Abstract
Atopic dermatitis (AD) is a common, chronic inflammatory skin disease characterized by impaired barrier function, eczematous dermatitis, and chronic pruritus. Mouse models have been heavily utilized to deepen our understanding of complicated disease mechanisms in AD, and also provide a pre-clinical platform prior to performing clinical interventional research on novel therapeutic agents in humans. However, what aspects of human AD these mouse AD models faithfully recapitulate is insufficiently understood. Herein, we categorized mouse AD models into three groups; 1) Inbred models, 2) genetically engineered mice in which genes of interest are overexpressed or deleted in a specific cell type, 3) models induced with topical application of exogenous agents. In order to maximize benefits from current murine AD models, understanding the strength or limitation of each model is essential to select a system suitable for the research question. We describe known and emerging AD mouse models and discuss the utilities and pitfalls of each system.
INTRODUCTION
Atopic dermatitis (AD) is a common, chronic inflammatory skin disease with underlying barrier impairment and is accompanied by severe pruritus and associated with type 2/22-mediated inflammation. Recent studies have begun to unveil and dissect the complex pathophysiology in AD, including the genetic basis for barrier impairment, diverse aspects of the dysregulated immune system, and the involvement of commensal microbiota, in particular, Staphylococcus aureus. Numerous AD mouse models have been generated over the years, each recapitulating one or more aspects of human AD (Fig. 1a). However, a considerable gap remains between what has been learned in mouse models and what information can be translated into humans. Better understanding of each AD mouse model may enable researchers to perform studies directly relevant to human AD pathogenesis and to identify or validate novel therapeutic targets. In order to reflect the spectrum of inflammation involved in classic and monogenic AD, as well as in AD mouse models, skin inflammation discussed herein is referred to as “eczematous dermatitis”.
Figure 1.
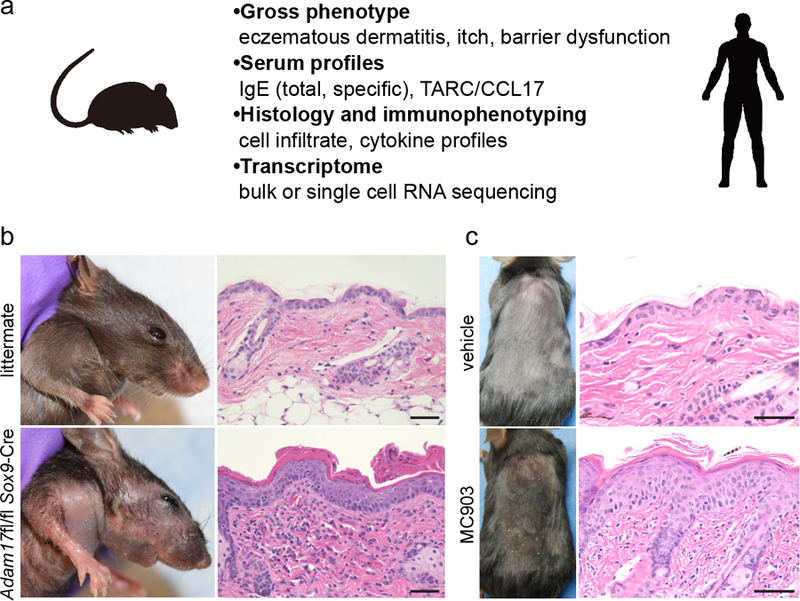
Examples of AD mouse models. (a) Features of AD mouse models that may be taken into consideration for phenotype analyses. (b) Gross phenotype and histology of an 8-week-old Adam17fl/fl Sox9-Cre mouse and a littermate control. (c) Phenotype and histology of MC903-induced AD-like inflammation. 45 μM of MC903 in ethanol was applied onto back skin of a C57BL/6 mouse every other day for 14 days. Scale bars = 50 μm.
