Abstract
Mosquito collections were conducted in Zika Forest near Entebbe, Uganda from July 2009 through June 2010 using CO2-baited light traps, ovitraps and human-baited catches. A total of 163,790 adult mosquitoes belonging to 10 genera and 61 species were captured. Of these, 24 species (39%) were captured in Zika Forest for the first time. All the new records found in the forest in this study had previously been captured in other regions of Uganda, implying that they are native to the country and do not represent new introductions. Twenty species previously collected in Zika forest were not detected in our collections and this may suggest a change in the mosquito fauna during the past 40 years or variation in species composition from year to year. Arboviruses of public health importance have previously been isolated from more than 50% of the 61 mosquito species captured in Zika forest which suggests a high potential for transmission and maintenance of a wide range of arboviruses in Zika Forest.
Keywords: Zika Forest, Uganda, Mosquito Species Composition, Arbovirus Vectors
INTRODUCTION
Investigations of the mosquitoes of Zika Forest near Entebbe, Uganda were initiated in 1946 (Kirya et al. 1977) following a human yellow fever (YF) sero-survey (Sawyer and Whitman 1936) which showed that YF was endemic throughout Uganda. The investigations intensified in 1960 when a 36.6 m (120 ft) steel tower was relocated from Mpanga Forest to Zika Forest to study the vertical stratification of mosquito activity, especially the important sylvan yellow fever virus (YFV) vector Aedes (Stegomyia) africanus Theobald (Kirya et al. 1977). In the course of these investigations, the mosquito species composition for Zika Forest was described (Haddow et al. 1964, Corbet 1964). In addition, a wealth of information was gained on the biology, biting behaviour and oviposition activity for the different sylvan species. Lastly, several arboviruses were isolated from the mosquitoes collected in Zika Forest including Zika virus (Haddow et al 1964b), Uganda S virus (Dick and Haddow 1952) and YF (Kirya et al. 1977) which demonstrated the medical importance of some mosquito species in Zika Forest and enhanced our understanding of the transmission and maintenance cycles of these arboviruses.
The routine mosquito collections at Zika Forest were discontinued in the early 1970s, and the mosquito species composition records have not been updated for more than 40 years. Forty years ago Zika Forest was isolated from all human settlement and virtually unaffected by human activities. Currently, Zika Forest is surrounded by new homes and/or crop fields and plantations and the ecosystems adjacent to the forest have significant modifications as a result of human activity. Studies have shown that forest modification and clearing have a negative impact on biodiversity (Chazdon et al. 2009, Nichols et al. 2007, Koh 2007, Gardner et al. 2008). These studies reported that protected areas are influenced directly or indirectly by many land uses including; road construction, hunting, cattle grazing, agricultural incursions and over harvesting of non-timber products. All land uses around the forest greatly exploit the forest habitat and indirectly affect the mosquito community. These effects have been further compounded by the ever changing climate around the country. The aim of this study was to describe the current mosquito species composition in Zika Forest and compare it to what was observed in the past.
MATERIALS AND METHODS
Study area
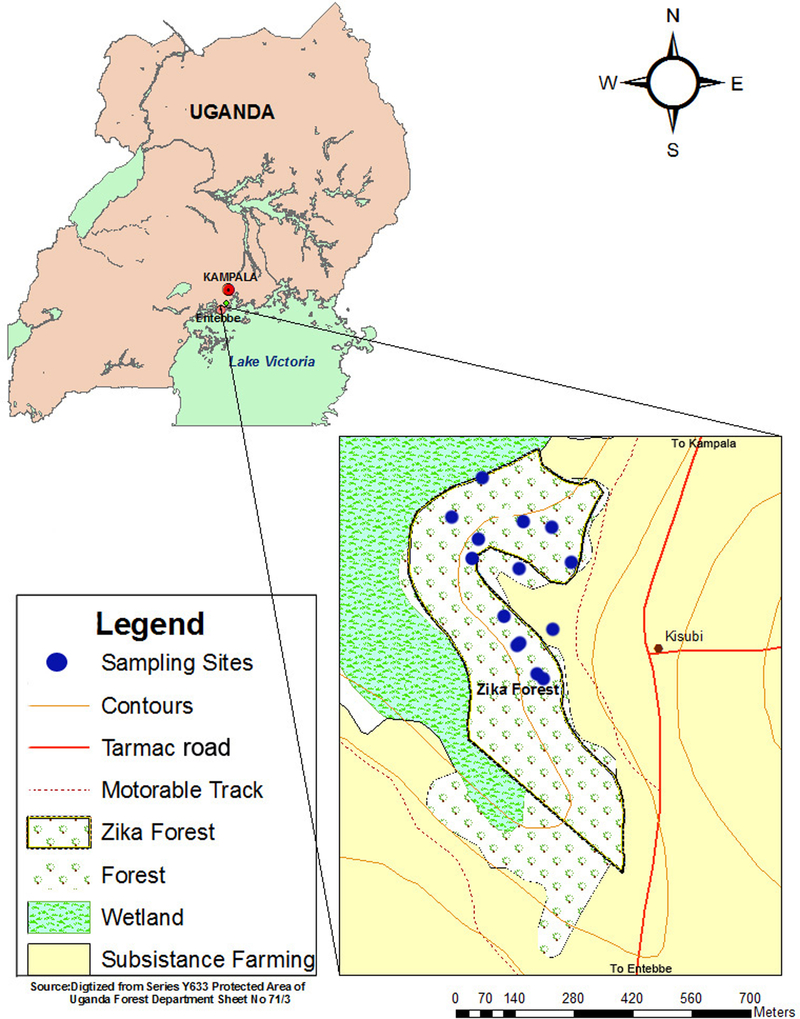
Zika Forest is a small isolated tropical forest located between coordinates (32” 30’ E and 0” 7’ N) and approximately 6 km (3.7 mi) from Entebbe. Zika Forest is a property of the Uganda Virus Research Institute, Entebbe (UVRIE) and it is protected and restricted to scientific research activities. The forest covers approximately 25 hectares (61.8 acres) and forms part of a narrow sinuous strip skirting the extensive grass and papyrus swamps of Waiya Bay, a sheltered inlet of Lake Victoria near Entebbe (Fig. 1). There is a 36.6 m (120 ft) steel tower located within the forest that was set up in 1960 to study the stratification of mosquito activities especially the principal YFV sylvan vector Ae. africanus (Kirya et al. 1977). Zika Forest is particularly suitable for mosquito work because it combines several ecosystems including hill-slope forest and very wet swamp-forest which comprises of a wide variety of mosquito habitats (Corbet 1961) and it is easily accessible; only 6 km from UVRIE.
