Abstract
Precise control of in vivo transport of anticancer drugs in normal and cancerous tissues with engineered nanoparticles is key to future successes of cancer nanomedicines in clinics, which demands us to fundamentally understand how engineered nanoparticles impact the targeting-clearance and permeation-retention paradoxes in the anticancer drug delivery. Using a widely used anticancer drug, doxorubicin, as model, we systematically investigated how renal-clearable gold nanoparticles (AuNPs) affect the permeation, distribution and retention of drug in both cancerous and normal tissues. Our results show that renal-clearable AuNPs retain the strength of free drug in rapid tumor targeting with high tumor vascular permeability. Along with the enhanced tumor delivery and efficacy, the renal-clearable AuNPs also accelerated body clearance of “off-target” drug via renal elimination. These results clearly indicate that diverse in vivo transport behaviors of engineered nanoparticles can be used to reconcile long-standing paradoxes in the anticancer drug delivery.
Keywords: drug delivery, enhanced and permeability effect, gold nanoparticles, renal clearance, tumor targeting
Graphical Abstract
Long-standing paradoxes in cancer nanomachines, high tumor targeting vs. rapid clearance and high permeability vs. low systemic toxicity can be addressed with renal-clearable gold nanoparticles.
Fundamental understanding on how nanoparticles impact the targeting and clearance of anticancer drugs in vivo is of importance to their future success in the clinical practice[1]. Rapid blood elimination and high vascular permeability of small-molecule drugs (i.e., doxorubicin, DOX) have been considered major reasons for the low tumor targeting, modest therapeutic efficacies as well as severe side effects[2]. Thus, using non-renal-clearable engineered nanoparticles to alter in vivo transport of anticancer drugs has been a major strategy for improving efficacy and safety[3]. For example, PEGylated liposomes have been successfully used in the clinic to increase blood retention and tumor accumulation of DOX through enhanced permeability and retention (EPR) effect[4], and to reduce cardiotoxicity by minimizing drug extravasation to cardio vasculature[5]. Not limited to DOX, many other small-molecule anticancer drugs have also been delivered by similar nano-formulations in the clinics or under preclinical investigation[6].
While these nanoparticle-based drug delivery systems (DDSs) successfully enhance therapeutic efficacy and reduce side effects of anticancer drugs, some limitations have recently been recognized as well. The tumor targeting of these DDSs with large sizes is slowed down due to the limited tumor extravasation and penetration[1b], and body retention of “off-target” drugs is increased because of nanoparticle accumulation in liver and other reticuloendothelial system (RES) organs[7]. For instance, PEGylated liposome encapsulated doxorubicin (Doxil) cannot extravasate in tumor environment as rapidly and efficiently as free DOX[1b, 8], resulting in limited efficacy to tumors with lower vascular permeability[9]. Meanwhile, the “off-target” Doxil cannot be rapidly eliminated out of the body, further inducing side effects such as hand-foot syndrome and liver toxicity[10]. As a result, free doxorubicin currently remains the first-line treatment for a variety of solid tumors in clinics. In addition to PEGylated liposomes, many other nanoparticle-based DDSs have encountered similar challenges in their clinical translation, which demands us to revisit these paradoxes at a more fundamental level[1a]. Do we have to sacrifice rapid clearance of anticancer drugs for the high tumor targeting? Do we have to compromise rapid tumor targeting for the long blood retention and strong EPR effect? Do we have to give up high vascular permeability of anticancer drugs to reduce their non-specific accumulation in and toxicity to healthy tissues?
