Highlights
-
•
We measured focused attention via task-evoked pupil dilation during a visual working memory task in 13-month-old infants.
-
•
Pupil dilation during encoding significantly correlated with subsequent memory performance.
-
•
This relationship between focused attention and memory was present both on the individual and the trial-by-trial level.
-
•
Pupillometry is a promising new tool to measure focused attention with a high temporal resolution in infants.
Keywords: Visual working memory, Focused attention, Pupillometry, Task-evoked pupil responses, Infants, Cognitive effort, Eye-tracking
Abstract
Attention turns looking, into seeing. Yet, little developmental research has examined the interface of attention and visual working memory (VWM), where what is seen is maintained for use in ongoing visual tasks. Using the task-evoked pupil response – a sensitive, real-time, involuntary measure of focused attention that has been shown to correlate with VWM performance in adults and older children – we examined the relationship between focused attention and VWM in 13-month-olds. We used a Delayed Match Retrieval paradigm, to test infants’ VWM for object-location bindings – what went where – while recording anticipatory gaze responses and pupil dilation. We found that infants with greater focused attention during memory encoding showed significantly better memory performance. As well, trials that ended in a correct response had significantly greater pupil response during memory encoding than incorrect trials. Taken together, this shows that pupillometry can be used as a measure of focused attention in infants, and a means to identify those individuals, or moments, where cognitive effort is maximized.
1. Introduction
In the first two years of life, infants actively interact with the environment, rapidly picking up new skills through observation and reasoning (Gweon and Schulz, 2011; Stahl and Feigenson, 2015; Téglás et al., 2011; Xu et al., 2005). A critical system supporting this is visual working memory (VWM) – a mechanism that allows for the storage and manipulation of visual information during cognitive tasks (Baddeley and Hitch, 1974). Alongside this, abundant evidence has suggested that focused attention is a critical component underlying VWM in adults (Baddeley, 1996; Kane and Engle, 2002). Pupillometry has emerged as a sensitive, fast, non-intrusive method to quantify the relationship between focused attention and VWM. Studies have settled on the ‘task-evoked’ pupil response - phasic dilations before or during stimulus presentation - as a marker of greater focused attention that can distinguish correct versus incorrect responses, and high and low performers, in visual working memory tasks (Klingner et al., 2011; Porter et al., 2007; Unsworth and Robison, 2015, 2017). So far, this connection between focused attention and VWM has been missing in infant work. This means that we are both missing insight into the development of the frontal circuitry connecting attention and memory (Colombo and Cheatham, 2006), but also that we may be systematically underestimating VWM ability in infants (in those epochs, or individuals, when attention is focused on task-relevant stimuli and events, estimates of VWM capacity, and/or fidelity, should be maximized). Here we use pupillometry similarly to how it is used in adult work, to isolate moments of focused attention and quantify their effect on VWM.
1.1. The emergence of focused attention
Visual attention is defined as the selection of behaviorally relevant information, prioritizing its processing, while inhibiting irrelevant information (Treue, 2003); the mechanism that “turns looking into seeing” (Carrasco, 2011). Posner and Petersen (Petersen and Posner, 2012; Posner and Petersen, 1990) described three systems of attention: (1) orienting/arousal, (2) selective attention, and (3) executive attention. Development of orienting/arousal appears from birth to 2 months, while selective attention starts to emerge from 2 to 6 months. Executive attention comes online latest, developing across infancy and toddlerhood (Colombo and Cheatham, 2006; Courage and Richards, 2008; Martinez-Alvarez et al., 2017). Attention goes through a qualitative shift from infancy to early childhood, from being driven by exogenous cues to being driven in a more proactive, purposeful manner (Martinez-Alvarez et al., 2017; Wright and Vlietstra, 1975).
The emergence of attentional control is a critical step in the development of the child as an active seeker of information. To capture this, we use the term ‘focused attention’ (Ruff, 1986) similarly to Kahneman: “[The] intensive aspects of attention (…) must be distinguished from arousal. Thus, the schoolboy who pays attention… is performing work, expending his limited resources, and the more attention he pays, the harder he works.” (Kahneman, 1973, p. 4). (Attentional control (Astle and Scerif, 2011), and the regulation of cognitive resources in sustained attention (Karatekin et al., 2007) are overlapping concepts.) Focused attention is driven endogenously and directed proactively (Colombo and Cheatham, 2006).
1.2. The role of focused attention in memory
VWM is a central cognitive mechanism that is intimately tied to attentional processes (A. Baddeley, 1996; Chun et al., 2011; Cowan et al., 2005; Kane and Engle, 2003). It has been proposed that the integration of memory and attention system emerges between 6–15 months of age, as a result of the maturation of frontal circuitry (Colombo and Cheatham, 2006). To measure attention behaviorally, developmental researchers have relied on gaze data, particularly fixation latencies and looking duration, reflecting how a stimulus can attract and hold visual attention, respectively (Cohen, 1972; Frick et al., 1999).
A challenge to relying on gaze data to infer attention is that looking and attending are partially separable (Kowler et al., 1995); one may look without attending, or attend without looking. Early developmental work established heart rate as a marker of attentional focus: when infants’ heart rate decelerated from a pre-trial baseline, they were less likely to get distracted from a fixated stimulus by the presentation of a second stimulus; researchers defined this period as “sustained attention phase” (Richards, 1985, Richards, 1987). Contrarily, when infants’ heart rate accelerated back to prestimulus baseline, infants were more easily distracted. Using heart rate fluctuation then to define attentional phases, Richards found that the quality of recognition memory was affected by these phases: when 4-6-month-olds were shown a stimulus for 5 s during sustained attention phase (as indexed by reduced heart rate versus the pre-trial baseline), it was sufficient to elicit a novelty preference equivalent to an exposure time of 20 s when stimulus presentation was not targeted to phases of sustained attention (Richards, 1997).
This link was demonstrated using electrophysiological measures as well: greater amplitude of Negative central (Nc) responses were associated with sustained attention (as defined by heart rate deceleration) (Richards, 2003), and differences in object exploration (as indexed by frontal theta band) could predict subsequent object recognition performance in 11-month-olds (Begus et al., 2015). Interestingly, this relationship between focused attention and recognition memory can help explain the result that infants who fixate for less time during familiarization show evidence of better recognition memory (Colombo and Mitchell, 1990): short-looking 6–7.5-month-old infants showed greater late slow wave ERP responses to novel (as opposed to familiar) stimuli, while longer-lookers did not (Reynolds et al., 2011).
1.3. Pupillometry as a measure of focused attention
Recent neurophysiological research has elucidated the mechanisms involved in the control of focused attention. In particular, the locus coeruleus–noradrenergic (LC-NE) neuromodulatory system plays a causal role in regulating task engagement and optimizing performance (Aston-Jones and Cohen, 2005; Sara, 2009). The LC is a small nucleus in the brainstem and is the sole source of cortical noradrenaline (NE). It projects to widely distributed areas of the brain, most prominently, the frontal cortex (Aston-Jones et al., 1986). The causal linkage between LC-NE system activity and task performance was best established through direct manipulation of the LC (Usher et al., 1999). In monkeys, local microinfusion of clonidine to increase LC phasic activity increases performance on a visual task, while a suppressive agent (pilocarpine) has the opposite effect (Aston-Jones and Cohen, 2005). In humans, administration of modafinil to increase LC activity yields task-related activity in cognitive control areas (shown by fMRI), and improves performance on a cognitive task (Minzenberg et al., 2008). According to Aston-Jones and Cohen’s (2005) integrative theory of adaptive gain control, in the phasic mode, LC cells exhibit activation when processing task-relevant stimuli, and this mode is associated with high levels of task performance (Aston-Jones et al., 1994; Bouret et al., 2003).
