Abstract
Background
Nivolumab showed a survival benefit for advanced gastric cancer (AGC). However, an acceleration of tumour growth during immunotherapy, (hyperprogressive disease, HPD) has been reported in various cancers. This study reviewed the HPD in patients with AGC treated with nivolumab or irinotecan.
Methods
The subjects of this retrospective study were patients with AGC with measurable lesions, and their tumour growth rates (TGR) during nivolumab or irinotecan were compared with those during prior therapy. HPD was defined as an increase in TGR more than twofold.
Results
34 and 66 patients received nivolumab and irinotecan in third or later line between June 2009 and September 2018 at our hospital; 22 patients receiving nivolumab had prior treatment with irinotecan, and one patient received irinotecan after nivolumab. Nivolumab and irinotecan showed no differences in disease control rates (38.2% and 34.8%) and in progression-free survival (PFS) (HR 1.1, 95% CI 0.7 to 1.6, p=0.802). The incidence of HPD was slightly higher after nivolumab (29.4%) than after irinotecan (13.5%) (p=0.0656), showing no differences in background between the patients with and without HPD. Compared between HPD and PD other than HPD after nivolumab, the HRs for PFS and overall survival (OS) were 1.1 (95% CI 0.5 to 2.7; p=0.756), and 2.1 (95% CI 0.7 to 5.8; p=0.168), but such clear difference in OS was not observed after irinotecan.
Conclusions
HPD was observed more frequently after nivolumab compared with irinotecan, which was associated with a poor prognosis after nivolumab but not so clearly after irinotecan.
Keywords: hyper-progressive disease, gastric cancer, nivolumab, irinotecan
Key questions.
What is already known about this subject?
While hyperprogressive disease (HPD) was observed in 9%–29% of patients with various advanced cancers during treatment with programmed cell death (PD-1)/PD-L-1 inhibitors, PD-1/PD-L-1 therapy induces HPD more frequently than single-agent cytotoxic chemotherapy in lung cancer. HPD was also reported to be associated with worse prognosis.
What does this study add?
In our study, HPD was observed in 29.4% of patients after nivolumab, more frequently than after irinotecan (13.5%) as in third—or later lines chemotherapy for advanced gastric cancer. HPD induced after nivolumab was associated with poor survival, but not so clearly after irinotecan. Progression-free survival (PFS) of patients with HPD was shorter after nivolumab than that after irinotecan, and same trend has been also seen in patients with PD other than HPD. On the contrary, among the patients who obtained disease control (partial response and stable disease), nivolumab showed longer PFS than irinotecan.
How might this impact on clinical practice?
It is clinically important to take the risk of hyperprogression during treatment with nivolumab into consideration when selecting third-line chemotherapy.
Introduction
Gastric cancer represents the fifth most commonly diagnosed cancer and the third-leading cause of cancer-related deaths worldwide. The incidences are markedly high in Eastern Asia.1 At the time of diagnosis, about half of patients present with unresectable, locally advanced or metastatic disease except in Japan and Korea which has a nationwide screening system for gastric cancer. Although various chemotherapeutic agents have been developed to improve prognosis, the clinical outcome of advanced gastric cancer (AGC) remains poor with a median overall survival (OS) of 1 year.
Combination of platinum compounds and fluoropyrimidines is the most common first-line treatment for patients with AGC as recommended by the treatment guidelines,2–5 and trastuzumab is added if tumour shows overexpression of the HER2 protein.6 After failure of the first-line treatment, combination therapy of paclitaxel and the vascular endothelial growth factor receptor 2 antibody ramucirumab is recommended as a second-line treatment regardless of HER2 expression.7 There was no global consensus on a standard third-line treatment until 2017, while irinotecan monotherapy was recommended for some cases according to the patient’s condition in the Japanese treatment guideline.4
Nivolumab is a programmed cell death (PD)-1 immune checkpoint inhibitor, which enhances antitumour T-cell activity. The phase III trial, ATTRACTION-2, showed a survival benefit of nivolumab over placebo as the third-line or later-line treatment for patients with AGC (HR 0.63; 95% CI 0.51 to 0.78; p<0.0001).8 The median OS was 5.26 months in the nivolumab group and 4.14 months in the placebo group. The 12 months OS rates were 26.2% with nivolumab and 10.9% with placebo. In 2017, nivolumab was approved for AGC with refractory to, or intolerant of at least two previous chemotherapy regimens in Japan. However, the response rate (RR) was as low as 10% and progression-free survival (PFS) curve of more than half of the patients overlapped with the placebo.
The tumour growth rate (TGR) is used to estimate the increase in tumour volume.9 Progression with TGR larger than double compared with that during previous treatment is called hyperprogressive disease (HPD).10 There are some reports of HPD during anti-PD-1/PD-L1 therapy, which was identified in 9% of 131 patients with various types of cancers including melanoma, lung, renal and colorectal cancer,10 in 29% of 34 patients with head and neck cancer,11 and in 14% of 406 patients with lung cancer.12 HPD was reported to be associated with worse prognosis in various types of cancers.
However, the frequency and clinical outcomes of HPD in patients with AGC treated with immunotherapy and cytotoxic agents are little known. The aim of this study was to review the prevalence, background and clinical outcomes of HPD in patients with AGC treated with nivolumab or irinotecan. In addition, we compared the treatment outcomes of HPD between the two cohorts treated with nivolumab and irinotecan.