Fig. 1:
Location of Zika forest (study area) in Entebbe, Uganda showing sampling sites.
Sampling
Mosquitoes sampling was conducted from July 2009 through June 2010 and the mosquitoes were collected following 3 different sampling protocols. In the first protocol we collected mosquitoes at different heights in the forest from 6.1 to 36.6 m (20 – 120 ft) above the ground. These studies were conducted on the steel tower. In the second and third protocols mosquitoes were collected at the ground level, approximately 1 m above the ground. In the second protocol collections were conducted at different locations inside the forest and in the third protocol mosquitoes were collected along the forest edge. Three different methods were used to collect mosquitoes, carbon dioxide (CO2)-baited CDC-light trap and human landing collections were conducted weekly throughout the sampling period, and ovitrap collections were conducted once every 3 months. Eggs collected by the ovitraps were reared to adults and identified to species. All identifications were conducted by using the keys of Edwards (1941), de Meillon (1947), Gillett (1972), Gilles and Coetzee (1987) and Jupp (1996). Climate parameters including temperature, rainfall and humidity were recorded at each time of sampling. Rainfall and temperature data was obtained from a weather station at Kisubi, located approximately 500 m (0.3 mi) from Zika forest. The relative humidity was measured by using a thermometer hygrometer (Viking AB, Sweden).
Statistical analysis
Analysis for mosquito composition was done using the program R 2.10 (R Development team 2010) and Past (Hammer et al. 2001). Differences in abundance of mosquitoes during the year were evaluated using non-parametric multivariate analysis of Variance (NPMANOVA) and the SIMPER test (Analysis of Similarity and Differences between Sites). Means were compared by (NPMANOVA) and when significantly different, they were exposed to the SIMPER test for the analysis of differences between mosquito species in the months and sites. Correlations between temperature, rainfall, relative humidity and numbers of mosquitoes collected over the year and sampling sites were determined using non parametric (Pearson-product moment) correlation analysis.
RESULTS
A total of 163,790 adult mosquitoes belonging to 10 genera and 61 species were collected (Table 1). The highest proportion of the collections were from CDC light traps (97.8%), followed by human landing catches (1.28%) and ovitraps (0.95%). Of the 61 species collected, 56 were collected using CDC light traps, 24 in ovitraps and 9 in humanlanding catches. Six species, Coquillettidia (Coquillettidia) fuscopennata (Theobald), Coquillettidia (Coquillettidia) metallica (Theobald), Culex (Culex) annulioris Theobald, Coquillettidia (Coquillettidia) pseudoconopas (Theobald), Coquillettidia (Coquillettidia) aurites (Theobald) and Coquillettidia (Coquillettidia) maculipennis (Theobald) were collected in relatively high abundance in the light traps (Table 1).
Table 1.
Mosquito species collected in Zika forest, Uganda, from July 2009 through August 2010 by using CO2-baited light traps, human-baited catches and ovitraps.
| Genus | Subgenus | Species | Light traps | Human | Ovitraps | Totals |
|---|---|---|---|---|---|---|
| Aedes | (Aedimorphus) | albocephalus (Theobald) | 2 | 0 | 0 | 2 |
| Aedes | (Aedimorphus) | argenteopunctatus (Theobald) | 8 | 0 | 0 | 8 |
| Aedes | (Aedimorphus) | cumminsii (Theobald) | 12 | 0 | 0 | 12 |
| Aedes | (Aedimorphus) | marshalii (Theobald) | 1 | 0 | 0 | 1 |
| Aedes | (Aedimorphus) | tarsalis (Newstead) | 15 | 0 | 0 | 15 |
| Aedes | (Aediomyia) | furfurea (Enderlein) | 32 | 1 | 0 | 33 |
| Aedes | (Dunnius) | spp | 1 | 0 | 0 | 1 |
| Aedes | (Finlaya) | ingrami Edwards | 19 | 108 | 0 | 127 |
| Aedes | (Finlaya) | longipalpis (Gruenberg) | 4 | 0 | 0 | 4 |
| Aedes | (Neomelaniconion) | circumluteolus (Theobald) | 25 | 0 | 0 | 25 |
| Aedes | (Neomelaniconion) | luridus McIntosh | 1 | 0 | 0 | 1 |
| Aedes | (Neomelaniconion) | mcintoshi Huang | 36 | 0 | 0 | 36 |
| Aedes | (Stegomyia) | aegypti formosus (Walker) | 0 | 0 | 10 | 10 |
| Aedes | (Stegomyia) | africanus (Theobald) | 98 | 1380 | 1199 | 2678 |
| Aedes | (Stegomyia) | apicoargenteus (Theobald) | 9 | 0 | 79 | 88 |
| Aedes | (Stegomyia) | metallicus (Edwards) | 0 | 1 | 0 | 1 |
| Anopheles | (Anopheles) | coustani Laveran | 109 | 0 | 0 | 109 |
| Anopheles | (Anopheles) | implexus (Theobald) | 1908 | 0 | 0 | 1908 |
