Abstract
Background
No tools to predict the probability of extrahepatic disease progression (ePD) of initially unresectable, liver-limited metastatic colorectal cancer (mCRC) are currently available. To estimate the likelihood to develop ePD and to identify clinical and molecular factors that could predict extrahepatic progression-free survival (ePFS), we conducted an observational, retrospective, multicentre cohort study.
Methods
We retrospectively identified a cohort of 225 patients with initially unresectable liver-limited disease (LLD), treated from January 2004 to December 2017 with first-line doublets or triplet plus a biological agent at two Italian institutions.
Results
173 (77%) patients experienced ePD which occurred within 1, 2 or 3 years from the diagnosis of mCRC in 15%, 49% and 66% of patients, respectively. Globally, 164 (73%) patients underwent a liver resection at some point of their disease history, and 54 (33%) of them underwent a subsequent locoregional treatment. Age > 70 years, locoregional nodal involvement at diagnosis of colorectal cancer and ≥4 liver metastases were significantly associated with higher risk of ePD while liver resections were associated with reduced risk of ePD. In the multivariable model, number of liver metastases (subdistribution HR, SHR 1.63, 95% CI 1.12 to 2.36; p = 0.01) and liver resections (SHR 0.43, 95% CI 0.29 to 0.63; p = 0.001) were still associated with ePD. Number of liver metastases < 4, no nodal involvement at diagnosis and liver resections were also associated with prolonged ePFS.
Conclusions
The identified clinical factors could help physicians in personalising the intensity and aggressiveness of liver-directed treatments in patients with mCRC with initially unresectable LLD.
Keywords: metastatic colorectal cancer, liver-limited disease, extrahepatic disease progression
Key questions.
What is already known about this subject?
Liver is the only site of metastases in one-third of patients with metastatic colorectal cancer (mCRC).
Increasing evidence supports the crucial role of the multidisciplinary management of these patients in order to offer them the most appropriate integration of systemic and locoregional interventions with curative intent.
No tools to predict the probability of extrahepatic disease progression (ePD) of initially unresectable, liver-limited mCRC are currently available.
What does this study add?
To estimate the likelihood to develop ePD and to identify clinical and molecular factors that could predict extrahepatic progression-free survival, we conducted an observational, retrospective, multicentre cohort study.
Age >70 years, locoregional nodal involvement at diagnosis of colorectal cancer and ≥4 liver metastases were significantly associated with higher risk of ePD while liver resections were associated with reduced risk of ePD.
How might this impact on clinical practice?
The identified clinical factors could help physicians in personalising the intensity and aggressiveness of liver-directed treatments in an oligometastatic setting.
Introduction
Liver is the most frequent site of metastatic spread from colorectal cancer (CRC) and appears as the only site of metastases in one-third of cases. Increasing evidence supports the crucial role of the multidisciplinary management of these patients in order to offer them the most appropriate integration of systemic and locoregional interventions.
Surgery is regarded as the gold standard, since the radical resection of liver metastases is associated with prolonged overall survival (OS) as compared with non-curative or no liver resection,1 with a 5-year survival rate of 25%2 and a similar 10-year survival rate,3 4 demonstrating that a small but not negligible fraction of patients achieves meaningful long-term benefit5 and sometimes cure from this approach.
However, about two-thirds of patients undergoing liver surgery for metastatic CRC (mCRC) experience disease recurrence and derive limited benefit from surgical approaches.
Several scoring systems have been developed to predict the risk of recurrence after the surgical resection of liver metastases based on clinical and pathological prognostic factors.2 6–19
In the last years, the therapeutic scenario for patients with mCRC with liver-limited disease (LLD) has notably changed. Indeed, active systemic regimens are now able to convert to resection a consistent percentage of initially unresectable patients with a 10-year survival rate around 20%.4 In these patients, not only inducing cytoreduction but also achieving an adequate control of the micrometastatic disease is crucial to allow subsequent locoregional interventions and long-term disease control. Moreover, the options provided by the toolbox of locoregional therapies beyond surgical resection are more and more numerous, including thermoablation, intra-arterial chemotherapy, chemoembolisation or radioembolisation and stereotactic radiotherapy.20
The development of multiple tools and the lack of high levels of evidence about their proper use highlight the need to identify those patients who may benefit from these strategies in the modern therapeutic landscape. In fact, clinical experience reveals that patients with LLD at the time of metastases diagnosis may display heterogeneous disease behaviours: while in some patients, the metastases are confined to the liver during the natural history of the disease and across different lines of therapy; in other cases, the extrahepatic spread occurs early. By a clinical perspective, the adoption of locoregional approaches, including liver surgery, in the therapeutic route of patients with mCRC would be especially indicated in the first scenario, and less effective in the latter.
So far, the weight of clinical and molecular prognostic factors on the probability to develop extrahepatic disease progression (ePD) has not been investigated. The present study aimed at estimating the likelihood to develop ePD and at identifying clinical, pathological and molecular factors associated with extrahepatic progression-free survival (ePFS) in patients with initially unresectable LLD mCRC.