Herein, we report that using renal-clearable gold nanoparticles (AuNPs) as a novel delivery system and DOX as a model drug, we not only enhanced renal clearance of DOX by nearly 4 times, but also increased its tumor targeting and retention by more than 5 times over free drug. The renal-clearable DOX-loaded AuNPs (DOX@AuNPs) exhibited significantly improved tumor inhibition and survival rate than free DOX among several primary tumors and lung metastasis of breast cancer. This indicates the enhancement in tumor targeting and body elimination of anticancer drugs can be achieved concurrently with renal-clearable AuNPs as delivery vector. This paradigm shift in our understanding on the paradox between kidney elimination and tumor targeting fundamentally originates from high vascular permeability of renal-clearable ultrasmall AuNPs, which enables DOX to rapidly and efficiently target tumors in the early delivery phase. This is distinct from current large-size nanomedicines that require long blood circulation to enhance drug accumulation in tumor, compared to free drug[11]. In addition, dense and disordered tumor vasculatures further enhanced the tumor retention of DOX carried by the renal clearable AuNPs through EPR effect. With the enhanced renal clearance of “off-target” drug, the loaded DOX on the AuNPs was eliminated more rapidly from heart, lung and muscle, and also induced much less renal and hepatic toxicity than free DOX. These findings suggest that renal-clearable AuNPs can render anticancer drugs in vivo transport (targeting and clearance) kinetics completely different from conventional non-renal-clearable DDSs, opening up a new path to reconciling many long-standing paradoxes in the anticancer drug delivery.
With reduced size (<6 nm) and surface passivation, ultrasmall nanoparticles can be highly resistant to serum protein and renally clearable[12]. Renal-clearable nanoparticles readily cross inter-endothelial junctions of normal blood vessels[13] (~6 nm) like small molecular agents[14], escape uptake by liver (Fig. S1) and other RES organs[15]. More recently, we found that renal-clearable PEGylated AuNPs (PEG-AuNPs) targeted tumors passively with high efficiency >8 %ID/g (percentage of injected dose per gram of tumor)[16]. Thus, it is important to further explore how renal-clearable AuNPs will alter the delivery of anticancer drugs. To efficiently load drug (DOX) onto renal-clearable AuNPs, we intentionally incorporated a secondary ligand, 4-mercaptobenzoic acid (MBA), on the PEG-AuNPs through ligand exchange reaction (Methods and Fig. S2). By utilizing π-π interaction[17] between DOX and the aromatic MBA ligand, we loaded 33 ± 7 DOX molecules onto individual dual-ligand-coated AuNP (PEG/MBA-AuNPs; Fig. 1a), increasing loading capacity 5-time over unmodified PEG-AuNPs (Fig. S3 a and b). The characteristic absorbance of DOX at ~500 nm was readily observed in the DOX@AuNPs (Fig. 1b), where the 8-nm red-shift is attributed to dipole-dipole interactions among the loaded DOX molecules [18]. Further increasing drug loading resulted in aggregation of particles (Fig. S3c), therefore, we mainly focused on the AuNPs with an average loading of 33 DOX in this study.
Figure 1. Renal-clearable AuNPs enhanced renal elimination and retained cytotoxicity of DOX.
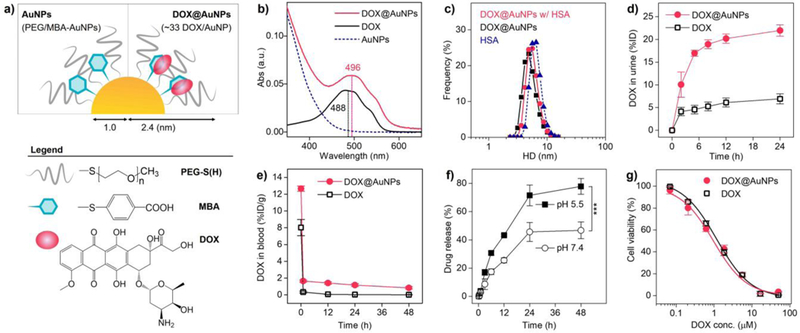
a) Scheme of renal-clearable DOX@AuNPs, which consists of the gold core (2.06 ± 0.18 nm in diameter, Fig. S2), surface coating with poly(ethylene glycol) thiol (PEG-SH, MW. 800 Da) and mercaptobenzoic acid (MBA), and loaded DOX molecules. b) UV-Vis absorbance spectra in aqueous solution. The loaded DOX exhibited 8-nm redshift (from 488 to 496 nm) compared with free DOX, indicating the formation of J-aggregates. c) Hydrodynamic diameters of DOX@AuNPs (5 μM) in PBS with and without human serum albumin (HSA, 6 nm, 5 μM) at 37 °C for 3 h. d) Renal clearance of DOX after intravenous injection of DOX@AuNPs and free DOX (n = 3). Error bar indicates s.d. e) Blood DOX concentration in the MCF-7 tumor-bearing mice (n = 4). f) In vitro drug release study in neutral (pH 7.4) and acidic (pH 5.5) physiological environment. ***P<0.005 (n = 3, Student’s t-test). g) In vitro cytotoxicity study with MCF-7 cancer cells (n = 6). To be noted, the AuNPs without DOX loading showed very low toxicity to the cells (Fig. S4d).