Methods
Patients and treatment
The source of the subjects of this retrospective study was patients with AGC who were treated with nivolumab or irinotecan as the third or later line at our institution between June 2009 and September 2018, including patients who received both nivolumab and irinotecan.
The selection criteria were as follows: (1) 20 years or older; (2) histologically confirmed unresectable advanced or recurrent gastric/gastro-oesophageal junction adenocarcinoma; (3) at least one measurable lesion according to RECIST V.1.1 which had to be assessed at least three times (during prior therapy, immediately before and after initiating nivolumab or irinotecan) and (4) refractory to, or intolerant to at least two previous chemotherapy regimens (must be refractory to immediately previous chemotherapy). Patients who had received previous immunotherapy were excluded from the cohort treated with nivolumab
Tumour growth rate
To calculate TGR, information of CT scans assessing measurable disease before and during treatment with nivolumab or irinotecan was used. TGR was calculated using the following formula: TG=3 Log(Dt/D0)/t, where t is the interval time (months) between two CT scans and 0 means baseline; D is the sum of the largest diameters of the target lesions as per RECIST V.1.1 (new lesions and non-measurable lesion were not included). Where R is the radius of one virtual sphere lesion with size D, the tumour volume (V) is approximated by V=4/3 x π x R3. Assuming the tumour growth follows an exponential law, the tumour volume at time t (Vt) can be calculated by the formula: Vt=V0 exp(TG.t), where V0 is the volume at baseline and TG is the growth rate. Finally, TGR, represented as a percentage increase in tumour volume during 1 month, is obtained by the following transformation: TGR=100 [exp(TG)−1], where exp(TG) represents the exponential of TG.10 13–15 Finally, TGR was compared before and after nivolumab or irinotecan treatment, and more than twofold increase in the TGR after nivolumab or irinotecan compared with prior therapy was defined as HPD.10 Tumour response was assessed according to RECIST V.1.1. Responses other than HPD including complete and partial response (PR), stable disease (SD), PD with TGR less than twofold increase was defined as non-HPD.
Statistical analysis
Background characteristics at the initiation of treatment between nivolumab and irinotecan, and those between and HPD and non-HPD in each therapy were compared using the Fisher's exact test, t-test. OS was defined as the time from initiation of nivolumab or irinotecan until the date of death from any cause or censored at the latest follow-up for surviving patients. PFS was defined as the time from initiation of nivolumab or irinotecan until detection of disease progression or death and survivors without disease progression were censored at the last contact. OS and PFS curves were estimated using the Kaplan-Meier method and compared by the log-rank test. HR was estimated using the Cox proportional hazards model. Statistical analyses were performed using the EZR software for Windows version V.1.37
Results
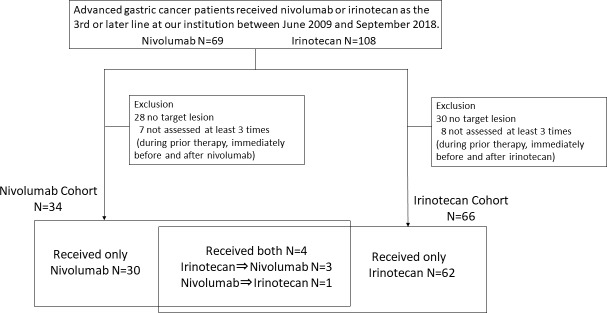
The source of the subjects was 69 and 104 patients treated with nivolumab and irinotecan at our hospital between June 2009 and September 2018, and a total of 34 patients receiving nivolumab and 66 patients receiving irinotecan whose TGR just before and during nivolumab or irinotecan could be calculated were included in this study (figure 1). Within the 34 patients receiving nivolumab, 22 had received irinotecan as prior treatment. Nineteen of these 22 patients had received irinotecan at other institutions and their TGR before irinotecan could not be obtained. The remaining three patients who received nivolumab after irinotecan and another patient who received irinotecan after nivolumab at our hospital were included in both cohorts. The backgrounds of the patients at the initiation of each treatment are listed in the table 1. There were more prior chemotherapy regimens at initiating nivolumab than irinotecan (p<0.0001).
Figure 1.
Flow chart of study selection process.
Table 1.
Patient characteristics at initiation of nivolumab and irinotecan treatments
| Total | Nivolumab N=34 |
Irinotecan N=66 |
P value (Fisher's exact test) |
| Sex | |||
| Male | 26 (76.5%) | 53 (80.3%) | 0.796 |
| Female | 8 (23.5%) | 13 (19.7%) | |
| Age | |||
| Median (range) | 67 (51–84) | 66 (41–77) | |
| Performance status (PS) | |||
| PS 0 | 3 (8.8%) | 9 (13.6%) | 0.746 |
| >1 | 31 (91.2%) | 57 (86.4%) | |
| Histological type | |||
| Intestinal | 21 (61.8%) | 37 (56.0%) | 0.83 |
| Diffuse | 13 (38.2%) | 26 (44.0%) | |
| HER2 status | |||
| Negative | 24 (80.0%) | 34 (69.4%) | 0.307 |
| Positive | 6 (20.0%) | 15 (30.6%) | |
| Disease status | |||
| Recurrent | 18 (52.9%) | 19 (28.8%) | 0.0281 |
| Stage IV | 16 (47.1%) | 47 (71.2%) | |
| Peritoneal metastasis | |||
| Absent | 16 (47.1%) | 38 (57.6%) | 0.398 |
| Present | 18 (52.9%) | 28 (42.4%) | |
| No of metastatic sites | |||
| 1 | 4 (11.8%) | 12 (18.2%) | 0.285 |
| ≥2 | 30 (88.2%) | 54 (81.8%) | |
| No of prior chemotherapy lines | |||
| <3 | 10 (29.4%) | 62 (93.9%) | <0.0001 |
| ≥3 | 24 (70.6%) | 4 (6.1%) | |
| Agents contained in prior chemotherapy | |||
| Fluoropyrimidine | 34 (100%) | 66 (100%) | |
| Platinum | 31 (91.2%) | 60 (90.9%) | 1.000 |
| Taxane | 33 (97.1%) | 62 (93.9%) | 0.659 |
| Ramucirumab | 13 (38.2%) | 20 (30.3%) | 0.502 |
| Irinotecan | 22 (64.7%) | 0 (0%) | |
| Immune check point inhibitor | 0 (%) | 1 (1.5%) | |
| ALP (U/L) | |||
| <360 | 17 (50.0%) | 38 (57.6%) | 0.528 |
| ≥360 | 17 (50.0%) | 28 (42.4%) |
ALP, alkaline phosphatase.
