Abstract
Background
The aim of this retrospective study was to identify perioperative variables predictive of the development of delirium in older surgical patients after spine surgery.
Methods
We collected pre-, intra- and postoperative data on patients ≥ 65 years of age having spine surgery between July 1, 2015 and March 15, 2017. The primary outcome was the development of postoperative delirium. Data were analyzed using univariate and multivariable analysis.
Results
Among the 716 patients included in this study 127 (18%) developed postoperative delirium. On multivariable analysis, independent predictors of postoperative delirium included older age (OR = 1.04 [95% (CI) 1.00 to 1.09]; P = 0.048), American Society of Anesthesiologists physical status > 2 (OR = 1.89 [95% CI 1.04 to 3.59]; P = 0.042), metabolic equivalents of task < 4 (OR = 1.84 [95% CI 1.10 to 3.07]; P = 0.019), depression (OR = 2.01 [95% CI 1.21 to 3.32]; P = 0.006), non-elective surgery (OR = 4.81 [95% CI 1.75 to 12.79]; P = 0.002), invasive surgical procedures (OR = 1.97 [95% CI 1.10 to 3.69]; P = 0.028) and higher mean pain scores on postoperative day 1 (OR = 1.28 [95% CI 1.11 to 1.48]; P < 0.001).
Conclusions
Postoperative delirium is a common complication in older patients after spine surgery, and there are several perioperative risk factors associated with its development.
Summary Statement:
Age, ASA physical status, metabolic equivalents of task, invasiveness, BIS monitoring an pain score on postoperative day 1 were predictive of postoperative delirium in older patients undergoing spine surgery.
Introduction
Improved social conditions and medical advances have resulted in marked increases in global life expectancy. Worldwide, 8.5% of the population is over the age of 65 years and this number is expected double over the next 30 years with 88 million people in the United States expected to be 65 years of age or older by 2050.1 This is important as nearly half of all surgical procedures are performed on patients over the age of 65 years.1,2 Spine surgery is one of the most commonly performed surgical procedures in older patients but is associated with an increased risk of adverse postoperative outcomes and mortality in older surgical patients.3,4
Delirium is among one of the most common complications following surgery and has been associated with worse surgical outcomes including postoperative complications, longer hospital length of stay, institutionalization at discharge, increased medical costs and increased postoperative mortality.5 The incidence of postoperative delirium after spine surgery varies, and may occur in up to 41% of older patients.6 Preoperative risk factors such as older age, baseline cognitive impairment, pain, depression, number of medications, neurologic diseases, anemia, and weight loss have all been found to be independent predictors of postoperative delirium.6–8 Intraoperatively, hypotension, blood transfusion, aggressive fluid administration and longer durations of surgery have been identified as risk factors for the development of postoperative delirium after spine surgery.6,9 Older patients that develop delirium in the postoperative period after spine surgery are more likely to have an increased hospital length of stay, institutionalization, 30-day hospital readmission and 30-day mortality.7,8,10
Although the incidence and risk factors for the development of delirium have been widely studied in other settings, there are few studies6–9 addressing this topic in older spine patients that have more geriatric conditions such as institutionizilation at baseline, marital status, chronic pain, anxiety, depression, polypharmacy and physical disabilities.11 The primary outcome of this retrospective study was to identify pre-, intra- and postoperative variables that are predictors of postoperative delirium in older surgical patients after spine surgery. Secondary outcomes were to identify pre-, intra- and postoperative predictors of other in-hospital complications, hospital length of stay, discharge to place other than home, 30-day hospital readmission and 30-day mortality.
Materials and Methods
The Partners Institutional Review Board (IRB) approved this study, and waived the need for patient consent. Medical records of all patients ≥ 65 years of age that had spine surgery (cervical, thoracic, lumbar or sacral/pelvic) at the Brigham and Women’s Hospital between July 1, 2015 and March 15, 2017 were identified and reviewed. We excluded from the analysis incomplete anesthesia records, outpatient procedures and reoperations resulting from the primary surgery. Accordingly, a total of 716 patients were available for analysis.
We defined the primary outcome as postoperative delirium assessed by comprehensive chart review by three independent investigators using published criteria12 (including review of all entries written in the medical record suggesting an acute onset and fluctuating course, inattention (easily distractable) and either an altered level of consciousness (e.g. agitation, drowsiness) or disorganized thinking or a formal cognitive assessment for delirium) or discharge diagnosis using ICD9 or ICD10 codes (“Delirium due to known physiological condition” (ICD10-F05), “Acute delirium” (ICD9–293), “Alcohol dependence with withdrawal delirium” (ICD10- F10.231) and “Alcohol withdrawal delirium” (ICD9–291)). To avoid bias we excluded from the analysis for the primary outcome any patient with alcohol withdrawal delirium and otherwise included all patients that did not meet our a priori exclusion criteria. Secondary outcomes included other in-hospital cardiopulmonary (myocardial infarction, congestive heart failure, cardiac arrest, new onset arrhythmia, pulmonary embolism, reintubation and deep venous thrombosis), infectious (wound infections, pneumonia, sepsis and urinary tract infection), renal (acute renal injury), or cerebrovascular (stroke and transient ischemic accident) complications; hospital length of stay after surgery, discharge to place other than home (those living elsewhere before surgery were excluded from this analysis), 30-day hospital readmission and 30-day mortality.