With successful drug loading, the DOX@AuNPs retained an ultrasmall hydrodynamic diameter (HD, 4.70 ± 0.89 nm, Fig. S4a) for efficient renal elimination. And the DOX@AuNPs showed no observable binding to human serum albumin (HSA, Fig. 1c) and resulting size increase, due to the antifouling nature of renal-clearable AuNPs, whereas, free DOX binds to HSA efficiently at a binding constant of 1.1 (± 0.3)× 104/M and a DOX-to-HSA ratio of 1.5[19]. After the intravenous administration of DOX@AuNPs into mice, 56.0% AuNPs were excreted in the urine at 24 h p.i. (post-injection), comparable to free PEG/MBA-AuNPs (52.7%) and PEG-AuNPs (50.9%, Fig. S4b). More importantly, with renal-clearable AuNPs as delivery vector, more than 22 %ID of the injected DOX was eliminated into urine in 24 h, which was 3.6 times higher than that of free DOX (~6 %ID, Fig. 1d). By comparing the renally cleared DOX and Au amount, we further found that the loaded DOX was stable in the body in a short term and was partially released with increased time (Fig. S4c). Together with the efficient renal clearance, blood DOX retention of DOX@AuNPs (63.7 %ID·h/g, 48-h AUC, area under the curve, Fig. 1e) was still nearly 10 times higher than that of free DOX (7.7%ID·h/g), which was because the renal-clearable AuNPs reduced serum protein binding of DOX and its hepatobiliary elimination[20]. Similar to many other DDSs where drugs were loaded via molecular interactions[21], DOX@AuNPs gradually released drug within a reasonable time period in physiological condition. The DOX release was accelerated in a slightly acidic pH (77.8% in 48 h, Fig. 1f) due to increased protonation[17], compared to the neutral environment (46.7%), which was favorable for drug release in tumor environment in vivo. Using MCF-7 human breast cancer cells for study, we found that the IC50 (half maximal inhibitory concentration) of DOX loaded on the AuNPs was 0.95 μM, comparable to that of free DOX (1.25 μM, Fig. 1g), indicating retained cytotoxicity of loaded drug.
Based on prevailing understanding on drug delivery, efficient renal clearance and short blood retention made it very hard for renal-clearable nanoparticles to target tumor effectively. However, our recent study reported that renal-clearable PEG-AuNPs targeted tumors passively with high efficiency (>8 %ID/g for MCF-7 human adenocarcinoma xenograft)[16]. By using the MCF-7 tumor as model, we next conducted the therapeutic studies with successive treatments of PBS, free DOX, and DOX@AuNPs (equivalent DOX, 5 mg/kg body weight, ×5, see Methods and Fig. S5 for dose schedule). From the tumor growth curves, the DOX@AuNPs showed significant tumor inhibition (compared to PBS control) as high as 77.6 ± 1.7 % (Fig. 2a); whereas, free DOX showed limited efficacy with tumor inhibition of 40.7 ± 6.7 %. Therefore, the survival rate was significantly increased by DOX@AuNPs over free DOX (Fig. 2b). Moreover, similar tumor inhibition by DOX@AuNPs was also observed in primary murine mammary carcinoma (4T1, Fig. 2c and d) and human triple-negative breast cancer (MDA-MB-231, Fig. S7) xenograft. In addition, since the 4T1 primary tumor showed rapid lung metastasis aggressively[22], we further conducted the therapeutic study on 4T1 lung metastasis. As a result, DOX@AuNPs not only effectively inhibited the lung metastasis (nodule count of 4.0 ± 2.7 per mouse, Fig. 2e and f), with 15.7, 21.2 times fewer nodule counts than free DOX and PBS, respectively, but also decreased the nodule sizes down to 0.83 ± 0.43 mm, significantly smaller than those treated with DOX and PBS (1.64 ± 0.77 and 1.95 ± 0.90 mm, respectively, Fig. 2g). These results indicate DOX@AuNPs significantly improved antitumor efficacy than free DOX together with the efficient renal elimination of “off-target” drug.
