Abstract
Isolevuglandins are 4-ketoaldehydes formed by peroxidation of arachidonic acid. Isolevuglandins react rapidly with primary amines including the lysyl residues of proteins to form irreversible covalent modifications. This review highlights evidence for the potential role of isolevuglandin modification in the disease processes, especially atherosclerosis, and some of the tools including small molecule dicarbonyl scavengers utilized to assess their contributions to disease.
Keywords: Lipid peroxidation, reactive lipid species, isolevuglandins, cardiovascular disease, HDL
Introduction
Lipid peroxidation is a constant process within living cells, as a wide variety of cellular processes produce reactive oxygen species (ROS). However, initiating events of pathological processes often exacerbate rates of lipid peroxidation, resulting in accumulation of lipid peroxidation products that contribute to the progression of disease. While abundant in phospholipids of cellular membranes, polyunsaturated fatty acids (PUFAs) are highly susceptible to peroxidation by ROS such as the hydroxyl radical. Peroxidation of linoleic acid and arachidonic acid generates a variety of stable as well as reactive products (Figure 1). This mini-review will focus on one particular family of lipid peroxidation products, the isolevuglandins (IsoLG), and their role in cardiovascular disease.
Figure 1.
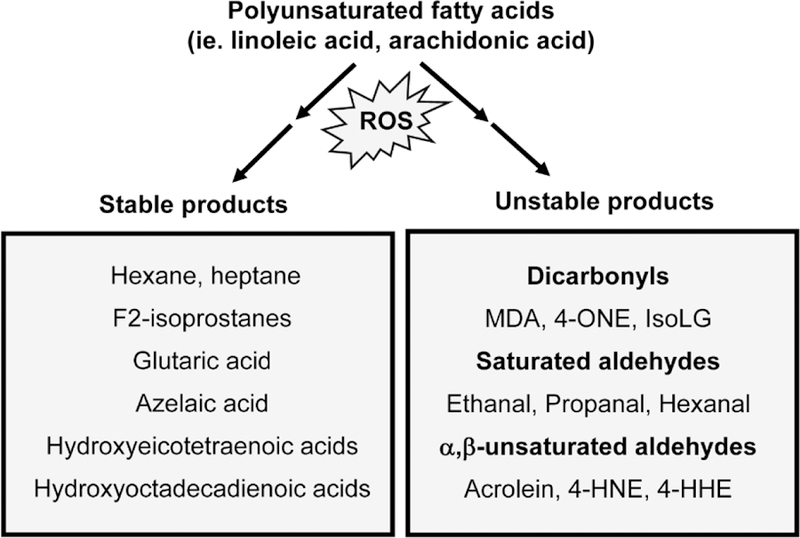
Peroxidation of polyunsaturated fatty acids (PUFAs) such as linoleic acid and arachidonic acid by ROS generates a variety of stable and reactive products.
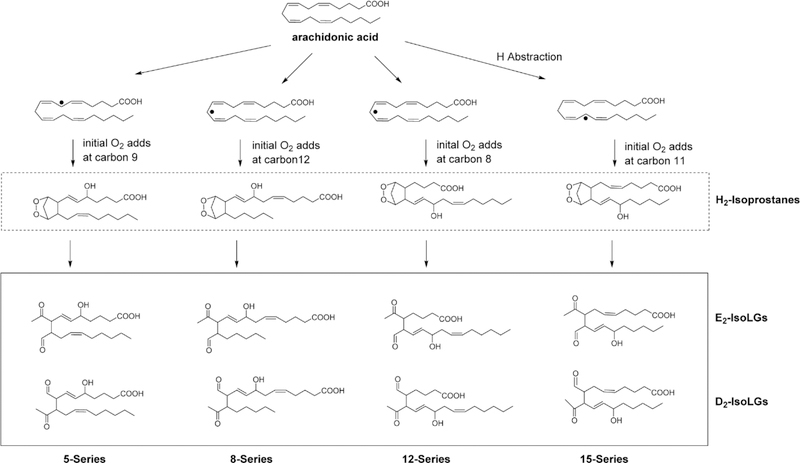
IsoLGs are a family of 4-ketoaldehydes (alternatively referred to as isoketals) that form from the peroxidation of arachidonic acid through bicycloendoperoxide intermediates (termed H2-isoprostanes) and undergo concerted rearrangement of the bicycloendoperoxide group to form the 4-ketoaldehyde1, 2. Because abstraction of the initial hydrogen can occur at 3 different positions on arachidonic acid, subsequent rearrangement and addition of oxygen give rise to four regioisomers of H2-isoprostanes. Each of these regioisomers can give rise to two distinct 4-ketoaldehyde isomers, generating a total of eight regioisomers (and 64 stereoisomers) of IsoLG (Figure 2). While all regioisomers form to some extent, the formation of certain regioisomers are favored (e.g. 15-E2-IsoLG and 5-E2-IsoLG versus 8-E2-IsoLG and 12-E2-IsoLG).
Figure 2. Formation of isolevuglandins (IsoLGs) from arachidonic acid via H2-isoprostane intermediates.
Radicals such as the hydroxyl or a lipid peroxyl radical can abstract a hydrogen from any one of three potential positions on a molecule of arachidonic acid to form a lipid radical. Because these carbon centered radicals form between two double bonds, the resulting electron is conjugated across the five adjacent carbons. Depending on at which position that molecular oxygen (a diradical) adds to these conjugated carbon centered radical, any one of four different regioisomers of the bicyclic endoperoxides (H2-isoprostanes) can form. Each of these regioisomers can give rise to two 4-ketoaldehyde regioisomers (D2-and E2-IsoLGs), generating a family of eight IsoLG regioisomers (and 64 stereoisomers since each regioisomer has 3 chiral carbons). These 4-ketoaldehydes then spontaneously react with primary amines such as lysyl residues of proteins or phosphatidylethanolamines.
A number of biological enzymes generate IsoLG, with the most significant being myeloperoxidase, NADPH oxidase (NOX), and cyclooxygenase. Myeloperoxidase is an abundant heme protein secreted by phagocytes in response to stimulation that serves as an enzyme catalyst for lipid peroxidation and lipoprotein oxidation in vivo3. Formation of IsoLG adducts by the myeloperoxidase/H2O2 system of leukocytes was found in a Candida sepsis murine model of inflammation4. Myeloperoxidase forms IsoLG adducts in lysophosphatidylethanolamines5 and also in proteins and phosphatidylethanolamines in high density lipoprotein6. The primary function of the NOX family is the generation of ROS, which directly leads to IsoLG formation via free radicals7. However, NOX activation can also lead to cyclooxygenase activation8 and thereby produce IsoLG via that pathway. Unlike the other enzymes mentioned, cyclooxygenase can not only generate a lipid peroxide directly (rather than indirectly by reaction between ROS and PUFA) but also generate prostaglandin (PG) H2. If not rapidly converted to other prostaglandins by PG synthases, PGH2 will undergo spontaneous non-enzymatic rearrangement to form levuglandin E2 and levuglandin D2, which are two specific stereoisomers of 15-E2-IsoLG and 15-D2-IsoLG, respectively. Following cyclooxygenase activity, IsoLG forms protein adducts in cells9 and in tissues10 as well as bind to histones11.
