Abstract
Exposures to persistent environmental pollutants like polychlorinated biphenyls (PCBs) has been associated with liver diseases such as toxicant-associated steatohepatitis (TASH). However, previously published PCB hepatotoxicity studies evaluated mostly male animal models. Moreover, epidemiologic studies on PCB-exposed cohorts evaluating sex differences are scarce. Therefore, the objective of this study was to examine hepato-toxicological responses of PCB exposures in the context of sex- dependent outcomes. Male and female C57Bl/6 mice were exposed to Aroclor 1260 (20 mg/kg), and PCB126 (20 μg/kg), by gavage for two weeks. Female mice appeared to be more sensitive to PCB-induced hepatotoxic effects as manifested by increased liver injury markers, namely, hepatic Serpine1 expression. Additionally, compared to their male counterparts, PCB-exposed females exhibited dysregulated hepatic gene expression favoring lipid accumulation rather than lipid breakdown; accompanied by dyslipidemia. Sex differences were also observed in the expression and activation of PCB targets such as the epidermal growth factor receptor (EGFR) while PCB-induced pancreatic toxicity was similar in both sexes. Importantly, PCB exposure appeared to cause pro-androgenic, anti-estrogenic along with sex-dependent thyroid hormone effects. The overall findings demonstrated that the observed PCB-mediated hepatotoxicity was sex-dependent; confirming the existence of sex differences in environmental exposure-induced markers of TASH and warrants further investigation.
Keywords: PCBs, sex differences, metabolic, endocrine, TASH
1. Introduction
Depending on sex, the health outcomes arising from environmental exposures to toxicants such as persistent organic pollutants (POPs) can vary in pathophysiology, disease progression and severity. Men and women can exhibit different responses from toxicant exposures because a number of factors influence i) cellular and molecular processes, and ii) interactions between environmental chemicals and physiological molecules (Federman, 2006). For example, environmental exposures in a woman’s lifespan are confounded by biological processes that are absent in men; such as menstruation, pregnancy, lactation and menopause. Biological sex plays a critical role in predicting risks and disease consequences. For instance, compared to men, women are at greater risk for developing cardiovascular disorders as a result of metabolic syndrome (Bentley-Lewis et al., 2007). However, because studies related to sex differences in environmental health and toxicology are limited, it is important to address such discrepancies (Mennecozzi et al., 2015). To clarify, the authors recognize the term ‘sex’ as pertaining to biological construct and physiological characteristics dependent on the presence of sex chromosomes; ‘gender’ as pertaining to orientation and identity of the subject dependent on socio-environmental and cultural factors (Torgrimson and Minson, 2005).
Polychlorinated biphenyls (PCBs) are a class of POPs previously produced commercially in the United States and worldwide for use in industrial and electrical applications (Erickson and Kaley, 2011). There were up to 130 individual PCB congeners manufactured accounting for approximately 1.3 million tons of global PCB production prior to their prohibited use (Breivik et al., 2002) Depending on the substitution of the chlorine atoms in the biphenyl ring, PCBs are categorized as either coplanar or non-coplanar PCBs. Coplanar PCBs are ‘dioxin-like’ in nature, inducing toxicity resembling that of 2,3,7,8-tetrachlorodibenzo-p-dioxin, whereas non-coplanar congeners are ‘non-dioxin-like’ in structure (Safe, 1994). Although they have been banned for over three decades, PCBs still persist in the environment due to their high thermodynamic stability and bio-accumulation in people and other living organisms. Environmental exposure to PCBs today is primarily through ingestion of contaminated water and food such as PCB-laden fish (Fernandez-Gonzalez et al., 2015).
Previous work from our laboratory group and others demonstrated that exposure to PCBs exacerbated non-alcoholic fatty liver disease and caused toxicant-associated steatohepatitis (TASH) in male animal models (Gadupudi et al., 2018; Gadupudi et al., 2016; Wahlang et al., 2017a; Wahlang et al., 2013; Wahlang et al., 2017b; Wahlang et al., 2016; Wahlang et al., 2014b). Moreover, studies on the Anniston Community Health Survey participants reported a positive association between plasma PCB levels and biomarkers of TASH in this population (Clair et al., 2018). Additionally, using in-vitro and knockout mouse models, our group demonstrated that depending on their structure and planarity, PCBs can interact with hepatic xenobiotic receptors including the arylhydrocarbon receptor (AhR), constitutive androstane receptor (CAR), and pregnane- xenobiotic receptor (PXR) to induce target gene batteries and toxicological responses (Wahlang et al., 2014a; Wahlang et al., 2016). These nuclear receptors, along with endobiotic nuclear receptors, including the peroxisome-proliferator activated receptors (PPAR) and hepatocyte nuclear factor 4 alpha (HNF4A) play distinct roles in regulating energy metabolism/homeostasis and liver function (Cave et al., 2016; Hang et al., 2017; Zhao et al., 2014). PCB exposure is therefore an important ‘hit’ driving liver disease progression in vulnerable and susceptible populations, with PCB-receptor interactions playing a mechanistic role in mediating pathological processes.
The liver is a sexually dimorphic organ and attention needs to be paid to sex differences when evaluating metabolic and liver disease endpoints. The liver’s sex-specific responses impacting experimental animal models and human studies were first reported over three decades ago (Roy and Chatterjee, 1983). Lu et al. reported that most hepatic cytochrome P450 enzymes and their corresponding transcriptional regulators are strongly regulated by sex as well as circadian rhythmicity (Lu et al., 2013). These differences in basal levels of receptor expression translate into reported differences in drug efficacy, safety and metabolism between men and women due to variations in pharmacokinetics and pharmacodynamics. In fact, a recent gender specific analysis on FDA-approved drugs showed that women reported more side effects than men for 53% of the drugs included in the study (Labots et al., 2018). Given that sexual dimorphism for organs such as the liver has been established and because toxicants like PCBs interact with multiple hepatic molecules and sex hormones, and are endocrine- disrupting in nature (Mimoto et al., 2017); it is important to evaluate the hepatic and metabolic toxicity of PCB exposure in both sexes, and to compare and contrast such findings to better optimize risk assessment in exposed populations.
Therefore, the objective of the current study was to investigate the effects of a short- term PCB exposure in male and female mice to identify sex differences arising from PCB exposure with emphasis on hepatic and metabolic disease endpoints. The findings demonstrated that sex played a significant role in dictating the effects of PCBs with female mice showing higher sensitivity to hepatotoxicity at the dose and duration used in the current study.
2. Materials and Methods
2.1. Mouse model
The animal protocol was approved by the University of Louisville Institutional Animal Care and Use Committee. All animals were housed in a temperature- and light- controlled room (12 h light; 12 h dark) with food and water ad libitum. Male and female C57Bl/6 mice (males-8 week old, females-7 week old) were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). All mice were fed a low-fat, synthetic diet consisting of 20.0%, 69.8%, and 10.2% of total calories from protein, carbohydrate, and fat respectively (TD06416, Envigo, Indianapolis, IN, USA) in this 2 week study. On week 0, mice were randomly divided into four groups (n=10, total=40) based on sex and exposure utilizing a 2X2 design (Male-CON, Male-PCBs, Female-CON, Female-PCBs), and allowed to acclimatize. The initial body weights (mean ± SD) at the beginning of the study were as follows: Male-CON = 25.23 ± 1.12, Male-PCBs = 25.65 ± 1.58, Female- CON = 18.63 ± 0.65, Female-PCBs = 18.52 ± 0.63. Five mice were housed per cage. At the beginning of week 1, mice were administered via oral gavage either the vehicle control (corn oil) or a mix of PCBs (purchased from AccuStandard, CT, USA) solubilized in corn oil. The PCB mix comprised of the commercial PCB mixture, Aroclor 1260 (20 mg/kg) and PCB126 (10 μg/kg). On week 2, mice were gavaged with a second dose of PCB126 (10 μg/kg) alone and a glucose tolerance test was performed. A schematic diagram depicting the experimental design, timeline and dosing regimen is provided in Supplementary Figure 1. The dose of PCBs used in the current study reflected doses used by our groups in previous experiments and were designed to mimic PCB bioaccumulation patterns in humans (Shi et al., 2019; Wahlang et al., 2016; Wahlang et al., 2014b). Mice were euthanized at the completion of week 2 (i.e. one week after the second PCB gavage) using ketamine/xylazine (100/20 mg/kg body weight, i.p.). Plasma and tissue samples were collected as previously described (Wahlang et al., 2017b).
2.2. Glucose tolerance test
On week 2, one day after the second PCB gavage, mice were fasted for 6 h (6 AM-12 PM). After 6 h, fasting blood glucose levels were measured with a hand-held glucometer (ACCU-CHECK Aviva, Roche, Basel, Switzerland) using 1–2 μL blood collected via tail snip. Glucose was then administered (1 mg glucose/g body weight, sterile saline, i.p.), and blood glucose was measured at 15, 30, 60, 90 and 120 min post injection. Diabetic parameters including insulin resistance and insulin sensitivity were calculated by the homeostasis model assessment of insulin resistance (HOMA-IR) and homeostasis model assessment for pancreatic beta cell function (HOMA-β) as described previously (Wahlang et al., 2017b).
2.3. Histological studies
Tissue sections were fixed in 10% neutral buffered formalin for 72 h and embedded in paraffin for routine histological examination as done previously (Wahlang et al., 2017a).
2.4. Cytokine, adipokine and lipoprotein measurements.
The Milliplex Map Mouse Adipokine Magnetic Bead Panels (Millipore Corp, Billerica, MA, USA) were utilized to measure plasma cytokines, adipokines, steroids and thyroid hormones on a Luminex 100 system (Luminex Corp, Austin, TX, USA), as per the manufacturer’s instructions. Insulin was measured using the Millipore Rat/Mouse Insulin ELISA Kit as per the manufacturer’s instructions. Plasma aspartate transaminase (AST), alanine transaminase (ALT), cholesterol, triglycerides, high and very low density lipoprotein (HDL, vLDL) were measured with the Piccolo Xpress Chemistry Analyzer using Lipid Panel Plus reagent disks (CLIAwaived Inc, San Diego, CA, USA) as described previously (Wahlang et al., 2017a).