| Coquillettidia | (Coquillettidia) | aurites (Theobald) | 11212 | 157 | 0 | 11369 |
| Coquillettidia | (Coquillettidia) | fraseri (Theobald) | 3970 | 5 | 0 | 3975 |
| Coquillettidia | (Coquillettidia) | fuscopennata (Theobald) | 38599 | 162 | 0 | 38761 |
| Coquillettidia | (Coquillettidia) | maculipennis (Theobold) | 9199 | 18 | 0 | 9217 |
| Coquillettidia | (Coquillettidia) | metallica (Theobald) | 29560 | 2 | 0 | 29652 |
| Coquillettidia | (Coquillettidia) | pseudoconopas (Theobald) | 19710 | 7 | 0 | 19717 |
| Culex | (Culex) | antennatus (Becker) | 246 | 3 | 0 | 249 |
| Culex | (Culex) | neavei Theobald | 179 | 0 | 0 | 179 |
| Culex | (Culex) | perfscus Edwards | 12 | 0 | 0 | 12 |
| Culex | (Culex) | pipiens Linnaeus | 280 | 58 | 53 | 391 |
| Culex | (Culex) | quinquefasciatus Say | 10 | 0 | 0 | 10 |
| Culex | (Culex) | univittatus Theobald | 1891 | 0 | 0 | 1891 |
| Culex | (Culex) | vansomereni Edwards | 93 | 0 | 0 | 93 |
| Culex | (Culex) | spp. | 46 | 20 | 12 | 78 |
| Culex | (Culiciomyia) | cinerellus Edwards | 321 | 1 | 0 | 321 |
| Culex | (Culiciomyia) | cinereus Theobald | 218 | 15 | 184 | 417 |
| Culex | (Culiciomyia) | nebulous Theobald | 497 | 0 | 0 | 497 |
| Culex | (Eumelanomyia) | horridus Edwards | 3 | 0 | 0 | 3 |
| Culex | (Eumelanomyia) | insignis (Carter) | 1922 | 3 | 0 | 1925 |
| Culex | (Eumelanomyia) | kingianus Edwards | 1 | 0 | 0 | 1 |
| Culex | (Kitzmillera) | moucheti Evans | 166 | 0 | 11 | 177 |
| Culex | (Lutzia) | tigripes De Grandpre & De Charmoy | 5 | 7 | 0 | 12 |
| Culex | (Oculeomyia) | annulioris (Edwards) | 22974 | 8 | 0 | 22982 |
| Culex | (Oculeomyia) | bitaeniorynchus Giles | 1 | 0 | 0 | 1 |
| Culex | (Oculeomyia) | poicilipes (Theobald) | 121 | 10 | 0 | 131 |
| Eretmapodites | chrysogaster Graham | 15 | 0 | 5 | 20 | |
| Hodgesia | (Hodgesia) | cyptopus Theobald | 129 | 0 | 0 | 129 |
| Hodgesia | (Hodgesia) | spp. | 390 | 2 | 0 | 392 |
| Mansonia | (Mansoniodes) | africana (Theobald) | 3138 | 32 | 0 | 3170 |
| Mansonia | (Mansoniodes) | africana nigerrima Theobald | 4053 | 0 | 0 | 4053 |
| Mansonia | (Mansoniodes) | uniformis (Theobald) | 1510 | 33 | 0 | 1543 |
| Mimomyia | (Mimomyia) | hispida (Theobald) | 252 | 0 | 0 | 252 |
| Mimomyia | (Mimomyia) | mimomyiaformis (Newstead) | 28 | 0 | 0 | 28 |
| Mimomyia | (Mimomyia) | splendens Theobald | 1 | 0 | 0 | 1 |
| Mimomyia | (Mimomyia) | plumosa (Theobald) | 4 | 0 | 0 | 4 |
| Mimomyia | (Etorleptiomyia) | mediolineata (Theobald) | 4 | 0 | 0 | 4 |
| Toxorhynchites | (Toxorhynchites) | brevipalpis Theobald | 0 | 0 | 8 | 8 |
| Uranotaenia | (Uranotaenia) | alboabdominalis Theobald | 4001 | 0 | 0 | 4001 |
| Uranotaenia | (Uranotaenia) | connali Edwards | 210 | 0 | 0 | 210 |
| Uranotaenia | (pseudofilcabia) | mashonaensis Theobald | 1037 | 0 | 0 | 1037 |
| Uranotaenia | (pseudofilcabia) | nigromaculata Edwards | 30 | 0 | 0 | 30 |
| Uranotaenia | (pseudofilcabia) | nivipous Theobald | 30 | 0 | 0 | 30 |
| Uranotaenia | (Uranotaenia) | spp. | 1766 | 0 | 0 | 1766 |
| Total | 160,134 | 2,095 | 1,561 | 163,790 | ||
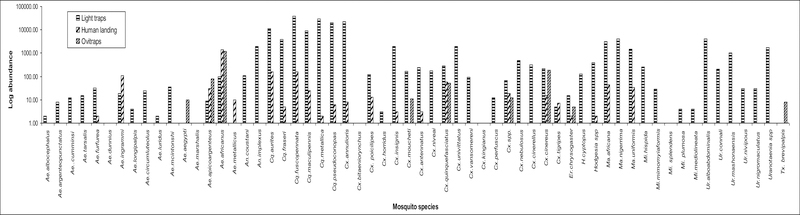
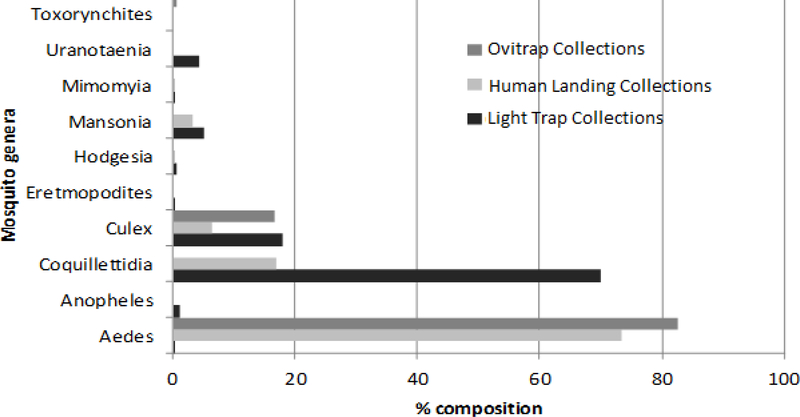
The most abundant species in human landing collections were Ae. africanus which comprised 65.9% of the total collections followed by Cq. fuscopennata (7.7%), Cq. aurites (7.5%) and Aedes (Finlaya) ingrami Edwards (5.2%). The most abundant species in ovitrap collections were Ae. africanus which comprised 76.8% of the total collections followed by Culex (Culiciomyia) cinereus Theobald (11.8%) and Aedes (Stegomyia) apicoargenteus (Theobald) (5.1%). All the other species combined were less than 5% of the total ovitrap collections. Only three species were collected by all the three methods, namely; Ae. africanus, the Culex (Culex) quinquefasciatus Say and Cx. cinereus (Table 1). Of the 61 species collected, 21 were collected in both light traps and human-baited catches, but only one species, Culex (Culex) moucheti Evans, was collected in light traps and ovitraps (Table 1). Mosquitoes in the genera Culex, Hodgesia and Mansonia were frequently captured in human-baited catches and light trap collections however species in the genera Mimomyia and Uranotaenia were only collected in light traps (Table 1). The Aedes species were collected in very low numbers in the light traps compared to human-bait and ovitrap methods. Toxorynchites species were only collected in ovitraps (Table 1).
