Abstract
Clinical Trial Registration: Clinicaltrials.gov NCT01756391
Keywords: Asthma, school, fungus, mold, inner-city, environmental exposure
INTRODUCTION
Asthma is the most common chronic disease among children. Poorly controlled asthma can cause disturbed sleep, limited activity and missed school days. The environment is one of many factors that influence asthma morbidity1, 2. Several studies and meta-analyses have recognized that home fungus exposure may be associated with development or worsening of asthma3–9. Children spend a large portion of their day in school; however, few studies have provided a comprehensive assessment of fungus in the school classroom and the possible effects on students with asthma10–13. We have previously shown that inner-city school classrooms have highly diverse fungal populations and significant intra-classroom variation14. The objective of this study was to evaluate the association of school-based fungal spore exposures on asthma symptom days in children with asthma.
METHODS
Study Population
The School Inner City Asthma Study (SICAS) is a single center longitudinal prospective study of a cohort of children with persistent asthma who attended schools in the Northeast US from 2008 to 2013. It was designed to evaluate the role of indoor allergen and toxin exposures specific to the inner-city classroom environment and asthma morbidity. The study design has been previously reported15. Briefly, inclusion criteria consisted of a history of physician-diagnosed asthma and either current symptoms of cough, wheezing, shortness of breath or whistling in the chest in the past 12 months; daily controller use; or unscheduled medical visits for asthma in the past year. The study population was made up of children, aged 4 to 13 years, attending an elementary school where permission for environmental sampling was obtained. Children were excluded from the study if they had a chronic lung disease other than asthma and cardiovascular disease. The study was approved by the Boston Children’s Hospital institutional review board and the participating schools.
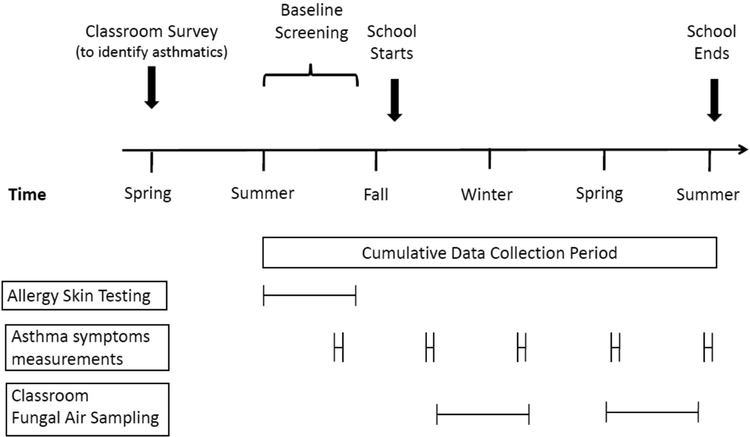
Each school year approximately 75 students were recruited from 8 to 10 participating schools. Children were recruited during the spring and phenotypically characterized at a baseline visit in the summer prior to the academic year. Assessments included a detailed questionnaire, allergy testing, spirometry and exhaled nitric oxide. Follow-up surveys were collected at 3, 6, 9 and 12 months after the baseline assessments (Figure 1).
Figure 1.
Annual schema for recruitment, screening and study procedures.
Fungus Sensitization
Skin prick testing (MultiTest device; Lincoln Diagnostics) was performed with Aspergillus, Alternaria tenius, Penicillium, and Cladosporium (Greer). Sensitization was defined as a wheal diameter ≥3mm larger than the negative control read 15 minutes after placement.
Fungal Air Sampling and Analysis
Burkard Indoor Recording Air Samplers (Burkard Mfg. Co., Rickmansworth, Herts., U.K.) were used to collect airborne spores of all fungi, including common indoor molds, in each classroom linked to the student. The segment of the slide representing the school day (8:00am until 4:00pm) was marked and a portion scanned at 1000x magnification and all fungal spores encountered were identified and counted. Raw counts were converted to airborne concentrations using the sampler flow rate, exposure time and percent of the collection surface analyzed. Results were reported as spores per cubic meter of air (spores/m3) for the 8-hour collection period. Two consecutive 8-hour days were averaged for each classroom. This method has been previously described14, 16.