2.5. Hepatic lipid analysis
Liver tissues were rinsed in neutral 1X phosphate buffered saline (PBS) and homogenized in 50 mM NaCl solution. Hepatic lipids were extracted using a solution of chloroform and methanol (2:1) according to Bligh and Dyer method (Bligh and Dyer, 1959). Triglycerides and free fatty acid contents were measured using commercial kits (Infinity Liquid Stable Reagents, Thermo Fisher Scientific, Wilmington, DE, USA) according to the manufacturer’s instructions.
2.6. Real-time PCR
RNA isolation, quantification and cDNA synthesis were performed as previously described (Wahlang et al., 2017b). RT-PCR was performed on the CFX384 TM Real- Time System (Biorad, Hercules, CA, USA) using iTaq Universal Probes Supermix and Taqman probes (Supplementary Table 1). Gene expression levels were calculated according to the 2−ΔΔCt method. The levels of mRNA were normalized relative to the levels of housekeeping genes and mean expression levels in unexposed, male mice which were set at 1.
2.7. Western blot
Liver tissue was homogenized in RIPA Buffer, supplemented with protease, and phosphatase inhibitors (Sigma-Aldrich, St. Louis, MO, USA). Protein concentration was determined by Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific). Protein (30 mg) was separated on a 4–15% gradient SDS Gel (BioRad, Hercules, CA), transferred, blocked and incubated with primary antibody according to the manufacturer’s instructions. The membranes were incubated in Pierce™ ECL agents (Thermo Fisher Scientific, Waltham, MA) and luminescent signals were captured with BioRad Chemidoc Imaging System. Western blot bands were quantified using BioRad Image Software. Primary antibodies for phosphorylated epidermal growth factor receptor (EGFR) Y1173 and Actin were obtained from Cell Signaling Technology (Danvers, MA, USA), total EGFR from Santa Cruz (Santa Cruz, CA, USA) and hepatocyte nuclear factor 4 alpha (HNF4A) from Abcam (Cambridge, MA, USA).
2.8. Statistical Analysis
Graphs were plotted using GraphPad Prism version 7.02 for Windows (GraphPad Software Inc., La Jolla, CA, USA). Numerical values were reported up to one significant digit. Statistical analyses were performed using the SAS statistical software (The SAS System V9. Cary, NC: SAS Institute Inc, 2003). Initially, ANOVA analysis was conducted for two factors; ‘sex’ and ‘PCBs’; this was followed by contrast test analyses in the model for different subgroup comparisons (Benjamini and Hochberg, 1995). The p-values were reported upto three significant digits and p <0.05 was considered statistically significant for the main factors. The adjusted p-values for the interaction and the four within group comparisons using Bonferroni method were considered statistically significant for p <=0.01. Because of the exploratory nature of the study, multiple comparisons for p <0.05 were also reported for relevant outcomes and considered as trending towards significance. All statistical analyses were overseen by biostatisticians at the University of Louisville.
3. Results
3.1. PCB effects on body weight and organ weight were dependent on sex
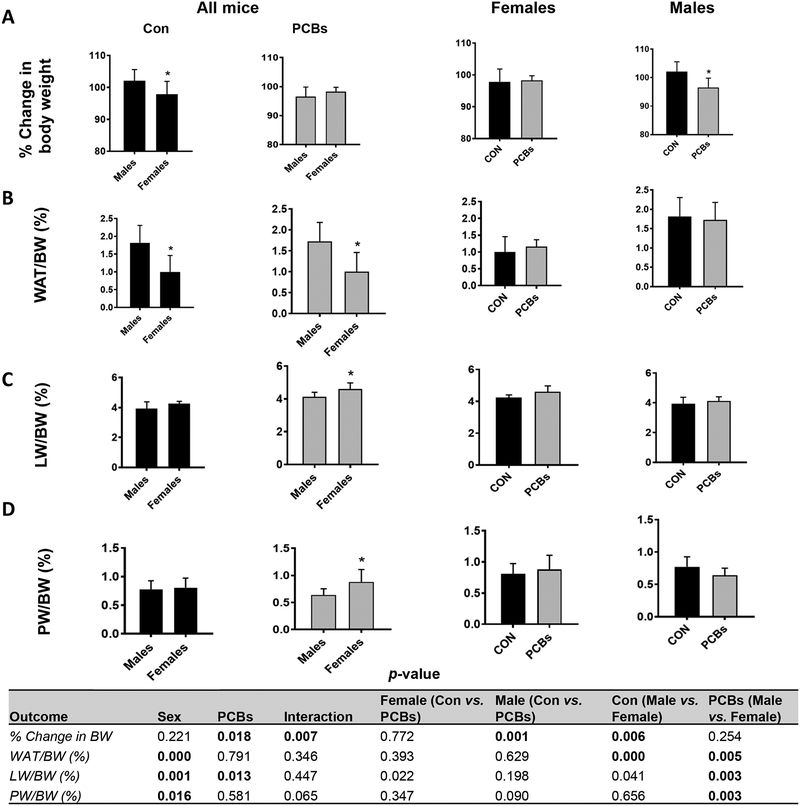
Initially, the impact of oral PCB administration on body weight was examined. As expected (Hong et al., 2009), male mice exhibited higher body weights relative to female mice and unexposed male mice had a significantly higher % increase in body weight gain compared to female mice (Fig. 1A). However, upon PCB exposure, male mice showed significantly decreased % body weight gain relative to unexposed male mice while there was no PCB effect on the weight of female mice. In terms of body fat composition, measured by the white adipose tissue (WAT) weight to body weight (BW) ratio, irrespective of exposure, female mice showed significantly lower fat composition compared to their male counterparts. PCBs had no significant impact on the WAT/BW ratio within either sex (Fig. 1B). PCB exposure led to significantly higher liver weight (LW) to BW ratio only in female mice (Fig. 1C). Interestingly, PCB-exposed female mice also showed significantly increased pancreatic weight (PW) to BW ratio, an effect that was absent in exposed, male mice (Fig. 1D).
Figure 1. PCB effects on body weight and fat composition.
(A) Body weight was measured throughout the two week study period and the percent increase or decrease in body weight relative to the initial body weight taken at the beginning of the study (considered as 100%) was calculated. (B) The body fat composition was measured by calculating the white adipose tissue weight to body weight ratio. (C & D) The liver and pancreas were isolated, weighed and their respective weights relative to body weight were calculated for each mouse. Values are mean ± SD. Values for p <0.05 are in bold.
3.2. Female mice were more sensitive to PCB-induced hepatic injury
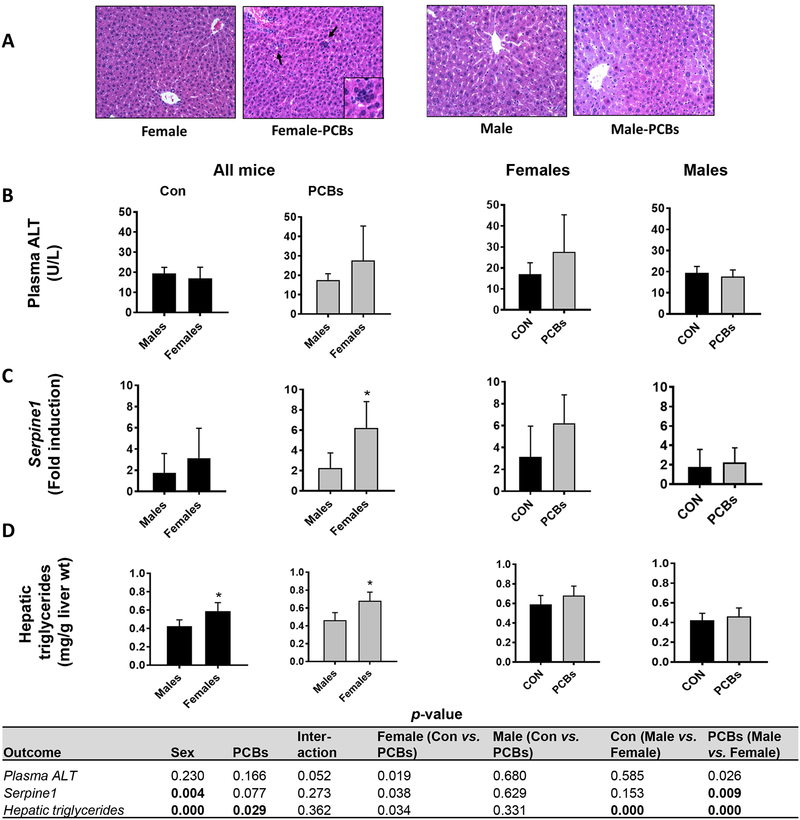
Liver sections of mice were examined for histological evidence of steatosis and inflammation. No histological steatosis was observed in any group by H&E staining (Fig. 2A). However, inflammatory foci were observed in PCB-exposed female mice, suggesting that female mice were more sensitive to PCB-induced inflammation versus males. Indeed, assessment of additional pro-inflammatory and hepatic injury markers demonstrated that PCB-exposed, female mice showed a trend for increased circulating liver enzyme levels namely ALT (Fig. 2B, p =0.026) and AST (Supplementary Figure 2A, p =0.033). PCB-exposed, female mice also showed significantly increased hepatic gene expression for plasminogen activator inhibitor (PAI-1, Serpine1) and a trend for increased expression of tumor necrosis factor (Tnf) (Fig. 2C and Supplementary Figure 2B). Further, female mice had significantly higher hepatic triglyceride levels than males, and PCB exposure exacerbated this effect (Fig. 2D), a finding reflective of the observed PCB-stimulated increase in LW/BW ratio in females (Fig. 1D). In contrast, the PCB- exposed, female mice exhibited significantly lower hepatic cholesterol levels than unexposed females (Supplementary Fig. 2C), suggesting dysregulation of hepatic lipid metabolism.
Figure 2. Female mice were more sensitive to PCB-induced hepatic injury.