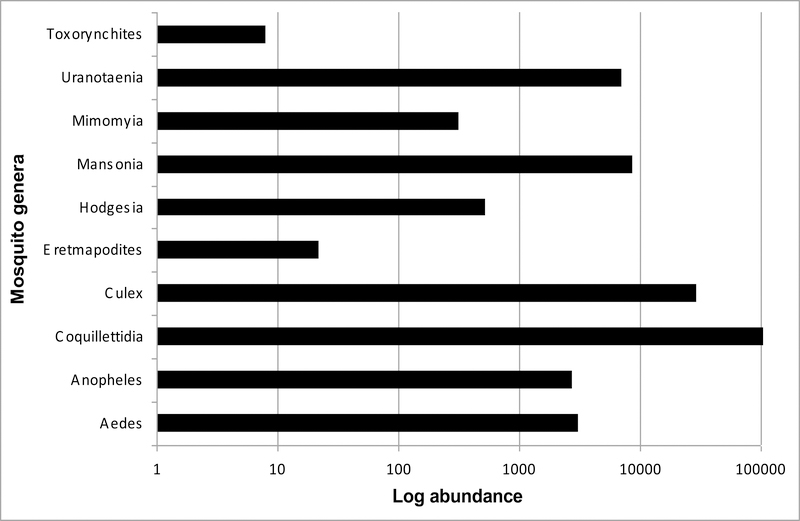
Aedes africanus was the most frequently captured species in human-baited catches and in ovitrap collections. The highest number of genera, species and number of mosquitoes were collected in light traps. The highest number of mosquitoes collected was in the genus Coquillettidia and mostly in light traps collections (Fig. 2, 3 and 4), while the majority of the Aedes species were collected in human-baited catches and in ovitraps (Fig. 2). Overall, the most abundant mosquito species collected was Cq. fuscopennata which was 24% of the total collections followed by Cq. metallica (18%), Cx. annulioris (14%) and Cq. pseudoconopas (12%) (Table 1). Coquillettidia metallica was the most frequently collected species from April through June 2010 (Fig. 5). In human-baited catches and ovitrap collections, the most frequently collected species were in the genus Aedes and the most abundant species in these collections was Ae. africanus, The Aedes (Stegomyia) aegypti group and Toxorhynchites (Toxorhynchites) brevipalpis Theobald were only collected in ovitraps (Fig. 2).
Fig 2:
Abundance of mosquito species collected in Zika forest in 2009 and 2010. Oviposition trap, Human landing & Carbon dioxide-baited light trap collections.
Fig. 3:
Mosquito genera collected in Zika forest in 2009 and 2010.
Fig. 4:
Percentage composition of mosquito collections in the light trap, human landing and ovitrap collections in Zika forest, 2009–2010.
Fig 5:
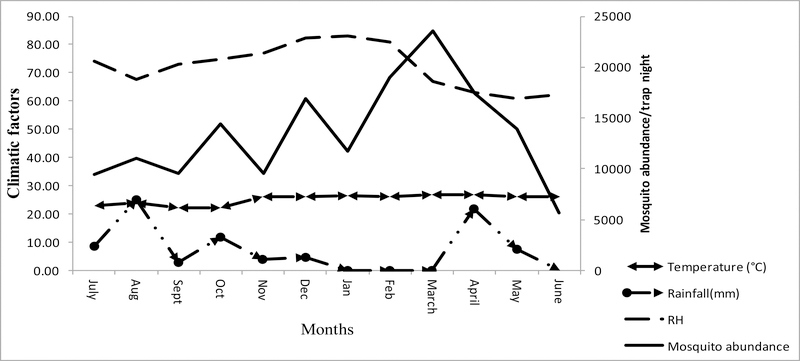
Change in mosquito abundance with climatic parameters (temperature, rainfall and relative humidity) over the sampling period.
More mosquitoes were collected between January and May 2010 (Fig. 5) and there were significant differences between mosquitoes collected over the months (NPMANOVA, p <0.005, p=0.0001). The highest numbers of mosquitoes were collected at Sites 1 – 4 at ground level inside the forest and the least number of mosquitoes were collected at 30.5 m (100 ft) and 36.6 m (120 ft) stations on the tower. Relative humidity (r = 0.065, P ≤ 0.05) and temperature (r = 0.396, P ≤ 0.05) showed positive correlations with mosquito abundance which suggests that both humidity and temperature increased mosquito activity. On the other hand, rainfall had a significant negative effect (r = −0.017, P ≤ 0.05) on the number of mosquitoes collected suggesting that thunderstorms inhibited mosquito activity.