Importantly, for our purposes, through the release of NE, the LC regulates the pupil (Koss, 1986; Samuels and Szabadi, 2008). Single-cell recordings in primates provided evidence of a strong relationship between pupil responses and LC activity (Joshi et al., 2016; Rajkowski et al., 1994). More recently, the same coupling has been demonstrated in humans as well: two fMRI studies found that pupil diameter tightly co-varied with BOLD activity in the LC (Alnæs et al., 2014; Murphy et al., 2014). The pupil therefore provides an online, non-invasive window into the activity of the LC-NE system, and therefore attentional state (Eldar et al., 2013; Gilzenrat et al., 2010; Malecek and Poldrack, 2013). Indeed, models of infants’ attentional development have incorporated the role of the LC-NE system (Colombo and Cheatham, 2006; Richards, 2001).
1.4. Measuring the relationship between focused attention and VWM
Given that pupil dilation is synchronized to changes in cognitive effort due to real-time variation in task demands (the ‘task-evoked pupil response’, TEPR) (Aston-Jones and Cohen, 2005; Beatty, 1982; Laeng et al., 2012), pupillometry has a long history in cognitive psychology. Hess and Polt were the first to show that the pupil dilated as a function of task difficulty with higher peak dilation in more difficult mental multiplication tasks (Hess and Polt, 1964). The TEPR can also signal surprise that is generated as the result of detecting a mismatch between expectations and the perceived world (O’Reilly et al., 2013; Preuschoff et al., 2011). This is true in infants as well (Hepach and Westermann, 2016; Laeng et al., 2012). For instance, infants showed higher pupil dilation to implausible physical events (Jackson and Sirois, 2009; Sirois and Jackson, 2012), or unexpected outcomes in social situations (Geangu et al., 2011; Gredebäck and Melinder, 2010).
In the memory domain, Kahneman and Beatty demonstrated that adults’ pupil dilation gradually increased with the number of items needed to be retained in a verbal short-term memory task (Kahneman and Beatty, 1966). This relationship has been shown in visual WM tasks as well (Klingner et al., 2011). Just as in verbal WM tasks, increases in attentional demand, associated with greater VWM load, lead to larger pupil dilation. As well, individual differences in adults’ VWM capacity were found to correlate with online attentional allocation and fluctuations in attention, as indexed by the pupil response (Unsworth and Robison, 2015).
In memory development, pupillometry has been identified as a rich measure (see Eckstein et al. (2017) for a review). For instance, a recent study demonstrated that differences in VWM capacity between 10-year-olds and adults were related to differences in task engagement during encoding as shown by pupil dilation (Johnson et al., 2014). In this study, just as in adults, children’s pupil diameter increased with the number of to-be-encoded items (unlike adults though, when VWM capacity limit was exceeded the pupil started to constrict, suggesting that when children met their cognitive limit they began to disengage from the task). As well, a recent study showed that TEPR can reflect novelty versus familiarity in 7-month-olds, similarly to adults, in a long-term memory task (Hellmer et al., 2016). Yet, to our knowledge, beyond preliminary work from our group (Cheng et al., 2016), a direct study of the relationship between focused attention and VWM in infants using pupillometry has not been conducted.
2. Method
2.1. Participants
Twenty-two full-term, healthy 13-month-old infants (12 girls) were recruited from the Greater Boston area, and tested at University of Massachusetts Boston (M = 13.3 months, SD = 1.26, age range: 11 months 6 days to 14 months 26 days). Caregivers received a small gift and $20 compensation for their time and travel expenses. All parents gave informed consent before the experiment and the protocol was approved by the Institutional Review Board of the University of Massachusetts Boston.
To be included in subsequent analyses, each participant had to have at least 3 trials (from the block of 12 total) where they looked at both of the to-be-remembered cards during the ‘encoding phase’, and had a valid response (i.e., looked at one of those two cards) during the ‘response phase’. All 22 infants met this requirement for performance analyses. Pupil analyses further required that pupil data were of sufficient quality (see Data Analysis). Two infants did not have at least three trials that met these requirements, and were excluded from pupil analyses.
2.2. Apparatus and stimuli
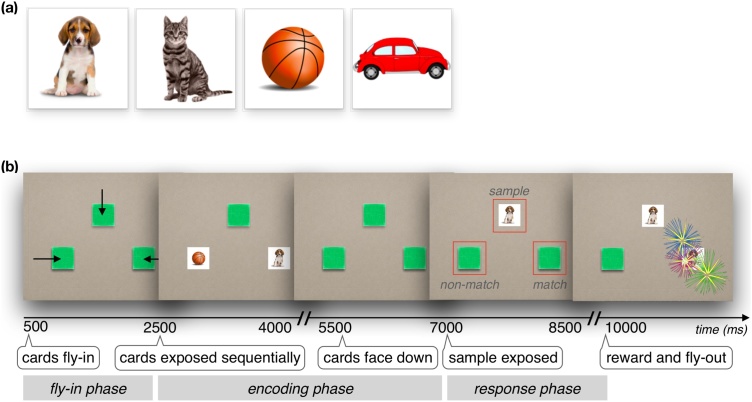
Parents sat in a chair holding their infants in their laps in front of a Tobii T120 eye-tracker (Tobii Technology, Stockholm, Sweden) in a dimly lit testing room. Parents were asked to wear a visor to cover their eyes and not to interact with their infants during the study. A standard Tobii 5-point infant calibration was used. The experimental stimuli were virtual ‘cards’ that could be shown face up, revealing a familiar object (car, ball, cat, dog), or be flipped face-down, occluding the object. In each trial, two of the four objects were randomly chosen (Fig. 1a). Cards each subtended 5 × 5 deg and were arranged symmetrically with their centers 5 deg from the center of the screen.
Fig. 1.
(a) Memory ‘card’ stimuli, face-up, showing to-be-remembered objects. Two of the four cards were randomly chosen on each test trial. One was used as both the Sample and Match cards, and the other as the Non-Match card. (b).
Schematic of test trial events. Anticipatory gaze responses were analyzed during the 2.5 s response phase, where VWM performance was measured as the percentage of trials where the infants’ first anticipatory look was to the Match card. Areas of Interest (AOIs) for the Match, Non-Match, and Sample cards are indicated by red frames (not shown during testing). Pupil diameter was measured throughout the trial, and the task-evoked pupil response (TEPR) during the encoding phase was evaluated as a predictor of task performance.
2.3. Design and Procedure
The procedure was a variant of our Delayed Match Retrieval task (Kaldy et al., 2016). Each trial began with the display of an empty background (1 s) after which three cards (the Match, Non-Match, and Sample) entered the screen, face-down, forming a triangular arrangement. The Match and Non-Match were at the bottom corners of the triangle, and the Sample was always on the top. During the encoding phase of the trial, the Match and Non-Match were flipped face up, sequentially. First, one of the two cards, randomly chosen, was exposed, then, after 1.75 s, the other card was exposed, then, after another 1.75 s, they both were flipped face-down at the same time and remained face-down for 1 s (comprising a total encoding phase of 4.5 s). Following that, the Sample card, which had an identical face to the Match card, was flipped face up marking the end of encoding phase and the beginning of the 2.75 s response phase, during which anticipatory gaze responses were recorded. After the response phase, the Match card was exposed, accompanied by a brief reward/feedback animation at its location (e.g., a colorful burst of fireworks). The Match then moved next to the Sample, touched it, then all three cards flew off screen (Fig. 1b). To maintain engagement, we added unique sounds to on-screen events (e.g., flipping, moving, and touching) and alternated between three different reward animations (fireworks, sparkles, flashbulbs). (See Supplementary Movie 1.)
