Abstract
Purpose:
The presented protocol for pediatric intermediate-risk Hodgkin lymphoma evaluated the use of a dose-intensive chemotherapy regimen (ABVE-PC [doxorubicin, bleomycin, vincristine, etoposide, cyclophosphamide, prednisone]) with response-based therapy augmentation (addition of DECA [dexamethasone, etoposide, cisplatin, cytarabine]) or therapy reduction (elimination of radiation).
Methods and Materials:
A central review of the radiation therapy data for quality assurance was performed, and the association between radiation protocol deviation (RPD) and relapse was assessed in the pediatric group (age <15 years) and adolescent and young adult (AYA) group (age ≥15-21 years). Involved-field radiation therapy (IFRT) planning was reviewed before the start of treatment and at treatment completion. The records were reviewed through the Quality Assurance Review Center’s central review to identify RPD, classified according to dose deviation (DD), volume deviation (VD), undertreatment (UT), and overtreatment (OT). DDs and VDs were further classified as major or minor.
Results:
Of the 1712 patients enrolled, 1155 received IFRT, of whom, 216 (18.7%) had RPDs. The DD and VD patterns were similar between the pediatric and AYA groups. Minor VDs were most common. UT RPDs accounted for 69% in the pediatric group and 75% in the AYA group. Of the 35 patients with relapse and a RPD, 29 had an undertreatment RPD. Among the patients who received IFRT, a significant difference was found in the cumulative incidence rates of relapse between the pediatric and AYA groups (P = .03); however, no significant difference was found between patients with and without RPD (P = .2).
Conclusions:
Most RPDs were minor and consisted of UT in the AYA and pediatric populations both. No difference was observed in RPDs between the pediatric and AYA patients. Thus, in a well-defined and standardized protocol, the RPD distributions for AYA patients will be similar to those for pediatric population. However, the increased cumulative incidence of relapse in the AYA patients who had received IFRT compared with the pediatric population requires further exploration, given the potential differences in clinical outcomes in the AYA population.
Summary
Because the Children’s Oncology Group AHOD0031 study is the largest phase III study to date on intermediate-risk Hodgkin lymphoma in pediatric patients, we investigated the patterns of radiation protocol deviation and relapse between the pediatric and adolescent and young adult patient cohorts. We found no statistically significant differences in deviation patterns between the 2 cohorts, although of those who had received involved-field radiation therapy, a significant difference was found in the cumulative incidence rates of relapse between the 2 groups.
Introduction
Hodgkin lymphoma (HL) comprises 15% of pediatric and adolescent cancers and is the most commonly diagnosed cancer among adolescents aged 15 to 19 years (1). The observed 15-year survival has been close to 91% (1). These excellent outcomes have led to a shift in the focus to balance efficacy with toxicity, using risk-adapted therapy centered on response-based therapy to attenuate the late effects and maintaining the excellent outcomes (2–17).
Thus, the presented protocol was designed to evaluate the role of early chemotherapy response in tailoring subsequent therapy, including radiation therapy (RT), to avoid treatment-associated risks that jeopardize overall survival and quality of life (18–20). The present trial used central review to ensure the standardization of RT to minimize treatment deviations and improve patient outcomes, because deviations can be independent predictors of poor outcomes (21–24). The study was also unique because of the use of real-time review of anatomic and metabolic images for a central review evaluation of the response (25).
The adolescent and young adult (AYA) population has emerged in recent years as a distinct age group with unique medical and psychosocial needs. AYA patients have not been treated consistently, and the therapy for AYA patients provider (eg, whether treated by an adult or pediatric oncologist). Data exist to suggest that AYA patients have improved clinical outcomes when treated at Children’s Oncology Group (COG) institutions (26). Cancer deaths have declined more dramatically in all age groups, except for the 15- to 29-year age group (27, 28). This population has demonstrated unique challenges and clinical characteristics that have prevented them from benefitting equally in outcomes compared with their pediatric and adult counterparts; hence, the AYA survival gap (26–36).
The purpose of the present investigation was to examine the patterns of radiation protocol deviation (RPD) and relapse between the pediatric (age <15 years) and AYA (age ≥15-21 years) patient cohorts in the Children’s Oncology Group AHOD0031 study to determine whether the clinical approaches and outcomes between these 2 age groups differed in the context of standardized therapy.