Status of mosquito fauna before the 1970’s compared with present
In Table 2 we summarize mosquito species that have previously been collected at Zika forest and the species we collected in 2010. Over 82 mosquito species were collected from Zika forest from 1955 to 1965 (Table 2). In the present study only 61 mosquito species were captured, but of these 24 were captured in Zuka Forest for the first time, including: Aedes (Aedimorphus) albocephalus (Theobald), Aedes (Aedimorphus) argenteopunctatus (Theobald), Aedes (Aedimorphus) marshalii (Theobald), Aedes (Neomelaniconion) luridus McIntosh, Aedes (Neomelaniconion) mcintonshi Huang, Aedes (Stegomyia) aegypti formosus (Walker), Aedes (Stegomyia) metalicus (Edwards), Culex (Culex) antennatus (Becker), Culex (Culex) neavei Edwards, Culex (Culex) perfuscus Edwards, Culex (Culex) pipiens Linnaeus, Cx. quiquefasciatus, Culex (Culex) vansomereni Edwards, Culex (Eumelanomyia) horridus Edwards, Culex (Eumelanomyia) kingianus Edwards, Culex (Oculeomyia) bitaeniorynchus Giles, Mimomyia (Mimomyia) hispida (Theobald), Mimomyia (Mimomyia) mimomyiaformis (Newstead), Mimomyia (Mimomyia) plumosa (Theobald), Mimomyia (Mimomyia) splendens Theobald, Mimomyia (Etorleptiomyia) mediolineata (Theobald), Uranotaenia (Uranotaenia) connali Edwards, Uranotaenia (Pseudofilcabia) mashonaensis Theobald, Uranotaenia (Pseudofilcabia) nigromaculata Edwards and Uranotaenia (Pseudofilcabia) nivipous Theobald were captured in Zika for the first time (Table 2). However, 20 species previously reported for Zika Forest were not represented in our collections, including: Anopheles (Anopheles) paludis Theobald, Coquillettidia (Coquillettidia) versicolor (Edwards), Culex (Eumelanomyia) fimbriforceps Edwards, Culex (Culex) guiarti Blanchard, Culex (Culex) ingrami Edwards, Culex (Culiciomyia) macfiei Edwards, Culex (Culiciomyia) semibrunneus Edwards, Culex (Eumelanomyia) subrima Edwards, Mimomyia (Mimomyia) femorata (Edwards), Ficalbia circumtestacea (Theobald), Eretmapodites vansomereni Hamon, Eretmapodites oedipodeius Graham, Toxorhynchites (Toxorhynchites) kaimosi (Van someren), Aedes (Neomelaniconion) taeniorostris (Theobald), Uranotaenia (Pseudoficalbia) fusca Theobald, Uranotaenia (Uranotaenia) pallidocephala Theobald, Aedes (mucidus) nigerrimus (Theobald), Aedes (Aedimorphus) domesticus (Theobald), Eretmapodites quinquevittatus Theobald, Aedes (mucidus) grahamii (Theobald) previous collected in Zika (Corbet 1961, Haddow et al. 1964, Haddow 1964, Haddow et al. 1968) (Table 2).
Table 2.
Mosquito species collected in the present study, 2009–2010, compared to species collected in earlier years in Zika forest, Uganda.
| Period | |||||||
|---|---|---|---|---|---|---|---|
| Genus | Subgenus | Species | 1955–57 | 1960–61 | 1961–62 | 1964–65 | 2009–10 |
| Aedes | Aedimorphus | abnormalis (Theobald) a,c,f | - | - | + | - | - |
| albocephalus (Theobald) | - | - | - | - | + | ||
| argenteopunctatus (Theobald) | - | - | - | - | + | ||
| cumminsii (Theobald) c,d,f | - | + | - | - | + | ||
| domesticus (Theobald)a,c,f | + | - | - | - | - | ||
| marshalii (Theobald) | - | - | - | - | + | ||
| tarsalis (Newstead) c,f | - | - | + | - | + | ||
| Aediomyia | furfurea (Enderlein) c,f | - | - | + | - | + | |
| Dunnius spp. | - | - | - | - | + | ||
| Finlaya | ingrami Edwards a,b, c, d, f, g, h | + | + | + | + | + | |
| longipalpis (Guenberg) a,c,f,h | + | - | + | - | + | ||
| Mucidus | nigerrimus (Theobald) a,c,f,g,h | + | + | + | + | - | |
| grahamii (Theobald) a,c,h, | + | + | + | - | - | ||
| scatophagoides (Theobald)c,f | - | - | - | + | - | ||
| Neomelaniconion | circumluteolus (Theobald) a,c,f,h | + | - | + | - | + | |
| luridus McIntosh | - | - | - | - | + | ||
| mcintonshi Huang | - | - | - | - | + | ||
| Stegomyia | aegypti (Walker) | - | - | - | - | + | |
| africanus (Theobald) a,b,c,f,gh | + | + | + | + | + | ||
| apicoargenteus (Theobald) a,b,c,d,f,g,h | + | + | + | + | + | ||
| metallicus (Edwards) | - | - | - | - | + | ||
| Anopheles | Anopheles | coustani Laveran | - | + | - | - | + |
| implexus (Theobald)a,b,c,f,h | + | + | + | - | + | ||
| obscurus (Grunberg)c,f | - | - | + | - | - | ||
| paludis Theobald a,b,c,d,f,g | - | - | + | + | - | ||
| Cellia | gambiae Giles a,c,f | - | + | - | - | - | |
| marshallii (Theobald) c,f | - | - | + | - | - | ||
| moucheti Evans, g | - | - | - | + | - | ||
| wellcomei Theobald c | - | - | + | - | - | ||
| Coquillettidia | Coquillettidia | aurites (Theobald) a,b,c,d,f,g,h | + | + | + | + | + |
| fraseri (Theobald)a,b,c,d,f,g | - | + | + | - | + | ||
| fuscopennata (Theobald) a,b,c,d,f,g,h | + | + | + | + | + | ||
| maculipennis (Theobald) a,b,c,d,f,g,h | + | + | + | + | + | ||
| metallica (Theobald) a,b,c,d,f,g,h | + | + | + | + | + | ||
| pseudoconopas (Theobald) a,b,c,d,f,g,h | + | + | + | + | + | ||
| Culex | Culex | antennatus (Becker) | - | - | - | - | + |
| guiarti Blanchard a,b,d,h | + | - | - | - | - | ||
| macfiei Edwards b,e | - | - | - | + | - | ||
| neavei Edwards | - | - | - | - | + | ||
| perfuscus Edwards | - | - | - | - | + | ||
| quiquefasciatus Say | - | - | - | - | + | ||
| subrima Edwards b | - | - | - | + | - | ||