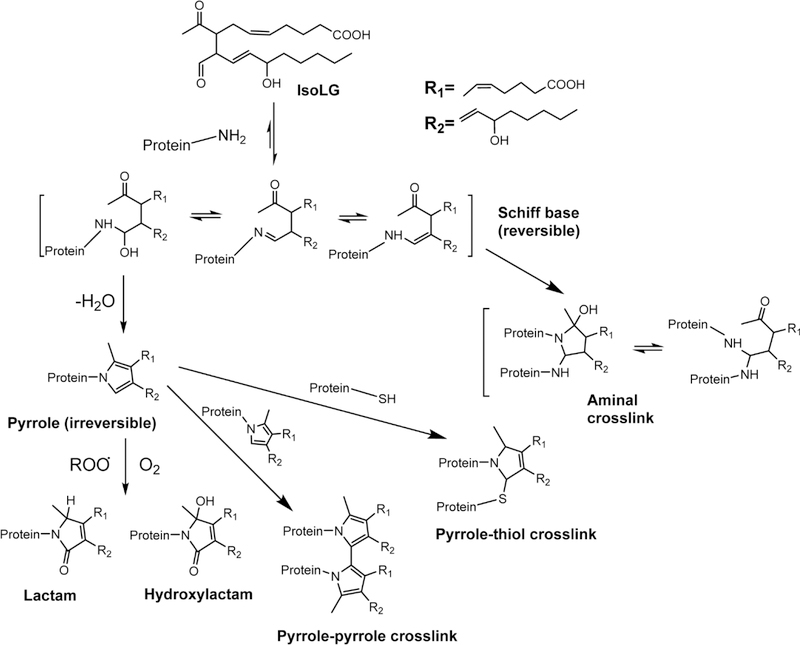
Two characteristics distinguish IsoLGs from other common lipid peroxidation species such as 4- hydroxynonenal (HNE), acrolein, and malondialdehyde (MDA). First, while all of these lipid peroxidation products spontaneously react with nucleophiles, IsoLGs are at least an order of magnitude more reactive with proteins than MDA or HNE12. Second, IsoLG almost exclusively react with primary amines12 (e.g. lysine and phosphatidylethanolamine) and not other nucleophiles like thiols (e.g. cysteine) or indoles (e.g. histidine). Both characteristics can be rationalized based on the mechanism of their chemical reaction. Characteristic of aldehydes, the aldehyde group of IsoLGs rapidly react with primary amines (not other nucleophiles like thiols) to form a highly reversible imine adduct (Schiff Base) (Figure 3). The 4-keto moiety of IsoLG subsequently reacts with the nitrogen of the imine, generating a dihydropyrrolidine adduct which then loses two water molecules to form the essentially irreversible pyrrole adduct13. Under oxidizing conditions, this pyrrole adduct can either react with molecular oxygen to form lactam and hydroxylactam adducts or with other nucleophiles such as thiols or nearby pyrroles to form intermolecular crosslinks. The latter reaction explains the high propensity of IsoLGs to crosslink proteins which distinguishes them from other reactive lipids species like HNE. Due to their rapid reaction rates, their propensity to form protein crosslinks, and the formation of unique adduct structures, IsoLGs often exert very different effects than other products of lipid peroxidation. The following sections of this review will discuss how IsoLG modification of various proteins including plasma lipoproteins appears to play an important role in the development of diseases related to oxidative stress.
Figure 3. Mechanism of IsoLG-adduct formation.
The aldehyde group of IsoLG spontaneously reacts with primary amines to form a highly reversible imine adduct (Schiff base). The 4-keto moiety of IsoLG subsequently reacts with the nitrogen of the imine, generating a dihydropyrrolidine adduct which then loses two water molecules to form the essentially irreversible pyrrole adduct. Under oxidizing conditions, this pyrrole
Risk factors for cardiovascular disease elevate IsoLG levels
Any claim that the formation of IsoLG adducts plays a causative role in disease processes requires the following evidence: 1) IsoLG adducts are elevated during the development of disease, 2) preventing the formation of these adducts attenuates disease, and 3) IsoLG exerts detrimental effects relevant to the development of disease. Furthermore, the ability to accurately quantify levels of IsoLG-adducts is critical. In this section, we will briefly review current approaches to measure IsoLG-protein adducts and evidence to support that IsoLG adduct formation contributes to cardiovascular diseases.
Two major approaches have been used to quantitate IsoLG-protein adducts: isotope dilution mass spectrometry and antibody-based approaches such as enzyme-linked immunosorbent assay (ELISA), immunohistochemistry, and flow cytometry. Isotope dilution mass spectrometry is a highly specific and quantitative method, where protein precipitated from tissues are digested to constituent IsoLG-modified lysines and measured against an isotopically-labeled internal standard12, 14. Similar mass spectrometry approaches are used to measure IsoLG-phosphatidylethanolamine adducts5. However, one disadvantage is that this approach cannot identify the specific protein modified by IsoLG nor determine the spatial location of adducts in tissue. These questions could potentially be addressed by mass spectrometry techniques used for detecting post-translational modifications on whole proteins or tryptic digests or by Matrix Assisted Laser Desorption/Ionization (MALDI) imaging techniques. However, such strategies have not been proved feasible for IsoLG adducts in tissues, so further development of these techniques is needed. To address questions of spatial localization of adducts in tissue, investigators have generated antibodies against IsoLG adducts15, 16. These antibodies have been particularly useful in immunochemistry to identify cellular sites of adduct formation and in flow cytometry to measure specific cell types where IsoLG adducts are produced. However, one challenge with these approaches is that current antibodies are of relatively low affinity, making approaches such as immunoprecipitation infeasible. For these reasons, the development of additional anti-IsoLG adduct antibodies is critically needed.