Methods and Materials
Patients
A total of 1712 eligible patients aged <22 years from 256 institutions in 7 countries were entered in the Children’s Oncology Group Study AHOD0031 protocol, which included newly diagnosed, biopsy proven HL, Ann Arbor stage IAE/IIAE (1.6%), IB (0.9%), IIB (21.3%), IIIA (20.6%), IVA (14.8%) without bulk disease, and IA (4.8%) or IIA (35.9%) with bulk disease. Overall, 1257 patients (73.4%) presented with bulk disease and 435 (25.4%) did not; the status of the remaining 20 patients was not reported. The histologic pathologic type included nodular sclerosis (80.8%), mixed cellularity (9.1%), lymphocyte predominant (5.7%), lymphocyte depleted (0.2%), and not specified (4.2%). The study participant characteristics are detailed further in Table 1. The trial opened to accrual on September 23, 2002 and closed permanently on October 9, 2009. Of the 1712 eligible patients, 1155 received involved-field RT (IFRT), of whom 216 had a RPD.
Table 1.
Characteristics of study participants
| Characteristic | n (%) |
|---|---|
| Age (y) | |
| 0-4 | 29 (1.7) |
| 5-9 | 147 (8.6) |
| 10-14 | 641 (37.4) |
| 15-21 | 895 (52.3) |
| Sex | |
| Male | 908 (53.0) |
| Female | 804 (47.0) |
| Stage | |
| I | 98 (5.7) |
| II | 1006 (58.8) |
| III | 354 (20.7) |
| IV | 254 (14.8) |
| Histologic type | |
| Nodular sclerosis | 1383 (80.8) |
| Mixed cellularity | 156 (9.1) |
| Lymphocyte predominant | 97 (5.7) |
| Lymphocyte depleted | 4 (0.2) |
| Unknown/unreported | 72 (4.2) |
| B symptoms | |
| Yes | 381 (22.2) |
| No | 1327 (77.5) |
| Not reported | 4 (0.2) |
| Bulk disease | |
| Yes | 1257 (73.4) |
| No | 435 (25.4) |
| Not reported | 20 (1.2) |
Treatment
The patients received 2 cycles of ABVE-PC (doxorubicin, bleomycin, vincristine, etoposide, cyclophosphamide, prednisone). The early response was assessed by computed tomography and classified as a rapid early response (RER) or a slow early response (SER). Patients with a RER received 2 additional cycles of ABVE-PC and were again assessed for a complete response (CR) (18). If a CR was achieved, they were randomly assigned to IFRT or observation. Patients with a RER without a CR were assigned to IFRT. Patients with a SER were randomly assigned to therapy augmentation with 2 cycles of DECA (dexamethasone, etoposide, cisplatin, cytarabine), followed by 2 additional cycles of ABVE-PC or 2 cycles of ABVE-PC without DECA. Both SER arms were assigned to IFRT.
IFRT was delivered in 14 fractions of 1.5 Gy for a total dose of 21 Gy that began within 4 weeks of completing chemotherapy. RT was limited to the involved areas of disease at presentation. Any lymph node measuring >1.5 cm in a single axis on computed tomography was included in the gross tumor volume and clinical target volume included the anatomic compartment containing the involved lymph nodes. To account for patient motion and setup variability, planning target volume included a 1.0-cm margin around the clinical target volume, although modification was left to the discretion of the treating radiation oncologist to allow for concern of extended treatment of normal tissue.
Protocol compliance and quality assurance
To ensure validity and generalizability in this large multinational trial involving a multiarm protocol schema, high-quality protocol methods were used, which has been the practice for cooperative group trials, including the COG. The Quality Assurance Review Center (QARC) was established to facilitate quality assurance (QA) for RT and superior outcomes, promoting confidence in study results.
Thus, treating centers were required to submit IFRT plans to the QARC for central review before the start of treatment, at which time suggestions were made to ensure compliance with protocol-specified fields. On therapy completion, plans were again required to be submitted to evaluate any revised documentation or modifications that occurred after the initial, rapid, pretreatment review.
