Abstract
BACKGROUND:
Bullying has been shown to increase the risk of developing a psychotic disorder. To date, no studies have examined brain behavior relationships within the context of bullying victimization in clinical high-risk (CHR) youth, a group characterized by both gray and white matter abnormalities. The present study employed multimodal neuroimaging to examine possible neural mechanisms associated with bullying victimization.
METHODS:
CHR and healthy volunteers underwent clinical interviews, parent reports and MRI scans. Regions of Interest (ROIs) were picked based on sensitivity to environmental stress, including hippocampal, amygdala, and orbitofrontal cortex (OFC) structural ROIs, and uncinate fasciculus white matter integrity.
RESULTS:
CHR individuals were more exposed to bullying victimization than healthy volunteers, and bullying was associated with depressive symptoms across the whole sample. CHR individuals exhibited smaller volumes in OFC, but not in other ROIs. Increased bullying exposure was associated with lower medial OFC volumes in CHR and HV groups independently. Results ought to be interpreted as preliminary, as they did not survive correction at the whole brain level.
DISCUSSION:
Bullying victimization may affect or be affected by volumetric OFC differences in both healthy and CHR individuals. However, given CHR showed greater exposure to bullying as well as underlying vulnerability (e.g. lower volumes), results also point to etiological clues and novel intervention targets, though future replication is needed in better powered samples.
Keywords: Clinical High Risk, Bullying, DTI, MRI, Orbital Frontal Cortex
Introduction
Exposure to environmental risk factors plays an important role in the pathogenesis of schizophrenia (van Os et al., 2010; Brown, 2011). Recent work has examined peer relationships and bullying. For example, studies have found individuals with a psychotic disorder to be approximately twice as likely to report bullying victimization compared to controls (Trotta et al., 2013). There is some evidence that clinical high-risk (CHR) individuals (i.e., those showing recently emergent attenuated positive symptoms and corresponding cognitive/functional decline, that are at imminent risk for transitioning to psychosis) are also more likely to be exposed to bullying victimization (Valmaggia et al., 2015). However, neural mechanisms underlying bullying victimization have seldom been explored in general, and never before in this population. This is a particularly relevant question as CHR individuals are typically in the adolescent-young adult window when peer evaluation and the role of social support play critical roles in development as well as in shaping life-time behaviors (Valmaggia et al., 2015; McDonnell et al., 2018) and further, this population is often characterized by pervasive gray (Borgwardt et al., 2007; Wood et al., 2008) and white-matter (Carletti et al., 2012; Cho et al., 2016) pathology. As both gray and white matter changes reflect differing neurodevelopmental mechanisms (Matsuzawa et al., 2001; Konrad et al., 2013; Fuhrmann et al., 2015), they may also speak to distinct vulnerabilities, each with unique susceptibility to environmental stressors (Konrad et al., 2013; Whittle et al., 2013; Fuhrmann et al., 2015). However, to date there have been no studies that explore possible neural mechanisms of bullying victimization in CHR and further, no investigations that have incorporated critical perspectives from both gray and white matter pathology. This study examines neural correlates of bullying victimization, as well as links with symptoms within and across CHR and healthy volunteer (HV) groups. Studying CHR individuals offers a unique opportunity to understand the role of a novel and potentially potent risk factor and aids in understanding the etiology of the disorder.
Bullying victimization may contribute to increased vulnerability to develop a psychotic disorder (Trotta et al., 2013; Catone et al., 2015; Moffa et al., 2017). Large prospective studies found that the risk of developing psychotic symptoms was increased among victims of bullying, even when controlling for confounding factors such as prior psychopathology, SES, and IQ (Schreier et al., 2009; Catone et al., 2015). Given this evidence, it is of great importance to understand the emergence of this possible vulnerability prior to a formal psychosis diagnosis; these early markers may inform intervention and prevention models. Recent studies also suggest that a history of bullying is more common in individuals at CHR for developing psychosis (Valmaggia et al., 2015). The adolescence period for CHR and typically developing individuals confers a heightened vulnerability period: these individuals are undergoing a critical period of brain development and hormonal changes, along with increased social and occupational demands, all resulting in increased stress exposure (Konrad et al., 2013; Fuhrmann et al., 2015). In addition, CHR individuals are likely to possess pre-existing neurobiological vulnerabilities, further increasing risk for developing psychopathology (Valmaggia et al., 2015; McDonnell et al., 2018). Thus, studying these populations offers a critical opportunity to examine whether normative and pathological responses to environmental stressors can be distinguished with regards to bullying victimization.
Understanding symptoms and neural underpinnings of bullying victimization in CHR individuals during a dynamic developmental period that may be particularly sensitive to environmental stressors is a valuable opportunity. Indeed, exposure to adverse environmental factors such as chronic victimization has been shown to alter emotion regulation and reward processing (Nusslock and Miller, 2016; Rudolph et al., 2016; Teicher and Samson, 2016; Hiser and Koenigs, 2018; Telzer et al., 2018). Further, with regards to symptoms and disease progression, bullying victimization has been numerously linked to mood instability and depressive symptoms in psychosis, CHR and non-clinical populations (Catone et al., 2015; Valmaggia et al., 2015; Moffa et al., 2017). It has also been linked to positive symptoms including hallucinations, and to paranoid ideation (Moffa et al., 2017). Given that studies found the link between bullying and positive symptoms was partially mediated by depression, investigating these could be etiologically informative with regards to understanding effects of bullying victimization (Moffa et al., 2017).
To date, research in psychosis populations investigating neural mechanisms associated with bullying victimization has been rather limited, despite promising research in non-clinical populations. For example, in typically developing adolescent girls, a study found that chronic peer victimization possibly compromised the reward system, altering neural sensitivity to risk taking as well as affective sensitivity (Telzer et al., 2018). Chronic stress more generally has been shown to cause gray matter volume decreases and cortical thinning, especially during the critical periods of adolescence and young adulthood (Lenroot and Giedd, 2006; Baker et al., 2013; Tottenham, 2014; Fuhrmann et al., 2015). However, evidence of effects of bullying in psychosis populations has focused on links with symptom severity and functional outcomes; neural mechanisms are largely unexplored (Catone et al., 2015; Upthegrove, 2015; Valmaggia et al., 2015; Moffa et al., 2017).
