better performance of diode laser is its mode of delivery, which is 200 µm fiber optic tip, with the length of 14 mm.[26] Penetration of endodontic irrigant is limited due to the presence of smear layer in root dentin. Amin et al.[27] showed that smear layer removal of diode laser is much better when compared with EDTA and ultrasonic.
When passive ultrasonic irrigant activation and laser–assisted activation is compared, penetration depth in ultrasonic group is lesser than that in laser group. This may be due to shorter contact time between ultrasonic endo file and root canal.[28]
Various anatomical complexities of dentin may limit the irrigant penetration into the dentinal tubules. In coronal and middle third of root canal, tubules are larger and densely packed and tubules are narrower in the apical third of root canal.[29] These contribute to better irrigant penetration in the coronal and middle third of root in our study.
CONCLUSION
Within the limitations of the given study, it is concluded that diode laser–assisted irrigant activation technique had better penetration depth in all the three aspects of root dentin.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hulsmann M, Peters OA, Dummer PMH. Mechanical preparation of root canals: Shaping goals, techniques and means. Endod Topics. 2005;10:30–76. [Google Scholar]
- 2.Zehnder M. Root canal irrigants. J Endod. 2006;32:389–98. doi: 10.1016/j.joen.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Blum JY, Abadie MJ. Study of the Nd:YAP laser. Effect on canal cleanliness. J Endod. 1997;23:669–75. doi: 10.1016/S0099-2399(97)80398-6. [DOI] [PubMed] [Google Scholar]
- 4.Siqueira JF, Jr, Rôças IN. Clinical implications and microbiology of bacterial persistence after treatment procedures. J Endod. 2008;34:1291–1301.e3. doi: 10.1016/j.joen.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 5.Zou L, Shen Y, Li W, Haapasalo M. Penetration of sodium hypochlorite into dentin. J Endod. 2010;36:793–6. doi: 10.1016/j.joen.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Taşman F, Cehreli ZC, Oğan C, Etikan I. Surface tension of root canal irrigants. J Endod. 2000;26:586–7. doi: 10.1097/00004770-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Gopikrishna V, Ashok P, Kumar AP, Narayanan LL. Influence of temperature and concentration on the dynamic viscosity of sodium hypochlorite in comparison with 17% EDTA and 2% chlorhexidine gluconate: An in vitro study. J Conserv Dent. 2014;17:57–60. doi: 10.4103/0972-0707.124142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bahcall J, Oslen FK. Clinical introduction of a plastic rotary endodontic finishing file. Endo Prac. 2007;10:17–20. [Google Scholar]
- 9.Paragliola R, Franco V, Fabiani C, Mazzoni A, Nato F, Tay FR, et al. Final rinse optimization: Influence of different agitation protocols. J Endod. 2010;36:282–5. doi: 10.1016/j.joen.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Koba K, Kimura Y, Matsumoto K, Takeuchi T, Ikarugi T, Shimizu T. A histopathological study of the morphological changes at the apical seat and in the periapical region after irradiation with a pulsed Nd:YAG laser. Int Endod J. 1998;31:415–20. doi: 10.1046/j.1365-2591.1998.00177.x. [DOI] [PubMed] [Google Scholar]
- 11.Minamisako MC, Kinoshita JL, Matsumoto K, Stolf DP, Marques JL. A study on root canal cleaning by Nd:YAG laser with black dye solution. J Oral Laser Applications. 2009;9:101–9. [Google Scholar]
- 12.Gu Y, Perinpanayagam H, Kum DJ, Yoo YJ, Jeong JS, Lim SM, et al. Effect of different agitation techniques on the penetration of irrigant and sealer into dentinal tubules. Photomed Laser Surg. 2017;35:71–7. doi: 10.1089/pho.2016.4125. [DOI] [PubMed] [Google Scholar]