The earliest evidence for increased IsoLG adducts in cardiovascular disease came from ELISA studies performed with anti-IsoLG adduct antibodies on plasmas of patients with and without verified atherosclerosis. IsoLG-protein adducts were found to be elevated two-fold in diseased patients17. IsoLG adducts are also elevated in conditions that increase risk for cardiovascular disease including sepsis, hypertension, hypercholesterolemia, obesity, chronic kidney disease. Poliakov et al. showed that mice with sepsis induced by Candida had a 3-fold elevation in plasma IsoLG-protein adduct levels4. As detailed in their article in this issue, Kirabo et al. demonstrated that levels of IsoLG adducts were increased in several murine models of hypertension and elucidated some mechanisms whereby IsoLG contributes to hypertension18–20. We found that IsoLG adducts of both protein and phosphatidylethanolamine were elevated in high density lipoprotein (HDL) isolated from humans with familial hypercholesterolemia compared to normal control subjects21, 22. The consequences of this modification on the function of HDL is discussed in more detail below. We also found increases in IsoLG-phosphatidylethanolamine adducts in livers of diet-induced obese mice22. Salomon and colleagues found increased IsoLG adducts in patients with chronic kidney disease23. They also found increased IsoLG adducts in mice chronically exposed to alcohol5. Although more studies are needed to fully elucidate the relationship between elevations in IsoLG adducts and development of cardiovascular disease, these studies support the notion that risk factors for cardiovascular disease elevate IsoLG adduct levels and may therefore play a causal role, rather than simply being a tombstone marker of well-established disease.
The formation of IsoLG adducts is not isolated to cardiovascular disease only. Any tissue with significant arachidonic acid content is prone to oxidative damage and will likely have elevated levels of IsoLG adducts. Other conditions where increased IsoLG adducts have been detected include cancer24, Alzheimer’s disease25, idiopathic pulmonary fibrosis7, and retinopathy26.
Development of dicarbonyl scavengers to assess the role of IsoLG in disease
Determining the contribution of IsoLG to disease processes requires effective interventions that selectively reduce levels of IsoLG adducts. In theory, increasing tissue levels of antioxidants could reduce IsoLG formation. However, IsoLG formation in tissues appears to be driven by ROS producing enzymes (such as myeloperoxidase and NOX), so that simply increasing tissue concentrations of antioxidants is unlikely able to combat the formation of IsoLG that result from these activated enzymes. Even with supraphysiological levels of antioxidants that may block IsoLG formation, this would also block the generation of hydrogen peroxide and a plethora of other lipid peroxidation products. Such broad approach would not only make interpreting the mechanism of IsoLG difficult, it would also drive adverse physiological effects since hydrogen peroxide acts as an intracellular signaling molecule that initiates several protective responses27. Such adverse effects may underlie the failure in clinical trials using dietary antioxidants like vitamin C and vitamin E in producing significant improvements in endpoints of cardiovascular disease28, 29.
To effectively inhibit the formation of IsoLG adducts, we have focused on developing small molecule primary amines that act as IsoLG scavengers30–32. These scavengers do not alter the formation of IsoLG or other lipid peroxidation products like F2-isoprostanes, nor do they block the formation of important signaling molecules like hydrogen peroxide. Rather, these scavengers react with IsoLG faster than IsoLG is able to react with the lysyl residues of proteins or with phosphatidylethanolamines, thereby significantly reducing IsoLG adduct levels (Figure 4).
Figure 4. Scavenging of IsoLG by 2-aminomethyphenols.
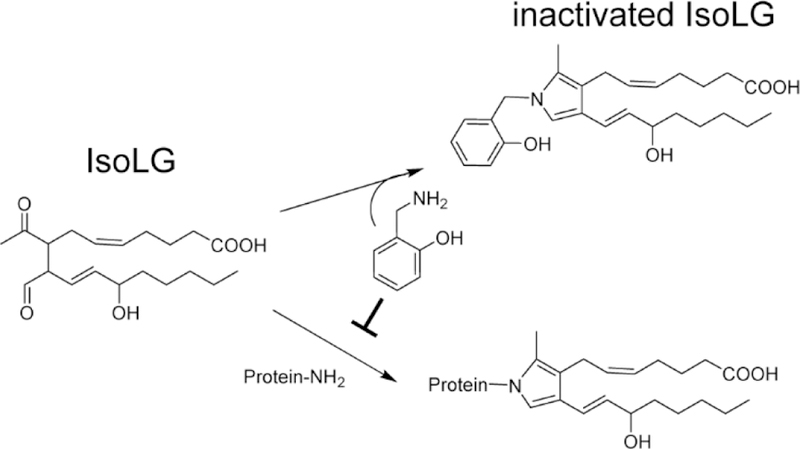
The reaction of 2-HOBA with IsoLG forms a stable adduct that is poorly reactive, thereby preventing IsoLG from reacting with proteins or phosphatidylethanolamine.
To develop IsoLG scavengers, we screened small molecule primary amines with known bioavailability for their ability to prevent formation of IsoLG-[3H] lysine adducts when incubated with IsoLG and [3H]lysine in vitro 30. Of the screened compounds, only pyridoxamine (PM), a water soluble vitamin B6 vitamer, was able to markedly reduce IsoLG-[3H]lysine formation30. Subsequent structure-activity relationship studies of structural analogs of PM using 4-oxopentanal (OPA) to model the reaction of 4-ketoaldehydes, showed that the second-order reaction rate of PM with OPA was more than 2,000 times faster than that of lysine with OPA30. Additionally, compounds which shared the 2-methylaminophenol structure of PM including salicylamine (SAM, or also known as 2-hydroxybenzylamine, 2-HOBA), showed similar high reaction rates with OPA. Importantly, the high rate of reactivity of 2-HOBA with OPA was ablated if the 2-hydroxy moiety was methylated or shifted to the para-position (4-HOBA)30, 32. Modification of other structural features of PM such as alkylation of the methylhydroxy group at the 5’position of pyridoxamine did not reduce the high reactivity rate with OPA.
A mechanism for the high reactivity of 2-aminomethylphenols with IsoLG (and other 4-ketoaldehydes such as OPA) was proposed based on these structure-activity relationships. In essence, the high reactivity of these compounds was postulated to result from two key considerations: 1) the 2-hydroxy groups serves to attract the proton from the amine group, making the amine group more basic and a better nucleophile, and 2) after the initial formation of an imine adduct with IsoLG, the 2-hydroxy group of 2-HOBA helps stabilize the 4-keto group of IsoLG (through hydrogen bonding), positioning it to react with the nitrogen group of the imine and thereby form the IsoLG-2-HOBA pyrrolidine adduct, which is the rate limiting step in pyrrole formation.