Interestingly, regions that are commonly linked to reward processing and affect regulation, including the orbitofrontal cortex (OFC), amygdala, and hippocampus, are also known to be particularly vulnerable to the effects of early life stress and adverse environmental factors (Haber and Knutson, 2010; Thomaes et al., 2010; Dannlowski et al., 2012; Baker et al., 2013; Samplin et al., 2013; Chaney et al., 2014; Mychasiuk et al., 2016; Teicher and Samson, 2016; Teicher et al., 2016; Wikenheiser and Schoenbaum, 2016; Hiser and Koenigs, 2018). Well-replicated findings include reduced hippocampal volume and impaired amygdalar affect regulation in individuals with maltreatment histories (Dannlowski et al., 2012; Baker et al., 2013; Samplin et al., 2013), as well as lower OFC volumes and resting blood flow in children with maltreatment history or threatening event exposure (Chugani et al., 2001; Hanson et al., 2010; Thomaes et al., 2010; De Brito et al., 2013). Further, research has demonstrated that the OFC and hippocampus work together to mediate responses to stressful experiences and regulate the central nervous system (McEwen, 2007; Mychasiuk et al., 2016; Wikenheiser and Schoenbaum, 2016), and lesions of both amygdala and OFC have been known to impair affective processing in rhesus monkeys (Schoenbaum, 2004). Thus, it is possible that these regions would be further impacted by bullying victimization as an adverse environmental factor and stressor.
Finally, with regards to white matter integrity, the uncinate fasciculus white matter tract links critical emotional areas, such as medial temporal lobe (MTL) hippocampus and amygdala and prefrontal regions including OFC/ventromedial areas (Ebeling and von Cramon, 1992; Kim et al., 2011). This tract has also been found to be altered in psychotic disorders (Kubicki et al., 2002; Kawashima et al., 2009). Given the critical role of communication between prefrontal and MTL areas in affect regulation and reward processing (Quirk and Beer, 2006; Teicher and Samson, 2016; Hiser and Koenigs, 2018), examining myelination between these regions could further aid understanding of symptom presentation in individuals affected by bullying victimization.
In the present study, a multimodal approach was adopted, using structural volumetric and white matter ROIs. Hippocampus, amygdala, and OFC gray matter along with uncinate fasciculus white matter integrity were chosen as ROI candidates. We assessed CHR and HV participants to determine (1) whether there are differences between diagnoses in exposure to bullying victimization, and if so, (2) whether these differences are associated with depression and positive symptoms as found in previous studies (Moffa et al., 2017). Finally, given the previous literature we aimed to determine (3) if exposure predicts lower brain region volumes and uncinate fasciculus white matter integrity.
Methods
Adolescent and young adult HV and CHR subjects were recruited to the Adolescent Development and Preventive Treatment (ADAPT) research program. Exclusion criteria for both groups included head injury, presence of a neurological disorder, lifetime substance dependence, and history/presence of an Axis I psychotic disorder. For HV, presence of a psychotic disorder in a first-degree relative and presence of an Axis I disorder were also exclusion criteria. Parents gave written consent for subjects younger than 18; subjects 18 years or older gave written consent themselves. Of 112 subjects with data on the A-TAC and SRS, 8 were excluded from bullying-specific analyses due to having missing data on one or more of the three items of interest. Therefore, 104 (53 HV and 51 CHR) were included in analyses examining group differences in bullying exposure and relationships with symptoms. Of this initial sample (n = 112), 98 subjects (49 HV and 49 CHR) had successfully normalized/segmented neuroimaging scans and were included in analyses examining group (CHR vs HV) differences in volume (see below for imaging data processing). Finally, of these, 89 (48 HV and 41 CHR) had both bullying data for all 3 items and successfully normalized/segmented neuroimaging scans and were included in analyses examining relationships between volumes and bullying. analyses (see below for imaging data processing).
Measures
Bullying Victimization Measures.
Exposure to victimization was measured using three items taken from two parent-report measures. Multiple items were chosen to increase reliability and validity. Two of these items were from the Autism-Tics, ADHD, and other Co-morbidities inventory (A-TAC), one of which asks “Is he/she easily teased?” and the other of which asks “Is or has she/he been bullied by other children in school?” (Larson et al., 2013). The third item was acquired from the Social Responsiveness Scale (SRS), and asks whether the child “gets teased a lot” (Constantino et al., 2003). Exposure to these items was dummy coded, such that a score of 1 indicated exposure, and 0 indicated no exposure. A cumulative score was calculated by adding the items that were endorsed (see Table 1); scores were treated as rank-order variables. Given that 2 of the items measure “teasing,” endorsement of one or the other was counted once; therefore, cumulative exposure scores ranged from 0–2. Previous studies using cumulative risk scores have proven successful in better capturing the potency and pervasiveness of an environmental risk/stressor, especially when there is variability between endorsement of one versus multiple items (Evans et al., 2013; Evans and Cassells, 2014). Cronbach’s alpha among the 3 items was 0.794. Removing any of the items resulted in a lower Cronbach’s alpha estimate.
Table 1.
Demographic Characteristics.
| HV (1) | CHR (2) | Group Diff. | |
|---|---|---|---|
| Demographics | |||
| Gender | 48.1% male | 62.1% male | 1 = 2 |
| Age | 17.57 (2.87) | 18.47 (1.87) | 1 = 2 |
| Years of education | 11.57 (2.78) | 11.95 (2.40) | 1 = 2 |
| Symptoms | |||
| Positive a | 0.76 (1.46) | 11.81 (4.88) | 1 < 2* |
| Depressive mood b | 3.82 (5.24) | 16.85 (9.95) | 1 < 2* |
| Victimization c | |||
| Overall exposure | 14 (26.4%) | 25 (49.01%) | 1 < 2* |
Note: Mean (SD), 2 = CHR, 1 = HV
p < 0.05
Measured by SIPS battery.
Measured by the Beck Depression Inventory.
Count of subjects endorsing exposure to victimization (percentage of sample). Measured by sub-items of A-TAC and SRS scales.
Clinical Assessments.
The Structured Interview for Prodromal Syndromes (SIPS) was administered to CHR and HV participants to rule out the presence of CHR syndromes in HVs, and to diagnose the presence of a risk syndrome in CHR individuals and assess symptoms (Miller et al., 1999). Subjects were administered the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) to rule out psychotic disorders in both groups and assess history of mood and anxiety (Lobbestael et al., 2011). The Beck Depression Inventory (BDI), a reliable and valid assessment of depressive symptoms in adolescents and young adults, was administered (Steer et al., 1997). These measures have demonstrated strong interrater reliability in adolescents (Martin et al., 2000). Training of advanced doctoral student interviewers was conducted over a 2-month period; interrater reliabilities exceeded the minimum study criterion of κ ≥80.
Structural Imaging.