Preoperatively we collected data on age, sex, baseline living situation (independent housing or nursing home/facility), marital status (married/partner or other), body mass index, American Society of Anesthesiology (ASA) physical status (< 3 or ≥ 3), metabolic equivalents of task (< 4 or ≥ 4), depression (diagnosis of or prescription for anti-depressant medication), anxiety (diagnosis of or prescription for anti-anxiety medication), total number of medications, preoperative opioid use, number of prior surgical procedures and case classification (elective or non-elective) from the patients medical record.
The type of surgical procedure was categorized by invasiveness into 4 tiers: tier 1, microdiscectomy; tier 2, lumbar laminectomy, anterior cervical procedures or minimally invasive fusions; tier 3, lumbar fusion, trauma, or posterior cervical fusion procedures; and tier 4, tumor, infection, deformity, or combined anterior and posterior cervical procedures.13 For the analysis, we grouped the tiers into 2 categories: tier 1 and tier 2 or tier 3 and tier 4. Other intraoperative variables included in the analysis included type of anesthesia (total intravenous, total intravenous plus volatile or volatile anesthesia), Bispectral Index (BIS) use, estimated blood loss (< 500 ml, 501 to 999 ml and >1000 ml), transfusion of blood products (red blood cells, plasma or platelets), hospital length of stay after surgery and opioid administration expressed as intravenous morphine equivalent’s calculated using standard conversion rates in a web-based calculator from data gathered from the patients electronic medical record.14,15 Postoperatively, we collected data on the mean pain score on postoperative day 1 (Numeric Rating Scale for pain from 0 to 10)16 and opioid requirements on postoperative day 1 expressed as intravenous morphine equivalents as described above. Postoperative outcomes were identified either by documentation identified in the patients medical record or Partners Research Patient Data Registry (RPDR) which gathers data from hospital systems and stores it in the research registry.
Study data were collected and managed using REDCap electronic data capture tools hosted at Brigham and Women’s Hospital.17 REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies.
Statistical analysis
The perioperative variables included in the multivariable analysis for the primary outcome, postoperative delirium, were identified using univariate analyses. Continuous variables (age, body mass index, total number of medications, number of past previous surgeries, length of surgery, intraoperative opioid administration, mean pain score on postoperative day 1 and opioid requirements on postoperative day 1) were evaluated using the Student’s t test or Wilcoxon rank sum test for non-normal variables to compare the differences between delirium and no delirium groups. Categorical variables (sex, baseline living situation, marital status, ASA physical status, metabolic equivalents of task, depression, anxiety, preoperative opioid use, case classification, invasiveness, type of anesthesia, BIS use, estimated blood loss and transfusion of blood products) were compared using χ2 test or Fisher’s exact test for small samples between the two groups. All the covariates with P < 0.1 in the univariate analysis were entered into the multiple logistic model for delirium. The final model was based on Akaike Information Criterion (AIC), likelihood ratio test, and the significance threshold with P < 0.05. This procedure was similarly performed for having any other in-hospital complications, discharge to other place than home and 30-day readmission. Model-fitting of the logistic models were evaluated using the Hosmer-Lemeshow goodness of fit test. A generalized linear model with the Gamma distribution and log link was used for modeling hospital length of stay after surgery.
After analyzing the results, we identified that BIS use during anesthesia was associated with the development of postoperative delirium. To understand which variables were associated with the use of BIS monitoring during anesthesia we performed a post-hoc univariate analysis to compare the groups. We also performed a post-hoc analysis comparing the group of patients who had missing variables to those who did not, to address potential bias.
All analyses were performed with statistical software R version 3.4.1 (R Foundation, Vienna, Austria).
Results
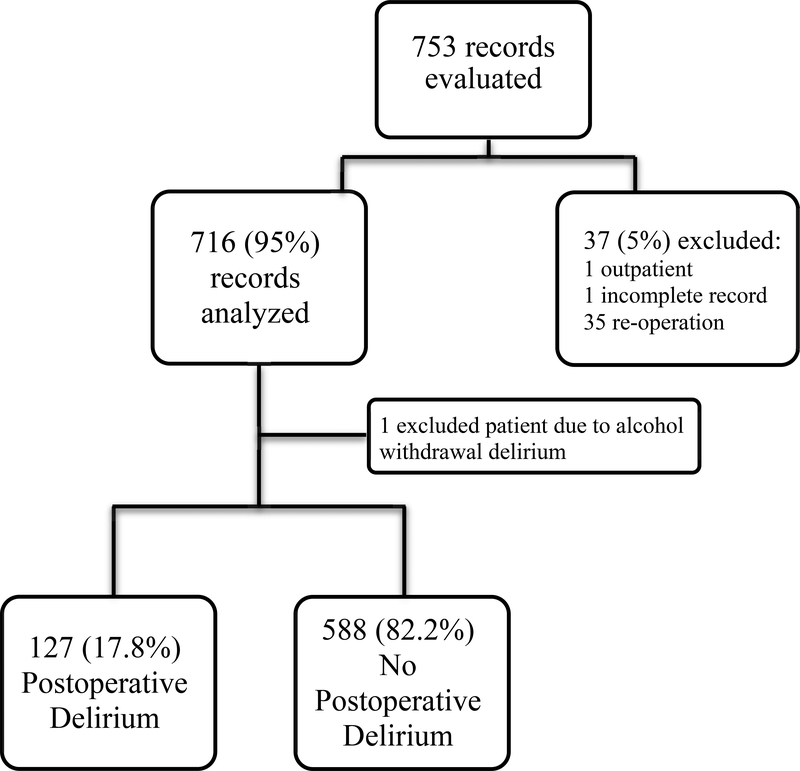
We identified 753 potential patients ≥ 65 years of age and excluded those with incomplete records (n = 1), outpatient procedures (n = 1) and reoperations resulting from the primary surgery (n = 35) accordingly, 716 patients were included in this analysis (Figure 1).