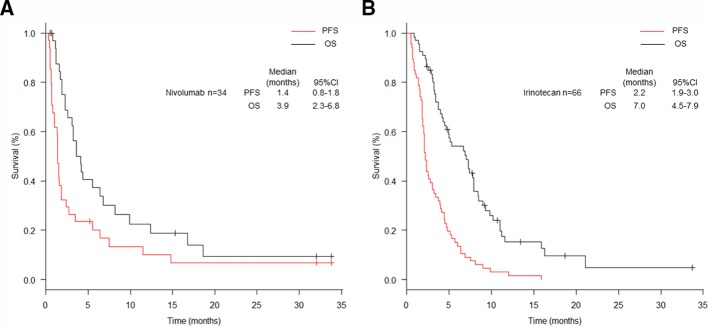
After initiation of nivolumab, the median follow-up period of survivors was 4.4 months (range: 0.5–33.8). The median OS was 3.9 months (95% CI 2.3 to 6.8), and the median PFS was 1.4 months (95% CI 0.8 to 1.8) (figure 2). Two PR were achieved in the two out of 34 patients receiving nivolumab, resulting in an RR of 5.9% (95% CI 0.7% to 19.7%). A total of 21 patients (61.8%) showed PD, 10 of which were classified as HPD (29.4%) (table 2). There was no difference in baseline characteristics at the initiation of nivolumab between patients with HPD and non-HPD (table 3). Six patients with non-HPD received post-treatment, and one patient with HPD did. We investigated prognosis according to the response: HPD, PD other than HPD, SD, PR (figure 3). The median PFS was 0.9 months (95% CI 0.3 to 1.4) in patients with HPD, 0.8 months (95% CI 0.5 to 1.5) with PD other than HPD, 5.5 months (95% CI 1.4 to 11.5) with SD and 14.8 months (95% CI 14.8 to not available (NA)) with PR. The median OS was 2.1 months (95% CI 1.2 to 3.6) in patients with HPD, 3.1 months (95% CI 1.1 to 6.8) with PD other than HPD, 8.2 months (95% CI 3.6 to 16.8) with SD and 18.6 months (95% CI 18.6 to NA) with PR. Compared between HPD and PD other than HPD, the HRs for PFS and OS were 1.1 (95% CI 0.5 to 2.7; p=0.756), and 2.1 (95% CI 0.7 to 5.8; p=0.168). The median PFS and OS of the all patients with non-HPD were 1.7 months (95% CI 1.0 to 5.5) and 5.5 months (95% CI 3.2 to 9.9). Compared between HPD and non-HPD, the HRs for PFS and OS were 3.4 (95% CI 1.5 to 8.0; p=0.00426) and 4.7 (95% CI 1.8 to 12.7; p=0.00195) (online supplementary figure 1).
Figure 2.
Kaplan-Meier plots for progression-free (PFS) and overall (OS) survival. Red lines indicate PFS and black lines indicate OS. (A) All patients after initiation of nivolumab treatment. (B) All patients after initiation of irinotecan treatment.
Table 2.
Summary of responses
| Total | Nivolumab N=34 (%) | Irinotecan N=66 (%) |
| PR | 2 (5.9) | 4 (6.1) |
| SD | 11 (32.4) | 19 (28.8) |
| PD | 21 (61.8) | 43 (65.2) |
| HPD | 10 (29.4) | 9 (13.5) |
| RR (95% CI) | 5.9% (0.7 to 19.7) | 6.1% (1.7 to 14.8) |
| DCR (95% CI) | 38.2% (22.2 to 56.4) | 34.8% (23.5 to 47.6) |
DCR, disease control rate; HPD, hyperprogressive disease;PD, progressive disease; PR, partial response;RR, response rate; SD, stable disease.
Table 3.