Methods
Patients’ population
We retrospectively identified a cohort of consecutive patients with mCRC with initially unresectable LLD treated with first-line chemotherapy doublets or triplet associated, when appropriate, with a biological agent, in two Italian institutions (Azienda Ospedaliera-Universitaria Pisana, Pisa, Italy and Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy) from January 2004 to December 2017.
Resectability of liver metastases was assessed at baseline by multidisciplinary teams at participating institutions considering both technical and biological criteria. Not only patients judged not technically resectable because of a high tumour burden but also those with technically resectable disease but with poor prognostic factors were included.
Data regarding clinical, molecular, and pathological features were collected at baseline. Information about treatments received, response including early tumour shrinkage and deepness of response obtained, and metastatic sites were collected from medical clinical records at baseline and along the whole disease history until death or last follow-up.
Patients with extrahepatic involvement including suspected distant nodal involvement at diagnosis, concurrent or previous other malignancy within 5 years from mCRC diagnosis or with inadequate follow-up were excluded.
Additional information about liver resections, including applied techniques and surgical outcomes, and further liver procedures (surgery or other locoregional treatments) were collected.
All clinical investigations detailed were conducted in accordance with the Declaration of Helsinki in compliance with Good Clinical Practice.
Statistical analysis
Baseline characteristics in patients undergoing at least one resection versus unresected patients were compared by means of χ2 test and Fisher’s exact test (when appropriate). The association of these characteristics with the probability of experiencing ePD was investigated by means of a competing risk assessment model at univariable and multivariable analyses based on Fine and Grey’s proportional subhazards method to estimate the absolute risk of the occurrence of ePD through a cumulative incidence function increasing with time, considering as a competing event the occurrence of intrahepatic disease progression only.
ePFS was calculated from the day of mCRC diagnosis to the first observation of extrahepatic disease. Survival curves were estimated by the Kaplan-Meier method and compared with the log-rank test.
The impact of the collected information on ePFS was first assessed in univariate analyses. Significantly prognostic variables (p<0.10) were included in a multivariable Cox proportional hazard model.
Moreover, to overcome a possible selection bias due to the retrospective nature of the observation and to the occurrence of liver resection at different times during patients’ disease histories, the Kernel smoothed hazard model was applied to better estimate the relative contribution of liver resection.
Results
Patients’ population
A cohort of 225 patients with initially unresectable liver-limited mCRC treated from January 2004 to December 2017 was identified. Among them, 187 (75%) were younger than 70 years, 195 (87%) had an Eastern Cooperative Oncology Group Performance Status (ECOG-PS) 0, 194 (86%) of them presented with synchronous metastases, 114 (51%) had a documented locoregional nodal involvement at diagnosis, 135 (60%) had more than three liver metastases, 161 (71%) had less than six liver segments involved and in 134 (60%) patients the maximum diameter of liver lesions was higher than 30 mm. Information about RAS/BRAF status were available for 199 (89%) patients. Of these, 92 (41%) had RAS/BRAF wild-type disease, whereas 99 (50%) and 8 (4%) harboured RAS-mutated or BRAF-mutated tumours, respectively. Microsatellite status was known in only 57 (25%) cases, three (1%) of whom were defined as microsatellite instability (MSI) high and 54 (24%) were microsatellite stable (MSS)/MSI low. Patients’ characteristics at the time of diagnosis of liver-limited mCRC are summarised in table 1. In all, 61 (27%) patients did not receive any liver-directed treatment in their therapeutic route, 164 (73%) underwent at least one locoregional liver treatment during their disease history and 54 (24%) of them underwent also a subsequent locoregional treatment (figure 1A).
Table 1.