These initial studies provided three compounds, PM, 2-HOBA, and PPM, that potentially could be used as IsoLG scavengers (Figure 5A). When tested for their ability to reduce cyclooxygenase-driven IsoLG adduct formation in platelets, 2-HOBA and PPM were found to have greater efficacy than PM31. Both also showed greater efficacy than PM in protecting HEK293 cells against hydrogen peroxide induced cytotoxicity31. The greater efficacy of 2-HOBA and PPM was likely due to their hydrophobicity, allowing penetration and localization within cellular membranes where IsoLGs are formed. Based on these studies, several other hydrophobic 2-aminomethylphenol compounds have been generated and shown efficacious in reducing IsoLG adducts in cells31, 33. In contrast, closely related phenolic compounds such as 4-HOBA (described earlier in this review), N-methyl-2-HOBA, and 5’-O-pentylpyridoxine (PPO) (Figure 5B), which are not effective dicarbonyl scavengers in vitro also fail to reduce IsoLG adducts in proteins or cells18, 21. Use of such compounds are important controls to rule out nonspecific antioxidant-like effects of 2-aminomethylphenols.
Figure 5. Dicarbonyl scavengers and their inactive analogs.
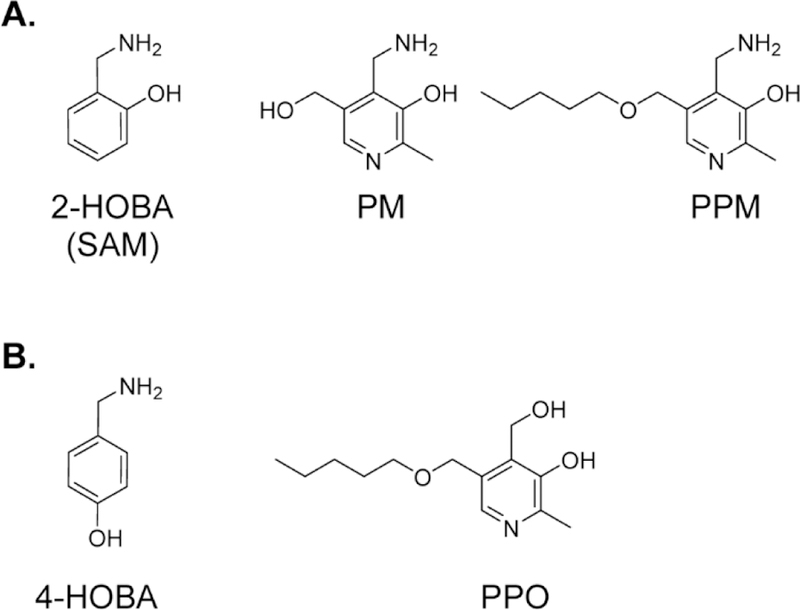
A. Notable small molecule primary amines that act as IsoLG scavengers include 2-hydroxybenzylamine (2-HOBA or SAM), pyridoxamine (PM), and 5’-O-pentyl-pyridoxamine (PPM). B. Analogues that are not effective scavengers include 4-hydroxybenzylamine (4-HOBA) and 5’-O-pentyl-pyridoxine (PPO).
While 2-HOBA and PPM are often referred to as IsoLG scavengers, their proposed mechanisms suggest that they should be effective against other 1,4-dicarbonyls (such as 4-oxononenal, 4-oxopentanal, and succinaldehyde) as well as against 1,3-dicarbonyls (such as such as MDA). This mechanism also predicts that 2-aminomethylphenols should not be effective scavengers of 4-hydroxynonenal and other α,β-unsaturated carbonyls. Subsequent studies demonstrated that while 2-HOBA and PPM most effectively scavenge IsoLG during conditions of lipid peroxidation, they also effectively scavenge MDA34, but not HNE30. Based on these results, 2-HOBA, PPM, and their analogs are best described as dicarbonyl scavengers.
The primary value of dicarbonyl scavengers is their ability to be used in cellular assays and in vivo conditions where oxidative injury occurs35. A number of studies show that these scavengers not only can protect cells or tissues from dicarbonyl-induced damage but also that these scavengers have reasonable bioavailability and do not show signs of toxicity at doses that achieve efficacy36, 37. For instance, 2-HOBA has been demonstrated to protect sodium channel function from inhibition by tert-butyl hydroperoxide38 and to prevent formation of preamyloid oligomers in rapidly paced atrial cells39. Initial pharmacokinetic studies showed that 2-HOBA was orally bioavailable with a half-life of 62 minutes40. Other dicarbonyl scavengers including PPM and analogs such as PPO and 4-HOBA are also orally bioavailable (unpublished studies). Dose studies showed that up to 1 g/L of 2-HOBA in drinking water provided plasma and tissue concentrations in the range required for effectively scavenging IsoLG without toxicity40. Administration of 1 g/L 2-HOBA to mice expressing human apoE4, a model of Alzheimer’s disease, protected against age-induced loss of working spatial memory41. As discussed in their article in this issue, Kirabo et al. have demonstrated the efficacy of 2-HOBA and a number of other dicarbonyl scavengers in a variety of models of hypertension18. A recent pre-clinical study demonstrated that short-term (28 day) oral administration of 2-HOBA at levels up to 1000mg/kg body weight did not cause adverse effects in rodents42. Additional studies are needed to evaluate long term effects, but the use of 2-HOBA has recently entered clinical trials.
Role of IsoLG modification of plasma lipoproteins in development of cardiovascular disease
In atherosclerosis, lipid modification of low density lipoprotein (LDL) triggers the uptake of LDL by macrophages which transforms to macrophage foam cells. Hoppe et al. showed that modification of LDL by IsoLG led to its uptake and degradation by macrophages43. This is similar to what is seen with MDA44 or HNE45 modification but at much lower concentrations.
While these studies suggest that IsoLG could contribute to atherosclerosis via modifying LDL, a key finding by Salomon et al. led us to consider if IsoLG might contribute by other mechanisms. They showed that only about 20% of IsoLG protein adducts isolated from plasma were associated with LDL17. Combining this finding with our subsequent finding that IsoLG adducts are increased in HDL of patients with familial hypercholesterolemia21 led us to examine the role of IsoLG modification in altering HDL function.
HDL, known in the lay press as ‘good cholesterol,’ protects against atherosclerosis through diverse functions, including reverse cholesterol transport of cholesterol from tissues such as the aortic wall to the liver for metabolism and excretion, and the inhibition of inflammatory response of macrophages to stimulus like bacterial endotoxins46. Both these functions are thought to be primarily driven by ApoAI47, 48, the major apolipoprotein of HDL (accounting for about 70% of its protein mass). In diabetics, increasing myeloperoxidase levels correlate with increased atheroma volume49. Incubation of HDL with myeloperoxidase leads to oxidative modifications of ApoAI and a dramatic reduction in the capacity of HDL to carry out reverse cholesterol transport50–52. We have found that incubation of HDL with myeloperoxidase also leads to modification of both phosphatidylethanolamine22 and protein21 by IsoLG and leads to ApoAI protein crosslinking21. Co-incubation of myeloperoxidase HDL complexes with the dicarbonyl scavenger PPM almost completely blocks IsoLG modification and crosslinking of ApoAI in HDL, whereas co-incubation with PPO, the inactive analog of PPM, has no effect on IsoLG modification or crosslinking. These findings demonstrate that IsoLG is produced downstream of an oxidative enzyme known to contribute to oxidative modification of HDL within the atherosclerotic lesion51.
