Abstract
RhoA is a founding member of Rho GTPase family and is a well-recognized regulator of actin cytoskeleton dynamics. Signal pathways transduced by RhoA are involved in cell migration, polarity, morphogenesis, proliferation, survival, and cell fate decision. Conditional gene targeting of Rhoa in murine blood system induces acute hematopoietic failure due to the loss of multilineage hematopoietic progenitor cells (HPC) caused by a cytokinesis defect and necrosis. Here we describe a method to conditionally induce Rhoa gene knockout in murine blood cells and a rescue by exogenous RhoA expression with lentivirus in HPCs, an approach that has general applicability in studying in vivo function of Rho GTPases and their regulators/ effectors by gene targeting.
Keywords: RhoA, Hematopoiesis, Cell fate, Gene targeting, Rescue, Viral transduction, Transplantation
1. Introduction
Hematopoietic stem cells (HSCs) can give rise to all mature blood cell lineages by a differentiation hierarchy through multipotent hematopoietic progenitor cells (HPCs). As intermediate populations in HSC differentiation, HPCs are critical for hematopoiesis and the proper homeostasis of the blood system. As a result, they are tightly controlled by multiple factors and signaling pathways involved in cell proliferation and differentiation. Dysregulation of the involved pathways can be associated with diverse hematologic diseases, including anemia, bone marrow failure, myelodysplastic syndromes, myeloproliferative neoplasms, leukemia, and lymphoma [1–7]. Dissecting the underlying mechanisms of blood development is important for improving the diagnosis and treatment of related hematologic disorders.
RhoA is a founding member of Rho GTPase family, critical for the regulation of actin cytoskeleton dynamics. Further, signaling pathways regulated by RhoA participate in multiple cell functions including cell adhesion, migration, proliferation, survival, cell cycle, and gene transcription [8–17]. Inhibition of RhoA activity by expression of a dominant RhoA mutant decreases HPC migration and increases engraftment of HSCs [16]. Conditional knockout in blood cells causes pancytopenia and bone marrow failure. However, loss of Rhoa does not impact stem cell engraftment; rather, it affects HPC function by inducing cytokinesis arrest and necrosis in multi-potent progenitors [17]. Here we describe a method to inducibly delete Rhoa from mouse bone marrow blood cells, and to validate RhoA-specific function by restoring Rhoa expression in Rhoa−/− HSPCs in vivo, which rescues the blood phenotypes caused by Rhoa loss.
2. Materials
2.1. Buffers and Reagents
FBS-PBS: Phosphate-buffered saline (PBS) (ready solution available from various commercial suppliers) with 2% fetal bovine serum (FBS) (Atlanta Biologicals).
Lysing buffer (BD).
Poly I:C (Amgen Inc.).
Recombinant fibronectin CH-296 (Takara Bio Inc.).
FuGENE 6 Transfection reagent (Promega).
Lineage depletion kit (Miltenyi Biotec).
Plasmids: Retrovirus packaging plasmids viral Gag-Pol and VSV-G envelope (Addgene); retroviral vectors REW13 (GFP expressing) and REW13-GFP/RhoA are described in ref. 17.
Antibodies (from BD): Biotin-labeled anti-B220 (RA3–6B2), CD3e (145–2C11), CD4 (RM4–5), CD8 (53–6.7), CD11b (M1/70), Gr-1 (RB6–8C5), Ter-119 (TER-119); PE-Cy7--conjugated anti-Sca-1 (D7), Percp-Cy5.5-conjugated streptavidin, PE-Cy7-conjugated anti-CD45.2 (104), APC-conjugated anti-c-Kit (2B8), and APC-conjugated anti-BrdU.
4′,6-Diamidino-2-phenylindole (DAPI) (Invitrogen).
7-Aminoactinomycin D (7-AAD) (BD).
2.2. Media
Dulbecco modified Eagle medium (DMEM) (Corning).
