Abstract
Background
Dengue is a common and important mosquito‐borne viral infection. In many low‐ and middle‐income countries it is endemic and is an important public health problem. Severe dengue is an important cause of death in children. There is no specific treatment for dengue, but observational studies suggest corticosteroids may have a benefit in dengue‐related shock, and some people believe corticosteroids may prevent the progression to severe illness if given early in the course of the illness.
Objectives
To compare treatment of dengue with and without use of corticosteroids or placebo in relation to preventing shock‐related death and disease progression in children and adults.
Search methods
We searched the Cochrane Infectious Disease Group Centralized Register; CENTRAL; MEDLINE; EMBASE; and LILACS, up to 6 January 2014. We screened reference lists and contacted the relevant study authors for additional information where required.
Selection criteria
Randomized controlled trials or quasi‐randomized controlled trials comparing corticosteroids with placebo or no corticosteroids in patients diagnosed with dengue‐related shock, or patients in an early symptomatic state of dengue with positive serology.
Data collection and analysis
Two researchers independently screened eligibility of records, extracted data and assessed quality of the studies. We presented findings in meta‐analysis and summary of findings tables and evaluated the quality of evidence using GRADE.
Main results
We included eight studies enrolling 948 participants in this review.
Paitents with dengue‐related shock
Four studies enrolled children younger than 15 years with dengue‐related shock at hospitals in Southeast Asia and evaluated intravenous corticosteroids. The trials did not detect an effect on death (four trials, 284 participants, very low quality evidence), the need for blood transfusion (two trials, 89 participants, very low quality evidence), pulmonary haemorrhage (one trial, 63 participants, very low quality evidence), convulsions (one trial, 63 participants, very low quality evidence), or duration of hospitalization (one trial, 63 participants, very low quality evidence). The body of evidence is too small to confidently prove or exclude clinically important effects. Furthermore, the trials are more than 20 years old with several methodological limitations.
Patients with dengue at an early stage
Four studies enrolled 664 children and adults with dengue at an early stage of infection (without shock) in Columbia, India, Sri Lanka and Vietnam. In these participants there were no evidence of effects of oral or intravenous corticosteroids on mortality (four trials, 664 participants, low quality evidence), or on the development of complications of severe dengue such as shock (two trials, 286 participants, very low quality evidence), severe bleeding (two trials, 425 participants, very low quality evidence), severe thrombocytopaenia (one trial, 225 participants, very low quality evidence), ascites (one trial, 178 participants, very low quality evidence) and intensive care unit (ICU) admissions (two trials, 286 participants, very low quality evidence).
Authors' conclusions
The evidence from trials using corticosteroids in dengue is inconclusive and the quality of evidence is low to very low. This applies to both the use of corticosteroids in dengue‐related shock and for dengue at an early stage. There is insufficient evidence to evaluate the effects of corticosteroids in the treatment of early stage dengue fever and dengue‐related shock outside of the context of a randomized controlled trial.
Keywords: Adult; Child; Humans; Adrenal Cortex Hormones; Adrenal Cortex Hormones/therapeutic use; Blood Transfusion; Blood Transfusion/statistics & numerical data; Randomized Controlled Trials as Topic; Severe Dengue; Severe Dengue/drug therapy; Severe Dengue/mortality; Shock, Hemorrhagic; Shock, Hemorrhagic/drug therapy; Shock, Hemorrhagic/mortality
Corticosteroids for treating dengue infection in children and adults
Dengue is a disease caused by a virus transmitted by mosquitoes, occurring in many resource‐limited countries, and children are often most severely affected. Most infected patients will recover with mild symptoms, but a few progress to severe dengue and may die. There is no specific treatment for dengue, but some clinicians provide corticosteroids at an early stage to prevent progression to severe dengue disease; and some treat patients with dengue‐related shock with corticosteroids to improve survival. It is important to summarise the effects of corticosteroids in dengue.
We conducted a search up to 6 January 2014 and included eight studies which enrolled 948 participants in total. Four studies of corticosteroids in the treatment of dengue‐related shock assessed if corticosteroids could improve survival, but these studies were small and older than 20 years. The evidence we found is of very low quality and do not provide any reliable evidence for corticosteroids for treating dengue‐related shock. Four trials evaluated whether corticosteroids provided at an early stage of dengue could prevent development of complications of severe dengue. These trials were more recent, but data were insufficient to be sure whether corticosteroids have an effect on the course of the disease.
Summary of findings
Summary of findings for the main comparison.
Corticosteroid for dengue‐related shock
| Corticosteroid for dengue‐related shock | ||||||
|
Patient or population: Patients with dengue‐related shock Settings: Endemic settings Intervention: Corticosteroid Outcome: Complications of severe dengue | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Corticosteroid | |||||
| Death | 21 per 100 | 15 per 100 (9 to 24) | RR 0.68 (0.42 to 1.11) | 284 (4 studies) | ⊕⊝⊝⊝ very low1,2,3,4 | |
| Need for blood transfusion | 24 per 100 | 26 per 100 (12 to 54) | RR 1.08 (0.52 to 2.24) | 89 (2 studies) | ⊕⊝⊝⊝ very low5,6,7,8 | |
| Pulmonary haemorrhage | 3 per 100 | 3 per 100 (0 to 48) | RR 0.97 (0.06 to 14.82) | 63 (1 study) | ⊕⊝⊝⊝ very low9,10,11 | |
| Convulsions | 0 per 100 | 0 per 100 (0 to 0) | RR 6.79 (0.36 to 126.24) | 63 (1 study) | ⊕⊝⊝⊝ very low9,10,11 | |
| Days in hospital | The mean duration of hospital stay in the control group was 6 days | The mean duration of hospital stay in the intervention group was 1.1 days higher (1.83 lower to 4.03 higher) | 63 (1 study) | ⊕⊝⊝⊝ very low9,10,11 | ||
| *The basis for the assumed risk (eg the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded by 1 for serious risk of bias: Three out of four studies were at unclear risk of selection bias, as sequence generation and allocation concealment were not reported in sufficient detail. Blinding was adequate in two out of four studies. The only study that was at low risk of bias did not find an effect. 2 No serious inconsistency: Statistical heterogeneity was low. Three out of four studies found no evidence of a benefit with corticosteroids. Only the oldest study which was at unclear risk of bias suggested a benefit. 3 No serious indirectness: All trials were performed in children aged below 15 years in hospitals in Southeast Asia, and the data may not be easily generalizable to other populations or settings. All diagnoses were confirmed by a laboratory test, and an intravenous corticosteroid was used in all studies. Not downgraded. 4 Downgraded by 2 for very serious imprecision: The 95% CI is wide and includes a clinically important effect and no effect. The trials are too small to detect an effect. To confidently detect a 25% relative reduction in mortality would require a sample size of more than 1700 participants. 5 Downgraded by 1 for serious risk of bias: Sequence generation, allocation concealment and blinding was only reported adequately in one of the two studies. This study at low risk of bias did not find an effect. 6 No serious inconsistency. No statistical heterogeneity. 7 No serious indirectness: Both trials were conducted in children in referral hospitals in Thailand. They used viral and serologic diagnostic tests and a similar total dose of intravenous corticosteroids. The data may not be easily generalizable to other populations or settings. Not downgraded. 8 Downgraded by 2 for very serious imprecision: The 95% CI of RR was wide and the trials too small to detect an effect. To confidently detect a 25% relative reduction in need for transfusion would require a sample size of more than 1400 participants. 9 No serious risk of bias: The one trial in this comparison reported adequate sequence generation, blinding and allocation concealment. 10 Downgraded by 1 for serious indirectness: Only a single small study evaluated this outcome. Further studies in different patient groups are needed to have confidence in the results. 11 Downgraded by 2 for very serious imprecision: The 95% CI of the RR was wide and the trials too small to detect an effect. To confidently detect a 25% relative reduction would require a sample size of more than 10,000 participants.
Summary of findings 2.
Corticosteroid for dengue at an early stage
| Corticosteroid for dengue at an early stage | ||||||
|
Patient or population: Patients with dengue infection at an early stage Settings: Endemic settings Intervention: Corticosteroids Outcome: Complications of dengue | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Corticosteroid | |||||
| Death | ‐ | ‐ | ‐ | 664 (4 studies) | ⊕⊕⊝⊝ low1,2,3 | No deaths occurred |
| Severe dengue: shock | 7 per 100 | 9 per 100 (3 to 23) | RR 1.30 (0.48 to 3.51) | 286 (2 studies) | ⊕⊝⊝⊝ very low4,5,6 | |
| Severe dengue: severe bleeding | 1 per 100 |
1 per 100 (0 to 5) |
RR 1.51 (0.24 to 9.43) |
425 (2 studies) |
⊕⊝⊝⊝7,8,9,10 very low | Kularatne 2009 reported that no bleeding complications occurred |
| Severe thrombocytopaenia11 | 3 per 100 |
4 per 100 (1 to 19) |
RR 1.51 (CI (0.31 to 7.28) | 225 (1 study) | ⊕⊝⊝⊝ very low12,13,14 | |
| Ascitis | 4 per 100 | 1 per 100 (0 to 9) | RR 0.12 (0.01 to 2.13) | 178 (1 study) | ⊕⊝⊝⊝ very low12,15,16 | |
| ICU admission | 8 per 100 | 8 per 100 (8 to 106) | RR 0.88 (0.38 to 1.99) | 286 (2 studies) | ⊕⊝⊝⊝ very low4,5,6 | |
| *The basis for the assumed risk (eg the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 No serious risk of bias: Three out of four trials reported adequate random sequence generation, adequate allocation concealment and blinding of clinicians and participants. Two out of four trials reported adequate blinding of outcome assessors. One of the trials was available as conference abstract only and we got relevant information by email with the author.
2 No serious indirectness: The studies were conducted in different settings in South Asia (India, Sri Lanka), Southeast Asia (Vietnam) and Latin America (Colombia) in both adults and children. 3 Downgraded by 2 for very serious imprecision: No deaths were reported in either the intervention or placebo/no intervention group. 4 Downgraded by 1 for serious risk of bias: One study reported adequately on sequence generation, allocation concealment and blinding of participants, clinicians and outcome assessors. Another study was an open‐label trial, and did not describe allocation concealment. 5 Downgraded by 1 for serious indirectness: These studies were conducted in different settings in India and Vietnam, which may not be easily generalizable to a variety of settings. 6 Downgraded by 2 for very serious imprecision: One trial reported that results for this outcome did not show a statistically significant difference and might be underpowered to detect an effect. Another trial reported no case of shock in either the treatment or control groups. The 95% CI of the RR was wide and the trials too small to detect an effect. To confidently detect a 25% relative reduction would require a sample size of more than 8000 participants.