Figure 1.
Flow diagram based on primary outcome (Postoperative Delirium)
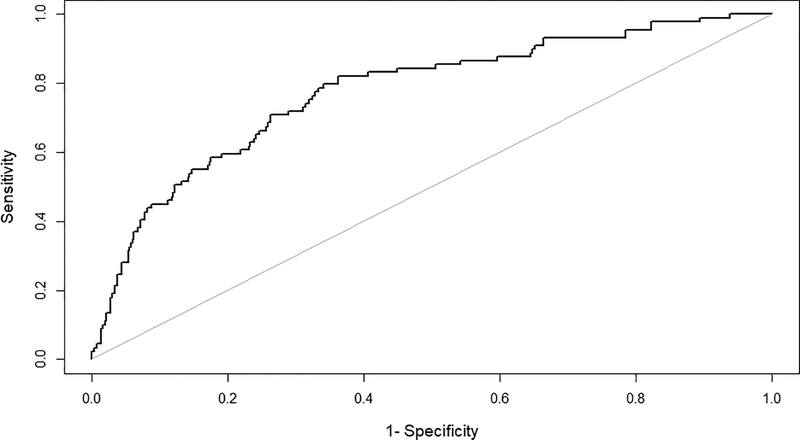
Baseline characteristics of the population by postoperative delirium status are shown in Table 1. The mean age ± standard deviation of the cohort was 74 ± 6 years old and 49% were male. One hundred and twenty-seven (17.8%) developed postoperative delirium (primary outcome measure) (Table 1). Only one case of alcohol withdrawal delirium was identified and that patient was excluded from the analysis of delirium. There were a number of preoperative (Table 1), intraoperative and postoperative (Table 2) predictors of postoperative delirium. On multivariable analysis, adjusting for other independent predictors of postoperative delirium older age (Odds Ratio (OR) = 1.04 [95% Confidence Interval (CI) 1.00 to 1.09]; P = 0.048) for every one year increase in age, ASA physical status ≥ 3 (OR = 1.89 [95% CI 1.04 to 3.59]; P = 0.042) relative to ≤ 2, metabolic equivalents of task < 4 (OR = 1.84 [95% CI 1.10 to 3.07]; P = 0.019) relative to ≥ 4, depression (OR = 2.01 [95% CI 1.21 to 3.32]; P = 0.006) relative to those without depression, non-elective surgery (OR = 4.81 [95% CI 1.75 to 12.79]; P = 0.002) relative to elective surgery, more invasive surgical procedure (OR = 1.97 [95% CI 1.10 to 3.69]; P = 0.028) relative to less invasive surgery, BIS monitoring during anesthesia (OR = 2.09 [95% CI 1.22 to 3.70]; P = 0.009) relative to those without BIS monitoring and higher mean pain score on postoperative day 1 (OR = 1.28 [95% CI 1.11 to 1.48]; P < 0.001) for each point increase in pain score (Table 3) were independent predictors of postoperative delirium. The receiver operating characteristic (ROC) curve is shown in Figure 2. The area under the cuve of the final model was 0.78 (95% CI 0.72 to 0.83).
Table 1.