These records were reviewed by study-affiliated or QARC-affiliated radiation oncologists to identify dose deviations (DDs) and volume deviations (VDs). A DD was classified as minor if a 6% to 10% deviation had occurred from the protocol specification and major if the deviation were >10%. A VD was considered “minor” if the volume margins were less than the protocol specification and major if the fields had transected disease-bearing areas. Our specific review, separate from the previous central review, further categorized any DD and VD as undertreatment (UT) or overtreatment (OT). UT was defined as less than the specified dose or volume and OT as more than the specified dose or volume.
Dharmarajan et al (20) recently reported on the use of the centralized review in the COG AHOD0031 trial titled, “Radiotherapy Quality Assurance Report from Children’s Oncology Group AHOD0031.” They found that 88% of patient plans underwent an interventional review and 98% of plans, a final review (20). A protocol guideline compliance rate of 100% was obtained when the recommended modifications made by the review center were incorporated by the treating institution. Patients were considered to have a RPD in the case of failure to submit plans for pretreatment review or if the plans were submitted but were not compliant with the recommended modifications.
Statistical analysis
Our primary research aim was to evaluate the differences in the prevalence of RPDs between pediatric (age <15 years) and AYA (age ≥15-21 years) patients in the COG AHOD0031 trial. The prevalence of all forms of RPDs in aggregate was compared across the pediatric and AYA groups using the χ2 test. Subanalyses were used to assess the prevalence of each form of RPD individually (ie, minor VDs, major VDs, minor DDs, major DDs) between the 2 age groups using χ2 and Fischer’s exact tests, as appropriate. Further subset analyses used the χ2 test to compare the prevalence of UT and OT between the 2 age groups. A secondary analysis assessed the association between RPDs and relapse among the pediatric and AYA populations using χ2 and Fisher’s exact tests, as appropriate. All data analysis was performed using SAS, version 9.4 (SAS Institute Inc, Cary, NC).
Results
Overall characteristics
Of the 1712 eligible patients, 1155 (476 pediatric, 679 AYA) received IFRT. A total of 220 RPDs (80 pediatric patients, 140 AYA patients) were recorded by the QARC, representing 216 patients (18.7%; 78 pediatric patients, 138 AYA patients).
DDs versus VDs
The patterns of deviation were examined between the pediatric and AYA groups. The deviations were categorized as minor VDs, major VDs, minor DDs, and major DDs using the specific criteria explained in the Methods and Materials section. When comparing the 2 groups, the DD and VD patterns were similar. Of the pediatric and AYA patients, 16% (78 of 476) and 20% (138 of 679) had a deviation recorded during the study (P = .09). Of the 220 RPD cases, representing 216 individual patients, 90% were categorized as VDs. A minor VD was the most common deviation type, with no statistically significant difference between the pediatric and AYA patients (56% [45 of 80] vs 60% [84 of 140; P = .59). A difference was not observed between the number of patients with a RPD in the pediatric and AYA cohorts (16% [78 of 476] vs 19% [138 of 679]; P = .09) nor among the patterns of deviation. The categorization of deviation patterns between the 2 age groups are summarized in Table 2.
Table 2.
Categorization of deviations between pediatric and AYA patients*
| Variable | Pediatric group (n = 80) | AYA group (n = 140) |
|---|---|---|
| Minor VD | 45 (56) | 84 (60) |
| Minor DD | 6 (8) | 10 (7) |
| Major VD | 27 (34) | 43 (31) |
| Major DD | 2 (3) | 3 (2) |
Abbreviations: AYA = adolescent and young adult; DD = dose deviation; VD = volume deviation.
Data presented as n (%).
P > .05.
UT versus OT
Further analysis included categorizing the type of deviation as either UT, defined as less than the specified dose or volume, and OT, defined as more than the specified dose or volume. UT RPDs accounted for 69% (54 of 78) of the deviations in the pediatric group and 75% (103 of 138) in the AYA group. OT RPD accounted for 26% (20 of 78) of the deviations in the pediatric group and 22% (30 of 138) in the AYA group, with 5% (4 of 78) of the pediatric patients and 4% (5 of 138) of the AYA patients having both UT and OT deviations. No difference was observed regarding UT or OT between the 2 groups. The categorization of the UT and OT patterns between the 2 age groups has been further summarized in Table 3.
