Abstract
The purposes of this study were to 1) evaluate the intra-rater reliability of estimating Achilles tendon mechanical properties with continuous shear wave elastography (cSWE), 2) propose an equivalent shear modulus comparable to Supersonic Shear Imaging, 3) demonstrate construct validity of cSWE, and 4) explore relationships between tensile and shear properties. Achilles tendon mechanical properties were estimated with cSWE at four time points throughout a four-hour period and at a 2-week follow up. Additionally, properties were estimated with cSWE across four different ankle positions. In these four positions, B-mode ultrasound imaging and dynamometry were used to quantify Young’s modulus. Intra-rater reliability was fair-to-excellent for Achilles tendon mechanical properties estimated with cSWE. Construct validity was demonstrated with increased ankle dorsiflexion leading to increased mechanical properties. Linear relationships were found between tensile and shear mechanical properties. Findings demonstrate that cSWE has sufficient intra-rater reliability and validity for estimating Achilles tendon mechanical properties.
Keywords: Viscoelastic Properties, Ultrasound Imaging, Tendon, Sonoelastography
INTRODUCTION & LITERATURE
The Achilles tendon is a highly-organized, densely-packed collagenous tissue that acts to transfer loads between muscle and bone. Furthermore, the Achilles tendon has a mechanical function that allows energy to be stored and released into the musculoskeletal system during dynamic activities. Achilles tendon injury is characterized by disorganization and buckling of collagen fibers, which alters the tendon’s mechanical properties (Järvinen et al. 1997; Kannus and Józsa 1991). Consequently, mechanical properties (e.g. Young’s modulus and shear modulus) have recently been proposed as biomarkers for tendon injury and recovery (Cortes et al. 2015; Dirrichs et al. 2018; Schepull et al. 2012).
Traditionally, real-time ultrasound imaging is synchronized with dynamometry to evaluate tendon tensile stiffness and Young’s modulus (Arya and Kulig 2010; Kongsgaard et al. 2011; Maganaris and Paul 1999). Although this technique accurately measures tensile properties in healthy tendon, it relies on volitional contractions, places large tensile loads on the tendon, and assumes that the tendon is homogenous. These features may negatively affect the accuracy of measuring tensile properties in patients with Achilles tendinopathy because of tissue heterogeneity and alterations in muscle activity (Baur et al. 2011; Wyndow et al. 2013). Also, because of the large tensile loads, this technique is contraindicated in patients early after Achilles tendon rupture. Therefore, there is a need to develop valid and reliable techniques for measuring tendon mechanical properties in injured populations.
In recent years, various shear wave elastography (SWE) techniques have been developed and commercialized to estimate Achilles tendon shear properties (e.g. shear elasticity, shear modulus). These properties represent the tendon’s resistance to a shear force, which is a different mechanical behavior compared to traditional tensile testing. However, measuring shear properties may improve our understanding of tendon injury since non-uniform shear forces between tendon fascicles has been identified as a possible explanation of pathogenesis(Arndt et al. 2012; Franz et al. 2015) and shear forces are thought to stimulate tenocytes to promote tendon healing(Sun et al. 2015). Additionally, these techniques put minimal tension on the tendon and allow for spatial variation of shear properties to be measured (Dirrichs et al. 2018; Zhang et al. 2016). Although SWE techniques appear ideal for measuring tendon shear properties, there are several limitations. Saturation of the elastogram (i.e. the elasticity map) is a major limitation of many commercially-available elastography techniques, especially when evaluating healthy tendons (Chen et al. 2013; DeWall et al. 2015). Saturation occurs when the elasticity of the tendon exceeds the upper limit of the elasticity scale that is used to render the elastogram. This is problematic since measurements taken from a saturated elastogram yield underestimated mechanical properties. Anisotropy is another limitation of SWE that may influence the accuracy of measuring mechanical properties. The calculations used to estimate mechanical properties with SWE assume the tendon is an isotropic material, which does not match the well-known elastic anisotropy of the tendon. However, the mechanical properties calculated represent an overall/average measurement of the elasticity of the tendon. By standardizing ultrasound probe alignment (i.e. parallel to tendon fibers based on B-mode images), patient positioning, and the region of interest, SWE mechanical properties may be reliably measured over time. Despite these limitations, it appears that critical information about tendon injury and recovery could be gained from developing a valid and reliable elastography technique that is safe for evaluating injured populations and is capable of measuring tendon mechanical properties without saturation.
We recently developed an ultrasound elastography method named continuous Shear Wave Elastography (cSWE) to measure tendon mechanical properties (static shear modulus and viscosity) in vivo (Cortes et al. 2015). cSWE is a modification of the Supersonic Shear Imaging (SSI) technique that uses an external actuator to generate shear waves across a specified range of frequencies. Frequency and shear wave speed data measured with cSWE is then analyzed using the Voigt model to estimate the static shear modulus and viscosity of the tendon. Other techniques use transient shear waves, which estimate a dynamic shear modulus of the tissue. cSWE has been found to measure shear wave velocities in healthy tendon above the capacity of some commercially-available scanners, which negates the concern of saturating the elastogram (Cortes et al. 2015). It has also been applied to individuals with healthy Achilles tendons (Suydam et al. 2015), Achilles tendinopathy (Cortes et al. 2015), Achilles tendon rupture (Zellers et al. 2016), and hamstring tendon (Suydam et al. 2017). Test-retest reliability during a single experimental session has been previously reported (Suydam et al. 2015). However, intra-rater reliability of cSWE over longer durations has not been investigated, which is critical to evaluate before measuring changes in tendon mechanical properties over time. Furthermore, criterion validity has been demonstrated by using agarose gels with known mechanical properties to compare measurements between cSWE and magnetic resonance elastography (Cortes et al. 2015), but validation in tendon tissue is limited to observations of side-to-side differences in individuals with tendon pathology (Cortes et al. 2015; Zellers et al. 2017). The use of cSWE for evaluating human tendon tissue would be further supported by demonstrating construct validity (i.e. the ability of a tool to measure the construct it was designed to measure). Additionally, since mechanical properties measured with cSWE (static shear modulus and viscosity) represent different mechanical behaviors compared to commercial elastography techniques (shear elasticity) and traditional tensile testing (Young’s modulus), it is necessary to calculate an equivalent/dynamic shear modulus that more closely relates to measures obtained with the SSI technique.
The purposes of this study were to evaluate the intra-rater reliability and stability of measuring mechanical properties with cSWE in healthy Achilles tendons, propose a measure of dynamic shear modulus determined with cSWE that is equivalent to shear elasticity measured with SSI, demonstrate construct validity of cSWE, and explore relationships between shear and tensile properties. Findings from this investigation are important to ascertain prior to evaluating Achilles tendon mechanical properties with cSWE over time or exploring relationships between these properties and clinical, functional and patient-reported outcomes.
MATERIALS & METHODS
Study Design
The study was performed in two separate parts – reliability and validation. All individuals included were without Achilles tendon pathology, as determined through a clinical examination performed by a licensed physical therapist, and showed no signs of tendon degeneration during screening with B-mode ultrasound imaging (tendon thickening or hypoechoic areas). Both parts of the study were approved by the institutional review board at the University of Delaware and subjects provided written consent after being informed of experimental procedures.
