Abstract
Duality of iron as an essential cofactor of many enzymatic metabolic processes and as a catalyst of poorly controlled redox-cycling reactions defines its possible biological beneficial and hazardous role in the body. In this review, we discuss these two “faces” of iron in a newly conceptualized program of regulated cell death, ferroptosis. Ferroptosis is a genetically programmed iron-dependent form of regulated cell death driven by enhanced lipid peroxidation and insufficient capacity of thiol-dependent mechanisms (glutathione peroxidase 4, GPX4) to eliminate hydroperoxy-lipids. We present arguments favoring the enzymatic mechanisms of ferroptotically engaged non-heme iron of 15-lipoxygenases (15-LOX) in complexes with phosphatidylethanolamines binding protein 1 (PEBP1) as a catalyst of highly selective and specific oxidation reactions of arachidonoyl- (AA) and adrenoyl-phosphatidylethanolamines (PE). We discuss possible role of iron chaperons as control mechanisms for guided iron delivery directly to their “protein clients” thus limiting non-enzymatic redox-cycling reactions. We also consider opportunities of loosely-bound iron to contribute to the production of proferroptotic lipid oxidation products. Finally, we propose a two-stage iron-dependent mechanism for iron in ferroptosis by combining its catalytic role in the 15-LOX-driven production of 15-hydroperoxy-AA-PE (HOO-AA-PE) as well as possible involvement of loosely-bound iron in oxidative cleavage of HOO-AA-PE to oxidatively truncated electrophiles capable of attacking nucleophilic targets in yet to be identified proteins leading to cell demise.
Keywords: Ferroptosis, Iron, Iron chaperons, Lipid peroxidation, 15-lipoxygenase, Hydroperoxy-arachidonoyl-phosphatidylethanolamine, Glutathione, GPX4
Graphical Abstract
“At once he became an enigma. One side or the other of his nature was perfectly comprehensible; but both sides together were bewildering.” ― Jack London, The Sea Wolf
Introduction.
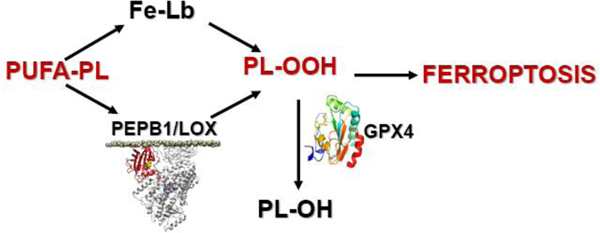
Cooling of the Earth’s crust during the Archean eon created conditions compatible with the utilization of iron for life-sustaining processes - electron transfer, biochemical catalysis and, later, binding and transport of oxygen [1, 2]. The role of reduced iron as the electron donor in many reactions, including photosynthesis, and oxidized iron as the universal terminal electron acceptor in respiratory chains, as well as the catalytic propensities of iron as a key cofactor for a variety of enzymatic reactions determined its irreplaceable functions in all living organisms [3–7]. The newly emerging field of radical enzymology points to a possibly important role of iron in primordial hydrogen abstraction reactions occurring anaerobically in primitive organism through tightly coordinated alkyl radical driven mechanisms [8]. The essential high chemical reactivity of iron also imposed the creation of highly specialized, complex mechanisms of competition for iron between host cells and invading bacterial pathogens [1], along with those for transport and control of extra- and intra-cellular iron, based on tight binding of this metal by specialized proteins. Paradoxically, the same high catalytic activity of iron may represent the threat to cell’s existence through catalysis of poorly controlled Fedependent reactions or, as has been recently established, via regulated death programs (eg, apoptosis, ferroptosis) (Figure 1;[9, 10]). This duality of the iron-driven mechanisms, at times representing bewildering conceptual and experimental challenges, is the main subject of the review
Figure 1.
Three pillars of ferroptosis.
Mishandling of iron, pathogenic mechanisms.
In mammalian cells, both deficiency and excess of iron perturb the homeostasis, and physiological conditions can be restored by iron supplementation or chelation, respectively [3, 11, 12]. Release of iron from transport and storage proteins and formation of low molecular mass iron complexes with cellular ligands (Fe-Lb) is viewed as a toxicologically significant event [13–15]. Acute poisoning in humans with iron has been associated with liver, kidney and lung toxicity [11]. In models of chronic iron overload in rodents, it has been observed that the content of iron markedly increases in liver, spleen and kidney with concomitant accumulation of aliphatic aldehydes, which are markers of lipid peroxidation [16–18]. Recent studies indicate that an iron storage protein, ferritin, can release its payload in acidic environment of lysosomes hence create conditions potentially leading to enhanced pro-oxidant effects. This mishandling of iron has been associated with directly or indirectly with the pathogenesis of worsening of a number of disease conditions including neurodegenerative diseases such as Alzheimer’s, Parkinson’s and Huntington’s diseases [19]. It is tempting to speculate this dysregulation of iron handling may cause ferroptotic cell death as a pathogenic factor [20, 21].
Enzymatic and non-enzymatic iron-catalyzed (bio)chemical reactions.
The wealth of biochemical reactions that are mediated by iron reflects its facile redox interconversion between oxidation states and its ability to form complexes with various cellular ligands. Although the oxidation state of iron can vary from −2 to +7, in biological systems this metal predominantly exists in the relatively stable 2+ (ferrous; Fe(II)) and 3+ (ferric; Fe(III)) oxidation states. In addition, reactive Fe(IV)-oxo species can be formed as short-lived intermediates in a number of biological reactions [22]. Many iron-containing enzymes have been structurally and functionally characterized; however, reliable and accurate protocols for analyzing the cellular distribution of Fe(II) and Fe(III) in Fe-Lb have not been established thus far. Hence, the mechanistic and derivative chemistry of Fe-Lb remains poorly understood and their biological role is often deduced from analyses of the chemical properties of low molecular mass iron complexes with non-physiological ligands.
Since the late 1950s [23, 24], a considerable research effort has been directed towards understanding the mechanisms of iron-induced lipid peroxidation in biological membranes. It is commonly believed that non-enzymatic lipid peroxidation is the consequence of the attack on polyunsaturated lipids by hydroxyl radical (HO•) generated in so-called Fenton reaction (Fe(II) + H2O2 → Fe(III) + HO• + HO–[25, 26]); a search in the PubMed database with keywords “iron”, “hydroxyl radical”, “lipid peroxidation”, and “Fenton reaction” renders 198,911, 18,942, 67,330, and 3,795 entries, respectively. Detailed studies of lipid peroxidation in isolated mitochondria and membranes of the endoplasmic reticulum (microsomal fraction) catalyzed by exogenously added “free” or “loose” iron have been published [27–31]. However, the relevance of these data to physiological iron-driven mechanisms of lipid peroxidation with a strict control of available iron remains elusive.
Cells consistently produce H2O2 [32], in many reactions of redox metabolism, particularly those yielding superoxide anion radicals that readily dismutate to H2O2 [33]. Due to its high reactivity, HO• is one of the most toxic species that can be formed in biological systems. HO• reacts at diffusion-controlled rates with the majority of biomolecules [34], including lipids [35]. Thus, HO• cannot “travel” far from the site of its generation, no further than the nearest molecule. This remarkably high reactivity of HO• imposes kinetic restrictions on the ability of potential exogenous protectors from oxidation of essential biomolecules by HO•. Assuming that a radical scavenger reacts with HO• at a diffusion-controlled rate, the concentration of such an effective protector should be at least one order of magnitude higher than that of the intracellular targets. This is well illustrated by cellular thiols, which are present at mM concentrations [36]. As many synthetic candidate radical scavengers exert significant cytotoxicity at such high concentrations, this sets the limit for their applications as protectors against HO•. Mother Nature has devised several alternative mechanisms to counteract the high reactivity of HO•, mostly based on the prevention of the Fenton chemistry via the elimination of its major precursor, H2O2 [37, 38].
Extra- and intra-cellular mechanisms of iron delivery control its redox activity.
Given the high and likely detrimental redox activity of loose iron in cells and extracellular compartments, transportation and delivery of this “high risk” catalyst to its final destinations remains under constant control of specialized proteins (Figure 2; [3–5, 7, 12, 39–42]). Extracellular iron in the plasma is almost exclusively in the ferric form and bound to circulating transferrin (reviewed in [43]). Transferrin bound iron (TBI) initially binds to transferrin receptor 1 (Tfr1) on the cell surface, which triggers endocytosis of the TBI-Tfr1 complex. In the acidic environment of the endosome, iron is released from transferrin and reduced by ferric reductases of the Steap family [44, 45]. Endosomal iron can then be transported across the membrane to the cytosol by a dedicated ferrous-iron transmembrane transporter, Divalent Metal Transporter 1 (DMT1) [46] or by a multispecific metal transporter, ZRT/IRT-like Protein (ZIP) 14 or 8 [47, 48]. In some settings, iron presented to the cell surface is not bound to transferrin and can be directly transported into the cell without endocytosis by transporters on the cell surface. Uptake of dietary iron at the apical surface of the intestinal epithelial cell is the most notable example of this type of uptake. In some physiological conditions, e.g. after a large dietary bolus of iron, or pathophysiological settings, e.g. when transferrin is saturated in hereditary iron overload, non-transferrin-bound iron may be present in the portal or systemic circulation and be taken up by transporters on the cell surface. Again, ferric reductase activities encoded by Steaps or the intestinal ferric reductase, duodenal cytochrome b (dCytB) [49] are required prior to transport by DMT1 or ZIP8/14 [50, 51].
Figure 2.