Baseline characteristics
| Characteristics | Overall population N=225 N (%) |
Never resected N=61 N (%) |
At least one liver treatment N=164 N (%) |
P value* |
| Age (years) | 0.04 | |||
| ≥70 | 38 (15) | 16 (26) | 22 (13) | |
| <70 | 187 (75) | 45 (74) | 142 (87) | |
| ECOG PS | < 0.0001 | |||
| 1 | 30 (13) | 49 (80) | 18 (11) | |
| 0 | 195 (87) | 12 (20) | 146 (89) | |
| Timing | 0.69 | |||
| Synchronous | 194 (86) | 54 (89) | 140 (85) | |
| Metachronous | 31 (14) | 7 (11) | 24 (15) | |
| Resected primary tumour | 0.16 | |||
| Yes | 151 (67) | 36 (59) | 115 (70) | |
| No | 74 (33) | 25 (41) | 49 (30) | |
| Sidedness | 0.21 | |||
| Left | 159 (71) | 39 (64) | 120 (73) | |
| Right | 65 (29) | 22 (36) | 43 (26) | |
| Not available | 1 (0) | 0 (0) | 1 (1) | |
| Nodal involvement | 0.10 | |||
| Yes | 114 (51) | 32 (53) | 82 (50) | |
| No | 53 (24) | 8 (13) | 45 (27) | |
| Not available | 58 (26) | 21 (34) | 37 (23) | |
| Baseline CEA level | 0.54 | |||
| >200 ng/mL | 49 (22) | 16 (26) | 33 (20) | |
| ≤200 ng/mL | 128 (57) | 34 (56) | 94 (57) | |
| Not available | 48 (21) | 11 (18) | 37 (23) | |
| Number of liver metastases | 0.002 | |||
| ≥4 | 135 (60) | 45 (74) | 90 (55) | |
| <4 | 69 (31) | 8 (13) | 61 (37) | |
| Not available | 21 (9) | 8 (13) | 13 (8) | |
| Liver metastases maximum diameter | 0.69 | |||
| >30 mm | 134 (60) | 38 (62) | 96 (58) | |
| ≤30 mm | 65 (29) | 16 (26) | 49 (30) | |
| Not available | 26 (11) | 7 (12) | 19 (12) | |
| Bilobar involvement | 0.22 | |||
| Yes | 153 (68) | 43 (71) | 110 (67) | |
| No | 63 (28) | 12 (20) | 51 (31) | |
| Not available | 9 (4) | 6 (9) | 3 (2) | |
| Liver involvement | < 0.0001 | |||
| >6 segments | 49 (22) | 26 (43) | 23 (14) | |
| ≤6 segments | 161 (71) | 26 (43) | 135 (82) | |
| Not available | 15 (7) | 9 (14) | 6 (4) | |
| RAS/BRAF status | 0.13 | |||
| Mutated | 107 (48) | 36 (59) | 71 (43) | |
| Wild type | 92 (41) | 21 (34) | 71 (43) | |
| Not available | 26 (11) | 4 (7) | 22 (14) | |
| Microsatellite status | 1.00 | |||
| MSI High | 3 (1) | 0 (0) | 3 (2) | |
| MSI low/MSS | 54 (24) | 9 (14) | 45 (27) | |
| Not available | 168 (75) | 52 (86) | 116 (71) | |
| Induction CT | 0.31 | |||
| Triplet | 126 (56) | 38 (62) | 88 (54) | |
| Doublet | 99 (44) | 23 (38) | 76 (46) | |
| Biological agent | 0.09 | |||
| Anti-EGFR | 57 (25) | 13 (21) | 44 (27) | |
| Anti-VEGF | 132 (59) | 38 (62) | 94 (57) | |
| Both | 11 (5) | 6 (10) | 5 (3) | |
| None | 25 (11) | 4 (7) | 21 (13) | |
*P value for χ2 and Fisher’s exact test
Anti-EGFR, anti-epidermal growth factor receptor; Anti-VEGF, anti-vascular endothelial growth factor; CEA, carcinoembryonic antigen;CT, chemotherapy; ECOG, Eastern Cooperative Oncology Group;MSI, microsatellite instability;MSS, microstatellite stable;PS, Performance Status.
Figure 1.
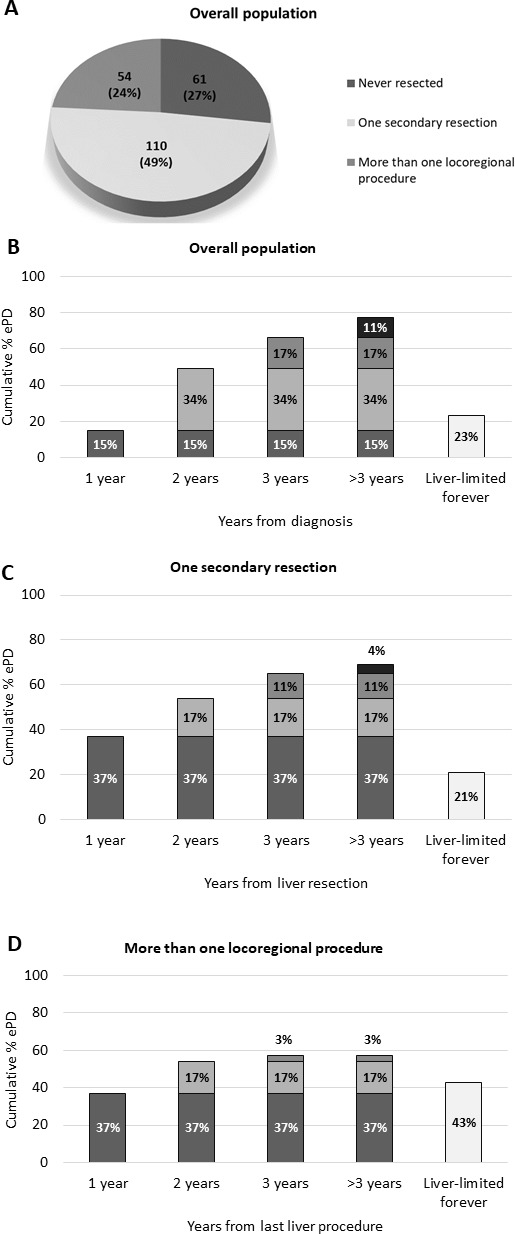
ePD distribution. (A) Description of overall population. (B) ePD cumulative distribution through each year from diagnosis in overall population. (C) ePD cumulative distribution through each year from first liver resection in patients undergoing one secondary resection. () ePD cumulative distribution through each year from last liver procedure in patients undergoing more than one locoregional procedure. ePD, extrahepatic disease progression.