Each participant was presented with a block of 12 trials. Within a block, half of the trials had the left card exposed first (and the other half, the right), half had the Match card exposed first (and the other half, Non-Match), and there was an equal number of trials (4) for each of three possible reward animations. The order of these trials was randomized for each participant’s block, with the restriction that no more than two consecutive trials presented the match card on the same side. Each test trial was followed by an attention grabber (a cartoon sun rotating in the center of the screen for 4 s, with a sound effect), to attract infants’ gaze towards the screen. At the beginning of the block, before the sequence of test trials, infants were presented with four brief familiarization trials, during which a pair of matching cards entered the screen, approached each other, jiggled, and then exited the screen (this sequence was repeated once for each of the four object types).
2.4. Data analysis
2.4.1. Gaze data analysis
Each 5 × 5 deg card was surrounded by a 7 × 7 deg Area of Interest (AOI), as larger AOI’s are recommended by Tobii to help make gaze measurements robust against modest calibration errors (“Tobii Pro Support,” 2016). Fixations were identified using the (default) Tobii Fixation Filter1 . We measured performance based on whether the Match or the Non-Match card received the first fixation within the 2.75 s response phase. Which card received the longer look (based on the total accumulated looking time) was also analyzed. Analyses of gaze data were done using custom MATLAB scripts.
2.4.2. Pupil data analysis
Pupil data were collected from the Tobii T120 eye-tracker, sampled at 60 Hz from each eye. The eye-tracker adjusted pupil measurements according to the distance between the eye and the sensor, and the position of the eyes, to estimate the actual, external physical pupil size throughout the trial. Analyses on this output, including functional data analyses, were done using custom MATLAB scripts adapted from Jackson and Sirois (2009). Permutation analyses used scripts developed by Mohinish Shukla, based on the analysis described in (Oakes et al., 2013). Preprocessing of pupil data consisted of the following steps: 1) trials where the participants did not fixate both of the cards during the encoding phase were discarded (this helps to equalize gaze behavior and retinal illumination across trials and participants; 8% of trials were excluded in this way); 2) raw pupil dilation values from the two eyes were averaged (if both were present); 3) outlier values (e.g., spurious spikes in pupil estimates due to blinks) based on median absolute deviation from the local median within a sliding, 500 ms window, were discarded and replaced with nearest-neighbor values; 4) missing data were interpolated with nearest-neighbor values; 5) the trace was smoothed using a robust quadratic regression over 500 ms windows; 6) subtractive baseline correction was done by determining the median pupil size during the initial 500 ms of each trial (i.e. during the initial phase of the trial when only an empty background was displayed), and then subtracting that value from the trace (Mathôt et al., 2018); 7) the encoding phase of the trace was isolated (i.e. the 4.5 s period beginning with the reveal of the first to-be-remembered Match/Non-Match card to just before the reveal of the Sample); and, 8) the trace was discarded if there had been a gap of missing data that exceeded a threshold (here, instead of an arbitrary data quality threshold, we used a biologically plausible one corresponding to a pupillary response latency of 220 ms, i.e. the minimal time window in which a task-evoked change in pupil could be expected to occur (Mathôt et al., 2015); any trace with a gap of that size or larger during baseline or encoding phases was discarded). For those analyses requiring combining traces across the trials of a participant, this was done on the basis of medians (which is more robust than the mean to extreme values due to potential sources of noise such as blinks, eye movements, equipment error, or off-screen glances).
3. Results
3.1. Behavioral performance
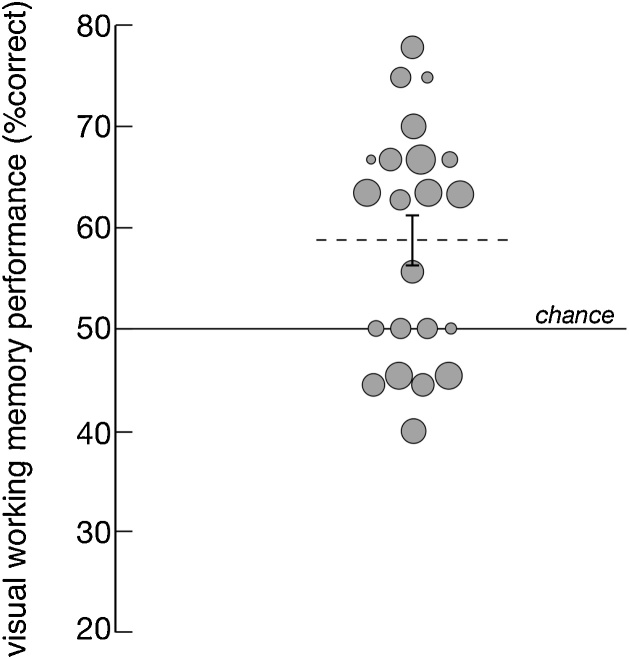
On average, participants completed 8.5 out of 12 test trials. For each infant participant, we calculated VWM performance as the percent of correct responses based on the dependent variable of first look, that is, which of the two face-down cards, Match or Non-Match was fixated first during the response phase (i.e., after the Sample was revealed). (The latencies themselves for this first look, given that participants were still fixating the just-exposed Sample, were relatively long, at 1539 ms (SD = 220 ms) and 1418 ms (SD = 429 ms) for the Match and Non-Match cards, respectively; a non-significant difference (t(38) = 1.12, p = 0.27, d = 0.35). If the infant did not look at either of the AOIs during the response phase, the trial was excluded from further analysis. Infants’ average performance was 59% correct, which was significantly above chance (50% correct) according to a one- sample t-test (t(21) = 3.58; p = 0.002, d = 1.56) (Fig. 2). The overall pattern of performance was the same based on which of the two cards (Match or Non-Match) garnered the longer look during the response phase (58% correct; t(21) = 2.42, p = 0.025, d = 1.06); accumulated total fixation duration itself reflected this as well, averaging 359 ms (SD = 181 ms) and 252 ms (SD = 120 ms) for the Match and Non-Match cards, respectively. All subsequent analyses rely on results based on the first look measure.
Fig. 2.
Individual and group average VWM performance (percent correct responses based on first looks). Each data point represents an infant participant, and the size of the point corresponds to the number of valid trials the infant contributed. Dotted line indicates average performance (error bar reflects SEM). Solid line indicates chance level (50%).
We tested the effects of the side of the Match card (left/right), whether the Match was presented first or second, and the object on the card. None of these effects were significant (all χ² < 0.76, p > 0.51).