Scans were acquired with a 3-Tesla Siemens Tim Trio magnetic resonance imaging scanner (Siemens Healthineers, Erlangen, Germany) and a standard 12-channel head coil. Structural images were collected with a T1-weighted 3D magnetization prepared rapid gradient multi-echo sequence (saggital plane; repetition time (TR) = 253- ms; echo times (TE) = 1.64, 3.5, 5.36, 7.22, and 9.08 ms; GRAPPA parallel imaging factor 2; 1 mm3 isomorphic voxels, 192 interleaved slices; FOV = 256 mm; flip angle = 7°, time = 6:03 min). A T2 weighted acquisition (axial oblique aligned with anterior commissure-posterior commissure line; TR = 3720 ms; TE = 89 ms; GRAPPA parallel imaging factor 2; 0.9 mm × 0.9 mm voxels; FOV = 240 mm; ip angle: 120°; 77 interleaved 1.5 mm slices; time = 5:14) was collected to check for incidental pathology. MRI technologists identified possible image quality issues and forwarded images of concern to radiologists for a formal review. MRI data were visually inspected for quality of gray matter. ROI gray matter volumes were extracted from Freesurfer defined OFC, hippocampus, and amygdala (Fischl, 2012).
Diffusion Tensor Imaging.
Diffusion-weighed scans were collected [71 gradient directions; TR 9600 ms; TE 86 mm; GRAPPA parallel imaging factor 2; β value 1000 s/mm2; FOV 256 mm; 72 slices; 2 mm3 isomorphic voxels; 7 βo images] and processed with TRActs Constrained by UnderLying Anatomy (TRACULA) (Yendiki et al., 2011). TRACULA calculates probabilistic tractography to diffusion data using subcortical segmentation labels from a FreeSurfer anatomical atlas of white matter tracts (Fischl et al., 2002; Fischl et al., 2004). TRACULA uses an algorithm for automated global probabilistic tractography that estimates the posterior probability of primary white matter pathways to calculate fractional anisotropy (FA) in the uncinate fasciculus. MNI coordinates were defined in standard space for the uncinate fasciculus ROI using the ICBM-DTI-81 white matter atlas and selected in FSLview.
Data analyses
Sample Characteristics.
One-way analysis of variance and chi-square tests were used to test for demographic differences and victimization exposure between diagnostic groups, as appropriate. Non-parametric tests were used to test associations between victimization exposure, BDI depression symptoms and positive symptoms (Spearman correlations). Links with depression were evaluated continuously, as these symptoms tend to occur across clinical and control groups. However, due to limited variability in the controls with respect to positive symptoms, associations with positive symptoms were tested in the CHR group alone.
ROI sMRI and DTI Analytic Strategy.
To test the omnibus effect for OFC and hippocampal volumes between diagnoses, 2 separate repeated-measures ANCOVAs were run (one for OFC and the other for hippocampal regions) specifying hemisphere (left versus right) and OFC/hippocampal regions (lateral versus medial for OFC and hippocampal versus parahippocampal for hippocampal regions) as within-subjects factors, and group (CHR versus HV) as between-subjects factor including age, gender, and estimated intracranial volume (ICV) as nuisance variables. These models will test whether there are group differences between hemispheres, as well as between lateral and medial OFC and hippocampal and parahippocampal regions. To test the omnibus effect for the amygdala on group, a repeated-measures ANCOVA was run specifying hemisphere (left versus right) as within-subjects factor, including age, gender, and ICV as nuisance variables. Finally, to test uncinate fasciculus fractional anisotropy differences between diagnoses, a mixed-model ANOVA explored the relationship between hemisphere (left versus right) uncinate fasciculus FA on group (CHR versus HV), accounting for age and gender as nuisance variables. In the case that main effects of hemisphere or interactions of hemisphere and group were observed in the omnibus test, ensuing analyses examining the association with bullying victimization were run for both left and right regions/tracts. If these were not observed in the omnibus test, analyses examining the association with bullying victimization did not take laterality into account. Due to the lack of normality of the distribution for the cumulative bullying variable, non-parametric tests were employed to test for associations with brain ROIs. Spearman correlations explored the relationship between cumulative bullying exposure and volumes in the whole sample for the gray matter ROIs, controlling for ICV. Spearman correlations explored the relationship between cumulative bullying victimization and uncinate fasciculus FA. Associations were evaluated across the entire sample. However, given interest in distinct effects within each group, in the case that these were different within diagnoses, this was noted as well.
Follow-up Exploratory Whole Brain Volume Analyses.
In addition to the above-mentioned ROI approach, in order to provide a conservative approach more conducive to replicability, whole-brain analyses were undertaken. These analyses explored cortical volume in CHR and healthy individuals, as well as in those exposed to bullying victimization within those two groups. These results were conducted as follow-up exploratory analyses given results observed with morphometry. Analyses were conducted for overall group differences, as well as for exposed versus not exposed to bullying victimization. Analyses of exposed versus non-exposed were conducted within the CHR and HV groups separately, and also across both groups. Statistical maps were created using Freesurfer’s Query, Design, Estimate, Contrast (QDEC) interface. The QDEC interface is a single-binary application that is part of the FreeSurfer distribution used to perform group averaging and inference on the cortical morphometric data produced by the FreeSurfer processing stream. The CHR group was first compared to the HV group. For each hemisphere, the General Linear Model (GLM) was computed vertex-by-vertex for analysis of cortical volume, using gender, age and intracranial volume as nuisance variables. QDEC creates the design matrix automatically using DODS (different offsets, different slopes), which assumes different morphometric measures for all groups (different offsets), as well as a different impact of the variable of interest between groups (different slope). Cortical maps were smoothed using a 10 mm full width at half maximum Gaussian kernel. Results were visualized by overlaying significant cortical areas onto semi-inflated cortical surfaces. Multiple comparisons were corrected using a Monte Carlo Simulation with a p-value set at <0.05.
Results
There were no differences between groups with regards to age, gender, or years of education (see Table 1).
Group Differences in Bullying Victimization.
As hypothesized, CHR individuals had greater parent-reported exposure to victimization than healthy individuals, F (1, 103) = 6.008, p = 0.016 (see Figure 1). There was meaningful variability in terms of number of items endorsed. Of the CHR sample, 51% did not endorse any items, 23.5% endorsed one item, and 25.5% endorsed both teasing and bullying. Of the HV sample, 73.5% did not endorse any items, 15% endorsed one item, and 11% endorsed both bullying and teasing.
Figure 1.