7 No serious risk of bias: The two trials that reported on this outcome both described adequate allocation concealment and blinding of clinicians and participants. The method of sequence generation was not clear in one study. 8 No serious inconsistency. No statistical heterogeneity. 9 Downgrade by 1 for serious indirectness: The two trials that reported on this outcome were conducted in Sri Lanka and Vietnam. The studies used different diagnostic criteria for dengue and for the diagnosis of haemorrhage and gave different doses of Prednisolone.
10Downgraded by 2 for very serious imprecision: Only four events were reported in the corticosteroid group, with only event in the placebo group. One of the two studies, conducted in Sri Lanka, did not report any events. The 95% CI of the RR was wide and the trials too small to detect an effect. To confidently detect a 25% relative reduction would require a sample size of more than 70,000 participants.
11 Severe thrombocytopaenia: platelet nadir < 10,000/μl.
12 No serious risk of bias: The one trial in this comparison reported adequate sequence generation, blinding and allocation concealment.
13Downgraded by 1 for serious indirectness: The one study reporting on this outcome was conducted in Vietnam, which may not be easily generalizable to a variety of settings.
14Downgraded by 2 for very serious imprecision: The single trial reporting on this outcome reported few events. The 95% CI of the RR was wide and the trials too small to detect an effect. To confidently detect a 25% relative reduction would require a sample size of more than 2000 participants.
15 Downgraded by 1 for serious indirectness: This single study from Colombia may not easily be generalizable to a variety of settings.
16Downgraded by 2 for very serious imprecision: The single trial that reported on this outcome reported few events. The 95% CI of the RR was wide and the trials too small to detect an effect. To confidently detect a 25% relative reduction would require a sample size of more than 9000 participants.
Background
Description of the condition
Dengue is a viral infection that causes fever, malaise (general feeling of discomfort and illness) and is occasionally fatal. There are in total four different strains of the dengue virus. The bite of the female infected Aedes mosquito, most commonly Aedes aegypti, transmits the virus to humans (WHO 2009; Simmons 2012).
Onset of the illness is sudden after an incubation period of three to 14 days (average four to seven days). In the early phases of the illness people have non‐specific flu‐like symptoms, nausea and vomiting, and half of them have a rash. The course of dengue is usually mild and people recover. However, sometimes complications occur in the critical phase when fever resolves on the third to seventh day of illness, usually on the fifth day (WHO 2009; Simmons 2012).
Before 2009, the WHO classified dengue fever (DF) and four stages of dengue haemorrhagic fever (DHF) according to clinical manifestations as shock or bleeding. Grade I and II were termed non‐shock DHF while grade III and IV were defined as dengue shock syndrome (DSS) (WHO 1997). This classification did not reliably identify severely sick patients, therefore the WHO introduced a new classification in 2009. This new classification differentiates between dengue, an uncomplicated disease with full recovery; and severe dengue. In severe dengue, an effect of the infection on the capillaries causes complications: the permeability of the capillaries increases and fluid leaks from the vessels into the tissue, causing a fall in blood pressure and shock. The WHO defines severe disease as:
plasma leakage leading to shock or breathing difficulties, or both;
severe bleeding;
severe organ impairment (WHO 2009).
Signs of dengue‐related shock are a narrow pulse pressure of 20 mmHg or less, and poor skin perfusion (Simmons 2012). Blood tests show increasing concentration of the blood and low levels of platelets and protein. The mechanisms leading to plasma leakage and the role of the immune system in the development of shock are unclear. Researchers suggest that the immune response can result in increased permeability of the capillaries. No animal models exist (WHO 2009; Simmons 2012).
Epidemiology
Dengue is the most common vector born viral infection in humans and the most rapidly spreading viral disease globally, and an important public health problem in low‐ and middle‐income countries in the tropics where most of the dengue infection happens The dispersal of the efficient mosquito vectors across much of the tropical and subtropical latitudes is crucial for the cause of public health problems. The primary vector has been widely distributed across these tropics (Simmons 2012). And the dengue epidemics are closely related to the seasonal climatic change and there are epidemics waves following each rainy season (Rajapakse 2012).
Over 40% of the world's population (about 2.5 billion) live in dengue‐endemic areas, and about 50 to 100 million people are infected with the dengue virus every year. Cases across the Americas, Southeast Asia and the Western Pacific exceeded 2.3 million in 2010. Its incidence has "multiplied many times over the last five decades at an alarming rate"(Rajapakse 2012). An estimated 500,000 people with severe dengue, of which a large proportion are children, require hospitalization every year. About 2.5% of these patients die of the disease (WHO 2009, WHO 2012a).
The Southeast Asia and the Western Pacific regions carry more than 75% (about 1.8 billion population) of the disease burden. These two regions plus the Americas are the three most seriously affected regions. Many tropical diseases are more common in rural areas, but dengue infections are acquired mostly in urban and semi‐urban areas, which puts tourists at higher risk and is an important reason for its increasing endemicity (WHO 2009).
All age groups are affected, but infants and young children are at greater risk of dengue‐related shock which is proved by epidemiological studies. The potential mechanism may explain this. Further risk factors include female sex, high body‐mass index (BMI), infection with certain virus strains, or individual genetic susceptibility (Greenfacts 2012; Simmons 2012; WHO 2012b).
Diagnosis
Clinicians diagnose severe dengue (including dengue‐related shock) on clinical grounds. Laboratory tests confirm the clinical diagnosis. Blood tests support diagnosis and guide management (WHO 2009). Current diagnostic methods include the detection of virus (or virus isolation), viral nucleic acid and viral antigen, and the detection of dengue‐specific antibodies in the blood.
Management
Currently there is no evidence‐base for a specific drug for dengue. Medical interventions remain supportive instead of curative. Patients receive fluid transfusions when hospitalized. Platelets are given when platelet counts drop too low. Severe anaemia in dengue is treated with blood transfusions. For dengue‐related shock , the WHO provides detailed recommendations for intravenous fluid resuscitation (WHO 2009; Simmons 2012). Non‐steroidal anti‐inflammatory drugs such as ibuprofen should be avoided as they can worsen bleeding tendency.
Description of the intervention
Corticosteroids are potent anti‐inflammatory agents with multiple effects on the immune system and a wide range of applications. In the twentieth century, some researchers conducted studies on the effect of intravenous corticosteroids on dengue‐related shock. More recently, researchers began to investigate whether intravenous or oral corticosteroids were effective in preventing disease progression from dengue at an early stage of infection to severe dengue (Kularatne 2009; Shashidhara 2013; Tam 2012; Villar 2009). Currently, the WHO does not recommend the use of corticosteroids either in severe dengue or in dengue at an early stage of infection (WHO 2009).
How the intervention might work
The mechanism in dengue resulting in plasma leakage is still unclear. Complications such as dengue‐related shock are occasionally reported during primary infection (the first time a person is infected by a dengue virus), but are strongly associated with secondary infection (the second time a person is infected, possibly by a different serotype of the dengue virus). This indicates an involvement of the immune system, and the mechanism is mediated and enhanced by antibodies against the dengue virus (WHO 2009). Clinicians give corticosteroids when they think the host immune response contributes to the disease process. In some areas, corticosteroids have been used for the treatment of dengue‐related shock or at an early stage of dengue infection. Observational studies have suggested corticosteroids may benefit people with dengue‐related shock (WHO 2009; Kularatne 2009; Tam 2012 ).
Clinicians have employed the anti‐inflammatory effect of corticosteroids for the treatment of shock caused by sepsis. Patients with sepsis have elevated blood levels of pro‐inflammatory cytokines for weeks after clinical resolution of infection, during which shock may become manifest. Studies that have used lower doses of hydrocortisone (≤ 300 mg per day or equivalent) for longer durations have reported earlier reversal of shock and improved survival (Sprung 2008). A recent systematic review demonstrated that corticosteroids had no clear benefit on mortality in severe sepsis and septic shock (Annane 2009).
However, recent trials have investigated corticosteroids for treating dengue at an early stage because researchers assumed that corticosteroids might be effective in preventing complications when given early in the course of infection (Tam 2012).
Why it is important to do this review
Dengue poses a relevant global public health problem in resource‐poor settings. It is important to evaluate effective treatment options to establish the best possible therapy, and to identify ineffective treatment options which consume limited resources and might do harm.
With the revision of the WHO classification in 2009, the case definition of severe dengue has become broader(WHO 2009). Therefore, all cases classified as Dengue shock syndrome according to the previous, stricter WHO definition (WHO 1997) will also meet the criteria for severe dengue of the WHO definition of 2009.
Objectives
To compare treatment of dengue with and without use of corticosteroids or placebo with regards to prevention of shock‐related death and disease progression in children and adults.
To assess the effects of corticosteroids for treating patients with dengue‐related shock
To assess the effects of corticosteroids in preventing severe dengue in patients with dengue at an early stage
Methods
Criteria for considering studies for this review
Types of studies
Randomized and quasi‐randomized controlled trials.
Types of participants
Children and adults patients diagnosed with dengue (at an early stage and in patients with dengue‐related shock).
Types of interventions
Intervention
Intravenous or oral corticosteroids (methylprednisolone, hydrocortisone, dexamethasone or any other kind of corticosteroid mentioned in the trial).
Control
Placebo or no corticosteroids.
Types of outcome measures
Primary outcomes
Death
Secondary outcomes
For patients with dengue‐related shock, measures of severity including:
Blood transfusion
Complications, such as pulmonary haemorrhage and convulsion
Duration of shock (hours)
Days in hospital or duration of hospitalization (days)
Adverse events
For patients with dengue at an early stage, measures of the onset of severe dengue including:
Dengue‐related shock
Severe bleeding
Severe thrombocytopaenia
Ascites
Intensive care uni (ICU)) admission
Any bleeding
Hospital admission (frequency of hospitalization)
Platelet count
Haematocrit
White blood cell count
Adverse events
Patients with serious adverse events
Patients with any adverse events
Patients with drug‐related adverse events
Patients with other reported events
Patients with adverse events (for different comparisons: low‐dose corticosteroids versus placebo; high‐dose corticosteroids versus placebo; low‐dose versus high‐dose corticosteroid)
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press and in progress).
Electronic searches
We searched the following databases up until 6 January 2014 using the search terms and strategy described in Appendix 1: Cochrane Infectious Diseases Group Specialized Register; the Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library; MEDLINE(via Pubmed); EMBASE; LILACS. We also searched the metaRegister of Controlled Trials (mRCT) using "dengue" AND ("steroid* OR corticosteroid*") as search terms.
Searching other resources
We screened the reference lists of existing reviews and of all trials identified by the above mentioned searches.