Patient characteristics at initiation of nivolumab treatment according to HPD status
| Total | HPD N=10 |
Non-HPD N=24 |
P value (Fisher's exact test) |
OR (adjusted) |
95% CI | P value |
| Sex | ||||||
| Male | 8 (80.0%) | 18 (75.0%) | 1.000 | |||
| Female | 2 (20.0%) | 6 (25.0%) | ||||
| Age | ||||||
| Median (range) | 66 (55–83) | 67 (51–84) | ||||
| Performance status (PS) | ||||||
| PS 0 | 1 (10.0%) | 2 (8.3%) | 1.000 | |||
| >1 | 9 (90.0%) | 22 (91.7%) | ||||
| Histological type | ||||||
| Intestinal | 6 (60.0%) | 15 (62.5%) | 1.000 | |||
| Diffuse | 4 (40.0%) | 9 (37.5%) | ||||
| HER2 status | ||||||
| Negative | 7 (70.0%) | 18 (85.7%) | 0.358 | |||
| Positive | 3 (30.0%) | 3 (14.2%) | ||||
| Disease status | ||||||
| Recurrent | 4 (40.0%) | 14 (58.3%) | 0.457 | Reference | ||
| Stage IV | 6 (60.0%) | 10 (41.7%) | 0.396 | 0.02 to 8.51 | 0.554 | |
| Peritoneal metastasis | ||||||
| Absent | 5 (50.0%) | 14 (58.3%) | 1.000 | |||
| Present | 5 (50.0%) | 10 (41.7%) | ||||
| No of metastatic sites | ||||||
| 1 | 1 (10.0%) | 3 (12.5%) | 1.000 | |||
| ≥2 | 9 (90.0%) | 21 (87.5%) | ||||
| No of prior chemotherapy lines | ||||||
| <3 | 4 (40.0%) | 6 (25.0%) | 0.431 | Reference | ||
| ≥3 | 6 (60.0%) | 18 (75.0%) | 1.520 | 0.18 to 12.80 | 0.702 | |
| Agents contained in postchemotherapy | ||||||
| Fluoropyrimidine | 0 (0%) | 2 (8.3%) | 1.000 | |||
| Platinum | 0 (0%) | 2 (8.3%) | 1.000 | |||
| Taxane | 0 (0%) | 1 (4.2%) | 1.000 | |||
| Ramucirumab | 0 (0%) | 4 (16.7%) | 0.296 | |||
| Irinotecan | 1 (10.0%) | 0 (0%) | 0.294 | |||
| ALP (U/L) | ||||||
| <360 | 3 (30.0%) | 14 (58.3%) | 0.259 | Reference | ||
| ≥360 | 7 (70.0%) | 10 (41.7%) | 0.174 | 0.01 to 2.73 | 0.213 | |
ALP, alkaline phosphatase; HPD, hyperprogressive disease.
Figure 3.
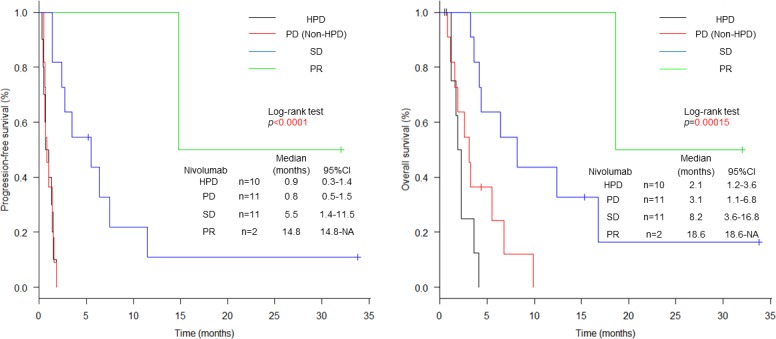
Kaplan-Meier plots for progression-free survival (PFS) and overall survival (OS). Black lines indicate patients with HPD, red lines indicate patients with PD other than HPD, blue lines indicate patients with SD and green lines indicate patients with PR. PFS and OS curves after initiation of nivolumab treatment according to HPD, PD, SD and PR. HPD, hyperprogressive disease; PD, progressive disease; PR, partial response; SD, stable disease.
esmoopen-2019-000488supp001.tif (116.9KB, tif)
After initiation of irinotecan, the median follow-up period of survivors was 5.3 months (range: 2.4–33.7). The median OS was 7.0 months (95% CI 4.5 to 7.9) and the PFS was 2.2 months (95% CI 1.9 to 3.0) (figure 2). A total of 4 out of 66 patients receiving irinotecan achieved response resulting in an RR of 6.1% (95% CI 1.7% to 14.8%). A total of 43 patients (65.2%) showed PD, nine of which were classified as HPD (13.5%) (table 2). No significant differences in baseline characteristics at initiation of irinotecan were observed between patients with HPD and non-HPD (table 4). Although the frequency was low, 3 patients with HPD and 13 patients with non-HPD patients received post-treatment. Prognosis according to the response: HPD, PD other than HPD, SD and PR are shown in figure 4. The median PFS was 2.1 months (95% CI 0.5 to 2.3) in patients with HPD, 1.8 months (95% CI 1.4 to 2.1) with PD other than HPD, 4.5 months (95% CI 3.9 to 5.3) with SD and 11.0 months (95% CI 8.1 to NA) with PR. The median OS was 7.3 months (95% CI 1.0 to 8.5) in patients with HPD, 4.2 months (95% CI 3.2 to 5.0) with PD other than HPD, 8.4 months (95% CI 7.3 to 11.0) with SD and 16.4 months (95% CI 11.0 to NA) with PR. Compared between HPD and PD other than HPD, the HRs for PFS and OS were 1.0 (95% CI 0.5 to 2.1; p=0.975), and 0.9 (95% CI 0.4 to 2.0; p=0.742). The median PFS and OS of non-HPD were 2.3 months (95% CI 1.8 to 3.7) and 7.0 months (95% CI 4.5 to 8.4). Compared between HPD and non-HPD, the HRs for PFS and OS were 2.1 (95% CI 1.0 to 4.4; p=0.0547) and 1.5 (95% CI 0.7 to 3.4; p=0.341) (online supplementary figure 2).