Figure 2. DOX@AuNPs enhanced anti-tumor efficacy of DOX among primary and metastatic tumor models.
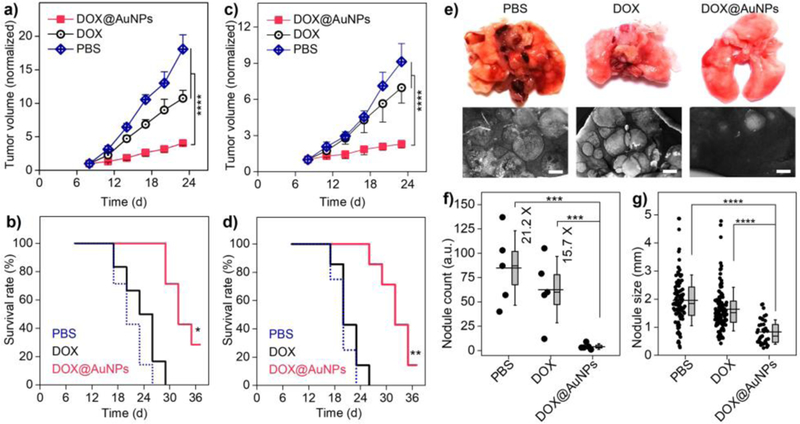
a–d) Normalized tumor growth curves and survival rates of the MCF-7 tumor-bearing nude mice (a, b) and 4T1 tumor-bearing balb/c mice (c, d) during successive treatments. For a and c, ****P<0.0001 (Student’s t-test). For b, * indicates <0.005, <0.0005 for DOX@AuNPs versus free DOX and PBS, respectively; For d, ** indicates P <0.001, <0.0005 for DOX@AuNPs versus free DOX and PBS, respectively (Kaplan-Meier, n = 6 – 8). To be noted, the AuNPs without DOX loading showed no tumor inhibition (Fig. S6). e) Images of mice lungs after the successive treatments (day-23). Scale bar, 1 mm. f) Nodule counts of the metastatic lung tumors after treatments. ***P<0.005 (n = 5). Box indicates median and s.e.m. g) Lung tumor nodule sizes. ****P<0.0001. Box indicates median and 25–75% interquartile range (Student’s t-test).
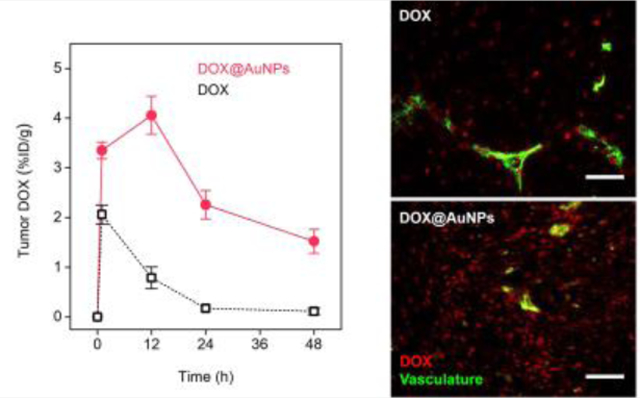
The further tumor targeting study unveiled the origin of the enhanced antitumor efficacy from DOX@AuNPs versus free drug. As shown in Fig. 3a, tumor accumulation of DOX delivered by the AuNPs rapidly increased to 3.35 ± 0.17 %ID/g at the early phase (1 h), which was 1.6 times higher than that of free DOX (2.06 ± 0.19 %ID/g). With time proceeded, DOX carried by the AuNPs reached its maximal tumor accumulation (4.06 ± 0.38 %ID/g) at 12 h and maintained delivery efficiency above 2 %ID/g for about 48 h, showing prolonged tumor retention. However, tumor accumulation of free DOX rapidly decreased to 0.17 %ID/g in 24 h, which was 12 times lower than that of DOX@AuNPs. As a result, the 48-h AUCtumor of DOX delivered by DOX@AuNPs (125.7 %ID•h/g) was 4.8 times higher than that of free DOX (26.0 %ID•h/g), indicating the high tumor targeting and prolonged tumor retention. This conclusion was further confirmed by drug distribution in tumor microenvironment. The fluorescent microscopy images shown that free DOX had low tumor distribution at 12 h p.i. (Fig. 3b) while more DOX carried by renal-clearable AuNPs extravasated from tumor vasculature and penetrated deeply in tumor environment (Fig. 3c). In comparison, it has shown in multiple reports that the intra-tumor delivery with large-sized liposomes as Doxil would have more heterogenous drug distribution and limited efficacy, due to the inefficient extravasation and the limited tumor penetration[1b, 23]. In addition, DOX release from the AuNPs within the tumor microenvironment was also observed after colocalizing the fluorescence images of DOX and renal-clearable AuNPs (Fig. S8).