(A) Hepatic sections were analyzed using hematoxylin-eosin (H&E) staining to determine the presence of steatosis and inflammation. (B) Plasma ALT levels was measured using the Piccolo Xpress Chemistry Analyzer (C) RT-PCR was performed to measure hepatic mRNA levels of Serpine1. (D) Hepatic lipids were extracted and triglycerides levels were measured using calorimetric assays. Values are mean ± SD. Values for p <0.05 are in bold.
3.3. Assessing sex- and PCB-dependent effects on plasma adipo-cytokines and lipid levels
To further examine the extent of PCB-induced hepatic injury and metabolic dysregulation, plasma levels of adipo-cytokines and lipids were evaluated (Table 1). Circulating levels of the inflammatory marker PAI-1 shown a trend for upregulation in the PCB-exposed, female group (Table 1, p =0.012) in concordance with hepatic Serpine1 levels (Fig. 2C). For circulating adipokines (Table 1), female mice had higher basal adiponectin levels while PCB-exposed, females showed a trend for decreased plasma adiponectin levels (p =0.015). In contrast, there was no differences in leptin levels or on the adiponectin to leptin ratio, a useful estimate of adipose tissue dysfunction (Fruhbeck et al., 2018), in either sex. Furthermore, PCB-exposed female mice showed a trend for higher levels of resistin versus their male counterparts (Table 1, p =0.017).
Table 1.
Plasma levels of adipo-cytokines, lipids and lipoproteins were measured. Values are mean ± SD. Values for p <0.05 are in bold. HDL-high density lipoproteins, VLDL- very low density lipoproteins.
| Outcome (Analyte) | Female-CON | Female-PCBs | Male-CON | Male-PCBs |
|---|---|---|---|---|
| PAI-1 (pg/mL) | 605.2 ± 124.9 | 927.4 ± 372.5 | 842.9 ± 244.8 | 724.5 ± 208.6 |
| Adiponectin (pg/mL) | 33466.0 ± 5042.0 | 26161.0 ± 8085.9 | 27581.0 ± 5815.3 | 22452.0 ± 6191.8 |
| Leptin (pg/mL) | 16.4 ± 11.2 | 13.1 ± 5.7 | 31.3 ± 6.1 | 64.5 ± 78.8 |
| Adiponectin to Leptin Ratio | 3291.0 ± 2947.4 | 2066.0 ± 895.3 | 2288.0 ± 2082.0 | 1948.0 ± 3611.7 |
| Resistin (pg/mL) | 1015.0 ± 297.0 | 763.3 ± 103.0 | 632.3 ± 202.5 | 518.1 ± 155.0 |
| Cholesterol (mg/dL) | 64.7 ± 3.6 | 58.2 ± 13.4 | 104.8 ± 9.5 | 83.4 ± 9.9 |
| Triglycerides (mg/dL) | 64.0 ± 11.1 | 62.9 ± 14.3 | 94.11 ± 8.43 | 84.2 ± 7.9 |
| HDL (mg/dL) | 47.4 ± 3.6 | 42.7 ± 13.0 | 83.7 ± 9.7 | 69.4 ± 9.3 |
| VLDL (mg/dL) | 12.9 ± 2.1 | 12.5 ± 2.8 | 18.9 ± 5.0 | 14.8 ± 4.1 |
| p-value | |||||||
|---|---|---|---|---|---|---|---|
| Outcome | Sex | PCBs | Inter-action | Female (Con vs. PCBs) | Male (Con vs. PCBs) | Con (Male vs. Female) | PCBs (Male vs. Female) |
| PAI-1 | 0.760 | 0.244 | 0.012 | 0.012 | 0.288 | 0.037 | 0.114 |
| Adiponectin | 0.023 | 0.004 | 0.593 | 0.015 | 0.081 | 0.047 | 0.202 |
| Leptin | 0.082 | 0.352 | 0.267 | 0.908 | 0.094 | 0.592 | 0.072 |
| Adiponectin/Leptin | 0.136 | 0.106 | 0.941 | 0.326 | 0.157 | 0.248 | 0.313 |
| Resistin | 0.000 | 0.010 | 0.316 | 0.015 | 0.225 | 0.000 | 0.017 |
| Cholesterol | 0.000 | 0.000 | 0.023 | 0.146 | 0.000 | 0.000 | 0.000 |
| Triglycerides | 0.000 | 0.391 | 0.491 | 0.902 | 0.282 | 0.002 | 0.021 |
| HDL | 0.000 | 0.004 | 0.125 | 0.275 | 0.002 | 0.000 | 0.000 |
| VLDL | 0.001 | 0.062 | 0.121 | 0.807 | 0.019 | 0.001 | 0.165 |
Plasma cholesterol levels were significantly lower in the PCB-exposed, female group versus the male group although PCB exposure itself reduced plasma cholesterol levels in male mice (Table 1). Female mice showed a trend for lower plasma triglyceride levels irrespective of PCB exposure, a finding in opposition to the higher hepatic triglyceride levels in females (Fig. 2D). Additionally, PCB exposure led to significantly lower plasma HDL levels and a trend for decrease (p =0.019) for VLDL levels only in male mice, although PCB-exposed female mice still had lower HDL levels than the exposed male mice (Table 1). In sum, it appears that both PCB exposure and sex affected plasma levels of biomarkers for liver injury and metabolic dysregulation.
3.4. Sex differences and PCB effects in hepatic lipid metabolism
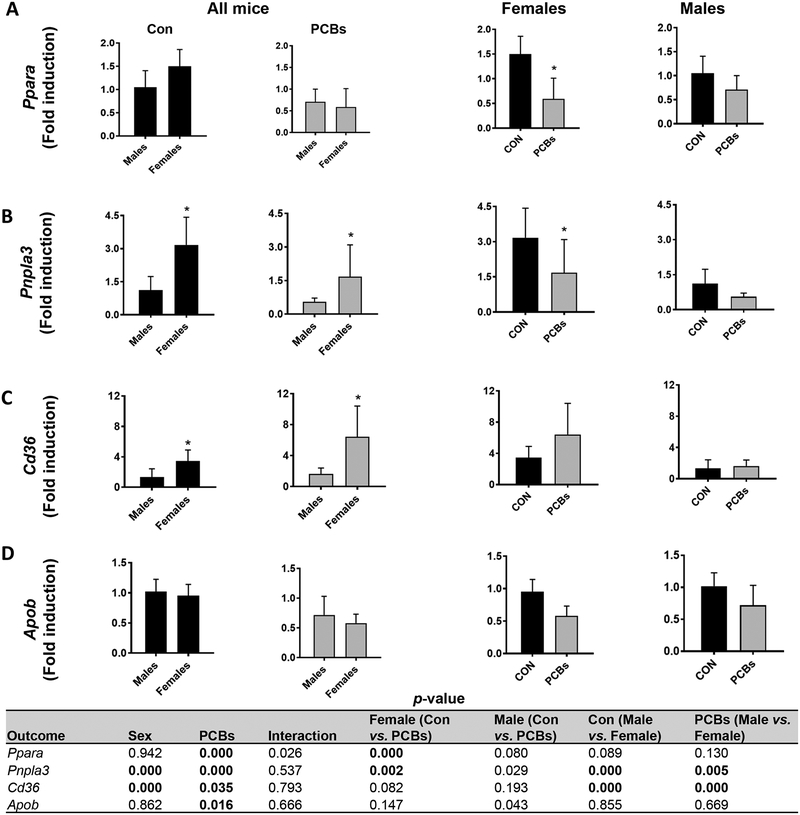
Due to the differences in hepatic triglyceride levels and known PCB effects on hepatic lipogenic and lipolytic genes (Wahlang et al., 2017a), hepatic expression of genes that play crucial roles in lipid synthesis, transport, and metabolism were measured. With respect to lipogenic gene expression (Supplementary Table 2), PCB exposure significantly decreased mRNA levels of fatty acid synthase (Fasn) in males, and PCB- exposed, males showed a trend for decreased sterol regulatory element binding transcription factor 1 (Srebf1) versus exposed females (p =0.017). In addition, PCB exposure significantly decreased stearoyl-Coenzyme A desaturase 1 (Scd1) only in females; thereby suggesting reduced hepatic de novo fatty acid and mono unsaturated fatty acid synthesis in males and females respectively. With regards to lipid breakdown, PCB exposure significantly reduced gene expression of Ppara, the hepatic nuclear receptor gene involved in lipid breakdown and mitochondrial fatty acid oxidation (Fig. 3A) only in females, while the PPAR gene target, carnitine palmitoyltransferase 1a (Cpt1a) was decreased in PCB-exposed females versus males (Supplementary Table 2). In addition, patatin-like phospholipase domain containing 3 (Pnpla3) expression was significantly higher in females than males and was significantly decreased with PCB exposure in females (Fig. 3B). Markers of hepatic lipid uptake such as the fatty acid transporter Cd36 and fatty acid binding protein 1 (Fabp1) were significantly upregulated in PCB-exposed females compared to males (Fig. 3C & Supplementary Table 2), suggesting that increases in hepatic triglycerides and decreases in plasma triglycerides may be due to increased hepatic efflux in females. Moreover, the hepatic expression of apolipoprotein B (Apob), a major constituent of plasma lipoproteins, showed a PCB effect and a trend for downregulation in PCB-exposed, male mice (Fig. 3D), consistent with the observed lower plasma VLDL levels in this group. Together these data suggest that PCB exposure disrupted hepatic lipid homeostasis with more pronounced effects in female mice due to the higher number of genes affected.
Figure 3. Sex differences and PCB effects in hepatic lipid metabolism.
Hepatic mRNA expression for genes involved in fatty acid breakdown including (A) Ppara (mitochondrial oxidation) and (B) Pnpla3 involved in lipolysis were measured using RT- PCR. Expression for genes involved in hepatic lipid uptake and lipoprotein synthesis, namely, (C) Cd36 and (D) Apob were also measured. Values are mean ± SD. Values for p <0.05 are in bold.