Table 4.
Patient characteristics at initiation of irinotecan treatment according to HPD status
| Total | HPD N=9 |
Non-HPD N=57 |
P value (Fisher's exact test) |
OR (adjusted) |
95% CI | P value |
| Sex | ||||||
| Male | 6 (88.9%) | 47 (82.5%) | 0.364 | |||
| Female | 3 (11.1%) | 10 (17.5%) | ||||
| Age | ||||||
| Median (range) | 64 (53–75) | 66 (41–77) | ||||
| Performance status (PS) | ||||||
| PS 0 | 1 (11.1%) | 8 (14.0%) | 1.000 | |||
| >1 | 8 (88.9%) | 49 (86.0%) | ||||
| Histological type | ||||||
| Intestinal | 4 (44.4%) | 33 (61.1%) | 1.000 | |||
| Diffuse | 5 (55.6%) | 21 (38.9%) | ||||
| HER2 status | ||||||
| Negative | 3 (50.0%) | 31 (72.1%) | 0.469 | |||
| Positive | 3 (50.0%) | 12 (27.9%) | ||||
| Disease status | ||||||
| Recurrent | 2 (22.2%) | 17 (29.8%) | 1.000 | Reference | ||
| Stage IV | 7 (77.8%) | 40 (70.2%) | 2.320 | 0.38 to 14.10 | 0.361 | |
| Peritoneal metastasis | ||||||
| Absent | 7 (77.8%) | 41 (71.9%) | 0.282 | |||
| Present | 2 (22.2%) | 16 (28.1%) | ||||
| No of metastatic sites | ||||||
| 1 | 2 (22.2%) | 12 (21.1%) | 1.000 | |||
| ≥2 | 7 (77.8%) | 45 (78.9%) | ||||
| No of prior chemotherapy lines | ||||||
| <3 | 9 (100%) | 53 (94.7%) | 1.000 | Reference | ||
| ≥3 | 0 (0%) | 4 (5.3%) | <0.0001 | 0.00 to Inf | 0.994 | |
| Agents contained in postchemotherapy | ||||||
| Fluoropyrimidine | 2 (22.2%) | 5 (8.8%) | 0.241 | |||
| Platinum | 2 (22.2%) | 4 (7.0%) | 0.186 | |||
| Taxane | 0 (0%) | 3 (5.3%) | 1.000 | |||
| Ramucirumab | 1 (11.1%) | 4 (7.0%) | 0.531 | |||
| Immune check point inhibitor | 0 (%) | 4 (7.0%) | 1.000 | |||
| ALP (U/L) | ||||||
| <360 | 7 (77.8%) | 31 (54.4%) | 0.282 | Reference | ||
| ≥360 | 2 (22.2%) | 26 (45.6%) | 0.215 | 0.04 to 1.26 | 0.09 | |
ALP, alkaline phosphatase; HPD, hyperprogressive disease.
Figure 4.
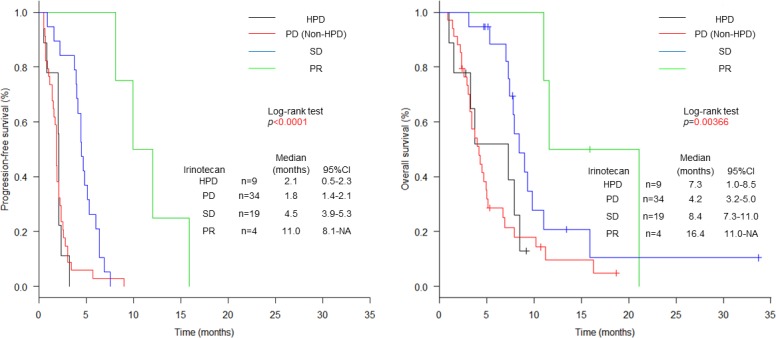
Kaplan-Meier plots for progression-free survival (PFS) and overall survival (OS). Black lines indicate patients with HPD, red lines indicate patients with PD other than HPD, blue lines indicate patients with SD and green lines indicate patients with PR. PFS and OS curves after initiation of irinotecan treatment according to HPD, PD, SD and PR. HPD, hyperprogressive disease; PD, progressive disease; PR, partial response; SD, stable disease.
esmoopen-2019-000488supp002.tif (116.6KB, tif)
Comparison between nivolumab and irinotecan
While the proportions of PD in overall population after both treatments were similar (table 2), the incidences of HPD were slightly higher after nivolumab (29.4%) than after irinotecan (13.5%) (p=0.0656). Similarly, there was no difference in PFS between nivolumab and irinotecan in overall population (median PFS: 1.4 months (95% CI 0.8 to 1.8) after nivolumab and 2.2 months (95% CI 1.9 to 3.0) after irinotecan, HR 1.1, 95% CI 0.7 to 1.6, p=0.802) (table 5). Interestingly, PFS was shorter after nivolumab than after irinotecan in the patients with HPD (median PFS: 0.9 months (95% CI 0.3 to 1.4) after nivolumab and 2.1 months (95% CI 0.5 to 2.3) after irinotecan, HR 8.7 (95% CI 1.8 to 41.8), p=0.00671), which showed similar trend in the patients with PD other than HPD (median PFS: 0.8 months (95% CI 0.5 to 1.5) after nivolumab and 1.8 months (95% CI 1.4 to 2.1) after irinotecan, HR 3.7 (95% CI 1.7 to 8.0), p=0.00098). On the contrary, although not significantly, PFS was longer after nivolumab than after irinotecan in the patients with disease control (PR and SD) (median PFS: 6.4 months (95% CI 2.4 to 14.8) after nivolumab and 4.8 months (95% CI 4.1 to 6.4) after irinotecan, HR 0.6 (95% CI 0.3 to 1.2), p=0.155).