MOUSE MODELS OF AD
Mouse AD models can be categorized into three groups; 1) inbred strains of mice that develop AD-like phenotypes, 2) genetically-engineered models with either ablation or overexpression of a single gene, either ubiquitously or in a certain cell lineage, and 3) AD-like phenotypes induced by exogenous agents. Understanding the strengths and limitations of each model would allow researchers to select a system that is suitable for a particular research question and to be aware of the caveats that need be considered.
Inbred models
Impaired skin barrier is a fundamental component of AD pathogenesis. Genetic studies have linked several chromosomal loci or genes involved in epidermal differentiation to risk of AD. FLG mutations (a genetic cause for ichthyosis vulgaris) contribute to barrier defect and represent a major predisposing factor for AD development in humans (Brown et al., 2012, Kezic et al., 2011). The flaky tail mice (ma/ma, Flgft/ft) harbor mutations in genes involved solely in keratinocyte homeostasis. These mice develop spontaneous eczematous dermatitis under specific pathogen-free (SPF) conditions with enhanced immune responses against percutaneous antigens (Fallon et al., 2009). Mutations in Flg and Tmem79 have been identified, the latter causing a defect in a component of lamellar granule assembly machineries, conferring both matted hair and spontaneous AD-like phenotypes (Sasaki et al., 2013, Saunders et al., 2013). Interestingly, segregation of the two mutated genes determined Tmem79, but not Flg, as the causative gene mutation that drove eczematous dermatitis. Consistently, genomic ablation of Flg is not sufficient for spontaneous onset of the AD-like phenotype, either under SPF conditions or upon S. aureus inoculation (Kobayashi et al., 2015), further indicating that at least one additional defect is required for the development of eczematous dermatitis (Kawasaki et al., 2012, Sasaki et al., 2013).
Another inbred strain is the NC/Nga mouse, in which pruritic skin lesions develop when they are maintained under conventional housing conditions (Matsuda et al., 1997). NC/Nga mice, like the flaky tail mice, exhibit pronounced type 2 immune responses. The genetic determinant in these mice appears to be localized in chromosome 9, which includes genes involved in immunity such as Thy1, Cd3d, Cd3e, Cd3g, Il10ra, Il18, and Csk (Kohara et al., 2001). Thus, these mice, in contrast to the flaky tail mice, might reflect the altered immune component of AD. Although the spontaneous nature of inbred mice may reflect the natural course in human AD, it is not trivial to pinpoint the underlying genetic defect. It should also be noted that genetic background-unique modifiers may either attenuate or aggravate phenotypes in any mouse model. Therefore, it is important that researchers be cognoscente about the genetic backgrounds of the mice and choose appropriate controls.
Genetically-engineered models
The overall complexity of AD pathogenesis and the vast numbers of secondary gene changes downstream of chronic inflammation hampers the narrowing down of genes that play central roles in AD pathogenesis. In this regard, transgenic and knockout (KO) or conditional KO (cKO) mice are valuable in elucidating the biological significance of the targeted molecules. Genetically-engineered mice with altered expression of AD-related genes would provide an approach to investigate the biological function of each molecule. The generation of genetically-engineered mice is time-consuming and costly, requiring strategic planning. A list of selected mouse strains with genetic modification is shown in Table 1.
Table 1.