Table 3.
Categorization of undertreatment and overtreatment between pediatric and AYA patients among RPD patients*
| Variable | Pediatric group (n = 78) | AYA group (n = 138) |
|---|---|---|
| Less than specified dose or volume | 54 (69) | 103 (75) |
| More than specified dose or volume | 20 (26) | 30 (22) |
| Both | 4 (5) | 5 (4) |
Abbreviation: AYA = adolescent and young adult.
Data presented as n (%).
P > .67.
Relapse patterns
Of the 216 patients with RPD, 35 experienced disease relapse. The cumulative incidence rates of relapse were compared between the 2 groups. Among the patients who had received IFRT, a statistically significant difference was found in the cumulative incidence rates of relapse between the pediatric and AYA groups (P = .03). However, no statistically significant difference was found between patients with and without RPD (P = .2). The comparisons of the cumulative incidence rates of relapse are shown in Figures 1 and 2.
Fig. 1.
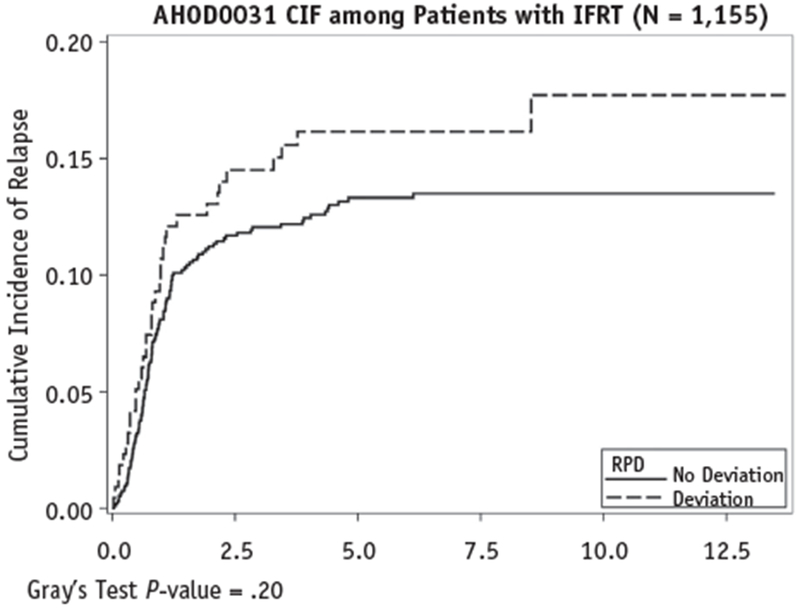
Comparison of cumulative incidence of relapse rates between patients with and without radiation protocol deviation (RPD). Abbreviations: CIF = cumulative incidence frequency; IFRT = involved-field radiation therapy.
Fig. 2.
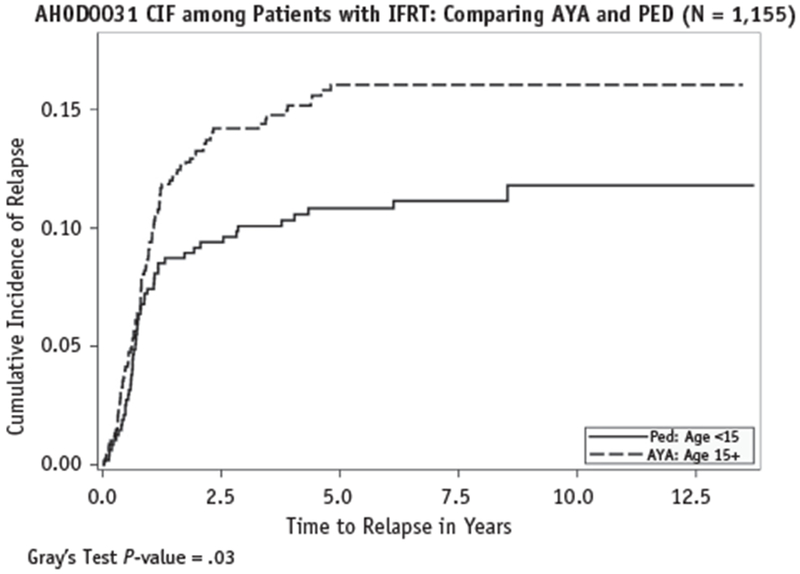
Comparison of cumulative incidence of relapse rates between pediatric (PED; Ped) and adolescent and young adult (AYA) patients. Abbreviations: CIF = cumulative incidence frequency; IFRT = involved-field radiation therapy.
