Abstract
Titanium dioxide is the only known material that can enable gas-phase CO2 photocatalysis in its anatase and rutile polymorphic forms. Materials engineering of polymorphism provides a useful strategy for optimizing the performance metrics of a photocatalyst. In this paper, it is shown that the less well known rhombohedral polymorph of indium sesquioxide, like its well-documented cubic polymorph, is a CO2 hydrogenation photocatalyst for the production of CH3OH and CO. Significantly, the rhombohedral polymorph exhibits higher activity, superior stability and improved selectivity towards CH3OH over CO. These gains in catalyst performance originate in the enhanced acidity and basicity of surface frustrated Lewis pairs in the rhombohedral form.
Subject terms: Heterogeneous catalysis, Photocatalysis, Nanoparticles
Polymorphs, compounds with identical chemical stoichiometries yet different atomic configurations, expand the range of potential chemical properties and new applications. Here, authors show rhombohedral indium oxides to be highly active and selective for photocatalytic CO2 hydrogenation.
Introduction
A breakthrough in CO2 photocatalysis often begins with materials discovery, and then, the challenges of optimizing its performance metrics by materials engineering follows. Through human intelligence and experiential learning, complemented by artificial intelligence and machine learning, one can hone the chemical and physical properties of a material to achieve the desired catalyst optimization for a targeted technology.
In the case of gas-phase heterogeneous hydrogenation of CO2 to chemicals and fuels, performance optimization usually involves fine tuning the chemical and physical properties of the (photo)catalyst1–3. This can be achieved by doping4,5, isomorphous and aliovalent substitution6,7, size control8,9, morphology changes10,11, heterostructuring12–14, and support and promoter effects15–17.
In this endeavor, a strategy rarely employed in catalyst optimization is polymorph selection, whereby the crystal structure of the catalyst material is changed, whereas its composition is retained. The challenge today is moving beyond titanium dioxide anatase and rutile polymorphs and discovering other compositions that exhibit polymorphism and enable CO2 (photo)catalysis.
Indium-based semiconductor materials have been reported as promising photocatalysts in terms of their geometric structures and electronic configurations, which can possibly enhance the mobility and separation efficiency of charge carriers and improve photocatalytic activity18,19. Numerous attempts have been made in recent years to develop various indium-based photocatalysts, such as oxides or mixed oxides20,21, binary and ternary sulfides22,23, hydroxides and oxyhydroxides24,25, In-MOF26, and so on. Among them, indium oxide (In2O3) is an n-type semiconductor with mainly two polymorphic phases, cubic and metastable rhombohedral phases27. Pan et al.28 and co-workers have demonstrated that cubic In2O3 nanobelts coated by carbon layer showed highly enhanced photocatalytic reduction of CO2 to CO and CH4 in aqueous solution with Pt as co-catalyst. Our group has reported that the cubic indium oxide nanocrystals with surface defects in the form of oxygen vacancies and hydroxyl groups, denoted as In2O3-x(OH)y, can be utilized as an active catalyst towards photocatalytic gaseous CO2 hydrogenation owing to advantageous surface, optical, and electronic properties29. The CO2 hydrogenation performance of the cubic In2O3-x(OH)y polymorph can be further boosted by assembling nanocrystals into rod-like superstructures, which gives rise to an improved conversion rate for the reverse water gas shift reaction and a champion rate for solar methanol production at atmospheric pressure10,30.
Herein, we have discovered that the rhombohedral form of indium oxide, like its cubic polymorph, is a highly active and selective heterogeneous (photo)catalyst for hydrogenating gaseous CO2 to CO and CH3OH. Significantly, the rhombohedral polymorph outperforms the cubic polymorph in terms of its catalytic activity, long-term stability, and selectivity towards CH3OH. These performance enhancements stem from an increase in the acidity and basicity of surface frustrated Lewis pairs (SFLP) in the rhombohedral compared with the cubic polymorph.
