BACKGROUND
Hip and knee arthroplasty are common surgical procedures that have excellent long-term outcomes. The demand for these procedures continues to rise both in the United States and around the world.1,2 With advancements in knowledge and technology, the implants used in total joint arthroplasty continue to undergo modifications to improve functional outcomes.3,4 In recent years, there has been a growing interest in new manufacturing techniques that include custom implants and patient-specific instrumentation.5,6 Although the literature is mixed on the outcomes of the early iterations of custom devices,7 interest in them is unlikely to abate. In particular, additive manufacturing (AM) is a field that has been rapidly expanding in many industries and will likely play a major role in the future of total joint arthroplasty.
AM has become popular for producing lightweight and complex geometries (eg, hollow parts and cellular structures), offering an unparalleled level of design freedom, while also reducing material waste and the weight of components. This technology contrasts with traditional machining processes in which excess material is removed to produce the final part. This design flexibility makes AM attractive to the biomedical, aerospace, automotive, and energy sectors. For instance, General Electric invested in 2 major metal AM manufacturers—Arcam AB and Concept Laser GmbH—in 2016 to advance their AM business and research.8 Given the current trends, investments in this technology are only expected to grow in the coming years.9
ADDITIVE MANUFACTURING BASICS
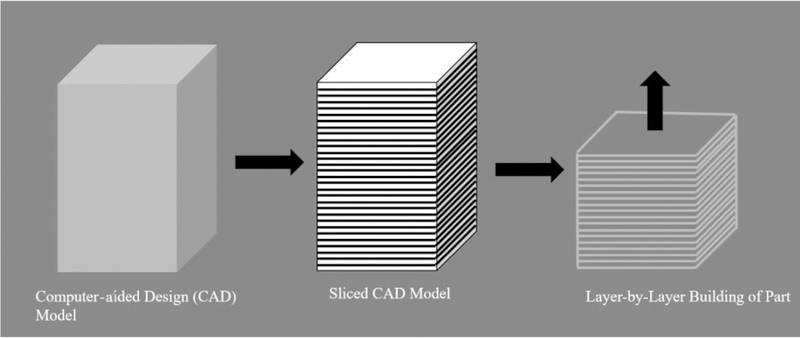
As the name suggests, AM is a process by which a final part is constructed by adding material successively in a layer-by-layer fashion, as shown in Fig. 1. A computer-aided design (CAD) model is first sliced into layers that are on the order of tens of microns and material is deposited into each layer successively, resulting in a complete part. This part is then postprocessed to result in a finished component. Current commercially available technologies can facilitate 3-D printing using a variety of materials, such as ceramics, composites, metals, plastics, and sand. Furthermore, active research is being conducted in the area of bioprinting to print cells, biomaterials, and biomolecules.10
Fig. 1.
Layer-by-layer building of the component in AM.
From a classification standpoint, AM processes can be put into various categories depending on the materials (eg, polymers and metals) and the process used for fabrication. Polymers are widely used in different industries, primarily for prototyping purposes. Because the focus of this review is on arthroplasty applications, which primarily use metal alloy systems, metal AM is emphasized in this discussion. Metal AM can be further classified into nanoparticle jetting, binder jetting, powder bed fusion, and directed energy deposition processes.11 The details of these processes and their applications in arthroplasty are discussed later.
Nanoparticle Jetting
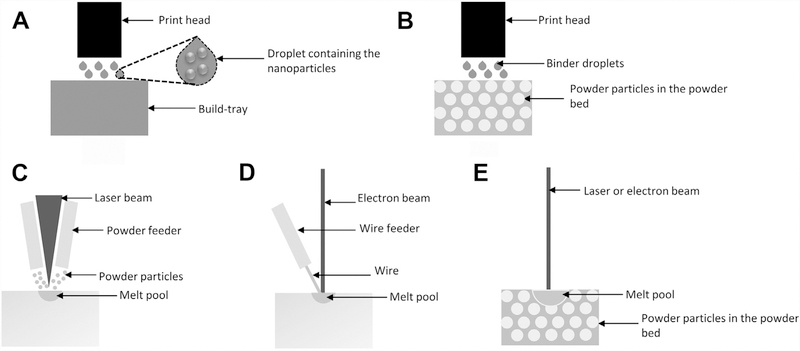
In the nanoparticle jetting process, droplets containing the nanoparticles are first deposited onto the build-tray shown in Fig. 2A. Then, very high temperatures in the build envelope evaporate the liquid surrounding the nano-sized particles within a droplet, which brings the particles together. This process repeats at every layer, resulting in a final component, which then goes through a sintering process. The sintering process is performed to fuse the particles together, which fill any voids in the as-fabricated component. Nanoparticle jetting can be used to fabricate components with fine features and better surface finish compared with the processes that use larger powder sizes, such as powder bed fusion processes. XJet patented this technology and commercially manufactures the nanoparticle jetting machines.12 This process can be used to additively manufacture both ceramic and metal parts. For example, medical materials such as zirconia can be used.