Of the 35 relapses, 29 (83%) occurred in patients with an UT RPD, which was not significantly different statistically from the 71% (128 of 181) of the nonrelapse group categorized as having an UT RPD (P = .38). The comparison of the UT RPD proportions are presented in Table 4. Further subcategorization comparing the relapse and UT/OT between the 2 age groups resulted in diminishing categorical values. The same analysis was conducted for the RER subpopulation and non-RER subpopulation to compare the UT RPD proportions between the relapse and nonrelapse groups. Among the RPD and non-RER patients, 6 of the 8 relapses (75%) occurred among the UT group. In contrast, 28 of the 39 nonrelapse patients (72%) had an UT RPD (P = .33). A similar nonsignificant association was found among the RER patients comparing the UT proportions among the relapse and nonrelapse patients: 23 of the 27 patients with relapse (85%) versus 100 of the 142 patients (70%) without relapse (P = .3). In addition, no significant differences in the cumulative incidence rates of relapse between the AYA and pediatric groups were found for either the RER subpopulation (P = .23) or the non-RER subpopulation (P = .54). Owing to the decreasing group numbers with further classification of the 27 patients with relapse, statistical analysis could not be performed.
Table 4.
OT and UT association to relapse*
| Variable | Relapse group (n = 35) | Nonrelapse group (n = 181) |
|---|---|---|
| Less than specified dose or volume (UT) | 83 (29/35) | 71 (128/181) |
| More than specified dose or volume (OT) | 17 (5/35) | 25 (45/181) |
| Both | 3 (1/35) | 4 (8/181) |
Abbreviations: OT = overtreatment; UT = undertreatment.
Data presented as n (%).
P > .34.
Discussion
The COG AHOD0031 study evaluated the outcomes of early response-based therapy in intermediate-risk pediatric HL patients. Central quality control in real time was critically performed to ensure therapy standardization. As such, the present collection of data is of great quality for analysis. The association of poor outcomes with the occurrence RPDs has been reported in several studies, underlining the importance of QA, not only in clinical trials, but also in everyday practice.
Of particular interest is that deviation patterns between the pediatric and AYA cohorts did not demonstrate any differences. The wide majority of deviations were minor, which could have resulted from several inherent characteristics of clinical practice and QA review. As such, a central review further reduced the small probability that the treating clinician created a plan with a major deviation. The observation that 93% of all deviations resulted from volume discrepancies reflects a cognizance to avoid the toxic late effects of therapy. In general, a similar percentage of pediatric and AYA patients had deviations from the RT protocol, and those deviations, whether volume or dose and major or minor, were similar between the 2 groups.
When the type of deviations were further categorized into UT and OT to assess for differences in the approach to treatment for pediatric and AYA patients, one would presume that the younger patients might be predisposed to UT, because the concern for late effects is increased. However, this was not the case; a similar percentage of the AYA population was also undertreated. Several confounding issues could have influenced this observation, including, but not limited to, the referral patterns of these patients, the familiarity with treating this specific population, and the definition of such age categories at different institutions.
These results suggest that the QA process was consistent regardless of age and that the treating clinicians did not approach RT plans differently according to the patient’s age. Successful adherence to the RT protocol was achieved, which, in turn, could improve the outcomes for the patients in the study, especially those in the AYA improvement gap.
A recent report by Dharmarajan et al (19) evaluated the relapse patterns in the COG AHOD0031 study. They evaluated 198 of the total 244 patient relapses—46 were not fully evaluable for review because the patients had developed progression during treatment or lacked imaging studies of the relapse. The median time to relapse was 12.8 months. They found that 94% of the recurrences developed within the irradiated sites, consistent with other data, implying that little rationale exists for expanding fields even in the higher risk patients. This suggests that treating involved fields is sufficient and that potential dose escalation can be re-evaluated in specific patients with less favorable disease (8, 37–39). Ongoing studies using involved-site radiation therapy will further identify the optimal dose and volume targets for these patients.