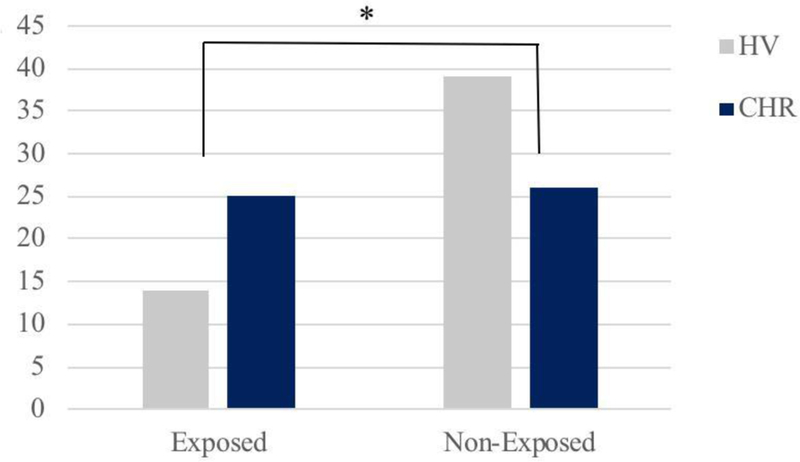
Count of subjects exposed (endorsed at least one item) versus not exposed to bullying victimization by group, * p < 0.05
Bullying Victimization and Symptoms.
As predicted, increased parent-reported bullying victimization was associated with greater depressive symptoms (Spearman r = 0.35, p < 0.001) in both healthy and CHR groups (see Figure 2). BDI scores were positively skewed, however log transforming BDI scores to correct for skewness did not affect results. There was no significant association between bullying victimization and positive symptoms in the CHR group (Spearman r = 0.09, p = 0.26).
Figure 2.
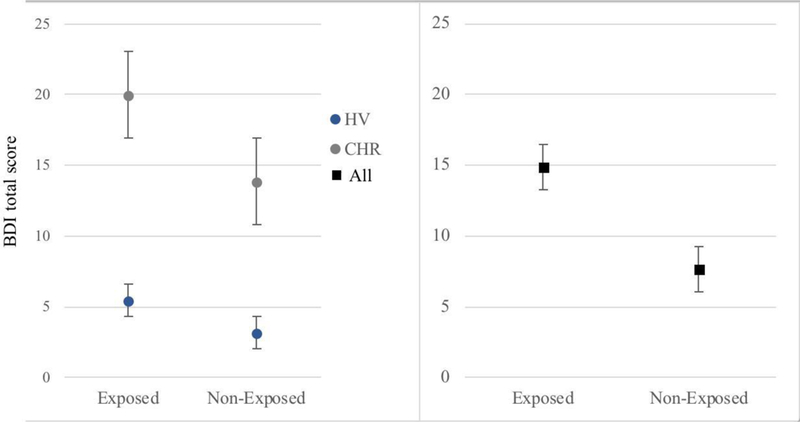
Depressive symptoms in exposed (endorsed at least one item) versus non-exposed subjects (EMM & SE controlling for age and gender).
Gray Matter Volume in OFC.
The between-subjects omnibus model of group was significant [F (6, 93) = 5.53, p = 0.021]. A main effect of hemisphere was not observed, [F (1, = 0.24, p = 0.878]. There was, however, a significant hemisphere by group interaction, [F (1, 93) = 4.85, p = 0.03]. Concurrently, there was a main effect of OFC [F (1, 93) = 6.53, p = 0.012]. As with hemisphere, there was a significant OFC and group interaction, [F (1, 93) = 4.886, p = 0.03]. Given the significant interactions between hemisphere and group, as well as OFC and group, OFC subregion-specific analyses were run for left and right lateral and medial OFC regions included in the omnibus model. These showed significant group effects such that CHR individuals showed lower volumes relative to HVs for the right lateral [F (1,98) = 6.22, p = 0.014] and right medial [F (1,98) = 4.68, p = 0.033] areas, as well as for the left lateral OFC [F (1, 98) = 5.44, p = 0.022], but not for the left medial area [F (1,98) = 0.175, p = 0.68].
Gray Matter Volume in Hippocampal Areas.
The between-subjects omnibus model of group was significant [F (6, 93) = 4.29, p = 0.041]. A main effect of hemisphere was not observed, [F (1, 93) = 0.3, p = 0.584]. There were no significant interactions between hemisphere and group [F (1, 98) = 0.327, p = 0.569]. However, there was a main effect of hippocampal/parahippocampal regions [F (1, 98) = 19.56, p < 0.001]. There was a significant interaction between hippocampal/parahippocampal regions and group [F (1, 98) = 7.772, p = 0.018]. Given lack of interaction between hemisphere and group, bilateral region-specific analyses were run for bilateral volumes included in the omnibus model. These showed significant group effects such that CHR individuals showed lower volumes relative to HVs for bilateral hippocampal volume [F (1,98) = 5.44, p = 0.022], but not for bilateral parahippocampal volume [F (1,98) = 0.011, p = 0.916].
Gray Matter Volume in Amygdala.
The between-subjects omnibus model of group was not significant [F (6, 94) = 3.33, p = 0.07]. A main effect of hemisphere was not observed, [F (1, 98) 0.22, p = 0.882]. There were no significant interactions between hemisphere and group [F (1, 98) = 0.681, p = 0.411].
White Matter Integrity in the Uncinate Fasciculus.
The between-subjects omnibus model of group was not significant [F (5, 95) = 0.873, p = 0.352]. A main effect of hemisphere was observed, [F (1, 98) =5.78, p = 0.018]. There were no significant interactions between hemisphere and group [F (1, 98) = 0.195, p = 0.66].
Brain ROIs and Bullying Victimization.
See Table 2 for significant results across the whole sample. Interestingly, increased bullying victimization was associated with smaller medial OFC volumes (see Figure 3). For OFC, hippocampal and uncinate fasciculus ROIs, observed patterns did not differ when associations were evaluated in the CHR and HV groups separately. In the case of amygdalar volumes, however, the association was no longer significant when examined within HV and CHR groups separately. Given high co-incidence of trauma and childhood adverse events (Nilsson et al., 2012), presence of a PTSD diagnosis in the CHR sample (n = 3) was controlled for, and results remained unchanged; therefore, results were reported as originally intended.
Table 2.
Correlations between cumulative bullying victimization and regions of interest across CHRd and HV sample.
| Region | Spearman’s r | p |
|---|---|---|
| OFC | ||
| Right Medial volume | −0.22* | 0.02 |
| Right Lateral volume | −0.13 | 0.11 |
| Left Medial volume | −0.13 | 0.11 |
| Left Lateral volume | −0.11 | 0.15 |
| Medial temporal regions | ||
| Hippocampal volume | −0.11 | 0.16 |
| Parahippocampal volume | −0.08 | 0.24 |
| Amygdala volume | −0.21* | 0.03 |
| White matter tract ROIs | ||
| Right uncinate fasciculus FA | 0.055 | 0.595 |
| Left uncinate fasciculus FA | 0.02 | 0.852 |
p < 0.05
Figure 3.