Figure 3. DOX@AuNPs increased tumor targeting and retention by improving tumor extravasation and penetration.
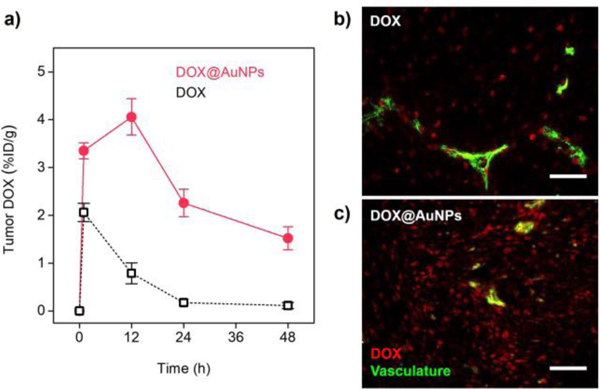
a) Drug delivery efficiencies to the MCF-7 tumors (n = 4). b-c) Fluorescence microscopy images of MCF-7 tumor tissues at 12 h post-injection of free DOX (b) and DOX@AuNPs (c). Red, DOX; green, stained vasculature (lectin-FITC). Scale bar, 50 μm.
Together with the improved drug delivery and efficacy, loading DOX on the AuNPs also reduced the hepatic and renal toxicity of drug. Liver metabolism is the major pathway for the body to eliminate free DOX (as well as many anticancer drugs), which is known to induce liver injury among 40% patients treated with DOX[20]. Blood chemistry tests showed that free DOX increased aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels (Fig. 4a and b), indicating the acute impairment to hepatocytes[24]. In addition, free DOX induced elevation in blood urea nitrogen (BUN) and creatinine (CREA) levels (Fig. 4c and d), indicating acute kidney injury by damaging glomerular podocytes[20, 25]. In contrast, DOX@AuNPs treatment showed the minimized elevation of AST, ALT, BUN and creatinine levels. This meant the DOX@AuNPs significantly reduced toxicity to both liver and kidneys, even though more DOX carried by the AuNPs was found in the liver and kidneys than free DOX (Fig. 4e) in 24 h. This is because the drug loading on the AuNPs minimized the interaction between DOX and the tissues, accelerating the DOX elimination.