Results
Synthesis and structural characterizations of rhombohedral In2O3-x(OH)y
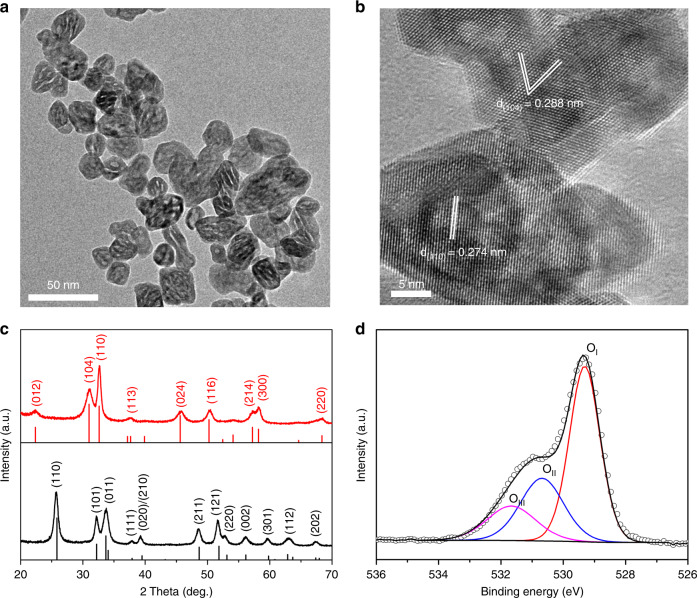
The rhombohedral In2O3-x(OH)y nanocrystals (annealing at 350 °C and denoted as rh-In2O3-x(OH)y) were synthesized via a thermal dehydration of an InOOH precursor, which was initially synthesized from a simple solvothermal system31. The obtained InOOH precursor is composed of monodispersed nanoparticles with the size of ~ 20 nm (Supplementary Fig. 1). After thermal treatment at 350 °C, the resultant rh-In2O3-x(OH)y nanocrystals maintained a similar particle size and exhibited a walnut shell-like morphology, which contained considerable nanopores in each nanocrystal (Fig. 1a). Such unique morphology could be caused by the loss of water from the lattice of the InOOH nanocrystal, which was confirmed by thermogravimetry (TG) and derivative thermogravimetry (DTG) analysis (Supplementary Fig. 2). In this case, the presence of these nanopores did not contribute much to the specific surface area of rh-In2O3-x(OH)y. As a result, the as-prepared rh-In2O3-x(OH)y has a specific surface area of 56 m2 g−1 and a pore size of 11 nm (Supplementary Fig. 3). The high-resolution transmission electron microscopy (HRTEM) images (Fig. 1b and Supplementary Fig. 4) indicate well-defined lattice fringes with inter-planar distances of 0.274 nm and 0.288 nm, which correspond to the (110) and (104) facets of rhombohedral In2O3, respectively. The powder X-ray diffraction (PXRD) patterns confirm that the precursor is orthorhombic InOOH, and leads to the formation of the corundum structure type of In2O3 (Fig. 1c). An obvious shift in binding energy was observed from the high-resolution X-ray photoelectron spectroscopy (XPS) of In 3d core level spectra for samples before and after annealing, indicating the conversion from InOOH to In2O3-x(OH)y (Supplementary Fig. 5). The O 1 s core level XPS spectra (Fig. 1d) could be fitted into three peaks at 529.3 eV, 530.7 eV, and 531.8 eV, which can be assigned to oxides, oxygen vacancies, and hydroxyl groups, respectively29. The existence of oxygen vacancies is further evidenced by a strong diagnostic luminescent peak centered at ca. 500 nm in the PL spectrum (Supplementary Fig. 6). These results confirm the formation of oxygen vacancies as well as the coexistence of oxides, vacancies, and hydroxyl groups in rh-In2O3-x(OH)y, which play a key role in the formation of SFLP32–34.
Fig. 1.
Structural characterizations of InOOH precursor and rhombohedral In2O3-x(OH)y nanocrystals. a TEM image of rh-In2O3-x(OH)y nanocrystals. b HRTEM image of rh-In2O3-x(OH)y nanocrystals. c PXRD patterns of InOOH precursor (black) and rh-In2O3-x(OH)y nanocrystals (red). d High-resolution O 1 s core level XPS spectra for rh-In2O3-x(OH)y nanocrystals
CO2 hydrogenation performance
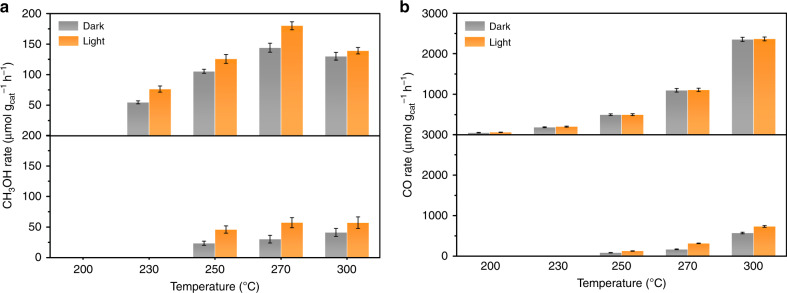
The catalytic properties of the as-obtained rh-In2O3-x(OH)y nanocrystals toward CO2 hydrogenation with and without solar irradiation were evaluated in a flow reactor at different temperatures, under atmospheric pressure with a mixed feed gas of CO2 and H2 (H2: CO2 = 3: 1) (Fig. 2a, b). To investigate the polymorph effect of indium oxide on CO2 hydrogenation performance, the cubic In2O3-x(OH)y nanocrystals (annealing at 300 °C and denoted as c-In2O3-x(OH)y) with similar porous morphology and crystalline size (Supplementary Fig. 7) were synthesized based on previous studies29 and utilized as a reference material for comparison. The catalytic performance of the selected photocatalysts was tested from 200 °C to 300 °C. Only the as-prepared rh-In2O3-x(OH)y was found to be able to catalyze the reverse water gas shift (RWGS) reaction at 200 °C with a CO rate of 51 μmol gcat−1 h−1 and 60 μmol gcat−1 h−1 for dark and light conditions, respectively. When the reaction temperature was increased to 230 °C, the rh-In2O3-x(OH)y showed an increased CO formation rate of 185 μmol gcat−1 h−1 (in dark) and 201 μmol gcat−1 h−1 (in light). Furthermore, at this specific temperature, only the rh-In2O3-x(OH)y was found to be capable of synthesizing CH3OH with a rate of 55 μmol gcat−1 h−1 (in dark) and 76 μmol gcat−1 h−1 (in light).
Fig. 2.