Summary of the representative preclinical mouse models of human atopic dermatitis
| Category | advantages /limitations | Examples | Characteristics | References |
|---|---|---|---|---|
| Inbred models | ||||
| Pros: resembles natural course of human AD; enhanced percutaneous sensitization to haptens and allergens | Flaky tail (ma/ma, Flgft/ft) |
|
(Fallon et al., 2009, Sasaki et al., 2013, Saunders et al., 2013) | |
| Cons: lack of genetic information in some strains; variable induction protocols when combined with hapten- or allergen-challenges; some models do not spontaneously develop dermatitis under SPF conditions | NC/Nga |
|
(Kohara et al., 2001, Matsuda et al., 1997) | |
| Genetically-engineered models | ||||
| Pros: useful in elucidating gene-specific functions in vivo; powerful when crossed with other strains |
Overexpression IL-4 (K14-IL4 Tg) IL-13 (K5-tTA-IL13 Tg) |
|
(Chan et al., 2006, Zheng et al., 2009) | |
| Cons: time-consuming and expensive to generate; undesirable effect by unexpected gene expression or alteration (i.e. variable penetrance, inefficiency of Cre) | IL-31 (EF1α-IL31 or Eμ-Lck-IL31 Tg) |
|
(Dillon et al., 2004) | |
| TSLP (K5-rtTA-TSLP Tg) IL-18 (K14-IL18 Tg) IL-33 (K14-IL33 Tg) |
|
(Imai et al., 2013, Konishi et al., 2002, Yoo et al., 2005) | ||
| JAK1 (Jak1spade/spade) |
|
(Yasuda et al., 2016) | ||
|
Ablation ADAM17 (Adam17fl/fl Sox9Cre) |
|
(Kobayashi et al., 2015) | ||
| CARMA-1 (unmodulated) |
|
(Jun et al., 2003) | ||
| Induced models by exogenous agents | ||||
| Pros: time-controlled induction; applicable to various mouse strains Cons: non-standardized products for some allergens; variable protocols (doses and durations); labor-intensive (daily applications) |
Hapten- induced (ex. oxazolone, TNCB) Allergen-induced (ex. ovalbumin, house dust mite) |
|
(Kitagaki et al., 1995, Kitagaki et al., 1997, Matsuoka et al., 2003, Spergel et al., 1998, Wang et al., 2007) | |
| MC903 (calcipotriol)- induced |
|
(Kim et al., 2013, Li et al., 2006, Myles et al., 2016, Naidoo et al., 2018, Oetjen et al., 2017) | ||
Transgenic mice overexpressing type 2 cytokines, interleukin (IL)-4 or IL-13 in epidermis, develop spontaneous pruritus and chronic dermatitis. In both strains, skin lesions are characterized by prominent infiltration of T cells, mast cells, eosinophils, and macrophages, and total IgE and IgG1 are elevated in serum (Chan et al., 2001, Zheng et al., 2009). These models also recapitulate chronic epithelial and stromal changes observed in human AD such as acanthosis or dermal remodeling with fibrosis and increased vasculature. The efficacy of dupilumab, a monoclonal antibody that blocks the binding of these cytokines to their cognate receptor, emphasizes that these transgenic mice are effective AD models. Importantly, however, lymphoid cells, rather than keratinocytes, produce IL-4 and IL-13 in both mice and humans under physiological conditions.
IL-31, the predominant source of which are Th2 cells, is associated with pruritus and disruption of the physical skin barrier, and has recently gained attention as a novel therapeutic target in AD (Dillon et al., 2004, Feld et al., 2016, Ruzicka et al., 2017). Transgenic mice overexpressing IL-31, driven by the ubiquitous promoter for elongation factor-1α (EF1α), develop hair loss by 2 months of age and display dermatitis with prominent scratch behavior (Dillon et al., 2004).
Keratinocyte-derived cytokines may also play crucial roles during atopic inflammation. Thymic stromal lymphopoietin (TSLP) is a keratinocyte-derived, type 2 cytokine. A doxycycline-inducible, keratinocyte-specific transgenic expression of TSLP (K5-TSLP) in mice leads to the onset of AD-like skin lesions after 2–3 weeks of doxycycline treatment, with concomitant increase in serum total IgE and the type 2 immunity-associated chemokine, CCL17 (Yoo et al., 2005). Keratinocyte-specific expression of the IL-1 family of cytokines, IL-18 and IL-33, each also exhibit AD-like phenotypes (Imai et al., 2013, Konishi et al., 2002). Given the fact that TSLP transgenic mice lacking conventional T cells (K5-TSLP, TCRβ−/−) still develop skin inflammation and that the three keratinocyte-derived cytokines, TSLP, IL-18 and IL33, are important tissue-derived cytokines that activate group 2 innate lymphoid cells (ILC2), these models might be useful in studying the crosstalk between keratinocytes and innate immunity. An anti-IL-33 antibody is currently under clinical trial (NCT03738423, NCT03736967). While IL18 has not been associated with human AD in GWAS studies, loci including IL18R1 and IL18RAP have been reported, implicating the involvement of this cytokine (Tamari and Hirota, 2014).