Iscove’s modified Dulbecco’s medium (IMDM) (Corning).
StemSpan Serum-free Expansion Medium (SFEM) medium (STEMCELL Technologies): Complete medium is supplemented with 2% FBS, 50 U/mL penicillin, 50 μg/mL streptomycin, 50 ng/mL recombinant murine SCF (PeproTech), 10 ng/mL mouse IL-3 (PeproTech), 1 mM deoxyribonucleotide triphosphates (dNTP), and 40 μg/mL low-density lipoprotein (Sigma).
2.3. Equipment
Ultracentrifuge (Beckman or equivalent).
Desktop centrifuge with plate adaptors (Beckman with SX4750A rotor and adaptors, or equivalent).
Fluorescence-activated cell sorter: FACSCanto II (BD), FACSAria II (BD), or equivalent.
Cesium irradiator.
Agate mortar and pestle.
Plasticware: 10 cm Cell culture dishes, 96-well plates, 50 mL conical tubes with 70 μm filters.
2.4. Cell Lines and Mice
HEK293T (ATCC).
NIH3T3 (ATCC).
6–8-Week-old RhoAfl/fl; Mx-Cre+ and RhoAfl/fl; Mx-cre− mice: RhoAfl/fl mice in C57Bl/6 background are crossed with Mx-cre mice in similar background.
6–8-Week-old CD45.1+ and CD45.2+ congenic C57BL/6J mice (Jackson Laboratories).
3. Methods
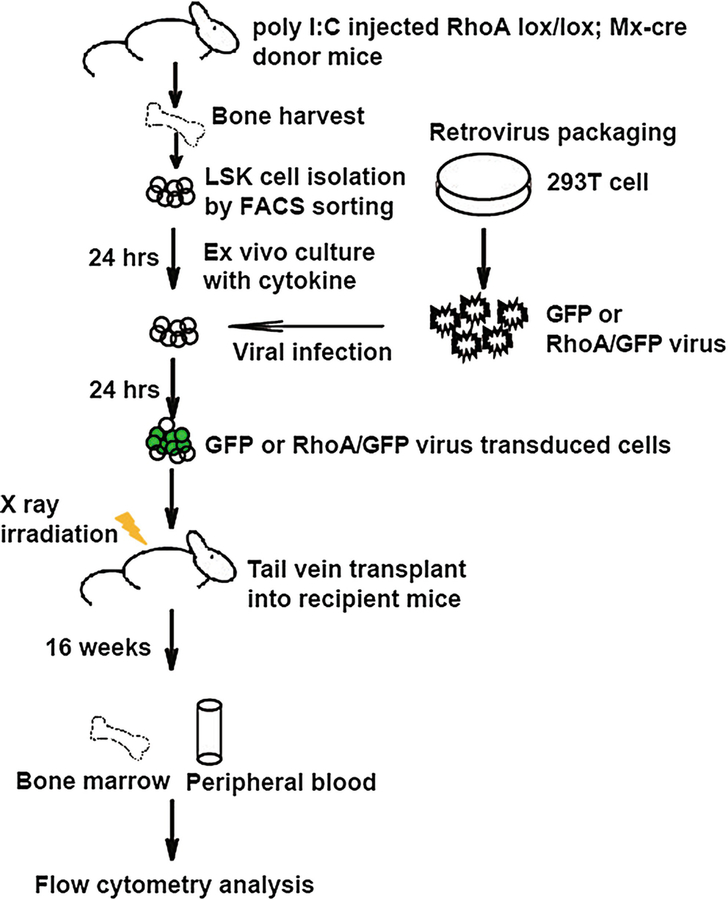
A bone marrow hematopoietic stem and progenitor cell (HSPC) manipulation and transplantation protocol is applied to evaluate HSPC functions in vivo (Fig. 1). Normal or lentiviral or retroviral transduced HSCs can reconstitute lethally irradiated mice in all blood lineages, including T, B, myeloid, and red blood cells, allowing an in vivo functional assessment of genes of interests. The wild-type or Rhoa knockout HSPCs (Lin−Sca1+Kit+ (LSK) bone marrow cells) with or without a reconstituted Rhoa gene by retroviral transduction are isolated from competitive transplantation recipients. These are mixed populations of CD45.1+ and CD45.2+ in phenotypes. The percentage of CD45.2+ myeloid cells, B cells, and T cells in the GFP+ population is determined by flow cytometry.