Catalytic performance of rh-In2O3-x(OH)y (up) and c-In2O3-x(OH)y (down). a CH3OH production rate at different reaction temperatures with and without solar irradiation. b CO production rate at different reaction temperatures with and without solar irradiation
When the temperature was increased to 250 °C, the rh-In2O3-x(OH)y exhibited a CH3OH rate of 105 μmol gcat−1 h−1 (in dark) and 126 μmol gcat−1 h−1 (in light). Thereafter, increasing the reaction temperature to 270 °C resulted in a CH3OH formation rate of 180 μmol gcat−1 h−1 and 144 μmol gcat−1 h−1 with and without light irradiation. Such a solar powered CH3OH rate of 180 μmol gcat−1 h−1 is a performance record35–38 and about two times higher than the best reported solar CH3OH maker (c-In2O3-x(OH)y nanorods)30 and 3.5 times higher than the reference c-In2O3-x(OH)y nanocrystals at similar reaction conditions. If catalytic performance is normalized on the basis of the specific surface area, the rh-In2O3-x(OH)y would show a solar CH3OH rate of 3.2 μmol h−1 m−2, which is ~ 5.8 times higher than that of c-In2O3-x(OH)y nanorods, or 8.6 times higher than that of c-In2O3-x(OH)y nanocrystals. A control experiment shows that the CH3OH selectivity of the commercial thermal catalyst (alumina supported copper zinc oxide) under the same photothermal reaction conditions and ambient pressure is ∼ 0.4 %, which is 32 times lower than rh-In2O3-x(OH)y. Although the CH3OH productivity of rh-In2O3-x(OH)y under high pressure needs further research, the rh-In2O3-x(OH)y shows great potential for the future development of a solar fuels economy. Compared with traditional thermocatalytic technologies, solar-driven photocatalytic and photothermal catalytic CO2 hydrogenation are proving to be promising strategies as they enable the utilization of abundant and clean solar energy, and during the catalytic process the photoexcitation of electrons into higher-energy states can lower the energy barrier of reaction39.
Owing to the exothermic nature of the CH3OH synthesis from H2-CO2, the CH3OH rate at 300 °C decreases slightly to 139 μmol gcat−1 h−1 (in light) and 130 μmol gcat−1 h−1 (in dark). The enhancement of the CH3OH rate with light irradiation can be attributed to the lower activation energy of the photocatalytic process as compared with the thermochemical process40. The Arrhenius plots over rh-In2O3-x(OH)y (Supplementary Fig. 8a) yield the apparent activation energy for CH3OH production reaction, photocatalytically, at 38.4 kJ mol−1, much smaller than 59.8 kJ mol−1 for the thermochemical reaction. Moreover, the activation energy for CH3OH production over rh-In2O3-x(OH)y is much lower than that of c-In2O3-x(OH)y under the same conditions (Supplementary Fig. 8b) and also lower than the reported value (103 kJ mol−1) for cubic indium oxide under dark condition41.
To further support that CH3OH production mainly proceeds through a photochemistry process, we also examined the dependence of CH3OH rate on the wavelength of incident light. The results showed that the CH3OH rate decreased with decreasing wavelength of the light (Supplementary Fig. 9), which matches well with the optical absorption spectra of rh-In2O3-x(OH)y. The photo-enhancement for CH3OH production almost disappeared when a 500 nm cutoff filter near the absorption edge was applied for the same light intensity.
Conversely, owing to the endothermic nature of the RWGS reaction, the CO rate is dramatically enhanced with increasing temperature and reaches about 2.4 mmol gcat−1 h−1 at 300 °C. Such a CO formation rate is comparable to some of the most active noble metal decorated catalysts13,42 and ~ 3.2 times higher than that of the reference c-In2O3-x(OH)y. Unlike CH3OH performance, which can be enhanced by the light irradiation, CO performance was only enhanced by ∼ 0.5 % under light irradiation (activation energy of ∼ 84.8 kJ mol−1 for both light and dark), which results in an enhanced solar CH3OH selectivity (Supplementary Fig. 10). The distinct light-dependent trend reveals that the rate determining step for the production of CO appears to occur mainly by a thermochemical pathway in the electronic ground state, whereas the production of methanol has a contributing photochemical pathway involving electrons and holes in the electronic excited state thereby enhancing the activity of the SFLP. Moreover, to confirm the veracity of the CO and CH3OH products from CO2, isotope tracing experiments were conducted, where the 13CO2 feedstock was utilized and the product gases were analyzed by gas chromatography–mass spectrometry (GC-MS), which confirmed the presence of 13CO and 13CH3OH and the verity of the CO2 derived products (Supplementary Fig. 11).