Baseline characteristics and postoperative delirium (univariate analysis)
| Postoperative Delirium Status | ||||
|---|---|---|---|---|
| Total n = 715 (%) | Yes n = 127 (17.8) | No n = 588 (82.2) | P-value | |
| Preoperative variables | ||||
| Age, years, mean ± SD | 73.6 ± 6.0 | 75.0 ± 6.6 | 73.3 ± 5.9 | 0.0051 |
| Male, n (%) | 351 (49.1) | 60 (47.2) | 292 (49.5) | 0.6462 |
| Baseline living situation, n (%), n = 648 | ||||
| Independent | 619 (95.5) | 115 (92.0) | 504 (96.4) | |
| Nursing home/ Facility | 29 (4.5) | 10 (8.0) | 19 (3.6) | 0.0342 |
| Marital status, n (%), n = 705 | ||||
| Married / Partner | 490 (69.5) | 85 (68.0) | 405 (69.8) | |
| Other | 215 (30.5) | 40 (32.0) | 175 (30.2) | 0.6872 |
| Body mass index, Kg/m2, mean ± SD, n = 706 | 29.0 (5.5) | 29.2 (6.1) | 28.9 (5.4) | 0.5911 |
| ASA physical status, n (%) | ||||
| < 3 | 243 (34.0) | 18 (14.2) | 225 (38.3) | |
| ≥ 3 | 472 (66.0) | 109 (85.8) | 363 (61.7) | <0.0012 |
| Metabolic Equivalents of Task, n (%), n = 612 | ||||
| < 4 | 169 (27.6) | 44 (46.8) | 125 (24.1) | |
| ≥ 4 | 443 (72.4) | 50 (53.2) | 393 (75.9) | <0.0012 |
| Depression, n (%), n = 701 | ||||
| No | 461 (65.8) | 61 (49.6) | 400 (69.2) | |
| Yes | 240 (34.2) | 62 (50.4) | 178 (30.8) | <0.0012 |
| Anxiety, n (%), n = 703 | ||||
| No | 512 (72.8) | 78 (63.4) | 434 (74.8) | |
| Yes | 191 (27.2) | 45 (36.6) | 146 (25.2) | 0.0102 |
| Total number of medications median [25th-75th], n = 712 | 7 [5–11] | 8 [6–12] | 7 [5–11] | 0.0023 |
| Preoperative opioid use, n (%), n = 712 | ||||
| No | 449 (63.1) | 65 (51.6) | 384 (65.5) | |
| Yes | 263 (36.9) | 61 (48.4) | 202 (34.5) | 0.0032 |
| Number of past previous surgeries median [25th-75th], n = 664 | 4 [2–6] | 4 [2–7] | 4 [2–6] | 0.2753 |
| Case classification, n (%), n = 710 | ||||
| Elective | 655 (92.3) | 98 (79.0) | 557 (95.1) | |
| Non-elective | 55 (7.8) | 26 (21.0) | 29 (5.0) | <0.0012 |
SD: standard deviation, ASA: American Society of Anesthesiologists physical status
T-tests were used.
Chi-square tests were used
Wilcoxon rank sum tests were used
Fisher’s exact tests were used
Table 2.
Intra- and postoperative variables and postoperative delirium (univariate analysis)
| Postoperative Delirium Status | ||||
|---|---|---|---|---|
| Total n = 716 (%) | Yes n = 127 (17.7) | No n = 589 (82.3) | P-value | |
| Intraoperative variables | ||||
| Invasiveness *, n (%) | ||||
| Tier 1 and 2 | 255 (35.6) | 23 (9.0) | 232 (91.0) | |
| Tier 3 and 4 | 461 (64.4) | 104 (22.6) | 357 (77.4) | <0.0012 |
| Type of anesthesia, n (%) | ||||
| TIVA | 98 (13.7) | 26 (26.5) | 72 (73.5) | |
| TIVA + Volatile | 285 (39.8) | 58 (20.4) | 227 (79.6) | |
| Volatile | 333 (46.5) | 43 (12.9) | 290 (87.1) | 0.0032 |
| BIS monitoring, n (%) | ||||
| No | 273 (38.1) | 28 (10.3) | 245 (89.7) | |
| Yes | 443 (61.9) | 99 (22.3) | 344 (77.7) | <0.0012 |
| Estimated blood loss, n (%), n = 693 | ||||
| < 500 ml | 600 (86.6) | 92 (15.3) | 508 (84.7) | |
| 501–999 ml | 45 (6.5) | 15 (33.3) | 30 (66.7) | |
| > 1000 ml | 48 (6.9) | 18 (37.5) | 30 (62.5) | <0.0012 |
| Transfusion of blood products, n (%) | ||||
| No | 659 (92.0) | 106 (16.1) | 553 (83.9) | |
| Yes | 57 (8.0) | 21 (36.8) | 36 (63.2) | <0.0012 |
| Length of surgery, min median [25th-75th] | 129.0 [97.0–187.2] | 162.0 [120.5–233.0] | 124 .0 [95.0–178.0] | <0.0013 |
| Intraoperative MEA median [25th-75th] | 15.6 [12.4–24.0] | 18.0 [12.8–27.6] | 14.4 [12.4–24.0] | 0.0093 |
| Postoperative variables | ||||
| Mean pain score on postoperative day 1 median [25th-75th], n = 697 | 4.6 [3.3–6.0] | 5.5 [4.0–6.8] | 4.5 [3.3–5.7] | <0.0013 |
| MEA on postoperative day 1, median [25th-75th] | 22.2 [12.0–33.4] | 23.0 [13.8–38.2] | 22.0 [12.0–32.4] | 0.0543 |
TIVA: Total Intravenous Anesthesia, BIS: Bispectral Index; MEA: morphine equivalents amount
Invasiveness: Tier 1, microdiscectomy, lumbar laminectomy or anterior cervical procedures, minimally invasive fusions; Tier 2, lumbar fusion, trauma, or posterior cervical fusion procedures, tumor, infection, deformity, or combined anterior and posterior cervical procedures.
T-tests were used.
Chi-square tests were used
Wilcoxon rank sum tests were used
Fisher’s exact tests were used
Table 3.