Data collection and analysis
Selection of studies
Two authors (Fan Zhang,FZ; Veronika Christine Kramer, VK) independently screened the titles and abstracts identified by the above search strategy and retrieved full‐text articles of potentially relevant trials. For the identified full‐text articles, we also independently used an eligibility form to decide on the final list of included studies. We listed excluded studies with the reason for exclusion. We contacted trial authors for clarification if eligibility was unclear. We resolved disagreements by discussion with Paul Garner (PG) and David Sinclair (DS).
Data extraction and management
Two authors independently extracted data using a pre‐specified, pre‐piloted data extraction form. For each outcome we extracted, where available, the number of participants randomized, the number of participants analyzed in each treatment group, the main methods, as well as detail about the interventions and outcomes. For dichotomous outcomes, we recorded the number of participants experiencing the event and the number assessed in each intervention group. For continuous outcomes, we extracted arithmetic means and standard deviations for each intervention group, together with the numbers assessed in each group. Two authors independently extracted and cross‐checked data to minimize errors. We contacted the trial authors for clarification or missing data where necessary.
Assessment of risk of bias in included studies
For this review update, we applied the latest Cochrane Collaboration methods for assessing risk of bias in included studies (Higgins 2011). FZ and VK independently assessed the risk of bias for each trial. We followed the guidance to assess whether adequate steps had been taken to reduce the risk of bias across six domains: sequence generation; allocation concealment; blinding (of participants, personnel, and outcome assessors); incomplete outcome data; selective reporting; and other sources of bias. We categorized the risk of bias as 'high', 'low', or 'unclear' as per the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Where our judgment was unclear, we attempted to contact the trial authors for clarification. We resolved any disagreements by discussion with PG or DS. We presented this information in a risk of bias summary table and used it for our interpretation of results. We used the GRADE approach to assist our assessment of the quality of evidence and to prepare of 'Summary of findings' tables.
Measures of treatment effect
We calculated results using the risk ratio (RR) for dichotomous data, and the mean difference (MD) for continuous data. These effect estimates were presented with 95% confidence intervals (CI).
Unit of analysis issues
The unit of analysis in all included trials was individual participants. One of our included studies, Tam 2012 had multiple intervention arms with 150 patients in each arm including 75 in high‐dose group and 75 in low‐dose group and 75 in the placebo group. When we did data analysis, we treat the study as two comparisons: one comparing high‐dose (75) with placebo (37) while another one comparing low‐dose (75) with placebo (38). We proportionately divided the placebo into two parts.
Dealing with missing data
Where no data was missing or unclear, we analyzed data according to the intention‐to‐treat principle (ie all randomized participants were analyzed in the groups to which they were originally assigned). Where there was a discrepancy between the number randomized and the number analyzed, we calculated and presented the percentage loss to follow‐up for each intervention group. We contacted trial authors for missing or unclear data. Where we got no response from them, we carried out the complete‐case analysis (ie only the available data was analyzed and the missing data ignored).
Assessment of heterogeneity
We looked for statistical heterogeneity by inspecting the forest plots for overlapping confidence intervals, applying the Chi² test (P value < 0.10 considered to be statistically significant), and the I² statistic (I² value of 50% used to denote moderate levels of heterogeneity).
Assessment of reporting biases
The number of trials per meta‐analysis was insufficient to construct a funnel plot to assess publication bias.
Data synthesis
We analyzed participants with dengue‐related shock and participants with dengue at an early stage separately.
One author (FZ) analyzed the data using Review Manager 5.2, and where appropriate we combined studies using a fixed‐effect model. If we detected heterogeneity, but still considered it was clinically meaningful to combine studies, we used a random‐effects model.
Subgroup analysis and investigation of heterogeneity
The number of the trials was insufficient to carry out a meaningful subgroup analysis. According to our protocol, we would consider potential sources of heterogeneity and do possible subgroup analysis, such as study design, study setting and location, patient characteristics,, and the intervention given (different drug regimens).
Sensitivity analysis
The number of the trials was insufficient to carry out a meaningful sensitivity analysis. We have planned to do the sensitivity analysis with regarding to the risk of bias.
Results
Description of studies
All eight of the included studies (948 participants in total) were randomized controlled trials.
For further detail refer to the tables Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The previous review authors conducted the original search in January 2006 and included four unique trials which assessed the effects of corticosteroids on dengue shock syndrome. An update search in August 2009 did not yield any new trials.
We conducted an update search with a broader scope in June 2013, and repeated this search in January 2014. We identified four new studies which explored the effects of corticosteroids in an early stage of dengue infection rather than in dengue‐related shock. Figure 1 shows the study flow diagram. We contacted authors of five studies which provided email addresses of the contact author or we found from other methods, and the contact author of three studies(Tam 2012, Villar 2009, Tassniyom 1993) replied and provided additional information (see 'Characteristics of included studies').
Figure 1.
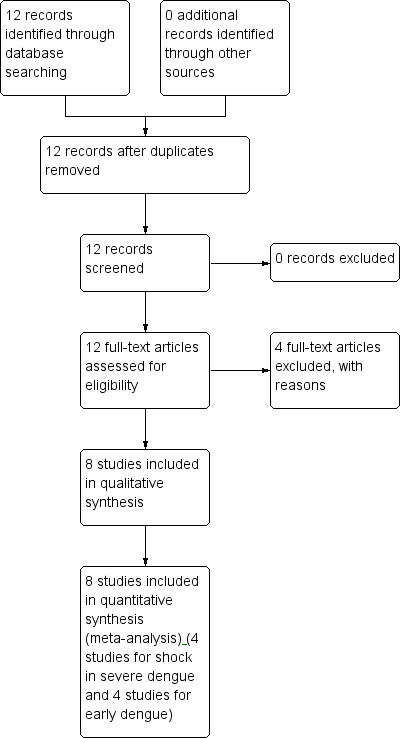
Study flow diagram.
Included studies
Dengue‐related shock
Four randomized controlled trials (Min 1975; Pongpanich 1973; Sumarmo 1982; Tassniyom 1993) including 284 participants met the inclusion criteria (see 'Characteristics of included studies') The participants enrolled were all children less than 15 years old.
All four included trials originated from Southeast Asia: two were conducted in Thailand (Pongpanich 1973; Tassniyom 1993), one in Myanmar (Min 1975), and one in Indonesia (Sumarmo 1982). All studies were hospital‐based. Tassniyom 1993 enrolled patients across two hospitals while the other three trials had patients enrolled in one hospital each.
Three trials compared intravenous hydrocortisone hemisuccinate with no intervention (Pongpanich 1973; Min 1975) or with placebo (Sumarmo 1982), and one compared intravenous methylprednisolone with placebo (Tassniyom 1993).
All four trials reported on death, two reported the number needing a blood transfusion (Pongpanich 1973; Tassniyom 1993), and one reported complications (pulmonary haemorrhage and convulsion) and duration of hospitalisation (Tassniyom 1993). None of these studies reported adverse events as an outcome (Table 5).
Table 1.
Adverse events reported in the included studies
| Study ID | Number of participants | Methods to monitor adverse events | Blinding | Comment on AE | |
| Participants | Clinicians | ||||
| Min 1975 | 48 | No comment | unclear | unclear | No comment |
| Pongpanich 1973 | 7 | No comment | unclear | unclear | No comment |
| Sumarmo 1982 | 47 | No comment | blinded | unclear | No comment |
| Tassniyom 1993 | 32 | Physical examination, blood test No specific recording of adverse events |
blinded | blinded | No significant difference between treatment and control groups in occurrence of
fever after shock, pneumonia, convulsion, cardiac arrest, pulmonary haemorrhage,
and positive hemo‐culture No specific comments on adverse events |
| Kularatne 2009 | 100 | Clinical signs recorded at baseline: mean axillary temperature, headache, nausea, flush, pulse rate, blood pressure Laboratory tests: hematocrit and white blood cell count (day 0 to 4) No specific recording of adverse events |
blinded | unclear | No comment |
| Shashidhara 2013 | 61 | No comment | not blinded | not blinded | No comment |
| Tam 2012 | 150 | Prospective adverse events reporting active surveillance of patient‐reported symptoms and laboratory results Laboratory tests: full blood count and random glucose level daily, with a fasting glucose test performed if the random glucose test showed a high level Biochemistry and coagulation profiles, heparan sulfate (HS) at day 0 (enrolment) day, days 5 to 6, and at follow‐up 2 to 3 weeks after discharge Recording: by trained study physicians using standardized structured case report form (severity, relatedness of study drugs). daily recording throughout the disease course Reporting: regularly to the Data and Safety Monitoring Board (DSMB). |
blinded | blinded | Transient hyperglycaemia in a small number of cases, but no significant clinical or virological adverse events detected |
| Villar 2009 | 87 | Recording: systematic evaluation of adverse events during and after drug
administration and monitoring Reporting: to an independent committee to evaluate the safety and efficacy by interim analysis. These reports are available immediately, and the final report after 30 days (from protocol). |
blinded | blinded | No adverse events reported. Adverse events reported to the committee were defined as fatal serious adverse effects, life‐threatening or clinically significant |
One trial was funded by the WHO (Sumarmo 1982), one by the Rockefeller Foundation (Tassniyom 1993), and funding was not specified for the other two trials (Min 1975; Pongpanich 1973).
Dengue at an early stage
Four randomized controlled trials (Kularatne 2009; Shashidhara 2013; Tam 2012; Villar 2009) including 664 participants (children and adults) met the inclusion criteria (see 'Characteristics of included studies') for the review.
Among these four studies, two were conducted in South Asia(Sri Lanka (Kularatne 2009) and India (Shashidhara 2013); one in Southeast Asia (Vietnam, Tam 2012), and the fourth in Colombia, Latin America (Villar 2009).
Two trials used intravenous corticosteroids, and two evaluated oral corticosteroids. Shashidhara 2013 compared intravenous dexamethasone with a control group which received no treatment. Kularatne 2009 compared intravenous dexamethasone with placebo. Tam 2012 compared oral prednisolone with placebo. Villar 2009 compared oral methylprednisolone with placebo.
Shashidhara 2013 enrolled adults (aged above 18 years) only. Tam 2012 enrolled children aged between ten and 15 years. The other two trials (Kularatne 2009; Villar 2009) recruited both adults and children.
Villar 2009 reported on the clinical outcomes death, ascites and frequency of hospitalization. Tam 2012 reported on shock, shock‐related complications and thrombocytopaenia. Shashidhara 2013 reported on death, shock and shock‐related complications and platelet counts. Kularatne 2009 reported on laboratory parameters (platelet count, haematocrit and white blood cell count).
Three trials (Kularatne 2009; Tam 2012; Villar 2009) reported either spontaneous haemorrhage, severe bleeding or any bleeding.
Two trials reported on adverse events (Tam 2012, Villar 2009). Tam 2012 used prospective, active surveillance to monitor adverse events in a double‐blind study (Table 5). Villar 2009 monitored adverse events prospectively, according to the trial protocol, but did not describe the method of data collection. Clinicians and participants were blinded. The author stated in the trial protocol that events were reported to an independent committee as fatal serious adverse effects, life‐threatening or clinically significant adverse events (Table 5).