Reliability
Subjects
Twenty subjects (11 males, 9 females) with a mean (SD) age of 29 (4) years, height of 177 (8) cm, and body mass of 82 (18) kg participated in the reliability portion of this study.
Experimental Procedures
Continuous shear wave elastography (cSWE) was used to estimate Achilles tendon static shear modulus and viscosity (Cortes et al. 2015). Minor differences existed between the experimental procedures for cSWE in the current study and the originally described technique (Cortes et al. 2015). The ultrasound scanner used in the current study was a SonixMDP Q+ (Ultrasonix, Vancouver, Canada), which is an upgraded model of the SonixMDP scanner used in the original study. Both studies however used the same L14–5/38 ultrasound transducer. The frame rate in the current study was 6438 frames/s compared to 6450 frames/s in the original study. In order to maintain an integer fraction of frames per wave period, excitation frequencies were adjusted. Additionally, raw radiofrequency (RF) data were acquired for twelve frequencies, instead of six. The excitation frequencies were 322, 339, 358, 379, 402, 429, 460, 495, 536, 585, and 643 Hz compared to the original six frequencies of 323, 340, 358, 379, 403, and 430 Hz. RF data were post-processed as described previously, except every other frequency was used to allow for a wider range of frequencies to better estimate viscoelastic properties. For highly compliant and stiff tendons, the first six or last six frequencies are used to keep the wavelength comparable to the size of the region of interest (ROI). However, throughout both parts of the current study, every other frequency was used. Static shear modulus and viscosity were calculated on a pixel-wise basis and averaged across each pixel within the central 50% of the ROI. The original method included all pixels within the ROI. However, inaccuracies associated with the Local Frequency Estimation (LFE) method for estimating wave speed are minimized by ignoring pixels within 25% of each lateral border. In addition to these technical modifications, foot positioning was altered from 0 degrees of ankle plantarflexion to −10 degrees. The dorsiflexed position ensured that the tendon was within the linear elastic region (i.e. where the tendon has a constant elastic modulus). To maintain a consistent position and limit muscle activity, the subject’s feet were secured against a platform with straps across the dorsum of the feet. This modification has previously been used (Suydam et al. 2015) and was selected for the current study due to difficulties with designing splints that accommodate different lower leg shapes and sizes.
All 20 subjects completed two experimental sessions with two weeks between sessions. During the first session, data was collected at four time points, which can be described as baseline, 30 minutes after baseline, 2 hours after baseline, and 4 hours after baseline. The second session was a single time point. During each time point, three trials of cSWE were performed by a single experienced evaluator to one Achilles tendon and the average static shear modulus and viscosity were used for analysis. A computer-generated randomization scheme was used to select which Achilles tendon (right or left side) would be used throughout testing. Data was not collected bilaterally since Suydam et al. (2015) showed there are no side-to-side differences in Achilles tendon mechanical properties estimated with cSWE. The randomization led to cSWE being performed on the dominant leg for 11 individuals and non-dominant leg for 9 individuals. Leg dominance was determined by subject-reported preferred kicking leg. To reduce the effects of activities performed between the four time points, subjects were allowed to perform everyday tasks (e.g. walking, sitting) between time points, but were asked to refrain from activities thought to impact the mechanical properties of the tendon (e.g. stretching, jumping, weightlifting, running). Additionally, since regional differences exist in mechanical properties along the length of the Achilles tendon (DeWall et al. 2015), all measurements were taken at a location in the midportion of the tendon just distal to the myotendinous junction of the soleus. This location was reproduced during each session by recording the distance from the proximal calcaneal insertion to the region of interest (Silbernagel et al. 2016).
Dynamic Shear Modulus
As stated above, cSWE uses a series of continuous shear waves ranging from 322 Hz to 643 Hz. By analyzing the change of wave speed in that frequency range, two viscoelastic properties are calculated: static shear modulus and viscosity. The SSI technique measures the group velocity of a broadband shear wave traveling through the tissue. Dispersion analysis of the transient shear wave generated by SSI in the Achilles tendon shows that shear wave has an approximately frequency range from 300 Hz to 700 Hz, and a stronger intensity at about 400 Hz (Brum et al. 2014). Consequently, we define a dynamic shear modulus, from cSWE measurements, using the static shear modulus, viscosity and the Voigt model as follows:
where is the dynamic shear modulus at a frequency, f,μ1 is the static shear modulus, and μ2 the viscosity. In order to have a dynamic shear modulus comparable to that obtained by SSI, a frequency of 400 Hz was selected. Intra-rater reliability of this new measure of dynamic shear modulus was evaluated using data obtained from the reliability portion of this study. Additionally, using data from the validation portion of this study, the relationship between dynamic shear modulus and Young’s modulus was explored.
Validation
Subjects
Six subjects (3 males, 3 females) with a mean (SD) age of 21 (1) years, height of 170 (11) cm, and body mass of 62 (12) kg participated in the validation portion of this study. These subjects did not participate in the reliability portion of this study.
Experimental Procedures
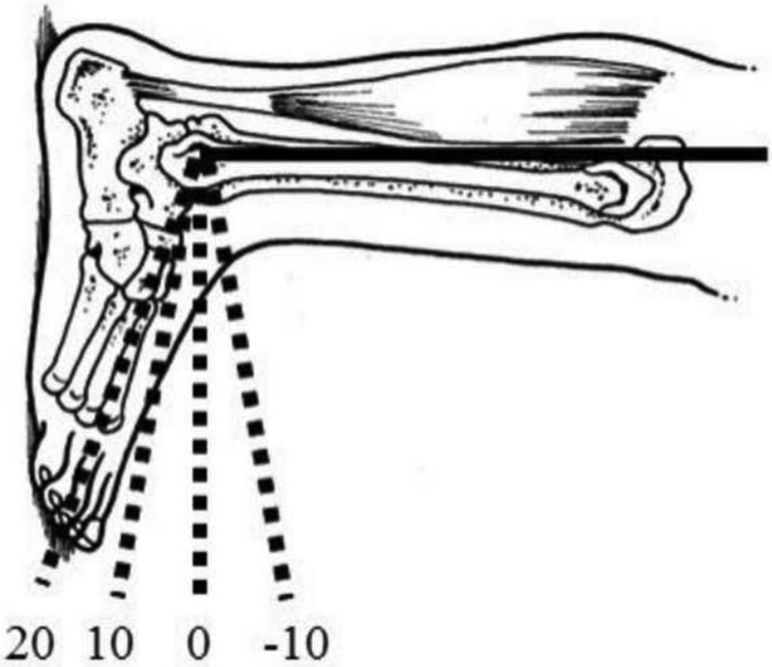
Subjects were positioned in prone with their knee fully extended and foot secured in a KinCom dynamometer (Model 500H, Isokinetic International, Chattanooga, TN, USA) at four foot positions, which included 20°, 10°, 0°, and −10° of ankle plantarflexion (Figure 1). The order of foot positions was randomized and subjects were given a 2-minute break between foot positions to walk around the room. Only right legs were tested, which led to procedures being performed on 5 dominant legs and 1 non-dominant leg. Static shear modulus, viscosity, and dynamic shear modulus were determined as described in the reliability portion of this study. The only difference was that the technique was performed in the four different ankle positions.