Predictors of postoperative delirium by multivariable analysis
| Postoperative Delirium | ||
|---|---|---|
| OR (95% CI) | P-value | |
| Age | 1.04 (1.00, 1.09) | 0.048 |
| ASA physical status ≥ 3 | 1.89 (1.04, 3.59) | 0.042 |
| METs < 4 | 1.84 (1.10, 3.07) | 0.019 |
| Depression | 2.01 (1.21, 3.32) | 0.006 |
| Non-elective surgery | 4.81 (1.75, 12.79) | 0.002 |
| Invasiveness Tier 3 or 4 | 1.97 (1.10, 3.69) | 0.028 |
| BIS Monitoring | 2.09 (1.22, 3.70) | 0.009 |
| Mean pain score postoperative day 1 | 1.28 (1.11, 1.48) | < 0.001 |
OR: Odds ratio, CI: Confidence interval, ASA: American Society of Anesthesiologists physical status, METs: Metabolic equivalents of task, BIS: Bispectral Index
Figure 2.
Receiver operating characteristic (ROC) curve of the final model for postoperative delirium.
Not surprisingly, on univariate analysis, postoperative delirium was associated with the development of other in-hospital complications (P < 0.001), longer hospital length of stay (P < 0.001), discharge to place other than home (P < 0.001), 30-day hospital readmission (P = 0.002) and 30-day mortality (P = 0.002. (Table 4)
Table 4.
Outcomes associated with postoperative delirium (univariate analysis)
| Postoperative Delirium Status | ||||
|---|---|---|---|---|
| Total n = 715 (%) | Yes n = 127 (17.8) | No n = 588 (82.2) | P-value | |
| Other complications, n (%) | ||||
| No | 621 (86.9) | 83 (65.4) | 538 (91.5) | |
| Yes | 94 (13.1) | 44 (34.6) | 50 (8.5) | <0.0012 |
| Hospital length of stay median [25th-75th], n = 710 | 3.0 [2.0–4.0] | 6.0 [3.0–8.0] | 3.0 [2.0–4.0] | <0.0013 |
| Discharge to other place than home n (%), n = 614 | ||||
| No | 361 (58.8) | 27 (24.6) | 334 (66.3) | |
| Yes | 253 (41.2) | 83 (75.4) | 170 (33.7) | <0.0012 |
| 30-day readmission, n (%), n = 710 | ||||
| No | 657 (92.5) | 104 (85.3) | 553 (94.1) | |
| Yes | 53 (7.5) | 18 (14.7) | 35 (6.0) | 0.0012 |
| 30- day mortality, n (%) | ||||
| No | 703 (98.3) | 120 (94.5) | 583 (99.2) | |
| Yes | 12 (1.7) | 7 (5.5) | 5 (0.8) | 0.0024 |
T-tests were used
Chi-square tests were used
Wilcoxon rank sum tests were used
Fisher’s exact tests were used
Ninety-four patients (13.1%) had postoperative complications other than delirium during their hospital stay (Table 4). On multivariable analysis, predictors of other postoperative complications included the development of postoperative delirium (adjusted OR = 3.52 [95% CI 2.08 to 5.91]; P < 0.001), living in a nursing home or facility (adjusted OR = 3.38 [95% CI 1.40 to 7.87]; P = 0.021), non-elective surgery (adjusted OR = 3.29 [95% CI 1.65 to 6.46]; P = 0.002) and intraoperative transfusion of blood products (adjusted OR = 2.75 [95% CI 1.37 to 5.40]; P = 0.015) (Table 5A). (See Supplementary Table 1 for univariate analysis).
Table 5.
Predictors of (A) other complications, (B) hospital length of stay, (C) discharge to place other than home, (D) 30-d hospital readmission after surgery and anesthesia.
| A. | Other complications | ||
|---|---|---|---|
| OR (95% CI) | P-value | Adjusted p-value | |
| Postoperative delirium | 3.52 (2.08, 5.91) | <0.001 | <0.001 |
| Age | 1.05 (1.01, 1.09) | 0.014 | 0.055 |
| Nursing home / Facility | 3.38 (1.40, 7.87) | 0.005 | 0.021 |
| Non-elective surgery | 3.29 (1.65, 6.46) | <0.001 | 0.002 |
| Transfusion of blood products | 2.75 (1.37, 5.40) | 0.004 | 0.015 |
| B. | Hospital length of stay | ||
| Exponentiated estimate (95% CI) | P-value | Adjusted p-value | |
| Postoperative delirium | 1.60 (1.43, 1.80) | <0.001 | <0.001 |
| Age | 1.01 (1.00, 1.01) | 0.041 | 0.165 |
| Nursing home / Facility | 1.30 (1.05, 1.61) | 0.016 | 0.063 |
| ASA physical status ≥ 3 | 1.11 (1.02, 1.22) | 0.022 | 0.088 |
| Non-elective surgery | 1.68 (1.43, 1.99) | <0.001 | <0.001 |
| Invasiveness Tier 3 or 4 | 1.32 (1.20, 1.44) | <0.001 | <0.001 |
| Preoperative opioid use | 1.10 (1.00, 1.20) | 0.040 | 0.160 |
| Transfusion of blood products | 1.36 (1.16, 1.60) | <0.001 | <0.001 |
| Mean pain score on postoperative day 1 | 1.04 (1.01, 1.06) | 0.002 | 0.008 |
| C. | Discharge to other place than home | Adjusted p-value | |
| OR (95% CI) | P-value | ||
| Postoperative delirium | 4.51 (2.35, 8.93) | <0.001 | <0.001 |
| Age | 1.11 (1.06, 1.16) | <0.001 | <0.001 |
| Marital Status (other than married/partner) | 2.17 (1.31, 3.61) | 0.003 | 0.010 |
| Body mass index | 1.05 (1.00, 1.10) | 0.036 | 0.144 |
| ASA physical status ≥ 3 | 1.88 (1.11, 3.20) | 0.019 | 0.078 |
| Total number of medications | 1.06 (1.00, 1.13) | 0.049 | 0.195 |
| Invasiveness Tier 3 or 4 | 2.38 (1.42, 4.06) | 0.001 | 0.005 |
| Length of Surgery | 1.00 (1.00, 1.01) | 0.004 | 0.016 |
| Estimated blood loss | |||
| >1000 ml | 7.56 (2.55, 28.11) | <0.001 | 0.003 |
| D. | 30-day readmission | Adjusted | |
| OR (95% CI) | P-value | p-value | |
| METs < 4 | 2.23 (1.14, 4.33) | 0.018 | 0.071 |
| BIS monitoring | 2.88 (1.31, 7.25) | 0.014 | 0.056 |
OR: Odds ratio, CI: Confidence interval, ASA: American Society of Anesthesiologists physical status, METs: Metabolic equivalents of task, BIS: Bispectral Index, Adjusted p-value: Bonferroni adjusted p-values.