A total fungi category was calculated as the sum of all fungus groupings. The three largest fungal categories were mitospores, basidiospores and ascospores. The mitospore category consisted of Alternaria, Botrytis, Cladosporium, Bipolaris, Epicoccum, Penicillium/Aspergillus, Periconia, Pithomyces, Stachybotrys and “other mitospores”. Penicillium and Aspergillus were reported together as they are too similar in morphology to differentiate by direct microscopy. The basidiospores category consisted of basidiospores small hyaline, Coprinus, Ganoderma and “other basidiospores”. The ascospores category consisted of Leptosphaeria, Xylariaceae, Chaetomium, Diatrype-like, Paraphaeosphaeria michotii, and “other ascospores”. More than 25 genera of fungi were measured.
Outcome Measure
The primary outcome was asthma symptom days per 2-week period (ASD) as used in prior urban home–based studies.17–20. It is the primary outcome in many pediatric asthma studies19, 21, 22, including those that investigate the impact of environmental exposures on asthma9, 23–25. This way of ascertaining asthma symptom days is validated, and allows comparability across studies.
ASD is comprised of the following variables in the 2 weeks prior to each follow up survey: (1) number of days with wheezing, chest tightness or cough; (2) number of days on which the child had to slow down or discontinue play activities due to wheezing, chest tightness or cough; (3) nights with wheezing, chest tightness or cough leading to disturbed sleep. The largest result of these 3 variables was used as the outcome of ASD with a range between 0 to 14 days.
Secondary outcome measurements included the following: number of days the child missed school due to asthma; health care use, defined as the number of hospitalizations and unscheduled health care visits for asthma; number of days the caregiver changed plans because of the child’s asthma; number of nights the caregiver lost sleep because of the child’s asthma; and lung function based on percentage of predicted pre-bronchodilator forced expiratory volume in 1 second (FEV1).
Statistical Analysis
Geometric means were calculated for each fungal spore grouping and total spores. In order to account for a high frequency of zero values, as was the case where certain fungal groups were rarely recovered, a value of 1 spore/m3 was added to all concentrations and then subtracted from the calculated geometric mean26. Asthma symptom days were linked to the closest measured fungal exposures from the student’s classroom during the academic school year. A priori, we defined fungal exposure as a binary “high” vs. “low” exposure based on whether the measured grouping was greater than or equal to the 75th percentile of exposure or below. We used generalized estimating equations to model asthma symptom days (binomial family, logit link) with an exchangeable correlation structure, robust variance estimates and an overdispersion parameter. Independent variables included fungal exposure, fungal sensitization, interaction term of exposure and sensitization, age, sex, race, season, mouse allergen and endotoxin level. Fungal sensitization was defined as any sensitization to fungus for models with the larger fungal categories; within specific spore categories, fungal sensitization was based on specific sensitizations for the specific exposure models (i.e., Alternaria sensitization interaction with Alternaria exposure on the health outcome). Season was defined as the number of days since school started and was modeled with linear and quadratic terms. For each model we reported the effect of fungal exposure for subjects stratified by their sensitization status. Our strategy for statistical testing was as follows: We first investigated the relationship between total fungus and the three large fungal groupings (mitospores, ascospores, and basidiospores) and asthma symptom days. In the event of a significant association between asthma symptom days and exposure to a larger fungus category, we then tested the association between asthma symptom days and each of the constituent fungi from that larger category. Analyses were performed using STATA 13.1 (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP.). All tests were 2-tailed, and P < .05 was considered significant.
RESULTS
In total, 351 students from 38 schools completed the baseline phenotypic screening and were enrolled in the study. Of those, 280 children from 37 schools (mean age, 7.9 years; 146 male and 134 female) met the requirements of having allergy testing, classroom fungal exposure data and follow up symptom data during the school year. The baseline characteristics of the study population are found in Table 1. The majority of students were Black or Hispanic. Forty percent of students were from homes with an annual household income less than $25,000 and 72% of students were from homes with an annual household income less than $45,000. Eighty one percent of students had a family history of asthma. Over half (55%) of students used maintenance asthma medication. Approximately 1 in 5 (18.9%) of the students were sensitized to at least one fungus with Aspergillus (9.9%) being the most common, followed by Alternaria (9.2%), Cladosporium (7.0%) and Penicillium (4%). The mean number of asthma symptom days per 2-week period was 2.9.