Figure 4. Renal-clearable AuNPs enhanced body clearance and minimized toxicity of the “off-target” DOX. a-d),
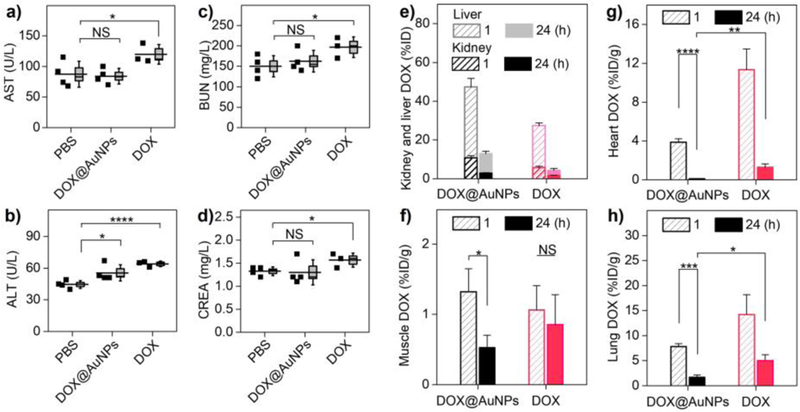
Blood chemistry analysis after successive treatments, indicating the acute toxicity of drug to liver function (aspartate transaminase, AST (a), alanine aminotransferase, ALT (b)) and kidney function (blood urea nitrogen, BUN (c), creatinine, CREA (d)). *P<0.05, ****P<0.0001, NS, not significant (n = 4 for DOX@AuNPs and PBS; n = 3 for free DOX, where one animal died prior to the end of the study). e) DOX distribution in liver and kidney for the injected DOX@AuNPs and free DOX (n = 3). f) Muscle DOX distribution at 1 and 24 h post-treatment (n = 3). *P<0.05. g-h) DOX distribution in vital organs as heart (g) and lungs (h) (n = 3). *P<0.05, **P<0.01, ***P<0.005, ****P<0.0001 (Student’s t-test).
While the administrated DOX@AuNPs held smaller size than interendothelial junctions and remained highly permeable across normal vasculature like free drug, DOX accumulation in muscle (gastrocnemius) decreased significantly from 1.32 ± 0.33 %ID/g at 1 h to 0.53 ± 0.18 %ID/g at 24 h p.i. (Fig. 4f), whereas free DOX showed much slower muscle elimination (from 1.06 %ID/g to 0.85 %ID/g). Surprisingly, the AuNPs also accelerated DOX elimination from heart with minimal accumulation (0.09 ± 0.04 %ID/g, Fig. 4g) at 24 h p.i., 14 times lower than that of free DOX (1.26 ± 0.37 %ID/g), which was greatly important to minimize the cardiotoxicity of DOX. In addition, the lung accumulation of DOX carried by AuNPs (1.66 ± 0.51 %ID/g, Fig. 4h) was also 3.0 times lower than that of free DOX (5.03 ± 1.17 %ID/g) at the same time point. The observed rapid DOX clearance from skeletal muscle, heart and lung after being loaded on the renal-clearable AuNPs is mainly because the antifouling nature of renal-clearable AuNPs reduces DOX interaction with these tissues and enhances its kidney elimination. These findings clearly indicated that DOX retention in these healthy tissues was much shorter after being loaded on the AuNPs even though DOX@AuNPs remained highly permeable to normal tissues and organs.
In the clinical translation of cancer nanomedicines, many paradoxes such as efficient kidney elimination versus high tumor targeting[26], rapid targeting versus long blood retention for strong EPR effect[27], and high vasculature permeability versus low systemic toxicity[7a] have been often encountered when anticancer drugs are delivered by non-renal-clearable DDSs. Using renal-clearable AuNPs as a delivery system and the doxorubicin as model, we found that these paradoxes can be reconciled. The enhancement in both tumor targeting and kidney elimination of DOX can be achieved concurrently with renal-clearable AuNPs. With high tumor permeability, the ultrasmall DOX@AuNPs can rapidly target tumor and retain in tumor much longer than free DOX through EPR effect, indicating that effective therapeutic treatment can be achieved through rapid tumor targeting with no need of prolonged blood retention. Moreover, the renal-clearable AuNPs significantly minimized the DOX-induced hepatic and renal toxicity by increasing renal elimination of “off-target” drugs as well as minimizing the drug interaction with and retention in healthy tissues. This further indicates that high vascular permeability and low systemic toxicity can be simultaneously achieved with renal-clearable AuNPs. These findings suggest that newly emerged renal clearable nanoparticles[12] hold great promise address many critical paradoxes between targeting and clearance, therapeutic efficacy and systematic toxicity in the clinical translation of cancer nanomedicines, which is expected to shift the paradigm in the anticancer drug delivery.
Methods
Methods and any associated references are available in the supporting information.