Tuning of SFLP toward CO2 hydrogenation on rhombohedral In2O3-x(OH)y
The structural parameters including concentration of oxygen vacancies and hydroxides are of great importance in forming the SFLP, and subsequently influence the photocatalytic performance. Accordingly, a series of rh-In2O3-x(OH)y nanocrystals were synthesized from the InOOH precursor with different annealing temperatures of 250, 300, 350, and 400 °C, and denoted as rh-250, rh-300, rh-350, and rh-400, respectively. PXRD patterns are found to be similar for samples annealed above 300 °C and could be assigned to the corundum structure type of In2O3 (Supplementary Fig. 12). Interestingly, the sample annealed at 250 °C exhibited a similar PXRD pattern to the InOOH precursor, which indicated a similar structure to InOOH, but with more oxygen vacancies and less hydroxide groups. The grain sizes for the prepared rh-In2O3-x(OH)y nanocrystals showed a gradual decrease with elevated annealing temperatures, along with more nanopores generated during the dehydroxylation reaction (Supplementary Figs. 13 and 14). The specific surface areas of the rh-In2O3-x(OH)y nanocrystals are slightly larger than the precursor InOOH, but are lower than the c-In2O3-x(OH)y. All rh-In2O3-x(OH)y samples show the same In(III) oxidation state, and the concentration of oxygen vacancies increases with increasing annealing temperatures, whereas the concentration of hydroxides decreases simultaneously (Supplementary Fig. 15). The band-edge absorption for all rh-In2O3-x(OH)y nanocrystals and c-In2O3-x(OH)y is located at ~ 450 nm, indicating similar electronic band structures (Supplementary Fig. 16).
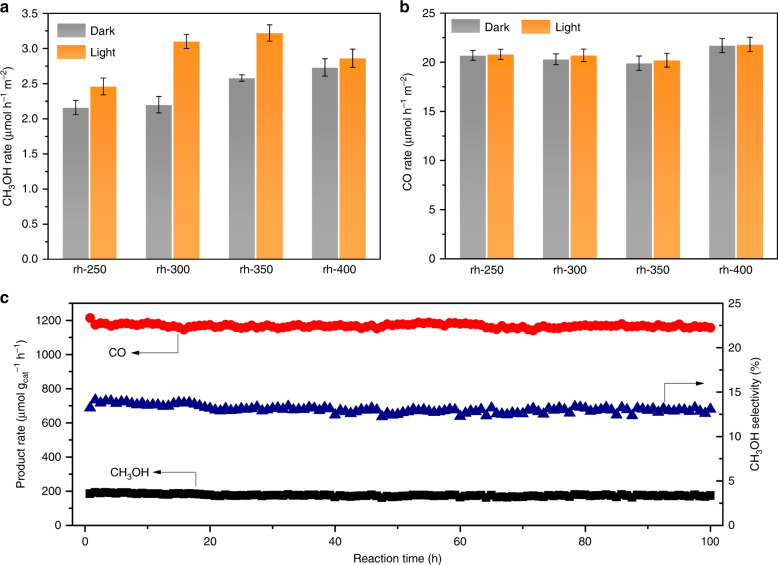
The normalized catalytic activity for the various surface tuned rh-In2O3-x(OH)y nanocrystals toward CO2 hydrogenation, at 270 °C and 300 °C, with and without illumination, are shown in Fig. 3a, b, Supplementary Fig. 17 and summarized in Table 1. Although the rh-250 still showed similar PXRD patterns to InOOH, the thermal treatment was able to remove some lattice oxygen as well as hydroxide groups, which may result in the formation of SFLP and exhibit catalytic performance toward CO2 hydrogenation. Other than the rh-250, the as-prepared rh-300, rh-350 and rh-400 exhibited diagnostic PXRD patterns, which can be assigned to the rhombohedral In2O3 structure with major exposed facets of (110) and (104) (Supplementary Fig. 18). The calculated grain size of rh-In2O3-x(OH)y were ~ 11.5 ± 1.5 nm for all selected samples. All samples exhibited similar structural and morphological properties as well as photocatalytic performance, whereas the rh-350 showed the best catalytic performance.
Fig. 3.
Catalytic performance of various rh-In2O3-x(OH)y nanocrystals. a Normalized CH3OH production rate at 270 °C with and without light irradiation. b Normalized CO production rate at 270 °C with and without light irradiation. c Long-term (100 h) catalytic stability of rh-In2O3-x(OH)y nanocrystals (rh-350) in catalyzing hydrogenation of CO2 with light irradiation; reaction condition: 270 °C, 6 ml min−1 H2 and 2 ml min−1 CO2
Table 1.
Summary of properties of various In2O3-x(OH)y samples
| Sample | Da | Ab | Egc | Ovd | OHe | RCOf | RMethanolg |
|---|---|---|---|---|---|---|---|
| rh-250 | 13 | 44 | 3.15 | 20.01 | 31.87 | 55.57 | 2.47 |
| rh-300 | 13 | 54 | 3.01 | 21.35 | 20.64 | 44.87 | 3.10 |
| rh-350 | 11 | 56 | 2.89 | 26.88 | 17.96 | 42.14 | 3.21 |
| rh-400 | 10 | 51 | 2.88 | 30.00 | 16.40 | 38.31 | 2.86 |
| c-In2O3-x(OH)y | 9.5 | 138 | 2.89 | 23.37 | 19.09 | 5.31 | 0.37 |
aGrain size calculated from PXRD patterns (nm)
bSpecific surface area obtained from BET measurement (m2 g−1)
cBand gap energy (eV) calculated by fitting the reflectance spectra using K–M theory
dConcentration of oxygen vacancies calculated from XPS (at. %)
eConcentration of hydroxide groups calculated from XPS (at. %)
fNormalized CO rate with solar irradiation obtained at 300 °C (μmol h−1 m−2)
gNormalized CH3OH rate with solar irradiation obtained at 270 °C (μmol h−1 m−2)
The CO2 adsorption properties were further investigated by CO2-TPD experiments. As shown in Supplementary Fig. 19 and Table 1, the desorption temperature of CO2 molecules on c-In2O3-x(OH)y (~ 157 °C) is higher than that of all rhombohedral samples (~ 140 °C), indicating that the CO2 molecules are more tightly absorbed on the surface of c-In2O3-x(OH)y. Moreover, the c-In2O3-x(OH)y shows a larger peak area, reflecting an enhanced CO2 adsorption. The reason for the higher CO2 adsorption capacity of c-In2O3-x(OH)y may simply be due to its larger surface area as compared with rhombohedral samples, so that the c-In2O3-x(OH)y could provide a larger population of capture sites for CO2. Even so, the enhanced CO2 adsorption capacity of c-In2O3-x(OH)y cannot contribute much to its photocatalytic CO2 hydrogenation performance as the activity of rhombohedral samples greatly exceeded that of c-In2O3-x(OH)y, and indicates the SFLP site served as the catalytically active site for CO2 hydrogenation.