Our study did not show a clear association among the occurrence of relapse, deviation, and UT or OT. One confounding factor could be that the AHOD0031 study analysis found that RT did not demonstrate a benefit for patients with RER with a CR in the overall study population. Further analysis excluding patients with a RER with a CR still did not demonstrate any significance when comparing the 2 groups. However, among the patients who received RT, we identified a significant difference in the cumulative incidence rates of relapse between the pediatric and AYA groups, although no significant difference was found between patients with and without RPD. This finding likely reflects the potential biological differences in the AYA population compared with their younger counterparts with similar diagnoses (40).
Ultimately, higher doses might be required to achieve local control in the setting in which only unfavorable patients, such as those with bulky disease or a slow response, are referred for RT. The challenge of present studies will be to identify the optimal balance between maintaining the efficacy of therapy, especially for those patients who continue to have suboptimal event-free survival such as patients with a SER, and minimizing long-term iatrogenic morbidity.
Conclusions
A RPD occurred in the few patients with intermediate-risk HL undergoing IFRT in the COG AHOD0031 study. Most RPDs were VDs and were minor and related to UT. No difference was observed in the RPD patterns between the pediatric and AYA cohorts, demonstrating that the QA process was consistent, regardless of patient age. The AYA patients experienced a greater cumulative incidence rate of relapse compared with the pediatric patients, warranting further exploration, given that no difference was found between those with and without RPDs.
Acknowledgments
This work was supported by the American Society for Radiation Oncology through the ASTRO Summer Minority Fellowship Award.
Footnotes
Conflict of interest: none.
References
- 1.Ward E, DeSantis C, Robbins A, et al. Childhood and adolescent cancer statistics. CA Cancer J Clin 2014;64:83–103. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz CL, Constine LS, Villaluna D, et al. A risk-adapted, response-based approach using ABVE-PC for children and adolescents with intermediate- and high-risk Hodgkin lymphoma: The results of P9425. Blood 2009;114:2051–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donaldson SS, Link MP, Weinstein HJ, et al. Final results of a prospective clinical trial with VAMP and low-dose involved-field radiation for children with low-risk Hodgkin’s disease. J Clin Oncol 2007; 25:332–337. [DOI] [PubMed] [Google Scholar]
- 4.Dörffel W, Lüders H, Rühl U, et al. Preliminary results of the multicenter trial GPOH-HD 95 for the treatment of Hodgkin’s disease in children and adolescents: Analysis and outlook. Klin Padiatr 2003; 215:139–145. [DOI] [PubMed] [Google Scholar]
- 5.Hutchinson RJ, Fryer CJ, Davis PC, et al. MOPP or radiation in addition to ABVD in the treatment of pathologically staged advanced Hodgkin’s disease in children: Results of the Children’s Cancer Group phase III trial. J Clin Oncol 1998;16:897–906. [DOI] [PubMed] [Google Scholar]
- 6.Kelly KM, Sposto R, Hutchinson R, et al. BEACOPP chemotherapy is a highly effective regimen in children and adolescents with high-risk Hodgkin lymphoma: A report from the Children’s Oncology Group. Blood 2011;117:2596–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kung FH, Schwartz CL, Ferree CR, et al. POG 8625: A randomized trial comparing chemotherapy with chemoradiotherapy for children and adolescents with stages I, IIA, IIIA1 Hodgkin disease: A report from the Children’s Oncology Group. J Pediatr Hematol Oncol 2006; 28:362–368. [DOI] [PubMed] [Google Scholar]
- 8.Nachman JB, Sposto R, Herzog P, et al. Randomized comparison of low-dose involved-field radiotherapy and no radiotherapy for children with Hodgkin’s disease who achieve a complete response to chemotherapy. J Clin Oncol 2002;20:3765–3771. [DOI] [PubMed] [Google Scholar]