Supplementary Material
Acknowledgements
This study was supported by the NIH (1R01DK103363), CPRIT (RP160866), and Welch Research Foundation [AT-1974–20180324 (JZ)], and Cecil H. and Ida Green Professorship (JZ) from The University of Texas at Dallas.
Footnotes
Conflict of interest
The authors declare no conflict of interests.
References
- [1].a Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WC, Nature Reviews Materials 2016, 1, 16014; [Google Scholar]; b Jain RK, Stylianopoulos T, Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Allen TM, Cullis PR, Science 2004, 303, 1818–1822. [DOI] [PubMed] [Google Scholar]
- [3].Petros RA, DeSimone JM, Nat. Rev. Drug Discov. 2010, 9, 615. [DOI] [PubMed] [Google Scholar]
- [4].Iyer AK, Khaled G, Fang J, Maeda H, Drug Discov. Today 2006, 11, 812–818. [DOI] [PubMed] [Google Scholar]
- [5].Gabizon A, Shmeeda H, Barenholz Y, Clin. Pharmacokinet. 2003, 42, 419–436.www [DOI] [PubMed] [Google Scholar]
- [6].a Shi J, Kantoff PW, Wooster R, Farokhzad OC, Nat. Rev. Cancer 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]; b Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR, Pharm. Res. 2016, 33, 2373–2387. [DOI] [PubMed] [Google Scholar]
- [7].a Blanco E, Shen H, Ferrari M, Nat. Biotechnol. 2015, 33, 941; [DOI] [PMC free article] [PubMed] [Google Scholar]; b Tavares AJ, Poon W, Zhang Y-N, Dai Q, Besla R, Ding D, Ouyang B, Li A, Chen J, Zheng G, Robbins C, Chan WCW, Proc. Natl. Acad. Sci. U.S.A. 2017, 114, E10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Baish JW, Stylianopoulos T, Lanning RM, Kamoun WS, Fukumura D, Munn LL, Jain RK, Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 1799–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fabel K, Dietrich J, Hau P, Wismeth C, Winner B, Przywara S, Steinbrecher A, Ullrich W, Bogdahn U, Cancer: Interdisciplinary International Journal of the American Cancer Society 2001, 92, 1936–1942. [DOI] [PubMed] [Google Scholar]
- [10].a Dams ET, Laverman P, Oyen WJ, Storm G, Scherphof GL, van der Meer JW, Corstens FH, Boerman OC, J. Pharmacol. Exp. Ther. 2000, 292, 1071–1079; [PubMed] [Google Scholar]; b Lorusso D, Di Stefano A, Carone V, Fagotti A, Pisconti S, Scambia G, Ann. Oncol. 2007, 18, 1159–1164. [DOI] [PubMed] [Google Scholar]
- [11].Gabizon AA, Pappo O, Goren D, Chemla M, Tzemach D, Horowitz AT, Liposome Res J. 1993, 3, 517–528. [Google Scholar]
- [12].a Soo Choi H, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV, Nat. Biotechnol. 2007, 25, 1165–1170; [DOI] [PMC free article] [PubMed] [Google Scholar]; b Burns AA, Vider J, Ow H, Herz E, Penate-Medina O, Baumgart M, Larson SM, Wiesner U, Bradbury M, Nano Lett. 2008, 9, 442–448; [DOI] [PMC free article] [PubMed] [Google Scholar]; c Zhou C, Long M, Qin Y, Sun X, Zheng J, Angew. Chem. Int. Ed. 2011, 50, 3168–3172; [DOI] [PMC free article] [PubMed] [Google Scholar]; d Kang H, Gravier J, Bao K, Wada H, Lee JH, Baek Y, El Fakhri G, Gioux S, Rubin BP, Coll J-L, Choi HS, Adv. Mater. 