Patients’ outcomes
Overall, 52 (23%) patients including those who never progressed after a curative treatment were ePD free, during the whole period of observation and 173 (77%) experienced ePD which occurred within 1, 2 or 3 years from the diagnosis of mCRC in 15%, 49% and 66% of patients, respectively (figure 1B).
Among patients who were ePD free, five had obtained a complete response during the first-line treatment without experiencing disease progression and 22 never progressed after a curative liver resection. Among 198 patients who experienced at least one disease progression, 96 (48%) did not experienced ePD at first disease progression, and 51 (53%) of them did not experienced ePD also at the second disease progression. In all, 34 (80%) of them did not experienced again ePD at the third disease progression. Baseline characteristics of the 34 patients with ePD free at third disease progression are summarised in online supplementary material table 1.
esmoopen-2019-000496supp001.pdf (546.9KB, pdf)
Among patients who received only one liver resection, ePD occurred within 1, 2 or 3 years from the first liver procedure in 37%, 54% and 65% of cases, respectively (figure 1C); similarly, in patients who underwent more than one locoregional procedure, ePD occurred in 37%, 54% and 57% of patients within 1, 2 or 3 years, respectively, from the last liver procedure (figure 1D).
Competing risk analysis and determinants of ePFS
Univariate analyses for competing risk revealed that age over 70 years (subdistribution HR, SHR 1.50, 95% CI 1.04 to 2.18; p=0.03), nodal involvement at diagnosis (SHR 1.50, 95% CI 1.01 to 2.22; p=0.04) and more than four liver metastases at baseline (SHR 1.79, 95% CI 1.28 to 2.52; p=0.001) were significantly associated with higher risk for ePD. On the contrary, undergoing a liver resection was associated with lower risk of ePD (SHR 0.38, 95% CI 0.26 to 0.56; p=0.001). In the multivariable model, number of liver metastases (SHR 1.63, 95% CI 1.12 to 2.36; p=0.01) and liver resections (SHR 0.43, 95% CI 0.29 to 0.63; p=0.001) were still associated with ePD (online supplementary material table 2).
At the univariate analyses assessing the association between baseline characteristics and ePFS, age over 70 years (HR 1.43, 95% CI 0.93-2.18; p=0.07), ECOG PS 1 (HR 1.45, 95% CI 0.89 to 2.33; p=0.08), nodal involvement at diagnosis (HR 1.55, 95% CI 1.08 to 2.23; p=0.03), more than four liver lesions (HR 1.67, 95% CI 1.22 to 2.29; p=0.003), highest diameter of liver lesions >30 mm (HR 1.35, 95% CI 0.96 to 1.86; p=0.09), more than six liver segments involved (HR 1.40, 95% CI 0.94 to 2.09; p=0.06) and MSI high (HR 2.41, 95% CI 0.42 to 13.88; p=0.03) were associated with significantly shorter ePFS. On the contrary, undergoing a liver resection (HR 0.39, 95% CI 0.26 to 0.59; p<0.001) and subsequent liver retreatments (HR 0.27, 95% CI 0.19 to 0.39; p<0.001) were associated with significantly longer ePFS. In the multivariable model, ECOG PS (HR 2.04, 95% CI 1.11 to 3.72; p=0.02), number (HR 1.83, 95% CI 1.15 to 2.90; p=0.01), diameter of liver lesions (HR 1.98, 95% CI 1.23 to 3.20; p=0.005) and undergoing a liver resection (HR 0.37, 95% CI 0.21 to 0.65; p<0.001) were associated with ePFS (table 2).
Table 2.