One trial was funded by the Wellcome Trust (Tam 2012). Funding was not specified for the other three trials (Kularatne 2009; Shashidhara 2013; Villar 2009).
Excluded studies
Four trials (Futrakul 1981; Futrakul 1987; Sumarmo 1975; Sumarmo 1987) detected by the search specifications were excluded from the review (see 'Characteristics of excluded studies').
Risk of bias in included studies
See Figure 2 for a summary of the risk of bias assessment. Further details are presented in the Characteristics of included studies tables.
Figure 2.
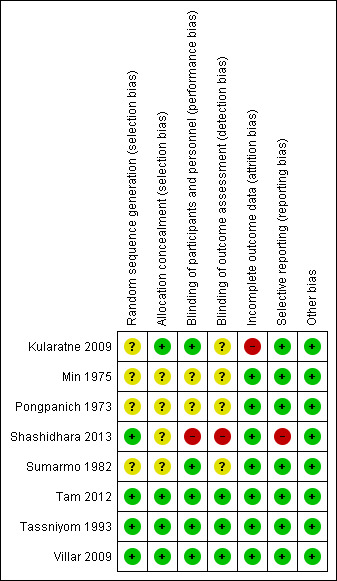
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
1) Dengue‐related shock
For dengue‐related shock, sequence generation and allocation concealment were adequate in one trial (Tassniyom1993) while the other three trials did not describe sequence generation in sufficient detail to allow a judgment.
Blinding was adequate in two trials (Tassniyom 1993; Sumarmo 1982), and unclear in the two other trials. No loss to follow‐up occurred in these four hospital‐based trials. We found no evidence of selective outcome reporting and identified no other sources of bias.
2) Dengue at an early stage
Sequence generation was adequate in three trials (Shashidhara 2013; Tam 2012; Villar 2009) while a fourth trial did not describe sequence generation in sufficient detail to allow a judgment Kularatne 2009).
Allocation concealment and blinding of participants and personnel were adequate in three trials (Kularatne 2009; Tam 2012; Villar 2009) and inadequate in the fourth trial (Shashidhara 2013). Blinding of outcome assessment was adequate in two trials (Tam 2012; Villar 2009) and unclear in one trial (Kularatne 2009). The fourth trial was described as an open‐label study (Shashidhara 2013)
Villar 2009 clearly stated that they used the intention‐to‐treat principle and gave the number of participants originally randomized into two groups. Shashidhara 2013 reported no loss to follow‐up. Tam 2012 reported a small loss of follow‐up. Kularatne 2009 was the only study with a high loss of follow‐up which was due to poor patient compliance in comparatively healthy children. Both studies (Kularatne 2009; Tam 2012) reported the number of participants originally randomized.
Only one trial (Shashidhara 2013) did not report all the pre‐specified outcomes. No evidence of selective outcome reporting was detected in the other three trials (Kularatne 2009; Tam 2012; Villar 2009).
No other sources of bias were identified.
Effects of interventions
1) Corticosteroids for treating dengue‐related shock
Death
Corticosteroid treatment in dengue‐related shock does not reduce mortality significantly when compared to placebo (four trials, 284 participants, I2=0%, Analysis 1.1). In Pongpanich 1973, no one died either in the corticosteroid or in the placebo group. In Min 1975, treatment with corticosteroids significantly reduced the risk of death. In Sumarmo 1982 and Tassniyom 1993, corticosteroids showed no statistically significant benefit.
Blood transfusion
There was no statistically significant difference between the groups in the number of participants needing blood transfusion (two trials, 89 participants, Analysis 1.2) (Pongpanich 1973; Tassniyom 1993).
Complications
Tassniyom 1993 reported no statistically significant difference between the corticosteroids and placebo groups for pulmonary haemorrhage and convulsions (63 participants, Analysis 1.3).
Days in hospital
Tassniyom 1993 reported an average stay of 7.3 days in hospital in the corticosteroid group and 6.2 days in the placebo group, without statistically significant difference between two groups (63 participants, Analysis 1.4).
Adverse events
None of the studies reported on adverse event monitoring.
Other outcomes: duration of shock
Data for outcomes we could not present as meta‐analysis were summarized in Table 6.
Table 2.
Duration of dengue‐related shock as reported in the relevant included studies
| Study ID | Measure | Duration of shock | P value (statistical significance) | Comments | |
| Corticosteroid group | Control group | ||||
| Min 1975 | Average hours Sample size |
4.8 N = 48 |
13.5 N = 50 |
P < 0.01 (significant) |
Hydrocortisone hemisuccinate: single dose of 25 mg/kg intravenous on day 1, 15
mg/kg on day 2, 10 mg/kg on day 3 2. No medication Quote: "There was a significant excess in the duration of shock in the non‐steroid group" |
| Pongpanich 1973 | Average hours (range) Sample size |
13.5 (3 to30) N = 7 |
10.3 (1 to23) N =19 |
P > 0.5 (not significant) |
1. Hydrocortisone hemisuccinate: intravenous 25 mg/kg/day; 5 mg/kg at start,
rest given in divided doses every four to six hours 2. No medication Quote: "Duration of shock showed no statistically significant difference between the two programs of treatment" |
| Study ID | Time period | Participants per time period of shock (N) | P value | Comments | |
| Corticosteroid group (N = 47) |
Control group (N = 50) |
||||
| Sumarmo 1982 | 0.5 to 2.4h 2.5 to 4.4h 4.5 to 6.4h 6.5+h |
31 12 4 3 |
28 13 3 3 |
Not reported | 1. Hydrocortisone hemisuccinate: 50 mg/kg, single intravenous dose 2. Placebo: sodium chloride 0.9% Quote: "there was no significant differences between the treatment groups" |
One out of two studies (Min 1975) reported a significantly shorter duration of shock in the corticosteroid group than in the placebo group, while the second study (Pongpanich 1973) found no difference. These studies were small (124 participants across two trials). Another study (Sumarmo 1982) reported the number of participants who recovered from shock within a certain time period after corticosteroid or placebo treatment, and differences between the two groups appeared small (97 participants, one study). Statistical significance was not reported.
2) Corticosteroids for treating dengue at an early stage
Death
No deaths in either the treatment or control (placebo or no treatment) group were reported in any of the trials (664 participants, four trials, Analysis 2.1).
Severe dengue: dengue‐related shock
Two trials (Shashidhara 2013; Tam 2012) reported no difference in patient numbers developing dengue‐related shock after preventive treatment with corticosteroids when compared to placebo or no corticosteroid (286 participants, Analysis 2.1).
Analysis 2.1.
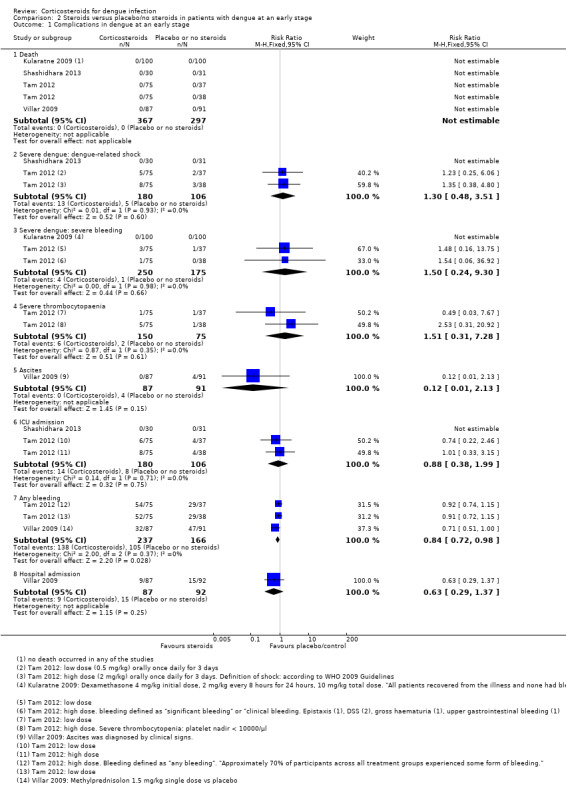
Comparison 2 Steroids versus placebo/no steroids in patients with dengue at an early stage, Outcome 1 Complications in dengue at an early stage.
One trial (Tam 2012) with three study arms detected no significant difference in the risk of dengue‐related shock after low‐dose corticosteroids, high‐dose corticosteroids or placebo. Eleven per cent of participants in the high‐dose corticosteroid group and 7% in each of the low‐dose and placebo groups developed shock. Another study reported no case of shock occurred in either the corticosteroid or in the control group (Shashidhara 2013).
Severe dengue: severe bleeding
Two trials reported that corticosteroids did not significantly reduce the risk of bleeding complications when compared to placebo (425 participants, Analysis 2.1).
Significant bleeding or bleeding complications were rare. Kularatne 2009 observed no bleeding complications in either the corticosteroid or placebo group. Tam 2012 detected four cases (2.67%) of significant or clinical bleeding in the group receiving corticosteroids (one in high‐dose and three in low‐dose group). Two patients had dengue‐related shock, one developed gross haematuria, and one suffered isolated upper gastrointestinal bleeding. One case (1.33%) was detected in the placebo group.
Severe thrombocytopaenia
Corticosteroids did not significantly reduce the incidence of severe thrombocytopaenia when compared to placebo in the one trial that reported this outcome (225 participants, Analysis 2.1). Severe thrombocytopaenia occurred in 4% of participants in the corticosteroid group and in 1% in the placebo group.
Ascitis
One trial (Villar 2009) reported that corticosteroids did not reduce the risk to develop ascites (178 participants, Analysis 2.1). While no one developed ascites in the corticosteroid group, four participants (4.40%) presented with ascites in the control group. However, the difference was not statistically significant (178 participants, Analysis 2.1) and the study was not powered to detect an effect.
Intensive care unit (ICU) admission
Two trials (Shashidhara 2013; Tam 2012) reported on ICU admissions (286 participants, Analysis 2.1). Shashidhara 2013 recorded no ICU admissions either in the corticosteroid or control group. Tam 2012 did not find a significant difference in ICU admissions between study arms (8% in the low‐dose group, 10% in both the high‐dose and the placebo groups).
Any bleeding
Corticosteroids reduced the risk of any bleeding by 15% when compared to the placebo (RR 0.85, 95% CI 0.72 to 0.99, 403 participants, two studies, Analysis 2.1 ). Definitions of "any bleeding" differed between the two studies: Tam 2012 found that approximately 70% of patients across all intervention groups experienced some form of bleeding as minor skin bleedings (petechiae), skin bruising or mild to moderate mucosal bleeding. Villar 2009 defined bleeding as "spontaneous haemorrhage", and reported proportions of 36% (in the corticosteroid group) to 50% (in the placebo group) suffered bleeding. The study protocol (Villar 2009) specified gastrointestinal bleeding or oral bleeding, nosebleed, blood in the urine (haematuria), minor skin bleedings (petechiae), bruising or purple skin as spontaneous bleeding manifestations.