Fig. 2.
Schematic of the metal AM processes: (A) nanoparticle jetting; (B) binder jetting; (C) Laser engineered net shaping; (D) electron beam wire feed; and (E) laser or electron beam powder bed fusion. (Data from Refs.11,12,15,16)
Binder Jetting
In the binder jetting process, liquid binder droplets are selectively deposited onto a powder bed within the boundaries described by the CAD model to bind the powder particles together, as shown in Fig. 2B.13 This process is repeated for every layer in the part. At the end of the process, the part consists of powder particles that are held together by a binding agent. This is typically referred to as a “green part,” which is then transferred to a curing oven to set the glue and further into a furnace for sintering and/or infiltration. During the sintering process, binder material evaporates and the powder particles sinter together, leaving the metal component behind. This results in shrinkage of approximately 30% to 40%. During the infiltration process, the bronze melts and is wicked into the part. On a comparative scale, infiltration results in less shrinkage (approximately 2%) than when just the sintering process is used. ExOne is the commercial manufacturer of the binder jetting machines to print components from metallic materials.14 This process can be useful for prototyping and demonstration purposes in arthroplasty because it is cheaper than other metal AM processes.
Directed Energy Deposition
In the directed energy deposition process, the heat source is typically a laser or an electron beam. A traveling heat source is used to form a melt pool, and feedstock material is fed into the melt pool. This is similar to welding processes. Feedstock material can be in the form of powder or wire. Typically, when a laser beam is used as the heat source, the feedstock material is a powder. This powder is fed into the melt pool at every layer of the part during the deposition process as shown in Fig. 2C. This process is known as laser engineered net shaping (LENS).15 Sandia National Labortories developed this process and Optomec commercially manufactures LENS equipment. On the other hand, when the electron beam is the heat source, feedstock material is a metal wire, and this process is known as the electron beam wire feed (EBF3) process, as depicted in Fig. 2D.16 National Aeronautics and Space Administration at Langley Research Center (Hampton, Virginia) developed this process and Sciaky commercially manufactures EBF3 equipment. EBF3 is a large-scale process suitable for medical equipment rather than implants. Materials, such as stainless steel and titanium, can be processed. On the other hand, LENS is used for surface treatment of implants to improve their biocompatibility and performance.17–19 It can also be used for fabrication of patient-specific implants and suitable for potential multimaterial applications and location-specific composition control of materials.20,21 A variety of arthroplasty materials, such as stainless steel, titanium, and other composites, can be used for fabrication in the LENS process.
Powder Bed Fusion
This category of AM processes is widely used in both industrial production and research. In powder bed fusion processes, the feedstock material is a metal powder. Powder is spread onto a build plate, also known as a start plate, on which the parts are fabricated. At each layer, the powder is melted selectively as per the CAD model, using a laser or electron beam, followed by rapid solidification of the molten material. A schematic of this process is shown in Fig. 2E. The process of spreading the powder, melting, and solidification occurs at each layer until the part fabrication is complete. If a laser beam is used to melt the powder layer, the process is referred to as laser powder bed fusion (LPBF). If an electron beam is used to melt the powder layer, the process is known as electron beam melting (EBM). There are various companies commercializing the LPBF process (eg, EOS, SLM Solutions, Concept Laser GmbH, and TRUMPF). On the other hand, Arcam AB is currently the only commercial manufacturer of the EBM process. These processes can be used for various applications extending from prototyping to actual implants and medical devices. Fig. 3 shows a Food and Drug Administration (FDA)-approved acetabular component with a rougher surface finish on the bone interface and smoother surface on the liner interface. Likewise, Fig. 4 demonstrates the batch fabrication of knee implants using the EBM process. These illustrations demonstrate the applications of AM for patient-specific implants and for tailoring the part properties such as surface finish.