- 9.Friedmann AM, Hudson MM, Weinstein HJ, et al. Treatment of unfavorable childhood Hodgkin’s disease with VEPA and low-dose, involved-field radiation. J Clin Oncol 2002;20:3088–3094. [DOI] [PubMed] [Google Scholar]
- 10.Wolden SL, Chen L, Kelly KM, et al. Long-term results of CCG 5942: A randomized comparison of chemotherapy with and without radiotherapy for children with Hodgkin’s lymphoma—A report from the Children’s Oncology Group. J Clin Oncol 2012;30:3174–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dieckmann K, Potter R, Hofmann J, et al. Does bulky disease at diagnosis influence outcome in childhood Hodgkin’s disease and require higher radiation doses? Results from the German-Austrian Pediatric Multicenter trial DAL-HD-90. Int J Radiat Oncol Biol Phys 2003;56:644–652. [DOI] [PubMed] [Google Scholar]
- 12.Fryer CJ, Hutchinson RJ, Krailo M, et al. Efficacy and toxicity of 12 courses of ABVD chemotherapy followed by low-dose regional radiation in advanced Hodgkin’s disease in children: A report from the Children’s Cancer Study group. J Clin Oncol 1990;8: 1971–1980. [DOI] [PubMed] [Google Scholar]
- 13.Mauz-Körholz C, Hasenclever D, Dörffel W, et al. Procarbazine-free OEPA-COPDAC chemotherapy in boys and standard OPPA-COPP in girls have comparable effectiveness in pediatric Hodgkin’s lymphoma: The GPOH-HD-2002 study. J Clin Oncol 2010;28:3680–3686. [DOI] [PubMed] [Google Scholar]
- 14.Tebbi CK, Mendenhall NP, London WB, et al. Response-dependent and reduced treatment in lower risk Hodgkin lymphoma in children and adolescents, results of P9426: A report from the Children’s Oncology Group. Pediatr Blood Cancer 2012;59:1259–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiner MA, Leventhal B, Brecher ML, et al. Randomized study of intensive MOPP-ABVD with or without low-dose total-nodal radiation therapy in the treatment of stages IIB, IIIA2, IIIB, and IV Hodgkin’s disease in pediatric patients: A Pediatric Oncology Group study. J Clin Oncol 1997;15:2769–2779. [DOI] [PubMed] [Google Scholar]
- 16.Carde P, Koscielny S, Franklin J, et al. Early response to chemotherapy: A surrogate for final outcome of Hodgkin’s disease patients that should influence initial treatment length and intensity? Ann Oncol 2002;13(1 Suppl):86–91. [DOI] [PubMed] [Google Scholar]
- 17.Metzger ML, Weinstein HJ, Hudson MM, et al. Association between radiotherapy vs no radiotherapy based on early response to VAMP chemotherapy and survival among children with favorable-risk Hodgkin lymphoma. JAMA 2012;307:2609–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman DL, Chen L, Wolden S, et al. Dose-intensive response-based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate-risk Hodgkin lymphoma: A report from the Children’s Oncology Group Study AHOD0031. J Clin Oncol 2014;32:3651–3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dharmarajan KV, Friedman DL, Schwartz CL, et al. Patterns of relapse from a phase 3 study of response-based therapy for intermediate-risk Hodgkin lymphoma (AHOD0031): A report from the Children’s Oncology Group. Int J Radiat Oncol Biol Phys 2015; 92:60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dharmarajan KV, Friedman DL, FitzGerald TJ, et al. Radiotherapy quality assurance report from Children’s Oncology Group AHOD0031. Int J Radiat Oncol Biol Phys 2015;91:1065–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abrams RA, Winter KA, Regine WF, et al. Failure to adhere to protocol specified radiation therapy guidelines was associated with decreased survival in RTOG 9704-A phase III trial of adjuvant chemotherapy and chemoradiotherapy for patients with resected adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys 2012; 82:809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peters LJ, O’Sullivan B, Giralt J, et al. Critical impact of radiotherapy protocol compliance and quality in the treatment of advanced head and neck cancer: Results from TROG 02.02. J Clin Oncol 2010;28: 2996–3001. [DOI] [PubMed] [Google Scholar]