Univariable and multivariable analyses for ePFS in the overall population
| Characteristics | Univariable analyses | Multivariable analyses | ||||
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age ≥70 years versus <70 years | 1.43 | 0.93 to 2.18 | 0. 07 | 1.02 | 0.99 to 1.04 | 0.12 |
| ECOG PS 1 versus 0 | 1.45 | 0.89 to 2.33 | 0.08 | 2.04 | 1.11 to 3.72 | 0.02 |
| Synchronous versus metachronous | 1.31 | 0.87 to 1.98 | 0.24 | – | – | – |
| Resected primary tumour versus unresected | 0.84 | 0.61 to 1.15 | 0.26 | – | – | – |
| Left versus right primary | 0.89 | 0.64 to 1.26 | 0.52 | – | – | – |
| Nodal involvement versus no involvement | 1.55 | 1.08 to 2.23 | 0.03 | 1.48 | 0.91 to 2.40 | 0.12 |
| Baseline CEA level >200 versus ≤200 ng/mL | 1.19 | 0.80 to 1.77 | 0.37 | – | – | – |
| Number of liver metastases ≥4 versus <4 | 1.67 | 1.22 to 2.29 | 0.003 | 1.83 | 1.15 to 2.90 | 0.01 |
| Liver metastases maximum diameter >30 versus ≤30 mm | 1.35 | 0.96 to 1.86 | 0.09 | 1.98 | 1.23 to 3.20 | 0.005 |
| Bilobar involvement yes versus no | 1.14 | 0.82 to 1.58 | 0.44 | – | – | – |
| Liver involvement >6 versus ≤6 segments | 1.40 | 0.94 to 2.09 | 0.06 | 0.69 | 0.36 to 1.33 | 0.27 |
| RAS/BRAF mutation versus RAS/BRAF wild type | 1.11 | 0.82 to 1.51 | 0.67 | – | – | – |
| MSI high versus MSS | 2.41 | 0.42 to 13.88 | 0.03 | -* | -* | -* |
| Triplet versus doublet induction | 1.20 | 0.89 to 1.62 | 0.22 | – | – | – |
| Anti-EGFR versus anti-VEGF | 1.04 | 0.73 to 1.47 | 0.37 | – | – | – |
| Liver secondary resection | 0.39 | 0.26 to 0.59 | < 0.001 | 0.37 | 0.21 to 0.65 | < 0.001 |
| Liver retreatments | 0.27 | 0.19 to 0.39 | < 0.001 | – | – | – |
*Too small sample size for multivariable model
†Bold values represent statistically significant values.
Anti-EGFR, anti-epidermal growth factor receptor; Anti-VEGF, anti-vascular endothelial growth factor receptor; CEA, carcinoembryonic antigen; ECOG, Eastern Cooperative Oncology Group; MSI, microsatellite instability; MSS, microstatellite stable; PS, Performance Status; ePFS, extrahepatic progression-free survival.
A more accurate estimation of the impact of baseline characteristics on ePFS was obtained through the Kernel smoothed hazard estimator model to overcome the selection bias introduced by the occurrence of liver resection at different times during patients’ disease histories. Consistently with previously reported results, hepatic resections, no nodal involvement at diagnosis and less than four liver metastases were independently associated with prolonged ePFS when adjusted for the other significant variables (age, ECOG PS, diameter of liver lesions, number of involved segments) (figure 2).
Figure 2.
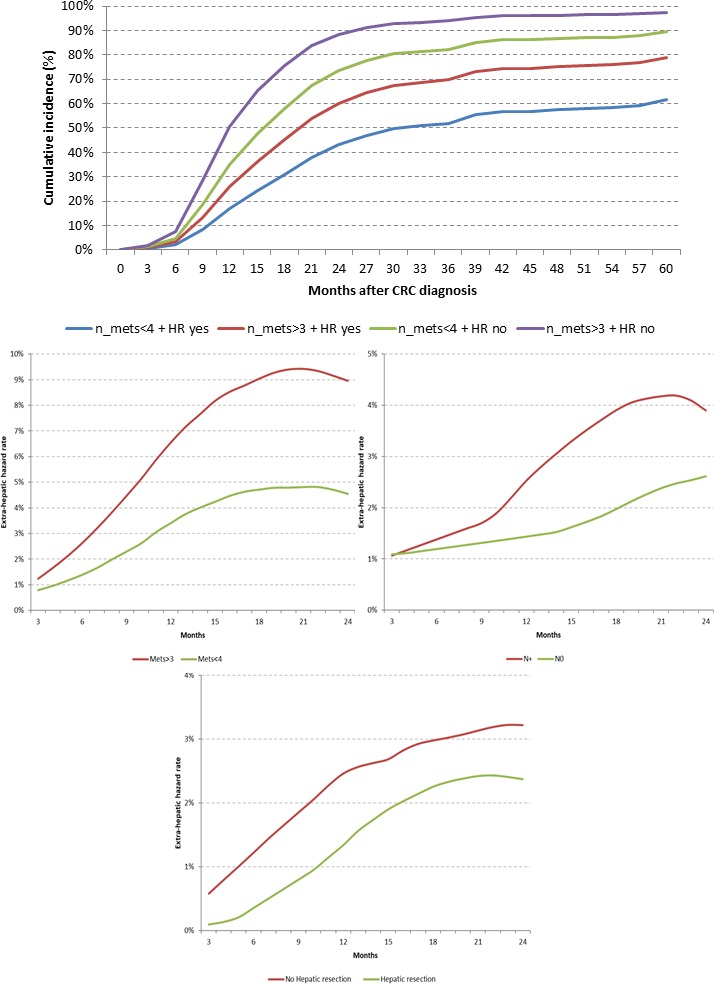
Cumulative incidence of ePD according to number of liver lesions and secondary resection and kernel smoothed hazard estimator for ePFS. CRC, colorectal cancer; ePFS, extrahepatic progression-free survival; n, number; Mets, liver metastases; HR, hepatic resection; N, nodal involvement.