On multivariable analysis, postoperative delirium increased hospital stay by 60% (Exponentiated estimate = 1.60 [95% CI 1.43 to 1.80]; P < 0.001) (Table 5B). Other perioperative variables that were associated with an increased hospital length of stay on multivariable analysis included non-elective surgery (Exponentiated estimate = 1.68 [95% CI 1.43 to 1.99]; P < 0.001), more invasive surgical procedure (Exponentiated estimate = 1.32 [95% CI 1.20 to 1.44]; P < 0.001), intraoperative transfusion of blood products (Exponentiated estimate = 1.36 [95% CI 1.16 to 1.60]; P < 0.001) and higher postoperative day 1 mean pain score (Exponentiated estimate = 1.04 [95% CI 1.01 to 1.06]; P = 0.008) (Table 5B). (See Supplementary Table 2 for univariate analysis)
Two hundred and fifty-four (41.3%) patients living at home before surgery were discharged to other place than home after surgery (Table 4). On multivariable analysis, postoperative delirium increased the odds of discharge to other place than home (OR = 4.51 [95% CI 2.35 to 8.93]; P < 0.001) (Table 4). Older age (OR = 1.11 [95% CI 1.06 to 1.16]; P < 0.001), not being married or living with a partner (OR = 2.17 [95% CI 1.31 to 3.61]; P = 0.010), higher body mass index (OR = 1.05 [95% CI 1.00 to 1.10]; P = 0.144), ASA physical status ≥ 3 (OR = 1.88 [95% CI 1.11 to 3.20]; P = 0.078), more preoperative medications (OR = 1.06 [95% CI 1.00 to 1.13]; P = 0.195), more invasive surgical procedure (OR = 2.38 [95% CI 1.42 to 4.06]; P = 0.005), longer surgery (OR = 1.00 [95% CI 1.00 to 1.01]; P = 0.016) and estimated blood loss > 1000 ml (OR = 7.56 [95% CI 2.55 to 28.11]; P = 0.003) were independent predictors of discharge to other place than home on multivariable analysis (Table 5C). (See Supplementary Table 3 for univariate analysis)
The 30-day readmission rate was 7.5% (Table 4) and none of the variables were significant in the multivariable logistic model (See supplementary Table 4 for univariate analysis).
Of 716 patients in this study, 12 patients (1.7%) died within 30 days of surgery. (Supplementary Table 5). None of the variables were significant in the logistic model due to small sample size (Results not shown).
We performed a post-hoc analysis to study the differences between the group of patients with missing variables and those with complete variables and found that only patients undergoing non-elective surgery had significantly more missing information (P < 0.001).
A post-hoc univariate analysis demonstrated that the use of BIS monitoring was associated with ASA physical status ≥ 3 (P = 0.013), metabolic equivalents of task < 4 (P = 0.042), greater estimated blood loss (P = 0.041), more invasive surgical procedures (P = 0.027), total intravenous anesthesia (P < 0.001), intraoperative transfusion of blood products (P = 0.006) and longer surgery (P < 0.001).
Discussion
Postoperative delirium was the most common complication in this cohort of older adults undergoing spine surgery. Older age, functional impairment, depression, non-elective and more invasive surgery, BIS use and poorly controlled postoperative pain were predictors of developing postoperative delirium. The development of postoperative delirium was associated with worse outcomes after surgery including an increased risk of other postoperative in-hospital complications, longer hospital length of stay and discharge to place other than home.