Table I:
Baseline characteristics of the study population (n = 280)
| Male | 146 | (52.1%) |
| Age, years - Mean (Range) | 7.9 | (4–13) |
| Race | ||
| White | 13 | (4.6%) |
| Black | 97 | (34.6%) |
| Hispanic | 101 | (36.1%) |
| Other | 69 | (24.6%) |
| Annual Household Income | ||
| < $25,000 | 112 | (40.0%) |
| < $45,000 | 170 | (71.7%) |
| Family History of Asthma | 227 | (81.1%) |
| Asthma Medications | ||
| SABA only | 126 | (45.0%) |
| ICS and/or montelukast | 154 | (55.0%) |
| Allergen Sensitization Rates | ||
| ANY Fungus | 53 | (18.9%) |
| Aspergillus | 27 | (9.9%) |
| Alternaria | 25 | (9.2%) |
| Penicillium | 13 | (4.8%) |
| Cladosporium | 19 | (7.0%) |
| Maximum Symptoms Days (Past 2 Weeks) | ||
| 0–1 Days | 147 | (52.5%) |
| 2–3 Days | 56 | (20.0%) |
| 4–9 Days | 48 | (17.1%) |
| 10–14 Days | 29 | (10.4%) |
| Mean (Std Dev) | 2.9 | (± 4.1) |
Abbreviations: SABA: inhaled Short-Acting Beta-Agonist, ICS = Inhaled CorticoSteroids, FEV1 = Forced Expiratory Volume in 1 second
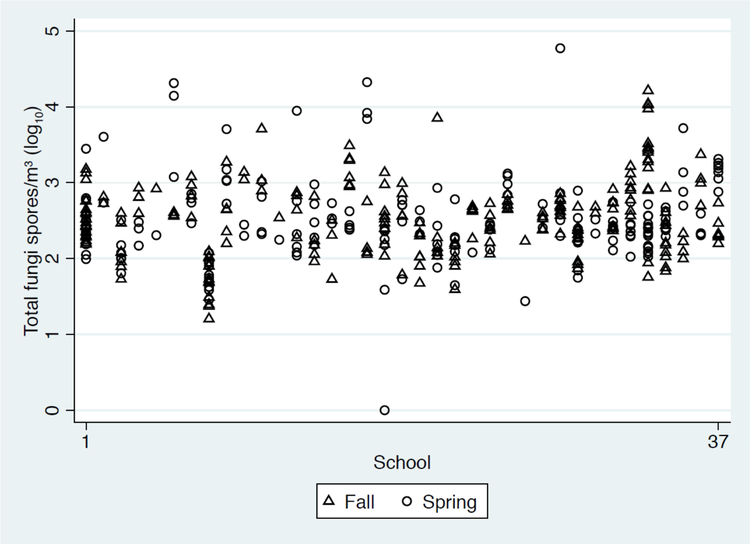
The prevalence, quantity and range of the fungal groupings with ≥20% prevalence are summarized in Table 2. Fungal spores were present in all 438 classroom samples. The geometric mean of the total fungi was 316.9 spores/m3 and ranged from 15.0 to 59,345.7 spores/m3. Mitospores were the most commonly detected fungal grouping. Within this group Cladosporium, Penicillium/Aspergillus, and Alternaria were the most prevalent. Cladosporium had the highest geometric mean of 29.3 spores/m3 and was found in 93% of samples. Regarding the traditional fungi associated with dampness and moisture damage, Penicillium/Aspergillus were detected in 86% of samples with a geometric mean of 18.9 spores/m3. Alternaria was not as prevalent or abundant. It was found in 22% of samples with a geometric mean of 0.6 spores/m3. When including only classroom samples with Alternaria present, the geometric mean was 7.5 spores/m3 (range of 3 – 47 spores/m3). The other major fungal groupings were basidiospores and ascospores. Basidiospores were detected in 81% of classroom samples and ascospores in 57% of samples. There was substantial variability in total fungus quantity between schools and classrooms within the same school (Figure 2).
