Abstract
Triple-negative breast cancer (TNBC) is associated with a high risk of recurrence and generally a bad prognosis. More than one-third of patients with TNBC will present distant metastases during the course of their disease. Although chemotherapy has been the main treatment option for metastatic TNBC for a long time, this scenario has changed recently with the advent of the polyadenosine diphosphate-ribose polymerase inhibitors (PARPis) for patients harbouring a mutation in the BRCA genes (BRCAmut) and also with the results of immunotherapy in patients with PD-L1-positive tumours. The present manuscript proposes a treatment algorithm for patients with metastatic TNBC based on the currently available, most relevant literature on the topic. For patients with a BRCAmut and able to tolerate chemotherapy, we recommend initiating treatment with platins (carboplatin/cisplatin) and to start PARPis at disease progression. For patients with PD-L1-positive tumours (PD-L1 expression on tumour-infiltrating immune cells ≥1%), we recommend first-line treatment with nab-paclitaxel and atezolizumab, when available. In patients without a BRCA mutation and with PD-L1-negative tumours, we recommend single-agent chemotherapy with taxanes (paclitaxel or docetaxel) as a first-line treatment. In patients with a high disease burden or who are very symptomatic, combinations such as anthracyclines plus cyclophosphamide or platins with taxanes are valid options. Chemotherapy should be maintained until the occurrence of disease progression or limiting toxicities. After progression to first-line chemotherapy, anthracyclines are an option for patients who received taxanes and vice versa. For patients who progressed to taxanes and anthracyclines, or who present contraindications to these agents, fluorouracil/capecitabine, eribulin, gemcitabine, cisplatin/carboplatin, vinorelbine and ixabepilone are alternatives. The treatment of TNBC is constantly evolving, and the inclusion of patients in ongoing trials evaluating new targeted agents, immunotherapy and predictive biomarkers should be encouraged, in an attempt to improve metastatic TNBC treatment outcomes.
Keywords: “triple-negative breast cancer”, “chemotherapy”, “immunotherapy”, “parp inhibitors”
Introduction
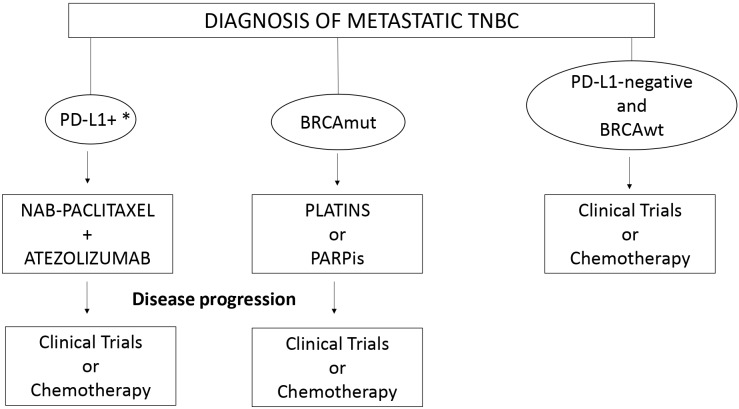
Around 15% of breast cancers are classified as triple-negative (TNBC), this subtype being associated with an aggressive clinical behaviour and a poor prognosis.1 More than one-third of patients with TNBC will present distant metastases, either recurrent or de novo metastatic disease.1 Chemotherapy has been the only active treatment for metastatic TNBC for a long time; however, this scenario has recently changed with the incorporation of polyadenosine diphosphate-ribose polymerase inhibitors (PARPis) for patients harbouring BRCA mutations (BRCAmut) and also with the positive results of the combination of chemotherapy and immunotherapy in patients with PD-L1-positive tumours (PD-L1 expression on tumour-infiltrating immune cells ≥1%). In the present manuscript, we will propose an algorithm for the first-line treatment of patients with metastatic TNBC based on the currently available, most relevant literature on the topic, considering the advent of PARPis and immunotherapy (figure 1).
Figure 1.