Figure 1.
Foot position naming convention
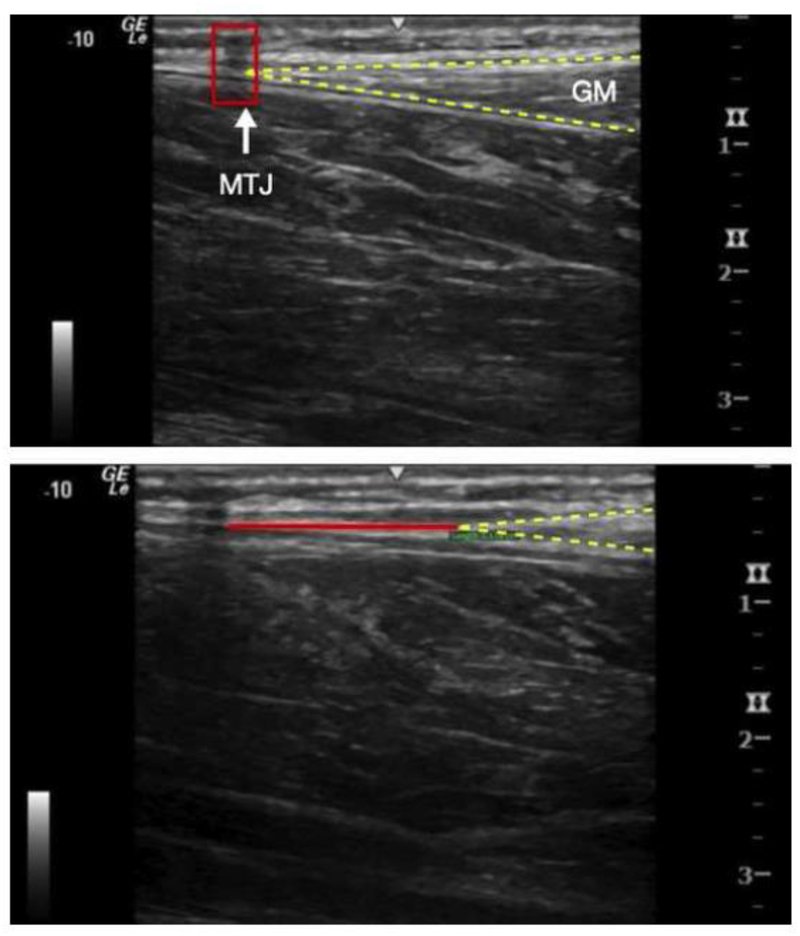
Young’s modulus of the Achilles tendon was estimated in the four foot positions using data obtained from ultrasound imaging and a KinCom dynamometer during three 5-second maximal voluntary isometric contractions (MVIC). The experimental procedures used in the current study were modified from previously described techniques (Arya and Kulig 2010; Kongsgaard et al. 2011). All ultrasound images were gathered with a LOGIQ e Ultrasound system (GE Healthcare, Chicago, IL, USA) with a wide-band linear array probe (5.0–13.0MHz). Achilles tendon length and cross-sectional area were obtained while the subject was prone with their foot hanging naturally over the end of the table. Tendon length was measured as the distance from the calcaneal osteotendinous junction to the myotendinous junction of the medial gastrocnemius using extended field of view settings (Ryan et al. 2013; Silbernagel et al. 2016). Tendon cross-sectional area was measured with short-axis ultrasound images immediately distal to the myotendinous junction of the soleus. Linear displacement of the tendon during each MVIC was measured with cine-loop ultrasound recordings. Peak displacement between a tape shadow positioned at rest and the myotendinous junction of the medial gastrocnemius during MVIC was measured (Figure 2).
Figure 2.
Measurement of displacement of the medial gastrocnemius (GM) myotendinous junction (MTJ). A. Prior to contraction B. During muscle contraction.
Peak force was measured during each MVIC using a KinCom dynamometer. The moment arm of the load cell was fixed to 19.5 cm for all subjects. Peak plantarflexion force created by the triceps surae was then estimated by dividing the measured external plantarflexion torque at the load cell by the Achilles tendon moment arm. The Achilles tendon moment arm was measured from the midpoint of the lateral malleolus to the most posterior aspect of the tendon with a tape measure. Three MVICs were performed with strong verbal encouragement in each foot position after performing two practice trials. The average peak displacement of the myotendinous junction of the medial gastrocnemius and average peak plantarflexion torque from three trials were used to estimate the Young’s Modulus.
Young’s Modulus was calculated using the following equation:
Where τ is peak plantarflexion torque at the load cell, MA is moment arm of the Achilles tendon, Lo is tendon length at rest, A is tendon cross-sectional area at rest, and ΔL is the peak displacement of the myotendinous junction of the medial gastrocnemius during MVIC.
Statistical Analysis
Intra-rater Reliability
Descriptive data are reported as mean (SD). Reliability analyses were performed using recommendations from Denegar and Ball (1993). Briefly, intra-rater reliability was assessed for each viscoelastic property during the first session using intraclass correlation coefficients (ICC2,3)(Denegar and Ball 1993). Values are reported as ICC (95% CI) and interpreted as poor (<0.40), fair-to-good (0.40–0.75), and excellent (>0.75) reliability as suggested by Shrout and Fleiss (1979).
Stability
Although an ICC provides an estimate of the reliability of a measurement, it does not necessarily reflect the stability of what is being measured over time. Therefore, to evaluate the stability of viscoelastic properties, standard error of measurement (SEM) and minimal detectable change (MDC95%) were calculated using data from the first session. A one-way repeated measures ANOVA was performed for each variable to analyze stability within the first session. A Greenhouse-Geisser correction was applied when assumptions of ANOVA were violated. Partial eta-squared effect sizes are reported and interpreted as suggested by Cohen (1988) as no effect (<0.01), small effect (<0.06), medium effect (<0.14), and large effect (≥0.14). Paired t-tests were performed using baseline data from the first session and data from the second session to evaluate stability over a two week period. Cohen’s d effect sizes are reported and interpreted as no effect (<0.2), small effect (<0.5), medium effect (<0.8), large effect (≥0.8) (Cohen 1988).
Validation
To demonstrate the construct validity of cSWE for measuring viscoelastic properties, values were compared across the four different foot positions. As the foot is moved from a plantarflexed position to a dorsiflexed position, the passive tension within the Achilles tendon increases. Since tension is directly related to wave speed propagation, viscoelastic properties are expected to increase with increased dorsiflexion. To demonstrate the capability of cSWE to detect this relationship, viscoelastic properties were compared across the four different foot positions using a one-way repeated measures ANOVA. In order to determine the sample size needed, a power analysis was performed using data obtained from the first five participants. With an effect size of η2partial=0.497 for static shear modulus, β=0.8, and α=0.05, it was determined the analysis would be adequately powered with 6 participants.