3.5. Sex differences and PCB effects in glucose metabolism
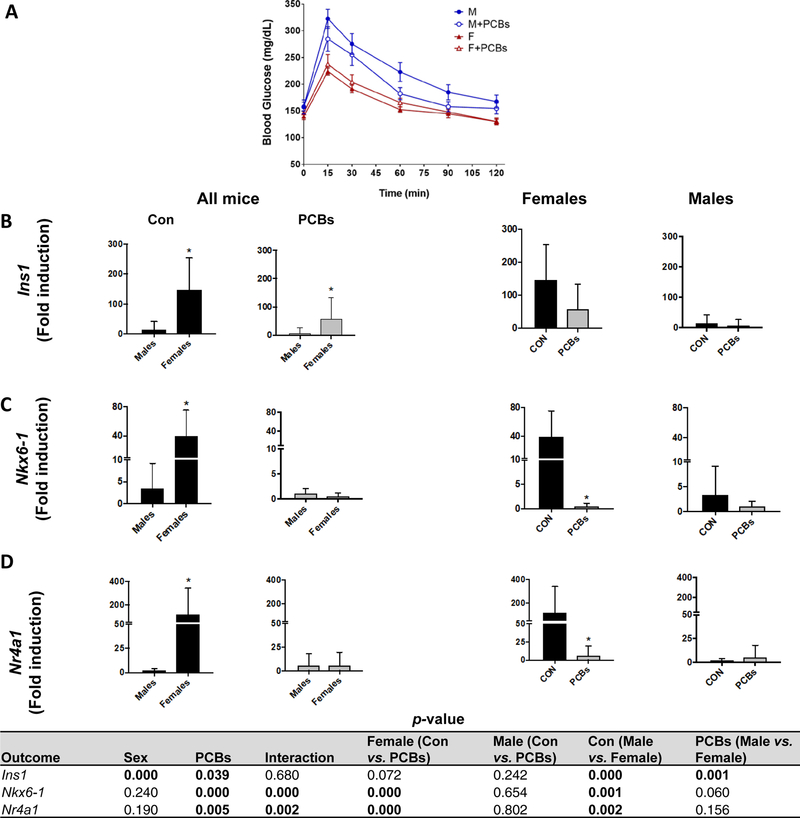
Previously, our group and others have reported that PCBs disrupt hepatic glucose regulation in animal and human studies with effects dependent on dose, congener type, and duration of exposure (Clair et al., 2018; Gadupudi et al., 2016; Wahlang et al., 2016; Wahlang et al., 2014b). Clearly, PCBs can alter both glucose and insulin levels, with the pancreas as an important target for PCB toxicity (Shi et al., 2019). In the current study, PCB exposure appeared to glucose metabolism in male mice as demonstrated by the glucose tolerance test (Fig. 4A). In general, female mice had a lower area under the curve (AUC) for the test (Supplementary Table 3), consistent with other reports (Macotela et al., 2009). PCB-exposed, male mice showed a trend for decreased AUC versus their unexposed counterparts (p =0.013). Nonetheless, there was no PCB effect on either fasting insulin or glucose levels (Supplementary Table 3). With regards to hepatic genes involved in glucose metabolism, the PCB-exposed, female group exhibited significantly lower hepatic transcript levels of solute carrier family 2 (Slc2a2), the glucose transporter between liver and blood (Supplementary Table 3). PCB exposure cause a trend for decreased hepatic expression of gluconeogenic genes, namely phosphoenolpyruvate carboxykinase 1 (Pck1), the rate- controlling enzyme in gluconeogenesis (p =0.018), and significantly decreased glucose- 6-phosphatase (G6Pc), an enzyme that hydrolyzes glucose-6-phosphate to free glucose in both sexes (Supplementary Table 3). Model assessments for determining insulin resistance (HOMA-IR) and insulin sensitivity (HOMA-β) showed that female mice had higher HOMA-IR levels regardless of exposure (Supplementary Table 2). In contrast, there was a trend towards increased HOMA- β levels in PCB-exposed male mice (p=0.051), consistent with previous findings (Wahlang et al., 2016). Gene expression for pancreatic b-cell markers was examined to better understand PCB and sex-dependent effects on pancreatic function (Fig. 4B, C & D). In general, females had significantly higher insulin 1 (Ins1) transcript levels than males in both PCB-exposed and unexposed groups. However, PCB exposure decreased transcript levels of pancreatic islet identity factors, namely, NK6 home box 1 (Nkx6–1) and the nuclear receptor subfamily 4, group A, member 1 (Nr4a1) only in female mice. There was a significant interaction for Nkx6–1 and Nr4a1 levels between PCBs and sex in female mice. These findings support previously reported findings of PCBs effects on pancreatic b-cell function in mice (Shi et al., 2019).
Figure 4. Sex-dependent, PCB effects in glucose metabolism.
(A) A glucose tolerance test was performed after the second PCB gavage and blood glucose levels were measured. Pancreatic mRNA expression for genes involved in insulin synthesis and pancreatic islet identity, namely (B) Ins1, (C) Nkx6–1 and (D) Nr4a1 were measured using RT-PCR. Values are mean ± SD. Values for p <0.05 are in bold.
3.6. Mechanistic analysis of PCB exposure and sex-dependent effects
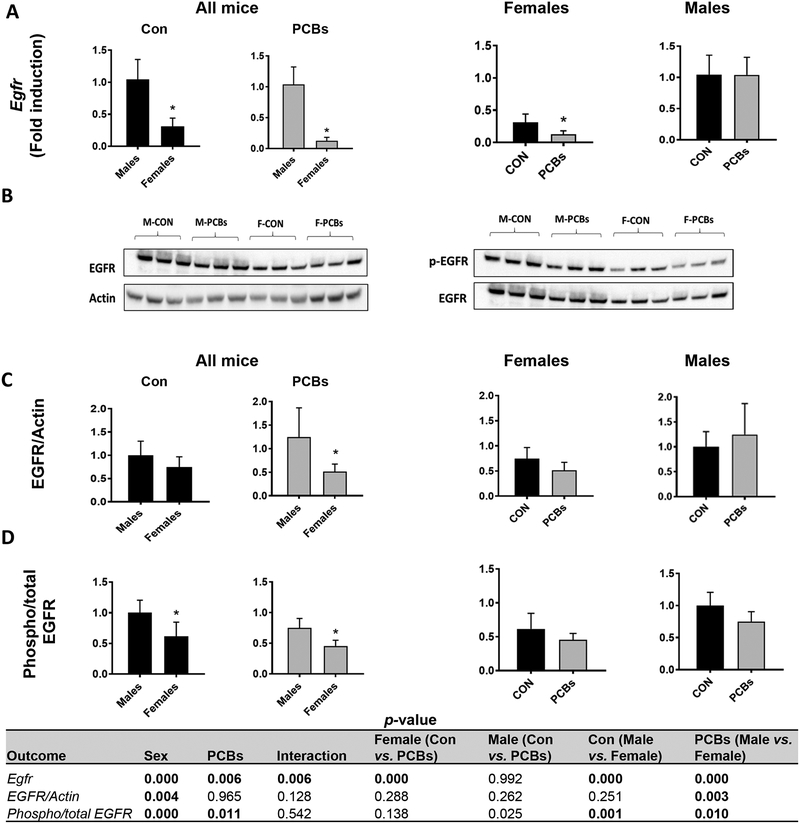
Thorough evaluation of known PCB-receptor interactions (Wahlang et al., 2014a) was performed, and comparisons were made dependent on sex. Initially, hepatic gene expression of known PCB targets was measured, namely cytochrome P450 Cyp1a2, an AhR target, Cyp3a11, a PXR target and Cyp2b10, a CAR target (Table 2). The dioxin and non-dioxin PCB mixture activated the AhR in both sexes as seen by significantly increased Cyp1a2 levels while Ahr gene expression significantly decreased with PCB exposure in female mice. This PCB mixture also significantly increased levels of Cyp3a11 in PCB-exposed females versus male mice while decreasing transcript levels of the PXR-encoding gene, Nr1i2, in female mice. CAR target genes (Cyp2b10 and Cyp2c29) were significantly increased with PCB exposure in both sexes. Critically, when compared to their respective basal levels, PCB-exposed male mice displayed higher Cyp2b10 fold induction versus PCB-exposed female mice (~80 fold vs. ~10 fold). PCB exposure significantly increased the hepatic expression of the CAR-encoding gene Nr1i3 in male mice. Hepatic gene expression and protein levels of EGFR, another protein that was recently characterized as a PCB target (Hardesty et al., 2017), was measured. Overall, hepatic Egfr levels were significantly lower in female mice (Fig. 5A) while PCB exposure further decreased Egfr levels. Concordantly, total EGFR protein levels were lower in female mice (Fig. 5B & C). EGFR phosphorylation was measured by western blot (Fig. 5B), and both PCB-exposed male and female mice showed a trend towards lower phosphorylated EGFR, indicating disrupted EGFR phosphorylation (Fig. 5D).
Table 2.
Hepatic gene expression for AhR, CAR and PXR and their targets was measured using RT-PCR. Values are mean ± SD. Values for p <0.05 are in bold.
| Outcome (Fold induction) | Female-CON | Female-PCBs | Male-CON | Male-PCBs |
|---|---|---|---|---|
| Cyp1a2 | 4.1 ± 8.3 | 33.8 ± 16.1 | 1.1 ± 0.6 | 23.1 ± 6.2 |
| AhR | 0.7 ± 0.3 | 0.4 ± 0.3 | 1.0 ± 0.3 | 0.7 ± 0.1 |
| Cyp3a11 | 2.5 ± 0.6 | 3.7 ± 2.1 | 1.2 ± 0.9 | 1.6 ± 0.4 |
| Nr1i2 | 1.8 ± 0.7 | 0.7 ± 0.4 | 1.3 ± 0.6 | 1.3 ± 0.3 |
| Cyp2b10 | 459.4 ± 261.0 | ±3027.0 | 9.1 ± 13.6 | 871.8 ±696.9 |
| Cyp2c29 | 3.9 ± 3.6 | 14.6 ± 11.2 | 1.1 ± 0.4 | 4.4 ± 2.4 |
| Nr1i3 | 1.2 ± 0.4 | 0.8 ± 0.5 | 1.1 ± 0.5 | 2.5 ± 0.7 |
| p-value | |||||||
|---|---|---|---|---|---|---|---|
| Outcome | Sex | PCBs | Inter-action | Female (Con vs. PCBs) | Male (Con vs. PCBs) | Con (Male vs. Female) | PCBs (Male vs. Female) |
| Cyp1a2 | 0.024 | 0.000 | 0.628 | 0.000 | 0.000 | 0.045 | 0.205 |
| Ahr | 0.002 | 0.000 | 0.180 | 0.000 | 0.052 | 0.142 | 0.002 |
| Cyp3a11 | 0.000 | 0.017 | 0.620 | 0.154 | 0.047 | 0.000 | 0.001 |
| Nr1i2 | 0.576 | 0.075 | 0.010 | 0.002 | 0.539 | 0.126 | 0.027 |
| Cyp2b10 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.007 |
| Cyp2c29 | 0.000 | 0.000 | 0.727 | 0.000 | 0.000 | 0.000 | 0.001 |
| Nr1i3 | 0.001 | 0.219 | 0.000 | 0.047 | 0.001 | 0.720 | 0.000 |
Figure 5. Sex-dependent, PCB effects on the EGFR.