3.2. Task-evoked pupil responses
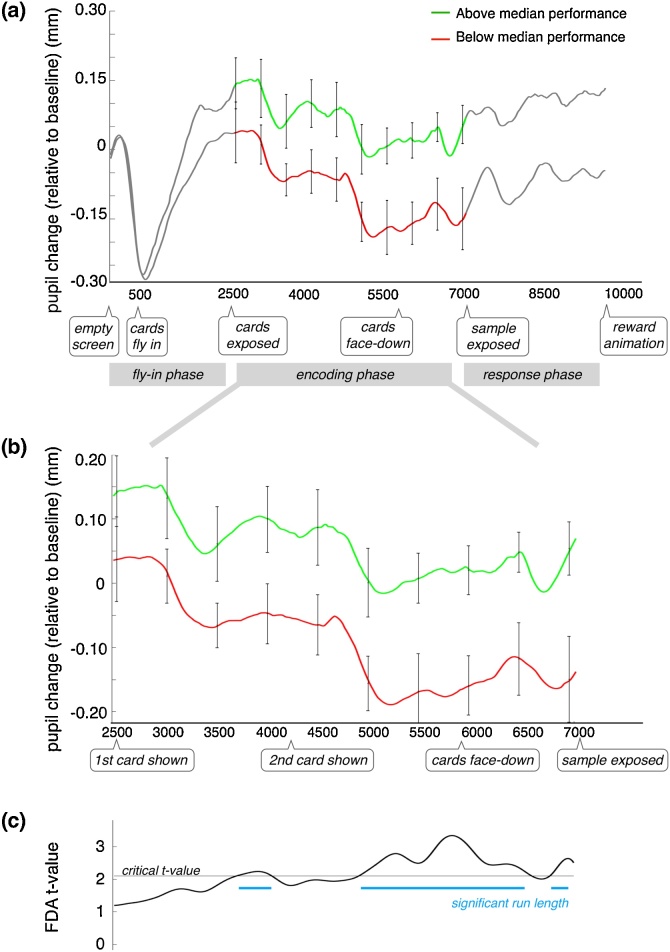
Here we addressed two questions about how the TEPR was related to VWM performance. 1) Is greater TEPR during memory encoding associated with higher-performing participants (and lesser, with lower-performing participants)? And, 2) is greater TEPR during encoding associated with correct trials (and lower, with incorrect)? To address the first question, we did a median split on participants based on performance. The pupil traces from a participant’s trials were combined (based on medians, see 2.4 Pupil data analysis) to generate a representative pupil trace for that participant’s encoding phase. The resulting traces for the 10 participants in the above-median group, and the 10 in the below-median group, were then averaged (Fig. 3a/b, green and red curves, respectively). The resulting laminar curves suggest that greater TEPR is associated with higher performance. Since TEPR is a continuous variable over time, Functional Data Analysis can be used to assess the difference between traces (Jackson and Sirois, 2009; Ramsay and Silverman, 1997). The curve in Fig. 3c shows the results of a functional t-test, with the value of the (uncorrected) t-statistic driven by the difference between the two pupil traces, over time2 . For reference, the curve is shown with respect to the two-tailed critical value for the t distribution. To test for a significant difference between the TEPR of the groups, we conducted a permutation analysis based on that of Oakes et al. (2013), which itself was derived from an approach used for EEG data (Groppe et al., 2011; Maris, 2012; Maris and Oostenveld, 2007).3 The permutation analysis revealed a significant difference between the groups that was most pronounced in the second half of the encoding phase (Fig. 3c). We used mean pupil diameter over that second half of the phase in a subsequent regression analysis (see below).
Fig. 3.
(a) Mean task-evoked pupil responses (TEPR), over time (ms), based on a median-split of individual VWM performance. Relevant trial events and phases are indicated below the time axis. The encoding phase is demarcated by the colored portion of the traces: the green curve reflects the mean pupil trace of the 10 above-median participants; red curve, the lower 10. Pupil values correspond to changes in (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
diameter (mm), relative to baseline (subtractive baseline correction was based on the first 500 ms of the trial). Error bars reflect SEM. (b) Data from (a), shown just for the encoding phase, to aid visualization. (c) Results of functional data analysis over the encoding phase, showing the uncorrected t-statistic based on the sets of median-split traces. The black horizontal line indicates the critical t-value (p < 0.05, two-tailed). A permutation test was applied; blue lines indicate regions deemed to have significantly long (p < 0.05) runs of above-critical tvalues (Oakes et al., 2013).
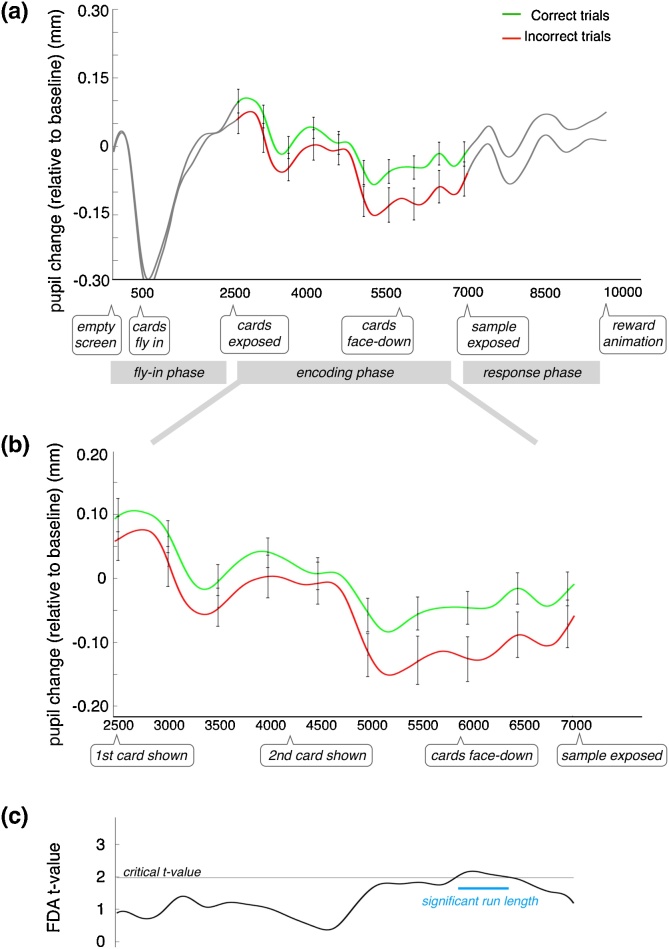
To address the second question, we averaged the individual traces from correct trials (n = 80), and those from incorrect trials (n = 52)4, drawn from the same 20 participants used in the previous analysis. The resulting laminar curves indicate that greater TEPR during memory encoding leads to a greater likelihood of a correct response on that particular trial (Fig. 4a/b). Here again, we performed a functional t-test, and tested for a significant difference with a permutation analysis. As with the median-split group analysis, there was a significant difference between the correct and incorrect trials, and this difference was most pronounced near the end of the encoding phase (Fig. 4c).
Fig. 4.
(a) Mean TEPR based on a binning of trials as either correct (N = 80) or incorrect (N = 52). Relevant events during the trial are indicated below the time axis. The encoding phase is demarcated by the colored portion of the traces: the green curve reflects the mean of all trials that ended in a correct response; red curve, incorrect. Error bars reflect SEM. (b) Data from (a), shown just for the encoding phase, to aid visualization. (c) The t-value from a functional t-test between the sets of correct/incorrect traces. The blue line indicates the results of the permutation test (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
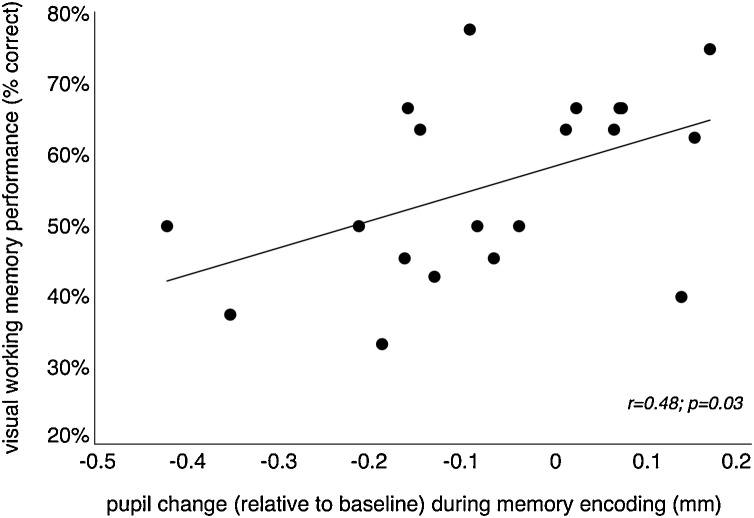
The FDA and permutation analyses on the TEPR of above- and below-median performing participants identified the second half of the encoding phase as the interval where differences were most pronounced (Fig. 3c). We used the mean TEPR value over that interval, for each participant, to further characterize the relationship between pupil response and performance. These exploratory analyses are not meant as hypothesis testing for a TEPR-performance relationship, per se, since the permutation analyses have already served that purpose, but instead, 1) to determine how well, relatively, the TEPR accounted for performance versus a more traditional measure of attention, i.e. total looking time to the cards during encoding; and, 2) as an exercise, to determine a function that relates mm’s of TEPR to increments in VWM performance. To address the first question, since it was possible that the amount of time participants spent looking at the Match and Non-Match cards during encoding (while the cards were face-up) influenced performance, we included total looking time as a factor in a stepwise multiple regression along with mean TEPR. As expected, results showed that mean TEPR over the second half of encoding phase was significantly related to VWM performance (F(1,18) = 5.36, p = 0.033), with a correlation coefficient R of 0.485 . However, the total looking time during encoding was deemed unnecessary as a predictor (t(18) = 0.57, p = 0.58). To address the second question, we quantified the relationship between each participant's mean TEPR versus their VWM performance (Fig. 5). The slope of the regression function was 0.38, and intercept was 0.58, thus the relationship between TEPR and performance can be expressed as Performance = 0.38 * TEPR + 0.58. In other words, every 0.1 mm increase in pupil dilation during (the latter half of) encoding was associated with a 3.8% increase in performance.