To directly assess the impact of IsoLG modification on HDL function, we incubated HDL with a range of IsoLG concentrations that produced levels of IsoLG protein adducts matching those found in patients with hypercholesterolemia or when myeloperoxidase is incubated with HDL (between 0.1 to 3 molar equivalence of IsoLG to ApoAI, respectively). We then assessed the effects of IsoLG modification on HDL functions such as its capacity to inhibit lipopolysaccharide (LPS)-induced macrophage inflammation, its exchangeability, and its macrophage cholesterol efflux capacity. While unmodified HDL inhibited LPS-induced cytokine release from primary murine macrophages, HDL modified with 0.1 molar equivalence of IsoLG not only completely abolishes its ability to inhibit inflammation but synergizes with LPS to produce a much greater proinflammatory cytokine expression.
This synergistic pro-inflammatory response is in contrast to the effects of PGE2, which is produced by macrophages in response to LPS, but that suppressing macrophage activation in part by inhibiting both MyD88-dependent53–56 and MyD88-independent57 cytokine expression. The inhibitory effects of PGE2 appear to be mediated by G protein-coupled receptors EP2 and/or EP455–60. Recently, PGE2 was shown to down-regulate the expression of COX-2 by increasing the expression of dual specificity phosphatase 1 (DUSP1), decreasing activity of MAPK p38, and enhancing the function of tristetraprolin 60. Since COX-2 in macrophages potentially produces both PGE2 and IsoLG, which appear to exert antagonistic effects, future studies are needed to understand how signaling downstream of these two compounds interact to regulate macrophage function. The signaling pathways by which IsoLG-modified HDL synergizes with LPS to further induce macrophage inflammation is not known and is currently under exploration.
We also found that modification of HDL with 1 molar equivalence or greater of IsoLG markedly reduced the exchangeability of ApoAI from HDL (termed ‘ApoAI-HDL exchange’61), a critical step in the ability of HDL to carry out reverse cholesterol transport functions. Not surprisingly, IsoLG modification markedly reduced the capacity of HDL to efflux [3H]-cholesterol from macrophages, a surrogate measure in cultured cells for in vivo reverse cholesterol transport. Interestingly, these modifications by IsoLG correlate with greater ApoAI protein crosslinking and the generation of a subpopulation of HDL of markedly increased diameter. Finally, we found that co-incubation of scavenger PPM was able to prevent IsoLG-induced protein crosslinking and the various described HDL dysfunctions.
Thus, modification of HDL in vitro with physiological relevant concentrations of IsoLG ablates its key anti-atherosclerotic functions (Figure 6), supporting the notion that the modification of HDL by IsoLG seen in vivo may play a key role in loss of HDL function and the development of atherosclerosis and cardiovascular disease.
Figure 6. Effects of IsoLG modification on HDL functions.
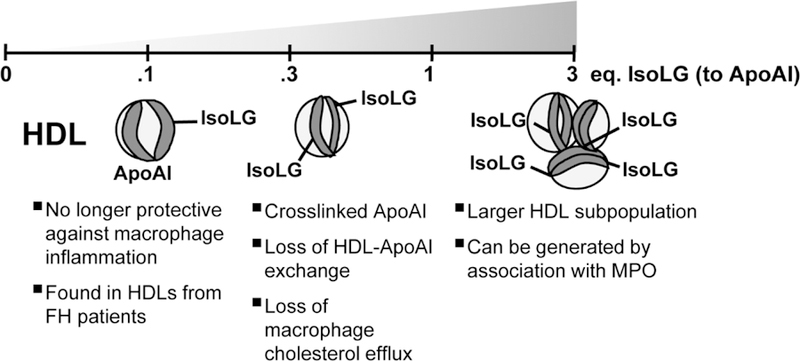
HDL isolated by density gradient ultracentrifugation was modified with a range of IsoLG (0.1–3 molar equivalence to ApoAI) which produces adduct levels matching those found in patients with familial hypercholesterolemia or by myeloperoxidase oxidation, respectively. At the lower range of 0.1 eq, IsoLG-modified HDL is no longer protective against LPS-induced macrophage inflammation. Approaching 1 eq. modification, IsoLG-modified HDL contains crosslinked ApoAI which loses its ability to exchange and consequently has lower macrophage cholesterol efflux capacity. At greater than 1 eq., IsoLG-modification yields an HDL subpopulation with greater diameter.
Conclusions & Future Directions
The development of methods to detect and analyze proteins or lipids modified by IsoLG, as well as the development of dicarbonyl scavengers to prevent modification has given rise to a number of studies in vitro and in vivo that support the notion that IsoLG adducts contribute to atherosclerosis and diseases related to increased oxidative stress. Future studies are needed to determine the efficacy of dicarbonyl scavengers in related models of disease where IsoLG adducts have been shown to be elevated. For instance, recent studies showed that IsoLG adducts formed by macrophages isolated from adipose tissues of mice promote pathogenic T-cell responses that may be important in insulin resistance and diabetes62, so studies are needed to examine whether dicarbonyl scavengers might to useful here. Future studies are also needed to elucidate the precise mechanisms whereby IsoLG modification exerts its effects. While it is easy to understand how IsoLG modification of enzymes at active site lysines or by crosslinking could lead to loss of enzyme function, IsoLG modification clearly exerts gain-of-function effects as well. One potential mechanism for such gain-of-function effects are that IsoLG modified proteins or PE act as ligands for receptors or otherwise activate cellular signaling. For example, IsoLG-PE adducts induce their inflammatory response in macrophages in part by activating the receptor for advanced glycation endproducts 22. Dissecting the precise signaling processes whereby IsoLG adducts exert their effects may identify additional molecular targets for drug development such as previously orphan receptors. Targeting IsoLG induced signaling pathways in combination with administrating dicarbonyl scavengers might prove especially beneficial. Future studies are also needed to examine whether specifically targeting dicarbonyl scavengers to likely sites of IsoLG formation could enhance their efficacy. For instance, mitochondria are well-known sites of ROS formation and IsoLGs induce mitochondrial dysfunction63, so that development of mitochondria-targeted scavengers might be especially valuable. Finally, future studies are needed to determine mechanism of metabolism and clearance of IsoLG modified proteins and PE in vivo. Enhancing catabolism of these modified macromolecules should reduce their levels and therefore potentially reduce disease. In summary, while studies to date have revealed important insights into the role of IsoLGs in cardiovascular disease and potential interventions, much work remains to be done to fully understand the mechanisms that lead to formation of IsoLG, the consequences of IsoLG modification, and the optimal strategies to prevent their adverse effects.