Table II.
Distribution of the most common fungal groupings in classrooms (n = 438 classroom samples from 37 schools; limited to >20% detectable)
| Fungal Grouping | Geomean (spore/m3) |
Detectable† | Min (spore/m3) |
Max (spore/m3) |
|---|---|---|---|---|
| Total Fungi | 316.9 | 100% | 15.0 | 59,345.7 |
| Mitospores** | 71.8 | 98% | 0 | 59,100.0 |
| Cladosporium | 29.3 | 93% | 0 | 1,525.7 |
| Penicillium/Aspergillus | 18.9 | 86% | 0 | 58,894.0 |
| Alternaria | 0.6 | 22% | 0 | 47.0 |
| Basidiospores* | 18.8 | 81% | 0 | 16,401.5 |
| Other Basidiospores* | 8.0 | 70% | 0 | 2,445.5 |
| Basidiospores small hyaline* | 5.1 | 51% | 0 | 16,017.5 |
| Coprinus* | 1.0 | 27% | 0 | 149.6 |
| Ganoderma* | 0.8 | 25% | 0 | 403.9 |
| Ascospores# | 4.5 | 57% | 0 | 8721.9 |
| Other Ascospores | 3.1 | 46% | 0 | 8612.3 |
| Hyphae | 70.9 | 99% | 0 | 571.2 |
| Unidentifiable spores | 33.6 | 98% | 0 | 563.7 |
| Smut spores (Ustilaginomycetes) | 12.1 | 83% | 0 | 639.4 |
| Bispora | 0.8 | 27% | 0 | 127.0 |
| Rust spores (Pucciniomycetes) | 0.6 | 23% | 0 | 29.8 |
| Myxomycetes | 0.6 | 22% | 0 | 291.7 |
Detectable is defined as percentage of classrooms where a particular fungal type was identified at least once.
Mitospores = Alternaria, Botrytis, Cladosporium, Bipolaris, Epicoccum, Penicillium/Asp, Periconia, Pithomyces, Stachybotrys and other Mitospores
Basidiospore = Basidiospores small hyaline, Coprinus, Ganoderma, Other Basidiospores
Ascospore = Leptosphaeria, Xylariaceae, Chaetomium, Diatrype-like, Paraphaeosphaeria michotii, other ascospores
Figure 2.
There is substantial variability in total fungus quantity between schools and classrooms within the same school. Total fungus (spores/m3) was not significantly higher in the Fall with a geometric mean of 340.2 (SD=3.2) and 307.3 (SD=3.5) in the Spring, p=0.39
For children with high exposure to total fungi, the odds of having an increase in asthma symptom days was elevated, though not statistically significant, for fungus sensitized children (OR =1.74, 95% CI =0.89–3.40, p=0.10), but was not elevated in children who were not sensitized to fungus (OR=0.91, 95%CI=0.63–1.30, p=0.6). The association between asthma symptoms days and mitospore exposure significantly varied by sensitization status (interaction p-value = 0.04). There was a significant association between exposure to mitospores and maximum symptom days in students with fungal sensitization (OR=2.00, 95% CI =1.06–3.76, p = 0.03), but not in students without fungal sensitization (OR=0.94, 95% CI=0.65–1.36). None of the secondary outcomes were significantly associated with mitospore exposure. There were no significant findings for exposure to ascospores (fungus sensitized OR=1.17, 95% CI=0.57–2.39, p = 0.67; fungus not-sensitized OR=1.12, 95% CI=0.79–1.60, p=0.52) or basidiospores (fungus sensitized OR=1.12, 95% CI=0.61–2.05, p = 0.72; fungus not-sensitized OR=0.91, 95% CI=0.64–1.29, p = 0.60).