(A) Hepatic mRNA expression for Egfr was measured. (B) Western blot was performed on liver protein to measure total and phosphorylated EGFR levels. (C) The densitometry ratios of total EGFR to the corresponding control protein, Actin and (D) phosphorylated to total EGFR were calculated. Values are mean ± SD. Values for p <0.05 are in bold.
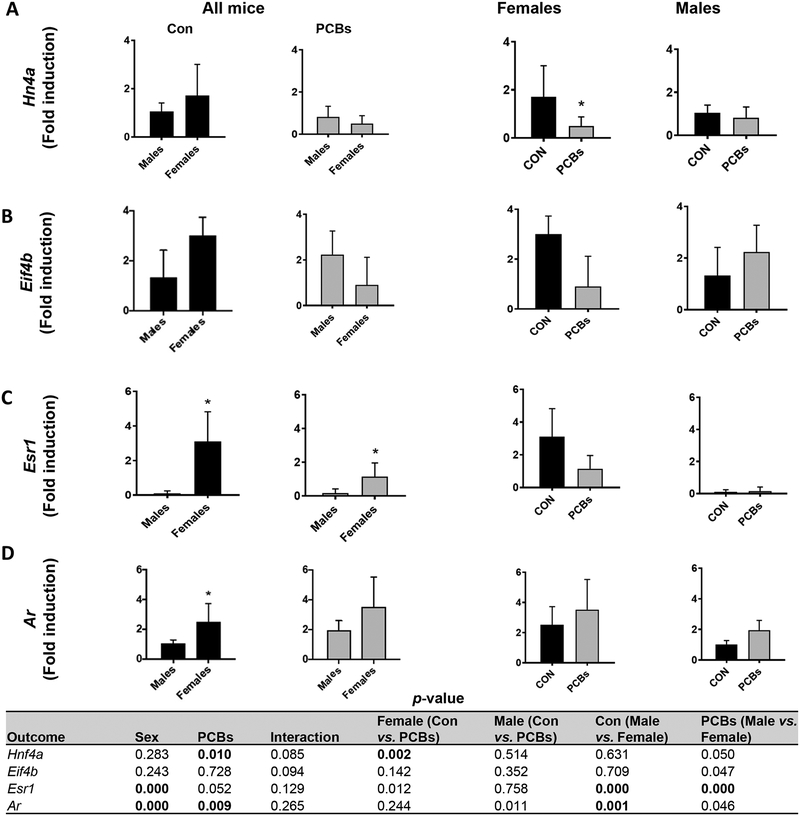
In addition to examining known PCB receptors, we investigated additional transcription factors and receptors pertaining to normal liver function and sexual dimorphism. The transcription factor HNF4A is crucial for the development and function of liver, pancreas, kidney and intestine (Babeu and Boudreau, 2014; Hang et al., 2017; Martovetsky et al., 2013). Female mice showed significantly higher basal levels of Hnf4a gene expression while PCB exposure downregulated Hnf4a in females but not males (Fig. 6A). However, HNF4A protein levels were similar in all groups (Supplementary Fig. 3). The hepatic expression of eukaryotic translation initiation factor 4B, Eif4b, a downstream target related to HNF4A function (Rouillard et al., 2016), showed a similar trend to Hnf4a (Fig. 6B). Because PCBs are known to affect the endocrine system and interact with sex hormone receptors (Takeuchi et al., 2017), hepatic expression of the estrogen receptor α (ERα)-encoding gene, estrogen receptor 1 (Esr1), and androgen receptor (Ar) was measured (Fig. 6C & D). In addition, circulating levels of sex-steroids and thyroid hormones were measured (Table 3). Female liver showed higher Esr1 transcript expression and PCB exposure showed a trend for decreased Esr1 transcript levels in female mice (p =0.012); accompanied by numerically higher plasma testosterone levels (Table 3). In contrast, PCB-exposed, male mice showed a trend for increased Ar mRNA levels (p =0.011), suggesting an anti-estrogenic and pro-androgenic activity with this PCB mixture. Further, compared to their male counterparts, the PCB-exposed, female mice had significantly higher levels of cortisol, the glucocorticoid associated with stress levels (Gong et al., 2015), and triiodothyronine (T3), while PCBs had the opposite effect in male mice for thyroxine (T4) levels (Table 3). Finally, glutathione-S-transferase pi (Gstp1, sexually dimorphic) and albumin (Alb, liver-specific) were evaluated (Supplementary Table 4). The GSTpi1 protein along with other GST isoforms are involved in cellular oxidative stress pathways and liver Gstp1 constitutive expression is higher in male versus female mice as observed in the current study (Knight et al., 2007; Nault et al., 2017). Importantly, PCB exposure significantly increased Gstp1 in females. In terms of the liver-specific gene, Alb, PCB exposure significantly increased hepatic Alb transcript levels only in female mice (Fig. 6F), which is yet another evidence of sex- dependent PCB effects in the liver.
Figure 6. Sex-dependent, PCB effects on additional targets.
Hepatic mRNA expression for (A) Hnf4a, (B) Eif4b, a downstream HNF4A target, (C) Esr1, the estrogen receptor 1 coding gene, and (D) Ar were measured using RT-PCR. Values are mean ± SD. Values for p <0.05 are in bold.
Table 3.
Plasma levels of circulating sex steroids, cortisol and thyroid hormones were measured. Values are mean ± SD. Values for p <0.05 are in bold.
| Outcome (Analyte) | Female-CON | Female-PCBs | Male-CON | Male-PCBs |
|---|---|---|---|---|
| Progesterone (pg/mL) | 1809.0 ± 1565.4 | 1499.0 ± 1200.7 | 361.5 ± 374.2 | 411.6 ± 426.0 |
| Testosterone (pg/mL) | 80.9 ± 40.7 | 247.5 ± 151.7 | 149.5 ± 54.8 | 466.4 ± 841.1 |
| Cortisol (ng/mL) | 12.2 ± 2.2 | 16.6 ± 9.2 | 10.6 ± 1.7 | 9.1 ± 2.1 |
| Triiodothyronine (T3) (pg/dL) | 563.1 ± 169.1 | 1393.1 ± 798.9 | 482.2 ± 158.2 | 407.1 ± 154.5 |
| Thyroxine (T4) (pg/dL) | 6196.0 ± 2764.5 | 7564.0 ± 3609.2 | 8418.0 ± 3191.4 | 3484.1 ± 2449.9 |
| p-value | |||||||
|---|---|---|---|---|---|---|---|
| Outcome | Sex | PCBs | Inter-action | Female (Con vs. PCBs) | Male (Con vs. PCBs) | Con (Male vs. Female) | PCBs (Male vs. Female) |
| Progesterone | 0.006 | 0.497 | 0.383 | 0.223 | 0.900 | 0.005 | 0.198 |
| Testosterone | 0.297 | 0.072 | 0.620 | 0.320 | 0.121 | 0.680 | 0.300 |
| Cortisol | 0.011 | 0.387 | 0.097 | 0.063 | 0.579 | 0.472 | 0.006 |
| Triiodothyronine (T3) | 0.001 | 0.014 | 0.004 | 0.000 | 0.731 | 0.683 | 0.000 |
| Thyroxine (T4) | 0.372 | 0.092 | 0.004 | 0.328 | 0.003 | 0.117 | 0.011 |
4. Discussion
The adverse health effects of PCB exposures are well-documented in various population studies and animal models, with disorders ranging from liver cancer to cardio-metabolic diseases such as diabetes, fatty liver disease, hypertension and stroke (Bergkvist et al., 2014; Goncharov et al., 2011; Zani et al., 2013). Given the major differences in the prevalence of metabolic disorders in males and females, it is important to address sex differences as another factor that can dictate the type and extent of PCB-induced toxicity. For instance, Ha et al. performed a gender-based study on the NHANES 1999–2002 population and found that dioxin-like and non-dioxin-like PCBs were positively associated with hypertension only in men and tended to be inversely correlated with hypertension in women (Ha et al., 2009). On the contrary, women were more prone to developing diabetes with PCB exposures in a 24-year follow up study on the ‘Yu-cheng’ cohort who were victims of a mass PCB poisoning in Taiwan (Wang et al., 2008). Additionally, a follow-up study on PCB-exposed farmers in Michigan reported that women experienced twice the incidence of self-reported type 2 diabetes with PCB exposure; however, there was no increased diabetic risk for PCB- exposed men (Vasiliu et al., 2006). PCB effects on obesity also appeared to be gender- based as evident from a children cohort study in the Faroes Island where girls tended to be more obese with prenatal PCB exposures compared to boys (Tang-Peronard et al., 2014). It is important to note that the anti-estrogenic or pro-androgenic effects of some PCB congeners may influence health outcomes; for example, breast cancer in women (Arrebola et al., 2016) and prostate cancer in men (Ali et al., 2016). Overall, the published literature provides preliminary evidence that sex differences exist in health outcomes related to human PCB exposures.