Relationships of mechanical properties
To explore the relationships between viscoelastic properties measured with cSWE and Young’s modulus measured with ultrasound and dynamometry, group averages at each foot position were calculated, plotted and investigated using Pearson product-moment correlations. All statistical procedures were performed using IBM SPSS v24 (Chicago, IL, USA) at a significance level of p<0.05.
RESULTS
Intra-rater Reliability
The mean (SD) static shear modulus, viscosity, and dynamic shear modulus for each time point are shown in Table 1. Shear modulus had fair-to-good reliability with an ICC of 0.697 (0.392–0.868), viscosity had excellent reliability with an ICC of 0.856 (0.717–0.937), and dynamic shear modulus had excellent reliability with an ICC of 0.855 (0.715–0.936).
Table 1.
Viscoelastic properties measured with cSWE for both experimental sessions.
| Baseline | 30 Minutes | 2 Hours | 4 Hours | 2 Weeks | |
|---|---|---|---|---|---|
| Static Shear Modulus (kPa) | 95.0 (16.0) | 94.6 (15.8) | 94.8 (15.4) | 94.0 (13.0) | 95.7 (13.9) |
| Viscosity (Pa*s) | 57.4 (13.4) | 555 (12.4) | 58.4 (13.3) | 58.5 (11.5) | 56.8 (11.3) |
| Dynamic Shear Modulus (kPa) | 226.8 (49.4) | 218.0 (46.2) | 230.7 (49.0) | 230.6 (42.2) | 224.8 (39.6) |
Stability
For static shear modulus, the SEM was 8.284 kPa, individual level MDC95% was 22.948 kPa, and the group level MDC95% was 5.131 kPa. Viscosity had a SEM of 4.797 Pa*s, an individual level MDC95% of 13.286 Pa*s, and a group level MDC95% of 2.971 Pa*s. Dynamic shear modulus had a SEM of 46.718 kPa, individual level MDC95% of 49.277 kPa, and group level MDC95% of 11.019 kPa.
During the first session, there were no significant differences between time points for static shear modulus (p=0.978; η2partial=0.001), viscosity (p=0.623; η2partial=0.030), and dynamic shear modulus (p=0.502; η2partial=0.040). Additionally, there were no differences for static shear modulus (p=0.854; d=0.050), viscosity (p=0.828; d=0.049), and dynamic shear modulus (p=0.844; d=0.043) between the baseline session and the 2-week follow up session.