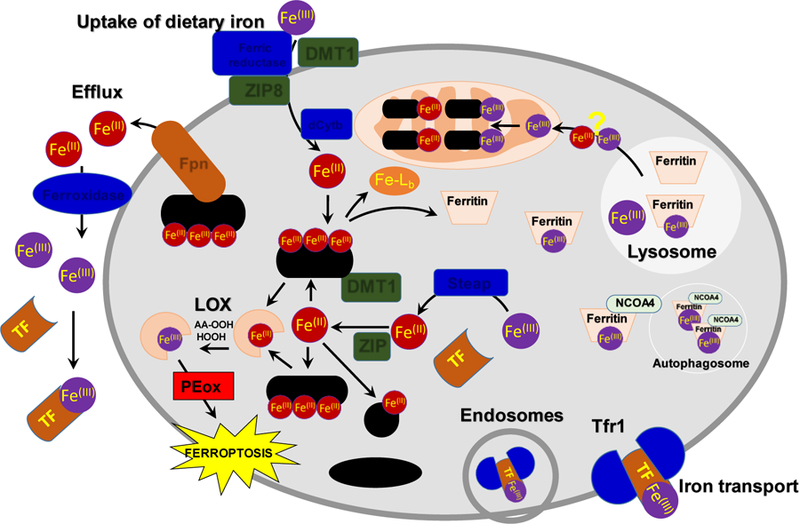
Schema of cellular iron homeostasis. The schema illustrates the various metalloproteins and metallochaperons involved in the influx of dietary iron into the cell, its import/transport into different organelles via different proteins, and its efflux from the cell. dCytB: Duodeneal cytochrome B, DMT1 (SLC11A2): Natural resistance-associated macrophage protein 2, Fe-Lb: Low molecular mass iron complexes with cellular ligands, FE-S: Iron-Sulphur clusters, Ferritin: Protein that stores Iron, Fpn (SLC40A1): Ferroportein-1 iron transporter, Ferric reductase: catalyses reduction of Fe(III) to Fe(II), Ferroxidase: catalyses oxidation of Fe(II) to Fe(III), GSH: Reduced Glutathione, HEME: Heme, NCOA4: Nuclear receptor coactivator 4, Autophagic cargo receptor for ferritin., LOX: Lipoxygenase, AA-OOH; Hydroperoxy-arachidonic acid, HOOH; hydrogen peroxide; PCBP1/2: Poly(rC)-binding protein-1, Iron chaperone, PE: Phosphatidylethanolamine lipid,Steap: Metalloreductase, TF: Transferrin, Tfr1: Transferrin Receptor protein 1, ZIP14/8 (SCL39A14): Zinc and iron permease.
Upon entering the cytosol, Fe(II) is likely coordinated by a pool of small and macromolecules that escort the iron to different sites within the cell. These molecules serve to limit the redox activity of iron and promote its interaction with appropriate targets within the cell. The major small molecule proposed to coordinate iron is reduced glutathione (GSH) [52]. GSH can coordinate Fe(II) through a single thiol ligand contributed by its reduced cysteine residue and therefore binds iron with relatively low affinity. Nevertheless, it is present in the cytosol at very high concentrations (2–10 mM), and speciation plots of Fe(II) and GSH suggest that this is the major small molecule ligand for iron in the cytosol.
Macromolecular ligands for cytosolic iron include the iron chaperones of the Poly rC Binding-Protein (PCBP) family [53]. These are multifunctional adaptor proteins that bind single-stranded nucleic acids, other proteins, and iron. The most abundant PCBPs are PCBP1 and PCBP2, both of which can bind Fe(II) in a 3:1 stoichiometry with low micromolar affinity [54, 55]. PCBP2 can directly interact with DMT1 to bind iron and facilitate its transfer to the cytosol [56]. A similar interaction with heme oxygenase, a membrane associated enzyme that liberates iron from heme, allows PCBP2 to capture iron released from heme [57]. PCBP2 can also interact with PCBP1, which binds cytosolic iron and delivers it to client proteins, such as ferritin, the iron storage protein [55], and to non-heme iron enzymes, such as 2-oxoglutarate-dependent dioxygenases (which have mononuclear iron centers)[58] and monooxygenases of the fatty acid hydroxylase/desaturase type (which have dinuclear iron centers) [59]. Cytosolic lipoxygenases implicated in ferroptosis contain mononuclear iron centers and, as such, are likely to receive iron from PCBP chaperones, although this has not been directly demonstrated. Under conditions of iron deficiency, iron stored in cytosolic ferritin can undergo recycling by autophagic turnover of ferritin in the lysosome [60–62]. The autophagic cargo receptor for ferritin is Nuclear Co-Activator 4 (NCOA4), another multifunctional protein that can modulate transcription of nuclear genes or bind to ferritin to direct it into the autophagosome [63–65]. Lysosomal Fe(III) released from degraded ferritin can be returned to the cytosol or directed to the mitochondria for incorporation into mitochondrial iron cofactors (Heme, Fe-S clusters, non-heme iron centers). Mechanisms of lysosomal iron transfer in mammalian cells are not completely understood as yet.
Finally, iron can exit cells through the sole iron transmembrane exporter, ferroportin (Fpn) [66–68]. PCBP2 may direct cytosolic iron to Fpn for export, as PCBP2 has been found to bind directly to Fpn and, in some cell types, PCBP2 depletion can impair Fpn-mediated iron efflux [69]. Fe(II) that exits the cell is rapidly converted to Fe(III) for loading onto transferrin, a reaction that is catalyzed by multicopper ferroxidases, such as the circulating enzyme ceruloplasmin and the membrane-bound hephaestin [70, 71]. The efflux of Fe(II) through Fpn appears to be coupled to the extracellular oxidation and loading of iron onto transferrin, as rates of Fe(II) efflux are augmented by the presence of extracellular ferroxidases and transferrin.
Iron is central to the execution of the ferroptotic death program.
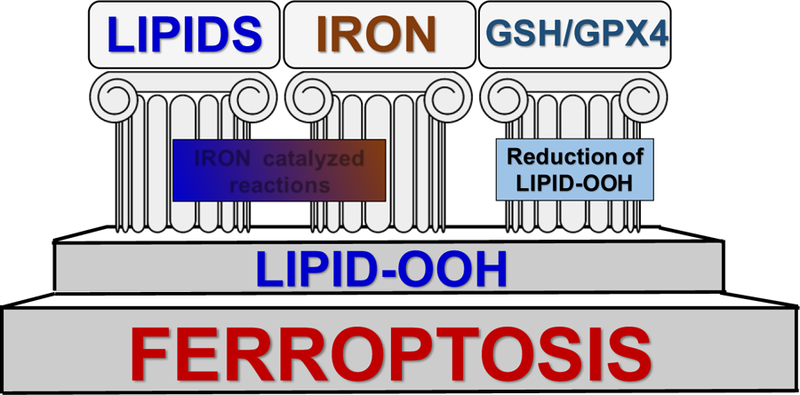
A new wave of attention to specific mechanisms of iron has been induced by its central role in a recently identified type of genetically programmed cell death, ferroptosis [72]. Three major pillars of ferroptosis – iron catalysis of lipid peroxidation yielding the hydroperoxy-products and their (also iron-catalyzed) cleavage to reactive, oxidativelytruncated electrophiles (instead of the effective reduction to alcohols by glutathione peroxidase type 4 (GPX4) – are the most important stages of the ferroptotic program (Fig. 1). Two major concepts on the role of iron-catalyzed reactions - stochastic free radical catalysis by Fe-Lb vs tightly regulated enzymatic process controlled by Feprotein(s) – have been considered as the mechanisms ultimately leading to the ferroptotic cell demise.
While these alternative mechanisms may seem a relatively narrow and even technical issue, in fact they reflect the general approach and philosophy of a very broad area of research in contemporary redox biology. Indeed, the success in understanding of free radical mechanisms of organic oxidation reactions in liquid phase inspired the transfer of these powerful concepts to the field of biology and encouraged the emergence of free radical biomedicine. Among the most popular concepts was iron-catalyzed free radical lipid peroxidation and membrane injury as one of the leading pathogenic mechanisms in a multitude of diseases, including major cardiovascular, neurodegenerative and neoplastic conditions [29]. The idea of uncontrolled free radical chain reactions as the major mechanism of injury and the opportunity of “fixing” it by antioxidants was so obvious and attractive that numerous experimental, pre-clinical and very expensive and time-consuming clinical trials have been funded and conducted. The results were disappointingly uniform - these studies did not reveal clinically significant positive results [73]. While meta-analysis indicated that a serious re-thinking of the underlying concepts and technological methodologies may be necessary [74], the tendency to directly describe and interpret biological phenomena in terms of random non-enzymatically controlled chemical reactions remains persistent.
Lipid peroxidation is a hallmark of ferroptosis.
The new concept of ferroptosis may represent a unique opportunity and testing ground for understanding the role of iron and radical-driven reactions in cell metabolism and fate. In cells, GPX4 catalyzes the reduction of fatty acid and phospholipid hydroperoxides to alcohols [75–77]. It is hypothesized that inhibition of GPX4 leads to reductive cleavage of hydroperoxides by Fe(II)-Lb to alkoxyl radicals (Scheme 1; [78]), which sets the stage for the formation of aliphatic aldehydes [78] that readily react with thiol and amino groups in proteins [79]. Random induction of lipid peroxidation can be envisioned as a process where Fenton-like reactions lead to a stochastic formation of lipid hydroperoxides. The paramount difference between random and enzymaticallyinduced lipid peroxidation in ferroptotic cells lays in the initial substrate specificity of the reactions, which would define the nature of the end-reaction products.