Our finding that postoperative delirium occurred in 18% of patients having spine surgery is consistent with the findings of others who have variably reported the incidence of postoperative delirium between 4% to 41%.6,10 The variability in the reported incidence of delirium may represent variability in the methods used to detect delirium,18 and variability in study inclusion criteria such as age, case classification and type of procedure.6,7,10,19
Identification of risk factors associated with postoperative delirium during the preoperative assessment of older adults may allow clinicians to implement strategies to reduce its incidence with the ultimate goal of improving patient outcomes.20 Prior studies have identified advanced age, lower metabolic equivalents of task, higher ASA physical status, non-elective surgery, depression and higher postoperative pain scores as risk factors for the development of postoperative delirium after surgery.21–28 We found it interesting that the use of BIS monitoring during anesthesia was associated with postoperative delirium, even though there is a growing body of evidence suggesting that intraoperative electroencephalogram monitoring may actually reduce the risk of postoperative delirium.29,30 However, this finding is confounded by the fact that BIS monitoring was used in patients at higher risk for the development of postoperative delirium including those with higher ASA physical status, lower metabolic equivalents of task and in those having more invasive, hemorrhagic and longer procedures, suggesting that practicing anesthesiologists may be more likely to use BIS monitoring in high risk patient populations.
It has been long known that postoperative delirium is associated with adverse outcomes including longer hospital length of stay, discharge to place other than home, and functional decline after surgery.31,32 Moreover, studies suggest that patients whose postoperative course is complicated by the development of delirium may have an increase long-term mortality, hospital re-admissions, cognitive impairment and worsening quality of life.32
This study has several limitations. Its retrospective nature impairs an accurate analysis of all potential perioperative risk factors associated with postoperative delirium and other adverse outcomes, mainly due to missing variables. We attempted to address this problem by performing a post-hoc analysis of the missing information and found that patients who had non-elective surgeries where the only group to be more likely to not having a complete medical record. There may be other potential confounders, mainly intra- and postoperative factors, including the elective use of a BIS monitor in older patients, more debilitated paients and the reliance on comprehensive chart review, although well validated, for the diagnosis of postoperative delirium.
In conclusion, our study reinforces existing evidence that postoperative delirium is a common complication in older patients after spine surgery and that there are several perioperative risk factors associated with delirium and other adverse outcomes. In addition, this study has identified social factors such as living in a care facility and marital status as potential factors in the development of adverse outcomes in older patients. Care providers should consider a focused multidisciplinary preoperative assessment that includes an evaluation of baseline medical comorbidities, social environment, and geriatric conditions that may identiy older patients that are at high risk for developing adverse postoperative outcomes and to develop individualized preoperative optimization strategies to enhance patient outcomes in older adults undergoing elective and non-elective spine surgery.33
Supplementary Material
Acknowledgments
Funding Statement: National Institutes of Health AG048522 (DJC); National Institutes of Health AG048637 (GC); and the Department of Anesthesiology, Perioperative, and Pain Medicine, Brigham and Women’s Hospital, Boston, MA
GC: Grant Funding: NIA, APSF, CRICO, Executive Section Editor: Neuroscience and Neuroanesthesiology, Anesthesia and Analgesia
DJC: Director of the American Board of Anesthesiology, Member ABMS Committee on Continuous Certification, ACGME – RRC ex-officio member, Executive Editor Anesthesiology, ASA committee member; Grant funding: APSF, NIA.
Footnotes
Conflicts of Interest:
MJS: None
SDS: Boston MSTAR (Medical Student Training in Aging Research) supported by a National Institutes of Aging/NIH T35 Research Training Grant
RHG: None
JDK: None
TRS: None
MWG: None
JHC: None
YL: None
MWG: None
JHC: K2M- advisory board/consultant, Medtronic consultant. Neither are related to the topic of the paper.
XX: None
This data was presented at the Harvard Department of Anesthesia Poster Session, 4th October 2017, Boston, MA, USA
References
- 1.Wan He DG, and Paul Kowal. U.S. Census Bureau, International Population Reports, P95/16–1, An Aging World: 2015. U.S. Government Publishing Office, Washington, DC, 2016 [Google Scholar]
- 2.Etzioni DA, Liu JH, Maggard MA, et al. : The aging population and its impact on the surgery workforce. Ann Surg 2003; 238: 170–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfuntner A, Wier LM, Stocks C: Most Frequent Procedures Performed in U.S. Hospitals, 2011: Statistical Brief #165, Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD), 2006 [Google Scholar]
- 4.Lagman C, Ugiliweneza B, Boakye M, et al. : Spine Surgery Outcomes in Elderly Patients Versus General Adult Patients in the United States: A MarketScan Analysis. World Neurosurg 2017; 103: 780–788 [DOI] [PubMed] [Google Scholar]
- 5.Marcantonio ER: Postoperative delirium: a 76-year-old woman with delirium following surgery. JAMA 2012; 308: 73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown CH, LaFlam A, Max L, et al. : Delirium After Spine Surgery in Older Adults: Incidence, Risk Factors, and Outcomes. J Am Geriatr Soc 2016; 64: 2101–2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fineberg SJ, Nandyala SV, Marquez-Lara A, et al. : Incidence and risk factors for postoperative delirium after lumbar spine surgery. Spine 2013; 38: 1790–6 [DOI] [PubMed] [Google Scholar]
- 8.Shi C, Yang C, Gao R, et al. : Risk Factors for Delirium After Spinal Surgery: A Meta-Analysis. World Neurosurg 2015; 84: 1466–72 [DOI] [PubMed] [Google Scholar]
- 9.Jiang X, Chen D, Lou Y, et al. : Risk factors for postoperative delirium after spine surgery in middle- and old-aged patients. Aging Clin Exp Res 2016; 29:1039–1044 [DOI] [PubMed] [Google Scholar]
- 10.Elsamadicy AA, Wang TY, Back AG, et al. : Post-operative delirium is an independent predictor of 30-day hospital readmission after spine surgery in the elderly (>/=65years old): A study of 453 consecutive elderly spine surgery patients. J Clin Neurosci 2017; 41: 128–131 [DOI] [PubMed] [Google Scholar]
- 11.Tong HC, Haig AJ, Geisser ME, et al. : Comparing pain severity and functional status of older adults without spinal symptoms, with lumbar spinal stenosis, and with axial low back pain. Gerontology 2007; 53: 111–5 [DOI] [PubMed] [Google Scholar]
- 12.Kuhn E, Du X, McGrath K, et al. : Validation of a consensus method for identifying delirium from hospital records. PLoS One 2014; 9: e111823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armaghani SJ, Lee DS, Bible JE, et al. : Preoperative opioid use and its association with perioperative opioid demand and postoperative opioid independence in patients undergoing spine surgery. Spine 2014; 39: E1524–30 [DOI] [PubMed] [Google Scholar]
- 14.SP. K: Opioid (Opiate) Equianalgesia Conversion Calculator, August 22. ClinCalc: http://clincalc.com/Opioids. Accessed August 22, 2017.