Fig. 5.
Correlation between infants’ (N = 20) mean task-evoked pupil response (TEPR) over the second half of the encoding phase (a permutation analysis revealed that this period showed the most pronounced difference between the groups; see Results) and their VWM performance (i.e., percent correct based on the percent of trials in which infants made an anticipatory saccade to the Match card before the Non-Match card during the response phase of a trial).
3.3. The potential effect of brightness differences
The luminance of on-screen elements can affect pupil dilation (i.e., the fundamental rise and fall of the pupil response over the trial). Much of this was sidestepped by the fact that the background of the display and the ‘backs’ of cards were physically isoluminant, both at approximately 48 cd/m2, thus, when the cards were face-down, the display was nominally isoluminant. The (spatially averaged) faces of cards were brighter than the background, but similar to one another at approximately 61 cd/m2. Some overall pupil changes over time, then, were due to factors like on-screen elements and events (e.g. the dip at the beginning of the trial, as seen in Fig. 3a, which corresponded to the onset of the display). However, such factors alone cannot account for the pupil response differences - between above-median and below-median performing participants, and between correct and incorrect trials - that we observed. That said, of concern is whether looking behavior, for instance, fixation patterns on the cards during encoding, could create a time-course of retinal illumination that would drive the pupillary light reflex in a way that could masquerade as a TEPR. In short, is the pupil larger in better performing participants, for instance, because they fixate the cards for less time? (It is relevant to note here that the brighter face of the cards would tend to contract the pupil, while the TEPR patterns we observe here show increases.)
To minimize this concern, we only analyzed pupil data on trials where the participant fixated both of the cards during encoding, allowing for a fairer comparison; any perturbation induced by the card-face luminance should be better equalized across trials and participants. As a check, we looked at total fixation duration on the Match and Non-Match cards6, during the first 3.5 s of encoding phase (the period that corresponded to the face up cards, without the final 1 s when they were face-down). The above-median performing group of participants averaged 2.925 s (SD = 0.219 s) of looking, while the below-median group averaged 2.928 ms (SD = 0.303 s), a non-significant difference (t(18) = -0.03, p = 0.98, d = 0.01). (The rest of this interval was spent looking at the face-down Sample card, with a total fixation duration of 0.52 s and 0.48 s for the above- and below-median groups, respectively). It is important to note that, in general, it is not unreasonable to expect that looking time to the cards during encoding could affect VWM performance (Colombo and Mitchell, 1990), but the context of the present experiment it did not seem to. This is likely because the engaging flipping animation of the Match and Non-Match cards over a short (3.5 s) interval rendered looking time effectively ‘at ceiling’ (at approximately 3 s); what was left to vary, then, was the allocation of focused attention during this period7 .
Most directly, we looked back at the regression analysis to see if there was a correlation between participants’ average total fixation duration on the cards during encoding, and his/her pupil response (i.e., the value used in our regression analyses above). There was no significant correlation (r = -0.17, p = 0.23). Along with the results of our stepwise regression analysis, where fixation duration was not deemed a necessary variable (but TEPR was), this was consistent with the conclusion that the TEPR differences we observed were driven by differences in focused attention.
4. Discussion
In the current study, we used task-evoked pupil responses (TEPR) to capture how moment-by-moment changes in focused attention influence visual working memory (VWM) performance in 13-month-old infants. We found that infants with greater focused attention during memory encoding better remembered object-location bindings in a subsequent test, based on a proactive, anticipatory saccadic response. While we look forward to replicating and expanding this finding with a larger sample in future work, the present study is the first, to our knowledge, to use TEPR to measure focused attention during an ongoing VWM task in infants.
Our results agree with prior infant EEG studies showing that focused attention can modulate VWM performance by the beginning of the second year of life (Begus et al., 2015). Further, we found that looking time at the items during encoding was not required as a predictive variable, suggesting it was the quality of encoding that mattered, not the quantity. (This is not to say that looking time is not an important and necessary index of engagement - but in our brief, dynamic encoding phase, participants’ looking time was effectively maximized, so the TEPR was the better, sufficient, index of focused attention.) It is important to note here that the differences we observed in TEPR were not limited to the encoding period - differences were present during a fly-in period that preceded encoding, and during a reward animation that followed it. However, those differences cannot be linked to infants’ better memory directly, since there was no information presented during those periods that could be used to solve the task.
Similarly to the findings in children that poor performance may reflect lower cognitive effort, rather than weaker ability (Chevalier, 2018), infants with lower performance in our study paid less (task-relevant) attention. By the second year, infants are able to recruit higher-level cognitive strategies, such as chunking by conceptual knowledge (Feigenson and Halberda, 2008), physical cues (Rosenberg and Feigenson, 2013), and social information (Stahl and Feigenson, 2014), to improve the effective capacity8 of their VWM. Our results suggest that in addition to these higher-level cognitive factors, the amount of task-relevant focused attention that is being allocated on a moment-to-moment basis modulates infants’ VWM performance.
Taking this into account helps establish whether an observed difference between groups or individuals was due to greater competence per se (in the present case, the potential for greater memory capacity), or because more focused attention was exerted (see e.g. Blaser et al., 2014). A full account of the abilities of a group or an individual should come from looking at those periods when focused attention is maximal. Focused attention has long been acknowledged as a crucial variable in studies of infant cognition. Researchers use varying, often loosely-defined assessments (fussiness, sleepiness, inability to habituate) as exclusion criteria (Slaughter and Suddendorf, 2007) and eliminate trials when the infant does not look at regions of interest. It is important to note that even when infants do look at those regions of interest, they may not be exerting focused attention, in other words, they may engage in a blank stare (Aslin, 2012). To date, there has been no formal way to track how focused attention varies as a function of trial, individual, or group; we propose that segmenting data by pupil dilation can facilitate this in future studies.
What determines whether a particular infant pays greater focused attention is outside the scope of the present study, but is a fruitful direction for future research. Several studies have looked at individual differences in different aspects of infants’ attention and their developmental trajectory. For example, Posner et al. (2012), found that visual orienting in free play to toys at 6-7-months of age predicted orienting scores in the Attention Network Test (ANT) at 4 years of age. As well, the speed of visual information processing in infancy predicts a variety of executive functions in childhood (Rose et al., 2012). More specifically, infants’ attentional focus has been demonstrated to be a crucial predictive factor for later executive functions (Joyce et al., 2018), and also has been shown to predict later self-regulatory behavior (Johansson et al., 2015). These findings suggest that the early ability to control focused attention has individual stability and longitudinal predictive value.
Author note
This research was supported by National Institutes of Health Grant R15HD086658. Preliminary results from this study were presented at the Annual Meeting of the Vision Sciences Society in May 2016 (Cheng et al., 2016). We would like to thank Sylvia Guillory for her contributions to a pilot study for this project (Guillory et al., 2014), and Sangya Dhungana and other members of the UMass Boston Baby Lab for their help with data collection.
Conflict of Interest
None.