Scheme 1.
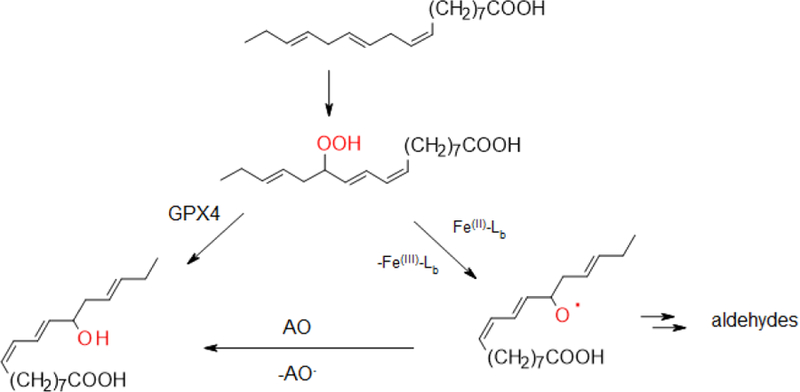
Inhibition of GPX4 leads to the formation of alkoxyl radicals.
15-LOX-driven enzymatic generation of ferroptotic signals.
The role of the enzymatically regulated process catalyzed by lipoxygenases [LOXes] (most likely 15-LOX) is supported by several lines of evidence, including selectivity towards peroxidation substrates and specificity of the oxidation products formed. Indeed, among thousands of molecular species of oxidizable phospholipids, two molecular species of arachidonoyl-PE and two of adrenoyl-PE were identified as substrates of 15LOX-catalyzed oxygenation reactions [80]. Moreover, only four hydroperoxy-PE - 15-HpETE- (and 17-HOO-AdA-PE) were identified as specific oxidation products catalyzed by 15-LOX in the presence of a scaffold protein, PEBP1 [81]. The latter forms a complex with both isoforms of 15-LOX – 15LO1 and 15-LO2 – in which the catalytic competence of the enzyme is changed from free AA to AA-PE. This is achieved via allosteric changes in the organization of the catalytic site of 15-LOX induced by PEBP1 as well as by the ability of the latter to bind up to nine free AA thus depleting free AA in the microenvironment of 15LOX. These features of AA-PE oxidation are compatible with the enzymatic nature and genetically conserved mechanisms of the overall ferroptotic process. Noteworthy, not only eukaryotic (mammalian) 15-LOXes but also prokaryotic (bacterial) 15-LOX can be involved in the selective and specific oxidation of AA-PE to 15-HpETE-PE and ferroptosis of the host organism [82]. For example, 15-LOX of a Gram-negative pathogen, P. aeruginosa, can oxidize AA-PE in the host bronchio-epithelial cells via a “theft-ferroptosis” pathway thus facilitating and propagating its effective colonization, particularly in immunecompromised organisms (eg, in cystic fibrosis and antiobiotic-resistant pneumonias) [82].
Further support to the notion for controlled lipid peroxidation is the strong dependence of ferroptosis on several lipid-metabolizing proteins specific for arachidonic and adrenic acids, such as ASCL4 required for the biosynthesis of oxidation substrates AA-PE and AdA-PE [80, 83]. In addition, a trans-acylase, LPCAT3, participating in the maintenance of sufficient levels of these oxidation substrates is also a significant required constituent of the ferroptotic program [80] (Figure 3).
Figure 3.
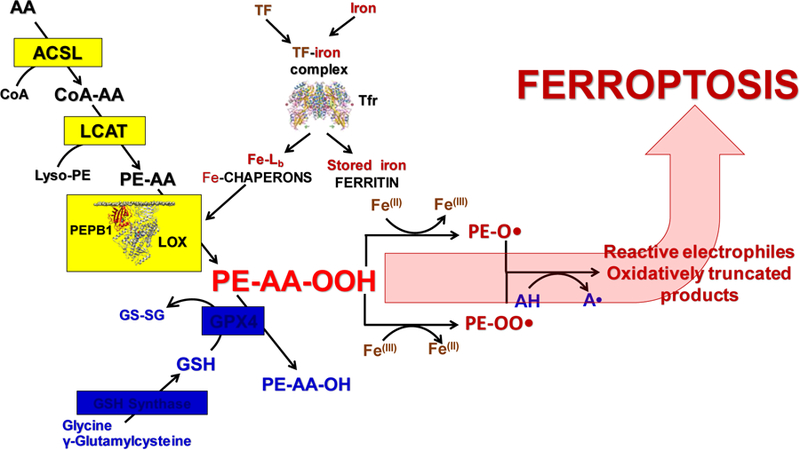
Schema illustrating three pillars of ferroptotic program:Lipid peroxidation, Iron handling pathways and GPX4-dependent reduction of hydroperoxy-phospholipids. ACSL, Acyl-CoA synthase; LCAT, Lyso-phospholipid acyl transferase; PEBP1, Phosphatidylethanolamine binding protein 1; LOX, Lipoxygenase; GSH, Glutathione; GS-SG, Oxidized glutathione; AA, arachidonic acid; PE-AA-OOH, Hydroperoxy-arachidonoyl phosphatidylethanolamine; PE-AA-OH, Hydroxy-arachidonoyl phosphatidylethanolamine;GPX4, Glutathione peroxidase 4; AH, Antioxidants (phenols, aromatic amines); Fe-Lb, Low molecular mass iron complexes with cellular ligands; TF, Transferrin; Tfr, Transferrin receptor.
Non-enzymatic iron-catalyzed free radical lipid peroxidation as a pro-ferroptotic mechanism.
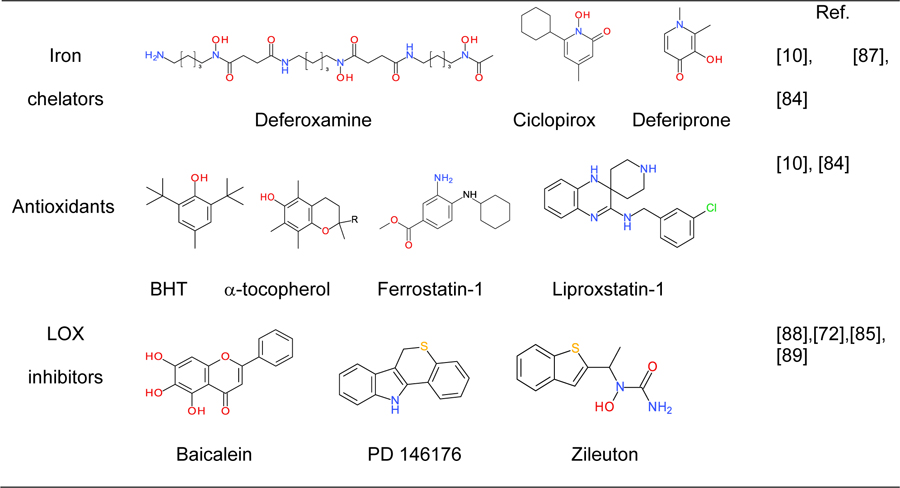
The anti-ferroptotic activity of a variety of free radical scavengers (Table I) has provided a foundation for the hypothesis that ferroptosis is triggered by random, Fe-Lb-catalyzed free radical reactions. Zilka et al. favored the random free radical pathway vs. LOXcatalyzed oxidation because, in liposomal suspensions, Ferrostatin-1 (Fer-1) and Liproxstatin-1 (Lip-1) proved better scavengers of ROO• than inhibitors of LOXs [84] as anti-ferroptotic agents [80, 85, 86].
Table 1.
The major types of small molecule inhibitors of ferroptosis.
 |
The authors argued that the observations made in their model system can be extrapolated to intact cells, as they are consistent with the greater potency of Fer-1 and Lip-1 relative to α -tocopherol and specific inhibitors of LOXs to impede ferroptosis. However, validation of this hypothesis would require assessment of the incorporation of α-tocopherol in liposomes as reported in ref. [84], as well as analysis of both the intracellular concentrations and the compartmentalization of Fer-1 and Lip-1 in experiments with intact cells.
Fer-1 contains an esterified carboxylic group that is most likely hydrolyzed by intracellular esterases. The latter reaction would maintain a continuous uptake of Fer-1 from the incubation medium and can lead to relatively high intracellular concentrations of the carboxylate anion of this amine. Furthermore, Fer-1 and Lip-1 are lipophilic amines/imines that may accumulate in acidic intracellular compartments, such as lysosomes, through a mechanism referred to as “ion trapping” [90, 91]. This mechanism includes the protonation of basic groups in lipophilic organic molecules and is exemplified by the compartmentalization of a number of drugs containing amino groups, whose lysosomal concentrations are orders of magnitude higher than those in the extracellular milieu [90, 91]. Hence, it would be interesting to assess (i) the cytosolic and lysosomal concentrations of both Fer-1 and Lip-1, with the notion that lysosomes are viewed as a major source of redox active Fe-Lb [92–95], including in cells undergoing ferroptosis [96], and (ii) whether at the concentrations found in cells Lip-1 and Fer-1 inhibit LOXs. Of note, different homologues of tocopherols and tocotrienols may act as inhibitors of LOX by occupying the substrate binding pocket [80]. It may be particularly important to assess the effects of tocopherols as inhibitors of AA-PE oxidation by 15-LOX/PEBP1 complexes involved in the execution of ferroptotic program [80]. It is also tempting to speculate that Fer-1 and Lip-1 can bind iron. Although iron complexes of Fer-1 and Lip-1 have not been characterized thus far, organic amines and imines, including benzene-1,2-diamine derivatives are known to chelate iron [97–101]. Some of these complexes have been found to exhibit catalase-like activities [102, 103].