Construct Validity
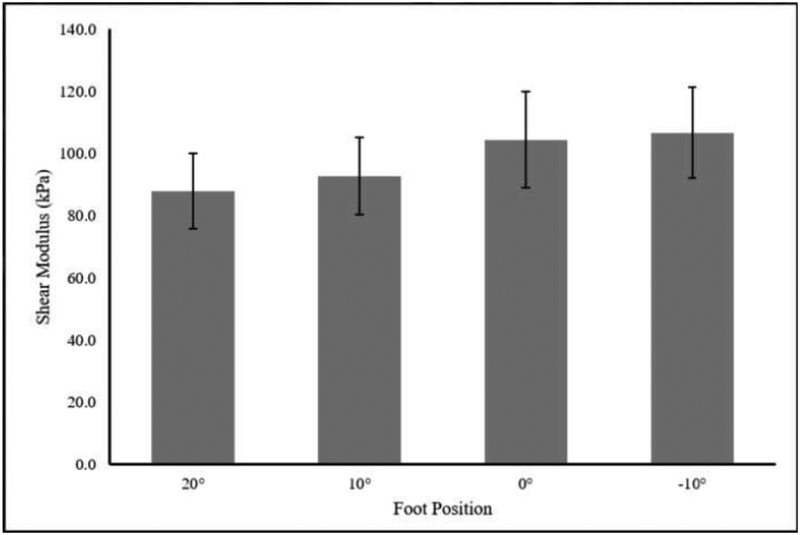
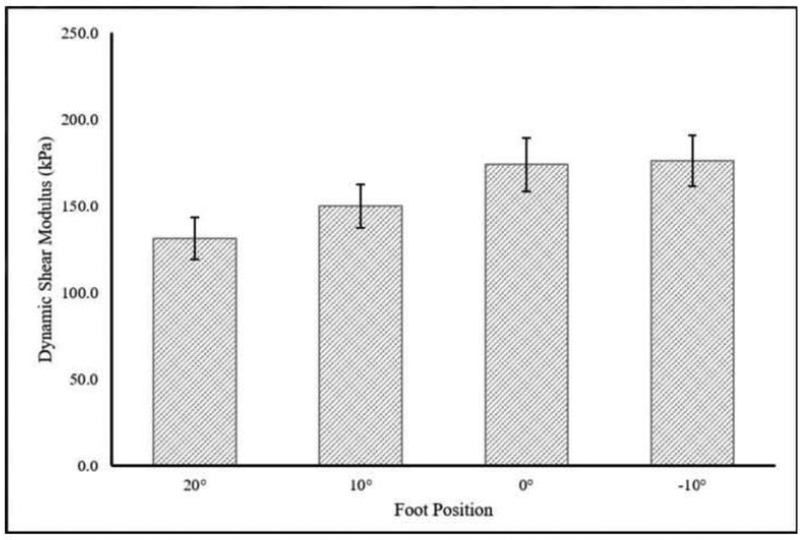
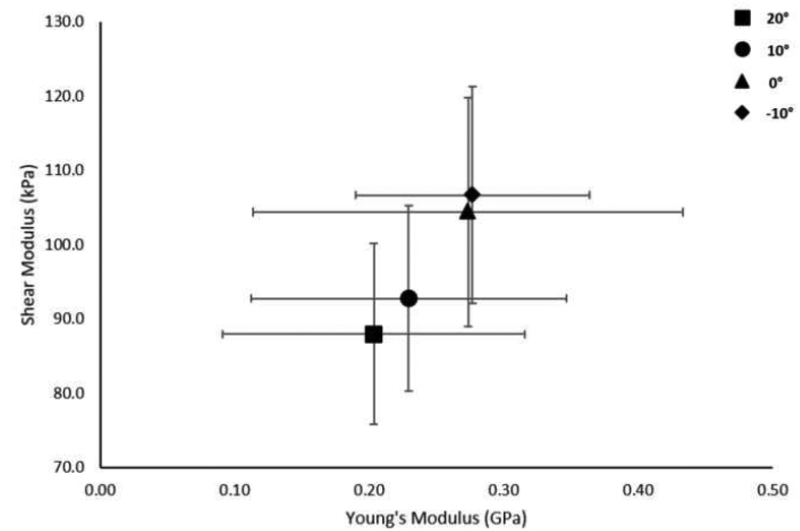
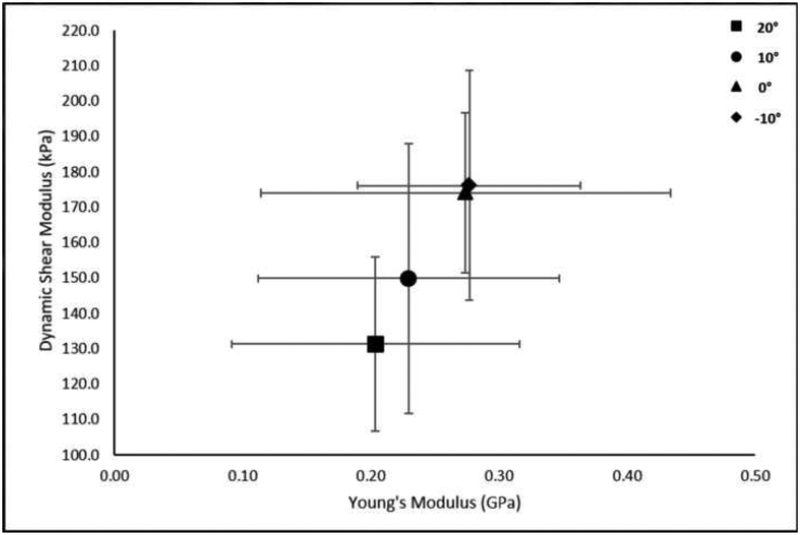
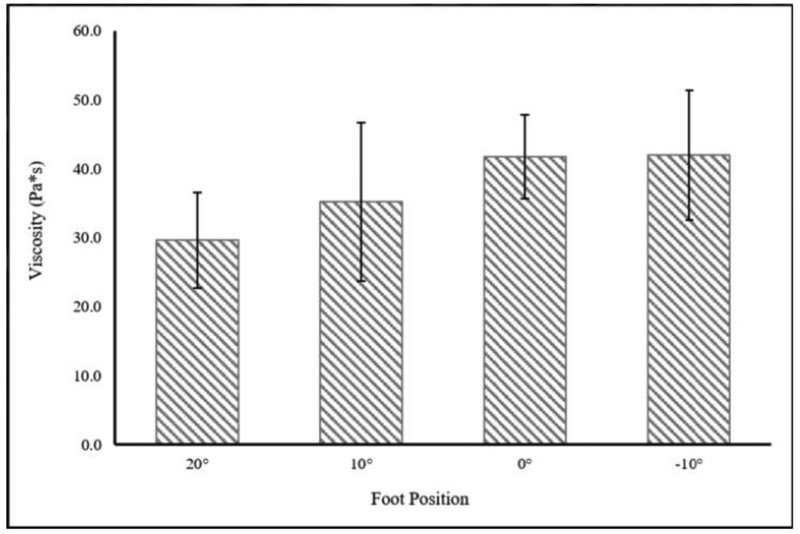
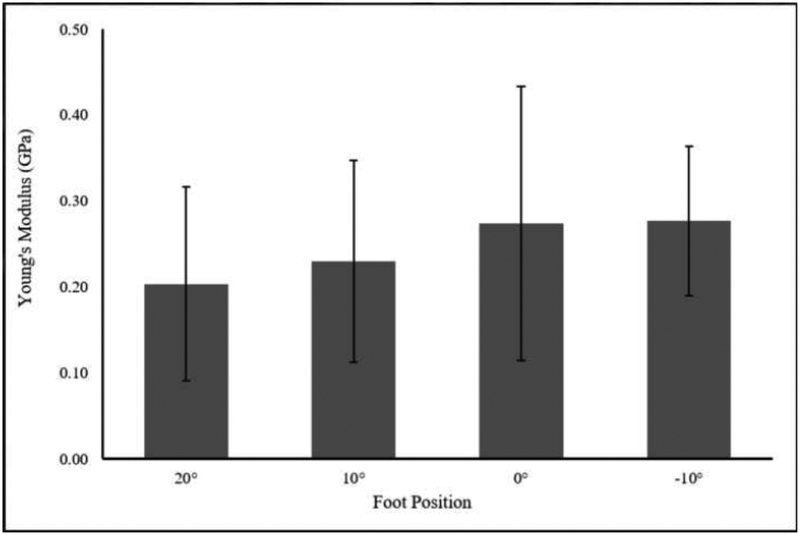
Mean (SD) tendon length to the medial gastrocnemius was 19.4 (3.4) cm and cross-sectional area distal to the myotendinous junction of the soleus was 0.406 (0.075) cm2. Static shear modulus, viscosity, dynamic shear modulus, and Young’s modulus increased with increasing amounts of dorsiflexion. There was a significant main effect of foot position on static shear modulus, viscosity, and dynamic shear modulus, but not on Young’s modulus (Table 2, Figure 3–6).
Table 2.
Tendon Mechanical Properties at different Foot Positions.
| Foot Angle | ||||||
|---|---|---|---|---|---|---|
| 20 degrees | 10 degrees | 0 degrees | -10 degrees | p-value | Effect size | |
| Static Shear Modulus (kPa) | 88.0 (12.2) | 92.7 (12.5) | 104.4 (15.4) | 106.6 (14.6) | 0.036* | 0.425 |
| Viscosity (Pa*s) | 29.7 (6.9) | 35.1 (11.5) | 41.7 (6.0) | 41.9 (9.5) | 0.019* | 0.476 |
| Dynamic Shear Modulus (kPa) | 131.3 (24.6) | 149.9 (38.2) | 174.1 (22.7) | 176.2 (32.4) | 0.013* | 0.502 |
| Young’s Modulus (GPa) | 0.20 (0.11) | 0.23 (0.12) | 0.27 (0.16) | 0.28 (0.09) | 0.118 | 0.316 |
Indicates p < 0.05. PF=Plantarflexion
Figure 3.
Change in Shear Modulus with Foot position. There was a significant effect of foot position on Shear Modulus (p=0.036; η2p=0.497)
Figure 6.
Change in Dynamic Shear Modulus (400Hz) with Foot Position. A significant effect of foot position on Dynamic Shear Modulus was observed (p=0.034; η2p=0.501).
Relationships of Mechanical Properties
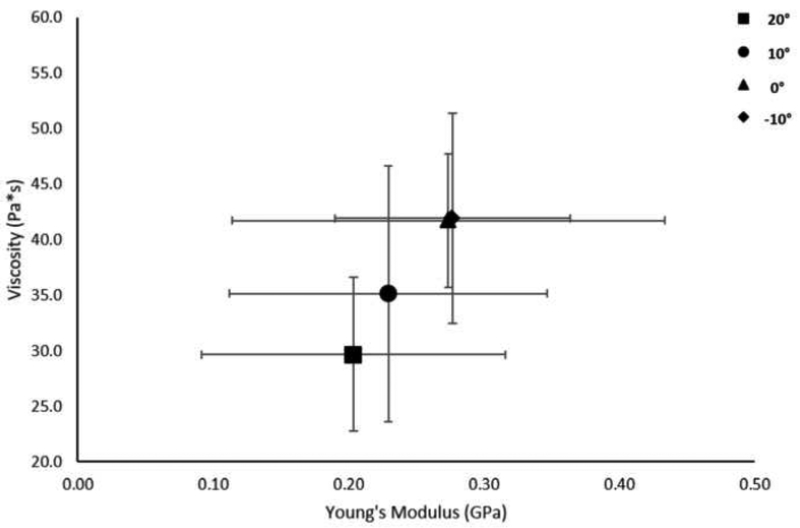
A linear relationship was found between Young’s modulus and static shear modulus (r=0.992, p=0.008), viscosity (r=0.994, p=0.006), and the proposed dynamic shear modulus (r=0.997, p=0.003) (Figures 7–9).