Highlights:
Isolevuglandins form stable pyrrole adducts with the lysyl residues of proteins
Levels of proteins modified by isolevuglandins are increased by cardiovascular risk factors.
Modification of HDL by isolevuglandin inhibits its protective functions.
Dicarbonyl scavengers block myeloperoxidase induced isolevuglandin modification of HDL.
Acknowledgments
Funding sources: This work was supported by the National Institutes of Health grants HL116263 (SSD) and HL138745 (LSZ).
References
- 1.Boutaud O, Brame CJ, Salomon RG, Roberts LJ 2nd and Oates JA Characterization of the lysyl adducts formed from prostaglandin H2 via the levuglandin pathway. Biochemistry 1999;38:9389–96. [DOI] [PubMed] [Google Scholar]
- 2.Salomon RG, Miller DB, Zagorski MG and Coughlin DJ. Solvent-induced fragmentation of prostaglandin endoperoxides. New aldehyde products from PGH2 and a novel intramolecular 1,2-hydride shift during endoperoxide fragmentation in aqueous solution. J Am Chem Soc 1984;106:12. [Google Scholar]
- 3.Daugherty A, Dunn JL, Rateri DL and Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest 1994;94:437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poliakov E, Brennan ML, Macpherson J, Zhang R, Sha W, Narine L, Salomon RG and Hazen SL. Isolevuglandins, a novel class of isoprostenoid derivatives, function as integrated sensors of oxidant stress and are generated by myeloperoxidase in vivo. FASEB J 2003;17:2209–20. [DOI] [PubMed] [Google Scholar]
- 5.Li W, Laird JM, Lu L, Roychowdhury S, Nagy LE, Zhou R, Crabb JW and Salomon RG. Isolevuglandins covalently modify phosphatidylethanolamines in vivo: detection and quantitative analysis of hydroxylactam adducts. Free Radic Biol Med 2009;47:1539–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo L, Chen Z, Amarnath V and Davies SS. Identification of novel bioactive aldehyde-modified phosphatidylethanolamines formed by lipid peroxidation. Free Radic Biol Med 2012;53:1226–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mont S, Davies SS, Roberts Second LJ, Mernaugh RL, McDonald WH, Segal BH, Zackert W, Kropski JA, Blackwell TS, Sekhar KR, Galligan JJ, Massion PP, Marnett LJ, Travis EL and Freeman ML. Accumulation of isolevuglandin-modified protein in normal and fibrotic lung. Sci Rep 2016;6:24919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sancho P, Martin-Sanz P and Fabregat I. Reciprocal regulation of NADPH oxidases and the cyclooxygenase-2 pathway. Free Radic Biol Med 2011;51:1789–98. [DOI] [PubMed] [Google Scholar]
- 9.Boutaud O, Li J, Zagol I, Shipp EA, Davies SS, Roberts LJ 2nd and Oates JA Levuglandinyl adducts of proteins are formed via a prostaglandin H2 synthase-dependent pathway after platelet activation. J Biol Chem 2003;278:16926–8. [DOI] [PubMed] [Google Scholar]
- 10.Boutaud O, Andreasson KI, Zagol-Ikapitte I and Oates JA. Cyclooxygenase-dependent lipid-modification of brain proteins. Brain Pathol 2005;15:139–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrier EJ, Zagol-Ikapitte I, Amarnath V, Boutaud O and Oates JA. Levuglandin forms adducts with histone h4 in a cyclooxygenase-2-dependent manner, altering its interaction with DNA. Biochemistry 2014;53:2436–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brame CJ, Salomon RG, Morrow JD and Roberts LJ 2nd. Identification of extremely reactive gamma-ketoaldehydes (isolevuglandins) as products of the isoprostane pathway and characterization of their lysyl protein adducts. J Biol Chem 1999;274:13139–46. [DOI] [PubMed] [Google Scholar]
- 13.DiFranco E, Subbanagounder G, Kim S, Murthi K, Taneda S, Monnier VM and Salomon RG. Formation and stability of pyrrole adducts in the reaction of levuglandin E2 with proteins. Chem Res Toxicol 1995;8:61–7. [DOI] [PubMed] [Google Scholar]
- 14.Davies SS, Amarnath V, Brame CJ, Boutaud O and Roberts LJ 2nd. Measurement of chronic oxidative and inflammatory stress by quantification of isoketal/levuglandin gamma-ketoaldehyde protein adducts using liquid chromatography tandem mass spectrometry. Nat Protoc 2007;2:2079–91. [DOI] [PubMed] [Google Scholar]
- 15.Salomon RG, Sha W, Brame C, Kaur K, Subbanagounder G, O’Neil J, Hoff HF and Roberts LJ 2nd. Protein adducts of iso[4]levuglandin E2, a product of the isoprostane pathway, in oxidized low density lipoprotein. J Biol Chem 1999;274:20271–80. [DOI] [PubMed] [Google Scholar]
- 16.Davies SS, Talati M, Wang X, Mernaugh RL, Amarnath V, Fessel J, Meyrick BO, Sheller J and Roberts LJ 2nd. Localization of isoketal adducts in vivo using a single-chain antibody. Free Radic Biol Med 2004;36:1163–74. [DOI] [PubMed] [Google Scholar]
- 17.Salomon RG, Batyreva E, Kaur K, Sprecher DL, Schreiber MJ, Crabb JW, Penn MS, DiCorletoe AM, Hazen SL and Podrez EA. Isolevuglandin-protein adducts in humans: products of free radical-induced lipid oxidation through the isoprostane pathway. Biochim Biophys Acta 2000;1485:225–35. [DOI] [PubMed] [Google Scholar]
- 18.Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, Saleh MA, Trott DW, Itani HA, Vinh A, Amarnath V, Amarnath K, Guzik TJ, Bernstein KE, Shen XZ, Shyr Y, Chen SC, Mernaugh RL, Laffer CL, Elijovich F, Davies SS, Moreno H, Madhur MS, Roberts J 2nd, and Harrison DG. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest 2014;124:4642–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu J, Saleh MA, Kirabo A, Itani HA, Montaniel KR, Xiao L, Chen W, Mernaugh RL, Cai H, Bernstein KE, Goronzy JJ, Weyand CM, Curci JA, Barbaro NR, Moreno H, Davies SS, Roberts LJ 2nd, Madhur MS and Harrison DG. Immune activation caused by vascular oxidation promotes fibrosis and hypertension. The Journal of clinical investigation 2016;126:1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dixon KB, Davies SS and Kirabo A. Dendritic cells and isolevuglandins in immunity, inflammation, and hypertension. Am J Physiol Heart Circ Physiol 2017;312:H368–H374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.May-Zhang LS, Yermalitsky V, Huang J, Pleasent T, Borja MS, Oda MN, Jerome WG, Yancey PG, Linton MF and Davies SS. Modification by isolevuglandins, highly reactive gamma-ketoaldehydes, deleteriously alters HDL structure and function. J Biol Chem 2018. [DOI] [PMC free article] [PubMed]