Further investigation of the individual mitospores revealed that exposure to higher levels of Alternaria was significantly associated with asthma symptom days in students sensitized to Alternaria (OR = 3.61, CI = 1.34–9.76, p=0.01), but not in children not-sensitized to Alternaria (Table 3). The association between asthma symptom days and Alternaria exposure significantly varied by sensitization status (interaction p-value = 0.02). This finding indicates that students sensitized to Alternaria and exposed to high levels were estimated to have 3.2 more symptom days per 2-week period as compared to students sensitized but exposed to low levels (5.3 vs. 2.1 symptom days) after adjusting for covariates. There was no difference in asthma symptom days for children not-sensitized to Alternaria, regardless of exposure. OR = 1.04 (0.72 – 1.49), pvalue=0.85). None of the secondary outcomes were significantly associated with Alternaria exposure (eTable 1). Finally, there were no associations between asthma symptoms and exposure to Cladosporium or Penicillium/Aspergillus, regardless of sensitization to these fungi (Table 3).
Table III.
Association of classroom fungal exposure on asthma morbidity in sensitized (specific to fungal genus) asthmatic children
| Sensitized to Specific Fungus | Not Sensitized to Specific Fungus | |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Alternaria | ||
| Asthma Symptom Day | ||
| Unadjusted# | 4.02 (1.43 – 11.28)* | 1.05 (0.74 – 1.48) |
| Adjusted | 3.61 (1.34 – 9.76)** | 1.04 (0.72 – 1.49) |
| Cladosporium | ||
| Asthma Symptom Day | ||
| Unadjusted# | 1.70 (0.46 – 6.26) | 1.19 (0.88 – 1.59) |
| Adjusted | 1.45 (0.41 – 5.17) | 1.10 (0.80 – 1.30) |
| Penicillium/Asoergillus | ||
| Asthma Symptom Day | ||
| Unadjusted# | 0.48 (0.17 – 1.29) | 0.80 (0.58 – 1.11) |
| Adjusted | 0.81 (0.58 – 1.13) | 0.49 (0.18 – 1.30) |
p=0.008
p=0.01
Adjusted for age, sex, race, season, classroom, mouse allergen, and classroom endotoxin levels.
DISCUSSION
This prospective study demonstrates that school classroom fungal exposure may be associated with increased asthma symptoms in sensitized children. Specifically, children with asthma who are sensitized to Alternaria and exposed to this fungus in their classroom have significantly more days with asthma symptoms than those who were sensitized and not exposed. There was no effect of exposure in those not sensitized to Alternaria. These findings were significant even when adjusting for co-exposure with known asthma triggers, including mouse allergen and endotoxin and when adjusted for variation in seasonal changes.
Students sensitized and exposed to Alternaria had 3.2 more symptom days per 2 week period as compared to students sensitized but not exposed to Alternaria. This difference would extrapolate to approximately 6 more asthma symptom days per month and approximately 50 more asthma symptom days per school year.
Sensitization to Alternaria has been found to be significantly higher among asthmatics than among subjects without asthma. Lehmann et al. investigated the prevalence of fungal sensitization in 203 German pediatric asthma patients aged 1–17 years and found that Alternaria sensitization was the most common among fungi at 17%27. In the Inner-City Asthma Study, Pongracic et al. found that Alternaria was the most common fungus sensitization, as well, with 36% of 469 asthmatic children sensitized to Alternaria species9. In our study, Alternaria was the second most common fungal sensitization with 9.2% of study participants having a positive skin prick test, only slightly behind Aspergillus with a sensitization prevalence of 9.9%. These differences in sensitization rates are likely related to varying environmental conditions regionally.
Our findings are consistent with the Inner-City Asthma Study (ICAS) which also found that home Alternaria exposure was one of a few fungi to have an effect on asthma symptom days9. Importantly, we extend the ICAS home-based assessment to identify the school environment as a significant source of fungal exposure-related asthma morbidity. The greater effect size of our findings compared with ICAS (3.2 versus 1.3 asthma symptom days per 2-week period) may be related to methodologic differences between the studies, such as the mode of sampling (direct microscopy versus volumetric culture-based), but may also reflect the intense and prolonged exposure microenvironment of the school classroom.