Hospital admission
Villar 2009 found that corticosteroids had no significant effect on the number of hospitalizations required (178 participants, Analysis 2.1). Ten percent of patients in the corticosteroid and 16% in the placebo group were hospitalized. The study was not powered to detect an effect.
Platelet count on days one to four
The combined results of two studies (Shashidhara 2013, Kularatne 2009) showed no statistically significant difference in platelet counts between corticosteroid and control group on the first, second, third or fourth day (261 participants, Analysis 2.2). The results was heterogenous on the second day.
Analysis 2.2.
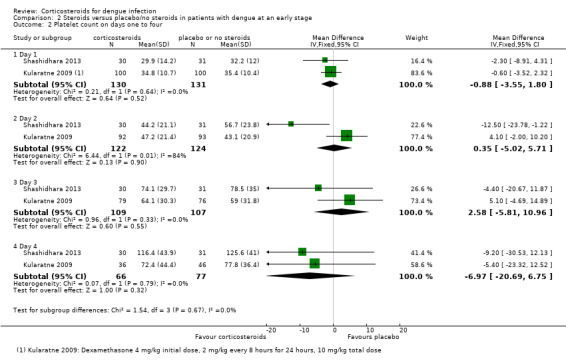
Comparison 2 Steroids versus placebo/no steroids in patients with dengue at an early stage, Outcome 2 Platelet count on days one to four.
Haematocrit on days one to four
The study(Kularatne 2009) showed no significant difference between the groups on days one, two, three or four (200 participants, one study, Analysis 2.3 ).
Analysis 2.3.

Comparison 2 Steroids versus placebo/no steroids in patients with dengue at an early stage, Outcome 3 Haematocrit on days one to four.
White blood cell count on days one to four
Kularatne 2009 found a significantly lower white blood cell count in the corticosteroid group on day 1 (mean difference, 4.8 cells/mcL for corticosteroid and 5.7 cells/mcL for placebo group) (200 participants, Analysis 2.4), but no significant difference between the groups was detected on days 2, 3 or 4.
Analysis 2.4.
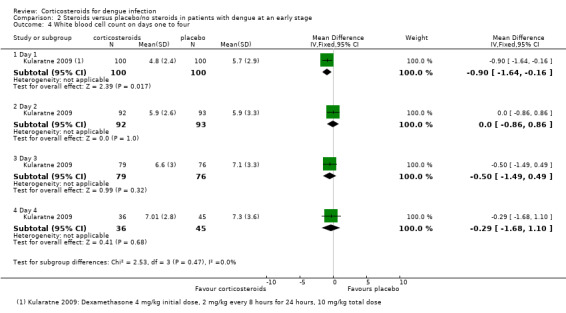
Comparison 2 Steroids versus placebo/no steroids in patients with dengue at an early stage, Outcome 4 White blood cell count on days one to four.
Patients with adverse events
Villar 2009 recorded adverse events prospectively and reported in the conference abstract that "no adverse or severe events" occurred.
Tam 2012 used prospective, active surveillance to monitor serious and any adverse events (Table 5).
Serious adverse events: There was no difference in the number of people who had serious adverse events after high‐dose corticosteroids, low‐dose corticosteroids or placebo (403 participants, Tam 2012, Analysis 2.5, Analysis 2.8, Analysis 2.9, Analysis 2.10).
Analysis 2.5.
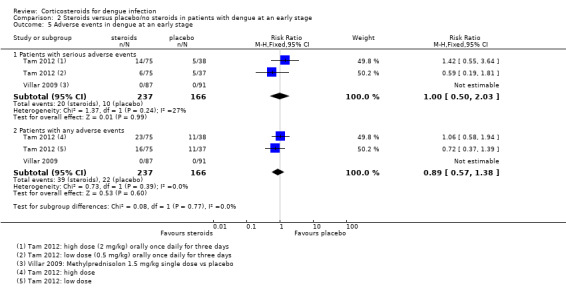
Comparison 2 Steroids versus placebo/no steroids in patients with dengue at an early stage, Outcome 5 Adverse events in dengue at an early stage.
Analysis 2.8.
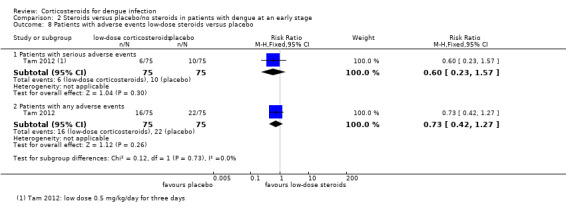
Comparison 2 Steroids versus placebo/no steroids in patients with dengue at an early stage, Outcome 8 Patients with adverse events low‐dose steroids versus placebo.
Analysis 2.9.
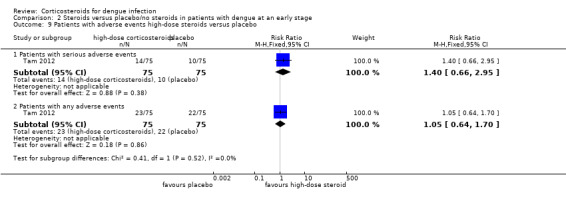
Comparison 2 Steroids versus placebo/no steroids in patients with dengue at an early stage, Outcome 9 Patients with adverse events high‐dose steroids versus placebo.
Analysis 2.10.
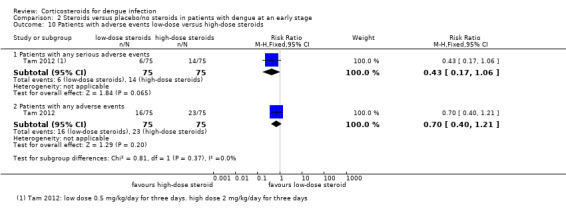
Comparison 2 Steroids versus placebo/no steroids in patients with dengue at an early stage, Outcome 10 Patients with adverse events low‐dose versus high‐dose steroids.
Any adverse event: There was no significant difference in participants who experienced any adverse events across treatment groups. The proportion of participants with any adverse events was high with 30% in both the high‐dose corticosteroid and placebo groups and 20% in the low‐dose corticosteroid group (403 participants, Tam 2012, Analysis 2.5, Analysis 2.8, Analysis 2.9, Analysis 2.10).
Drug‐related adverse events:Tam 2012 found no significant difference in the number of patients with hyperglycaemia, hypertension, pneumonia and upper respiratory tract infection after corticosteroid treatment when compared to placebo. The study authors thought these diagnoses to be possibly drug‐related (225 participants, Analysis 2.6). The study also reported three other adverse events, which they deemed not related to the study drug. Again, the study detected no significant difference between study arms (225 participants, Analysis 2.7).
Analysis 2.6.
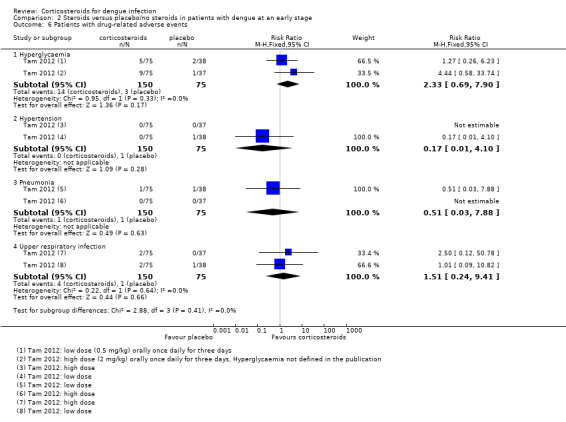
Comparison 2 Steroids versus placebo/no steroids in patients with dengue at an early stage, Outcome 6 Patients with drug‐related adverse events.
Analysis 2.7.
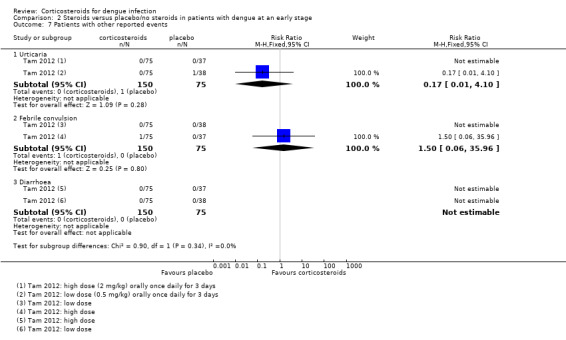
Comparison 2 Steroids versus placebo/no steroids in patients with dengue at an early stage, Outcome 7 Patients with other reported events.
Discussion
Summary of main results
Dengue‐related shock
The four trials evaluating corticosteroids in children with dengue‐related shock were conducted before 1988 and had small participant numbers a high risk of bias. The meta‐analysis was underpowered with very low quality evidence, and did not demonstrate an effect on mortality (four studies, 284 participants), the need for blood transfusion (two studies, 89 participants), pulmonary haemorrhage (one study, 63 participants), or convulsions (one study, 63 participants).
Dengue at an early stage
Intravenous or oral corticosteroids in children and adults with dengue infection have not been shown to reduce the risk of death (four trials, 664 participants, low quality of evidence) or the risk to develop complications of severe dengue, such as shock (two trials, 286 participants, very low quality of evidence), severe bleeding (two trials, 403 participants, very low quality of evidence), severe thrombocytopaenia (one trial, 225 participants, very low quality of evidence), ascites (one trial, 178 participants, very low quality evidence) and ICU admissions (two trials, 286 participants, very low quality of evidence).
Only four trials were included in this part of the review, and many of the outcomes were reported by one trial only.
Overall completeness and applicability of evidence
Dengue‐related shock
For the trials evaluating corticosteroids in shock the data was limited, and confined to studies in children only. All trials were conducted in Southeast Asia. These small trials were similar in terms of location, setting, and types of participants.
Dengue at an early stage
The body of evidence for corticosteroids in dengue at an early stage is limited. We identified four small trials from Colombia, India, Sri Lanka and Vietnam, which included both children and adults. They used different oral corticosteroids (prednisolone and methylprednisolone) (Tam 2012; Villar 2009) or intravenous dexamethasone in different doses (Kularatne 2009, Shashidhara 2013) and were conducted in different settings. Ten of our review's outcomes were reported by only one trial (Analysis 2.1‐Analysis 2.10). It is therefore difficult to generalize the findings.