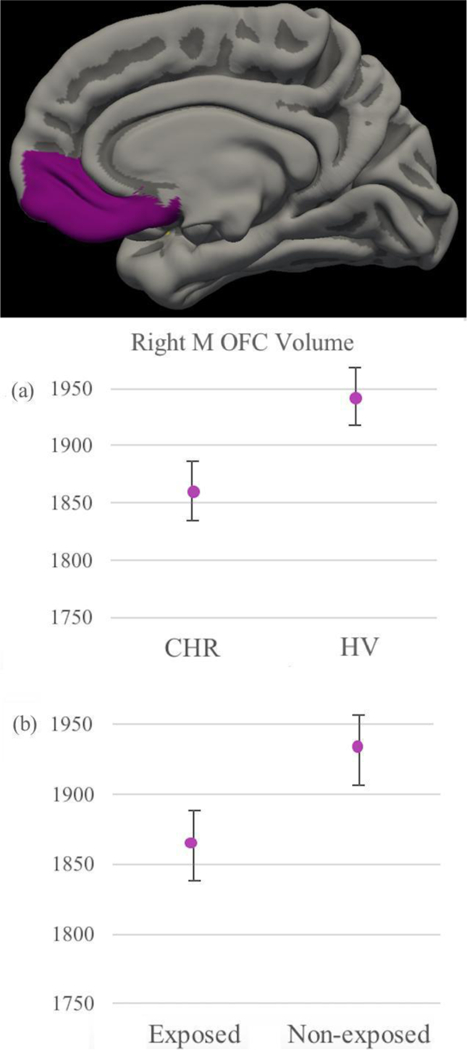
Gray Matter Volumetric Differences (EMM & SE, controlling for age, gender and ICV) Between Diagnoses (a) and between subjects exposed (endorsed at least one item) versus not exposed to bullying victimization (b) in Right Medial OFC.
Follow-up Exploratory Whole Brain Volume Analyses.
Differences surviving Monte Carlo Simulation multiple comparison corrections were not observed between diagnoses, nor were they observed between individuals whose parents reported had been exposed to bullying victimization, and those who had not. Running analyses within the CHR and HV groups independently did not alter results.
Discussion
The present study is the first to adopt a multimodal imaging approach to understanding correlates of bullying victimization during a critical period of neural plasticity in CHR and healthy populations. This subject is vitally important for further understanding how exposure to chronic and acute environmental stressors may interact with neurological vulnerabilities to increase risk for future adverse outcomes. As predicted, the CHR group showed greater exposure to bullying victimization compared to the HV group, as indicated by parent report. In addition, greater exposure was associated with increased depressive symptoms in both groups. With regards to gray matter structure, the CHR individuals showed lower volumes compared to HVs for hippocampal and OFC regions. Notably, lower volumes in medial OFC were associated with elevated parent-reported exposure to bullying victimization, across both CHR and HV groups (see Figure 3). Further, other areas differing between diagnoses were not significantly linked with bullying victimization, suggesting specific areas of susceptibility. Findings ought to be carefully interpreted as preliminary, given that they did not survive follow-up analyses using a multiple comparisons Monte Carlo Simulation correction at the whole-brain level. Nonetheless, taken together, results suggest victimization exposure is relevant for depressive symptom presentation and may affect or be affected by OFC structural differences in both healthy and CHR individuals. However, as bullying occurs more frequently in the clinical group, and CHR participants also show some vulnerability (e.g. smaller volumes), these results also point to important etiological clues and novel intervention targets.
This investigation aimed to determine whether there were group differences in exposure to bullying victimization, and if so, whether these differences were linked to depressive and positive symptoms. These aims are informative in establishing bullying victimization as a prevalent stressor in CHR individuals, as well as in establishing a relationship with symptom outcomes. Study results for both depressive symptoms and neural structural variables are notable in that associations are observable in both the CHR and HV samples, despite the fact that the CHR group exhibited greater parent-reported exposure to bullying victimization (see Figure 1). Findings support meta-analytic evidence suggesting bullying victimization may have adverse effects regardless of whether one is diagnosed with a psychiatric disorder (van Dam et al., 2012; Valmaggia et al., 2015). However, bullying has also been more specifically related to future development of psychosis spectrum symptoms, depression, and mood instability in non-clinical populations (van Dam et al., 2012; Valmaggia et al., 2015). There is frequent incidence of depressive symptoms during the CHR period (Azar et al., 2018), and since bullying exposure has predicted future development of these mood instability and depressive symptoms, perhaps it exacerbates depressive symptoms, which are common in this population (Catone et al., 2015; Moffa et al., 2017). Present findings lend support to this interpretation, as exposure to bullying was linked to an increase in depressive symptoms. Null findings of associations with positive symptoms in CHR are partially in line with previous findings of depressive symptoms mediating bullying prediction of psychosis symptoms (Moffa et al., 2017); future studies are needed to investigate this, but perhaps depressive symptoms predate effects of bullying on positive symptoms.
Of note, the present study sought to explore whether bullying would be associated not only with symptoms, but with brain morphometry and white matter integrity. This aim stands to inform theories of mechanisms underlying the effect of bullying victimization on symptoms and psychosis risk. Given that the literature examining underlying neural mechanisms of bullying victimization has been rather limited, the present investigation chose regions of interest previously shown to be sensitive to environmental risk factors more generally. Previously discussed findings with regards to symptoms are particularly interesting considering that lower OFC volumes were associated with higher parent-reported exposure to bullying victimization. Of note, the OFC is also critically involved in emotion regulation and reward processing (Chaney et al., 2014; Mychasiuk et al., 2016; Teicher and Samson, 2016; Teicher et al., 2016; Hiser and Koenigs, 2018). Studies have shown that lesions in the region disrupt sharing and understanding of others’ emotions (Hillis, 2014), causing socially inappropriate behavior (Jankowski and Takahashi, 2014) and impairment in affect regulation (Heide and Solomon, 2006). In addition, previous studies have found the OFC to be particularly sensitive to environmental influence during critical neurodevelopmental periods, along with being involved in modulating the neurological response to chronic stress (Thomaes et al., 2010; Chaney et al., 2014; Mychasiuk et al., 2016; Teicher et al., 2016; Teicher and Samson, 2016; Wikenheiser and Schoenbaum, 2016; Hiser and Koenigs, 2018). Specifically, lower OFC volumes have been numerously observed in individuals exposed to adverse environmental factors, including childhood maltreatment, deprivation, and exposure to threatening life events (Chugani et al., 2001; Hanson et al., 2010; Thomaes et al., 2010; De Brito et al., 2013). This supports the notion that bullying victimization may be one of many environmental factors effecting morphometry through dysregulation of areas associated with chronic stress and emotion regulation.