Fig. 3.
Acetabular cup fabricated with Ti-6Al-4V using a LPBF process. (left) Rougher surface finish on the bone interface. (right) Smoother surface on the liner interface. (EOS M290 machine). (Courtesy of EOS North America; with permission.)
Fig. 4.
Example of a knee implant prototype fabricated with Ti-6Al-4V using an EBM process (Arcam S12 machine). (Courtesy of NextManufacturing Center at Carnegie Mellon University, Pittsburgh, PA; with permission.)
Various processes and related arthroplasty-specific applications are reviewed. To realize and further advance the full potential of any technology, however, it is important to understand the advantages and limitations of that technology. Table 1 summarizes the major advantages and limitations of the state-of-the-art metal AM technologies.
Table 1.
Advantages and limitations of state-of-the-art metal additive manufacturing processes in relation to total joint arthroplasty
| Advantages | Limitations |
|---|---|
| • Allowing the fabrication of complex geometries that are otherwise not possible with traditional manufacturing methods for implants, such as porous mesh structures | • Machines and feedstock materials (if it is powder) are expensive24 and may currently be cost prohibitive in arthroplasty. |
| • Enabling the fabrication of materials that are difficult to machine and are useful in arthroplasty, such as Ti-6Al-4V | • Standardization is challenging due to part-to-part and machine-to-machine variability,25 which may be a barrier with regulatory agencies. |
| • Tailoring the microstructure and properties based on individual patient needs, such as adjusting the density of the implant to match with bone density22 | • Producing uniform microstructure and defect-free parts is a work in progress, which might require additional postprocessing that adds to the cost of the implant. |
| • Enabling the fabrication of lightweight parts that results in reduced material waste, such as hollow structures | • Successful fabrication of parts with new materials and design involves extensive experimentation, which is expensive and time consuming, and limits the utilization of the full potential of the technology.26 |
| • Enabling the consolidation of components to reduce the assembly time and create new design opportunities23 | • Postprocessing costs also contribute to the total fabrication costs, which can make the process expensive for applications, such as bearing surfaces, that require a smooth surface finish. |
APPLICATIONS IN TOTAL JOINT ARTHROPLASTY
According to the authors, the major current and potential applications of AM in arthroplasty can be divided into 3 main categories:
Patient-specific implants and instrumentation
Porous structures and functionally graded implants
Other novel applications
Because AM process-specific applications are discussed previously on different AM processes, the aim of this section is to summarize general applications of AM and their implications for total joint arthroplasty.
Patient-Specific Implants and Instrumentation
The concept of patient-specific instrumentation and implants has garnered much recent attention. The proposed potential benefits of patient-specific instrumentation in total knee arthroplasty include improving the accuracy of the bone cuts and thus the alignment of the implants and improving the operative time and efficiency (Fig. 5).27 Although most studies thus far do not support any advantage of patient-specific instrumentation over conventional instrumentation,7,28 improvements in the production and reliability of patient-specific instrumentation may increase their relevance and usefulness in the future, and AM technology could potentially provide this. AM has played a key role in the development of patient-specific implants in the field of craniomaxillofacial surgery and many researchers have explored this topic for different types of implants.29–32 Although there are still only small case series that have reported the use of additively manufactured patient-specific implants in the field of arthroplasty,33 there is much interest in this realm in the industrial sector, and surgeons will likely see an increased use for this technology moving forward. In particular, complex revision hip arthroplasty cases are an area where AM technology could be applied in the current environment but these applications will likely expand as the characteristics and potential benefits of additively manufactured constructs become better understood.34
Fig. 5.
Nylon 12 EOS PA2200 patient-specific cutting guide fabricated using an LPBF process (EOS P396 machine). (Courtesy of EOS North America; with permission.)