Fig. 1.
Experimental strategy of gene deletion and gene rescue assays in mice. LSK cells were purified from poly I:C-treated mice. After culture in the presence of a cytokine cocktail (24 h), cells were transduced with retrovirus for expression of RhoA/GFP or GFP. Next day all the cells were transplanted to lethally irradiated congenital recipient mice. Sixteen weeks later, bone marrow and peripheral blood samples were analyzed by FACS using relevant antibodies to assess engraftment and blood cell differentiation
3.1. Recombinant Retrovirus Packaging
Plate 4 × 106 HEK 293T cells in a 10 cm dish and culture them with DMEM medium supplemented with 10% FBS overnight.
Transfect HEK 293T cells with 10 μg of retroviral REW13 vector or REW13-GFP/RhoA plus 10 μg of packaging plasmids with FuGENE 6 following the manufacturer’s instructions.
Harvest virus supernatant 24 and 48 h after transfection.
Combine virus supernatants and concentrate them by ultracentrifugation at 20,000 × ℊ for 2 h at 4 °C; discard supernatant and suspend virus in IMDM supplemented with 10% FBS.
Titer virus with NIH 3T3 cells and analyze the infected cells by FACS next day to derive a multiplicity of infection (MOI) (see Note 1).
3.2. Isolation of HSPCs (Lin−Sca1+Kit+, LSK Cells)
Sacrifice 8-week-old Rhoa fl/fl; Mx-cre+ or Mx-Cre− mice and harvest bone marrow (BM) cells from femurs.
Tail vein inject lethally irradiated (split dose 1100 cGy 3 h apart) CD45.1+ C57BL/6J WT recipients (6–8 weeks old) with 3 × 106 CD45.2+ Rhoa fl/fl; Mx-cre+ or Mx-Cre− donor BM cells and equal amount of CD45.1+ WT competitor BM cells.
Eight weeks posttransplantation administer polyI:C to recipient mice by intraperitoneal injection, three injections, 10 μg/g body weight (see Note 2).
Two days after the last injection, sacrifice polyI:C-treated Mx-cre; Rhoa fl/fl and Rhoa fl/fl mice, and collect intact femurs and tibias with 1 mL of FBS-PBS; Rhoa-cKO stands for conditional knockout Rhoa in the following.
Crush bones in an agate mortar, transfer all the medium to a 50 mL conical tube with a 70 μm filter, wash the debris with 5 mL of FBS-PBS twice, combine pass-through, and spin down (500 × ℊ, 5 min, 4 °C). Resuspend cells in 400 μL of FBS-PBS.
- Lineage-negative (Lin−) cells are isolated following the instructions of the lineage depletion kit from Miltenyi Biotec.
- Incubate whole bone marrow cells with 100 μL of lineage cocktail (including all lineage markers) for 15 min on ice.
- Add 300 μL of FBS-PBS and 200 μL of beads and incubate for another 20 min.
- Wash off nonbinding antibodies and beads with 5 mL of FBS-PBS; spin down cells, and resuspend in 500 μL of FBS-PBS.
- Load cells onto magnet pre-resined column, and collect pass-through.
- Wash column with 3 mL of FBS-PBS three times.
- Combine pass-through and spin down; resuspend cells with 100 μL of FBS-PBS (see Note 3).
- Isolation of LSK cells:
- Stain Lin− cells with biotin-labeled B220, CD3e, CD4, CD8, CD11b, Gr1, and Ter119 antibodies for 15 min.