| univittatus Theobald a,b,g | - | - | - | + | + | ||
| vansomereni Edwards | - | - | - | - | + | ||
| Culiciomyia | cinerellus Edwardsf | - | - | + | + | + | |
| cinereus Theobald a,d,e | - | - | + | + | + | ||
| nebulosus Theobalda,b,g,h | + | - | - | + | + | ||
| Eumelanomyia | horridus Edwards | - | - | - | - | + | |
| insignis (Carter) a,c,f, g | - | + | + | + | + | ||
| kingianus Edwards a | - | - | - | - | + | ||
| Kitzmilleria | moucheti Evans a,b,h | + | - | - | - | + | |
| Lutzia | tigripes De Grandpre and De Charmoy ,h | + | - | - | - | + | |
| Oculeomyia | annulioris (Edwards) a,b,c,g,h | + | + | + | + | + | |
| bitaeniorynchus Giles | - | - | - | - | + | ||
| poicilipes (Theobald)c,f,g | - | - | + | + | + | ||
| Eretmapodites | chrysogaster Graham a,b,c,d,f,h | + | + | + | - | + | |
| oedipodeios Graham a,b,c,f,h | + | + | + | - | - | ||
| quinquevittatus Theobald a,c,f | - | - | + | - | - | ||
| Filcabia | uniformis (Theobald) h | + | - | - | - | - | |
| Hodgesia | Hodgesia | cyptopus Theobald a,b,c,d,f,g,h | + | + | + | + | + |
| Malaya | taeniorostris (Theobald) a,b | - | - | - | + | - | |
| Mansonia | Mansonoides | africana africana (Theobald) a,b,c,d,f,g,h | + | + | + | + | + |
| africana nigerrima Theobald a,b,c,d,f,g,h | - | - | - | - | + | ||
| uniformis (Theobald) a,b,c,d,f,g,h | + | + | + | + | + | ||
| Mimomyia | Mimomyia | hispida (Theobald) a | - | - | - | - | + |
| mimomyiaformis (Newstead) | - | - | - | - | + | ||
| plumosa (Theobald) a | - | - | - | - | + | ||
| splendens Theobald | - | - | - | - | + | ||
| Etorleptiomyia | mediolineata (Theobald) | - | - | - | - | + | |
| Toxorhynchites | Toxorhynchites | brevipalpis Theobald | - | - | - | - | + |
| Uranotaenia | Uranotaenia | alboabdominalis Theobald b,d,h | + | - | - | - | + |
| caeruleocephala Theobald h | + | - | - | - | - | ||
| connali Edwards | - | - | - | - | + | ||
| pallidocephala Theobald b,d | - | - | - | + | - | ||
| Pseudofilcabia | mashonaensis Theobald | - | - | - | - | + | |
| nigromaculata Edwards d | - | - | - | - | + | ||
| nivipous Theobald | - | - | - | - | + | ||
Goma 1964
Species without references were collected in Zika Forest for the first time in 2009–2010.
DISCUSSION
Our data shows variations in the species composition of Zika Forest from the last published species lists more than 40 years ago. Twenty-one of the 24 species collected in Zika Forest for the first time during our studies were captured using light traps (Tables 1 and 2). The primary collection method used in Zika Forest before the mid 1970s was human landing catches and this may have precluded these species from previous detection in Zika Forest. In addition, Ae. luridus, Ae. marshalii, Cx. kingianus, Cx. bitaeniorynchus, Mi. splendens, Mi. plumosa and Mi. mediolineata were each represented by less than 5 specimens in our collections (Table 1) suggesting that these species are extremely rare in Zika Forest or difficult to detect. However, some species especially in the genera Mimomyia and Uranotaenia were captured in relatively high abundance (Fig. 2) suggesting that these species are abundant in Zika Forest, but probably only readily collected in light traps. Three species detected for the first time in Zika Forest but captured by methods other than CO2-baited light traps included Ae. aegypti formosus. However, there is the possibility that the Ae. aegypti aegypti previously reported in Zika forest was Ae. aegypti formosus since the two species are morphologically very similar and all recent mosquito surveys have not detected Ae. aegypti aegypti in Uganda. The fact that 1% of Cx. antennatus were captured in human landing catches and the rest in CO2 baited light traps suggested that this species may have previously been collected in Zika Forest but, there was a tendency of lumping together and processing Culex mosquitoes collected in human landing catches as Culex species and not identifying them to species (Haddow et al. 1964). Aedes metallicus was only captured in human landing catches (Table 1) but, only one specimen was collected in twelve months (Table 1) suggesting that this species is extremely rare in Zika Forest. It is possible that Ae. metallicus has been present in Zika Forest but in low densities and has been undetected until our studies in 2009 – 2010. Alternatively, Ae. metallicus may have been recently introduced to the areas by the human activity around Zika Forest. However, the fact that all 24 species have previously been captured in other regions of the country (Corbet 1961, Haddow 1945, 1946, 1948, 1954, Simpson et al. 1965, Haddow and Ssenkubuge 1965, 1974, Lutwama 2000) shows that all these species are native to Uganda and their recent detections in Zika forest do not represent new introductions to the country.
The 20 species that were previous collected in Zika by Corbet (1961), Haddow et al. (1964) and Haddow et al. (1968) (Table 2) and not detected in our collections may reflect variations in species composition or variations in relative abundance from year to year in Zika Forest, or localized extinction in Zika Forest during the last 40 years.