Over the past 80 years, considerable progress has been made in the development of free radical chemistry [104]. However, the assessment of preponderant free radical reactions in complex biological matrices remains challenging. This is due, at least in part, to the limited methods and/or lack of specific molecular probes for analysis of short-lived species. Indeed, the discrimination of iron-, oxygen-centered radicals-, and LOXs-induced ferroptosis has proven difficult because of the overlapping reactivity of the probes used to analyze this death pathway (Table I): (i) the iron chelators and inhibitors of ferroptosis, Deferoxamine, Ciclopirox, and Deferiprone are good scavengers of free radicals [105–107] that also disrupt cellular processes such as DNA repair, cell division signals,and protein synthesis by mechanisms that are not fully understood [108]; (ii) in addition to reactions with oxygen-centered radicals, the antioxidants used to impede ferroptosis have the potential to reduce metal ions [100, 109, 110] and to act as substrates of peroxidases [111, 112]; (iii) most inhibitors of LOXs are efficient scavengers of free radicals [113]; and (iv) Fe(IV)-Lb-oxo complexes may account for the deleterious effects of Fe-Lb [114–116]. Recent studies with synthetic low molecular mass iron complexes have shown that they can utilize oxygen and H2O2 for inner-sphere hydroxylation of aromatic ligands [117, 118], C-H hydroxylation of alkanes [119–121], and epoxidation of alkenes [122–124]. However, the question of whether Fe-Lb-oxo complexes are formed in cells and contribute to the toxicity of iron remains to be resolved. It should also be mentioned that triggering of lipid peroxidation in ferroptotic cells may be preceded or paralleled by Fe-Ln- or Fe(iv)-Lb-oxo-induced modification of the enzymatic activities of redox-sensitive proteins [125–127].
Combination of enzymatic generation of HOO-AA-PE and their oxidative cleavage in ferroptosis.
The nature of the immediate lipid peroxidation products acting as proximate pro-ferroptotic signals still remains to be elucidated.
It is likely that the overall process includes two stages: (i) selective and specific, enzymatic production of 15-HOO-AA-PE by 15-LOX; and ii) oxidative cleavage of these initial HOO-derivatives to proximate electrophiles capable of interacting with protein targets to cause the formation of pores in plasma membranes, or to rupture them. One can envision that two types of oxidatively-truncated products can be formed from HOOAA-PE – with the carbonyl function either on the shortened AA-residue esterified into PE or on the leaving aldehyde (Scheme 2).
Scheme 2.
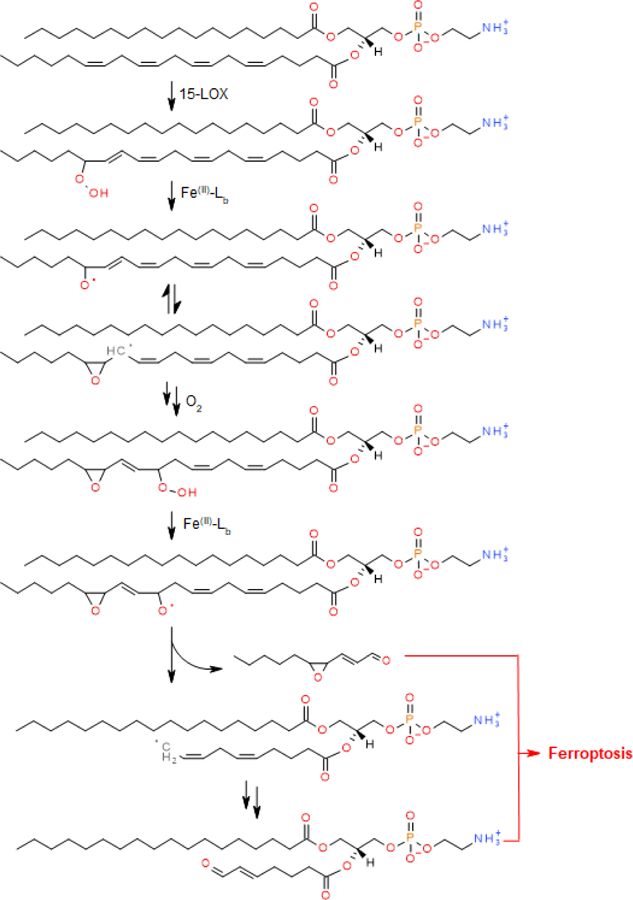
Proposed reaction sequence leading to ferroptosis. Reactions with two arrows denote multiple steps that are omitted from the reaction scheme.
In the first scenario, the specificity of PE-derived electrophiles will be retained – in contrast to the free aldehydes that can be formed during oxidative truncation of any (phospho)lipid. Much more work is necessary to identify the chemical nature of these proximal PE oxidation products, the mechanisms of their formation, as well as their protein targets and adducts. The specificity of the process of oxidative truncation of HOO-AA-PE catalyzed by Fe-protein complexes or by Fe-Lb needs to be thoroughly investigated. Furthermore, the role of coordination of iron by different intracellular chaperons and their contributory role in the strict control of iron redox-cycling activity via its tight “caging” within redox “silent” complexes needs to be established.
The pleiotropic mechanisms of action of ferroptosis inhibitors, the uncertainty of characteristic morphological features as well as biochemical biomarkers of ferroptosis makes it difficult to specifically ascertain the engagement of this cell death mechanism, particularly in vivo as illustrated by the following example. While iron is predominantly coordinated within proteins, it can also be present in metabolically available pools of kinetically labile iron, often referred to as the labile iron pool. The levels of the labile iron pool are regulated by iron-responsive factors that balance iron uptake and utilization with deposition into ferritin or efflux [128]. Increased levels of labile iron may create prooxidant conditions and cause cell injury and death, possibly ferroptosis. Much of the labile iron pool is coordinated by iron chaperones of the PCBP family. In the absence of these, the redox activity of the labile iron pool might be significantly altered.
We performed LC-MS-based lipidomics analysis on liver tissue from C57Bl6 mice specifically lacking PCBP1 in hepatocytes (C. Philpott, in preparation). These mice exhibit marked disturbances in lipid metabolism. We were interested in determining whether accumulation of pro-ferroptotic biomarkers of PE oxidation could be associated with PCBP1-deficiency. Redox phospholipidomics analysis of liver homogenates revealed 56 molecular species of oxygenated PE. However, the levels of HOO-AA-PE previously identified as biomarkers of ferroptosis [80], were not significantly elevated in the livers of PCBP1-deficient compared to WT mice (Fig. 4).
Figure 4.
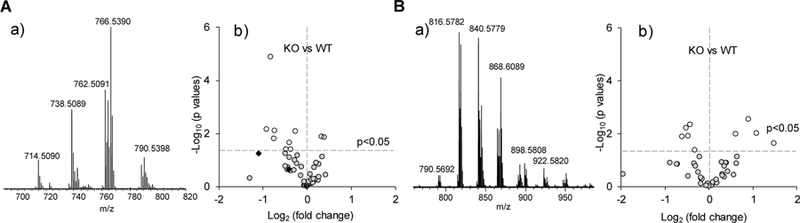
Assessment of oxygenated phospholipid species in mouse liver. Typical MS spectra of phosphatidylethanolamine (PE) (A-a) and phosphatidylcholine (PC) (B-a) obtained from WT mouse liver. Analysis of oxygenated PE species (A-b) and oxygenated PC (B-b) in liver of PCBP1-deleted (KO) vs. wild type (WT) mice. Male mice were 5–6 weeks old and maintained on a synthetic, defined diet containing adequate, but not elevated, amounts of iron (50 ppm). Ferroptotic cell death signals (hydroperoxy-PE species) are shown as closed diamonds. N=6.
We detected 53 oxidized species of the most abundant phospholipid, PC. Among those, the levels of nine species were different (p<0.05) between WT and PCBP1deficient samples, but only two species exhibited a >2-fold increase and these specifically accumulated in the PCBP1-deficient livers. However, neither of these PC oxidation products have been associated with the execution of the ferroptotic program. Because PCBP1 functions as an iron chaperone for other mononuclear iron enzymes, lipoxygenases that specifically modify AA-PE may have reduced activity in PCBP1deficient tissues. Furthermore, lipoxygenase 15 is expressed at very low levels in murine liver [129]. Thus, the GPX4 activity may be sufficient to reduce HOO-AA-PE to alcohols (HO-AA-PE), and prevent the triggering of the ferroptotic program. These observations suggest that perturbations in the reactivity of the labile iron pool may not be sufficient to trigger ferroptosis. This also illustrates the complexity of the task of revealing the involvement of ferroptosis in the aberrant reactions of lipid metabolism and lipid peroxidation in vivo.