In our study, “high fungus exposure” was defined as ≥75th percentile and “low fungal exposure” was defined as <75th percentile. For Alternaria, which was found in just 22% of classroom samples, these cutoffs were essentially consistent with “exposure” versus “non-exposure”. Alternaria was present in lesser quantities and in fewer classrooms as compared to Cladosporium and Penicillium/Aspergillus. However, we did not find associations between asthma morbidity and total fungal spores or other prevalent fungi such ascospores, basidiospores, Penicillium/Aspergillus and Cladosporium.
Studies from other countries have identified exposure to fungi in classrooms as a risk factor for asthma or asthma morbidity10–13, 28–30. However, these were cross sectional studies. The methods used to quantify fungus exposure included identification of visible fungus, measurement of fungal metabolites in dust, culture-based volumetric sampling and direct microscopy with a shorter collection time (40 minutes). Our study is unique in that it is a prospective longitudinal cohort study conducted in the United States in multiple inner-city schools. The population was comprised of urban children with known asthma and symptoms were evaluated at several time points during the year. We used continuous air sampling for two entire school days in two distinct seasons, which may reflect a more accurate exposure over long periods of time by averaging the short term spore concentration peaks and valleys. Direct microscopy was used for spore identification and quantification because it provided the opportunity to identify the most spore types regardless of culturability or viability. The absences of a standardized fungal measurement technique and lack of exposure thresholds make assessing health risks of fungal exposure a challenge. Quantification methods vary from direct microscopy to culture-based volumetric sampling to measurement of fungal cell wall components such as ergosterol or beta-D-glucan. Even newer techniques for evaluating fungal diversity such as metagenomic sequencing may offer different insight into characterization of the fungal microbiome.
A limitation of our study is that it was created to evaluate asthma health effects from fungi and not necessarily to determine the source of the fungus exposure. Our key finding is that fungi are present in the classroom and Alternaria, in particular, may be associated with cause a health effect in sensitized asthmatic students. It is unclear if the exposures to Alternaria spores were primarily due to indoor growing spores versus outdoor spores that penetrated the indoor environment. Although we do not have outdoor sampling available, the variability in classroom spore count within the same school (see Figure II) and the strong correlation of the indoor classroom Alternaria count with asthma symptoms in children within those classrooms suggests that the indoor exposure is responsible. If the effect were due to outdoor Alternaria exposures alone, we would assume that all the sensitized asthmatic children within the same school would be exposed to comparable outdoor concentrations and have similar symptoms. Our sampling was done during the Fall and Spring in the Northeast U.S. where conditions commonly include closed windows and reduced exposure to outdoor fungus. While some of the spores may have been carried in from the outdoors, some of the higher concentrations include fungi more characteristically known as important in indoor environments, suggesting that there are likely direct indoor sources of fungi in these classrooms. Regardless of the source of Alternaria exposure, we have shown that the presence of Alternaria in the indoor classroom environment was associated with increased asthma symptom days in sensitized asthmatic children. Furthermore, we recognize that the brief sampling time and variability of sampling date might not completely reflect the exposure. We acknowledge that our symptom measurement and outcome measurement may have been at different times and that this strategy might lead to exposure misclassification. Finally, determining the prevalence of fungal sensitization and quantifying exposure remains a challenge. Due to the lack of high-quality fungal extracts and the presence of cross-reactivity among fungi, the prevalence of fungal allergies are difficult to determine with certainty31
Conclusions
In summary, Alternaria exposure in school classrooms may play an important role in asthma morbidity in inner-city children. Further research is necessary to determine whether efforts to decrease fungus exposure in classrooms can improve asthma outcomes in vulnerable children. A follow up study by our lab is evaluating whether the use of air filters can reduce fungal levels and additionally whether a reduction in levels can reduce asthma morbidity (https://clinicaltrials.gov/ct2/show/NCT02291302).