- 22.Guo L, Chen Z, Amarnath V, Yancey PG, Van Lenten BJ, Savage JR, Fazio S, Linton MF and Davies SS. Isolevuglandin-type lipid aldehydes induce the inflammatory response of macrophages by modifying phosphatidylethanolamines and activating the receptor for advanced glycation endproducts. Antioxid Redox Signal 2015;22:1633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salomon RG, Kaur K and Batyreva E. Isolevuglandin-protein adducts in oxidized low density lipoprotein and human plasma: a strong connection with cardiovascular disease. Trends Cardiovasc Med 2000;10:53–9. [DOI] [PubMed] [Google Scholar]
- 24.Yan HP, Roberts LJ, Davies SS, Pohlmann P, Parl FF, Estes S, Maeng J, Parker B and Mernaugh R. Isolevuglandins as a gauge of lipid peroxidation in human tumors. Free Radic Biol Med 2017;106:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zagol-Ikapitte I, Masterson TS, Amarnath V, Montine TJ, Andreasson KI, Boutaud O and Oates JA. Prostaglandin H(2)-derived adducts of proteins correlate with Alzheimer’s disease severity. J Neurochem 2005;94:1140–5. [DOI] [PubMed] [Google Scholar]
- 26.Charvet CD, Saadane A, Wang M, Salomon RG, Brunengraber H, Turko IV and Pikuleva IA. Pretreatment with pyridoxamine mitigates isolevuglandin-associated retinal effects in mice exposed to bright light. J Biol Chem 2013;288:29267–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veal E and Day A. Hydrogen peroxide as a signaling molecule. Antioxid Redox Signal 2011;15:147–51. [DOI] [PubMed] [Google Scholar]
- 28.Vivekananthan DP, Penn MS, Sapp SK, Hsu A and Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet 2003;361:2017–23. [DOI] [PubMed] [Google Scholar]
- 29.Lonn E, Yusuf S, Dzavik V, Doris C, Yi Q, Smith S, Moore-Cox A, Bosch J, Riley W, Teo K and Investigators S. Effects of ramipril and vitamin E on atherosclerosis: the study to evaluate carotid ultrasound changes in patients treated with ramipril and vitamin E (SECURE). Circulation 2001;103:919–25. [DOI] [PubMed] [Google Scholar]
- 30.Amarnath V, Amarnath K, Amarnath K, Davies S and Roberts LJ 2nd. Pyridoxamine: an extremely potent scavenger of 1,4-dicarbonyls. Chem Res Toxicol 2004;17:410–5. [DOI] [PubMed] [Google Scholar]
- 31.Davies SS, Brantley EJ, Voziyan PA, Amarnath V, Zagol-Ikapitte I, Boutaud O, Hudson BG, Oates JA and Roberts LJ 2nd. Pyridoxamine analogues scavenge lipid-derived gamma-ketoaldehydes and protect against H2O2-mediated cytotoxicity. Biochemistry 2006;45:15756–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zagol-Ikapitte I, Amarnath V, Bala M, Roberts LJ 2nd, Oates JA and Boutaud O. Characterization of scavengers of gamma-ketoaldehydes that do not inhibit prostaglandin biosynthesis. Chem Res Toxicol 2010;23:240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han EH, Kim HG, Lee EJ and Jeong HG. Endosulfan Induces CYP1A1 Expression Mediated through Aryl Hydrocarbon Receptor Signal Transduction by Protein Kinase C. Toxicol Res 2015;31:339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zagol-Ikapite I, Sosa IR, Oram D, Judd A, Amarnath K, Amarnath V, Stec D, Oates JA and Boutaud O. Modification of platelet proteins by malondialdehyde: prevention by dicarbonyl scavengers. J Lipid Res 2015;56:2196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies SS and Zhang LS. Reactive Carbonyl Species Scavengers-Novel Therapeutic Approaches for Chronic Diseases. Curr Pharmacol Rep 2017;3:51–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zagol-Ikapitte I, Amarnath V, Jadhav S, Oates JA and Boutaud O. Determination of 3-methoxysalicylamine levels in mouse plasma and tissue by liquid chromatography-tandem mass spectrometry: application to in vivo pharmacokinetics studies. J Chromatogr B Analyt Technol Biomed Life Sci 2011;879:1098–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zagol-Ikapitte I, Matafonova E, Amarnath V, Bodine CL, Boutaud O, Tirona RG, Oates JA, Roberts LJ Ii and Davies SS. Determination of the Pharmacokinetics and Oral Bioavailability of Salicylamine, a Potent gamma-Ketoaldehyde Scavenger, by LC/MS/MS. Pharmaceutics 2010;2:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakajima T, Davies SS, Matafonova E, Potet F, Amarnath V, Tallman KA, Serwa RA, Porter NA, Balser JR, Kupershmidt S and Roberts LJ 3rd. Selective gamma-ketoaldehyde scavengers protect Nav1.5 from oxidant-induced inactivation. J Mol Cell Cardiol 2010;48:352–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sidorova TN, Yermalitskaya LV, Mace LC, Wells KS, Boutaud O, Prinsen JK, Davies SS, Roberts LJ 2nd, Dikalov SI, Glabe CG, Amarnath V, Barnett JV and Murray KT. Reactive gamma-ketoaldehydes promote protein misfolding and preamyloid oligomer formation in rapidly-activated atrial cells. J Mol Cell Cardiol 2015;79:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zagol-Ikapitte IA, Matafonova E, Amarnath V, Bodine CL, Boutaud O, Tirona RG, Oates JA, Roberts LJ 2nd, and Davies SS. Determination of the Pharmacokinetics and Oral Bioavailability of Salicylamine, a Potent gamma-Ketoaldehyde Scavenger, by LC/MS/MS. Pharmaceutics 2010;2:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davies SS, Bodine C, Matafonova E, Pantazides BG, Bernoud-Hubac N, Harrison FE, Olson SJ, Montine TJ, Amarnath V and Roberts LJ 2nd. Treatment with a gamma-ketoaldehyde scavenger prevents working memory deficits in hApoE4 mice. J Alzheimers Dis 2011;27:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pitchford LM, Smith JD, Abumrad NN, Rathmacher JA and Fuller JC Jr. Acute and 28-day repeated dose toxicity evaluations of 2-hydroxybenzylamine acetate in mice and rats. Regul Toxicol Pharmacol 2018;98:190–198. [DOI] [PubMed] [Google Scholar]