Concluding remarks:
Discovery and characterization of several programmed cell death mechanisms has already facilitated the identification of their regulators with potential therapeutic effects. Further work on their improvement requires exact knowledge of specific features and metabolic networks engaged in the execution of death programs. Interestingly, all three major pathways of regulated cell death – apoptosis, necroptosis and ferroptosis - are believed to include lipid peroxidation as an essential part of the program. Indeed, oxygenation of cardiolipins has been shown to be essential to apoptosis, whereas oxygenation of phosphatidylethanolamines is characteristic of ferroptosis. Lipid peroxidation has been postulated as an important part of necroptotic cell death program but specific lipid substrates have not been identified so far. While the name ferroptosis emphasizes the central role of iron-driven reactions in this type of regulated cell death, the nature of the mechanisms involved remains controversial. A wealth of experimental data support the contribution of strictly controlled “caged” iron-dependent processes catalyzed by 15-LOX/PEBP1 complexes with their high selectivity and specificity towards the substrates and products of the reactions. There are also results pointing to the role of loosely-bound iron in ferroptosis. These two seemingly opposing concepts may be resolved within the framework of a two-stage process: catalysis by 15LOX/PEBP1 complex yielding 15-OOH-AA-PEs and oxidative cleavage/truncation of hydroperoxy-products. The latter may include less specific participation of small molecule iron ligands or other yet to be identified Fe-peptides/proteins. The resolution of this conundrum strongly depends on the identification of the immediate ferroptosisinducing proteins responsible for the disruption of the cell membrane leading to cell demise. This task requires refined and detailed redox proteomics-lipidomics analyses aimed at detecting of protein conjugates with oxidatively truncated PEox products.
Highlights:
Phospholipid peroxidation in ferroptosis
Lipoxygenase oxidation of arachidonoyl phosphatidylethanolamine
Guided transportation of iron to target destinations in cells
GPX4 reduction of hydroperoxy-arachidonoyl-phosphatidylethanolamine
ACSL4 biosynthesis of arachidonoyl phosphatidylethanolamine is required for
Acknowledgements
This work was supported by NIH (U19AI068021, HL114453, NS076511, NS061817, HL136143, HL086884, and P41GM103712 and by Program 5–100 of Russian Federation.
References
- [1].Gutteridge JMC, Halliwell B, Mini-Review: Oxidative stress, redox stress or redox success?, Biochem Biophys Res Commun, 502 (2018) 183–186. [DOI] [PubMed] [Google Scholar]
- [2].Knoll AH, Bergmann KD, Strauss JV, Life: the first two billion years, Philos Trans R Soc Lond B Biol Sci, 371 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Anderson GJ, Frazer DM, Current understanding of iron homeostasis, Am J Clin Nutr, 106 (2017) 1559S–1566S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ganz T, Nemeth E, Regulation of iron acquisition and iron distribution in mammals, Biochim Biophys Acta, 1763 (2006) 690–699. [DOI] [PubMed] [Google Scholar]
- [5].Outten FW, Theil EC, Iron-based redox switches in biology, Antioxid Redox Signal, 11 (2009) 1029–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Theil EC, Goss DJ, Living with iron (and oxygen): questions and answers about iron homeostasis, Chem Rev, 109 (2009) 4568–4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wang JA, Pantopoulos K, Regulation of cellular iron metabolism, Biochem J, 434 (2011) 365–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Latham JA, Barr I, Klinman JP, At the confluence of ribosomally synthesized peptide modification and radical S-adenosylmethionine (SAM) enzymology, J Biol Chem, 292 (2017) 16397–16405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kim YM, Chung HT, Simmons RL, Billiar TR, Cellular non-heme iron content is a determinant of nitric oxide-mediated apoptosis, necrosis, and caspase inhibition, Journal of Biological Chemistry, 275 (2000) 10954–10961. [DOI] [PubMed] [Google Scholar]
- [10].Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR, Ferroptosis: an iron-dependent form of nonapoptotic cell death, Cell, 149 (2012) 10601072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Brown RJ, Gray JD, The mechanism of acute ferrous sulphate poisoning, Can Med Assoc J, 73 (1955) 192–197. [PMC free article] [PubMed] [Google Scholar]
- [12].Britton RS, Leicester KL, Bacon BR, Iron toxicity and chelation therapy, Int J Hematol, 76 (2002) 219–228. [DOI] [PubMed] [Google Scholar]
- [13].Toyokuni S, Uchida K, Okamoto K, Hattori-Nakakuki Y, Hiai H, Stadtman ER, Formation of 4-hydroxy-2-nonenal-modified proteins in the renal proximal tubules of rats treated with a renal carcinogen, ferric nitrilotriacetate, Proc Natl Acad Sci U S A, 91 (1994) 2616–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Harigae H, Iron metabolism and related diseases: an overview, Int J Hematol, 107 (2018) 5–6. [DOI] [PubMed] [Google Scholar]
- [15].Fleming RE, Ponka P, Iron overload in human disease, N Engl J Med, 366 (2012) 348–359. [DOI] [PubMed] [Google Scholar]
- [16].Sochaski MA, Bartfay WJ, Thorpe SR, Baynes JW, Bartfay E, Lehotay DC, Liu PP, Lipid peroxidation and protein modificationin a mouse model of chronic iron overload, Metabolism, 51 (2002) 645–651. [DOI] [PubMed] [Google Scholar]
- [17].Valerio LG Jr., Petersen DR, Characterization of hepatic iron overload following dietary administration of dicyclopentadienyl iron (Ferrocene) to mice: cellular, biochemical, and molecular aspects, Exp Mol Pathol, 68 (2000) 1–12. [DOI] [PubMed] [Google Scholar]
- [18].Kozlov AV, Bini A, Gallesi D, Giovannini F, Iannone A, Masini A, Meletti E, Tomasi A, ‘Free’ iron, as detected by electron paramagnetic resonance spectroscopy, increases unequally in different tissues during dietary iron overload in the rat, Biometals, 9 (1996) 98–103. [DOI] [PubMed] [Google Scholar]
- [19].Biasiotto G, Di Lorenzo D, Archetti S, Zanella I, Iron and Neurodegeneration: Is Ferritinophagy the Link?, Mol Neurobiol, 53 (2016) 5542–5574. [DOI] [PubMed] [Google Scholar]
- [20].Lane DJR, Ayton S, Bush AI, Iron and Alzheimer’s Disease: An Update on Emerging Mechanisms, J Alzheimers Dis, 64 (2018) S379–S395. [DOI] [PubMed] [Google Scholar]
- [21].Moreau C, Duce JA, Rascol O, Devedjian JC, Berg D, Dexter D, Cabantchik ZI, Bush AI, Devos D, F.-I.s. group, Iron as a therapeutic target for Parkinson’s disease, Mov Disord, 33 (2018) 568–574. [DOI] [PubMed] [Google Scholar]
- [22].Hohenberger J, Ray K, Meyer K, The biology and chemistry of high-valent ironoxo and iron-nitrido complexes, Nat Commun, 3 (2012). [DOI] [PubMed] [Google Scholar]
- [23].Golberg L, Martin LE, Batchelor A, Biochemical changes in the tissues of animals injected with iron. 3. Lipid peroxidation, Biochem J, 83 (1962) 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Golberg L, Smith JP, Changes associated with the accumulation of excessive amounts of iron in certain organs of the rat, Br J Exp Pathol, 39 (1958) 59–73. [PMC free article] [PubMed] [Google Scholar]
- [25].Lai CS, Piette LH, Spin-trapping studies of hydroxyl radical production involved in lipid peroxidation, Arch Biochem Biophys, 190 (1978) 27–38. [DOI] [PubMed] [Google Scholar]
- [26].Koppenol WH, The Haber-Weiss cycle−-70 years later, Redox Rep, 6 (2001) 229–234. [DOI] [PubMed] [Google Scholar]
- [27].Vladimirov YA, Free radical lipid peroxidation in biomembranes: Mechanism, regulation, and biological consequences, in: Johnson JE Jr., Walford R, Harman D, Miquel J (Eds.) Free Radicals, Aging, and Degenerative Diseases, Allan R.Liss,Inc., Place Published, 1986, pp. 141–195. [Google Scholar]
- [28].Vladimirov YA, Olenev VI, Suslova TB, Cheremisina ZP, Lipid peroxidation in mitochondrial membrane, Adv.Lipid Res. J1 - ALR, 17 (1980) 173–249. [DOI] [PubMed] [Google Scholar]
- [29].Koskenkorva-Frank TS, Weiss G, Koppenol WH, Burckhardt S, The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: insights into the potential of various iron therapies to induce oxidative and nitrosative stress, Free Radic Biol Med, 65 (2013) 1174–1194. [DOI] [PubMed] [Google Scholar]
- [30].Minotti G, Sources and role of iron in lipid peroxidation, Chem. Res. Toxicol, 6 (1993) 134–146. [DOI] [PubMed] [Google Scholar]
- [31].Vladimirov YA, Proskurnina EV, Free radicals and cell chemiluminescence, Biochemistry (Mosc), 74 (2009) 1545–1566. [DOI] [PubMed] [Google Scholar]