Porous Structures and Functionally Graded Implants
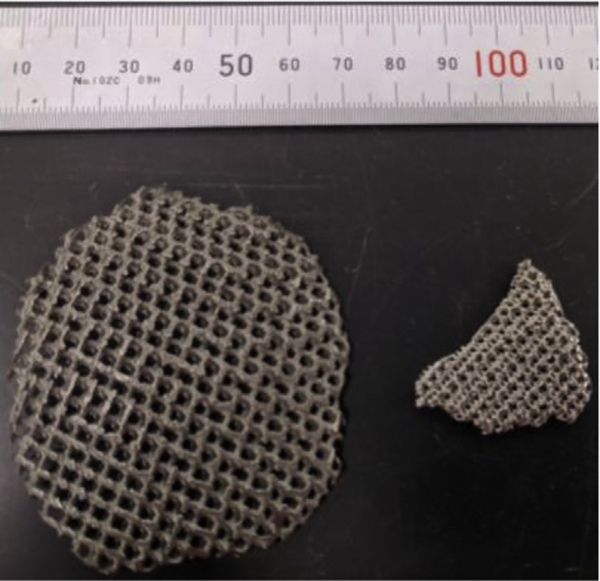
The layer-by-layer nature of AM makes it popular for fabricating complex geometries. This has proved especially advantageous for medical implants. Fig. 6 shows the possibility of fabricating fine mesh structures and the ability to tailor mesh structures to change the density and resulting stiffness of the fabricated part.35 Murr and colleagues22 demonstrated the concepts of variable density and stiffness in total knee and total hip implants using Ti-6Al-4V and Co-29Cr-6Mo via the EBM process. For instance, the inner surface of the Co-29Cr-6Mo femoral knee implant and outer surface of the Ti-6Al-4V acetabular cup are made porous to improve bone ingrowth and to modify the stiffness to address stress shielding. Arabnejad and colleagues implemented the tailoring of mesh structures to generate a functionally-graded femoral stem that has an optimum relative density distribution. In vitro tests revealed substantial reduction in stress shielding illustrating that tailoring densities and stiffness were critical for minimizing the stress shielding effects.36 For cementless orthopedic implants, Harrison and colleagues37 demonstrated that AM can be used to fabricate novel surface architectures to replace traditional surface coatings. In this study, hip stem components with OsteoAnchor surface architecture made out of Ti-6Al-4V were compared with the hip stems with standard plasma sprayed titanium coatings that are implanted in animals. Results suggested that the OsetoAnchor surface achieved with AM had superior primary fixation and better bone in-growth. This work suggests that AM can be used as a tool to fabricate and test customized surface architectures. Interested readers may want to review the vast literature on the impact of porous structures and functionally graded implants on the stability and the life of an implant.
Fig. 6.
Cellular mesh structures at different size scales fabricated with Ti-6Al-4V using an EBM process (Arcam S12 machine). Different meshes result in different part densities and mechanical properties. (Courtesy of NextManufacturing Center at Carnegie Mellon University, Pittsburgh, PA; with permission.)
Novel Applications
The layer-by-layer manufacturing of materials and the ability to fabricate with a variety of materials and geometries opens up opportunities to pursue new research. For instance, use of AM has been suggested as an alternative to the current procedures to treat periprosthetic infections.38 Kim and colleagues38 proposed using AM to print polylactic acid liners, which can either have antibiotics embedded into them or consist of reservoirs and microchannels for controlled drug delivery. Similarly, Bezuidenhout and colleagues39 suggested the possibility of using AM for drug delivery. In the same work, challenges related to the development of such implants are discussed, highlighting the need for collaboration among stakeholders to realize these novel applications. For in situ measurements of implant performance, Micolini and colleagues40 demonstrated the use of AM to fabricate a polymer sensor array with conductive polyaniline that can be embedded into implants for in-situ measurement of load transmission. Besides that, AM is also being used as a prototyping tool for testing new research approaches. As an example, Uklejewski and colleagues41 used selective laser melting to fabricate a biomimetic multispiked connecting scaffold in their efforts to develop noncemented stemless resurfacing hip arthroplasty endoprostheses. These examples demonstrate that AM holds potential to revolutionize the state-of-the-art in the implant industry and catalyze the industry toward improving health care.