Of the 61 mosquito species we collected in Zika Forest, arboviruses of public health importance have previously been isolated from at least 31 (50.8%). The arboviruses associated with these species include; Chikungunya virus (Weinbren et al. 1958), Zika virus (Weinbren and Williams 1958), Rift Valley Fever virus (Haddow et al. 1964), O’nyong-Nyong virus (Rwaguma et al. 1997, Lanciotti et al. 1998, Kiwanuka et al. 1999, Lutwama et al. 1999, Sanders et al. 1999), Sindbis virus (Woodall 1964), Bunyamwera virus (Smithburn et al. 1946), Wesselsbron virus and Banzi virus (Henderson et al. 1968), Ntaya virus (Smithburn and Haddow 1951), Semliki Forest virus (Finter 1964), West Nile virus and Usutu virus (Smithburn et al. 1940, Williams et al. 1964), Witwatersrand virus and Germiston virus (Monath et al. 1972), and Uganda S virus (Dick and Haddow 1952). This suggests a high potential for transmission and maintenance of a wide range of arboviruses of public health importance within Zika Forest.
Acknowledgements
We wish to thank F. Ssenfuka, T. Muwawu, J.-F. Lwanga, J. Mugga, and S. Wakaalo for their assistance in the field and in the laboratory. We thank R. Kading. M. Crabtree of the Centers for Disease Control and Prevention (CDC) and the entomology team at KEMRI, Nairobi for their assistance with mosquito identification. We thank the Uganda Virus Research Institute and the Department of Biological Sciences, Makerere University for financial assistance. This research was partly funded by in part by UVRIE, the CDC and by a grant from the Organization for Women in Science for the Developing World (OWSDW) formerly TWOWS to M. Kaddumukasa.
References Cited
- Chazdon RL, Harvey CA, Komar O, Griffith DM, Ferguson BG, Martínez‐Ramos M, Morales H, Nigh R, Soto‐Pinto L, and Van Breugel M 2009. Beyond reserves: A research agenda for conserving biodiversity in human‐modified tropical landscapes. Biotropica, 41: 142–153. [Google Scholar]
- Corbet PS 1961. Entomological studies from a high tower in Mpanga forest, Uganda. Trans. R. Entomol. Soc. Lond 113: 356–368. [Google Scholar]
- Corbet PS 1964. Observations on mosquitoes ovipositing in small containers in Zika Forest, Uganda. J. An. Ecol 141–164. [Google Scholar]
- De Meillon B 1947. The Anophelini of the Ethiopian geographical region South African Institute for Medical Research, Johannesburg, Repubulic of South Africa. [Google Scholar]
- Dick GW and Haddow AJ 1952. Uganda S virus; a hitherto unrecorded virus isolated from mosquitoes in Uganda. I. Isolation and pathogenicity. Trans. R. Soc. Trop. Med. Hyg 46: 600–618. [DOI] [PubMed] [Google Scholar]
- Edwards FW 1941. Mosquitoes of the Ethiopian Reqion. III. Culicine adults and pupae. British Museum (Natural History), London, United Kingdom. [Google Scholar]
- Gillett JD 1972. Common African mosquitoes and their medical importance. William Heinemann Medical Books Limited, London, United Kingdom. [Google Scholar]
- Gillies MT and Coetzee M 1987. A Supplement to the Anophelinae of Africa South of the Sahara (Afrotropical Region) The South African Institute of Medical Research, Johannesburg, South Africa. [Google Scholar]
- Goma LKH 1965. The flight activity of some East African mosquitos (Diptera, Culicidae). I.—Studies on a high steel tower in Zika Forest, Uganda. Bull. Entomol. Res 56: 17–35. [DOI] [PubMed] [Google Scholar]
- Haddow AJ 1945. On the mosquitoes of Bwamba County Uganda. I. Biting activity, with special reference to the influence of microclimate. Bull. Entomol. Res 36: 33–73. [Google Scholar]
- Haddow AJ 1946. The mosquitoes of Bwamba county, Uganda. IV. - Studies on the genus Eretmapodites, Theobald. Bull. Entomol. Res 37: 57–82. [Google Scholar]
- Haddow AJ 1948. The mosquitoes of Bwamba County, Uganda. VI. Mosquitoes breeding in plant axils. Bull. Entomol. Res 39: 185–212. [DOI] [PubMed] [Google Scholar]
- Haddow AJ 1954. Studies of the biting-habits of African mosquitos. An appraisal of methods employed, with special reference to the twenty four-hour catch. Bull. Entomol. Res 45: 199–242. [Google Scholar]
- Haddow AJ 1963. Entomology field studies at Zika. East African Virus Research Institute report 1964–1965. [Google Scholar]
- Haddow AJ, Williams MC, Woodall JP, Simpson DIH and Goma LKH 1964. Twelve Isolations of Zika Virus from Aedes (Stegomyla) africanus (Theobald) taken in and above a Uganda Forest. Bull. Wld. Hlth. Org 31: 57–69. [PMC free article] [PubMed] [Google Scholar]
- Haddow AJ 1964. Observations on the biting habits of mosquitoes in the forest canopy at Zika, Uganda, with special reference to crepuscular periods. Bull. Entomol. Res 55: 589–608. [Google Scholar]
- Haddow AJ and Ssenkubuge Y 1965. Entomological studies from a high steel tower in Zika Forest, Uganda. Part I The biting activity of mosquitoes and Tabanids as shown by twenty-four catches. East African Virus Research Institute Report 1964–1965. [Google Scholar]
- Haddow AJ and Ssenkubuge Y 1965. The mosquitoes of Bwamba County, Uganda. X. Observations on the biting behaviour of these species in the Entebbe area. Trans. R. Entomol. Soc. Lond 117: 215–243. [Google Scholar]
- Haddow AJ, Casley DJL, O’sullivan JP, Ardoin PML, Ssenkubuge Y and Kitama A 1968. Entomological studies from a high steel tower in Zika Forest,Uganda. Part II. The biting activity of mosquitoes above the forest canopy in the hour after sunset. Trans. R. Entomol. Soc. Lond 120: 212–236. [Google Scholar]