Figure 7.
Relationship of shear modulus and Young’s modulus at the group level. Data points represent group means at the different foot positions. Error bars represent ±1 SD.
Figure 9.
Relationship of Dynamic Shear Modulus and Young’s Modulus at the group level. Data points represent group means at the different foot positions. Error bars represent ±1 SD.
DISCUSSION
We found fair-to-excellent intra-rater reliability for measuring static shear modulus, viscosity, and dynamic shear modulus in healthy Achilles tendons with cSWE. Additionally, these viscoelastic properties appear to be stable over a 4-hour and 2-week period in healthy Achilles tendons. We also further validated cSWE by demonstrating that greater degrees of dorsiflexion lead to increases in viscoelastic properties. Furthermore, the dynamic shear modulus proposed in this study was more comparable to values reported using SSI (Chen et al. 2013) than previous reports of static shear modulus (Cortes et al. 2015; Suydam et al. 2015). Taken together, these findings provide support for the use of cSWE in quantifying Achilles tendon viscoelastic properties over time and using dynamic shear modulus as a new parameter to track these properties.
Reliability of Elastography
Recent evidence has shown that ultrasound elastography can be a useful tool in measuring tendon mechanical properties and appears to be more sensitive in detecting pathologic changes than structural measures obtained from conventional ultrasound (Dirrichs et al. 2018; Klauser et al. 2013; Ooi et al. 2014). These properties have further been suggested as possible biomarkers for diagnosis and tracking structural recovery in the presence of tendon pathology (Cortes et al. 2015; Dirrichs et al. 2018; Schepull et al. 2012). Despite the potential usefulness of elastography, methodological and computational differences exist between the different elastography techniques (i.e. cSWE, SSI, Compression elastography), which directly impacts the ability to compare results between studies and calls into question the reliability and validity of each technique.
Intra-rater reliability of SSI, cSWE, and compression elastography have been reported for measuring Achilles tendon mechanical properties (Drakonaki et al. 2009; Payne et al. 2017; Peltz et al. 2013; Suydam et al. 2015). SSI was found to have fair-to-good (ICC=0.42) intra-rater reliability after pooling data from both right and left tendons, but reliability was poor (ICC=0.17) on the right and fair-to-good (ICC=0.62) on the left (Peltz et al. 2013). Intra-rater reliability of static shear modulus (ICC=0.875) and viscosity (ICC=0.876) measured via cSWE has been shown to be excellent when analyzing three trials performed consecutively (Suydam et al. 2015). Poor intra-rater reliability has been reported for compression elastography when five trials are performed consecutively (ICC=0.11) and when a single trial is performed over five days (ICC=0.01) (Payne et al. 2017). Conversely, Drakonaki et al. (2009) found good-to-excellent (ICC=0.66–0.78) intra-rater reliability for compression elastography when five trials are performed. These results suggest that cSWE may have superior reliability compared to other elastography methods, as demonstrated by the higher intra-class correlation coefficient.
In the current study, we found fair-to-excellent intra-rater reliability for measuring viscoelastic properties over a 4-hour period and determined that these measures are stable in healthy Achilles tendons during a 4-hour and 2-week period. We speculate that these findings may be due to the standardization of experimental procedures (e.g. ultrasound probe alignment, subject positioning, and locating the ROI), minimization of operator-dependent factors (e.g. no pressure application or qualitative grading systems), and performing the study in a healthy population. It is difficult however to compare our findings to the reliability of other elastography techniques since our study used the average of three trials across multiple time points rather than multiple measures at a single time point. Future research is needed to compare the reliability of different elastography techniques for measuring tendon mechanical properties.
Comparison to Supersonic Shear Imaging (SSI)
The average dynamic shear modulus of the Achilles tendon at baseline was 226.8 kPa for the 20 healthy individuals included in the reliability portion of this study. This value represents the estimated modulus at 400 Hz instead of 0 Hz, which is proposed to be more comparable to measures obtained with SSI. Healthy human Achilles tendons have an average reported modulus ranging from 51.5 kPa and 779.5 kPa when measured with SSI (Arda et al. 2011; Aubry et al. 2013; Chen et al. 2013; Siu et al. 2016). These values are difficult to compare to cSWE since there is no consensus on foot position or calculations used to estimate modulus. Using data from a study that compared mechanical properties between ruptured and non-ruptured sides in patients within 1 year of Achilles tendon rupture (Zellers et al. 2017), dynamic shear modulus was determined to be 155 kPa on the ruptured side and 207 kPa on the non-ruptured side. In a study that tracked mechanical properties with SSI over a 48-week post-operative period in patients post-Achilles tendon rupture (Zhang et al. 2016), the average modulus increased from 187.7 kPa at 12 weeks to 289.6 kPa at 48 weeks. Taken together, dynamic shear modulus calculated from cSWE appears more comparable to SSI than static shear modulus. The current study found that estimating dynamic shear modulus with cSWE in healthy Achilles tendons has adequate intra-rater reliability and is stable over time. Therefore, it appears that dynamic shear modulus estimated with cSWE can be used to monitor Achilles tendon mechanical properties over time. Further research is needed to better understand if there is physiological or histological rationale for continuing to look at static shear modulus and viscosity independently.
Relationship between Elastography and Traditional in vivo Mechanical Testing
Validation of cSWE has been limited to comparisons to magnetic resonance elastography using agarose gels and observations of side-to-side differences in patients with tendon injury(Cortes et al. 2015; Zellers et al. 2016; Zellers et al. 2017). Similarly, detection of side-to-side differences in Achilles tendinopathy and rupture has also been reported with axial-strain elastography(De Zordo et al. 2010; Tan et al. 2012) and shear wave elastography(Aubry et al. 2015; Chen et al. 2013). Despite the clinical usefulness of detecting side-to-side differences, limited studies have validated measures obtained with elastography to bench-top testing(Haen et al. 2017; Yeh et al. 2013) and only one study has investigated the ability of elastography to identify histological changes(Klauser et al. 2013). Haen et al.(2017) found a strong relationship between shear modulus measured with ultrasound elastography and elastic modulus from bench-top testing in human cadaveric tendons, while Yeh et al.(Yeh et al. 2013) found a strong relationship between shear wave speed and elastic modulus in a porcine model. Klauser et al. (2013) showed that axial-strain elastography identified 100% of tendon samples with histological degeneration, which was superior to the 86% identified with B-mode ultrasound imaging. In the current study, we found a strong relationship between Young’s modulus and viscoelastic properties obtained with cSWE. For the elastography portion of this study, shear modulus was used rather than wave speed since much of the literature reports modulus and the two variables are directly related(Brum et al. 2014; Martin et al. 2015). Additionally, in our study there was a significant effect of foot position on shear modulus, which agrees with what is reported in the literature(Aubry et al. 2013; Haen et al. 2017; Helfenstein-Didier et al. 2016). This finding reflects the ability of cSWE to detect the linear nature of tendon that occurs with different amounts of strain. These findings further validate cSWE for quantifying Achilles tendon viscoelastic properties in vivo and supports its use when mechanical testing is not feasible.