The current study focused on identifying sex differences in TASH and associated metabolic disorders using a mouse model exposed to a mixture of ‘dioxin-like’ and ‘non- dioxin-like’ PCBs. The PCB mixture was designed to represent the higher molecular weight PCB congeners that bioaccumulate, consistent with current human PCB exposure patterns (Wahlang et al., 2014a). The overall findings clearly demonstrated that PCB exposure led to different toxicity outcomes in male and female mice. The PCB mixture used did not result in observable obesogenic effects. Instead, it decreased body weight gain in male mice only. With regards to liver toxicity, PCB-exposed female mice had higher triglycerides, inflammation and liver injury; an effect that was absent in male mice. Indeed, the PCB-exposed female mice also exhibited dysregulated hepatic gene expression which favored hepatic lipid accumulation (Cd36, Fabp1) and reduced hepatic lipid breakdown (Ppara, Pnpla3), with concurrent dysregulated circulating lipids (lower plasma triglycerides, HDLs). Importantly, PCB exposure also induced pancreatic toxicity in female mice as reflected by decreased expression of genes required for pancreatic b-cell development (Nkx61 and Nr4a1). In addition, these exposed female mice also showed a trend for insulin resistance (higher HOMA-IR) compared to male mice. These observations run in parallel to previous epidemiologic observations that reported women as more susceptible to PCB-associated diabetes compared to men (Kautzky-Willer et al., 2016).
In terms of PCB-receptor interactions, the PCB mixture used in the current study activated both the AhR (Cyp1a2 induction) and CAR (Cyp2b10, Cyp2c29 induction) at the dose and duration utilized. Intriguingly, male mice displayed a more robust CAR induction than female mice (Cyp2b10 fold increase) although female mice had a higher basal CAR activity. The degree of AhR activation appeared similar in both sexes. Previously, our group and others have demonstrated that activation of these hepatic receptors led to observed health effects, as illustrated by AhR-driven inflammation and PXR-associated steatosis (Angrish et al., 2013; He et al., 2013). A recent study using CAR and PXR knockout male mouse models failed to show protection against PCB- induced hepatic injury, thereby implicating the involvement of additional receptors/signaling pathways in mediating PCB liver toxicity (Wahlang et al., 2016). Indeed, ‘phenobarbital-like’ PCBs that are ‘non-dioxin-like’ in nature (like Aroclor 1260) and many other metabolism-disrupting chemicals interfere with normal EGFR signaling similar to phenobarbital (Hardesty et al., 2018; Hardesty et al., 2017). EGFR, a transmembrane receptor for EGR and other ligands, is a key regulator of numerous signaling processes required for energy metabolism and homeostasis (Tan et al., 2016). Disruption of the EGFR signaling cascade is one of many mechanisms by which metabolism-disrupting chemicals induce toxicity (Hardesty et al., 2018). In addition, drugs such as phenobarbital indirectly activate CAR through EGFR inhibition, as do PCBs (Mutoh et al., 2013). The lower Egfr transcript and protein levels in female mice could plausibly explained the observed sex-dependent effects associated with PCB exposure in our study. Another notable observation reported here that may be associated with the greater PCB-induced hepatic gene dysregulation in female mice was the lower gene expression and activity of HNF4A, a crucial transcription factor for hepatocyte function. Although the exact mechanism by which HNF4A was affected by PCBs was not explored in the current study, it is important to note that the altered Hnf4a expression could play a critical role in directing the dysregulated hepatic gene expression observed in this group.
In addition to acting as metabolism-disrupting chemicals, PCBs are also established endocrine-disrupting chemicals through their interactions with endocrine receptors (Luthe et al., 2008). Most low molecular weight PCBs that are not heavily chlorinated are known to activate estrogen receptors (ERα and ERβ) and antagonize AR (Pencikova et al., 2018). Higher molecular PCB congeners that are more heavily chlorinated are considered anti-estrogenic and this is relevant to sex-specific effects (Zhang et al., 2014). The high-molecular weight congeners present in the PCB mixture used in our study may account for the decreased hepatic Esr1 in PCB-exposed female mice while simultaneously upregulating testosterone levels. Estrogens at physiological levels play beneficial roles in lipid metabolism, bone formation, and fluid balance, in addition to their roles in development and maintenance of the reproductive system (Farzaneh and Zarghi, 2016). Hence, aberrant estrogen production and activity is related not only to breast and ovarian cancer, but also to metabolic disorders including liver and cardiovascular diseases and the metabolic syndrome. The current study also showed that PCB exposure modified plasma cortisol and thyroid levels with different effects in males and females, which is another striking observation. In general, females had higher circulating T3 levels consistent with previous reports (Segal et al., 1982); while PCB exposure increased T3 levels in females and decreased T4 levels in males. Importantly, PCBs are known to competitively bind to thyroid hormones receptors due to their structural resemblance and have been reported to decrease T4 production in animal models (Bansal and Zoeller, 2008; Chen et al., 2015). However, the fact that the PCB mixture in the current study increased T3 levels in females perhaps implicated the possible role of sex-dependent PCB effects on the hypothalamus-pituitary-thyroid axis and plausible cross-talk between sex and thyroid hormones (Duarte-Guterman et al., 2014). Although further investigations are needed to determine the genesis of these differences, the findings imply endocrine disruption as a key player influencing the observed PCB-exposure-driven metabolic disruption.
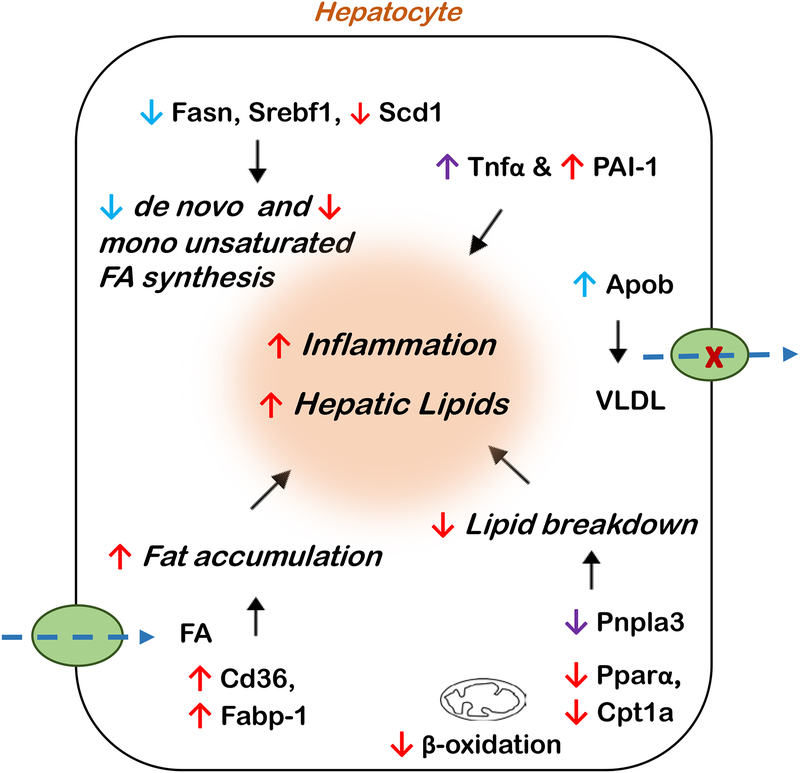
To summarize, the current study showed distinct sex differences with PCB exposure which can be attributed to factors including sex-dependent differences in basal levels of genes involved in regulating hepatic gene transcription and function, e.g. Egfr and Hnf4a, the expression levels of ERα and AR, as well as other nuclear receptors, and EGFR signaling. These differences, in part, could potentially influence PCB-modulated hepatic gene expression leading to more pronounced effects in females (Fig. 7). Due to growing epidemiological reports of sex differences in diseases associated with POPs exposures, the current study is of therefore of relevance to the field of environmental metabolic health. Notably, the existence of sexual dimorphism in chromatin structure in the liver (Sugathan and Waxman, 2013) provides a basis for sex differences in energy metabolism, consequently affecting PCB-mediated metabolic outcomes. Indeed, our findings demonstrate the impact of sexual dimorphism in regulating PCB effects on metabolic disruption and TASH. A potential limitation of the current study is that it focuses on short-term exposures and PCB effects on a low-fat diet whereas full-blown TASH and fibrosis are implicated in pollutant and high fat diet co-exposures occurring over a longer time period. Another limitation in the current study is that it did not account for the effect of estrous cycle in female mice which may have potentially influence data variability. Also, the study did not employ a power calculation for sample size selection to minimize variability within a group. Future studies will include i) investigating sex differences related to pollutant:nutrition interactions, and ii) performing high throughput omics studies to identify key cellular pathways and signaling processes driving sex differences in TASH to obtain more mechanistic insight. In conclusion, the current evidence of sex-dependent, metabolic disruption with environmental toxicants strongly supports the importance of considering sex differences when calculating risk assessment in exposed populations.
Figure 7.
A schematic diagram depicting the net results from the altered hepatic gene expression (both significantly as well as trending towards significance) induced by PCB exposure in male mice (indicated by blue), female mice (indicated by red), or in both (indicated by purple). FA-fatty acids.
Supplementary Material
Highlights.
Male and female mice were exposed to a mixture of polychlorinated biphenyls (PCBs)
PCB exposure resulted in disruption of hepatic metabolism in both sexes
PCBs caused sex-dependent endocrine disruption
Exposed female mice demonstrated liver injury and pronounced alterations in energy metabolism
Acknowledgements
The authors would like to acknowledge the University of Louisville Cancer Center biostatisticians, Drs. Shesh Rai and Jianmin Pan, for their assistance with the statistical analysis of the data.
Funding Support
The current study is supported by the National Institute of Environmental Health Sciences [R35ES028373, P42ES023716, T32ES011564]; the National Institute of General Medical Sciences [P20GM113226]; and the National Institute on Alcohol Abuse and Alcoholism [P50AA024337].