- Haddow AJ and Ssekubuge Y 1974. The mosquitoes of Bwamba county, Uganda. X. Observations on the biting behaviour of Anopheles spp. other than A. gambiae Giles, with notes on the behaviour of these species in the Entebbe area. Bull.Entomol. Res 64: 45–51. [Google Scholar]
- Hammer O, Harper DAT and Ryan PD 2001. Past: Paleontological statistics software package for education and data analysis. Educ. Pal. Elect 4: 1–9. [Google Scholar]
- Jupp PG 1996. Mosquitoes of Southern Africa: Culicinae and Toxorynchitinae. Ekoglide Publishers, Repubulic of South Africa. [Google Scholar]
- Kirya BG, Mukwaya LG and Sempala SDK 1977. A yellow fever epizootic in Zika Forest, Uganda, during 1972: Part 1: Virus isolation and sentinel monkeys. Trans. R. S. Trop. Med. Hyg 71: 254–260. [DOI] [PubMed] [Google Scholar]
- Kiwanuka N, Sanders EJ, Rwaguma EB, Kawamata J, Ssengooba FP, Najjemba R, Were WA, Lamunu M, Bagambisa G and Burkot TR 1999. O’Nyong-Nyong Fever in South-Central Uganda, 1996—1997: Clinical features and validation of a clinical case definition for surveillance purposes. Clin. Infect. Dis 29: 1243–1250. [DOI] [PubMed] [Google Scholar]
- Koh LP 2007. Impacts of land use change on South‐east Asian forest butterflies: a review. J. App. Ecol 44: 703–713. [Google Scholar]
- Lanciotti RS, Ludwig ML, Rwaguma EB, Lutwama JJ, Kram TM, Karabatsos N, Cropp BC and Miller BR 1998. Emergence of Epidemic O’nyong-nyong Fever in Uganda after a 35-Year Absence: Genetic Characterization of the Virus 1. Virology. 252: 258–268. [DOI] [PubMed] [Google Scholar]
- Lutwama JJ 2000. Type localities of Mosquito species in Uganda. Ins. Sci. Appl 20: 51–61. [Google Scholar]
- Lutwama JJ, Kayondo J, Savage HM, Burkot TR and Miller BR 1999. Epidemic O’Nyong-Nyong fever in southcentral Uganda, 1996–1997: entomologic studies in Bbaale village, Rakai District. Am. J. Trop. Med.Hyg 61:158–162. [DOI] [PubMed] [Google Scholar]
- Monath TPC, Henderson BE and Kirya GB 1972. Characterization of viruses (Witwatersrand and Germiston) isolated from mosquitoes and rodents collected near Lunyo Forest, Uganda, in 1968. Arch. Virol 38: 125–132. [DOI] [PubMed] [Google Scholar]
- Nichols E, Larsen T, Spector S, Davis A, Escobar F, Favila M, and Vulinec K 2007. Global dung beetle response to tropical forest modification and fragmentation: a quantitative literature review and meta-analysis. Biol. Cons. 137: 1–19 [Google Scholar]
- R Development Core Team. 2010. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: ISBN 3–900051-07–0, URL: http://www.R-project.org. R libraries and packages [Google Scholar]
- Rwaguma E, Lutwama J, Sempala S, Kiwanuka N, Kamugisha J, Okware S, Bagambisa G, Lanciotti R, Roehrig J and Gubler D 1997. Emergence of epidemic O’nyong-nyong fever in southwestern Uganda, after an absence of 35 years. Emerg. Infect. Dis. 3, 77–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders EJ, Rwaguma EB, Kawamata J, Kiwanuka N, Lutwama JJ, Ssengooba FP, Lamunu M, Najjemba R, Were WA and Bagambisa G 1999. O’nyong-nyong fever in south-central Uganda, 1996–1997: description of the epidemic and results of a household-based seroprevalence survey. J. Inf. Dis 180: 1436–1443. [DOI] [PubMed] [Google Scholar]
- Sawyer WA and Whitman L 1936. The yellow fever immunity survey of North, East and South Africa. Trans. R. Soc. Trop. Med. Hyg, 29, 397–412. [Google Scholar]
- Simpson DIH, Haddow AJ, Williams MC and Woodall JP 1965. Yellow fever in Central Uganda, 1964 Part IV. Investigations on blood-sucking dipteria and monkeys. Trans. R. Soc. Trop. Med. Hyg 59: 449–458. [DOI] [PubMed] [Google Scholar]
- Smithburn KC and Haddow AJ 1951. Ntaya Virus. A hitherto unknown agent isolated from mosquitoes collected in Uganda. Exp. Biol. Med 77: 130. [DOI] [PubMed] [Google Scholar]
- Smithburn KC, Haddow AJ and Mahaffy AF 1946. A neurotropic virus isolated from Aedes mosquitoes caught in the Semliki Forest. Am. J. Trop. Med. Hyg 1: 189–208. [DOI] [PubMed] [Google Scholar]
- Smithburn KC, Hughes TP, Burke AW and Paul JH 1940. A neurotropic virus isolated from the blood of a native of Uganda. Am. J. Trop. Med. Hyg 20: 471–492. [Google Scholar]
- Weinbren MP, Haddow AJ and Williams MC 1958. The occurrence of chikungunya virus in Uganda I. Isolation from mosquitoes. Trans. R. Soc. Trop. Med.Hyg 52: 253–262. [DOI] [PubMed] [Google Scholar]
- Weinbren MP and Williams MC 1958. Zika virus: further isolations in the Zika area, and some studies on the strains isolated. Trans. R. Soc. Trop. Medi. Hyg 52: 263–268. [DOI] [PubMed] [Google Scholar]
- Williams MC 1964. Studies on mosquitoes (Diptera, Culicidae) biting birds using twenty-four hour catches in the Entebbe area, Uganda. East African Virus Research Institute Report. 1963–1965. [Google Scholar]
- Williams MC, Simpson DI, Haddow AJ and Knight EM 1964. The isolation of West Nile Virus from man and of Usutu virus from the bird biting mosquito Mansonia aurites (Theobald) in the Entebbe area of Uganda. Ann. Trop. Med. Parasitol 58: 367–374. [DOI] [PubMed] [Google Scholar]
- Woodhall JP 1964. The viruses isolated from arthropods at the East African Virus Research Institute in the 26 years ending December 1963. Proc. E. Afric. Acad 2: 141–146. [Google Scholar]