Treatmentalgorithm for metastatic TNBC patients consideringthe incorporation of PARPis and immunotherapy. *Defined as PD-L1 expression on tumour-infiltratingimmune cells ≥1% of the tumour area. BRCAmut, BRCA mutations; BRCAwt, BRCA wild type; PARPis, polyadenosine diphosphate-ribose polymeraseinhibitors; PD-L1, programmed death receptor ligand 1; TNBC, triple-negative breast cancer.
Chemotherapy
The most active agents in the first-line setting are anthracyclines and taxanes, which can be used either as single agents or as part of combination regimens.2 While combinations increase response rates, they are also associated with more toxicities and do not provide any survival advantage in comparison with single agents.2 Therefore, to choose between single agent or combinations, variables such as performance status, risk of adverse events, prior chemotherapy regimens, disease burden and patient preferences must be considered. In line with international guidelines, for most patients we recommend single-agent chemotherapy with taxanes as a first-line treatment (paclitaxel or docetaxel).3 However, in patients with a high disease burden or who are very symptomatic, combinations such as anthracyclines with cyclophosphamide or platins with taxanes are valid options. Chemotherapy should be maintained until disease progression, limiting toxicities or according to patient preferences, and treatment pauses can be discussed on a case-by-case basis.3
After progression to first-line chemotherapy, anthracyclines, if not previously given, are an option for patients who received taxanes and vice versa.2 Several other agents are active in TNBC, such as fluorouracil/capecitabine, eribulin, gemcitabine, cisplatin/carboplatin, vinorelbine and ixabepilone.2 These agents are appropriate options for patients who progressed during treatment with anthracyclines and/or taxanes, or for those with contraindications to anthracyclines and/or taxanes in the first-line setting. The specific posology and safety profile of each agent shall be considered to choose the best treatment for each patient. Due to its oral administration, capecitabine is particularly interesting for patients who wish to avoid frequent visits to the hospital and are able to adhere to a self-administered treatment.2
Patients with BRCA mutations
A mutation in one of the BRCA genes (BRCA1 and BRCA2) is found in up to 20% of patients with TNBC.4 The proteins encoded by BRCA participate in DNA double-strand breaks repair as part of the homologous recombination pathway.5 Therefore, cells harbouring a deleterious BRCA mutation have an impaired DNA repair system. Platins are alkylating agents that exert their effect by binding to DNA and inducing multiple single-strand breaks, which result in apoptosis and cell death. The synergy of two different mechanisms that potentially induce DNA damage (platins causing single-strand breaks and BRCAmut inefficiently repairing double-strand breaks) is known as synthetic lethality, which is the rationale for a potential benefit of platins in BRCAmut patients. Supporting this hypothesis, in a single-arm phase II study with 20 patients with BRCA1mut metastatic breast cancer, the overall response rate with single-agent cisplatin (75 mg/m2 every 3 weeks for six cycles) was 80% and the median time to progression was 12 months.6 In the phase III “Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups -TNT trial”, 376 patients with metastatic TNBC were randomised 1:1 to receive carboplatin (area under the curve (AUC) 6 every 3 weeks) or docetaxel (100 mg/m2 every 3 weeks). The objective response rates (primary endpoint of the study) were similar between carboplatin and docetaxel (31.4% vs 34.0%, respectively; p=0.66). However, in the subgroup of BRCAmut patients (n=43), those who received carboplatin presented higher response rates (68% vs 33%; p=0.01) and a longer median progression-free survival (PFS) (6.8 months vs 4.4 months; p=0.002) in comparison with those who received docetaxel.7
The polyadenosine diphosphate-ribose polymerase (PARP) is a group of proteins that have an important role in the repair of DNA single-strand breaks.8 By binding to PARP and blocking its function, PARPis interfere with the repair of single-strand DNA breaks; therefore, the concept of synthetic lethality also applies for PARPis in BRCAmut patients.8 In two different phase III trials, PARPis improved the median PFS when compared with chemotherapy of investigator’s choice in metastatic BRCAmut and human epidermal growth factor receptor 2 (HER2)-negative patients: in the OLYMPIAD study (N=302), the median PFS was 7.0 months with olaparib (300 mg twice daily) vs 4.2 months with chemotherapy (HR 0.58; 95% CI 0.43 to 0.80; p<0.001); in the EMBRACA study (N=431), the median PFS was 8.6 months with talazoparib (1 mg once a day) vs 5.6 months with chemotherapy (HR 0.54; 95% CI 0.41 to 0.71; p<0.001).9 10 In