In the subgroup of patients who did not undergo any liver procedure, the competing risk model could not be applied as all of them experienced ePD during their disease history. In this specific subgroup, ECOG PS 1 (HR 2.19, 95% CI 0.95 to 5.06; p=0.01), baseline carcinoembryonic antigen levels over 200 ng/mL (HR 1.82, 95% CI 0.91 to 3.64; p=0.03), >4 liver metastases (HR 2.61, 95% CI 1.34 to 5.09; p=0.03) and highest diameter of liver lesions over 30 mm (HR 2.62, 95% CI 1.48 to 4.63; p=0.002) predicted shorter ePFS at univariate analyses. Left primary sidedness (HR 0.56, 95% CI 0.30 to 1.01; p=0.03) and previous primary resection (HR 0.60, 95% CI 0.34 to 1.05; p=0.05) were associated with prolonged ePFS. At the multivariate analysis, ECOG PS 1 (HR 2.68, 95% CI 1.14 to 6.30; p=0.02) and highest diameter of liver metastases >30 mm (HR 3.03, 95% CI 1.29 to 7.12; p=0.01) were still associated with shorter ePFS (online supplementary material table 3). In the subgroup of patients who underwent at least one liver-directed procedure, nodal involvement (HR 1.55, 95% CI 1.02 to 2.36; p=0.04) and MSI-high status (HR 3.03, 95% CI 0.43 to 21.15; p=0.049) were associated with shorter ePFS while undergoing subsequent locoregional retreatments was associated with longer ePFS (HR 0.31, 95% CI 0.19 to 0.52; p<0.001). In the multivariable model, liver retreatments were still associated with longer ePFS with a trend towards significance (HR 0.41, 95% CI 0.16 to 1.08; p=0.07) (online supplementary material table 4).
Discussion
The multidisciplinary management of mCRC substantially contributed to the improvement of patients’ life expectancy and especially affected the prognosis of those with initially unresectable LLD whose treatment options have been substantially changed by the development and adoption of locoregional approaches.20 The present retrospective analysis provides an updated snapshot of the contemporary therapeutic route of patients affected by colorectal liver metastases. Notably, at least one liver locoregional procedure was performed at some point of the disease history in the vast majority (73%) of cases, and in the 33% of these, further locoregional interventions were reported.
When putting our results in the frame of available literature data, these percentages are extremely high, thus confirming the evolving scenario of systemic and local interventions’ integration at two Italian high-volume, referral centres.
In a previous historical pivotal series of 1439 consecutive patients with liver-limited mCRC enrolled from 1988 to 1999, 1104 were considered as initially unresectable and were treated with systemic chemotherapy. Among them, only 138 (12.5%) underwent a liver resection demonstrating that systemic treatment allows a small fraction of rescue liver surgeries with an OS at 10 years of 23%.21
Nonetheless, all patients included in our series were fit to receive a combination regimen as upfront treatment and were, therefore, able to achieve benefit from the most active systemic regimens. Moreover, only patients with strictly liver-limited, and not with liver-dominant spread were included in this cohort.
Resection rates of 32%, 49% and 61% were reported with doublets plus cetuximab, FOLFOX plus bevacizumab and FOLFOXIRI plus bevacizumab, respectively, in contemporary phase II randomised trials investigating the efficacy of these regimens as conversion therapies.22 23
As compared with these controlled and prospective trials, not only patients who needed a relevant shrinkage to make liver resection feasible were included in our series but also those deemed initially unresectable based on biological considerations driven by disease aggressiveness and poor prognostic factors.
Based on our results, most of ePDs occurred within 3 years from the last liver procedure, thus suggesting that tailored surveillance protocols should be adopted and, in particular, the intensification of clinical and radiological assessments may be justified in the first 2 years from liver curative approaches.
The paramount relevance of surgery with radical intent in favourably affecting the natural history of the disease is confirmed also in terms of ePFS. Also when applying the Kernel smoothed hazard estimator model, patients undergoing a liver surgery are less likely to develop extrahepatic metastases than unresected ones. Of note, all patients in the never resected subgroup experienced ePD at some time of their disease history, whereas patients undergoing repeated/sequential locoregional approaches may achieve a definitive cure.24
Indeed, our results suggest that the probability of experiencing ePD through subsequent lines of treatment and disease progressions is numerically higher for those patients who never underwent liver resections compared with those who underwent at least one liver locoregional procedure.
With regard to other clinical and molecular factors, no nodal involvement at diagnosis and less than four liver metastases could affect the extrahepatic spread.
We acknowledge some limitations of our work, including the relatively small sample size and the retrospective collection of clinical data. Moreover, given the strong impact of a post-baseline variable, such as surgical resection, on the natural history of the disease and its progression patterns, this study did not allow developing and validating a score or a nomogram able to predict the risk of ePD at baseline. When focusing on the subgroup of patients who underwent at least one liver-directed procedure, the smaller sample size prevented to derive robust conclusions on ePD determinants, though highlighting the positive impact of subsequent liver re-treatments on the extrahepatic spread. The clinical relevance of locoregional tools in the therapeutic route of patients with oligometastatic CRC is well described in the latest version of the European Society for Medical Oncology guidelines20 underlining the contribution of disease localisation, treatment goal, patient-related comorbidities and treatment morbility to the choice of the best approach in each individual case.