CHALLENGES AND OPPORTUNITIES FOR FUTURE RESEARCH
AM applications in arthroplasty include prototyping, actual functional implants, and many potential novel applications that are otherwise not viable through traditional manufacturing. Although this sounds promising, there are challenges associated with the technology that are hindering its widespread adoption. From the authors’ perspective, the push for using AM has to be led by the surgeons and, more specifically, through their interest in customizable implants. Currently, due to the high costs of AM, this technology is mainly limited to usage for revision arthroplasty procedures. Reducing the cost of AM can potentially lead to a more widespread adoption of AM techniques beyond just revisions. Another practical challenge is the process of obtaining approval from the FDA, which can be extensive and cumbersome. Recently, the FDA issued technical considerations for additively manufactured devices to serve as a guide for industry as well as for FDA staff who are considering to use AM.42 Unlike for some of the established manufacturing practices, such as machining and casting, it is reasonable to say that for a new technology like AM, the burden of proof is higher.
The opportunities for future research lie in addressing these challenges. To address the higher costs of using AM, Laureijs and colleagues24 concluded that reducing the cost associated with feedstock material can reduce the manufacturing costs of the implants. One way to accomplish this is to develop methods to use inexpensive feedstock materials that can provide comparable performance to the current feedstock material. Additionally, cost can be reduced by minimizing the scrap material from the fabricated parts. Typically, scrap includes support structures and extra material added to the component to achieve tolerances after machining. Furthermore, cost can also be reduced by increasing the reuse of materials. For FDA approval purposes, standards can be developed by understanding the properties of the fabricated components and the effect of processing conditions on the part properties. It is also important to understand and control the process variability and this can be done by implementing in situ monitoring and controls.25,43 To encourage surgeons to use AM, new functionalities should be explored and incorporated into the additively manufactured implants and devices. Specifically, the advantages offered by AM, such as geometric freedom and location-specific control of properties, should be leveraged to address the challenges faced by surgeons. In conclusion, this review highlights the potential of AM to revolutionize the area of total joint arthroplasty and research opportunities that lie at the intersection of addressing the current challenges in AM and developing innovative approaches in arthroplasty.
KEY POINTS.
Additive manufacturing, popularly known as 3-D printing, is a layer-by-layer manufacturing process.
Some of the advantages of additive manufacturing include using nonmachinable materials, fabricating complex geometries, and tailoring part properties.
Currently available additive manufacturing processes, which fabricate parts with materials ranging from polymers to ceramics to metals, are discussed from the perspective of applicability in arthroplasty.
Some applications of additive manufacturing in total joint arthroplasty include custom, patient-specific instrumentation and implants in hip and knee arthroplasty with new functionalities.
There are gaps in additive manufacturing research, which, if addressed, have the potential to have a positive impact on the area of arthroplasty.
ACKNOWLEDGMENTS
Dr S.P. Narra is supported by the NextManufacturing Center at Carnegie Mellon University. Dr K.L. Urish is supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS K08AR071494), the National Center for Advancing Translational Sciences (NCATS KL2TR0001856), and the Orthopaedic Research and Education Foundation. The authors thank EOS North America for providing the images of the acetabular cup and patient-specific cutting guide. The authors would also like to thank graduate students Joseph Pauza, Ali Alp Gurer, Vignesh Viswanathan, Mahemaa Rajasekaran, Daming Ding, and Yumin Yan at Carnegie Mellon University for generously offering us images of the knee implant prototype used in this article. The authors would like to acknowledge the NextManufacturing Center at Carnegie Mellon University for providing the images of the components fabricated on the Arcam S12.
Footnotes
Disclosure Statement: None.
REFERENCES
- 1.Kurtz SM, Ong KL, Lau E, et al. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am 2014;96(8):624–30. [DOI] [PubMed] [Google Scholar]
- 2.Inacio MCS, Graves SE, Pratt NL, et al. Increase in total joint arthroplasty projected from 2014 to 2046 in Australia: a conservative local model with international implications. Clin Orthop Relat Res 2017;475(8):2130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lachiewicz PF, Kleeman LT, Seyler T. Bearing surfaces for total hip arthroplasty. J Am Acad Orthop Surg 2018;26(2):45–57. [DOI] [PubMed] [Google Scholar]
- 4.Nieuwenhuijse MJ, Nelissen RGHH, Schoones JW, et al. Appraisal of evidence base for introduction of new implants in hip and knee replacement: a Systematic review of five widely used device technologies. BMJ 2014;349:g5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen LCL, Lehil MS, Bozic KJ. Trends in total knee arthroplasty implant utilization. J Arthroplasty 2015;30(5):739–42. [DOI] [PubMed] [Google Scholar]
- 6.Berend ME, Berend KR, Lombardi AV, et al. The patient-specific Triflange acetabular implant for revision total hip arthroplasty in patients with severe acetabular defects: planning, implantation, and results. Bone Joint J 2018;100B(1):50–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sassoon A, Nam D, Nunley R, et al. Systematic review of patient-specific instrumentation in total knee arthroplasty: new but not improved. Clin Orthop Relat Res 2015;473(1):151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.GE makes significant progress with investments in additive equipment companies | GE Additive. Available at: https://www.ge.com/additive/press-releases/ge-makes-significant-progress-investments-additive-equipment-companies. Accessed July 1, 2018.