both studies, grade ≥3 haematological toxicities were more frequent with PARPis, whereas non-haematological toxicities were more frequent with chemotherapy.11 Notably, none of these trials compared a PARPi with a platin-based chemotherapy, although in both studies olaparib and talazoparib were active in patients previously exposed to platins (in the OLYMPIAD, previous [neo]adjuvant platins were allowed if a minimum of 12 months had elapsed since the last dose, whereas previous treatment with platins for metastatic disease was allowed if no disease progression occurred during therapy; in the EMBRACA study, previous [neo]adjuvant platins were allowed if the patient had a disease-free interval of at least 6 months after the last dose, whereas previous treatment with platins for metastatic disease was allowed if no disease progression occurred during treatment).9 10
For patients with metastatic BRCAmut TNBC, both platins and PARPis are appropriate treatment options. Platins have a reduced cost, although they have the inconveniences of intravenous administration and potential adverse events such as neuropathy, nausea, ototoxicity and haematological toxicities. On the other hand, PARPis have the advantage of being orally administered, although the elevated costs and the risks of haematological toxicities must be considered. For patients with BRCAmut TNBC with good performance status and no major uncontrolled comorbidities who are considered fit to tolerate chemotherapy, given the evidence that PARPis are active in patients previously exposed to platins, we recommend first-line treatment with platins (carboplatin or cisplatin single agent). However, starting treatment with PARPis is also a valid option, given there are no strong data to guide the sequencing of these agents. With the availability of new agents such as PARPis, the ideal treatment sequence in patients with BRCAmut TNBC needs to be further explored in future clinical trials.
Immunotherapy
TNBC has the highest tumour mutational burden among all breast cancer subtypes.12 More mutations can lead to the synthesis of more abnormal proteins, which may function as ‘neoantigens’ to be recognised by the antigen-presenting cells that can ultimately start an antitumour immune response.12 Supporting this hypothesis, tumour-infiltrating lymphocytes (TILs) are frequently present in TNBC samples, and increased levels of TILs are associated with a good prognosis.13 Therefore, TNBC is considered an interesting subset for the development of immunotherapy. In the Impassion 130 phase III study, 902 patients with metastatic TNBC with no previous treatment for metastatic disease were randomised 1:1 to receive nab-paclitaxel (100 mg/m2 on days 1, 8 and 15 every 28 days) combined with atezolizumab (840 mg intravenously on days 1 and 15 every 28 days) or placebo until disease progression or limiting toxicities. In the overall population, the addition of atezolizumab to nab-paclitaxel increased the median PFS (7.2 months with atezolizumab-nab-paclitaxel vs 5.5 months with placebo-nab-paclitaxel; HR 0.80; 95% CI 0.69 to 0.92; p=0.002), although it did not significantly improve overall survival (OS): 21.3 months with atezolizumab-nab-paclitaxel arm vs 17.6 months with the placebo-nab-paclitaxel (HR 0.84; 95% CI 0.69 to 1.02; p=0.08). However, in the subgroup of PD-L1-positive patients (defined as PD-L1 expression on tumour-infiltrating immune cells ≥1% of the tumour area), the median PFS (7.5 months vs 5.0 months; HR 0.62; 95% CI 0.49 to 0.78; p<0.001) and OS (25 months vs 15.5 months; HR 0.62; 95% CI 0.45 to 0.86) were improved with atezolizumab-nab-paclitaxel in comparison to placebo-nab-paclitaxel.14 The frequency of grade ≥3 adverse events was 48.7% in the atezolizumab-nab-paclitaxel group and 42.2% in the placebo-nab-paclitaxel group, with the most common events in both groups being neutropaenia, peripheral neuropathy, fatigue and anaemia. Grade ≥3 potentially immune-related toxicities occurred in 7.5% of the patients in the atezolizumab-nab-paclitaxel group and in 4.3% of the patients in the placebo-nab-paclitaxel group.14
Although the combination of nab-paclitaxel and atezolizumab is not yet available in clinical practice, it arises as a promising strategy to be considered for PD-L1-positive patients with metastatic TNBC. Ongoing studies are further evaluating new immunotherapy agents and potential biomarkers to predict immunotherapy response in patients with metastatic TNBC.15
Conclusions
Chemotherapy has been the cornerstone in the treatment of patients with metastatic TNBC for many years. However, potentially less toxic and more efficient strategies such as PARPis and immunotherapy are changing this paradigm. The development of new targeted agents, immunotherapy and predictive biomarkers is ongoing with the objective to optimise the treatment of patients with metastatic TNBC in the forecoming years.