Supplementary Material
Funding Source:
This study was supported by grants R01AI073964, R01AI073964–02S1, K24AI106822, K23AI106945, K23ES023700, K23AI104780, U10HL098102, U01AI110397 from the National Institutes of Health. This work was conducted with the support from Harvard Catalyst/The Harvard Clinical and Translational Science Center (NIH Award # 8UL1TR000170) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, the National Center for Research Resources, or the National Institutes of Health CTSU PI (Nagler).
Abbreviations used:
- SICAS
School Inner-City Asthma Study
- ICAS
Inner-City Asthma Study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest:
none
References
- 1.Gold DR, Adamkiewicz G, Arshad SH, Celedon JC, Chapman MD, Chew GL, et al. NIAID, NIEHS, NHLBI, and MCAN Workshop Report: The indoor environment and childhood asthma-implications for home environmental intervention in asthma prevention and management. J Allergy Clin Immunol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanchongkittiphon W, Mendell MJ, Gaffin JM, Wang G, Phipatanakul W. Indoor environmental exposures and exacerbation of asthma: an update to the 2000 review by the Institute of Medicine. Environ Health Perspect 2015; 123:6–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.In: Damp Indoor Spaces and Health Washington (DC); 2004. [PubMed] [Google Scholar]
- 4.Fisk WJ, Lei-Gomez Q, Mendell MJ. Meta-analyses of the associations of respiratory health effects with dampness and mold in homes. Indoor Air 2007; 17:284–96. [DOI] [PubMed] [Google Scholar]
- 5.Karvonen AM, Hyvarinen A, Korppi M, Haverinen-Shaughnessy U, Renz H, Pfefferle PI, et al. Moisture damage and asthma: a birth cohort study. Pediatrics 2015; 135:e598–606. [DOI] [PubMed] [Google Scholar]
- 6.Mendell MJ, Mirer AG, Cheung K, Tong M, Douwes J. Respiratory and allergic health effects of dampness, mold, and dampness-related agents: a review of the epidemiologic evidence. Environ Health Perspect 2011; 119:748–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quansah R, Jaakkola MS, Hugg TT, Heikkinen SA, Jaakkola JJ. Residential dampness and molds and the risk of developing asthma: a systematic review and meta-analysis. PLoS One 2012; 7:e47526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gent JF, Kezik JM, Hill ME, Tsai E, Li DW, Leaderer BP. Household mold and dust allergens: exposure, sensitization and childhood asthma morbidity. Environ Res 2012; 118:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pongracic JA, O’Connor GT, Muilenberg ML, Vaughn B, Gold DR, Kattan M, et al. Differential effects of outdoor versus indoor fungal spores on asthma morbidity in inner-city children. J Allergy Clin Immunol 2010; 125:593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meklin T, Potus T, Pekkanen J, Hyvarinen A, Hirvonen MR, Nevalainen A. Effects of moisture-damage repairs on microbial exposure and symptoms in schoolchildren. Indoor Air 2005; 15 Suppl 10:40–7. [DOI] [PubMed] [Google Scholar]
- 11.Simoni M, Cai GH, Norback D, Annesi-Maesano I, Lavaud F, Sigsgaard T, et al. Total viable molds and fungal DNA in classrooms and association with respiratory health and pulmonary function of European schoolchildren. Pediatr Allergy Immunol 2011; 22:843–52. [DOI] [PubMed] [Google Scholar]
- 12.Taskinen T, Hyvarinen A, Meklin T, Husman T, Nevalainen A, Korppi M. Asthma and respiratory infections in school children with special reference to moisture and mold problems in the school. Acta Paediatr 1999; 88:1373–9. [DOI] [PubMed] [Google Scholar]
- 13.Chen CH, Chao HJ, Chan CC, Chen BY, Guo YL. Current asthma in schoolchildren is related to fungal spores in classrooms. Chest 2014; 146:123–34. [DOI] [PubMed] [Google Scholar]