Though considering above-reported limitations, our findings may assist clinicians in intensifying liver-directed locoregional treatments in patients with LLD and could be useful also to select patients who may benefit from liver transplantation in the frame of ongoing prospective clinical studies. Similar considerations may be applied to other available liver-directed therapies in patients still deemed unresectable following conversion therapies. For example, radioembolisation prolongs intrahepatic PFS, but not intrahepatic plus extrahepatic overall PFS in patients with unresectable LLD with or without minimal extrahepatic disease.25
No association between molecular markers tested in the daily practice (ie, RAS/BRAF/MSI) and extrahepatic metastases was found. The association of BRAFV600E mutation with nodal and peritoneal spread is well known26 and previous series evidenced short relapse-free and OS following liver resection in the rare cases of LLD.27 28 Our results are in line with a previous pooled analysis of patients with initially unresectable liver-limited mCRC treated in prospective clinical trials with FOLFOXIRI plus bevacizumab29 that is regarded by major international guidelines20 30 as the preferred therapeutic option, when feasible, to counterbalance the intrinsic biological aggressiveness of BRAF-mutated tumours.
Nevertheless, shorter ePFS (HR 2.41, 95% CI 0.42 to 13.88; p=0.03) was reported in patients with MSI-high tumours versus MSS/MSI low. Due to the low prevalence of this alteration, especially in the case of LLD, these results should be cautiously interpreted, also taking into account the frequent co-occurrence of MSI and BRAF V600E mutation. Finally, the clarification of the surrogacy of ePFS for OS in liver-limited mCRC could be of interest in order to evaluate the potential role of ePFS as a new endpoint for clinical trials in this specific setting.
Footnotes
Contributors: All named authors have participated in the study to a sufficient extent to be named as authors, read and approved the final version of the manuscript, and agreed to the submission.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The study was approved by the local Ethics Committees of participating institutions and was performed in compliance with Good Clinical Practice and in accordance with the Declaration of Helsinki.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Steele G, Bleday R, Mayer RJ, et al. . A prospective evaluation of hepatic resection for colorectal carcinoma metastases to the liver: Gastrointestinal Tumor Study Group Protocol 6584. J Clin Oncol 1991;9:1105–12. 10.1200/JCO.1991.9.7.1105 [DOI] [PubMed] [Google Scholar]
- 2. Nordlinger B, Guiguet M, Vaillant JC, et al. . Surgical resection of colorectal carcinoma metastases to the liver. a prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer 1996;77:1254–62. [DOI] [PubMed] [Google Scholar]
- 3. Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. . Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol 2007;25:4575–80. 10.1200/JCO.2007.11.0833 [DOI] [PubMed] [Google Scholar]
- 4. Jones RP, Poston GJ. Resection of liver metastases in colorectal cancer in the era of expanding systemic therapy. Annu Rev Med 2017;68:183–96. 10.1146/annurev-med-062415-093510 [DOI] [PubMed] [Google Scholar]
- 5. Scheele J, Stang R, Altendorf-Hofmann A, et al. . Resection of colorectal liver metastases. World J Surg 1995;19:59–71. 10.1007/BF00316981 [DOI] [PubMed] [Google Scholar]
- 6. Rees M, Tekkis PP, Welsh FKS, et al. . Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg 2008;247:125–35. 10.1097/SLA.0b013e31815aa2c2 [DOI] [PubMed] [Google Scholar]
- 7. Malik HZ, Prasad KR, Halazun KJ, et al. . Preoperative prognostic score for predicting survival after hepatic resection for colorectal liver metastases. Ann Surg 2007;246:806–14. 10.1097/SLA.0b013e318142d964 [DOI] [PubMed] [Google Scholar]
- 8. Minagawa M, Yamamoto J, Miwa S, et al. . Selection criteria for simultaneous resection in patients with synchronous liver metastasis. Arch Surg 2006;141:1006–12. 10.1001/archsurg.141.10.1006 [DOI] [PubMed] [Google Scholar]
- 9. Konopke R, Kersting S, Distler M, et al. . Prognostic factors and evaluation of a clinical score for predicting survival after resection of colorectal liver metastases. Liver Int 2009;29:89–102. 10.1111/j.1478-3231.2008.01845.x [DOI] [PubMed] [Google Scholar]
- 10. Fong Y, Cohen AM, Fortner JG, et al. . Liver resection for colorectal metastases. J Clin Oncol 1997;15:938–46. 10.1200/JCO.1997.15.3.938 [DOI] [PubMed] [Google Scholar]