With regards to other regions of interest, differences between diagnoses were also found in hippocampal regions, though volumes were not associated with exposure to bullying victimization within CHR or HVs. This supports possible mechanistic specificity of the OFC with regards to sensitivity to exposure to bullying victimization, or in its effects with regards to affect processing symptoms. Contrary to study predictions, differences were not found between diagnoses in terms of gray matter amygdalar volume, or myelination in the uncinate fasciculus tract. This is consistent with the literature, as amygdalar volume differences in CHR individuals are not consistently found (Bartholomeusz et al., 2017). In addition, across the whole sample, reduced amygdalar volume was associated with greater exposure to bullying victimization. These findings are consistent with previous studies finding impaired amygdalar affect regulation in individuals with trauma histories (Dannlowski et al., 2012; Baker et al., 2013; Samplin et al., 2013). Overall, ROI findings may reflect lack of power in the current sample to detect an effect between diagnoses, though they may also lend support to mechanistic specificity of the OFC as a risk factor in CHR populations.
Perhaps bullying victimization effects OFC volume, thereby increasing vulnerability and risk for developing future symptoms and psychopathology. However, it is important to note that causality is not established: perhaps pre-existing reduced OFC volume differences put an individual at risk for exhibiting emotion regulation and social understanding deficits, therefore increasing the individual’s risk of being exposed to bullying victimization. Current study findings could lend support to either interpretation. Nonetheless, it is also likely that the association is bidirectional. In line with a neural-diathesis stress conceptualization of psychopathology, underlying neurobiological vulnerabilities may interact with environmental risk factors to exacerbate vulnerability and increase risk of developing psychopathology (Walker and Diforio, 1997; Pruessner et al., 2017). Current results suggest the right medial OFC may be particularly affected, which is consistent with it having a greater role in emotion regulation and reward processing (Hiser and Koenigs, 2018). However, future studies will be essential in order to explore whether the lateral and medial OFC are differentially affected.
Several limitations are important to highlight so that future studies may better improve our understanding of this critical subject. First, it is important to note the heterogeneity of CHR samples, as well as the fact that only a minority of the sample goes on to develop a psychotic disorder (Wood et al., 2008). Given the nature of this clinical group, caution should be exercised when considering whether findings provide clues of determinants of psychotic illness specifically. Unfortunately, specific timing of exposure was not collected. The age of exposure to environmental stressors and risk factors has been proven to be essential, especially during critical neurodevelopmental periods (Brown, 2011; McLaughlin et al., 2014; Nusslock and Miller, 2016; Teicher and Samson, 2016). Therefore, it will be important for future studies to explore whether exposure timing differentially affects outcomes. Given the limitations of retrospective reporting, ideally these studies would be longitudinal. Further, the use of parental reports in this investigation may have omitted some information that the parents were not aware of and therefore could not report. This could have led to an underestimation of presence of bullying across the sample. However, it is important to note that the information that the parents are aware of is likely to be a salient or potent influence in the child’s environment. Nonetheless, future studies would ideally collect both self-report and parent report data, and aggregate across these reports.
The current study undertook an ROI approach. Regions of interest were carefully chosen based on previous literature on effects of bullying on symptoms, as well as on neural regions affected by environmental stressors and risk factors. As a follow-up, we sought to confirm our analysis results using a whole-brain approach in order to more fully condone replicability. Upon undertaking a whole-brain approach, however, results did not survive the more conservative multiple comparisons corrected approach. This may be due to insufficient power in the current sample. It could also be the case, however, that current ROI results reflect Type 1 error. Going forward, future more well powered future studies would benefit from addressing this concern by undertaking a whole brain approach in order to attempt to replicate findings. As presently observed, results should be cautiously interpreted as preliminary.
Current results were not affected by SCID-assessed presence of post-traumatic stress. However, more well powered future studies would benefit from collecting data on bullying victimization exposure and comparing effects of other environmental risk factors. This would help distinguish whether bullying victimization is distinct, or mechanistically similar to other types of risk exposure and environmental stressors (Nusslock and Miller, 2016). Finally, our use of a cumulative bullying victimization score based on additive aggregation of item endorsement captured informative variability. Indeed, previous additive cumulative risk models in adolescent populations have proven successful in capturing potency of a given environmental stressor (Evans et al., 2013; Evans and Cassells, 2014). Nonetheless, previous studies have found a dose response effect between amount of environmental risk exposure and likelihood of developing psychosis (Shevlin et al., 2008); this suggests that further exploring cumulative risk models (including multiple environmental stressor types) would be a valuable undertaking for future investigations.
Acknowledgements
This work was supported by a Northwestern University Society Biology and Health Cluster fellowship (T.V.) and by grants R01MH112545, R21/R33MH103231 and R21MH110374 (V.A.M).
Role of funding source
This work was supported by a Northwestern University Society Biology and Health Cluster fellowship (T.V.) and by grants R01MH112545, R21/R33MH103231 and R21MH110374 (V.A.M).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors report no biomedical financial interests or conflicts of interest. V.A.M is a consultant with Takeda Pharmaceuticals.