- Add 1 mL of FBS-PBS and spin down; resuspend cells with 100 μL of FBS-PBS containing Percp-Cy5.5-conjugated streptavidin, PE-Cy7–Sca-1, and APC-conjugated anti-c-Kit antibodies.
- After 30-min incubation add 1 mL of FBS-PBS and spin down; then resuspend pellet with 150 μL of FBS-PBS containing 1 μg/mL DAPI.
- LSK cells are sorted to StemSpan SFEM medium supplemented with 2% FBS.
Culture LSK cells in complete StemSpan SFEM medium for 24 h (see Note 4).
3.3. Transduction of Isolated HSPCs by Retrovirus
Coat a 96-well plate with 20 μg/mL recombinant fibronectin, and incubate at room temperature for 1 h.
Remove the remaining material and rinse wells with distilled water.
Add REW13-GFP/RhoA or REW13 empty vector retrovirus (MOI: 25) and LSK cells with StemSpan SFEM medium to the coated plate.
Wrap the plates and spin at 1500 × ℊ for 90 min at 32 °C.
After centrifugation, return plates to the incubator (see Note 5).
Four to six hours after incubation spin down transduced cells, remove supernatant, resuspend them with fresh culture medium, and culture for another 24 h.
3.4. Transplantation of Manipulated HSPCs into Recipient Mice
Irradiate 20 CD45.1+ C57BL/6J recipient mice with split dose of 1100 cGy 3 h apart (see Note 6).
Spin down transduced LSK cells, resuspend them with FBS-PBS, and add 2 × 105 CD45.1+ whole bone marrow cells as support cells. The final concentration should be 50,000 transduced LSK and 1 × 106 CD45.1+ whole bone marrow cells in 1 mL.
Transplant 200 μL of mixed cells to each mouse by tail-vein injection. Transplant five mice for each genotype.
3.5. Evaluation of the Reconstituted HSPC Function in Transplant Recipients by Peripheral Blood Analysis
Bleed the recipient mice that received transduced LSK cells 16 weeks after transplantation and collect 80–100 μL of peripheral blood.
Lyse red blood cells with 1 mL of lysing buffer.
Suspend mononuclear cells with 100 μL of FBS-PBS.
Remove 50 μL of mononuclear cells and stain them with PE-Cy7-conjugated anti-CD45.2; biotin-conjugated anti-CD11b, Gr1, B220, CD3e, or Ter119; as well as Percp-Cy5.5-conjugated streptavidin for 30 min.
Wash off nonbinding antibodies and resuspend cells with 150 μL of FBS-PBS containing 1 μg/mL DAPI.
- Analyze stained cells by FACS:
- Gate Ter119− and DAPI− cells.
- Plot Gr1 and CD11b and gate Gr1+CD11b+ for myeloid cells.
- Plot CD3e and B220 with Gr1−CD11b− cells.
- Gate B220+ for B cells and CD3e+ for T cells.