The imbalance of skin commensal microbiota, termed dysbiosis, is now a recognized feature of human AD. While S. aureus colonization in AD skin has been known for over half a century, whether it contributed to pathogenesis, or was merely a result of chronic inflammation, had been debated. A mouse model that recapitulated this condition had been lacking. We recently reported using Adam17fl/fl Sox9-Cre mice, which lack a disintegrin and metalloproteinase 17 (ADAM17) in keratinocytes, that these mice spontaneously developed dysbiosis that was dominated by Corynebacterium species and S. aureus (Kobayashi et al., 2015). These mice display dry skin around 3–4 weeks after birth, then develop overt eczematous dermatitis at around 6 weeks (Fig. 1b). Eczematous dermatitis is preceded by the emergence of S. aureus, and targeting of the dysbiotic organisms with antibiotics extinguishes skin inflammation (Kobayashi et al., 2015). While eczematous dermatitis is less prominent in the absence of S. aureus in mice housed in facilities with stringent health status (unpublished observation), this can be taken advantage of by inoculating S. aureus to induce eczematous dermatitis in a time-controlled manner.
AD mouse models have also been established through screening libraries following chemical-induced, genome-wide mutagenesis. Heterozygous mutations in CARD11, encoding a scaffolding protein involved in lymphocyte receptor signaling, is linked with monogenic AD in humans (Ma et al., 2017). Growing evidence suggests a benefit of targeting Janus kinase in AD (Guttman-Yassky et al., 2018). In these contexts, two N-ethyl-N-nitrosourea-derived models, CARMA-1/Card11-mutant mice (Jun et al., 2003) and JAK1spade/spade mice (Yasuda et al., 2016) might be interesting models to understand atopic inflammation from the immune signaling perspective.
Models induced by epicutaneous application of exogenous agents
Induced mouse models are perhaps the most frequently used systems in fields of dermatologic research such as immunology and carcinogenesis. Although topical application can be labor-intensive, it enables time- and dose-controlled induction of a phenotype and can be used in a variety of mouse models including genetically modified mice.
Haptens are small molecules which penetrate intact mouse epidermis and provoke adaptive immune responses upon subsequent exposures, resulting in contact hypersensitivity responses that model allergic contact dermatitis in humans. Repeated hapten challenge is reported to induce AD-like dermatitis by shifting type 1 into type 2 responses (Kaplan et al., 2012, Kitagaki et al., 1995, Kitagaki et al., 1997). Note should be taken that allergic contact dermatitis and AD are distinct entities and whether dermatitis induced by chronic hapten application recapitulates eczematous dermatitis remains to be determined.
Sensitization to protein antigens is thought to occur in patients with AD that may contribute to the onset of food allergy and asthma, known as the atopic march. Multiple epicutaneous exposure to ovalbumin (OVA) can induce AD-like symptoms (Spergel et al., 1998) with OVA-specific IgG1, IgG2a and IgE humoral responses (Wang et al., 2007). Human AD-like symptoms can also be induced by applications of house dust mite (HDM) extract onto mouse skin (Matsuoka et al., 2003). Skin changes in both models are enhanced when the barrier disruption is induced mechanically or by using mice which exhibit spontaneous skin barrier perturbation such as NC/Nga or flaky tail mice. The relevance of skin inflammation induced in the HDM model has yet to be determined, as humans presumably are not exposed to high doses of HDM antigens percutaneously. It is also worth noting that commercially available HDM and OVA allergen product can vary in their allergen composition and concentration depending on how they are prepared (Casset et al., 2012).