- 11. Zakaria S, Donohue JH, Que FG, et al. . Hepatic resection for colorectal metastases: value for risk scoring systems? Ann Surg 2007;246:183–91. 10.1097/SLA.0b013e3180603039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamaguchi T, Mori T, Takahashi K, et al. . A new classification system for liver metastases from colorectal cancer in Japanese multicenter analysis. Hepatogastroenterology 2008;55:173–8. [PubMed] [Google Scholar]
- 13. Iwatsuki S, Dvorchik I, Madariaga JR, et al. . Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg 1999;189:291–9. 10.1016/S1072-7515(99)00089-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tan MCB, Castaldo ET, Gao F, et al. . A prognostic system applicable to patients with resectable liver metastasis from colorectal carcinoma staged by positron emission tomography with [18F]fluoro-2-deoxy-D-glucose: role of primary tumor variables. J Am Coll Surg 2008;206:857–68. 10.1016/j.jamcollsurg.2007.12.023 [DOI] [PubMed] [Google Scholar]
- 15. Schindl M, Wigmore SJ, Currie EJ, et al. . Prognostic scoring in colorectal cancer liver metastases: development and validation. Arch Surg 2005;140:183–9. 10.1001/archsurg.140.2.183 [DOI] [PubMed] [Google Scholar]
- 16. Tanaka K, Shimada H, Fujii Y, et al. . Pre-hepatectomy prognostic staging to determine treatment strategy for colorectal cancer metastases to the liver. Langenbecks Arch Surg 2004;389:371–9. 10.1007/s00423-004-0490-y [DOI] [PubMed] [Google Scholar]
- 17. Lise M, Bacchetti S, Da Pian P, et al. . Patterns of recurrence after resection of colorectal liver metastases: prediction by models of outcome analysis. World J Surg 2001;25:638–44. 10.1007/s002680020138 [DOI] [PubMed] [Google Scholar]
- 18. Ueno H, Mochizuki H, Hatsuse K, et al. . Indicators for treatment strategies of colorectal liver metastases. Annals of Surgery 2000;231:59–66. 10.1097/00000658-200001000-00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nagashima I, Takada T, Matsuda K, et al. . A new scoring system to classify patients with colorectal liver metastases: proposal of criteria to select candidates for hepatic resection. J Hepatobiliary Pancreat Surg 2004;11:79–83. 10.1007/s00534-002-0778-7 [DOI] [PubMed] [Google Scholar]
- 20. Van Cutsem E, Cervantes A, Adam R, et al. . ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386–422. 10.1093/annonc/mdw235 [DOI] [PubMed] [Google Scholar]
- 21. Adam R, Delvart V, Pascal G, et al. . Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg 2004;240:644–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Folprecht G, Gruenberger T, Bechstein W, et al. . Survival of patients with initially unresectable colorectal liver metastases treated with FOLFOX/cetuximab or FOLFIRI/cetuximab in a multidisciplinary concept (CELIM study). Ann Oncol 2014;25:1018–25. 10.1093/annonc/mdu088 [DOI] [PubMed] [Google Scholar]
- 23. Gruenberger T, Bridgewater J, Chau I, et al. . Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: the OLIVIA multinational randomised phase II trial. Ann Oncol 2015;26:702–8. 10.1093/annonc/mdu580 [DOI] [PubMed] [Google Scholar]
- 24. Dexiang Z, Li R, Ye W, et al. . Outcome of patients with colorectal liver metastasis: analysis of 1,613 consecutive cases. Ann Surg Oncol 2012;19:2860–8. 10.1245/s10434-012-2356-9 [DOI] [PubMed] [Google Scholar]
- 25. Wasan HS, Gibbs P, Sharma NK, et al. . First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-Global): a combined analysis of three multicentre, randomised, phase 3 trials. Lancet Oncol 2017;18:1159–71. 10.1016/S1470-2045(17)30457-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lipsyc M, Yaeger R. Impact of somatic mutations on patterns of metastasis in colorectal cancer. J Gastrointest Oncol 2015;6:645–9. 10.3978/j.issn.2078-6891.2015.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schirripa M, Bergamo F, Cremolini C, et al. . BRAF and RAS mutations as prognostic factors in metastatic colorectal cancer patients undergoing liver resection. Br J Cancer 2015;112:1921–8. 10.1038/bjc.2015.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Margonis GA, Buettner S, Andreatos N, et al. . Association of BRAF Mutations With Survival and Recurrence in Surgically Treated Patients With Metastatic Colorectal Liver Cancer. JAMA Surg 2018;153:e180996 10.1001/jamasurg.2018.0996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cremolini C, Casagrande M, Loupakis F, et al. . Efficacy of FOLFOXIRI plus bevacizumab in liver-limited metastatic colorectal cancer: a pooled analysis of clinical studies by Gruppo Oncologico del Nord Ovest. Eur J Cancer 2017;73:74–84. 10.1016/j.ejca.2016.10.028 [DOI] [PubMed] [Google Scholar]
- 30. Benson AB, Venook AP, Al-Hawary MM, et al. . NCCN guidelines insights: colon cancer, version 2.2018. J Natl Compr Canc Netw 2018;16:359–69. 10.6004/jnccn.2018.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2019-000496supp001.pdf (546.9KB, pdf)