Footnotes
Gaze position for each eye is collected at 60 Hz, and averaged between the two eyes to reduce noise. Then, missing data is interpolated (if the gap is under 100 ms). Following this, velocity peaks, within a sliding temporal window (of 5 frames @ 60 Hz, i.e. 83.8 ms), are identified. If a peak exceeds a set threshold value (0.42 pixels/ms) it is recorded as a new fixation (but if the distance between two candidate fixations is less than 35 pixels (<1 deg), they are merged). The duration of a fixation, then, is the elapsed time between peaks, and the position of the fixation is the median of the gaze coordinates during that interval (“Tobii Pro Support,” 2016).
Traces were fit with B-spline basis functions, with 24 bases (Jackson and Sirois, 2009).
Just as with EEG data, classic Bonferroni correction is considered inappropriately conservative for this type of (non-independent) time-course data. The permutation method repeatedly (here, 1000 times) shuffles the data, in time, and finds the length of the resulting runs of consecutive t-statistic values that exceed the critical value for the t-distribution (p<0.05, two-tailed), then compares the by-chance run lengths generated by this process to those observed in the original experimental data. In other words, it evaluates whether the observed runs from the experimental data should be expected by chance, or whether they were long enough to reject this hypothesis at p<0.05. For more detail, see Oakes et al. (2013).
If TEPR at this time point was an outlier (a value whose absolute deviation is more than 3.0 times the median absolute deviation), it was discarded (Leys et al., 2013). Data from three of the incorrect trials were discarded in this way.
Using the mean TEPR over the entire encoding phase, not just the second half where the difference between groups was most pronounced, still shows a marginally significant correlation with performance (R = 0.44; p = 0.053).
Latencies to (either of) the match and nonmatch cards at the start of the encoding phase averaged 206 ms (SD = 115 ms). Latencies were skewed to be short because participants typically have already planned or launched a saccade toward one of the (face down) Match/Non-Match cards, as the start of the encoding phase follows right after their fly-in.
This analysis also helps to allay concerns about the influence of gaze position on estimates of pupil dilation. The Tobii and other eye-trackers can have systematic biases in estimates of pupil size as a function of gaze position (Brisson et al., 2013). Our cards were were closely, and symmetrically, spaced around fixation, which should minimize biases, but more importantly, this analysis shows that participants, in both groups, spent nearly all of the encoding interval looking at the cards (3.45 s and 3.41 s out of 3.5s for the above- and below-median groups, respectively); i.e., gaze position distribution during encoding was indistinguishable between above- and below-median groups.
Here we use the term ‘effective capacity’ (Bick and Rabinovich, 2009) to refer to memory capacity after taking into account the effect of modulatory factors. We argue that focused attention has the power to modulate effective capacity from zero (as when, say, a participant is distracted) to some maximal level (given full, task-relevant attention).
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2019.100616.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Alnæs D., Sneve M.H., Espeseth T., Endestad T., van de Pavert S.H.P., Laeng B. Pupil size signals mental effort deployed during multiple object tracking and predicts brain activity in the dorsal attention network and the locus coeruleus. J. Vis. 2014;14(4):1–20. doi: 10.1167/14.4.1. [DOI] [PubMed] [Google Scholar]
- Aslin R.N. Infant eyes: a window on cognitive development. Infancy. 2012;17(1):126–140. doi: 10.1111/j.1532-7078.2011.00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astle D.E., Scerif G. Interactions between attention and visual short-term memory (VSTM): what can be learnt from individual and developmental differences? Neuropsychologia. 2011;49(6):1435–1445. doi: 10.1016/j.neuropsychologia.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G., Ennis M., Pieribone V.A., Nickell W.T., Shipley M.T. The brain nucleus locus coeruleus: restricted afferent control of a broad efferent network. Science. 1986;234(4777):734–737. doi: 10.1126/science.3775363. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G., Rajkowski J., Kubiak P., Alexinsky T. Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. J. Neurosci. 1994;14(7):4467–4480. doi: 10.1523/JNEUROSCI.14-07-04467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G., Cohen J.D. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Baddeley A. The fractionation of working memory. Proc. Natl. Acad. Sci. U.S.A. 1996;93(24):13468–13472. doi: 10.1073/pnas.93.24.13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A.D., Hitch G. Working memory. In: Bower Gordon H., editor. Volume 8. Academic Press; 1974. pp. 47–89. (Psychology of Learning and Motivation). [Google Scholar]
- Beatty J. Task-evoked pupillary responses, processing load, and the structure of processing resources. Psychol. Bull. 1982;91(2):276–292. [PubMed] [Google Scholar]
- Begus K., Southgate V., Gliga T. Neural mechanisms of infant learning: differences in frontal theta activity during object exploration modulate subsequent object recognition. Biol. Lett. 2015;11(5) doi: 10.1098/rsbl.2015.0041. 20150041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick C., Rabinovich M.I. Dynamical origin of the effective storage capacity in the brain’s working memory. Phys. Rev. Lett. 2009;103(21) doi: 10.1103/PhysRevLett.103.218101. [DOI] [PubMed] [Google Scholar]
- Blaser E., Eglington L., Carter A.S., Kaldy Z. Pupillometry reveals a mechanism for the autism Spectrum disorder (ASD) advantage in visual tasks. Sci. Rep. 2014;4:4301. doi: 10.1038/srep04301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret S., Duvel A., Onat S., Sara S.J. Phasic Activation of Locus Ceruleus Neurons by the Central Nucleus of the Amygdala. J. Neurosci: Off. J. Soc. Neurosci. 2003;23(8):3491–3497. doi: 10.1523/JNEUROSCI.23-08-03491.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson J., Mainville M., Mailloux D., Beaulieu C., Serres J., Sirois S. Pupil diameter measurement errors as a function of gaze direction in corneal reflection eyetrackers. Behav. Res. Methods. 2013;45(4):1322–1331. doi: 10.3758/s13428-013-0327-0. [DOI] [PubMed] [Google Scholar]
- Carrasco M. Visual attention: the past 25 years. Vision Res. 2011;51(13):1484–1525. doi: 10.1016/j.visres.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C., Kaldy Z., Blaser E. Accounting for cognitive effort in a visual working memory task in 13- and 15-month old infants. Presented at the Annual Meeting of the Vision Sciences Society; May, St. Pete Beach, FL; 2016. [Google Scholar]
- Chevalier N. Willing to think hard? The subjective value of cognitive effort in children. Child Dev. 2018;89(4):1283–1295. doi: 10.1111/cdev.12805. [DOI] [PubMed] [Google Scholar]
- Chun M.M., Golomb J.D., Turk-Browne N.B. A taxonomy of external and internal attention. Annu. Rev. Psychol. 2011;62:73–101. doi: 10.1146/annurev.psych.093008.100427. [DOI] [PubMed] [Google Scholar]
- Cohen L.B. Attention-getting and attention-holding processes of infant visual preferences. Child Dev. 1972;43(3):869–879. [PubMed] [Google Scholar]
- Colombo J., Cheatham C.L. Volume 34. Elsevier; 2006. The emergence and basis of endogenous attention in infancy and early childhood; pp. 283–322. (Advances in Child Development and Behavior). [DOI] [PubMed] [Google Scholar]