Quality of the evidence
Dengue‐related shock
We found the quality of evidence to be very low for all outcomes of this comparison. The four trials were conducted more than 25 years ago from 1960s to 1980s. They preceded newer guidance for transparent reporting on sequence generation and allocation concealment, and have methodological limitations. For our risk of bias assessment, please see Figure 2. These trials were conducted in hospital settings with low loss to follow‐up, and no evidence of selective outcome reporting. The trials were also underpowered and thus too small to reliably detect an effect in the outcomes that they measured. For an estimation for the sample sizes that would be required per study and per meta‐analysis please see Table 7.
Table 3.
Sample size calculations for corticosteroids in dengue‐related shock and in dengue at an early stage versus placebo or no intervention
| Outcome | Assumed risk | Source | Clinically important relative reduction | Sample size required1,2 | Sample size in meta‐analysis |
| Dengue‐related shock | |||||
| Death | 21.3% | Analysis 1.1 | 25% | 1.672 | 284 |
| Need for blood transfusion | 24.0% | Analysis 1.2 | 25% | 1.440 | 89 |
| Pulmonary haemorrhage | 3.2% | Analysis 1.3 | 25% | 13.348 | 63 |
| Convulsions | 1% (assumed, instead of 0%) | Analysis 1.3 | 25% | 43.576 | 63 |
| Dengue at an early stage | |||||
| Dengue‐related shock | 4.7% | Analysis 2.1 | 25% | 8.568 | 286 |
| Severe bleeding | 0.6% | Analysis 2.1 | 25% | 72.886 | 425 |
| Severe thrombocytopaenia | 2.6% | Analysis 2.1 | 25% | 16.518 | 225 |
| ICU admission | 7.5% | Analysis 2.1 | 25% | 5.318 | 286 |
| Ascitis | 4.4% | Analysis 2.1 | 25% | 9.600 | 178 |
| Death | 1% (assumed, instead of 0%) | Analysis 2.1 | 25% | 43.576 | 664 |
1 The sample size was calculated for binary outcomes for superiority trials. We estimated that a 25% reduction of risk to develop a complication in the intervention group when compared to the control group would be clinically important. The "assumed risk" is the risk in the control group. All calculations are based on: 2‐sided tests, with a ratio of 1:1, power of 0.8, and confidence level of 0.95. 2 All calculations were performed using the software available at http://www.sealedenvelope.com/power/binary‐superiority/.
Analysis 1.1.
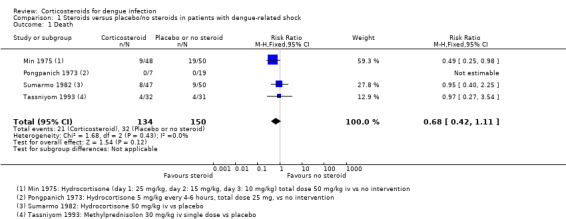
Comparison 1 Steroids versus placebo/no steroids in patients with dengue‐related shock, Outcome 1 Death.
Analysis 1.2.
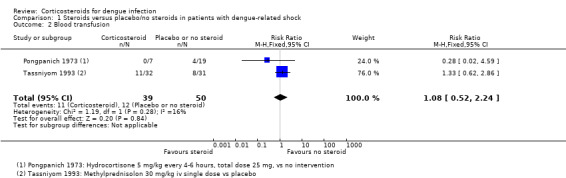
Comparison 1 Steroids versus placebo/no steroids in patients with dengue‐related shock, Outcome 2 Blood transfusion.
Analysis 1.3.
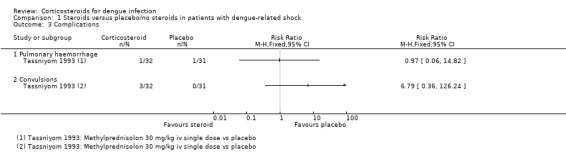
Comparison 1 Steroids versus placebo/no steroids in patients with dengue‐related shock, Outcome 3 Complications.
Dengue at an early stage
The quality of evidence for this comparison of the review was low for the outcome death, and very low for dengue‐related shock, ICU admission, severe bleeding, severe thrombocytopaenia and ascites. The four trials have lower risk of bias and were conducted more recently between 2004 and 2011. One out of four trials reported high loss to follow‐up. Nevertheless, they are seriously underpowered (Table 7) and cannot be easily generalized to various settings. This was our reason for downgrading the quality of evidence.
Agreements and disagreements with other studies or reviews
The findings of a former systematic review (Panpanich 2010) and two other literature reviews (Rajapakse 2009, Rajapakse 2012) that evaluated the effects of corticosteroids in dengue‐related shock are consistent with our findings. However, these reviews did not address the effects of corticosteroids in dengue at an early stage infection.
A study nested in the trial by Tam 2012 explored the effects of low‐dose corticosteroids, high‐dose corticosteroids and placebo on the immune response of patients with dengue at an early stage (Nguyen 2013). The study reported a limited effect of corticosteroids on patients' cytokine levels and immune modulation. This is consistent with results of the clinical trial (Tam 2012) which detected no significant clinical benefits after corticosteroids. The authors concluded that "early prednisolone therapy has little impact on the host immune response", even if the dose might be "too little or too late".
The current WHO strategy focusses on early diagnosis of dengue cases and on staff training for improved case management, but also emphasises the importance of a greater evidence‐base for interventions (WHO 2012c).
Authors' conclusions
At present, there is insufficient evidence to know if routinely using corticosteroids in treating dengue‐related shock in children and adults has an effect.
There is also insufficient evidence to know if using corticosteroids in treating dengue at an early stage influences the course of the disease.
At present, recommended treatment for severe dengue is restricted to supportive therapy. More research is needed to evaluate if drug therapies such as corticosteroids are effective and safe and should be recommended or not.
For corticosteroid use in people with dengue‐related shock, it would require a trial of probably more than 1672 people to identify an effect on death (sufficient sample size, Table 7). Whether this is a priority for care depends on balancing other potentially important interventions are worth testing for managing severe dengue infection.
For people with symptomatic dengue early in the illness, large, rigorously conducted randomized controlled trials that measure death or other severe dengue‐related complications as an outcome would be needed to justify the use of corticosteroids, but these would need to be even larger, with an estimate sample size of over 8000 people (Table 7).
Acknowledgements
We acknowledge Paul Garner and David Sinclair for their advice and constructive comments on our review. We are grateful to all the members of the Fellowship Programme run by the Cochrane Infectious Diseases Group for their help. We also thank Ratana Panpanich, P Sornchai and Kittika Kanjanaratanakorn, the authors of the previous Cochrane review "Corticosteroids for treating dengue shock syndrome" which we have updated.
The editorial base for the Cochrane Infectious Diseases Group is funded by UKaid from the UK Government for the benefit of low‐ and middle‐income countries.
Appendices
Appendix 1. Search strategy
| Search set | CIDG SRa | CENTRAL | MEDLINEb | EMBASEb | LILACSb |
| 1 | adrenal cortex hormone | adrenal cortex hormone | exp DENGUE | exp DENGUE | dengue |
| 2 | corticosteroids | corticosteroids | dengue | dengue | corticosteroids |
| 3 | hydrocortisone | hydrocortisone | HEMORRAGIC FEVER | HEMORRAGIC FEVER | dexamethasone |
| 4 | dexamethasone | dexamethasone | hemorrhagic fever | hemorrhagic fever | prednisolone |
| 5 | methylprednisolone | methylprednisolone | ('break‐bone fever').ti,ab | ('break‐bone fever').ti,ab | 2 or 3 or 4 |
| 6 | prednisolone | prednisolone | 1 or 2 or 3 or 4 or 5 | 1 or 2 or 3 or 4 or 5 | 1 and 5 |
| 7 | hemorrhagic fever | hemorrhagic fever | ADRENAL CORTEX HORMONES | adrenal cortex hormones | — |
| 8 | dengue fever | dengue fever | corticosteroids | corticosteroids | — |
| 9 | 1 or 2 or 3 or 4 or 5 or 6 | 1 or 2 or 3 or 4 or 5 or 6 | steroid* | steroid$ | — |
| 10 | 7 or 8 | 7 or 8 | cortisol* | cortisol$ | — |
| 11 | 9 and 10 | 9 and 10 | HYDROCORTISONE | HYDROCORTISONE | — |
| 12 | — | — | hydrocortisone | hydrocortisone | — |
| 13 | — | — | DEXAMETHASONE | DEXAMETHASONE | — |
| 14 | — | — | dexamethasone | dexamethasone | — |
| 15 | — | — | METHYLPREDNISOLONE | METHYLPREDNISOLONE | — |
| 16 | — | — | methylprednisolone | methylprednisolone | — |
| 17 | — | — | PREDNISOLONE | PREDNISOLONE | — |
| 18 | — | — | prednisolone | prednisolone | — |
| 19 | — | — | 7 ‐ 18/OR | 7‐ 18/OR | — |
| 20 | — | — | 6 and 19 | 6 and 19 | — |
| 21 | — | — | Limit 20 to human | Limit 20 to human | — |
aCochrane Infectious Diseases Group Specialized Register. bSearch terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Higgins 2011; upper case: MeSH or EMTREE heading; lower case: free text term.)
Data and analyses
Comparison 1.
Steroids versus placebo/no steroids in patients with dengue‐related shock
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 4 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.42, 1.11] |
| 2 Blood transfusion | 2 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.52, 2.24] |
| 3 Complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Pulmonary haemorrhage | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Convulsions | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Days in hospital | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Analysis 1.4.
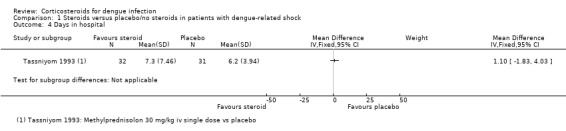
Comparison 1 Steroids versus placebo/no steroids in patients with dengue‐related shock, Outcome 4 Days in hospital.
Comparison 2.