References
- Azar M, Pruessner M, Baer LH, Iyer S, Malla AK, Lepage M, 2018. A study on negative and depressive symptom prevalence in individuals at ultra-high risk for psychosis. Early Interv Psychiatry 12(5), 900–906. [DOI] [PubMed] [Google Scholar]
- Baker LM, Williams LM, Korgaonkar MS, Cohen RA, Heaps JM, Paul RH, 2013. Impact of early vs. late childhood early life stress on brain morphometrics. Brain Imaging Behav 7(2), 196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomeusz CF, Cropley VL, Wannan C, Di Biase M, McGorry PD, Pantelis C, 2017. Structural neuroimaging across early-stage psychosis: Aberrations in neurobiological trajectories and implications for the staging model. Aust N Z J Psychiatry 51(5), 455–476. [DOI] [PubMed] [Google Scholar]
- Borgwardt SJ, Riecher-Rossler A, Dazzan P, Chitnis X, Aston J, Drewe M, Gschwandtner U, Haller S, Pfluger M, Rechsteiner E, D’Souza M, Stieglitz RD, Radu EW, McGuire PK, 2007. Regional gray matter volume abnormalities in the at risk mental state. Biol Psychiatry 61(10), 1148–1156. [DOI] [PubMed] [Google Scholar]
- Brown AS, 2011. The environment and susceptibility to schizophrenia. Prog Neurobiol 93(1), 23–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carletti F, Woolley JB, Bhattacharyya S, Perez-Iglesias R, Fusar Poli P, Valmaggia L, Broome MR, Bramon E, Johns L, Giampietro V, Williams SC, Barker GJ, McGuire PK, 2012. Alterations in white matter evident before the onset of psychosis. Schizophr Bull 38(6), 1170–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catone G, Marwaha S, Kuipers E, Lennox B, Freeman D, Bebbington P, Broome M, 2015. Bullying victimisation and risk of psychotic phenomena: analyses of British national survey data. Lancet Psychiatry 2(7), 618–624. [DOI] [PubMed] [Google Scholar]
- Chaney A, Carballedo A, Amico F, Fagan A, Skokauskas N, Meaney J, Frodl T, 2014. Effect of childhood maltreatment on brain structure in adult patients with major depressive disorder and healthy participants. J Psychiatry Neurosci 39(1), 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KI, Shenton ME, Kubicki M, Jung WH, Lee TY, Yun JY, Kim SN, Kwon JS, 2016. Altered Thalamo-Cortical White Matter Connectivity: Probabilistic Tractography Study in Clinical-High Risk for Psychosis and First-Episode Psychosis. Schizophr Bull 42(3), 723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani HT, Behen ME, Muzik O, Juhasz C, Nagy F, Chugani DC, 2001. Local brain functional activity following early deprivation: a study of postinstitutionalized Romanian orphans. Neuroimage 14(6), 1290–1301. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, Metzger LM, Shoushtari CS, Splinter R, Reich W, 2003. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord 33(4), 427–433. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Domschke K, Hohoff C, Ohrmann P, Bauer J, Lindner C, Postert C, Konrad C, Arolt V, Heindel W, Suslow T, Kugel H, 2012. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry 71(4), 286–293. [DOI] [PubMed] [Google Scholar]
- De Brito SA, Viding E, Sebastian CL, Kelly PA, Mechelli A, Maris H, McCrory EJ, 2013. Reduced orbitofrontal and temporal grey matter in a community sample of maltreated children. J Child Psychol Psychiatry 54(1), 105–112. [DOI] [PubMed] [Google Scholar]
- Ebeling U, von Cramon D, 1992. Topography of the uncinate fascicle and adjacent temporal fiber tracts. Acta Neurochir (Wien) 115(3–4), 143–148. [DOI] [PubMed] [Google Scholar]
- Evans GW, Cassells RC, 2014. Childhood Poverty, Cumulative Risk Exposure, and Mental Health in Emerging Adults. Clin Psychol Sci 2(3), 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, Li D, Whipple SS, 2013. Cumulative risk and child development. Psychol Bull 139(6), 1342–1396. [DOI] [PubMed] [Google Scholar]
- Fischl B, 2012. FreeSurfer. Neuroimage 62(2), 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM, 2002. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33(3), 341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM, 2004. Automatically parcellating the human cerebral cortex. Cereb Cortex 14(1), 11–22. [DOI] [PubMed] [Google Scholar]
- Fuhrmann D, Knoll LJ, Blakemore SJ, 2015. Adolescence as a Sensitive Period of Brain Development. Trends Cogn Sci 19(10), 558–566. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B, 2010. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35(1), 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson RJ, Pollak SD, 2010. Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. J Neurosci 30(22), 7466–7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heide KM, Solomon EP, 2006. Biology, childhood trauma, and murder: rethinking justice. Int J Law Psychiatry 29(3), 220–233. [DOI] [PubMed] [Google Scholar]
- Hillis AE, 2014. Inability to empathize: brain lesions that disrupt sharing and understanding another’s emotions. Brain 137(Pt 4), 981–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiser J, Koenigs M, 2018. The Multifaceted Role of the Ventromedial Prefrontal Cortex in Emotion, Decision Making, Social Cognition, and Psychopathology. Biol Psychiatry 83(8), 638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski KF, Takahashi H, 2014. Cognitive neuroscience of social emotions and implications for psychopathology: examining embarrassment, guilt, envy, and schadenfreude. Psychiatry Clin Neurosci 68(5), 319–336. [DOI] [PubMed] [Google Scholar]
- Kawashima T, Nakamura M, Bouix S, Kubicki M, Salisbury DF, Westin CF, McCarley RW, Shenton ME, 2009. Uncinate fasciculus abnormalities in recent onset schizophrenia and affective psychosis: a diffusion tensor imaging study. Schizophr Res 110(1–3), 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ, 2011. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res 223(2), 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad K, Firk C, Uhlhaas PJ, 2013. Brain development during adolescence: neuroscientific insights into this developmental period. Dtsch Arztebl Int 110(25), 425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Maier SE, Frumin M, Nestor PG, Salisbury DF, Kikinis R, Jolesz FA, McCarley RW, Shenton ME, 2002. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am J Psychiatry 159(5), 813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson T, Lundstrom S, Nilsson T, Selinus EN, Rastam M, Lichtenstein P, Gumpert CH, Anckarsater H, Kerekes N, 2013. Predictive properties of the A-TAC inventory when screening for childhood-onset neurodevelopmental problems in a population-based sample. BMC Psychiatry 13, 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN, 2006. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev 30(6), 718–729. [DOI] [PubMed] [Google Scholar]
- Lobbestael J, Leurgans M, Arntz A, 2011. Inter-rater reliability of the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID I) and Axis II Disorders (SCID II). Clin Psychol Psychother 18(1), 75–79. [DOI] [PubMed] [Google Scholar]
- Martin CS, Pollock NK, Bukstein OG, Lynch KG, 2000. Inter-rater reliability of the SCID alcohol and substance use disorders section among adolescents. Drug Alcohol Depend 59(2), 173–176. [DOI] [PubMed] [Google Scholar]