- Colombo J., Mitchell D.W. Individual Differences in Infancy: Reliability, Stability, Prediction; 1990. Individual Differences in Early Visual Attention: Fixation Time and Information Processing; pp. 193–227. [Google Scholar]
- Courage M.L., Richards J.E. Attention. In: Haith M.M., Benson J.B., editors. Encyclopedia of Infant and Early Childhood Development. Academic Press; San Diego: 2008. pp. 106–117. [Google Scholar]
- Cowan N., Elliott E.M., Scott Saults J., Morey C.C., Mattox S., Hismjatullina A., Conway A.R.A. On the capacity of attention: its estimation and its role in working memory and cognitive aptitudes. Cogn. Psychol. 2005;51(1):42–100. doi: 10.1016/j.cogpsych.2004.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein M.K., Guerra-Carrillo B., Miller Singley A.T., Bunge S.A. Beyond eye gaze: what else can eyetracking reveal about cognition and cognitive development? Dev. Cogn. Neurosci. 2017;25:69–91. doi: 10.1016/j.dcn.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar E., Cohen J.D., Niv Y. The effects of neural gain on attention and learning. Nat. Neurosci. 2013;16(8):1146–1153. doi: 10.1038/nn.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenson L., Halberda J. Conceptual knowledge increases infants’ memory capacity. Proc. Natl. Acad. Sci. U.S.A. 2008;105(29):9926–9930. doi: 10.1073/pnas.0709884105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick J.E., Colombo J., Saxon T.F. Individual and developmental differences in disengagement of fixation in early infancy. Child Dev. 1999;70(3):537–548. doi: 10.1111/1467-8624.00039. [DOI] [PubMed] [Google Scholar]
- Geangu E., Hauf P., Bhardwaj R., Bentz W. Infant pupil diameter changes in response to others’ positive and negative emotions. PLoS One. 2011;6(11) doi: 10.1371/journal.pone.0027132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilzenrat M.S., Nieuwenhuis S., Jepma M., Cohen J.D. Pupil diameter tracks changes in control state predicted by the adaptive gain theory of locus coeruleus function. Cogn. Affect. Behav. Neurosci. 2010;10(2):252–269. doi: 10.3758/CABN.10.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gredebäck G., Melinder A. Infants’ understanding of everyday social interactions: a dual process account. Cognition. 2010;114(2):197–206. doi: 10.1016/j.cognition.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Groppe D.M., Urbach T.P., Kutas M. Mass univariate analysis of event-related brain potentials/fields I: a critical tutorial review. Psychophysiology. 2011;48(12):1711–1725. doi: 10.1111/j.1469-8986.2011.01273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillory S.B., Blaser E., Kaldy Z. Task-evoked pupillary response predicts performance in a visual working memory task in 7-10-months-olds. Presented at the International Conference on Infant Studies; Berlin, Germany; 2014. [Google Scholar]
- Gweon H., Schulz L. BREVIA 16-Month-Olds rationally infer causes of failed actions. Science. 2011;332(6037):1524. doi: 10.1126/science.1204493. [DOI] [PubMed] [Google Scholar]
- Hellmer K., Söderlund H., Gredebäck G. The eye of the retriever: developing episodic memory mechanisms in preverbal infants assessed through pupil dilation. Dev. Sci. 2016;21:e12520. doi: 10.1111/desc.12520. [DOI] [PubMed] [Google Scholar]
- Hepach R., Westermann G. Pupillometry in infancy research. J. Cognit. Dev. 2016;17(3):359–377. [Google Scholar]
- Hess E.H., Polt J.M. Pupil size in relation to mental activity during simple problem-solving. Science. 1964;143(3611):1190–1192. doi: 10.1126/science.143.3611.1190. [DOI] [PubMed] [Google Scholar]
- Jackson I., Sirois S. Infant cognition: going full factorial with pupil dilation. Dev. Sci. 2009;12(4):670–679. doi: 10.1111/j.1467-7687.2008.00805.x. [DOI] [PubMed] [Google Scholar]
- Johansson M., Marciszko C., Gredebäck G., Nyström P., Bohlin G. Sustained attention in infancy as a longitudinal predictor of self-regulatory functions. Infant Behav. Dev. 2015;41:1–11. doi: 10.1016/j.infbeh.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Johnson E.L., Miller Singley A.T., Peckham A.D., Johnson S.L., Bunge S.A. Task-evoked pupillometry provides a window into the development of short-term memory capacity. Front. Psychol. 2014;5:218. doi: 10.3389/fpsyg.2014.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S., Li Y., Kalwani R.M., Gold J.I. Relationships between pupil diameter and neuronal activity in the locus coeruleus, Colliculi, and cingulate cortex. Neuron. 2016;89(1):221–234. doi: 10.1016/j.neuron.2015.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce A.W., Friedman D.R., Wolfe C.D., Bell M.A. Executive attention at 8 years: concurrent and longitudinal predictors and individual differences. Infant Child Dev. 2018;27(2):e2066. doi: 10.1002/icd.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D. Vol. 1063. Prentice-Hall; Englewood Cliffs, NJ: 1973. (Attention and effort). [Google Scholar]
- Kahneman D., Beatty J. Pupil diameter and load on memory. Science. 1966;154(3756):1583–1585. doi: 10.1126/science.154.3756.1583. [DOI] [PubMed] [Google Scholar]
- Kaldy Z., Guillory S.B., Blaser E. Delayed match retrieval: a novel anticipation-based visual working memory paradigm. Dev. Sci. 2016;19(6):892–900. doi: 10.1111/desc.12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane M.J., Engle R.W. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychon. Bull. Rev. 2002;9(4):637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Kane M.J., Engle R.W. Working-memory capacity and the control of attention: the contributions of goal neglect, response competition, and task set to Stroop interference. J. Exp. Psychol. Gen. 2003;132(1):47–70. doi: 10.1037/0096-3445.132.1.47. [DOI] [PubMed] [Google Scholar]
- Karatekin C., Marcus D.J., Couperus J.W. Regulation of cognitive resources during sustained attention and working memory in 10-year-olds and adults. Psychophysiology. 2007;44(1):128–144. doi: 10.1111/j.1469-8986.2006.00477.x. [DOI] [PubMed] [Google Scholar]
- Klingner J., Tversky B., Hanrahan P. Effects of visual and verbal presentation on cognitive load in vigilance, memory, and arithmetic tasks. Psychophysiology. 2011;48(3):323–332. doi: 10.1111/j.1469-8986.2010.01069.x. [DOI] [PubMed] [Google Scholar]
- Koss M.C. Pupillary dilation as an index of central nervous system alpha 2-adrenoceptor activation. J. Pharmacol. Methods. 1986;15(1):1–19. doi: 10.1016/0160-5402(86)90002-1. [DOI] [PubMed] [Google Scholar]
- Kowler E., Anderson E., Dosher B., Blaser E. The role of attention in the programming of saccades. Vision Res. 1995;35(13):1897–1916. doi: 10.1016/0042-6989(94)00279-u. [DOI] [PubMed] [Google Scholar]
- Laeng B., Sirois S., Gredebäck G. Pupillometry: a window to the preconscious? Perspectives on psychological science. Perspect. Psychol. Sci. 2012;7(1):18–27. doi: 10.1177/1745691611427305. [DOI] [PubMed] [Google Scholar]
- Leys C., Ley C., Klein O., Bernard P., Licata L. Detecting outliers: do not use standard deviation around the mean, use absolute deviation around the median. J. Exp. Soc. Psychol. 2013;49(4):764–766. [Google Scholar]
- Malecek N.J., Poldrack R.A. Beyond dopamine: the noradrenergic system and mental effort [Peer commentary on Kurzban et al.’s An opportunity cost model of subjective effort and task performance] Behav. Brain Sci. 2013;36:692–693. doi: 10.1017/S0140525X13001106. [DOI] [PubMed] [Google Scholar]
- Maris E. Statistical testing in electrophysiological studies. Psychophysiology. 2012;49(4):549–565. doi: 10.1111/j.1469-8986.2011.01320.x. [DOI] [PubMed] [Google Scholar]