- [32].Boveris A, Oshino N, Chance B, The cellular production of hydrogen peroxide, Biochem J, 128 (1972) 617–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jamieson D, Chance B, Cadenas E, Boveris A, The Relation of Free-Radical Production to Hyperoxia, Annu Rev Physiol, 48 (1986) 703–719. [DOI] [PubMed] [Google Scholar]
- [34].Buxton GV, Greenstock CL, Helman WP, Ross AB, Critical-Review of Rate Constants for Reactions of Hydrated Electrons, Hydrogen-Atoms and Hydroxyl Radicals (.Oh/.O-) in Aqueous-Solution, J Phys Chem Ref Data, 17 (1988) 513–886. [Google Scholar]
- [35].Kellogg EW, Fridovich I, Superoxide, Hydrogen-Peroxide, and Singlet Oxygen in Lipid Peroxidation by a Xanthine-Oxidase System, Journal of Biological Chemistry, 250 (1975) 8812–8817. [PubMed] [Google Scholar]
- [36].Hansen RE, Roth D, Winther JR, Quantifying the global cellular thiol-disulfide status, Proc Natl Acad Sci U S A, 106 (2009) 422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kozlov AV, Lancaster JR, Meszaros AT, Weidinger A, Mitochondria-meditated pathways of organ failure upon inflammation, Redox Biology, 13 (2017) 170–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Keilin D, Hartree EF, Decomposition of Hydrogen Peroxide by Catalase, Nature, 153 (1943) 626. [Google Scholar]
- [39].Toyokuni S, Ito F, Yamashita K, Okazaki Y, Akatsuka S, Iron and thiol redox signaling in cancer: An exquisite balance to escape ferroptosis, Free Radical Bio Med, 108 (2017) 610–626. [DOI] [PubMed] [Google Scholar]
- [40].Yanatori I, Richardson DR, Toyokuni S, Kishi F, The iron chaperone poly(rC)binding protein 2 forms a metabolon with the heme oxygenase 1/cytochrome P450 reductase complex for heme catabolism and iron transfer, Journal of Biological Chemistry, 292 (2017) 13205–13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Theil EC, Mining ferritin iron: 2 pathways, Blood, 114 (2009) 4325–4326. [DOI] [PubMed] [Google Scholar]
- [42].Gutteridge JM, Bleomycin-detectable iron in knee-joint synovial fluid from arthritic patients and its relationship to the extracellular antioxidant activities of caeruloplasmin, transferrin and lactoferrin, Biochem J, 245 (1987) 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lane DJ, Merlot AM, Huang ML, Bae DH, Jansson PJ, Sahni S, Kalinowski DS, Richardson DR, Cellular iron uptake, trafficking and metabolism: Key molecules and mechanisms and their roles in disease, Biochim Biophys Acta, 1853 (2015) 1130–1144. [DOI] [PubMed] [Google Scholar]
- [44].Ohgami RS, Campagna DR, Greer EL, Antiochos B, McDonald A, Chen J, Sharp JJ, Fujiwara Y, Barker JE, Fleming MD, Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells, Nat Genet, 37 (2005) 1264–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ohgami RS, Campagna DR, McDonald A, Fleming MD, The Steap proteins are metalloreductases, Blood, 108 (2006) 1388–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA, Cloning and characterization of a mammalian proton-coupled metal-ion transporter, Nature, 388 (1997) 482–488. [DOI] [PubMed] [Google Scholar]
- [47].Wang CY, Jenkitkasemwong S, Duarte S, Sparkman BK, Shawki A, Mackenzie B, Knutson MD, ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading, J Biol Chem, 287 (2012) 3403234043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zhao N, Gao J, Enns CA, Knutson MD, ZRT/IRT-like protein 14 (ZIP14) promotes the cellular assimilation of iron from transferrin, J Biol Chem, 285 (2010) 32141–32150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].McKie AT, Barrow D, Latunde-Dada GO, Rolfs A, Sager G, Mudaly E, Mudaly M, Richardson C, Barlow D, Bomford A, Peters TJ, Raja KB, Shirali S, Hediger MA, Farzaneh F, Simpson RJ, An iron-regulated ferric reductase associated with the absorption of dietary iron, Science, 291 (2001) 1755–1759. [DOI] [PubMed] [Google Scholar]
- [50].Jenkitkasemwong S, Wang CY, Mackenzie B, Knutson MD, Physiologic implications of metal-ion transport by ZIP14 and ZIP8, Biometals, 25 (2012) 643–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Liuzzi JP, Aydemir F, Nam H, Knutson MD, Cousins RJ, Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells, Proc Natl Acad Sci U S A, 103 (2006) 13612–13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hider RC, Kong XL, Glutathione: a key component of the cytoplasmic labile iron pool, Biometals, 24 (2011) 1179–1187. [DOI] [PubMed] [Google Scholar]
- [53].Philpott CC, Ryu MS, Frey A, Patel S, Cytosolic iron chaperones: Proteins delivering iron cofactors in the cytosol of mammalian cells, J Biol Chem, 292 (2017) 12764–12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Leidgens S, Bullough KZ, Shi H, Li F, Shakoury-Elizeh M, Yabe T, Subramanian P, Hsu E, Natarajan N, Nandal A, Stemmler TL, Philpott CC, Each member of the poly-r(C)-binding protein 1 (PCBP) family exhibits iron chaperone activity toward ferritin, J Biol Chem, 288 (2013) 17791–17802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Shi H, Bencze KZ, Stemmler TL, Philpott CC, A cytosolic iron chaperone that delivers iron to ferritin, Science, 320 (2008) 1207–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Yanatori I, Yasui Y, Tabuchi M, Kishi F, Chaperone protein involved in transmembrane transport of iron, Biochem J, 462 (2014) 25–37. [DOI] [PubMed] [Google Scholar]
- [57].Yanatori I, Richardson DR, Toyokuni S, Kishi F, The iron chaperone poly(rC)binding protein 2 forms a metabolon with the heme oxygenase 1/cytochrome P450 reductase complex for heme catabolism and iron transfer, J Biol Chem, 292 (2017) 13205–13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Nandal A, Ruiz JC, Subramanian P, Ghimire-Rijal S, Sinnamon RA, Stemmler TL, Bruick RK, Philpott CC, Activation of the HIF prolyl hydroxylase by the iron chaperones PCBP1 and PCBP2, Cell Metab, 14 (2011) 647–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Frey AG, Nandal A, Park JH, Smith PM, Yabe T, Ryu MS, Ghosh MC, Lee J, Rouault TA, Park MH, Philpott CC, Iron chaperones PCBP1 and PCBP2 mediate the metallation of the dinuclear iron enzyme deoxyhypusine hydroxylase, Proc Natl Acad Sci U S A, 111 (2014) 8031–8036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Asano T, Komatsu M, Yamaguchi-Iwai Y, Ishikawa F, Mizushima N, Iwai K, Distinct mechanisms of ferritin delivery to lysosomes in iron-depleted and iron-replete cells, Mol Cell Biol, 31 (2011) 2040–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kidane TZ, Sauble E, Linder MC, Release of iron from ferritin requires lysosomal activity, Am J Physiol Cell Physiol, 291 (2006) C445–455. [DOI] [PubMed] [Google Scholar]
- [62].Zhang Y, Mikhael M, Xu D, Li Y, Soe-Lin S, Ning B, Li W, Nie G, Zhao Y, Ponka P, Lysosomal proteolysis is the primary degradation pathway for cytosolic ferritin and cytosolic ferritin degradation is necessary for iron exit, Antioxid Redox Signal, 13 (2010) 999–1009. [DOI] [PubMed] [Google Scholar]
- [63].Dowdle WE, Nyfeler B, Nagel J, Elling RA, Liu S, Triantafellow E, Menon S, Wang Z, Honda A, Pardee G, Cantwell J, Luu C, Cornella-Taracido I, Harrington E, Fekkes P, Lei H, Fang Q, Digan ME, Burdick D, Powers AF, Helliwell SB, D’Aquin S, Bastien J, Wang H, Wiederschain D, Kuerth J, Bergman P, Schwalb D, Thomas J, Ugwonali S, Harbinski F, Tallarico J, Wilson CJ, Myer VE, Porter JA, Bussiere DE, Finan PM, Labow MA, Mao X, Hamann LG, Manning BD, Valdez RA, Nicholson T, Schirle M, Knapp MS, Keaney EP, Murphy LO, Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo, Nat Cell Biol, 16 (2014) 1069–1079. [DOI] [PubMed] [Google Scholar]
- [64].Mancias JD, Vaites L. Pontano, Nissim S, Biancur DE, Kim AJ, Wang X, Liu Y, Goessling W, Kimmelman AC, Harper JW, Ferritinophagy via NCOA4 is required for erythropoiesis and is regulated by iron dependent HERC2-mediated proteolysis, Elife, 4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC, Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy, Nature, 509 (2014) 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Abboud S, Haile DJ, A novel mammalian iron-regulated protein involved in intracellular iron metabolism, J Biol Chem, 275 (2000) 19906–19912. [DOI] [PubMed] [Google Scholar]
- [67].Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, Paw BH, Drejer A, Barut B, Zapata A, Law TC, Brugnara C, Lux SE, Pinkus GS, Pinkus JL, Kingsley PD, Palis J, Fleming MD, Andrews NC, Zon LI, Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter, Nature, 403 (2000) 776–781. [DOI] [PubMed] [Google Scholar]