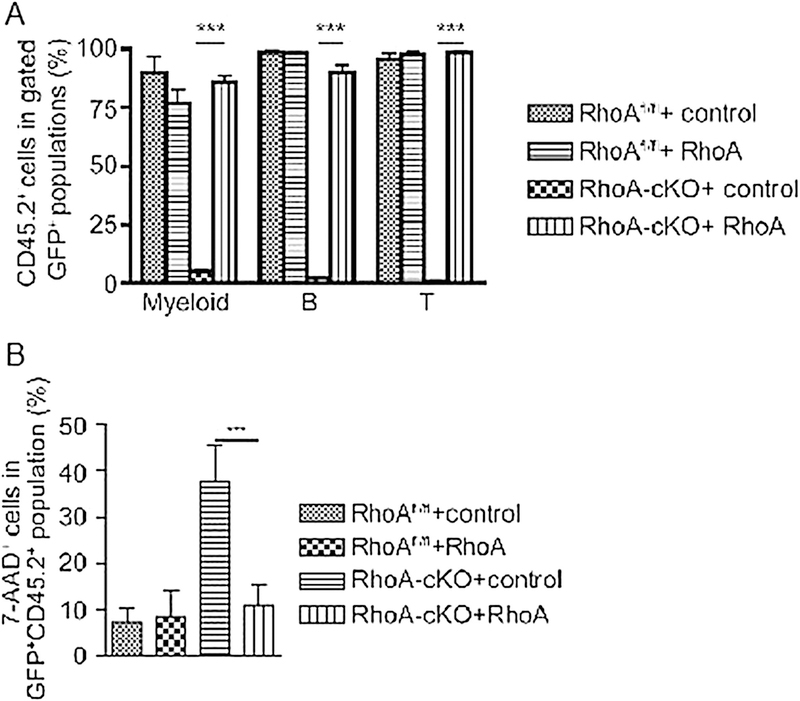
- Calculate the percentage of CD45.2+ of each lineage in GFP+ cells (Fig. 2a).
Fig. 2.
Following the experimental protocol of Fig. 1, peripheral blood cells (a) and LK cells from bone marrow (b) of recipient mice that received transduced LSK cells were analyzed by FACS. RhoAfl/fl + control: RhoAfl/fl LSK cells transduced with empty vector; RhoAfl/fl + RhoA: RhoAfl/fl LSK cells transduced with RhoA; RhoA-cKO + control: RhoA-cKO LSK cells transduced with empty vector; RhoA-cKO + RhoA: RhoA-cKO LSK cells transduced with RhoA. cKO conditional knockout allele. Significance was determined by unpaired two-tailed Student’s t-test. Error bars indicate SEM. ***p < 0.001. Adapted from ref. 17 with permission
3.6. Evaluation of the Reconstituted HSPC Function in Transplant Recipients
Harvest bone marrow cells as in Subheading 3.2, step 4.
Lyse red blood cells with 1 mL of lysing buffer.
Suspend mononuclear cells with 1000 μL of FBS-PBS.
Remove 50 μL of mononuclear cells and stain them with biotin-conjugated anti-CD11b, anti-Gr1, anti-B220, anti-CD3e, anti-Ter119, APC-Cy7-c-Kit, and Percp-Cy5.5-conjugated streptavidin, for 30 min.
Wash off nonbinding antibodies, and resuspend cells in 300 μL of FBS-PBS containing 1 μg/mL 7-AAD.
Analyze stained cells by FACS: gate Percp-Cy5.5− APC-Cy7+ GFP+ cells (i.e., GFP+ Lin−cKit+), and calculate the percentage of 7-AAD+ cells in this population (Fig. 2b). 7-AAD+ means necrosis.
3.7. Conclusion
As shown in Fig. 2, control LSK cells transduced with wild-type RhoA or vector could give rise to all blood lineages, whereas the LSK cells without RhoA expression failed to reconstitute the recipient mice as almost no donor-derived cells were detectable in peripheral blood 4 months after transplantation. When exogenous RhoA was delivered to RhoA-deficient HSPCs by retrovirus, the function of LSK cells was restored to generate myeloid cells, B cells, and T cells, comparable to that of control LSK cells (Fig. 2a). In bone marrow, LSK cells deficient of RhoA retained the engraftment in recipient mice, but RhoA deletion induced programmed necrosis, but not apoptosis, in the progenitors. Restoration of RhoA expression inhibited necrosis caused by RhoA loss (Fig. 2b). This set of rescue/transplant experiments showed that RhoA-deficient LSK cells retain engraftment ability in the bone marrow, but could not develop into myeloid, B, and T cells. Thus, RhoA is dispensable for hematopoietic stem cell engraftment but is required for progenitor cell differentiation at least in part through programmed necrosis [17]. Such a scheme of gene knockout and rescue experiments is necessary to functionally demonstrate the role of individual Rho GTPases and their regulators/effectors in mouse gene-targeted models.