- Maris E., Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods. 2007;164(1):177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Martinez-Alvarez A., Pons F., de Diego-Balaguer R. Endogenous temporal attention in the absence of stimulus-driven cues emerges in the second year of life. PLoS One. 2017;12(9) doi: 10.1371/journal.pone.0184698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathôt S., van der Linden L., Grainger J., Vitu F. The pupillary light response reflects eye-movement preparation. J. Exp. Psychol. Hum. Percept. Perform. 2015;41(1):28–35. doi: 10.1037/a0038653. [DOI] [PubMed] [Google Scholar]
- Mathôt S., Fabius J., Van Heusden E., Van der Stigchel S. Safe and sensible preprocessing and baseline correction of pupil-size data. Behav. Res. Methods. 2018;50(1):94–106. doi: 10.3758/s13428-017-1007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg M.J., Watrous A.J., Yoon J.H., Ursu S., Carter C.S. Modafinil shifts human locus coeruleus to low-tonic, high-phasic activity during functional MRI. Science. 2008;322(5908):1700–1702. doi: 10.1126/science.1164908. [DOI] [PubMed] [Google Scholar]
- Murphy P.R., O’Connell R.G., O’Sullivan M., Robertson I.H., Balsters J.H. Pupil diameter covaries with BOLD activity in human locus coeruleus. Hum. Brain Mapp. 2014;35(8):4140–4154. doi: 10.1002/hbm.22466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly J.X., Schüffelgen U., Cuell S.F., Behrens T.E.J., Mars R.B., Rushworth M.F.S. Dissociable effects of surprise and model update in parietal and anterior cingulate cortex. Proc. Natl. Acad. Sci. U.S.A. 2013;110(38):e3660–e3669. doi: 10.1073/pnas.1305373110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes L.M., Baumgartner H.A., Barrett F.S., Messenger I.M., Luck S.J. Developmental changes in visual short-term memory in infancy: evidence from eye-tracking. Front. Psychol. 2013;4:697. doi: 10.3389/fpsyg.2013.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen S.E., Posner M.I. The attention system of the human brain: 20 years after. Annu. Rev. Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter G., Troscianko T., Gilchrist I.D. Effort during visual search and counting: insights from pupillometry. Q. J. Exp. Psychol. 2007;60(2):211–229. doi: 10.1080/17470210600673818. [DOI] [PubMed] [Google Scholar]
- Posner M.I., Petersen S.E. The attention system of the human brain. Annu. Rev. Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posner M.I., Rothbart M.K., Sheese B.E., Voelker P. Control networks and neuromodulators of early development. Dev. Psychol. 2012;48(3):827–835. doi: 10.1037/a0025530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuschoff K., ’t Hart B.M., Einhäuser W. Pupil dilation signals surprise: evidence for noradrenaline’s role in decision making. Front. Neurosci. 2011;5:115. doi: 10.3389/fnins.2011.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowski J., Kubiak P., Aston-Jones G. Locus coeruleus activity in monkey: phasic and tonic changes are associated with altered vigilance. Brain Res. Bull. 1994;35(5-6):607–616. doi: 10.1016/0361-9230(94)90175-9. [DOI] [PubMed] [Google Scholar]
- Ramsay J.O., Silverman B.W. Springer-Verlag; New York: 1997. Functional Data Analysis. [Google Scholar]
- Reynolds G.D., Guy M.W., Zhang D. Neural Correlates of Individual Differences in Infant Visual Attention and Recognition Memory. Infancy: Off. J. Int. Soc. Infant Stud. 2011;16(4):368–391. doi: 10.1111/j.1532-7078.2010.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J.E. The Development of Sustained Visual Attention in Infants from 14 to 26 Weeks of Age. Psychophysiology. 1985;22(4):409–416. doi: 10.1111/j.1469-8986.1985.tb01625.x. [DOI] [PubMed] [Google Scholar]
- Richards J.E. Infant Visual Sustained Attention and Respiratory Sinus Arrhythmia. Child Dev. 1987;58(2):488–496. [PubMed] [Google Scholar]
- Richards J.E. Effects of attention on infants’ preference for briefly exposed visual stimuli in the paired-comparison recognition-memory paradigm. Dev. Psychol. 1997;33(1):22–31. doi: 10.1037//0012-1649.33.1.22. [DOI] [PubMed] [Google Scholar]
- Richards J.E. Handbook of Developmental Cognitive Neuroscience. 2001. Attention in young infants: a developmental psychophysiological perspective; pp. 321–338. [Google Scholar]
- Richards J.E. Attention affects the recognition of briefly presented visual stimuli in infants: an ERP study. Dev. Sci. 2003;6(3):312–328. doi: 10.1111/1467-7687.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S.A., Feldman J.F., Jankowski J.J. Implications of infant cognition for executive functions at age 11. Psychol. Sci. 2012;23(11):1345–1355. doi: 10.1177/0956797612444902. [DOI] [PubMed] [Google Scholar]
- Rosenberg R.D., Feigenson L. Infants hierarchically organize memory representations. Dev. Sci. 2013;16(4):610–621. doi: 10.1111/desc.12055. [DOI] [PubMed] [Google Scholar]
- Ruff H.A. Components of attention during infants’ manipulative exploration. Child Dev. 1986;57(1):105–114. doi: 10.1111/j.1467-8624.1986.tb00011.x. [DOI] [PubMed] [Google Scholar]
- Samuels E.R., Szabadi E. Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part II: physiological and pharmacological manipulations and pathological alterations of locus coeruleus activity in humans. Curr. Neuropharmacol. 2008;6(3):254–285. doi: 10.2174/157015908785777193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara S.J. The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci. 2009;10(3):211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Sirois S., Jackson I.R. Pupil dilation and object permanence in infants. Infancy. 2012;17(1):61–78. doi: 10.1111/j.1532-7078.2011.00096.x. [DOI] [PubMed] [Google Scholar]
- Slaughter V., Suddendorf T. Participant loss due to “fussiness” in infant visual paradigms: a review of the last 20 years. Infant Behav. Dev. 2007;30(3):505–514. doi: 10.1016/j.infbeh.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Stahl A.E., Feigenson L. Social knowledge facilitates chunking in infancy. Child Dev. 2014;85(4):1477–1490. doi: 10.1111/cdev.12217. [DOI] [PubMed] [Google Scholar]
- Stahl A.E., Feigenson L. Observing the unexpected enhances infants’ learning and exploration. Science. 2015;348(6230):91–94. doi: 10.1126/science.aaa3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Téglás E., Vul E., Girotto V., Gonzalez M., Tenenbaum J.B., Bonatti L.L. Pure reasoning in 12-month-old infants as probabilistic inference. Science. 2011;332(6033):1054–1059. doi: 10.1126/science.1196404. [DOI] [PubMed] [Google Scholar]
- Tobii Pro Support . 2016. Tobii Pro Support.https://www.tobiipro.com/learn-and-support/support/ Retrieved from. [Google Scholar]
- Treue S. Visual attention: the where, what, how and why of saliency. Curr. Opin. Neurobiol. 2003;13(4):428–432. doi: 10.1016/s0959-4388(03)00105-3. [DOI] [PubMed] [Google Scholar]
- Unsworth N., Robison M.K. Individual differences in the allocation of attention to items in working memory: evidence from pupillometry. Psychon. Bull. Rev. 2015;22(3):757–765. doi: 10.3758/s13423-014-0747-6. [DOI] [PubMed] [Google Scholar]
- Unsworth N., Robison M.K. A locus coeruleus-norepinephrine account of individual differences in working memory capacity and attention control. Psychon. Bull. Rev. 2017;24:1282. doi: 10.3758/s13423-016-1220-5. [DOI] [PubMed] [Google Scholar]
- Usher M., Cohen J.D., Servan-Schreiber D., Rajkowski J., Aston-Jones G. The role of locus coeruleus in the regulation of cognitive performance. Science. 1999;283(5401):549–554. doi: 10.1126/science.283.5401.549. [DOI] [PubMed] [Google Scholar]
- Wright J.C., Vlietstra A.G. Volume 10. Elsevier; 1975. The development of selective attention: from perceptual exploration to logical search; pp. 195–239. (Advances in Child Development and Behavior). [DOI] [PubMed] [Google Scholar]
- Xu F., Cote M., Baker A. Labeling guides object individuation in 12-month-old infants. Psychol. Sci. 2005;16(5):372–377. doi: 10.1111/j.0956-7976.2005.01543.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.