While rash observed during topical application of calcemic vitamin D3 analogs in psoriasis patients is clinically distinct from AD, topical application of MC903 (calcipotriol) to mouse skin recapitulates features of AD (Fig. 1c), such as inflammation, itch, and barrier dysfunction (Li et al., 2006, Naidoo et al., 2018). Mice treated with MC903 also have increased serum IgE. Conveniently, these AD-like responses can be induced regardless of genetic backgrounds, enabling the use of this model in mice that carry multiple transgenes without the necessity for backcrossing, which may facilitate their use in pre-clinical studies. Emerging concepts of AD pathogenesis such as innate lymphoid cells, sensory neuron, or microbiota have also been explored by utilizing this model (Kim et al., 2013, Myles et al., 2016, Oetjen et al., 2017).
Comparison of murine AD models to human AD
To date, the gross phenotypes of mouse models have been correlated with human AD by comparing clinical manifestations, histology, and expression of a limited number of markers. However, emerging cutting-edge technologies with transcriptomic analysis now deepen our understanding of each model and should allow us to compare complex molecular networks between species. Interestingly, comparison of gene expression data from mouse models to a differentially expressed list (“MADAD”) of 595 genes from human AD skin defined by meta-analysis revealed that the IL-23-injection model, a cytokine that is usually associated with psoriasis, exhibited the highest degree of overlap (Chan et al., 2006, Ewald et al., 2017). Our analysis of Adam17fl/fl Sox9-Cre mice showed overlap with the human AD transcriptome to a degree that was comparable to the IL-23-injected model (Woodring et al., 2018). However, the maximum overlap of genes remains under 40% in any model, suggesting that animal models each reflect limited aspects of human AD. These observations warrant further evaluations on the predictive power of each as preclinical models. One approach to identify a model that reflects human AD might be to test whether therapeutics with known clinical efficacy in humans are also effective in AD mouse models. It is possible that mouse models reflect certain subsets of classic human AD and that further clinical sub-categorization of AD is needed. Notably, over 20% of protein coding genes are not shared between mice and humans, suggesting that the two species may have developed unique immune systems after divergence from common ancestors. Future analysis may require the establishment of bioinformatics analytical frameworks with an evolutionary systems biology approach.
CONCLUSIONS AND FUTURE PERSPECTIVES
We have highlighted the diversity of current murine AD models and their advantages and limitations that should be considered when selecting a model that is appropriate for each research question or interpreting published studies. In order to increase the translatability of AD mouse models, it may be beneficial to establish phenotype criteria and accumulate transcriptome data, which should facilitate distinction of eczematous dermatitis from other forms of skin inflammation (Fig. 1a), such as psoriasis and contact hypersensitivity. Practical and reproducible approaches for evaluating the degree of inflammation are also essential, as ear thickness, transepidermal water loss, and other laboratory assays are variably utilized. A standardized clinical scoring system should be useful in reducing variability between studies (Kobayashi et al., 2015, Plant et al., 2012). Lastly, beyond mouse models, non-murine animal models for AD such as canine AD may better recapitulate human AD and thus be powerful models for preclinical studies (Cosgrove et al., 2013, Michels et al., 2016).
Supplementary Material
SUMMARY POINTS.
BENEFITS
Mouse AD models are valuable tools to deepen mechanistic insight into disease pathogenesis and to develop novel therapeutic agents in AD.
Recent advances in genetic engineering have accelerated our understanding of the biological significance of targeted genes in vivo.
LIMITATIONS
Each animal model reflects limited aspects of human AD.
There remains a considerable translational gap between AD mouse models and human AD.
ACKNOWLEDGEMENT
We apologize to those in the field whose work could not be included due to space constraints. This research was supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health.
Appendix
MULTIPLE CHOICE QUESTIONS
- The MC903 model used to study human atopic dermatitis represents which category of mouse model?
- Inbred model
- Genetically-engineered, transgenic model
- Genetically-engineered, knockout model
- Induced model by an exogenous agent
- Which of the following mutations is the most responsible for atopic dermatitis-like inflammation in flaky tail mice?
- Flg
- Tmem79
- Tslp
- Adam17
- Which of the following cytokines from keratinocytes is responsible for the activation of group 2 innate lymphoid cells 2 (ILC2)?