- 23.Ohri N, Shen X, Dicker AP, et al. Radiotherapy protocol deviations and clinical outcomes: A meta-analysis of cooperative group clinical trials. J Natl Cancer Inst 2013;105:387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fairchild A, Straube W, Laurie F, et al. Does quality of radiation therapy predict outcomes of multicenter cooperative group trials? A literature review. Int J Radiat Oncol Biol Phys 2013;87: 246–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.FitzGerald TJ. What we have learned: The impact of quality from a clinical trials perspective. Semin Radiat Oncol 2012;22:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bleyer A The quid pro quo of pediatric versus adult services for older adolescent cancer patients. Pediatr Blood Cancer 2010;54:238–241. [DOI] [PubMed] [Google Scholar]
- 27.Bleyer A Young adult oncology: The patients and their survival challenges. CA Cancer J Clin 2007;57:242–255. [DOI] [PubMed] [Google Scholar]
- 28.Bleyer A, Viny A, Barr R. Introduction In: Bleyer A, O’Leary M, Barr R, et al. , editors. Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 Years of Age, Including SEER Incidence and Survival: 1975-2000. Bethesda, MD: National Cancer Institute; 2006. p. 1–14. [Google Scholar]
- 29.Bleyer A, Morgan S, Barr R. Proceedings of a workshop: Bridging the gap in care and addressing participation in clinical trials. Cancer 2006; 107(S7 Suppl):1656–1658. [DOI] [PubMed] [Google Scholar]
- 30.Aben KK, van Gaal C, van Gils NA, et al. Cancer in adolescents and young adults (15—29 years): A population-based study in the Netherlands 1989—2009. Acta Oncol 2012;51:922–933. [DOI] [PubMed] [Google Scholar]
- 31.Adolescent and Young Adult Oncology Progress Review Group. Closing the gap: Research and care imperatives for adolescents and young adults with cancer. NIH Publication No. 06-6067 National Cancer Institute; 2006. Available at: http://www.cancer.gov/types/aya/research/ayao-august-2006.pdf Accessed June 11, 2016. [Google Scholar]
- 32.Eichenauer DA, Borchmann P, Engert A. Adolescents with Hodgkin lymphoma: Old children or young adults? Leuk Lymphoma 2012;53: 1257–1262. [DOI] [PubMed] [Google Scholar]
- 33.Jachimowicz RD, Engert A. The challenging aspects of managing adolescents and young adults with Hodgkin’s lymphoma. Acta Haematol 2014;132:274–278. [DOI] [PubMed] [Google Scholar]
- 34.Bleyer A, Budd T, Montello M. Adolescents and young adults with cancer: The scope of the problem and criticality of clinical trials. Cancer 2006;107(7 Suppl):1645–1655. [DOI] [PubMed] [Google Scholar]
- 35.Müller J, Illés A, Molnár Z, et al. Adolescent Hodgkin lymphoma: Are treatment results more favorable with pediatric than with adult regimens? J Pediatr Hematol Oncol 2011;33:e60–e63. [DOI] [PubMed] [Google Scholar]
- 36.Mass SJ, Patlak M. Identifying and Addressing the Needs of Adolescents and Young Adults With Cancer: Workshop Summary. Washington, DC: National Academies Press; 2013. [PubMed] [Google Scholar]
- 37.Dhakal S, Biswas T, Liesveld JL, et al. Patterns and timing of initial relapse in patients subsequently undergoing transplantation for Hodgkin’s lymphoma. Int J Radiat Oncol Biol Phys 2009;75:188–192. [DOI] [PubMed] [Google Scholar]
- 38.Shahidi M, Kamangari N, Ashley S, et al. Site of relapse after chemotherapy alone for stage I and II Hodgkin’s disease. Radiother Oncol 2006;78:1–5. [DOI] [PubMed] [Google Scholar]
- 39.Huynh-Le MP, Walker AJ, Kominers SD, et al. Patterns of failure after involved field radiation therapy for pediatric and young adult Hodgkin lymphoma. Pediatr Blood Cancer 2014;61:1210–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bleyer A, Barr R, Hayes-Lattin B, et al. Biology and Clinical Trials Subgroups of the US National Cancer Institute Progress Review Group in Adolescent and Young Adult Oncology. The distinctive biology ofcancer in adolescents and young adults. Nat Rev Cancer 2008;8:288–298. [DOI] [PubMed] [Google Scholar]