Table 5.
Comparison of PFS between nivolumab and irinotecan according to response
| Response | No of patients median PFS (month) (95% CI) |
HR (95% CI) |
P value | |
| Nivolumab | Irinotecan | |||
| All | N=34 1.4 (0.8 to 1.8) |
N=66 2.2 (1.9 to 3.0) |
1.1 (0.7 to 1.6) | 0.802 |
| Non-PD (PR+SD) | N=13 6.4 (2.4 to 14.8) |
N=23 4.8 (4.1 to 6.4) |
0.6 (0.3 to 1.2) | 0.155 |
| PR | N=2 14.8 (14.8 to NA) |
N=4 11.0 (8.1 to NA) |
0.3 (0.03 to 2.4) | 0.229 |
| SD | N=11 5.5 (1.4 to 11.5) |
N=19 4.5 (3.9 to 5.3) |
0.6 (0.2 to 1.3) |
0.188 |
| PD | N=21 0.8 (0.6 to 1.4) |
N=43 1.9 (1.6 to 2.1) |
4.3 (2.3 to 8.2) | <0.0001 |
| PD other than HPD | N=11 0.8 (0.5 to 1.5) |
N=34 1.8 (1.4 to 2.1) |
3.7 (1.7 to 8.0) | 0.00098 |
| HPD | N=10 0.9 (0.3 to 1.4) |
N=9 2.1 (0.5 to 2.3) |
8.7 (1.8 to 41.8) | 0.00671 |
HPD, hyperprogressive disease; PD, progressive disease; PFS, progression-free survival;PR, partial response;SD, stable disease.
Discussion
Head-to-head comparison between immune check inhibitor and cytotoxic agents has been conducted in the second-line treatment (Keynote-061 trial)16 and in the third-line treatment (Javelin Gastric 300 trial)17 could not show superiority of immune checkpoint inhibitor over cytotoxic agents. However, because both immune check point inhibitors showed equivalent efficacy to cytotoxic agents, it is very difficult to select an optimal treatment without established biomarker for selecting immune checkpoint inhibitors except for very limited fractions with Epstein-Barr virus positive or microsatellite instability high among patients with advanced gastric cancer. Therefore, it is very important in clinical practice to recognise the risks and benefits comparing between these two kinds of drugs.
In this retrospective study, HPD was observed in 29.4% of patients after nivolumab treatment, more frequently than after irinotecan treatment (13.5%). In previous reports, HPD during anti-PD-1/PD-L-1 therapy was observed in 9%–29% in various types of advanced cancers treated with PD-1/PD-L-1 inhibitors.10–12 While the proportion of HPD differed based on cancer types, the results shown here for gastric cancer seem to be consistent with previous reports for other cancer types. Among lung cancer, 14% of 406 patients treated with PD-1/PD-L-1 were classified as HPD and 5.1% of 59 patients treated with single-agent chemotherapy were classified as HPD.12 These results suggest that nivolumab therapy may induce HPD more frequently than single-agent cytotoxic chemotherapy also in patients with AGC.
Although HPD was reported to be associated with higher age,10 18 locoregional recurrence11 and high number of metastatic sites12 in other cancers. In this study, the average tumour burden and the level of alkaline phosphatase were not significantly different between patients with HPD and non-HPD either after nivolumab or after irinotecan. It is suggested that no particular patient characteristics were related with HPD in AGC.
In this study, patients with HPD showed shorter OS than non-HPD patients after nivolumab treatment, but there was no such clear difference observed after irinotecan treatment. Actually, a phase III study, comparing nivolumab with docetaxel in previously treated patients with advanced non-squamous non-small cell lung cancer (NSCLC), showed an initial benefit in favour of docetaxel regarding survival while 14 deaths were observed during the first 3 months in the nivolumab arm.19 Recently, Ferrara et al reported that advanced NSCLC patients treated with PD-1/PD-L1 inhibitors and experiencing HPD within the first 6 weeks showed significantly shorter OS compared with patients who showed PD other than non-HPD (median 3.4 vs 6.2 months).12 Although there were some differences in background between nivolumab and irinotecan, our results suggest that HPD induced after nivolumab may be associated with poor survival of patients with AGC, but not so clearly after ininotecan.
Several previous studies assessed the efficacy of irinotecan treatment as third line or later were reported20–22 and showed that the median OS was 4.0–6.6 months which looks similar to our results, while the efficacy of nivolumab in this study seemed slightly worse compared with the phase III trial because practically nivolumab was given to many patients even with poor condition who had been waiting for approval of nivolumab in 2017. Since this study included patients receiving irinotecan before approval of nivolumab, the nivolumab cohort had more prior chemotherapy including irinotecan. Therefore, it may not be appropriate to compare directly both treatments, especially for OS. However, it is important to note that the PFS of patients with HPD was worse after nivolumab than after irinotecan. Same trend has been also seen in patients with PD other than HPD. On the contrary, among the patients who obtained disease control (PR and SD), nivolumab showed longer PFS than irinotecan. Thus, while it is very difficult to speculate which is superior as the third or later lines treatment, nivolumab or irinotecan, for AGC, the risk and benefit of nivolumab should be taken into consideration when selecting third-line chemotherapy, either irinotecan or nivolumab. Biomarkers for selecting either nivolumab or irinotecan in terms of efficacy (disease control) and resistance (disease progression) should be established.