Acknowledgments
This work was supported in part by US NIH grants R01CA193350 and R01DK104814 to Y.Z.
4 Notes
A retrovirus packaging and titration protocol can be found in ref. 18.
In vivo, poly I:C will cause an inflammatory response in mice and subsequent floxed allele deletion by inducing Mx-Cre expression.
In the adult mouse HSCs reside in bone marrow, including pelvis, femur, sternum, and tibia bones. LSK stands for all lineage markers negative, Sca1+ and Kit+, which enriches HSPCs. For human, HSPCs are CD34+CD38− in phenotype. HSC transplantation can reconstitute lethally irradiated mice in all lineages. Usually transplantation of bone marrow cells is a functional assay for HSCs. After transplantation, the donor-derived cells can be tracked in peripheral blood and bone marrow including mature lineages and progenitor and stem cells. It can be used to determine the frequency of HSCs in bone marrow or function of HSCs.
The FACSAria II is used to purify LSK cells and can detect all the surface markers stained with different fluorochromes. Firstly, DAPI+ and APC-Cy7+ cells are gated out; then plot cells with PE-Cy7 and APC, and double-positive cells are sorted. Those are Lin−Sca1+Kit+ (LSK) cells derived from recipient mice and are mixed cells by CD45.1+ and CD45.2+ in phenotypes.
The ex vivo culture step is to stimulate LSK cells to proliferate, which will increase the viral transduction efficiency.
Radiation treatment can damage and eliminate host hematopoietic stem cells. Transplanted HSPCs can reconstitute the host hematological system upon engraftment. Sublethal dose irradiation followed by transplantation can preserve a part of endogenous HSPCs to produce a chimera with the donor-derived cells in the recipient mice.
References
- 1.Hu S, Qian M, Zhang H, Guo Y, Yang J, Zhao X, He H, Lu J, Pan J, Chang M, Du G, Lin TN, Kham SK, Quah TC, Ariffin H, Tan AM, Cheng Y, Li C, Yeoh AE, Pui CH, Skanderup AJ, Yang JJ (2017) Whole-genome non-coding sequence analysis in T-cell acute lymphoblastic leukemia identifies oncogene enhancer mutations. Blood 129:3264–3268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makishima H, Yoshizato T, Yoshida K, Sekeres MA, Radivoyevitch T, Suzuki H, Przychodzen B, Nagata Y, Meggendorfer M, Sanada M, Okuno Y, Hirsch C, Kuzmanovic T, Sato Y, Sato-Otsubo A, LaFramboise T, Hosono N, Shiraishi Y, Chiba K, Haferlach C, Kern W, Tanaka H, Shiozawa Y, Gómez-Seguí I, Husseinzadeh HD, Thota S, Guinta KM, Dienes B, Nakamaki T, Miyawaki S, Saunthararajah Y, Chiba S, Miyano S, Shih LY, Haferlach T, Ogawa S, Maciejewski JP (2017) Dynamics of clonal evolution in myelodysplastic syndromes. Nat Genet 49:204–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding LW, Sun QY, Tan KT, Chien W, Mayakonda A, Yeoh AEJ, Kawamata N, Nagata Y, Xiao JF, Loh XY, Lin DC, Garg M, Jiang YY, Xu L, Lim SL, Liu LZ, Madan V, Sanada M, Fernández LT, Hema Preethi SS, Lill M, Kantarjian HM, Kornblau SM, Miyano S, Liang DC, Ogawa S, Shih LY, Yang H, Koeffler HP (2017) Mutational landscape of pediatric acute lymphoblastic leukemia. Cancer Res 77:390–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, Potter NE, Heuser M, Thol F, Bolli N, Gundem G, Van Loo P, Martincorena I, Ganly P, Mudie L, McLaren S, O’Meara S, Raine K, Jones DR, Teague JW, Butler AP, Greaves MF, Ganser A, Döhner K, Schlenk RF, Döhner H, Campbell PJ (2016) Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med 