Advantages and Pitfalls of Continuous Shear Wave Elastography (cSWE)
There are many advantages of measuring tendon mechanical properties with cSWE compared to other elastography and in vivo techniques (e.g. ultrasound synchronized with dynamometry). One of the main advantages is that it can be safely completed throughout all stages of injury, including immediately after tendon rupture. This is because cSWE does not rely on muscle contractions that place large tensile loads on the tendon. Another advantage of cSWE is the ability to measure variations of mechanical properties within healthy and injured tendons without concerns of saturating the elastogram. This enhances the validity of cSWE and may provide future insights into tendon healing and pathogenesis. Contrary to these advantages, cSWE requires additional hardware (e.g. external actuator) and two testers to perform data collection. Another pitfall is that cSWE requires offline processing, which is time consuming compared to commercially-available elastography techniques that have processing in real time. Taking these advantages and disadvantages into consideration, it appears that cSWE is well-situated for measuring tendon viscoelastic properties, especially when conventional mechanical testing is not feasible.
Study Limitations
The current study is not without its limitations. A relatively small sample of only young, healthy Achilles tendons were included throughout both parts of this study. Although a healthy sample is critical for establishing reliability, uncertainty remains if the relationships between Young’s modulus and measures of viscoelastic properties differ in the presence of tendon injury. Additionally, the relationships between mechanical properties violate the assumption of Pearson product-moment correlation that data points are independent of each other. However, it was necessary to use the same subjects throughout testing due to large individual variability. Therefore, reported R- and p-values should be interpreted with caution. Another limitation is that measures of shear modulus and viscosity from this study cannot be directly compared to values previously reported with cSWE(Cortes et al. 2015; Suydam et al. 2015) due to procedural and computational modifications that are outlined in the material and methods section. It is unknown if the values obtained with cSWE are representative of the true shear modulus and viscosity of the tissue. However, the purpose of quantifying these values is to establish biomarkers that can be used to track structural integrity of tendon. Lastly, all testing procedures were performed by a single experienced investigator. Results may represent the reliability of cSWE performed by this individual and not accurately reflect the reliability if performed by another evaluator.
Conclusion
This study showed fair-to-excellent intra-rater reliability of cSWE for measuring tendon viscoelastic properties, demonstrated construct validity of cSWE by detecting differences in viscoelastic properties with changes in ankle position, and found strong relationships between mechanical properties obtained with cSWE and traditional mechanical testing. Additionally, as expected in healthy tendon, measurements of static shear modulus, viscosity, and dynamic shear modulus were stable over time. Collectively, findings suggest that cSWE can be used for measuring changes in Achilles tendon viscoelastic properties.
Figure 4.
Change in Viscosity with Foot Position. No significant effect of foot position on Viscosity (p=0.051; η2p=0.464) was observed.
Figure 5.
Change in Young’s Modulus with Foot Position. No significant effect of foot position on Young’s Modulus (p=0.246; η2p=0.308) was observed.
Figure 8.
Relationship of viscosity and Young’s modulus at the group level. Data points represent group means at the different foot positions. Error bars represent ±1 SD.
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute of General Medicine Sciences of the National Institutes of Health under Award Numbers R01-AR072034–01A1, R21-AR067390 and P30-GM103333. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This research was also supported by the Delaware Bioscience Center for Advanced Technology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arda K, Ciledag N, Aktas E, Aribas BK, Köse K. Quantitative assessment of normal soft-tissue elasticity using shear-wave ultrasound elastography. Am J Roentgenol 2011;197:532–536. [DOI] [PubMed] [Google Scholar]
- Arndt A, Bengtsson AS, Peolsson M, Thorstensson A, Movin T. Non-uniform displacement within the Achilles tendon during passive ankle joint motion. Knee Surgery, Sport Traumatol Arthrosc 2012;20:1868–1874. [DOI] [PubMed] [Google Scholar]
- Arya S, Kulig K. Tendinopathy alters mechanical and material properties of the Achilles tendon. J Appl Physiol 2010;108:670–675. [DOI] [PubMed] [Google Scholar]
- Aubry S, Nueffer J- P, Tanter M, Becce F, Vidal C, Michel F. Viscoelasticity in Achilles tendonopathy: quantitative assessment by using real-time shear-wave elastography. Radiology 2015;274:821–9. [DOI] [PubMed] [Google Scholar]
- Aubry S, Risson JR, Kastler A, Barbier-Brion B, Siliman G, Runge M, Kastler B. Biomechanical properties of the calcaneal tendon in vivo assessed by transient shear wave elastography. Skeletal Radiol 2013;42:1143–1150. [DOI] [PubMed] [Google Scholar]
- Baur H, Müller S, Hirschmüller A, Cassel M, Weber J, Mayer F. Comparison in lower leg neuromuscular activity between runners with unilateral mid-portion Achilles tendinopathy and healthy individuals. J Electromyogr Kinesiol, 2011;21:499–505. [DOI] [PubMed] [Google Scholar]
- Brum J, Bernal M, Gennisson JL, Tanter M. In vivo evaluation of the elastic anisotropy of the human Achilles tendon using shear wave dispersion analysis. Phys Med Biol 2014;59:505–523. [DOI] [PubMed] [Google Scholar]
- Chen X- M, Cui L- G, He P, Shen W- W, Qian Y- J, Wang J- R. Shear wave elastographic characterization of normal and torn achilles tendons: a pilot study. J Ultrasound Med 2013;32:449–55. [DOI] [PubMed] [Google Scholar]
- Cortes DH, Suydam SM, Silbernagel KG, Buchanan TS, Elliott DM. Continuous Shear Wave Elastography: A New Method to Measure Viscoelastic Properties of Tendons in Vivo. Ultrasound Med Biol 2015;41:1518–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Zordo T, Chhem R, Smekal V, Feuchtner G, Reindl M, Fink C, Faschingbauer R, Jaschke W, Klauser AS. Real-time sonoelastography: Findings in patients with symptomatic achilles tendons and comparison to healthy volunteers. Ultraschall der Medizin 2010;31:394–400. [DOI] [PubMed] [Google Scholar]
- De Zordo T, Fink C, Feuchtner GM, Smekal V, Reindl M, Klauser AS. Real-time sonoelastography findings in healthy Achilles tendons. Am J Roentgenol 2009;193:134–138. [DOI] [PubMed] [Google Scholar]