As SFLP can be considered as the active sites for the catalytic performance, the thermal treatment at 350 °C can efficiently remove lattice oxygen as well as hydroxide group to tune the surface into a ratio of 1:0.67 between oxygen vacancy and hydroxide group, which results in the optimal composition among all the samples tested. This can be further confirmed by the decreased activity of a H2 treated rh-350 sample, which possessed relatively more oxygen vacancies and less hydroxide groups (Supplementary Fig. 20). Furthermore, as compared with CO, the production of methanol was significantly improved when irradiating with light, and exhibited an enhanced methanol selectivity as well.
Owing to its resulting best photocatalytic performance, the rh-350 was selected for further long-term stability testing at 270 °C under atmospheric pressure with light irradiation. As shown in Fig. 3c, excellent stability resulted, with no significant change in production rate as well as selectivity in rh-350 over more than 100 h of continuous testing. As a result, methanol selectivity was maintained at 13% throughout the test, with the methanol rate of 170 μmol gcat−1 h−1 and CO rate of 1150 μmol gcat−1 h−1. Moreover, the used catalyst was also evaluated by XPS, PXRD, TEM, and no obvious oxidation state and structural changes were observed (Supplementary Figs. 21–23).
The SFLP on rhombohedral In2O3-x(OH)y
The surface vacancy breaks the stable Lewis acid–base adjuncts (bridging In–O or In–OH), which can then create novel surface Lewis acidic sites, and the nearby surface hydroxide groups can function as the Lewis basic sites. As such, the strong charge difference between the resulting unsaturated In atom and the hydroxide group can form the SFLP on the single component metal oxide, which could further activate the small H2 molecules. The determination of the presence of this SFLP on defect laden rh-In2O3-x(OH)y was performed as follows.
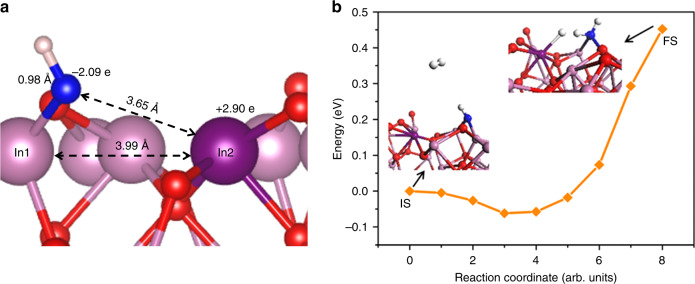
Density functional theory (DFT) simulations were carried out to investigate the properties of the rhombohedral In2O3 nanocrystal. Based on PXRD patterns and HRTEM images, both of the major facets, (110) and (104), were used for the determination of the removal of lattice oxygen and addition of OH (as hydroxide). The (110) facet has been chosen for further calculation and explanation owing to the much stronger Bader charge of the present SFLP. To investigate this further, the bulk rhombohedral In2O3 structure was cut along the direction of (110) and generated the corresponding surface. As shown in Supplementary Fig. 24, possible vacancy sites were determined on the (110) rhombohedral In2O3 surface, and the formation energies of vacancies were calculated by using a known approach from the literature40,43. All atomic removal steps were found to be endothermic in which the adsorption energy would range from 4.15 (for site 1) to 4.94 eV (for site 3) (Supplementary Table 2). To simulate the rh-In2O3-x(OH)y surfaces, which contain the OH group, the defected (110) rhombohedral In2O3-x surface was used, which has the vacancies at site 1 and site 3 as the most and least favorable adsorption sites. Then the OH group was added to the defected surface at vacancy sites to form In2O3-x(OH)y, and the adsorption energy and charge transfer of binding were calculated (Supplementary Fig. 25). The amounts of total free energy change due to the binding of OH at site 1 and 3 of the defected surface are − 3.97 eV and − 4.30 eV, respectively, which indicate that the formation of hydroxylated nanostructures can be highly exothermic. Previous studies for the (111) surface of the c-In2O3-x(OH)y nanostructure suggest that the O atom from the OH group and the near In atom (including + 1.66 e and − 1.50 e, respectively) can form an SFLP owing to the large charge difference40. Thus, Bader charge calculations were performed to probe the localized charges on surface constituents, showing that the related O and In pair involve atomic local charges of + 2.90 e and − 2.09 e, and + 2.90 e and − 2.03 e, for site 1 and site 3 of the rh-In2O3-x(OH)y nanostructure (Fig. 4a), respectively. The larger Bader charge between the Lewis acid and Lewis base on rh-In2O3-x(OH)y could be caused by the following contributing effects. (i) A larger geometrical distance between the active O atom and In atom (3.65 Å) than that of the c-In2O3-x(OH)y (3.20 Å), and (ii) a higher coordination number of the active In atom (6 in (110) facets) in rh-In2O3-x(OH)y than that in c-In2O3-x(OH)y (4 in (111) facets). Similar to the (110) facets, the SFLP can also be constructed by removing surface oxygen atom and introducing a hydroxide group on (104) facets of rhombohedral In2O3 (Supplementary Figs. 26 and 27). It reveals that the Lewis acidic In and Lewis basic O of the OH sites at the (104) surface possess Bader charges of + 1.61 e and − 1.12 e, respectively. In this scenario, the distance between Lewis acid and base is 3.75 Å.