- 14.Baxi SN, Muilenberg ML, Rogers CA, Sheehan WJ, Gaffin J, Permaul P, et al. Exposures to molds in school classrooms of children with asthma. Pediatr Allergy Immunol 2013; 24:697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phipatanakul W, Bailey A, Hoffman EB, Sheehan WJ, Lane JP, Baxi S, et al. The school inner-city asthma study: design, methods, and lessons learned. J Asthma 2011; 48:1007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muilenberg ML. Sampling devices. Immunol Allergy Clin North Am 2003; 23:337–55. [DOI] [PubMed] [Google Scholar]
- 17.Crain EF, Walter M, O’Connor GT, Mitchell H, Gruchalla RS, Kattan M, et al. Home and allergic characteristics of children with asthma in seven U.S. urban communities and design of an environmental intervention: the Inner-City Asthma Study. Environ Health Perspect 2002; 110:939–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gruchalla RS, Pongracic J, Plaut M, Evans R 3rd, Visness CM, Walter M, et al. Inner City Asthma Study: relationships among sensitivity, allergen exposure, and asthma morbidity. J Allergy Clin Immunol 2005; 115:478–85. [DOI] [PubMed] [Google Scholar]
- 19.Morgan WJ, Crain EF, Gruchalla RS, O’Connor GT, Kattan M, Evans R 3rd, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med 2004; 351:1068–80. [DOI] [PubMed] [Google Scholar]
- 20.Evans R 3rd, Gergen PJ, Mitchell H, Kattan M, Kercsmar C, Crain E, et al. A randomized clinical trial to reduce asthma morbidity among inner-city children: results of the National Cooperative Inner-City Asthma Study. J Pediatr 1999; 135:332–8. [DOI] [PubMed] [Google Scholar]
- 21.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med 2011; 364:1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med 1997; 336:1356–63. [DOI] [PubMed] [Google Scholar]
- 23.Lai PS, Sheehan WJ, Gaffin JM, Petty CR, Coull BA, Gold DR, et al. School Endotoxin Exposure and Asthma Morbidity in Inner-city Children. Chest 2015; 148:1251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsui EC, Perzanowski M, Peng RD, Wise RA, Balcer-Whaley S, Newman M, et al. Effect of an Integrated Pest Management Intervention on Asthma Symptoms Among Mouse-Sensitized Children and Adolescents With Asthma: A Randomized Clinical Trial. JAMA 2017; 317:1027–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheehan WJ, Permaul P, Petty CR, Coull BA, Baxi SN, Gaffin JM, et al. Association Between Allergen Exposure in Inner-City Schools and Asthma Morbidity Among Students. JAMA Pediatr 2017; 171:31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eudey LSH SH, Burge HA. Biostatistics and bioaerosols In: Inc. CP, editor. Bioaerosols Boca Raton, FL: Lewis Publishers; 1995. p. 269–307. [Google Scholar]
- 27.Lehmann S, Sprunken A, Wagner N, Tenbrock K, Ott H. Clinical relevance of IgE-mediated sensitization against the mould Alternaria alternata in children with asthma. Ther Adv Respir Dis 2017; 11:30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavaleiro Rufo J, Madureira J, Paciencia I, Aguiar L, Pereira C, Silva D, et al. Indoor fungal diversity in primary schools may differently influence allergic sensitization and asthma in children. Pediatr Allergy Immunol 2017; 28:332–9. [DOI] [PubMed] [Google Scholar]
- 29.Mi YH, Norback D, Tao J, Mi YL, Ferm M. Current asthma and respiratory symptoms among pupils in Shanghai, China: influence of building ventilation, nitrogen dioxide, ozone, and formaldehyde in classrooms. Indoor Air 2006; 16:454–64. [DOI] [PubMed] [Google Scholar]
- 30.Zhao Z, Sebastian A, Larsson L, Wang Z, Zhang Z, Norback D. Asthmatic symptoms among pupils in relation to microbial dust exposure in schools in Taiyuan, China. Pediatr Allergy Immunol 2008; 19:455–65. [DOI] [PubMed] [Google Scholar]
- 31.Larenas-Linnemann D, Baxi S, Phipatanakul W, Portnoy JM, Environmental Allergens W. Clinical Evaluation and Management of Patients with Suspected Fungus Sensitivity. J Allergy Clin Immunol Pract 2016; 4:405–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.