- 43.Hoppe G, Subbanagounder G, O’Neil J, Salomon RG and Hoff HF. Macrophage recognition of LDL modified by levuglandin E2, an oxidation product of arachidonic acid. Biochim Biophys Acta 1997;1344:1–5. [DOI] [PubMed] [Google Scholar]
- 44.Fogelman AM, Shechter I, Seager J, Hokom M, Child JS and Edwards PA. Malondialdehyde alteration of low density lipoproteins leads to cholesteryl ester accumulation in human monocyte-macrophages. Proc Natl Acad Sci U S A 1980;77:2214–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoff HF, O’Neil J, Chisolm GM 3rd, Cole TB, Quehenberger O, Esterbauer H and Jurgens G. Modification of low density lipoprotein with 4-hydroxynonenal induces uptake by macrophages. Arteriosclerosis 1989;9:538–49. [DOI] [PubMed] [Google Scholar]
- 46.Navab M, Reddy ST, Van Lenten BJ and Fogelman AM. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat Rev Cardiol 2011;8:222–32. [DOI] [PubMed] [Google Scholar]
- 47.Ma J, Liao XL, Lou B and Wu MP. Role of apolipoprotein A-I in protecting against endotoxin toxicity. Acta Biochim Biophys Sin (Shanghai) 2004;36:419–24. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y, Zanotti I, Reilly MP, Glick JM, Rothblat GH and Rader DJ. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation 2003;108:661–3. [DOI] [PubMed] [Google Scholar]
- 49.Kataoka Y, Shao M, Wolski K, Uno K, Puri R, Tuzcu EM, Hazen SL, Nissen SE and Nicholls SJ. Myeloperoxidase Levels Predict Accelerated Progression of Coronary Atherosclerosis in Diabetic Patients: Insights from Intravascular Ultrasound. Atherosclerosis 2014;232:377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hewing B, Parathath S, Barrett T, Chung WKK, Astudillo YM, Hamada T, Ramkhelawon B, Tallant TC, Yusufishaq MSS, DiDonato JA, Huang Y, Buffa J, Berisha SZ, Smith JD, Hazen SL and Fisher EA. Effects of native and myeloperoxidase-modified apolipoprotein A-I on reverse cholesterol transport and atherosclerosis in mice. Arteriosclerosis, thrombosis, and vascular biology 2014;34:779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bergt C, Pennathur S, Fu X, Byun J, O’Brien K, McDonald TO, Singh P, Anantharamaiah GM, Chait A, Brunzell J, Geary RL, Oram JF and Heinecke JW. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci U S A 2004;101:13032–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shao B, Oda MN, Bergt C, Fu X, Green PS, Brot N, Oram JF and Heinecke JW. Myeloperoxidase impairs ABCA1-dependent cholesterol efflux through methionine oxidation and site-specific tyrosine chlorination of apolipoprotein A-I. J Biol Chem 2006;281:9001–4. [DOI] [PubMed] [Google Scholar]
- 53.Kunkel SL, Spengler M, May MA, Spengler R, Larrick J and Remick D. Prostaglandin E2 regulates macrophage-derived tumor necrosis factor gene expression. J Biol Chem 1988;263:5380–4. [PubMed] [Google Scholar]
- 54.Kunkel SL, Wiggins RC, Chensue SW and Larrick J. Regulation of macrophage tumor necrosis factor production by prostaglandin E2. Biochem Biophys Res Commun 1986;137:404–10. [DOI] [PubMed] [Google Scholar]
- 55.Jing H, Vassiliou E and Ganea D. Prostaglandin E2 inhibits production of the inflammatory chemokines CCL3 and CCL4 in dendritic cells. J Leukoc Biol 2003;74:868–79. [DOI] [PubMed] [Google Scholar]
- 56.Vassiliou E, Jing H and Ganea D. Prostaglandin E2 inhibits TNF production in murine bone marrow-derived dendritic cells. Cell Immunol 2003;223:120–32. [DOI] [PubMed] [Google Scholar]
- 57.Xu XJ, Reichner JS, Mastrofrancesco B, Henry WL Jr., and Albina JE. Prostaglandin E2 suppresses lipopolysaccharide-stimulated IFN-beta production. J Immunol 2008;180:2125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Akaogi J, Yamada H, Kuroda Y, Nacionales DC, Reeves WH and Satoh M. Prostaglandin E2 receptors EP2 and EP4 are up-regulated in peritoneal macrophages and joints of pristane-treated mice and modulate TNF-alpha and IL-6 production. J Leukoc Biol 2004;76:227–36. [DOI] [PubMed] [Google Scholar]
- 59.Sakamoto A, Matsumura J, Mii S, Gotoh Y and Ogawa R. A prostaglandin E2 receptor subtype EP4 agonist attenuates cardiovascular depression in endotoxin shock by inhibiting inflammatory cytokines and nitric oxide production. Shock 2004;22:76–81. [DOI] [PubMed] [Google Scholar]
- 60.Tang T, Scambler TE, Smallie T, Cunliffe HE, Ross EA, Rosner DR, O’Neil JD and Clark AR. Macrophage responses to lipopolysaccharide are modulated by a feedback loop involving prostaglandin E2, dual specificity phosphatase 1 and tristetraprolin. Sci Rep 2017;7:4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Borja MS, Zhao L, Hammerson B, Tang C, Yang R, Carson N, Fernando G, Liu X, Budamagunta MS, Genest J, Shearer GC, Duclos F and Oda MN. HDL-apoA-I exchange: rapid detection and association with atherosclerosis. PLoS One 2013;8:e71541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McDonnell WJ, Koethe JR, Mallal SA, Pilkinton MA, Kirabo A, Ameka MK, Cottam MA, Hasty AH and Kennedy AJ. High CD8 T Cell Receptor Clonality and Altered CDR3 Properties are Associated with Elevated Isolevuglandins in Adipose Tissue During Diet-Induced Obesity. Diabetes 2018. [DOI] [PMC free article] [PubMed]
- 63.Stavrovskaya IG, Baranov SV, Guo X, Davies SS, Roberts LJ 2nd, and Kristal BS. Reactive gamma-ketoaldehydes formed via the isoprostane pathway disrupt mitochondrial respiration and calcium homeostasis. Free Radic Biol Med 2010;49:567–79. [DOI] [PMC free article] [PubMed] [Google Scholar]