Fig. 4.
Surface frustrated Lewis pairs on rh-In2O3-x(OH)y. a Side view of optimized configuration for (110) rh-In2O3-x(OH)y. b Reaction pathway and energy barrier of H2 dissociation on (110) rh-In2O3-x(OH)y. White, pink, red, purple, and blue spheres represent H, In, O, Lewis pair In, and Lewis pair O atoms, respectively
The larger charge difference between the Lewis acid and Lewis base pairs in the (110) rh-In2O3-x(OH)y structure compared with that of the (111) c-In2O3-x(OH)y surface, envisages the rh-In2O3-x(OH)y nanostructure would form more active Lewis acid–base pairs than the c-In2O3-x(OH) pair can muster, and therefore could strongly polarize H–H bonds and dissociate H2 molecules. This prediction can be evidenced by the optimized configuration of hydrogenated rh-In2O3-x(OH)y, in which the H–H distance of H2 is enlarged from 0.75 Å to 1.24 Å and the bond length of newly formed In–H and H–OH bonds are 1.86 and 1.07 Å, respectively (Supplementary Fig. 28). Furthermore, the relative activation energy barrier for H2 heterolysis over rh-In2O3-x(OH)y further confirms that the strong SFLP could enable more efficient H2 dissociation. The calculation of reaction pathway and energy barrier of H2 dissociation shows that H2 dissociation on (110) rh-In2O3-x(OH)y surface is endothermic with an activation energy barrier of 0.45 eV (Fig. 4b), which is much smaller than that (0.66 eV) on the (111) c-In2O3-x(OH)y surface40. More recently, experimental evidence for heterolysis of H2 on the SFLP of rh-In2O3-x(OH)y has also been observed by our group using a suite of five insightful spectroscopy probes including diffuse reflectance infrared Fourier-transform spectroscopy (DRIFTS), XPS, 1H solid state MAS NMR, EPR, and UV-Vis-NIR, which provides an in-depth understanding of how gaseous H2 interacts with nanostructured rh-In2O3-x(OH)y.
Investigation of the CO2 hydrogenation pathway
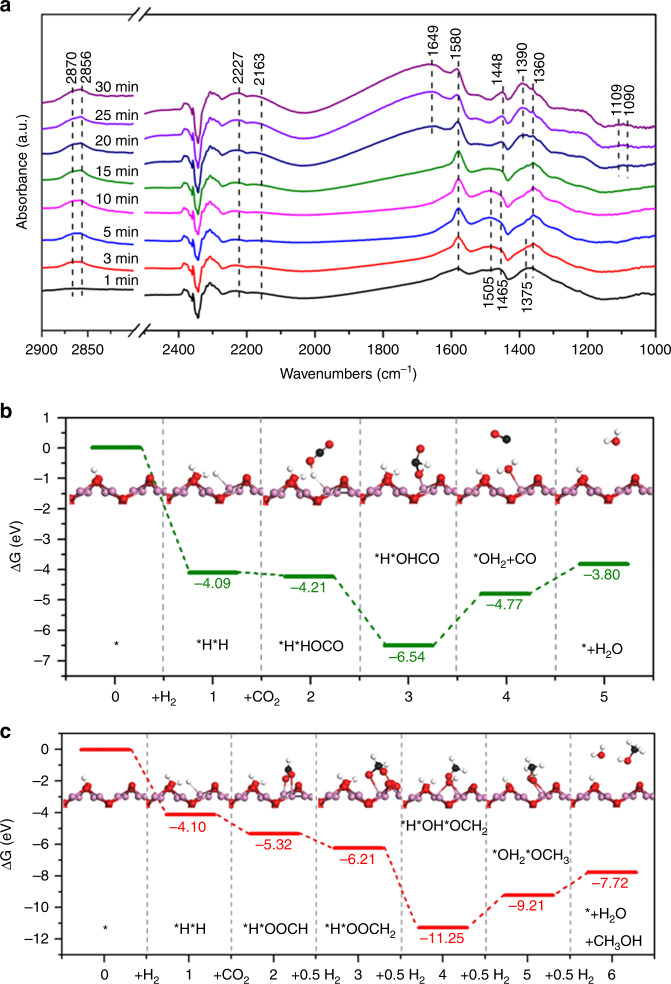
In order to understand the catalytic pathway for CO2 hydrogenation on rh-In2O3-x(OH)y nanocrystals, in situ DRIFTS measurements were performed in a flow cell under reaction operando conditions. In the initial reaction stage, two primary surface species were observed, as shown in Fig. 5a. The first kind of species with fingerprint modes at 1505, 1465, and 1375 cm−1 can be assigned to chemisorbed CO2 species including bicarbonate (HCO3−) and carbonate (CO32−)44–47. The second kind of species signaled by fingerprint modes at 1580 and 1360 cm−1 can be attributed to the asymmetric and symmetric OCO stretching vibrations of adsorbed bidentate formate (HCOO*) species. This assignment is supported by two additional modes at 2870 cm−1 and 1390 cm−1 that are attributed to the stretching vibration ν(CH) and bending vibration δ(CH) of the same species48–50. Along with the CO2 hydrogenation the bicarbonate and carbonate species could readily be transformed to formate species, as evidenced by the decrease and disappearance of these bands. Apart from the formation of formate species, another important intermediate appears with diagnostic peaks at 2856, 1448, 1109, and 1090 cm−1 in the spectra that are assigned to methoxy (H3CO*)48,49,51. From these DRIFT results, CO2 hydrogenation over rh-In2O3-x(OH)y may proceed via formate intermediates (Supplementary Fig. 29, formate pathway), which eventually produces CH3OH via the C-O bond cleavage and *HCO or *H2CO intermediates48,52. Simultaneously, we also observe the diagnostic vibrational modes of CO at bands 2227 and 2163 cm−1, and water at 1649 cm−1, indicative of another reaction pathway featuring a CO intermediate (Supplementary Fig. 29, RWGS pathway), which is produced from the RWGS reaction via carboxyl (*HOCO) intermediates50,53. However, such *HOCO intermediates are unstable and cannot be detected even at a low temperature (90 K)54. To corroborate these experimental observations, free energy profiles for CO2 hydrogenation