Steroids versus placebo/no steroids in patients with dengue at an early stage
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complications in dengue at an early stage | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Death | 4 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Severe dengue: dengue‐related shock | 2 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.48, 3.51] |
| 1.3 Severe dengue: severe bleeding | 2 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.24, 9.30] |
| 1.4 Severe thrombocytopaenia | 1 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.31, 7.28] |
| 1.5 Ascites | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.01, 2.13] |
| 1.6 ICU admission | 2 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.38, 1.99] |
| 1.7 Any bleeding | 2 | 403 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.72, 0.98] |
| 1.8 Hospital admission | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.29, 1.37] |
| 2 Platelet count on days one to four | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Day 1 | 2 | 261 | Mean Difference (IV, Fixed, 95% CI) | ‐0.88 [‐3.55, 1.80] |
| 2.2 Day 2 | 2 | 246 | Mean Difference (IV, Fixed, 95% CI) | 0.35 [‐5.02, 5.71] |
| 2.3 Day 3 | 2 | 216 | Mean Difference (IV, Fixed, 95% CI) | 2.58 [‐5.81, 10.96] |
| 2.4 Day 4 | 2 | 143 | Mean Difference (IV, Fixed, 95% CI) | ‐6.97 [‐20.69, 6.75] |
| 3 Haematocrit on days one to four | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Day 1 | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.54, 1.54] |
| 3.2 Day 2 | 1 | 185 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.70, 2.50] |
| 3.3 Day 3 | 1 | 155 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐2.29, 1.09] |
| 3.4 Day 4 | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐0.16, 4.16] |
| 4 White blood cell count on days one to four | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Day 1 | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.64, ‐0.16] |
| 4.2 Day 2 | 1 | 185 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.86, 0.86] |
| 4.3 Day 3 | 1 | 155 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.49, 0.49] |
| 4.4 Day 4 | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐1.68, 1.10] |
| 5 Adverse events in dengue at an early stage | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Patients with serious adverse events | 2 | 403 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.50, 2.03] |
| 5.2 Patients with any adverse events | 2 | 403 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.57, 1.38] |
| 6 Patients with drug‐related adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Hyperglycaemia | 1 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [0.69, 7.90] |
| 6.2 Hypertension | 1 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 4.10] |
| 6.3 Pneumonia | 1 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.03, 7.88] |
| 6.4 Upper respiratory infection | 1 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.24, 9.41] |
| 7 Patients with other reported events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Urticaria | 1 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 4.10] |
| 7.2 Febrile convulsion | 1 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.06, 35.96] |
| 7.3 Diarrhoea | 1 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Patients with adverse events low‐dose steroids versus placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Patients with serious adverse events | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.23, 1.57] |
| 8.2 Patients with any adverse events | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.42, 1.27] |
| 9 Patients with adverse events high‐dose steroids versus placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Patients with serious adverse events | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.66, 2.95] |
| 9.2 Patients with any adverse events | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.64, 1.70] |
| 10 Patients with adverse events low‐dose versus high‐dose steroids | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Patients with any serious adverse events | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.17, 1.06] |
| 10.2 Patients with any adverse events | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.40, 1.21] |
What's new
| Date | Event | Description |
|---|---|---|
| 15 November 2016 | Amended | corrected link in methods section |
History
Protocol first published: Issue 1, 2002 Review first published: Issue 3, 2006
| Date | Event | Description |
|---|---|---|
| 10 June 2014 | New search has been performed | Objectives: we included studies evaluating steroids given early in dengue to prevent the disease, and adjusted the inclusion criteria and search strategy.We improved the assessment of risk of bias and calculated the optimal sample size to help interpret our results. |
| 10 June 2014 | New citation required but conclusions have not changed | Authors: changed from Panpanich R, Sornchai P and Kanjanaratanakorn K to Zhang F and Kramer CV. |
Differences between protocol and review
In this review update, we focus not only on the dengue shock, but also the early dengue. The changing of the scope is the only difference.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: randomized controlled trial Study dates: June 2004 to February 2006 Diagnostics: clinical, serological (haemagglutinin inhibiting antibody essay (HIA), IgM and IgG ELISA) |
|
| Participants | Number of participants randomized: 200 enrolled (100 in corticosteroid
group; 100 in placebo group) Age: 12 to 65 years Inclusion criteria: with acute, serologically confirmed dengue, without any concomitant illnesses Exclusions: patients with evidence of bleeding and shock |
|
| Interventions | 1. Dexamethasone: intravenous 4 mg as the initial dose and 2 mg every 8
hours thereafter for 24 hours 2. Placebo: intravenous isotonic saline |
|
| Outcomes | 1. Platelet count 2. Haematocrit 3. White blood cell count (WBC) 4. Bleeding complications |
|
| Notes | Location: Central Hospital, Peradeniya , Sri Lanka Transmission: not specified Funding: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned patients", no more detail about sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "using sealed envelop method" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "placebo group received intravenous isotonic saline identical in appearance to the active drug according to the same regimen" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High losses to follow‐up on days 3 and 4 were reported Treatment group: day 1: 0, day 2: 7%, day 3: 24%, day 4: 54% Control group: day 1: 0, day 2: 8%, day 3: 21%, day 4: 64% |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
| Methods | Study design: randomized controlled trial Study dates: February 1973 to February 1974 Diagnostics: clinical, "serologically proven" |
|
| Participants | Number of participants randomized: 98 enrolled (48 in corticosteroid group;
50 in control group). Age: not reported Inclusion criteria: children diagnosed with dengue shock syndrome using serological confirmation Exclusions: not specified |
|
| Interventions | 1. Hydrocortisone hemisuccinate: single dose of 25 mg/kg intravenous on day
1, 15 mg/kg on day 2, 10 mg/kg on day 3 2. No medication (fluid replacement only) |
|
| Outcomes | 1. Death 2. Duration of shock |
|
| Notes | Location: Children's hospital, Rangoon, Burma (Myanmar) Transmission: not specified Funding: not specified Fluid replacement included normal saline, Ringer lactate solution, plasma, and blood products |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly selected in 2 groups after carefully matching them by age groups and sex", no detail about method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Double blind" No placebo given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Double blind" No placebo given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up were reported |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
| Methods | Study design: randomized controlled trial Study dates: 1969 to 1971 Diagnostics: clinical diagnosis and serological or virological. Haemagglutination inhibition (HI) test performed on paired sera; positive result was a fourfold rise in titre or a fixed level at 1:640 or more. |
|
| Participants | Number of participants: randomized: 26 enrolled (7 in corticosteroid group;
19 in control group) Age: 6.6 to 9.5 years Inclusion criteria: children diagnosed with dengue shock syndrome using serological confirmation Exclusions: not specified |
|
| Interventions | 1. Hydrocortisone hemisuccinate: intravenous 25 mg/kg/day; 5 mg/kg at
start, rest given in divided doses every four to six hours in addition to
fluid replacement 2. No medication (fluid replacement only) |
|
| Outcomes | 1. Death 2. Duration of shock 3. Number requiring fluid replacement 4. Number needing blood transfusion |
|
| Notes | Location: Ramathibodi Hospital, Bangkok, Thailand Transmission: not specified Funding: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A card was drawn at onset of shock to decide which treatment program would be given", no more detail about sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up were reported |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
| Methods | Study design: randomized controlled trial Study dates: June 2010 to June 2011 Diagnostics: serological (IgM ELISA) |
|
| Participants | Number of participants randomized: 61 enrolled (30 in corticosteroid group;
31 in control group) Age: above 18 years Inclusion criteria: aged above 18 years; serologically confirmed IgM ELISA, when platelet counts dropped below 50,000/cumm during the acute stage of illness Exclusions: patients with evidence of bleeding and shock; patients with HIV, autoimmune disease, connective tissue disorders and vasculitis, ITP, malignancy during direct examination and clinical investigations if necessary; patients with diabetes mellitus, hypertension, history of peptic ulcer, hypersensitivity to corticosteroids, total leucocyte counts of more than 11,000/cumm |
|
| Interventions | 1. Dexamethasone: intravenous 8 mg as the initial dose and 4 mg every 8
hours thereafter for 4 days (iv fluids were given whenever necessary) 2. Control: only intravenous fluids and antipyretics whenever necessary |
|
| Outcomes | 1. Platelet count 2. Death 3. Number developing dengue‐related shock 4. Number requiring transfusion |
|
| Notes | Location: JSS Hospital Mysore, Karnataka, India (tertiary medical care
centre) Transmission: not specified Funding: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "They were allotted randomly by blocked randomisation by using a fixed blocking method" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote:"open label" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote:"open label" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up were reported |
| Selective reporting (reporting bias) | High risk | Among all data recorded, only platelet counts was reported |
| Other bias | Low risk | No other bias identified |
| Methods | Study design: randomized controlled trial Study dates: February 1978 to May 1979 Diagnostics: clinical and serological or virological: Haemagluttinin Inhibition (HI) test on acute and convalescent paired sera, technique of Clarke and Casals. Virus isolation by mosquito inoculation, technique of Rose and Gubler for patients with a fourfold increase of the HI antibody titre or patients who died. |
|
| Participants | Number of participants: randomized: 97 enrolled (47 in corticosteroid
group; 50 in placebo group) Age: 0.9 to 10 years Inclusion criteria: children with serologically confirmed dengue shock syndrome Exclusions: not specified |
|
| Interventions | 1. Hydrocortisone hemisuccinate: 50 mg/kg, single intravenous dose in
addition to fluid replacement 2. Placebo: sodium chloride 0.9% with same colour and turbidity |
|
| Outcomes | 1. Death 2. Duration of shock 3. Requirement of fluid replacement (mL) |
|
| Notes | Location: Dr Cipto Mangunkusumo Hospital, Jakarta, Indonesia Transmission: not specified Funding: WHO Project ICPRPD 001 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "divided group using a simple random assignment method", no more detail about the method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double blind" and "Placebo packaged in indistinguishable coded vials" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up were reported |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
| Methods | Study design: randomized controlled trial Study dates: August 2009 to January 2011 Diagnostics: virological diagnosis (rapid test for dengue non‐structural protein NS1 silver strip) |
|
| Participants | Number of participants: randomized: 225 enrolled (150 in corticosteroid
group including 75 in high‐dose group and 75 in low‐dose