- 13.Ghorbanzadeh A, Aminsobhani M, Sohrabi K, Chiniforush N, Ghafari S, Shamshiri AR, et al. Penetration depth of sodium hypochlorite in dentinal tubules after conventional irrigation, passive ultrasonic agitation and Nd:YAG laser activated irrigation. J Lasers Med Sci. 2016;7:105–11. doi: 10.15171/jlms.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vadhana S, Latha J, Velmurugan N. Evaluation of penetration depth of 2% chlorhexidine digluconate into root dentinal tubules using confocal laser scanning microscope. Restor Dent Endod. 2015;40:149–54. doi: 10.5395/rde.2015.40.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siqueira JF, Rocas IN, Lopez HP. Patterns of colonization in primary root canal infection. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:174–8. doi: 10.1067/moe.2002.119910. [DOI] [PubMed] [Google Scholar]
- 16.Gu LS, Kim JR, Ling J, Choi KK, Pashley DH, Tay FR. Review of contemporary irrigant agitation techniques and devices. J Endod. 2009;35:791–804. doi: 10.1016/j.joen.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 17.de Gregorio C, Estevez R, Cisneros R, Heilborn C, Cohenca N. Effect of EDTA, sonic, and ultrasonic activation on the penetration of sodium hypochlorite into simulated lateral canals: An in vitro study. J Endod. 2009;35:891–5. doi: 10.1016/j.joen.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 18.van der Sluis LW, Versluis M, Wu MK, Wesselink PR. Passive ultrasonic irrigation of the root canal: A review of the literature. Int Endod J. 2007;40:415–26. doi: 10.1111/j.1365-2591.2007.01243.x. [DOI] [PubMed] [Google Scholar]
- 19.de Groot SD, Verhaagen B, Versluis M, Wu MK, Wesselink PR, van der Sluis LW. Laser-activated irrigation within root canals: Cleaning efficacy and flow visualization. Int Endod J. 2009;42:1077–83. doi: 10.1111/j.1365-2591.2009.01634.x. [DOI] [PubMed] [Google Scholar]
- 20.Kimura Y, Wilder-Smith P, Matsumoto K. Lasers in endodontics: A review. Int Endod J. 2000;33:173–85. doi: 10.1046/j.1365-2591.2000.00280.x. [DOI] [PubMed] [Google Scholar]
- 21.Pradhan S, Kamik R. Temperature rise on external root surface during laser endodontic therapy using 940nm diode laser: An in vitro study. Int J Laser Dent. 2011;1:29–35. [Google Scholar]
- 22.Sim TP, Knowles JC, Ng YL, Shelton J, Gulabivala K. Effect of sodium hypochlorite on mechanical properties of dentine and tooth surface strain. Int Endod J. 2001;34:120–32. doi: 10.1046/j.1365-2591.2001.00357.x. [DOI] [PubMed] [Google Scholar]
- 23.Kara Tuncer A, Tuncer S. Effect of different final irrigation solutions on dentinal tubule penetration depth and percentage of root canal sealer. J Endod. 2012;38:860–3. doi: 10.1016/j.joen.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Gharib SR, Tordik PA, Imamura GM, Baginski TA, Goodell GG. A confocal laser scanning microscope investigation of the epiphany obturation system. J Endod. 2007;33:957–61. doi: 10.1016/j.joen.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Kundabala M, Shenoy S, Thukral N, Rajeshwari, Hegde V, Kamath P. An evaluation of horizontal depth of penetration of various irrigants into the dentinal tubules when used alone and in combination with diode laser: An in vitro study. J Interdiscip Dentistry. 2014;4:130–4. [Google Scholar]
- 26.George R, Meyers IA, Walsh LJ. Laser activation of endodontic irrigants with improved conical laser fiber tips for removing smear layer in the apical third of the root canal. J Endod. 2008;34:1524–7. doi: 10.1016/j.joen.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 27.Amin K, Masoodi A, Nabi S, Ahmad P, Farooq R, Purra AR, et al. Effect of diode laser and ultrasonics with and without ethylenediaminetetraacetic acid on smear layer removal from the root canals: A scanning electron microscope study. J Conserv Dent. 2016;19:424–7. doi: 10.4103/0972-0707.190005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haapasalo M, Shen Y, Qian W, Gao Y. Irrigation in Endodontics. Dent Clin North Am. 2010;54:291–312. doi: 10.1016/j.cden.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Paqué F, Luder HU, Sener B, Zehnder M. Tubular sclerosis rather than the smear layer impedes dye penetration into the dentine of endodontically instrumented root canals. Int Endod J. 2006;39:18–25. doi: 10.1111/j.1365-2591.2005.01042.x. [DOI] [PubMed] [Google Scholar]