- [68].McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, Miret S, Bomford A, Peters TJ, Farzaneh F, Hediger MA, Hentze MW, Simpson RJ, A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation, Mol Cell, 5 (2000) 299–309. [DOI] [PubMed] [Google Scholar]
- [69].Yanatori I, Richardson DR, Imada K, Kishi F, Iron Export through the Transporter Ferroportin 1 Is Modulated by the Iron Chaperone PCBP2, J Biol Chem, 291 (2016) 17303–17318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Vulpe CD, Kuo YM, Murphy TL, Cowley L, Askwith C, Libina N, Gitschier J, Anderson GJ, Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse, Nat Genet, 21 (1999) 195–199. [DOI] [PubMed] [Google Scholar]
- [71].Cherukuri S, Potla R, Sarkar J, Nurko S, Harris ZL, Fox PL, Unexpected role of ceruloplasmin in intestinal iron absorption, Cell Metab, 2 (2005) 309–319. [DOI] [PubMed] [Google Scholar]
- [72].Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascon S, Hatzios SK, Kagan VE, Noel K, Jiang X, Linkermann A, Murphy ME, Overholtzer M, Oyagi A, Pagnussat GC, Park J, Ran Q, Rosenfeld CS, Salnikow K, Tang D, Torti FM, Torti SV, Toyokuni S, Woerpel KA, Zhang DD, Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease, Cell, 171 (2017) 273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Ghezzi P, Jaquet V, Marcucci F, Schmidt H, The oxidative stress theory of disease: levels of evidence and epistemological aspects, Br J Pharmacol, 174 (2017) 1784–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Egea J, Fabregat I, Frapart YM, Ghezzi P, Gorlach A, Kietzmann T, Kubaichuk K, Knaus UG, Lopez MG, Olaso-Gonzalez G, Petry A, Schulz R, Vina J, Winyard P, Abbas K, Ademowo OS, Afonso CB, Andreadou I, Antelmann H, Antunes F, Aslan M, Bachschmid MM, Barbosa RM, Belousov V, Berndt C, Bernlohr D, Bertran E, Bindoli A, Bottari SP, Brito PM, Carrara G, Casas AI, Chatzi A, Chondrogianni N, Conrad M, Cooke MS, Costa JG, Cuadrado A, Dang P. MyChan, De Smet B, Debelec-Butuner B, Dias IHK, Dunn JD, Edson AJ, El Assar M, El-Benna J, Ferdinandy P, Fernandes AS, Fladmark KE, Forstermann U, Giniatullin R, Giricz Z, Gorbe A, Griffiths H, Hampl V, Hanf A, Herget J, Hernansanz-Agustin P, Hillion M, Huang J, Ilikay S, Jansen-Durr P, Jaquet V, Joles JA, Kalyanaraman B, Kaminskyy D, Karbaschi M, Kleanthous M, Klotz LO, Korac B, Korkmaz KS, Koziel R, Kracun D, Krause KH, Kren V, Krieg T, Laranjinha J, Lazou A, Li H, Martinez-Ruiz A, Matsui R, McBean GJ, Meredith SP, Messens J, Miguel V, Mikhed Y, Milisav I, Milkovic L, Miranda-Vizuete A, Mojovic M, Monsalve M, Mouthuy PA, Mulvey J, Munzel T, Muzykantov V, Nguyen ITN, Oelze M, Oliveira NG, Palmeira CM, Papaevgeniou N, Pavicevic A, Pedre B, Peyrot F, Phylactides M, Pircalabioru GG, Pitt AR, Poulsen HE, Prieto I, Rigobello MP, Robledinos-Anton N, Rodriguez-Manas L, Rolo AP, Rousset F, Ruskovska T, Saraiva N, Sasson S, Schroder K, Semen K, Seredenina T, Shakirzyanova A, Smith GL, Soldati T, Sousa BC, Spickett CM, Stancic A, Stasia MJ, Steinbrenner H, Stepanic V, Steven S, Tokatlidis K, Tuncay E, Turan B, Ursini F, Vacek J, Vajnerova O, Valentova K, Van Breusegem F, Varisli L, Veal EA, Yalcin AS, Yelisyeyeva O, Zarkovic N, Zatloukalova M, Zielonka J, Touyz RM, Papapetropoulos A, Grune T, Lamas S, Schmidt H, Di Lisa F, Daiber A, European contribution to the study of ROS: A summary of the findings and prospects for the future from the COST action BM1203 (EU-ROS), Redox Biol, 13 (2017) 94–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Imai H, [Biological significance of lipid hydroperoxide and its reducing enzyme, phospholipid hydroperoxide glutathione peroxidase, in mammalian cells], Yakugaku Zasshi, 124 (2004) 937–957. [DOI] [PubMed] [Google Scholar]
- [76].May JM, Morrow JD, Burk RF, Thioredoxin reductase reduces lipid hydroperoxides and spares alpha-tocopherol, Biochem Biophys Res Commun, 292 (2002) 45–49. [DOI] [PubMed] [Google Scholar]
- [77].Bjornstedt M, Hamberg M, Kumar S, Xue J, Holmgren A, Human thioredoxin reductase directly reduces lipid hydroperoxides by NADPH and selenocystine strongly stimulates the reaction via catalytically generated selenols, J Biol Chem, 270 (1995) 11761–11764. [DOI] [PubMed] [Google Scholar]
- [78].Davies MJ, Slater TF, Studies on the metal-ion and lipoxygenase-catalysed breakdown of hydroperoxides using electron-spin-resonance spectroscopy, Biochem J, 245 (1987) 167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Dalleau S, Baradat M, Gueraud F, Huc L, Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance, Cell Death Differ, 20 (2013) 1615–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, Kapralov AA, Amoscato AA, Jiang J, Anthonymuthu T, Mohammadyani D, Yang Q, Proneth B, Klein-Seetharaman J, Watkins S, Bahar I, Greenberger J, Mallampalli RK, Stockwell BR, Tyurina YY, Conrad M, Bayir H, Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis, Nat Chem Biol, 13 (2017) 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wenzel SE, Tyurina YY, Zhao J, Croix CM St, Dar HH, Mao G, Tyurin VA, Anthonymuthu TS, Kapralov AA, Amoscato AA, Mikulska-Ruminska K, Shrivastava IH, Kenny EM, Yang Q, Rosenbaum JC, Sparvero LJ, Emlet DR, Wen X, Minami Y, Qu F, Watkins SC, Holman TR, VanDemark AP, Kellum JA, Bahar I, Bayir H, Kagan VE, PEBP1 Wardens Ferroptosis by Enabling Lipoxygenase Generation of Lipid Death Signals, Cell, 171 (2017) 628–641 e626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Dar HH, Tyurina YY, Mikulska-Raminska K, Shrivastava I, Ting H, Tyurin VA, Krieger J, Croix CM St., Watkins S, Bayir E, Mao G, Ambruster C, Kapralov A, Wang H, Parsek MR, Anthonymuthu TS, Ogunsola AF, Flitter BA, Freedman CJ, Gaston JR, Holman T, Pilewski JM, Greenberger JS, Mallampalli RK, Doi Y, Lee JS, Bahar I, Bomberger J, Bayır H, Kagan1 VE, Pseudomonas aeruginosa utilizes host polyunsaturated phosphatidylethanolamines to trigger theft-ferroptosis in bronchial epithelium, J. Clin. Invest, In Press (2018). [DOI] [PMC free article] [PubMed]
- [83].Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A, Prokisch H, Trumbach D, Mao G, Qu F, Bayir H, Fullekrug J, Scheel CH, Wurst W, Schick JA, Kagan VE, Angeli JP, Conrad M, ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition, Nat Chem Biol, 13 (2017) 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zilka O, Shah R, Li B, Friedmann Angeli JP, Griesser M, Conrad M, Pratt DA, On the Mechanism of Cytoprotection by Ferrostatin-1 and Liproxstatin-1 and the Role of Lipid Peroxidation in Ferroptotic Cell Death, ACS Cent Sci, 3 (2017) 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR, Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis, Proc Natl Acad Sci U S A, 113 (2016) E4966–4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Angeli JPF, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, Basavarajappa D, Radmark O, Kobayashi S, Seibt T, Beck H, Neff F, Esposito I, Wanke R, Forster H, Yefremova O, Heinrichmeyer M, Bornkamm GW, Geissler EK, Thomas SB, Stockwell BR, O’Donnell VB, Kagan VE, Schick JA, Conrad M, Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice, Nat Cell Biol, 16 (2014) 1180–U1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Hayano M, Yang WS, Corn CK, Pagano NC, Stockwell BR, Loss of cysteinyltRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation, Cell Death Differ, 23 (2016) 270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Xie Y, Song X, Sun X, Huang J, Zhong M, Lotze MT, Zeh HJR, Kang R, Tang D, Identification of baicalein as a ferroptosis inhibitor by natural product library screening, Biochem Biophys Res Commun, 473 (2016) 775–780. [DOI] [PubMed] [Google Scholar]
- [89].Li Q, Han X, Lan X, Gao Y, Wan J, Durham F, Cheng T, Yang J, Wang Z, Jiang C, Ying M, Koehler RC, Stockwell BR, Wang J, Inhibition of neuronal ferroptosis protects hemorrhagic brain, JCI Insight, 2 (2017) e90777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Goldman SDB, Funk RS, Rajewski RA, Krise JP, Mechanisms of amine accumulation in, and egress from, lysosomes, Bioanalysis, 1 (2009) 1445–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Kaufmann AM, Krise JP, Lysosomal sequestration of amine-containing drugs: Analysis and therapeutic implications, J Pharm Sci-Us, 96 (2007) 729–746. [DOI] [PubMed] [Google Scholar]