- 9.3D Printing Industry to 2027 | Market Reports | Smithers Pira. Smithers Pira. Available at: https://www.smitherspira.com/industry-market-reports/printing/the-future-of-3d-printing-to-2027. Accessed May 17, 2018.
- 10.Seol YJ, Kang HW, Lee SJ, et al. Bioprinting technology and its applications. Eur J Cardiothorac Surg 2014;46(3):342–8. [DOI] [PubMed] [Google Scholar]
- 11.3D HUBS. What is 3D Printing? The definitive guide to additive manufacturing. 3Dhubs.Com. 2017. Available at: https://www.3dhubs.com/what-is-3d-printing. Accessed March 3, 2017.
- 12.XJet. X-Jet technology. Available at: http://www.xjet3d.com/technology.html. Accessed March 3, 2017.
- 13.Sachs E, Cima M, Williams P, et al. Three dimensional printing: rapid tooling and prototypes directly from a CAD model. J Eng Ind 1992;114:481–8. [Google Scholar]
- 14.What is Binder Jetting? | ExOne. 2016. Available at: http://www.exone.com/Resources/Technology-Over-view/What-is-Binder-Jetting. Accessed March 3, 2017.
- 15.Griffith M, Keicher D. Free form fabrication of metallic components using laser engineered net shaping (LENS). In: Solid Freeform Fabrication Proceedings. Austin (TX); 1996:125–32. [Google Scholar]
- 16.Taminger K, Hafley R. Electron beam freeform fabrication: a rapid metal deposition process. In: Proceedings of the 3rd Annual Automotive Composites Conference Troy; 2003:9–10. Available at: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20040042496.pdf. Accessed March 3, 2017. [Google Scholar]
- 17.Emmelmann C, Scheinemann P, Munsch M, et al. Laser additive manufacturing of modified implant surfaces with osseointegrative characteristics. Phys Procedia 2011;12:375–84. [Google Scholar]
- 18.Janaki Ram G, Yang Y, Stucker BE. Deposition of Ti/TiC composite coatings on implant structures using laser engineered net shaping. In: Proceedings of Solid Freeform Fabrication Symposium. Austin (TX); 2007:527–39. [Google Scholar]
- 19.Roy M, Bandyopadhyay A, Bose S. In vitro antimicrobial and biological properties of laser assisted tricalcium phosphate coating on titanium for load bearing implant. Mater Sci Eng C 2009;29(6):1965–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vamsi Krishna B, Xue W, Bose S, et al. Engineered porous metals for implants. JOM 2008;60(5):45–8. [Google Scholar]
- 21.Das M, Balla VK, Kumar TSS, et al. Fabrication of biomedical implants using laser engineered net shaping (LENS™). Trans Indian Ceram Soc 2013; 72(3):169–74. [Google Scholar]
- 22.Murr LE, Gaytan SM, Martinez E, et al. Next generation orthopaedic implants by additive manufacturing using electron beam melting. Int J Biomater 2012; 2012:245727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmelzle J, Kline EV, Dickman CJ, et al. (Re) Designing for part consolidation: understanding the challenges of metal additive manufacturing. J Mech Des 2015;137(11):111404. [Google Scholar]
- 24.Laureijs RE, Roca JB, Narra SP, et al. Metal additive manufacturing: cost competitive beyond low volumes. J Manuf Sci Eng Trans ASME 2017; 139(8). [Google Scholar]
- 25.Herderick E Additive manufacturing of metals: a review. Columbus (OH): Materials Science and Technology (MS&T); 2011. p. 1413–25. [Google Scholar]
- 26.Beuth J, Fox J, Gockel J, et al. Process mapping for qualification across multiple direct metal additive manufacturing processes. In: Proceedings of Solid Freeform Fabrication Symposium. Austin (TX); 2013:655–5. [Google Scholar]
- 27.Urish KL, Conditt M, Roche M, et al. Robotic total knee arthroplasty: surgical assistant for a customized normal kinematic knee. Orthopedics 2016; 39(5):e822–7. [DOI] [PubMed] [Google Scholar]