- Matsuzawa J, Matsui M, Konishi T, Noguchi K, Gur RC, Bilker W, Miyawaki T, 2001. Age-related volumetric changes of brain gray and white matter in healthy infants and children. Cereb Cortex 11(4), 335–342. [DOI] [PubMed] [Google Scholar]
- McDonnell J, Stahl D, Day F, McGuire P, Valmaggia LR, 2018. Interpersonal sensitivity in those at clinical high risk for psychosis mediates the association between childhood bullying victimisation and paranoid ideation: A virtual reality study. Schizophr Res 192, 89–95. [DOI] [PubMed] [Google Scholar]
- McEwen BS, 2007. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 87(3), 873–904. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Lambert HK, 2014. Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci Biobehav Rev 47, 578–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM, Hoffman R, Davidson L, 1999. Symptom assessment in schizophrenic prodromal states. Psychiatr Q 70(4), 273–287. [DOI] [PubMed] [Google Scholar]
- Moffa G, Catone G, Kuipers J, Kuipers E, Freeman D, Marwaha S, Lennox BR, Broome MR, Bebbington P, 2017. Using Directed Acyclic Graphs in Epidemiological Research in Psychosis: An Analysis of the Role of Bullying in Psychosis. Schizophr Bull 43(6), 1273–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mychasiuk R, Muhammad A, Kolb B, 2016. Chronic stress induces persistent changes in global DNA methylation and gene expression in the medial prefrontal cortex, orbitofrontal cortex, and hippocampus. Neuroscience 322, 489–499. [DOI] [PubMed] [Google Scholar]
- Nilsson DK, Gustafsson PE, Svedin CG, 2012. Polytraumatization and trauma symptoms in adolescent boys and girls: interpersonal and noninterpersonal events and moderating effects of adverse family circumstances. J Interpers Violence 27(13), 2645–2664. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Miller GE, 2016. Early-Life Adversity and Physical and Emotional Health Across the Lifespan: A Neuroimmune Network Hypothesis. Biol Psychiatry 80(1), 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner M, Cullen AE, Aas M, Walker EF, 2017. The neural diathesis-stress model of schizophrenia revisited: An update on recent findings considering illness stage and neurobiological and methodological complexities. Neurosci Biobehav Rev 73, 191–218. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS, 2006. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol 16(6), 723–727. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Miernicki ME, Troop-Gordon W, Davis MM, Telzer EH, 2016. Adding insult to injury: neural sensitivity to social exclusion is associated with internalizing symptoms in chronically peer-victimized girls. Soc Cogn Affect Neurosci 11(5), 829–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samplin E, Ikuta T, Malhotra AK, Szeszko PR, Derosse P, 2013. Sex differences in resilience to childhood maltreatment: effects of trauma history on hippocampal volume, general cognition and subclinical psychosis in healthy adults. J Psychiatr Res 47(9), 1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, 2004. Affect, action, and ambiguity and the amygdala-orbitofrontal circuit. Focus on “combined unilateral lesions of the amygdala and orbital prefrontal cortex impair affective processing in rhesus monkeys”. J Neurophysiol 91(5), 1938–1939. [DOI] [PubMed] [Google Scholar]
- Schreier A, Wolke D, Thomas K, Horwood J, Hollis C, Gunnell D, Lewis G, Thompson A, Zammit S, Duffy L, Salvi G, Harrison G, 2009. Prospective study of peer victimization in childhood and psychotic symptoms in a nonclinical population at age 12 years. Arch Gen Psychiatry 66(5), 527–536. [DOI] [PubMed] [Google Scholar]
- Shevlin M, Houston JE, Dorahy MJ, Adamson G, 2008. Cumulative traumas and psychosis: an analysis of the national comorbidity survey and the British Psychiatric Morbidity Survey. Schizophr Bull 34(1), 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer RA, Ball R, Ranieri WF, Beck AT, 1997. Further evidence for the construct validity of the Beck depression Inventory-II with psychiatric outpatients. Psychol Rep 80(2), 443–446. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, 2016. Annual Research Review: Enduring neurobiological effects of childhood abuse and neglect. J Child Psychol Psychiatry 57(3), 241–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Anderson CM, Ohashi K, 2016. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci 17(10), 652–666. [DOI] [PubMed] [Google Scholar]
- Telzer EH, Miernicki ME, Rudolph KD, 2018. Chronic peer victimization heightens neural sensitivity to risk taking. Dev Psychopathol 30(1), 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomaes K, Dorrepaal E, Draijer N, de Ruiter MB, van Balkom AJ, Smit JH, Veltman DJ, 2010. Reduced anterior cingulate and orbitofrontal volumes in child abuse-related complex PTSD. J Clin Psychiatry 71(12), 1636–1644. [DOI] [PubMed] [Google Scholar]
- Tottenham N, 2014. The importance of early experiences for neuro-affective development. Curr Top Behav Neurosci 16, 109–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta A, Di Forti M, Mondelli V, Dazzan P, Pariante C, David A, Mule A, Ferraro L, Formica I, Murray RM, Fisher HL, 2013. Prevalence of bullying victimisation amongst first-episode psychosis patients and unaffected controls. Schizophr Res 150(1), 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upthegrove R, 2015. Bullying, victimisation, and psychosis. Lancet Psychiatry 2(7), 574–576. [DOI] [PubMed] [Google Scholar]
- Valmaggia LR, Day FL, Kroll J, Laing J, Byrne M, Fusar-Poli P, McGuire P, 2015. Bullying victimisation and paranoid ideation in people at ultra high risk for psychosis. Schizophr Res 168(1–2), 68–73. [DOI] [PubMed] [Google Scholar]
- van Dam DS, van der Ven E, Velthorst E, Selten JP, Morgan C, de Haan L, 2012. Childhood bullying and the association with psychosis in non-clinical and clinical samples: a review and meta-analysis. Psychol Med 42(12), 2463–2474. [DOI] [PubMed] [Google Scholar]
- van Os J, Kenis G, Rutten BP, 2010. The environment and schizophrenia. Nature 468(7321), 203–212. [DOI] [PubMed] [Google Scholar]
- Walker EF, Diforio D, 1997. Schizophrenia: a neural diathesis-stress model. Psychol Rev 104(4), 667–685. [DOI] [PubMed] [Google Scholar]
- Whittle S, Dennison M, Vijayakumar N, Simmons JG, Yucel M, Lubman DI, Pantelis C, Allen NB, 2013. Childhood maltreatment and psychopathology affect brain development during adolescence. J Am Acad Child Adolesc Psychiatry 52(9), 940–952 e941. [DOI] [PubMed] [Google Scholar]
- Wikenheiser AM, Schoenbaum G, 2016. Over the river, through the woods: cognitive maps in the hippocampus and orbitofrontal cortex. Nat Rev Neurosci 17(8), 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SJ, Pantelis C, Velakoulis D, Yucel M, Fornito A, McGorry PD, 2008. Progressive changes in the development toward schizophrenia: studies in subjects at increased symptomatic risk. Schizophr Bull 34(2), 322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki A, Panneck P, Srinivasan P, Stevens A, Zollei L, Augustinack J, Wang R, Salat D, Ehrlich S, Behrens T, Jbabdi S, Gollub R, Fischl B, 2011. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front Neuroinform 5, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]