Abbreviations
- ALT
Alanine transaminase
- Alb
albumin
- AR
androgen receptor
- ApoB
apolipoprotein B
- AhR
arylhydrocarbon receptor
- AST
aspartate transaminase
- Cpt1a
carnitine palmitoyltransferase 1a
- CAR
constitutive androstane receptor
- CD36
cluster of differentiation
- CYP
cytochrome P450
- EGFR
epidermal growth factor receptor
- ER
estrogen receptor
- Esr1
estrogen receptor 1
- Eif4b
eukaryotic translation initiation factor 4B
- FXR
farnesoid-X-receptor
- Fabp1
fatty acid binding protein 1
- Fasn
fatty acid synthase
- G6Pc
glucose-6-phosphatase
- Gstp1
glutathione-S- transferase pi 1
- H&E
hematoxylin and eosin
- HNF4A
hepatocyte nuclear factor 4 alpha
- HDL
high density lipoprotein
- HOMA-β
homeostasis model assessment for pancreatic beta cell function
- HOMA-IR
homeostasis model assessment of insulin resistance
- Ins1
insulin 1
- LW
liver weight
- Nkx6–1
NK6 homeobox 1
- Nr1i2
nuclear receptor subfamily 1, group I, member 2
- Nr1i3
nuclear receptor subfamily 1, group I, member 3
- Nr4a1
nuclear receptor subfamily 4, group A, member 1
- PW
pancreatic weight
- PNPLA3
patatin-like phospholipase domain containing 3
- PPAR
peroxisome-proliferator activated receptors
- POPs
persistent organic pollutants
- Pck1
phosphoenolpyruvate carboxykinase 1
- PAI-1, Serpine1
plasminogen activator inhibitor
- PCBs
polychlorinated biphenyls
- PXR
pregnane-xenobiotic receptor
- Slc2a2
solute carrier family 2
- Scd1
stearoyl- Coenzyme A desaturase 1
- Srebf1
sterol regulatory element binding transcription factor 1
- T4
thyroxine
- TASH
toxicant-associated steatohepatitis
- T3
triiodothyronine
- Tnf
tumor necrosis factor
- VLDL
very low density lipoprotein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
All authors declare no conflicts of interest.
References
- Ali I, Julin B, Glynn A, Hogberg J, Berglund M, Johansson JE, Andersson SO, Andren O, Giovannucci E, Wolk A, Stenius U, Akesson A, 2016. Exposure to polychlorinated biphenyls and prostate cancer: population-based prospective cohort and experimental studies. Carcinogenesis. 37, 1144–1151. doi: 10.1093/carcin/bgw105 [DOI] [PubMed] [Google Scholar]
- Angrish MM, Dominici CY, Zacharewski TR, 2013. TCDD-elicited effects on liver, serum, and adipose lipid composition in C57BL/6 mice. Toxicol Sci. 131, 108–115. doi: 10.1093/toxsci/kfs277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrebola JP, Fernandez-Rodriguez M, Artacho-Cordon F, Garde C, Perez-Carrascosa F, Linares I, Tovar I, Gonzalez-Alzaga B, Exposito J, Torne P, Fernandez MF, Olea N, 2016. Associations of persistent organic pollutants in serum and adipose tissue with breast cancer prognostic markers. Sci Total Environ. 566–567, 41–49. doi: 10.1016/j.scitotenv.2016.04.188 [DOI] [PubMed] [Google Scholar]
- Babeu JP, Boudreau F, 2014. Hepatocyte nuclear factor 4-alpha involvement in liver and intestinal inflammatory networks. World J Gastroenterol. 20, 22–30. doi: 10.3748/wjg.v20.i1.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal R, Zoeller RT, 2008. Polychlorinated biphenyls (Aroclor 1254) do not uniformly produce agonist actions on thyroid hormone responses in the developing rat brain. Endocrinology. 149, 4001–4008. doi: 10.1210/en.2007-1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological). 57, 289–300. [Google Scholar]
- Bentley-Lewis R, Koruda K, Seely EW, 2007. The metabolic syndrome in women. Nat Clin Pract Endocrinol Metab. 3, 696–704. doi: 10.1038/ncpendmet0616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergkvist C, Kippler M, Larsson SC, Berglund M, Glynn A, Wolk A, Akesson A, 2014. Dietary exposure to polychlorinated biphenyls is associated with increased risk of stroke in women. J Intern Med. 276, 248–259. doi: 10.1111/joim.12194 [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ, 1959. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 37, 911–917. doi: 10.1139/o59-099 [DOI] [PubMed] [Google Scholar]
- Breivik K, Sweetman A, Pacyna JM, Jones KC, 2002. Towards a global historical emission inventory for selected PCB congeners--a mass balance approach. 1. Global production and consumption. Sci Total Environ. 290, 181–198. [DOI] [PubMed] [Google Scholar]
- Cave MC, Clair HB, Hardesty JE, Falkner KC, Feng W, Clark BJ, Sidey J, Shi H, Aqel BA, McClain CJ, Prough RA, 2016. Nuclear receptors and nonalcoholic fatty liver disease. Biochim Biophys Acta. 1859, 1083–1099. doi: 10.1016/j.bbagrm.2016.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zhang X, Jia X, Li Q, Su Q, Wang W, Liu Z, 2015. Thyroid disrupting effects of polychlorinated biphenyls in ovariectomized rats: A benchmark dose analysis. Environ Toxicol Pharmacol. 40, 733–740. doi: 10.1016/j.etap.2015.08.021 [DOI] [PubMed] [Google Scholar]
- Clair HB, Pinkston CM, Rai SN, Pavuk M, Dutton ND, Brock GN, Prough RA, Falkner KC, McClain CJ, Cave MC, 2018. Liver Disease in a Residential Cohort With Elevated Polychlorinated Biphenyl Exposures. Toxicol Sci. 164, 39–49. doi: 10.1093/toxsci/kfy076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte-Guterman P, Navarro-Martin L, Trudeau VL, 2014. Mechanisms of crosstalk between endocrine systems: regulation of sex steroid hormone synthesis and action by thyroid hormones. Gen Comp Endocrinol. 203, 69–85. doi: 10.1016/j.ygcen.2014.03.015 [DOI] [PubMed] [Google Scholar]
- Erickson MD, Kaley RG 2nd, 2011. Applications of polychlorinated biphenyls. Environ Sci Pollut Res Int. 18, 135–151. doi: 10.1007/s11356-010-0392-1 [DOI] [PubMed] [Google Scholar]
- Farzaneh S, Zarghi A, 2016. Estrogen Receptor Ligands: A Review (2013–2015). Sci Pharm. 84, 409–427. doi: 10.3390/scipharm84030409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federman DD, 2006. The biology of human sex differences. N Engl J Med. 354, 1507–1514. doi: 10.1056/NEJMra052529 [DOI] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R, Yebra-Pimentel I, Martinez-Carballo E, Simal-Gandara J, 2015. A Critical Review about Human Exposure to Polychlorinated Dibenzo-p- Dioxins (PCDDs), Polychlorinated Dibenzofurans (PCDFs) and Polychlorinated Biphenyls (PCBs) through Foods. Crit Rev Food Sci Nutr. 55, 1590–1617. doi: 10.1080/10408398.2012.710279 [DOI] [PubMed] [Google Scholar]
- Fruhbeck G, Catalan V, Rodriguez A, Gomez-Ambrosi J, 2018. Adiponectin-leptin ratio: A promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte. 7, 57–62. doi: 10.1080/21623945.2017.1402151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadupudi GS, Elser BA, Sandgruber FA, Li X, Gibson-Corley KN, Robertson LW, 2018. PCB126 Inhibits the Activation of AMPK-CREB Signal Transduction Required for Energy Sensing in Liver. Toxicol Sci. 163, 440–453. doi: 10.1093/toxsci/kfy041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadupudi GS, Klaren WD, Olivier AK, Klingelhutz AJ, Robertson LW, 2016. PCB126-Induced Disruption in Gluconeogenesis and Fatty Acid Oxidation Precedes Fatty Liver in Male Rats. Toxicol Sci. 149, 98–110. doi: 10.1093/toxsci/kfv215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncharov A, Pavuk M, Foushee HR, Carpenter DO, Anniston Environmental Health Reseach, C., 2011. Blood pressure in relation to concentrations of PCB congeners and chlorinated pesticides. Environ Health Perspect. 119, 319–325. doi: 10.1289/ehp.1002830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Miao YL, Jiao GZ, Sun MJ, Li H, Lin J, Luo MJ, Tan JH, 2015. Dynamics and correlation of serum cortisol and corticosterone under different physiological or stressful conditions in mice. PLoS One. 10, e0117503. doi: 10.1371/journal.pone.0117503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha MH, Lee DH, Son HK, Park SK, Jacobs DR Jr., 2009. Association between serum concentrations of persistent organic pollutants and prevalence of newly diagnosed hypertension: results from the National Health and Nutrition Examination Survey 1999–2002. J Hum Hypertens. 23, 274–286. doi: 10.1038/jhh.2008.124 [DOI] [PubMed] [Google Scholar]
- Hang HL, Liu XY, Wang HT, Xu N, Bian JM, Zhang JJ, Xia L, Xia Q, 2017. Hepatocyte nuclear factor 4A improves hepatic differentiation of immortalized adult human hepatocytes and improves liver function and survival. Exp Cell Res. 360, 81–93. doi: 10.1016/j.yexcr.2017.08.020 [DOI] [PubMed] [Google Scholar]
- Hardesty JE, Al-Eryani L, Wahlang B, Falkner KC, Shi H, Jin J, Vivace BJ, Ceresa BP, Prough RA, Cave MC, 2018. Epidermal Growth Factor Receptor Signaling Disruption by Endocrine and Metabolic Disrupting Chemicals. Toxicol Sci. 162, 622–634. doi: 10.1093/toxsci/kfy004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardesty JE, Wahlang B, Falkner KC, Clair HB, Clark BJ, Ceresa BP, Prough RA, Cave MC, 2017. Polychlorinated biphenyls disrupt hepatic epidermal growth factor receptor signaling. Xenobiotica. 47, 807–820. doi: 10.1080/00498254.2016.1217572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Gao J, Xu M, Ren S, Stefanovic-Racic M, O’Doherty RM, Xie W, 2013. PXR ablation alleviates diet-induced and genetic obesity and insulin resistance in mice. Diabetes. 62, 1876–1887. doi: 10.2337/db12-1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J, Stubbins RE, Smith RR, Harvey AE, Nunez NP, 2009. Differential susceptibility to obesity between male, female and ovariectomized female mice. Nutr J. 8, 11. doi: 10.1186/1475-2891-8-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautzky-Willer A, Harreiter J, Pacini G, 2016. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr Rev. 37, 278–316. doi: 10.1210/er.2015-1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight TR, Choudhuri S, Klaassen CD, 2007. Constitutive mRNA expression of various glutathione S-transferase isoforms in different tissues of mice. Toxicol Sci. 100, 513–524. doi: 10.1093/toxsci/kfm233 [DOI] [PubMed] [Google Scholar]