- Denegar CR, Ball DW. Assessing reliability and precision of measurement: an introduction to intraclass correlation and standard error of measurement. J Sport Rehabil 1993;2:35–42. [Google Scholar]
- DeWall RJ, Slane LC, Lee KS, Thelen DG. Spatial variations in Achilles tendon shear wave speed. J Biomech 2015;47:2685–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirrichs T, Quack V, Gatz M, Tingart M, Rath B, Betsch M, Kuhl CK, Schrading S. Shear Wave Elastography (SWE) for Monitoring of Treatment of Tendinopathies. Acad Radiol 2018;25:265–272. [DOI] [PubMed] [Google Scholar]
- Drakonaki EE, Allen GM, Wilson DJ. Real-time ultrasound elastography of the normal Achilles tendon: reproducibility and pattern description. Clin Radiol 2009;64:1196–202. [DOI] [PubMed] [Google Scholar]
- Franz JR, Slane LC, Rasske K, Thelen DG. Non-uniform in vivo deformations of the human Achilles tendon during walking. Gait Posture 2015;41:192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haen TX, Roux A, Soubeyrand M, Laporte S. Shear waves elastography for assessment of human Achilles tendon’s biomechanical properties: An experimental study. J Mech Behav Biomed Mater, 2017;69:178–184. [DOI] [PubMed] [Google Scholar]
- Helfenstein-Didier C, Andrade RJ, Brum J, Hug F, Tanter M, Nordez A, Gennisson J- L. In vivo quantification of the shear modulus of the human Achilles tendon during passive loading using shear wave dispersion analysis. Phys Med Biol 2016;61:2485–2496. [DOI] [PubMed] [Google Scholar]
- Järvinen M, Józsa L, Kannus P, Järvinen TL, Kvist M, Leadbetter W. Histopathological findings in chronic tendon disorders. Scand J Med Sci Sports 1997;7:86–95. [DOI] [PubMed] [Google Scholar]
- Kannus P, Józsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am 1991;73:1507–1525. [PubMed] [Google Scholar]
- Klauser AS, Miyamoto H, Tamegger M, Faschingbauer R, Moriggl B, Klima G, Feuchtner GM, Kastlunger M, Jaschke WR. Achilles Tendon Assessed with Sonoelastography: Histologic Agreement. Radiology 2013;267:837–842. [DOI] [PubMed] [Google Scholar]
- Kongsgaard M, Nielsen CH, Hegnsvad S, Aagaard P, Magnusson SP. Mechanical properties of the human Achilles tendon, in vivo. Clin Biomech 2011;26:772–777. [DOI] [PubMed] [Google Scholar]
- Maganaris CN, Paul JP. In vivo human tendon mechanical properties. J Physiol 1999;521 Pt 1:307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JA, Biedrzycki AH, Lee KS, DeWall RJ, Brounts SH, Murphy WL, Markel MD, Thelen DG. In Vivo Measures of Shear Wave Speed as a Predictor of Tendon Elasticity and Strength. Ultrasound Med Biol 2015;41:2722–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi CC, Malliaras P, Schneider ME, Connell DA. “Soft, hard, or just right?” Applications and limitations of axial-strain sonoelastography and shear-wave elastography in the assessment of tendon injuries. Skeletal Radiol 2014;43:1–12. [DOI] [PubMed] [Google Scholar]
- Payne C, Webborn N, Watt P, Cercignani M. Poor reproducibility of compression elastography in the Achilles tendon: same day and consecutive day measurements. Skeletal Radiol 2017;46:889–895. [DOI] [PubMed] [Google Scholar]
- Peltz CD, Haladik JA, Divine G, Siegal D, Van Holsbeeck M, Bey MJ. ShearWave elastography: Repeatability for measurement of tendon stiffness. Skeletal Radiol 2013;42:1151–1156. [DOI] [PubMed] [Google Scholar]
- Ryan ED, Rosenberg JG, Scharville MJ, Sobolewski EJ, Thompson BJ, King GE. Test-Retest Reliability and the Minimal Detectable Change for Achilles Tendon Length: A Panoramic Ultrasound Assessment. Ultrasound Med Biol 2013;39:2488–2491. [DOI] [PubMed] [Google Scholar]
- Schepull T, Kvist J, Aspenberg P. Early E-modulus of healing Achilles tendons correlates with late function: Similar results with or without surgery. Scand J Med Sci Sport 2012;22:18–23. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychol Bull 1979;86:420–428. [DOI] [PubMed] [Google Scholar]
- Silbernagel KG, Shelley K, Powell S, Varrecchia S. Extended field of view ultrasound imaging to evaluate Achilles tendon length and thickness: a reliability and validity study. Muscles, Ligaments Tendons J 2016;6:104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu W- L, Chan C- H, Lam C- H, Lee C- M, Ying M. Sonographic evaluation of the effect of long-term exercise on Achilles tendon stiffness using shear wave elastography. J Sci Med Sport 2016;19:883–887. [DOI] [PubMed] [Google Scholar]
- Sun Y- L, Wei Z, Zhao C, Jay GD, Schmid TM, Amadio PC, An K- N. Lubricin in human achilles tendon: The evidence of intratendinous sliding motion and shear force in achilles tendon. J Orthop Res 2015;33:932–937. [DOI] [PubMed] [Google Scholar]
- Suydam SM, Cortes DH, Axe MJ, Snyder-Mackler L, Buchanan TS. Semitendinosus Tendon for ACL Reconstruction: Regrowth and Mechanical Property Recovery. Orthop J Sport Med 2017;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suydam SM, Soulas EM, Elliott DM, Silbernagel KG, Buchanan TS, Cortes DH, Gravare Silbernagel K, Buchanan TS, Cortes DH. Viscoelastic properties of healthy Achilles tendon are independent of isometric plantar flexion strength and cross-sectional area. J Orthop Res 2015;33:926–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S, Kudas S, Özcan AS, Ipek A, Karaoglanoglu M, Arslan H, Bozkurt M. Real-time sonoelastography of the Achilles tendon: Pattern description in healthy subjects and patients with surgically repaired complete ruptures. Skeletal Radiol 2012;41:1067–1072. [DOI] [PubMed] [Google Scholar]
- Wyndow N, Cowan SM, Wrigley TV., Crossley KM. Triceps surae activation is altered in male runners with Achilles tendinopathy. J Electromyogr Kinesiol 2013;23:166–172. [DOI] [PubMed] [Google Scholar]
- Yeh CL, Kuo PL, Li PC. Correlation between the shear wave speed in tendon and its elasticity properties. IEEE Int Ultrason Symp IUS 2013;9–12. [Google Scholar]
- Zellers JA, Cortes DH, Corrigan P, Pontiggia L, Silbernagel KG. Side-to-side differences in Achilles tendon geometry and mechanical properties following achilles tendon rupture. Muscles Ligaments Tendons J 2017;7:541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellers JA, Cortes DH, Silbernagel KG. From Acute Achilles Tendon Rupture To Return To Play – a Case Report Evaluating Recovery of Tendon Structure, Mechanical Properties, Clinical and Functional Outcomes. Int J Sports Phys Ther 2016;11:1150–1159. [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wan W, Wang Y, Jiao Z, Zhang L, Luo Y, Tang P. Evaluation of elastic stiffness in healing Achilles tendon after surgical repair of a tendon rupture using in vivo ultrasound shear wave elastography. Med Sci Monit 2016;22:1186–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]