- 15.D M: Narcotic analgesic converter. http://www.globalrph.com/narcoticonv.htm. Accessed August 22, 2017, 2013
- 16.Farrar JT, Young JP Jr., LaMoreaux L, et al. : Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001; 94: 149–58 [DOI] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, et al. : Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inouye SK: Delirium in older persons. N Engl J Med 2006; 354: 1157–65 [DOI] [PubMed] [Google Scholar]
- 19.Kawaguchi Y, Kanamori M, Ishihara H, et al. : Postoperative delirium in spine surgery. Spine J 2006; 6: 164–9 [DOI] [PubMed] [Google Scholar]
- 20.Inouye SK, Bogardus ST Jr., Charpentier PA, et al. : A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 1999; 340: 669–76 [DOI] [PubMed] [Google Scholar]
- 21.Raats JW, van Eijsden WA, Crolla RM, et al. : Risk Factors and Outcomes for Postoperative Delirium after Major Surgery in Elderly Patients. PLoS One 2015; 10: e0136071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scholz AF, Oldroyd C, McCarthy K, et al. : Systematic review and meta-analysis of risk factors for postoperative delirium among older patients undergoing gastrointestinal surgery. Br J Surg 2016; 103: e21–8 [DOI] [PubMed] [Google Scholar]
- 23.Culley DJ, Flaherty D, Fahey MC, et al. : Poor Performance on a Preoperative Cognitive Screening Test Predicts Postoperative Complications in Older Orthopedic Surgical Patients. Anesthesiology 2017; 127:765–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganai S, Lee KF, Merrill A, et al. : Adverse outcomes of geriatric patients undergoing abdominal surgery who are at high risk for delirium. Arch Surg 2007; 142: 1072–8 [DOI] [PubMed] [Google Scholar]
- 25.Leung JM, Sands LP, Mullen EA, et al. : Are preoperative depressive symptoms associated with postoperative delirium in geriatric surgical patients? J Gerontol A Biol Sci Med Sci 2005; 60: 1563–8 [DOI] [PubMed] [Google Scholar]
- 26.Knaggs JD, Larkin KA, Manini TM: Metabolic cost of daily activities and effect of mobility impairment in older adults. J Am Geriatr Soc 2011; 59: 2118–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ansaloni L, Catena F, Chattat R, et al. : Risk factors and incidence of postoperative delirium in elderly patients after elective and emergency surgery. Br J Surg 2010; 97: 273–80 [DOI] [PubMed] [Google Scholar]
- 28.Vaurio LE, Sands LP, Wang Y, et al. : Postoperative delirium: the importance of pain and pain management. Anesth Analg 2006; 102: 1267–73 [DOI] [PubMed] [Google Scholar]
- 29.Radtke FM, Franck M, Lendner J, et al. : Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth 2013; 110 Suppl 1: i98–105 [DOI] [PubMed] [Google Scholar]
- 30.Chan MT, Cheng BC, Lee TM, et al. : BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol 2013; 25: 33–42 [DOI] [PubMed] [Google Scholar]
- 31.Rudolph JL, Inouye SK, Jones RN, et al. : Delirium: an independent predictor of functional decline after cardiac surgery. J Am Geriatr Soc 2010; 58: 643–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin BJ, Buth KJ, Arora RC, et al. : Delirium: a cause for concern beyond the immediate postoperative period. Ann Thorac Surg 2012; 93: 1114–20 [DOI] [PubMed] [Google Scholar]
- 33.American Geriatrics Society Expert Panel on Postoperative Delirium in Older A: Postoperative delirium in older adults: best practice statement from the American Geriatrics Society. J Am Coll Surg 2015; 220: 136–48 e1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.