Footnotes
Contributors: All authors contributed to the writing and reviewing of this manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: EdA received honoraria from Roche-Genentech, research grant from Roche-Genentech (to the institution), and travel grants from Roche-Genentech and GlaxoSmithKline, outside the submitted work. ML served as a consultant for Teva and received honoraria from Theramex, outside the submitted work. RC has received speaker honoraria from Boehringer Ingelheim, AstraZeneca and Janssen, and travel grants from AstraZeneca, outside of the submitted work.
Patient consent for publication: Not required.
Provenance and peer review: Commissioned; internally peer reviewed.
References
- 1.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007;13:4429–34. 10.1158/1078-0432.CCR-06-3045 [DOI] [PubMed] [Google Scholar]
- 2.Zeichner SB, Terawaki H, Gogineni K. A review of systemic treatment in metastatic triple-negative breast cancer. Breast Cancer (Auckl) 2016;10:25–36. 10.4137/BCBCR.S32783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardoso F, Senkus E, Costa A, et al. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4)†. Ann Oncol 2018;29:1634–57. 10.1093/annonc/mdy192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez-Angulo AM, Timms KM, Liu S, et al. Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clin Cancer Res 2011;17:1082–9. 10.1158/1078-0432.CCR-10-2560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshida K, Miki Y. Role of BRCA1 and BRCA2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damage. Cancer Sci 2004;95:866–71. 10.1111/j.1349-7006.2004.tb02195.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrski T, Dent R, Blecharz P, et al. Results of a phase II open-label, non-randomized trial of cisplatin chemotherapy in patients with BRCA1-positive metastatic breast cancer. Breast Cancer Res 2012;14 10.1186/bcr3231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tutt A, Tovey H, Cheang MCU, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TnT trial. Nat Med 2018;24:628–37. 10.1038/s41591-018-0009-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashworth A, Lord CJ. Synthetic lethal therapies for cancer: what’s next after PARP inhibitors? Nat Rev Clin Oncol 2018;15:564–76. 10.1038/s41571-018-0055-6 [DOI] [PubMed] [Google Scholar]
- 9.Litton JK, Rugo HS, Ettl J, et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N Engl J Med 2018;379:753–63. 10.1056/NEJMoa1802905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robson M, Im S-A, Senkus E, et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med 2017;377:523–33. 10.1056/NEJMoa1706450 [DOI] [PubMed] [Google Scholar]
- 11.Poggio F, Bruzzone M, Ceppi M, et al. Single-agent PARP inhibitors for the treatment of patients with BRCA -mutated HER2-negative metastatic breast cancer: a systematic review and meta-analysis. ESMO Open 2018;3:e000361 10.1136/esmoopen-2018-000361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Z, Li M, Jiang Z, et al. A comprehensive immunologic portrait of triple-negative breast cancer. Transl Oncol 2018;11:311–29. 10.1016/j.tranon.2018.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 2018;19:40–50. 10.1016/S1470-2045(17)30904-X [DOI] [PubMed] [Google Scholar]
- 14.Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 2018;379:2108–21. 10.1056/NEJMoa1809615 [DOI] [PubMed] [Google Scholar]
- 15.Vikas P, Borcherding N, Zhang W. The clinical promise of immunotherapy in triple-negative breast cancer. Cancer Manag Res 2018;10:6823–33. 10.2147/CMAR.S185176 [DOI] [PMC free article] [PubMed] [Google Scholar]