group; 75 in placebo group) Inclusion criteria: children and young adults Age: 5 to 20 years (enrolled participants were 10 to 15 years of age) with clinically suspected dengue, supported by a positive antigen test for dengue, fever for ≤72 hours and negative pregnancy test Exclusions: those weighing < 20 kg, those with evidence for any dengue‐related complications, symptoms suggesting another infectious disease, or a past history of serious illness or chronic disease including psychiatric/behavioural problems, and those taking regular medications |
|
| Interventions | 1. prednisolone: low‐dose (0.5 mg/kg) orally once daily for three
days 2. prednisolone: high‐dose (2 mg/kg) orally once daily for three days 3. placebo: placebo group was additionally 1:1 randomized to low‐ or high‐dose placebo |
|
| Outcomes | 1. Number of complications: dengue shock syndrome; ICU admission; severe
thrombocytopaenia; significant bleeding; severe abdominal pain; increased
liver enzymes 2. Number of adverse events: hyperglycaemia, hypertension, pneumonia, upper respiratory infection Platelet nadir days 3 to 8, 109/L Laboratory endpoints: maximum haematocrit at days 3 to 8; %haemoconcentration Virological endpoints (not of interest for this review) Area under the curve (AUC) log viraemia at days 3 to 6, log 10 copies/mL; time to undetectable viraemia, number and % with undetectable viraemia; days from enrolment, median (interquartile range, IQR) to undetectable viraemia; time to negative NS1: number with negative NS1 %; days from enrolment, median (IQR) to negative NS1 |
|
| Notes | Location: Hospital for Tropical Diseases, Ho Chi Min City, Vietnam Transmission: not specified Funding: Wellcome Trust Tam 2012 defined platelet nadir < 10,000/μl L = SAE as severe thrombocytopaenia. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "block randomisation" |
| Allocation concealment (selection bias) | Low risk | Quote: "All participants and study staff were blind to the treatment
allocation" and "did not blind the dose allocation, but the
placebo group was additionally 1:1 randomized to low‐ or high‐dose placebo to maintain blinding of the drug" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "identical prednisolone and placebo were available", and "placebo group was additionally 1:1 randomized to low‐ or high‐dose placebo to maintain blinding of the drug". They were blind to intervention or placebo, but not to high or low dose. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Email correspondence with author: "The study staff were the outcome assessors and they were blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low losses to follow‐up were reported |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
| Methods | Study design: randomized controlled trial Study dates: 1987 to 1988 Diagnostics: viral isolation, serological diagnosis: haemagglutinin inhibition (HI) test, ELISA for dengue antibodies |
|
| Participants | Number of participants: randomized: 63 enrolled (32 in corticosteroid
group; 31 in placebo group) Age: 3 to 14 years Inclusion criteria: children diagnosed with dengue shock syndrome using World Health Organization clinical criteria and serological confirmation Exclusions: not specified |
|
| Interventions | 1. Methylprednisolone (Solumedrol, Upjohn): single intravenous dose of 30
mg/kg in addition to fluid replacement 2. Placebo: 5% dextrose in normal saline solution |
|
| Outcomes | 1. Death 2. Number needing blood transfusion 3. Duration of hospitalization 4. Number getting complications (separate data for each complication) |
|
| Notes | Location: Khon Kaen University Hospital, and Khon Kaen Provincial Hospital
in Khon Kaen, Thailand Transmission: not specified Funding: Rockefeller Foundation (grant RF 87006#95) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Block randomisation" and "Generation of allocation sequence: generated by statistician and running number put on drug package" |
| Allocation concealment (selection bias) | Low risk | Email correspondence with author: "By using sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double blind", "All specimens were identified only by code number" and "identical placebo" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Email reply from author: outcome assessors was blinded to the intervention, they "knew patients by code number" only |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up were reported |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
| Methods | Study design: randomized controlled trial Study dates: July 2006 to January 2009 Diagnostics: clinical diagnosis or immuno chromatography test |
|
| Participants | Number of participants randomized: 189 enrolled (87 in corticosteroid
group; 91 in placebo group) Age: not stated Inclusion criteria: fever over 48 hours but less than 120 hours, with clinical diagnosis of dengue or immuno chromatography test, and free of clinical signs of plasma leakage Exclusion criteria: leukocytosis; evidence of plasma leakage, hypotension, or major bleeding and bruising, hematemesis, melena, rectal bleeding and purple; oral intolerance; women of reproductive age with amenorrhoea than 4 weeks; cancer, HIV/AIDS or haematological disorders; breast‐feeding patients and those with a history of diseases; patients who have visited a malaria endemic area in the last 30 days |
|
| Interventions | 1. Metylprednisolone: single oral dose 1.5 mg/kg 2. Placebo |
|
| Outcomes | 1. Complications such as number developing spontaneous haemorrhages and
ascites 2. Frequency of hospitalization 3. Adverse or severe events or mortality |
|
| Notes | Location: Bucaramanga, Colombia Transmission: not specified Funding: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Email correspondence with author: "generated in the computer 4 lists of 25 randomly selected blocks with replacement" |
| Allocation concealment (selection bias) | Low risk | Email correspondence with author: "lists of numbers will be generated by project engineer, who will be the only person to know that each code contains measures of treatment during the period of collection of information" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "blinded for participants, care givers" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "blinded for outcome adjudicators" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low losses of follow‐up were reported |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No other bias identified |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Futrakul 1981 | Not a randomized or quasi‐randomized controlled trial |
| Futrakul 1987 | Not a randomized or quasi‐randomized controlled trial |
| Nguyen 2013 | The clinical outcomes of this trial are reported in Tam 2012 |
| Sumarmo 1975 | Not a randomized or quasi‐randomized controlled trial |
| Sumarmo 1987 | Duplicate data with an included trial |
Contributions of authors
Both review authors contributed to the development of the review, extraction of the data, data analysis, and presentation and interpretation of the results.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
External sources
Department of International Development, UK.
Declarations of interest
None known.
Unchanged
References
References to studies included in this review
- Kularatne SAM, Walathara C, Mahindawansa SI, Wijesinghe S, Pathirage MMK, Kumarasiri PVR, et al. Efficacy of low dose dexamethasone in severe thrombocytopenia caused by dengue fever: a placebo controlled study. Postgraduate Medical Journal 2009;85:525‐9. [DOI] [PubMed] [Google Scholar]
- Min M, Tin U, Aye M, Shwe TN, Swe T. Hydrocortisone in the management of dengue shock syndrome. Southeast Asian Journal of Tropical Medicine and Public Health 1975;6(4):573‐9. [PubMed] [Google Scholar]
- Pongpanich B, Bhanchet P, Phanichyakarn P, Valyasevi A. Studies on dengue hemorrhagic fever: an evaluation of steroids as a treatment. Journal of Medical Association of Thailand 1973;56(1):6‐14. [PubMed] [Google Scholar]
- ShaShidhara KC, SudharShan Murthy KA, BaSavana Gowdappa H, BhoGraj A. Effect of high dose of steroid on platelet count in acute stage of dengue fever with thrombocytopenia. Journal of Clinical and Diagnostic Research 2013;7(7):1397‐1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumarmo, Talogo W, Asrin A, Isnuhandojo B, Sahudi A. Failure of hydrocortisone to affect outcome in dengue shock syndrome. Pediatrics 1982;69(1):45‐9. [PubMed] [Google Scholar]
- Tam DTH, Ngoc TV, Tien NTH, Kieu NTT, Thuy TTT, Thanh LTC, et al. Effects of short‐course oral corticosteroid therapy in early dengue infection in Vietnamese patients: a randomized, placebo‐controlled trial. Clinical Infectious Diseases 2012:1‐9. [Hanh Tien TN, Nguyen THQ, Vu TT, Farrar J, Hoang TL, Dong THT, et al. Corticosteroids for dengue ‐ why don't they work? PLoS Neglected Tropical Diseases 2013;7(12):e2592.] [Google Scholar]
- Tassniyom S, Vasanawathana S, Chirawathul A, Rojanasuphot S. Failure of high‐dose methylprednisolone in established dengue shock syndrome: A placebo‐controlled, double‐blind study. Pediatrics 1993;92(1):111‐5. [PubMed] [Google Scholar]
- Villar LA, Martinez RA, Diaz FA, Villar JC, Rueda E. Effect of methylprednisolone in preventing dengue complications: a single‐center randomized placebo‐controlled trial. American Jounal of Tropical Medicine and Hygiene. ASTMH Washington, DE United States: 58th Annual Meeting of the American Society of Tropical Medicine and Hygiene, 2009. [Google Scholar]
References to studies excluded from this review
- Futrakul P, Vasanauthana S, Poshyachinda M, Mitrakul C, Cherdboonchart V, Kanthirat V. Pulse therapy in severe form of dengue shock syndrome. Journal of Medical Association of Thailand 1981;64(10):64. [PubMed] [Google Scholar]
- Futrakul P, Poshyachinda M, Mitrakul C, Kwakpetoon S, Unchumchoke P, Teranaparin C. Hemodynamic response to high‐dose methyl prednisolone an mannitol in severe dengue‐shock patients unresponsive to fluid replacement. Southeast Asian Journal of Tropical Medicine and Public Health 1987;18(3):373‐9. [PubMed] [Google Scholar]
- Hanh Tien TN, Nguyen THQ, Vu TT, Farrar J, Hoang TL, Dong THTet al. Corticosteroids for dengue why don't they work?. PLoS Neglected Tropical Diseases 2013;7(12):e2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumarmo, Widya M S, Martoatmodjo K. Clinical observations on dengue shock syndrome (an evaluation of steroid treatment). Paediatrica Indonesiana 1975;15(5‐6):151‐60. [PubMed] [Google Scholar]
- Sumarmo. The role of steroids in dengue shock syndrome. Southeast Asian Journal of Tropical Medicine and Public Health 1987;18(3):383‐9. [PubMed] [Google Scholar]
Additional references
- Annane D, Bellissant E, Bollaert PE, Briegel J, Confalonieri M, Gaudio RD, et al. Corticosteroids in the treatment of severe sepsis and septic shock in adults. JAMA 2009;301(22):2362‐74. [DOI] [PubMed] [Google Scholar]
- GreenFacts Scientific Board. WHO regions. www.greenfacts.org/glossary/wxyz/who‐regions.htm (accessed 4 October 2012).
- Higgins J P T, Green S (editors). Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions. 5.1.0. Chichester, West Sussex, England: The Cochrane Collaboration, March 2011. Available from www.cochrane‐handbook.org (accessed 4 October 2012):187‐235. [Google Scholar]
- Rajapakse S. Corticosteroids in the treatment of dengue illness. Transactions of the Royal Society of Tropical Medicine and Hygiene 2009;103:122‐6. [DOI] [PubMed] [Google Scholar]
- Rajapakse S, Chaturaka R, Rajapakse A. Treatment of dengue fever. Infection and Drug Resistance 2012;5:103‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons CP, Farrar JJ, Chau NVV, Wills B. Dengue. The New England Journal of Medicine 2012;366:1423‐32. [DOI] [PubMed] [Google Scholar]
- Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K. Hydrocortisone therapy for patients with septic shock. The New England Journal of Medicine 2008;358(2):111‐24. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Dengue haemorrhagic fever: diagnosis, treatment, prevention and control. 2nd Edition. Geneva: World Health Organization, 1997. [Google Scholar]
- World Health Organization. Dengue guidelines for diagnosis, treatment, prevention and control. New. Geneva: World Health Organization, 2009. [PubMed] [Google Scholar]
- World Health Organization. Dengue and severe dengue. Fact Sheet[January 2012]. http://www.who.int/mediacentre/factsheets/fs117/en/index.html (accessed 7 Feburary 2014).
- World Health Organization. What is dengue and how is it treated?. http://www.who.int/features/qa/54/en/index.html On line Q&A [January 2012](accessed 7 February 2014).
- World Health Organization. Global strategy for dengue prevention and control 2012‐2020. http://www.who.int/denguecontrol/9789241504034/en/. Geneva, (accessed 7 February 2014).
References to other published versions of this review
- Panpanich R, Sornchai P, Kanjanaratanakorn K. Corticosteroids for treating dengue shock syndrome. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD003488] [DOI] [PubMed] [Google Scholar]