- [92].Uchiyama A, Kim JS, Kon K, Jaeschke H, Ikejima K, Watanabe S, Lemasters JJ, Translocation of Iron from Lysosomes into Mitochondria Is a Key Event During Oxidative Stress-Induced Hepatocellular Injury, Hepatology, 48 (2008) 16441654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Yu ZQ, Persson HL, Eaton JW, Brunk UT, Intralysosomal iron: A major determinant of oxidant-induced cell death, Free Radical Bio Med, 34 (2003) 1243–1252. [DOI] [PubMed] [Google Scholar]
- [94].Persson HL, Yu Z, Tirosh O, Eaton JW, Brunk UT, Prevention of oxidantinduced cell death by lysosomotropic iron chelators, Free Radic Biol Med, 34 (2003) 1295–1305. [DOI] [PubMed] [Google Scholar]
- [95].Starke PE, Gilbertson JD, Farber JL, Lysosomal origin of the ferric iron required for cell killing by hydrogen peroxide, Biochem Biophys Res Commun, 133 (1985) 371379. [DOI] [PubMed] [Google Scholar]
- [96].Torii S, Shintoku R, Kubota C, Yaegashi M, Torii R, Sasaki M, Suzuki T, Mori M, Yoshimoto Y, Takeuchi T, Yamada K, An essential role for functional lysosomes in ferroptosis of cancer cells, Biochem J, 473 (2016) 769–777. [DOI] [PubMed] [Google Scholar]
- [97].Nam W, High-valent iron(IV)-oxo complexes of heme and non-heme ligands in oxygenation reactions, Acc Chem Res, 40 (2007) 522–531. [DOI] [PubMed] [Google Scholar]
- [98].Kuroda Y, Tanaka N, Goto M, Sakai T, Low-Spin Tetracyanoferrate(Ii) and Tetracyanoferrate(Iii) Complexes of Meso-Type 1,2-Diamines - Synthesis and Steric Effects on Chelate-Ring Conformation and Rate of Ligand Dehydrogenation, Inorg Chem, 28 (1989) 2163–2169. [Google Scholar]
- [99].Kiskin MA, Aleksandrov GG, Dobrokhotova ZV, Novotortsev VM, Shvedenkov YG, Eremenko IL, Transformations of high spin Mn-II and Fe-II polymeric pivalates in reactions with pivalic acid and o-phenylenediamines, Russ Chem B+, 55 (2006) 806–820. [Google Scholar]
- [100].Kuroda Y, Tanaka N, Goto M, Sakai T, Conformational Interconversion Rates of 1,2-Diamine Chelates: Determination by Paramagnetic NMR Spectra of Low-Spin Iron (III) Complexes, Inorg. Chem, 28 (1989) 977–1003. [Google Scholar]
- [101].Chlopek K, Bill E, Weyhermuller T, Wieghardt K, Molecular and electronic structure of five-coordinate complexes of iron(II/III) containing odiiminobenzosemiquinonate(1-) pi radical ligands, Inorg Chem, 44 (2005) 7087–7098. [DOI] [PubMed] [Google Scholar]
- [102].Paschke J, Kirsch N, Korth HG, de Groot H, Sustmann R, Catalase-like activity of a non-heme dibenzotetraaza[14]annulene-Fe(III) complex under physiological conditions, J Am Chem Soc, 123 (2001) 11099–11100. [DOI] [PubMed] [Google Scholar]
- [103].Rauen U, Kettler-Thiel T, de Groot H, Korth HG, Sustmann R, Conversion of the synthetic catalase mimic precursor TAA-1 into the active catalase mimic in isolated hepatocytes, Chem Biol Drug Des, 73 (2009) 494–501. [DOI] [PubMed] [Google Scholar]
- [104].Tōgō H, Advanced free radical reactions for organic synthesis, 1st ed., Elsevier, Place Published, 2004. [Google Scholar]
- [105].Sato E, Kohno M, Nakashima T, Niwano Y, Ciclopirox olamine directly scavenges hydroxyl radical, Int J Dermatol, 47 (2008) 15–18. [DOI] [PubMed] [Google Scholar]
- [106].Bartesaghi S, Trujillo M, Denicola A, Folkes L, Wardman P, Radi R, Reactions of desferrioxamine with peroxynitrite-derived carbonate and nitrogen dioxide radicals, Free Radic Biol Med, 36 (2004) 471–483. [DOI] [PubMed] [Google Scholar]
- [107].Davies MJ, Donkor R, Dunster CA, Gee CA, Jonas S, Willson RL, Desferrioxamine (Desferal) and superoxide free radicals. Formation of an enzymedamaging nitroxide, Biochem J, 246 (1987) 725–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Leem SH, Park JE, Kim IS, Chae JY, Sugino A, Sunwoo Y, The possible mechanism of action of ciclopirox olamine in the yeast Saccharomyces cerevisiae, Mol Cells, 15 (2003) 55–61. [PubMed] [Google Scholar]
- [109].Yamamoto K, Niki E, Interaction of alpha-tocopherol with iron: antioxidant and prooxidant effects of alpha-tocopherol in the oxidation of lipids in aqueous dispersions in the presence of iron, Biochim Biophys Acta, 958 (1988) 19–23. [DOI] [PubMed] [Google Scholar]
- [110].Cao Q, Dornan LM, Rogan L, Hughes NL, Muldoon MJ, Aerobic oxidation catalysis with stable radicals, Chem Commun (Camb), 50 (2014) 4524–4543. [DOI] [PubMed] [Google Scholar]
- [111].Thompson DC, Cha YN, Trush MA, The peroxidase-dependent activation of butylated hydroxyanisole and butylated hydroxytoluene (BHT) to reactive intermediates. Formation of BHT-quinone methide via a chemical-chemical interaction, J Biol Chem, 264 (1989) 3957–3965. [PubMed] [Google Scholar]
- [112].Fornera S, Yazawa K, Walde P, Spectrophotometric quantification of lactose in solution with a peroxidase-based enzymatic cascade reaction system, Anal Bioanal Chem, 401 (2011) 2307–2310. [DOI] [PubMed] [Google Scholar]
- [113].Czapski GA, Czubowicz K, Strosznajder RP, Evaluation of the antioxidative properties of lipoxygenase inhibitors, Pharmacol Rep, 64 (2012) 1179–1188. [DOI] [PubMed] [Google Scholar]
- [114].Rush JD, Maskos Z, Koppenol WH, Distinction between Hydroxyl Radical and Ferryl Species, Method Enzymol, 186 (1990) 148–156. [DOI] [PubMed] [Google Scholar]
- [115].Rush JD, Koppenol WH, Oxidizing intermediates in the reaction of ferrous EDTA with hydrogen peroxide. Reactions with organic molecules and ferrocytochrome c, J Biol Chem, 261 (1986) 6730–6733. [PubMed] [Google Scholar]
- [116].Rush JD, Maskos Z, Koppenol WH, Distinction between hydroxyl radical and ferryl species, Methods Enzymol, 186 (1990) 148–156. [DOI] [PubMed] [Google Scholar]
- [117].Makhlynets OV, Das P, Taktak S, Flook M, Mas-Balleste R, RybakAkimova EV, Que L, Iron-Promoted ortho- and/or ipso-Hydroxylation of Benzoic Acids with H2O2, Chem-Eur J, 15 (2009) 13171–13180. [DOI] [PubMed] [Google Scholar]
- [118].Jensen MP, Lange SJ, Mehn MP, Que EL, Que L, Biomimetic aryl hydroxylation derived from alkyl hydroperoxide at a nonheme iron center. Evidence for an Fe-IV=O oxidant, J Am Chem Soc, 125 (2003) 2113–2128. [DOI] [PubMed] [Google Scholar]
- [119].Kim YJ, Feng XD, Lippard SJ, Synthesis, structure, and properties of a mixedvalent triiron complex of tetramethyl reductic acid, an ascorbic acid analogue, and its relationship to a functional non-heme iron oxidation catalyst system, Inorg Chem, 46 (2007) 6099–6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Kleespies ST, Oloo WN, Mukherjee A, Que L, C-H Bond Cleavage by Bioinspired Nonheme Oxoiron(IV) Complexes, Including Hydroxylation of n-Butane, Inorg Chem, 54 (2015) 5053–5064. [DOI] [PubMed] [Google Scholar]
- [121].Kumar D, Hirao H, Que L, Shaik S, Theoretical investigation of C-H hydroxylation by (N4Py)Fe-IV=O2+ : An oxidant more powerful than p450?, J Am Chem Soc, 127 (2005) 8026–8027. [DOI] [PubMed] [Google Scholar]
- [122].Cusso O, Garcia-Bosch I, Ribas X, Lloret-Fillol J, Costas M, Asymmetric Epoxidation with H2O2 by Manipulating the Electronic Properties of Non-heme Iron Catalysts, J Am Chem Soc, 135 (2013) 14871–14878. [DOI] [PubMed] [Google Scholar]
- [123].Oloo WN, Que L, Bioinspired Nonheme Iron Catalysts for C-H and C=C Bond Oxidation: Insights into the Nature of the Metal-Based Oxidants, Accounts Chem Res, 48 (2015) 2612–2621. [DOI] [PubMed] [Google Scholar]
- [124].Shan XP, Que L, High-valent nonheme iron-oxo species in biomimetic oxidations, J Inorg Biochem, 100 (2006) 421–433. [DOI] [PubMed] [Google Scholar]
- [125].Stoyanovsky DA, Sparvero LJ, Amoscato AA, He RR, Watkins S, Pitt BR, Bayir H, Kagan VE, Improved spatial resolution of matrix-assisted laser desorption/ionization imaging of lipids in the brain by alkylated derivatives of 2,5dihydroxybenzoic acid, Rapid Commun Mass Spectrom, 28 (2014) 403–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Stoyanovsky DA, Salama G, Kagan VE, Ascorbate/iron activates Ca(2+)release channels of skeletal sarcoplasmic reticulum vesicles reconstituted in lipid bilayers, Arch Biochem Biophys, 308 (1994) 214–221. [DOI] [PubMed] [Google Scholar]
- [127].Li G, Pone EJ, Tran DC, Patel PJ, Dao L, Xu Z, Casali P, Iron inhibits activation-induced cytidine deaminase enzymatic activity and modulates immunoglobulin class switch DNA recombination, J Biol Chem, 287 (2012) 2152021529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Cabantchik ZI, Labile iron in cells and body fluids: physiology, pathology, and pharmacology, Front Pharmacol, 5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Kriska T, Cepura C, Magier D, Siangjong L, Gauthier KM, Campbell WB, Mice lacking macrophage 12/15-lipoxygenase are resistant to experimental hypertension, Am J Physiol Heart Circ Physiol, 302 (2012) H2428–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]