via the proposed RWGS and formate pathways over rh-In2O3-x(OH)y were calculated. The results suggest that via the RWGS pathway (Fig. 5b), except for product (CO, H2O) desorption, the preceding hydrogenation reactions steps 1–3 are all exothermic. Similarly, steps 1–4 also behave in an exergonic nature via the formate pathway (Fig. 5c). These are different from the previously reported c-In2O3-x(OH)y surface30,40, resulting in the possibility of enhanced catalytic activity for hydrogenation of CO2. Notably, the smaller free energy barrier for the RWGS pathway is consistent with our experimental data of a faster CO production rate.
Fig. 5.
CO2 hydrogenation mechanism on rh-In2O3-x(OH)y nanocrystals. a In situ DRIFTS spectra of surface species formed from CO2 hydrogenation. b Energy profiles for CO2 hydrogenation via the RWGS pathway. c Energy profiles for CO2 hydrogenation via the formate pathway. Insets are the corresponding structures of reaction intermediates. The zero energy corresponds to the total free energy of the rh-In2O3-x(OH)y nanocrystal
Discussion
As the first report that an SFLP in cubic In2O3-xOHy40, denoted InOH•••In, can facilitate heterolysis of H2 to form InOH2+•••InH−, a number of chemical strategies have been devised to modify its Lewis acidity and Lewis basicity in order to tune its activity and selectivity in CO2 (photo)catalysis.
To amplify, following heterolysis of H2 on the SFLP, InOH•••In → InOH2+•••InH−, the proton bound to the hydroxide Lewis base and hydride bound to the coordinately unsaturated indium can subsequently react with CO2 to form CO in an endothermic reverse water gas shift reaction (Eq. 1):
| 1 |
and CH3OH in an exothermic methanol forming reaction (Eq. 2):
| 2 |
in both cases with the desorption of co-product H2O.
The rate and selectivity of these two reactions, which proceed simultaneously and by different pathways, facilitated by the SFLP, can be tailored advantageously by engineering the properties of the SFLP. In practice, this requires chemical means of adjusting the geometry of the SFLP and the negative and positive charge on the Lewis base and Lewis acid sites, respectively (Fig. 4a).
Note that these approaches to engineering the Lewis acidity and basicity of the SFLP refer to the electronic ground state of In2O3-x(OH)y. In the photo-excited state of In2O3-x(OH)y, the In2 and In1OH sites of the InOH•••In SFLP serve as traps for electrons and holes, respectively, making the proton and hydride of the SFLP H2 heterolysis intermediate InOH2+•••InH− more acidic and basic towards subsequent reactions with CO232. These traps also serve to lengthen the electron and hole excited state lifetimes, thereby enhancing the probability of reactions of InOH2+•••InH− with CO233,34. To this end, the charge-transfer dynamics of all samples were further investigated by time-resolved fluorescence spectroscopy (Supplementary Fig. 30). The curves can be fitted well using a triple exponential function (Supplementary Table 3 and Table 4). Following bandgap photoexcitation, electron-hole pairs relax into PL midgap SFLP defect sites, [O]/In(III) located closer in energy to the CB and OH similarly to the VB33,34. This non-radiative process into SFLP sites, likely corresponds to the shortest ns lifetime τ3. The electron-hole pair residing in the SFLP can relax radiatively to yield the observed defect PL or non-radiatively to the electronic ground state as phonons or chemically reacting with reactants CO2/H2 (Supplementary Fig. 31). Notably, rh-350 with the longest average PL lifetime has the superior photocatalytic activity. This is another way of engineering the activity and selectivity of the SFLP. The solar advantage stems from the greater excited state Lewis acid and Lewis basicity of the SFLP of In2O3-x(OH)y, which is manifest experimentally in lower activation energies for the excited state pathway compared with the ground state.