- TSLP
- IL-18
- IL-33
- All of the above
- Which of the following microbes is responsible for the development of skin inflammation in Adam17fl/fl Sox9Cre mice?
- Cutibacterium acnes
- Malassezia furfur
- Pseudomonas aeruginosa
- Staphylococcus aureus
- Which of the following sentences highlight a lesson from a recent study which compared transcriptomic profiles between human atopic dermatitis (AD) and mouse models?
- Oxazolone-induced mouse model exhibited the highest degree of overlap with human AD.
- More than 50% of core signatures of the human AD transcriptome overlapped with the differential expression genes analyzed in all tested mouse models.
- Using available databases, less than 5% of protein coding genes are not shared in mice and humans AD.
- Each animal model reflects limited aspects of human AD.
CORRECT ANSWER
D. Repetitive application of MC903 (calcipotriol), a topical anti-psoriatic agent, onto mouse skin can induce AD-like inflammation.
B. A recent report with segregation of the two mutated genes identified Tmem79, but not Flg, as the causative gene mutation of skin inflammation in flaky tail mice.
D. Group 2 innate lymphoid cells (ILC2s) get activated in response to a variety of stimuli, including epithelial cytokines IL-18, IL-25, IL-33, and thymic stromal lymphopoietin (TSLP).
D. S. aureus is primarily responsible for driving skin inflammation in Adam17fl/fl Sox9Cre mice.
D. The IL-23-injection model has been reported to show the highest degree of overlap to human AD among the tested models. However, the maximum overlap of genes between human AD and mouse models remains under 40%. In general, over 20% of protein coding genes are not shared between mice and humans.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors state no conflict of interest.
REFERENCES
- Brown SJ, Kroboth K, Sandilands A, Campbell LE, Pohler E, Kezic S, et al. Intragenic copy number variation within filaggrin contributes to the risk of atopic dermatitis with a dose-dependent effect. The Journal of investigative dermatology 2012;132(1):98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casset A, Mari A, Purohit A, Resch Y, Weghofer M, Ferrara R, et al. Varying allergen composition and content affects the in vivo allergenic activity of commercial Dermatophagoides pteronyssinus extracts. International archives of allergy and immunology 2012;159(3):253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JR, Blumenschein W, Murphy E, Diveu C, Wiekowski M, Abbondanzo S, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. The Journal of experimental medicine 2006;203(12):2577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan LS, Robinson N, Xu L. Expression of interleukin-4 in the epidermis of transgenic mice results in a pruritic inflammatory skin disease: an experimental animal model to study atopic dermatitis. The Journal of investigative dermatology 2001;117(4):977–83. [DOI] [PubMed] [Google Scholar]
- Cosgrove SB, Wren JA, Cleaver DM, Walsh KF, Follis SI, King VI, et al. A blinded, randomized, placebo-controlled trial of the efficacy and safety of the Janus kinase inhibitor oclacitinib (Apoquel(R)) in client-owned dogs with atopic dermatitis. Veterinary dermatology 2013;24(6):587–97, e141–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld-Franklin M, Presnell SR, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nature immunology 2004;5(7):752–60. [DOI] [PubMed] [Google Scholar]
- Ewald DA, Noda S, Oliva M, Litman T, Nakajima S, Li X, et al. Major differences between human atopic dermatitis and murine models, as determined by using global transcriptomic profiling. The Journal of allergy and clinical immunology 2017;139(2):562–71. [DOI] [PubMed] [Google Scholar]
- Fallon PG, Sasaki T, Sandilands A, Campbell LE, Saunders SP, Mangan NE, et al. A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nature genetics 2009;41(5):602–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feld M, Garcia R, Buddenkotte J, Katayama S, Lewis K, Muirhead G, et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. The Journal of allergy and clinical immunology 2016;138(2):500–8.e24. [DOI] [PubMed] [Google Scholar]