HPD after anti-PD-1/PD-L-1 therapy might result from several physiological mechanisms derived from the pleiotropic effects of the factors involved in immunity and the redundancy of the immune signalling pathways.23 First, PD-L-1 blockade upregulates T-regulatory (Treg) cell activity.24 25 Second, compensatory T-cells are exhausted by PD-L1 blockade. In patients with lung cancer treated with anti-PD-1/PD-L-1 antibodies, aberrant proliferation of peripheral exhausted CD4 +T cells was observed in patients with HPD.26 Third, tumour-promoting cells are modulated. Interferon γ released by PD-1 blockade may have detrimental effects on immunity.27 28 In addition, tumour tissues with high myeloid-derived suppressor cells (MDSCs) infiltration are related to poor prognosis and resistance to various therapies. In our previous report of gastric cancer, patients with high granulocytic MDSCs showed significantly shorter PFS than those with low granulocytic MDSCs.29 Fourth, immune system-induced inflammation may contribute to tumour growth through angiogenesis and tissue remodelling by producing growth factors and matrix metalloproteinases.30–33 Fifth, an oncogenic pathway in cancer cells is activated by PD-1 blockade. Basic research using mice model showed that PD-1/PD-L-1 blockade can directly lead to tumour progression34 35. Although the mechanisms of HPD have been studied, there are many aspects to be elucidated. Thus, it is important that future studies include analysis of sample such as tumour biopsy or peripheral blood from patients with HPD before, during, and after treatment.
This study had several limitations due to its retrospective nature and that was based on a single institution. The sample size was also small and patient’s background could not be adjusted statistically enough. We are now conducting a multicentre retrospective study with a larger sample size investigating the clinical outcomes, including HPD, after nivolumab. Because many patients received nivolumab after irinotecan and some patients were included in both cohorts, the OS could not be compared between the two therapies, and the PFS might be affected by lines of therapy. Finally, we did not perform biomarker analysis using tissues and blood which could lead to the discovery of more HPD mechanisms. We are currently conducting a translational research collecting blood and tissue samples before, during, and at disease progression in patients receiving nivolumab in the West Japan Oncology Group.
In conclusion, HPD was observed more frequently after nivolumab compared with irinotecan, which was associated with a poor prognosis after nivolumab but not so clearly after irinotecan. Further research on a large population is needed in order to validate our results in gastric cancer and to find the molecular mechanisms of HPD.
Acknowledgments
We thank all the participants, physicians, nurses and staff members who supported this study.
Footnotes
Contributors: MA performed the research, analysed the data and wrote the manuscript. NB edited the manuscript. All authors approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: NB has received honoraria from Ono Pharmaceutical, Bristol-Myers Squibb, Taiho Pharmaceutical, Chugai Pharmaceutical, Eli Lilly and research grants from Ono Pharmaceutical, Bristol-Myers Squibb, Taiho Pharmaceutical. KK has received research grants from Ono Pharmaceutical, MSD, Merck Serono, Shionogi Pharmaceutical, Beigene.
Patient consent for publication: Not required.
Ethics approval: This study was approved by the ethics committee of the National Cancer Center.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. . Global Cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Ajani JA, D'Amico TA, Almhanna K, et al. . Gastric cancer, version 3.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2016;14:1286–312. 10.6004/jnccn.2016.0137 [DOI] [PubMed] [Google Scholar]
- 3.Smyth EC, Verheij M, Allum W, et al. . Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27(suppl 5):v38–49. 10.1093/annonc/mdw350 [DOI] [PubMed] [Google Scholar]
- 4.Japanese Gastric Cancer Association Japanese Gastric cancer treatment guidelines 2014 (VER. 4). Gastric Cancer 2017;20:1–19. 10.1007/s10120-016-0622-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen L, Shan Y-S, Hu H-M, et al. . Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol 2013;14:e535–47. 10.1016/S1470-2045(13)70436-4 [DOI] [PubMed] [Google Scholar]
- 6.Bang Y-J, Van Cutsem E, Feyereislova A, et al. . Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. The Lancet 2010;376:687–97. 10.1016/S0140-6736(10)61121-X [DOI] [PubMed] [Google Scholar]
- 7.Wilke H, Muro K, Van Cutsem E, et al. . Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (rainbow): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224–35. 10.1016/S1470-2045(14)70420-6 [DOI] [PubMed] [Google Scholar]
- 8.Kang Y-K, Boku N, Satoh T, et al. . Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet 2017;390:2461–71. 10.1016/S0140-6736(17)31827-5 [DOI] [PubMed] [Google Scholar]
- 9.Gomez-Roca C, Koscielny S, Ribrag V, et al. . Tumour growth rates and RECIST criteria in early drug development. Eur J Cancer 2011;47:2512–6. 10.1016/j.ejca.2011.06.012 [DOI] [PubMed] [Google Scholar]
- 10.Champiat S, Dercle L, Ammari S, et al. . Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res 2017;23:1920–8. 10.1158/1078-0432.CCR-16-1741 [DOI] [PubMed] [Google Scholar]
- 11.Saâda-Bouzid E, Defaucheux C, Karabajakian A, et al. . Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol 2017;28:1605–11. 10.1093/annonc/mdx178 [DOI] [PubMed] [Google Scholar]