- 28.Thienpont E, Schwab PE, Fennema P. A systematic review and meta-analysis of patient-specific instrumentation for improving alignment of the components in total knee replacement. Bone Joint J 2014;96 B(8):1052–61. [DOI] [PubMed] [Google Scholar]
- 29.Nickels L World’s first patient-specific jaw implant. Metal Powder Report 2012;67(2):12–4. [Google Scholar]
- 30.Jardini AL, Larosa MA, Filho RM, et al. Cranial reconstruction: 3D biomodel and custom-built implant created using additive manufacturing. J Craniomaxillofac Surg 2014;42(8):1877–84. [DOI] [PubMed] [Google Scholar]
- 31.Dérand P, Rännar L-E, Hirsch J-M. Imaging, virtual planning, design, and production of patient-specific implants and clinical validation in cranio-maxillofacial surgery. Craniomaxillofac Trauma Reconstr 2012;05(03):137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salmi M, Tuomi J, Paloheimo K, et al. Patient-specific reconstruction with 3D modeling and DMLS additive manufacturing. Rapid Prototyp J 2012; 18(3):209–14. [Google Scholar]
- 33.Colen S, Harake R, De Haan J, et al. A modified custom-made triflanged acetabular reconstruction ring (MCTARR) for revision hip arthroplasty with severe acetabular defects. Acta Orthop Belg 2013;79(1):71–5. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23547519. Accessed June 9, 2018. [PubMed] [Google Scholar]
- 34.Cheng A, Humayun A, Cohen DJ, et al. Additively manufactured 3D porous Ti-6Al-4V constructs mimic trabecular bone structure and regulate osteoblast proliferation, differentiation and local factor production in a porosity and surface roughness dependent manner. Biofabrication 2014;6(4): 045007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gibson LJ, Ashby MF. Cellular solids. Cambridge (UK): Cambridge University Press; 1997. 10.1017/CBO9781139878326 [DOI] [Google Scholar]
- 36.Arabnejad S, Johnston B, Tanzer M, et al. Fully porous 3D printed titanium femoral stem to reduce stress-shielding following total hip arthroplasty. J Orthop Res 2017;35(8):1774–83. [DOI] [PubMed] [Google Scholar]
- 37.Harrison N, Field JR, Quondamatteo F, et al. Preclinical trial of a novel surface architecture for improved primary fixation of cementless orthopaedic implants. Clin Biomech 2014;29(8): 861–8. [DOI] [PubMed] [Google Scholar]
- 38.Kim TWB, Lopez OJ, Sharkey JP, et al. 3D printed liner for treatment of periprosthetic joint infections. Med Hypotheses 2017;102:65–8. [DOI] [PubMed] [Google Scholar]
- 39.Bezuidenhout MB, Dimitrov DM, Van Staden AD, et al. Titanium-based hip stems with drug delivery functionality through additive manufacturing. Bio- med Res Int 2015;2015:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Micolini C, Holness F, Johnson J, et al. Assessment of embedded conjugated polymer sensor arrays for potential load transmission measurement in orthopaedic implants. Sensors (Basel) 2017;17(12): 2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uklejewski R, Rogala P, Winiecki M, et al. Preliminary results of implantation in animal model and osteoblast culture evaluation of prototypes of biomimetic multispiked connecting scaffold for noncemented stemless resurfacing hip arthroplasty endoprostheses. Biomed Res Int 2013; 2013:689089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.FDA. Technical Considerations for Additive Manufactured Medical Devices - Guidance for Industry and Food and Drug Administration Staff; 2017. Available at: https://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/Guidan-ceDocuments/UCM499809.pdf. Accessed May 16, 2018.
- 43.Frazier WE. Metal additive manufacturing: a review. J Mater Eng Perform 2014;23(6):1917–28. [Google Scholar]