- Guttman-Yassky E, Silverberg JI, Nemoto O, Forman SB, Wilke A, Prescilla R, et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: a phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study. Journal of the American Academy of Dermatology 2018. [DOI] [PubMed]
- Imai Y, Yasuda K, Sakaguchi Y, Haneda T, Mizutani H, Yoshimoto T, et al. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proceedings of the National Academy of Sciences of the United States of America 2013;110(34):13921–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JE, Wilson LE, Vinuesa CG, Lesage S, Blery M, Miosge LA, et al. Identifying the MAGUK protein Carma-1 as a central regulator of humoral immune responses and atopy by genome-wide mouse mutagenesis. Immunity 2003;18(6):751–62. [DOI] [PubMed] [Google Scholar]
- Kaplan DH, Igyarto BZ, Gaspari AA. Early immune events in the induction of allergic contact dermatitis. Nature reviews Immunology 2012;12(2):114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H, Nagao K, Kubo A, Hata T, Shimizu A, Mizuno H, et al. Altered stratum corneum barrier and enhanced percutaneous immune responses in filaggrin-null mice. The Journal of allergy and clinical immunology 2012;129(6):1538–46.e6. [DOI] [PubMed] [Google Scholar]
- Kezic S, O’Regan GM, Yau N, Sandilands A, Chen H, Campbell LE, et al. Levels of filaggrin degradation products are influenced by both filaggrin genotype and atopic dermatitis severity. Allergy 2011;66(7):934–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Science translational medicine 2013;5(170):170ra16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagaki H, Fujisawa S, Watanabe K, Hayakawa K, Shiohara T. Immediate-type hypersensitivity response followed by a late reaction is induced by repeated epicutaneous application of contact sensitizing agents in mice. The Journal of investigative dermatology 1995;105(6):749–55. [DOI] [PubMed] [Google Scholar]
- Kitagaki H, Ono N, Hayakawa K, Kitazawa T, Watanabe K, Shiohara T. Repeated elicitation of contact hypersensitivity induces a shift in cutaneous cytokine milieu from a T helper cell type 1 to a T helper cell type 2 profile. Journal of immunology (Baltimore, Md : 1950) 1997;159(5):2484–91. [PubMed] [Google Scholar]
- Kobayashi T, Glatz M, Horiuchi K, Kawasaki H, Akiyama H, Kaplan DH, et al. Dysbiosis and Staphylococcus aureus Colonization Drives Inflammation in Atopic Dermatitis. Immunity 2015;42(4):756–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara Y, Tanabe K, Matsuoka K, Kanda N, Matsuda H, Karasuyama H, et al. A major determinant quantitative-trait locus responsible for atopic dermatitis-like skin lesions in NC/Nga mice is located on Chromosome 9. Immunogenetics 2001;53(1):15–21. [DOI] [PubMed] [Google Scholar]
- Konishi H, Tsutsui H, Murakami T, Yumikura-Futatsugi S, Yamanaka K, Tanaka M, et al. IL-18 contributes to the spontaneous development of atopic dermatitis-like inflammatory skin lesion independently of IgE/stat6 under specific pathogen-free conditions. Proceedings of the National Academy of Sciences of the United States of America 2002;99(17):11340–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Hener P, Zhang Z, Kato S, Metzger D, Chambon P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proceedings of the National Academy of Sciences of the United States of America 2006;103(31):11736–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CA, Stinson JR, Zhang Y, Abbott JK, Weinreich MA, Hauk PJ, et al. Germline hypomorphic CARD11 mutations in severe atopic disease. Nature genetics 2017;49(8):1192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Watanabe N, Geba GP, Sperl J, Tsudzuki M, Hiroi J, et al. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. International immunology 1997;9(3):461–6. [DOI] [PubMed] [Google Scholar]