374:2209–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor J, Xiao W, Abdel-Wahab O (2017) Diagnosis and classification of hematologic malignancies on the basis of genetics. Blood 130:410–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel BJ, Przychodzen B, Thota S, Radivoyevitch T, Visconte V, Kuzmanovic T, Clemente M, Hirsch C, Morawski A, Souaid R, Saygin C, Nazha A, Demarest B, LaFramboise T, Sakaguchi H, Kojima S, Carr-away HE, Ogawa S, Makishima H, Sekeres MA, Maciejewski JP (2017) Genomic determinants of chronic myelomonocytic leukemia. Leukemia 31:2815–2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Vlierberghe P, Ferrando A (2012) The molecular basis of T cell acute lymphoblastic leukemia. J Clin Invest 122:3398–3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geh E, Meng Q, Mongan M, Wang J, Takatori A, Zheng Y, Puga A, Lang RA, Xia Y (2011) Mitogen-activated protein kinase kinase kinase 1 (MAP 3K1) integrates developmental signals for eyelid closure. Proc Natl Acad Sci U S A 108:17349–17354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson B, Peyrollier K, Pedersen E, Basse A, Karlsson R, Wang Z, Lefever T, Ochsenbein AM, Schmidt G, Aktories K, Stanley A, Quondamatteo F, Ladwein M, Rottner K, van Hengel J, Brakebusch C (2011) RhoA is dispensable for skin development, but crucial for contraction and directed migration of keratinocytes. Mol Biol Cell 22:593–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melendez J, Stengel K, Zhou X, Chauhan BK, Debidda M, Andreassen P, Lang RA, Zheng Y (2011) RhoA GTPase is dispensable for acto-myosin regulation but is essential for mitosis in primary mouse embryonic fibroblasts. J Biol Chem 286:15132–15137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pleines I, Hagedorn I, Gupta S, May F, Chakarova L, van Hengel J, Offermanns S, Krohne G, Kleinschnitz C, Brakebusch C, Nieswandt B (2012) Megakaryocyte-specific RhoA deficiency causes macrothrombocytopenia and defective platelet activation in hemostasis and thrombosis. Blood 119:1054–1063 [DOI] [PubMed] [Google Scholar]
- 12.Xiang SY, Vanhoutte D, Del Re DP, Purcell NH, Ling H, Banerjee I, Bossuyt J, Lang RA, Zheng Y, Matkovich SJ, Miyamoto S, Molkentin JD, Dorn GW, Brown JH (2011) RhoA protects the mouse heart against ischemia/reperfusion injury. J Clin Invest 121:3269–3276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang S, Zhou X, Lang RA, Guo F (2012) RhoA of the rho family small GTPases is essential for B lymphocyte development. PLoS One 7:e33773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zandvakili I, Lin Y, Morris JC, Zheng Y (2017) Rho GTPases: anti- or pro-neoplastic targets? Oncogene 36:3213–3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paul CD, Mistriotis P, Konstantopoulos K (2017) Cancer cell motility: lessons from migration in confined spaces. Nat Rev Cancer 17:131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghiaur G, Lee A, Bailey J, Cancelas JA, Zheng Y, Williams DA (2006) Inhibition of RhoA GTPase activity enhances hematopoietic stem and progenitor cell proliferation and engraftment. Blood 108:2087–2094 [DOI] [PubMed] [Google Scholar]
- 17.Zhou X, Florian MC, Arumugam P, Chen X, Cancelas JA, Lang R, Malik P, Geiger H, Zheng Y (2013) RhoA GTPase controls cytokinesis and programmed necrosis of hematopoietic progenitors. J Exp Med 210:2371–2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. https://www.addgene.org/viral-vectors/retrovirus/retro-guide/