2016, 28, 8162–8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].a Sarin H, J. Angiogenes. Res. 2010, 2, 14; [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zhou C, Long M, Qin Y, Sun X, Zheng J, Angew Chem Int Edit 2011, 50, 3168–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liu J, Yu M, Zhou C, Yang S, Ning X, Zheng J, J. Am. Chem. Soc. 2013, 135, 4978–4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].a Peng C, Gao X, Xu J, Du B, Ning X, Tang S, Bachoo RM, Yu M, Ge W-P, Zheng J, Nano Res. 2017, 10, 1366–1376; [DOI] [PMC free article] [PubMed] [Google Scholar]; b Yu M, Zheng J, ACS Nano 2015, 9, 6655–6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Liu J, Yu M, Ning X, Zhou C, Yang S, Zheng J, Angew. Chem. Int. Ed. 2013, 52, 12572–12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Liu Z, Sun X, Nakayama-Ratchford N, Dai H, ACS nano 2007, 1, 50–56. [DOI] [PubMed] [Google Scholar]
- [18].Yang X, Zhang X, Liu Z, Ma Y, Huang Y, Chen Y, The Journal of Physical Chemistry C 2008, 112, 17554–17558. [Google Scholar]
- [19].a Eksborg S, Ehrsson H, Ekqvist B, Cancer Chemother. Pharmacol. 1982, 10, 7–10; [DOI] [PubMed] [Google Scholar]; b Chassany O, Urien S, Claudepierre P, Bastian G, Tillement J-P, Cancer Chemother. Pharmacol. 1996, 38, 571–573; [DOI] [PubMed] [Google Scholar]; c Agudelo D, Bourassa P, Bruneau J, Bérubé G, Asselin É, Tajmir-Riahi H-A, PLoS One 2012, 7, e43814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tacar O, Sriamornsak P, Dass CR, J. Pharm. Pharmacol. 2013, 65, 157–170. [DOI] [PubMed] [Google Scholar]
- [21].a Lu Y, Hu Q, Lin Y, Pacardo DB, Wang C, Sun W, Ligler FS, Dickey MD, Gu Z, Nature communications 2015, 6, 10066; [DOI] [PMC free article] [PubMed] [Google Scholar]; b Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M, Terada Y, Kano M, Miyazono K, Uesaka M, Nat. Nanotechnol. 2011, 6, 815–823; [DOI] [PubMed] [Google Scholar]; c Fan Z, Chang Y, Cui C, Sun L, Wang DH, Pan Z, Zhang M, Nature communications 2018, 9, 2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].a Xu R, Zhang G, Mai J, Deng X, Segura-Ibarra V, Wu S, Shen J, Liu H, Hu Z, Chen L, Nat. Biotechnol. 2016, 34, 414; [DOI] [PMC free article] [PubMed] [Google Scholar]; b Shao S, Zhou Q, Si J, Tang J, Liu X, Wang M, Gao J, Wang K, Xu R, Shen Y, Nature Biomedical Engineering 2017, 1, 745. [DOI] [PubMed] [Google Scholar]
- [23].Manzoor AA, Lindner LH, Landon CD, Park J-Y, Simnick AJ, Dreher MR, Das S, Hanna G, Park W, Chilkoti A, Cancer Res. 2012, 72, 5566–5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].a Carvalho C, Santos RX, Cardoso S, Correia S, Oliveira PJ, Santos MS, Moreira PI, Curr. Med. Chem. 2009, 16, 3267–3285; [DOI] [PubMed] [Google Scholar]; b Gillet R, Grimber G, Bennoun M, de Fromentel CC, Briand P, Joulin V, Oncogene 2000, 19, 3498. [DOI] [PubMed] [Google Scholar]
- [25].Okuda S, Oh Y, Tsuruda H, Onoyama K, Fujimi S, Fujishima M, Kidney Int. 1986, 29, 502–510. [DOI] [PubMed] [Google Scholar]
- [26].Fang J, Nakamura H, Maeda H, Adv. Drug Del. Rev. 2011, 63, 136–151. [DOI] [PubMed] [Google Scholar]
- [27].a Chauhan VP, Stylianopoulos T, Martin JD, Popović Z, Chen O, Kamoun WS, Bawendi MG, Fukumura D, Jain RK, Nat. Nanotechnol. 2012, 7, 383–388; [DOI] [PMC free article] [PubMed] [Google Scholar]; b Chauhan VP, Jain RK, Nat. Mater. 2013, 12, 958. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