- Matsuoka H, Maki N, Yoshida S, Arai M, Wang J, Oikawa Y, et al. A mouse model of the atopic eczema/dermatitis syndrome by repeated application of a crude extract of house-dust mite Dermatophagoides farinae. Allergy 2003;58(2):139–45. [DOI] [PubMed] [Google Scholar]
- Michels GM, Ramsey DS, Walsh KF, Martinon OM, Mahabir SP, Hoevers JD, et al. A blinded, randomized, placebo-controlled, dose determination trial of lokivetmab (ZTS-00103289), a caninized, anti-canine IL-31 monoclonal antibody in client owned dogs with atopic dermatitis. Veterinary dermatology 2016;27(6):478–e129. [DOI] [PubMed] [Google Scholar]
- Myles IA, Williams KW, Reckhow JD, Jammeh ML, Pincus NB, Sastalla I, et al. Transplantation of human skin microbiota in models of atopic dermatitis. JCI insight 2016;1(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo K, Jagot F, van den Elsen L, Pellefigues C, Jones A, Luo H, et al. Eosinophils Determine Dermal Thickening and Water Loss in an MC903 Model of Atopic Dermatitis. The Journal of investigative dermatology 2018. [DOI] [PubMed]
- Oetjen LK, Mack MR, Feng J, Whelan TM, Niu H, Guo CJ, et al. Sensory Neurons Co-opt Classical Immune Signaling Pathways to Mediate Chronic Itch. Cell 2017;171(1):217–28.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant JD, Gortel K, Kovalik M, Polissar NL, Neradilek MB. Development and validation of the Canine Atopic Dermatitis Lesion Index, a scale for the rapid scoring of lesion severity in canine atopic dermatitis. Veterinary dermatology 2012;23(6):515–e103. [DOI] [PubMed] [Google Scholar]
- Ruzicka T, Hanifin JM, Furue M, Pulka G, Mlynarczyk I, Wollenberg A, et al. Anti-Interleukin-31 Receptor A Antibody for Atopic Dermatitis. The New England journal of medicine 2017;376(9):826–35. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Shiohama A, Kubo A, Kawasaki H, Ishida-Yamamoto A, Yamada T, et al. A homozygous nonsense mutation in the gene for Tmem79, a component for the lamellar granule secretory system, produces spontaneous eczema in an experimental model of atopic dermatitis. The Journal of allergy and clinical immunology 2013;132(5):1111–20.e4. [DOI] [PubMed] [Google Scholar]
- Saunders SP, Goh CS, Brown SJ, Palmer CN, Porter RM, Cole C, et al. Tmem79/Matt is the matted mouse gene and is a predisposing gene for atopic dermatitis in human subjects. The Journal of allergy and clinical immunology 2013;132(5):1121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, Geha RS. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. The Journal of clinical investigation 1998;101(8):1614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamari M, Hirota T. Genome-wide association studies of atopic dermatitis. The Journal of dermatology 2014;41(3):213–20. [DOI] [PubMed] [Google Scholar]
- Wang G, Savinko T, Wolff H, Dieu-Nosjean MC, Kemeny L, Homey B, et al. Repeated epicutaneous exposures to ovalbumin progressively induce atopic dermatitis-like skin lesions in mice. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology 2007;37(1):151–61. [DOI] [PubMed] [Google Scholar]
- Woodring T, Kobayashi T, Kim D, Nagao K. ADAM17-Deficient Mice Model the Transcriptional Signature of Human Atopic Dermatitis. The Journal of investigative dermatology 2018;138(10):2283–6. [DOI] [PubMed] [Google Scholar]
- Yasuda T, Fukada T, Nishida K, Nakayama M, Matsuda M, Miura I, et al. Hyperactivation of JAK1 tyrosine kinase induces stepwise, progressive pruritic dermatitis. The Journal of clinical investigation 2016;126(6):2064–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo J, Omori M, Gyarmati D, Zhou B, Aye T, Brewer A, et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. The Journal of experimental medicine 2005;202(4):541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng T, Oh MH, Oh SY, Schroeder JT, Glick AB, Zhu Z. Transgenic expression of interleukin-13 in the skin induces a pruritic dermatitis and skin remodeling. The Journal of investigative dermatology 2009;129(3):742–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