- Labots G, Jones A, de Visser SJ, Rissmann R, Burggraaf J, 2018. Gender differences in clinical registration trials: is there a real problem? Br J Clin Pharmacol. 84, 700–707. doi: 10.1111/bcp.13497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YF, Jin T, Xu Y, Zhang D, Wu Q, Zhang YK, Liu J, 2013. Sex differences in the circadian variation of cytochrome p450 genes and corresponding nuclear receptors in mouse liver. Chronobiol Int. 30, 1135–1143. doi: 10.3109/07420528.2013.805762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthe G, Jacobus JA, Robertson LW, 2008. Receptor interactions by polybrominated diphenyl ethers versus polychlorinated biphenyls: a theoretical Structure-activity assessment. Environ Toxicol Pharmacol. 25, 202–210. doi: 10.1016/j.etap.2007.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macotela Y, Boucher J, Tran TT, Kahn CR, 2009. Sex and depot differences in adipocyte insulin sensitivity and glucose metabolism. Diabetes. 58, 803–812. doi: 10.2337/db08-1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martovetsky G, Tee JB, Nigam SK, 2013. Hepatocyte nuclear factors 4alpha and 1alpha regulate kidney developmental expression of drug-metabolizing enzymes and drug transporters. Mol Pharmacol. 84, 808–823. doi: 10.1124/mol.113.088229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennecozzi M, Landesmann B, Palosaari T, Harris G, Whelan M, 2015. Sex differences in liver toxicity-do female and male human primary hepatocytes react differently to toxicants in vitro? PLoS One. 10, e0122786. doi: 10.1371/journal.pone.0122786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimoto MS, Nadal A, Sargis RM, 2017. Polluted Pathways: Mechanisms of Metabolic Disruption by Endocrine Disrupting Chemicals. Curr Environ Health Rep. 4, 208–222. doi: 10.1007/s40572-017-0137-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh S, Sobhany M, Moore R, Perera L, Pedersen L, Sueyoshi T, Negishi M, 2013. Phenobarbital indirectly activates the constitutive active androstane receptor (CAR) by inhibition of epidermal growth factor receptor signaling. Sci Signal. 6, ra31. doi: 10.1126/scisignal.2003705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nault R, Fader KA, Harkema JR, Zacharewski T, 2017. Loss of liver-specific and sexually dimorphic gene expression by aryl hydrocarbon receptor activation in C57BL/6 mice. PLoS One. 12, e0184842. doi: 10.1371/journal.pone.0184842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pencikova K, Svrzkova L, Strapacova S, Neca J, Bartonkova I, Dvorak Z, Hyzdalova M, Pivnicka J, Palkova L, Lehmler HJ, Li X, Vondracek J, Machala M, 2018. In vitro profiling of toxic effects of prominent environmental lower-chlorinated PCB congeners linked with endocrine disruption and tumor promotion. Environ Pollut. 237, 473–486. doi: 10.1016/j.envpol.2018.02.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouillard AD, Gundersen GW, Fernandez NF, Wang Z, Monteiro CD, McDermott MG, Ma’ayan A, 2016. The harmonizome: a collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database (Oxford). 2016. doi: 10.1093/database/baw100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Chatterjee B, 1983. Sexual dimorphism in the liver. Annu Rev Physiol. 45, 37–50. doi: 10.1146/annurev.ph.45.030183.000345 [DOI] [PubMed] [Google Scholar]
- Safe SH, 1994. Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit Rev Toxicol. 24, 87–149. doi: 10.3109/10408449409049308 [DOI] [PubMed] [Google Scholar]
- Segal J, Troen BR, Ingbar SH, 1982. Influence of age and sex on the concentrations of thyroid hormone in serum in the rat. J Endocrinol. 93, 177–181. [DOI] [PubMed] [Google Scholar]
- Shi H, Jan J, Hardesty JE, Falkner KC, Prough RA, Balamurugan AN, Mokshagundam SP, Chari ST, Cave MC, 2019. Polychlorinated biphenyl exposures differentially regulate hepatic metabolism and pancreatic function: Implications for nonalcoholic steatohepatitis and diabetes. Toxicol Appl Pharmacol. 363, 22–33. doi: 10.1016/j.taap.2018.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugathan A, Waxman DJ, 2013. Genome-wide analysis of chromatin states reveals distinct mechanisms of sex-dependent gene regulation in male and female mouse liver. Mol Cell Biol. 33, 3594–3610. doi: 10.1128/MCB.00280-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi S, Anezaki K, Kojima H, 2017. Effects of unintentional PCBs in pigments and chemical products on transcriptional activity via aryl hydrocarbon and nuclear hormone receptors. Environ Pollut. 227, 306–313. doi: 10.1016/j.envpol.2017.04.059 [DOI] [PubMed] [Google Scholar]
- Tan X, Lambert PF, Rapraeger AC, Anderson RA, 2016. Stress-Induced EGFR Trafficking: Mechanisms, Functions, and Therapeutic Implications. Trends Cell Biol. 26, 352–366. doi: 10.1016/j.tcb.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang-Peronard JL, Heitmann BL, Andersen HR, Steuerwald U, Grandjean P, Weihe P, Jensen TK, 2014. Association between prenatal polychlorinated biphenyl exposure and obesity development at ages 5 and 7 y: a prospective cohort study of 656 children from the Faroe Islands. Am J Clin Nutr. 99, 5–13. doi: 10.3945/ajcn.113.066720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgrimson BN, Minson CT, 2005. Sex and gender: what is the difference? J Appl Physiol (1985). 99, 785–787. doi: 10.1152/japplphysiol.00376.2005 [DOI] [PubMed] [Google Scholar]
- Vasiliu O, Cameron L, Gardiner J, Deguire P, Karmaus W, 2006. Polybrominated biphenyls, polychlorinated biphenyls, body weight, and incidence of adult-onset diabetes mellitus. Epidemiology. 17, 352–359. doi: 10.1097/01.ede.0000220553.84350.c5 [DOI] [PubMed] [Google Scholar]
- Wahlang B, Barney J, Thompson B, Wang C, Hamad OM, Hoffman JB, Petriello MC, Morris AJ, Hennig B, 2017a. Editor’s Highlight: PCB126 Exposure Increases Risk for Peripheral Vascular Diseases in a Liver Injury Mouse Model. Toxicol Sci. 160, 256–267. doi: 10.1093/toxsci/kfx180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlang B, Falkner KC, Clair HB, Al-Eryani L, Prough RA, States JC, Coslo DM, Omiecinski CJ, Cave MC, 2014a. Human receptor activation by aroclor 1260, a polychlorinated biphenyl mixture. Toxicol Sci. 140, 283–297. doi: 10.1093/toxsci/kfu083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlang B, Falkner KC, Gregory B, Ansert D, Young D, Conklin DJ, Bhatnagar A, McClain CJ, Cave M, 2013. Polychlorinated biphenyl 153 is a diet-dependent obesogen that worsens nonalcoholic fatty liver disease in male C57BL6/J mice. J Nutr Biochem. 24, 1587–1595. doi: 10.1016/j.jnutbio.2013.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlang B, Perkins JT, Petriello MC, Hoffman JB, Stromberg AJ, Hennig B, 2017b. A compromised liver alters polychlorinated biphenyl-mediated toxicity. Toxicology. 380, 11–22. doi: 10.1016/j.tox.2017.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlang B, Prough RA, Falkner KC, Hardesty JE, Song M, Clair HB, Clark BJ, States JC, Arteel GE, Cave MC, 2016. Polychlorinated Biphenyl- Xenobiotic Nuclear Receptor Interactions Regulate Energy Metabolism, Behavior, and Inflammation in Non-alcoholic-Steatohepatitis. Toxicol Sci. 149, 396–410. doi: 10.1093/toxsci/kfv250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlang B, Song M, Beier JI, Cameron Falkner K, Al-Eryani L, Clair HB, Prough RA, Osborne TS, Malarkey DE, Christopher States J, Cave MC, 2014b. Evaluation of Aroclor 1260 exposure in a mouse model of diet-induced obesity and non-alcoholic fatty liver disease. Toxicol Appl Pharmacol. 279, 380–390. doi: 10.1016/j.taap.2014.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SL, Tsai PC, Yang CY, Guo YL, 2008. Increased risk of diabetes and polychlorinated biphenyls and dioxins: a 24-year follow-up study of the Yucheng cohort. Diabetes Care. 31, 1574–1579. doi: 10.2337/dc07-2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zani C, Toninelli G, Filisetti B, Donato F, 2013. Polychlorinated biphenyls and cancer: an epidemiological assessment. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 31, 99–144. doi: 10.1080/10590501.2013.782174 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Lu M, Wang C, Du J, Zhou P, Zhao M, 2014. Characterization of estrogen receptor alpha activities in polychlorinated biphenyls by in vitro dual- luciferase reporter gene assay. Environ Pollut. 189, 169–175. doi: 10.1016/j.envpol.2014.03.001 [DOI] [PubMed] [Google Scholar]
- Zhao X, Cho H, Yu RT, Atkins AR, Downes M, Evans RM, 2014. Nuclear receptors rock around the clock. EMBO Rep. 15, 518–528. doi: 10.1002/embr.201338271 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.