- 12.Ferrara R, Mezquita L, Texier M, et al. . Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol 2018;4:1543–52. 10.1001/jamaoncol.2018.3676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Therasse P, Arbuck SG, Eisenhauer EA, et al. . New guidelines to evaluate the response to treatment in solid tumors. European Organization for research and treatment of cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–16. [DOI] [PubMed] [Google Scholar]
- 14.Eisenhauer EA, Therasse P, Bogaerts J, et al. . New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 15.Ferté C, Fernandez M, Hollebecque A, et al. . Tumor growth rate is an early indicator of antitumor drug activity in phase I clinical trials. Clin Cancer Res 2014;20:246–52. 10.1158/1078-0432.CCR-13-2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shitara K, Özgüroğlu M, Bang Y-J, et al. . Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. The Lancet 2018;392:123–33. 10.1016/S0140-6736(18)31257-1 [DOI] [PubMed] [Google Scholar]
- 17.Bang Y-J, Ruiz EY, Van Cutsem E, et al. . Phase III, randomised trial of avelumab versus physician's choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of javelin gastric 300. Ann Oncol 2018;29:2052–60. 10.1093/annonc/mdy264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato S, Goodman A, Walavalkar V, et al. . Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res 2017;23:4242–50. 10.1158/1078-0432.CCR-16-3133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borghaei H, Paz-Ares L, Horn L, et al. . Nivolumab versus docetaxel in advanced Nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627–39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makiyama A, Arimizu K, Hirano G, et al. . Irinotecan monotherapy as third-line or later treatment in advanced gastric cancer. Gastric Cancer 2018;21:464–72. 10.1007/s10120-017-0759-9 [DOI] [PubMed] [Google Scholar]
- 21.Nishimura T, Iwasa S, Nagashima K, et al. . Irinotecan monotherapy as third-line treatment for advanced gastric cancer refractory to fluoropyrimidines, platinum, and taxanes. Gastric Cancer 2017;20:655–62. 10.1007/s10120-016-0670-9 [DOI] [PubMed] [Google Scholar]
- 22.Kawakami T, Machida N, Yasui H, et al. . Efficacy and safety of irinotecan monotherapy as third-line treatment for advanced gastric cancer. Cancer Chemother Pharmacol 2016;78:809–14. 10.1007/s00280-016-3138-z [DOI] [PubMed] [Google Scholar]
- 23.Champiat S, Ferrara R, Massard C, et al. . Hyperprogressive disease: recognizing a novel pattern to improve patient management. Nat Rev Clin Oncol 2018;15:748–62. 10.1038/s41571-018-0111-2 [DOI] [PubMed] [Google Scholar]
- 24.Franceschini D, Paroli M, Francavilla V, et al. . PD-L1 negatively regulates CD4+CD25+FoxP3+ Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV. J Clin Invest 2009;119:551–64. 10.1172/JCI36604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnaba V, Schinzari V, Induction SV. Induction, control, and plasticity of Treg cells: the immune regulatory network revised? Eur J Immunol 2013;43:318–22. 10.1002/eji.201243265 [DOI] [PubMed] [Google Scholar]
- 26.Zuazo-Ibarra M, Arasanz H, Fernández-Hinojal G, et al. . Highly differentiated CD4 T cells unequivocally identify primary resistance and risk of Hyperprogression to PD-L1/PD-1 immune checkpoint blockade in lung cancer. bioRxiv 2018. [Google Scholar]
- 27.Peng W, Liu C, Xu C, et al. . PD-1 blockade enhances T-cell migration to tumors by elevating IFN-γ inducible chemokines. Cancer Res 2012;72:5209–18. 10.1158/0008-5472.CAN-12-1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakai S, Kauffman KD, Sallin MA, et al. . CD4 T cell-derived IFN-γ plays a minimal role in control of pulmonary Mycobacterium tuberculosis infection and must be actively repressed by PD-1 to prevent lethal disease. PLoS Pathog 2016;12:e1005667 10.1371/journal.ppat.1005667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shoji H, Tada K, Kitano S, et al. . The peripheral immune status of granulocytic myeloid-derived suppressor cells correlates the survival in advanced gastric cancer patients receiving cisplatin-based chemotherapy. Oncotarget 2017;8:95083–94. 10.18632/oncotarget.18297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo X, Zhai L, Xue R, et al. . Mast cell tryptase contributes to pancreatic cancer growth through promoting angiogenesis via activation of angiopoietin-1. Int J Mol Sci 2016;17 10.3390/ijms17060834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manson G, Norwood J, Marabelle A, et al. . Biomarkers associated with checkpoint inhibitors. Ann Oncol 2016;27:1199–206. 10.1093/annonc/mdw181 [DOI] [PubMed] [Google Scholar]
- 32.DeNardo DG, Andreu P, Coussens LM. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev 2010;29:309–16. 10.1007/s10555-010-9223-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin EY, Nguyen AV, Russell RG, et al. . Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med 2001;193:727–40. 10.1084/jem.193.6.727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wartewig T, Kurgyis Z, Keppler S, et al. . Erratum: PD-1 is a haploinsufficient suppressor of T cell lymphomagenesis. Nature 2018;553 10.1038/nature25142 [DOI] [PubMed] [Google Scholar]
- 35.Ludin A, Zon LI. Cancer immunotherapy: the dark side of PD-1 receptor inhibition. Nature 2017;552:41–2. 10.1038/nature24759 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2019-000488supp001.tif (116.9KB, tif)
esmoopen-2019-000488supp002.tif (116.6KB, tif)