Another point worth mentioning is the adsorption strength of intermediates and products to the SFLP, which can influence selectivity and conversion rates of the CO and CH3OH pathways. For the case of a formate intermediate, created by the reaction of hydride with CO2, weak binding to the SFLP will favor the CO pathway (Supplementary Fig. 29, RWGS pathway), whereas strong binding will enable successive hydride transfers to formate, thereby favouring the CH3OH pathway (Supplementary Fig. 29, formate pathway). In addition, the strongly adsorbing, surface coordinating products H2O and CH3OH, which have to be desorbed to make the CO2 hydrogenation reactions catalytic, will act as rate limiting if the Lewis acidity of the In2 site is too high. Clearly, optimizing the rate and selectivity of CO2 hydrogenation to CO and CH3OH by In2O3-x(OH)y requires a delicate balancing act of the Lewis acidity and Lewis basicity of the SFLP towards the binding of reactants, intermediates and products.
Approaches to SFLP engineering that have proven successful so far include control of oxygen vacancies and isomorphous substitution of the indium sites6,29. In the case of [O]v vacancies, the higher the substituting population is, the lower the oxygen coordination number around the In(III) sites. This makes the In1OH more Lewis basic and the coordinately unsaturated In2 more Lewis acidic. For isomorphous substitution of In(III) by a similar-sized yet more electronegative element like Bi(III), exchange of the In2 site renders it more Lewis acidic, whereas replacement of In1 makes the hydroxide less Lewis basic.
Another strategy for tailoring the SFLP would be to change the distance between the Lewis acid and Lewis base sites (Fig. 4a), which would alter their charges and modify how they interact with H2. The geometry of the SFLP will vary between different crystal facets, which to implement will require strict control of the crystal morphology. Geometry changes of the SFLP are also achievable by polymorph engineering, a more straightforward method in practice, and the subject of the experimental and computational studies described herein.
In summary, we have demonstrated a polymorph selection strategy to modify the Lewis acidity and Lewis basicity of rhombohedral In2O3-x(OH)y with a view to tuning activity and selectivity in gas-phase CO2 (photo)catalysis. Significantly, rh-In2O3-x(OH)y turns out to be a high performance photocatalyst, achieving champion CO2 hydrogenation rates to CH3OH and CO at atmospheric pressure. The superior catalytic performance appears to originate in the enhanced activity of surface Lewis acid–base pairs and strong propensity towards H2 dissociation. An operando DRIFT study and DFT calculation provide information on the surface chemistry responsible for the formation of CH3OH and CO, which appear to proceed by different reaction pathways. Based on the results and insight gained from this work, it should prove possible to optimize the Lewis acidity and Lewis basicity and enhance the photocatalytic performance of heterogeneous SFLP photocatalysts through polymorph selection. Furthermore, by understanding the distinct light-dependence of CO and CH3OH formation and the impact of the electronic structure on CO2 activation and H2 dissociation by cubic and rhombohedral In2O3-x(OH)y, these heterogeneous SFLP systems can be incorporated into multi-component catalytic systems exemplified by polymorphic heterostructures, with distinct structures yet continuously adjustable fractions, enabling efficient CO2 hydrogenation with front-line status.
Methods
Chemicals
All reagents used in the present study, including N,N-dimethylformamide (DMF), Indium(III) nitrate hydrate (In(NO3)3·4.5H2O, In 29%), and ethanol (C2H5OH) were analytical reagent grade and obtained from Sigma-Aldrich. All chemicals were used as received. Deionized water was used throughout the synthesis.
Synthesis of InOOH precursor and rhombohedral In2O3-x(OH)y nanocrystals
In a typical synthesis of InOOH precursor, 0.3 g of In(NO3)3·4.5H2O and 0.8 mL of distilled water were added to a 25 mL autoclave. DMF was then added to bring the total volume up to 17 mL. The aqueous solution was then heated at 150 °C for 24 h. After being cooled to room temperature, the white products were collected through centrifugation and washed with water and ethanol. The sample was finally dried at 60 °C. The dried InOOH precursors were then placed into an oven and treated at various temperatures (250–400 °C) in air for 4 h to obtain the final In2O3-x(OH)y samples.
Characterization
PXRD was performed on a Bruker D2-Phaser X-ray diffractometer, using Cu Ka radiation at 30 kV. The HRTEM measurement was conducted using a JEM–2010 microscope working at 200 kV. Nitrogen Brunauer–Emmet–Teller (BET) adsorption isotherms were obtained using an ASAP2020 M apparatus (Micromeritics Instrument Corp., USA). For BET surface area analyses, the samples were degassed in vacuum at 110 °C for 10 h and then measured at 77 K. The weight loss of InOOH precursor was carried out in a TA Instruments SDT Q600 thermogravimetric analyzer in an alumina pan under 100 mL min−1 flow of compressed air. The temperature was steadily increased from room temperature (25 °C) to 580 °C at a rate of 5 °C min−1. UV-visible diffuse reflectance spectra of the powders were obtained for the dry-pressed disk samples using a Cary 500 Scan Spectrophotometer (Varian, USA) over a range of 200–800 nm. BaSO4 was used as a reflectance standard in the UV-visible diffuse reflectance experiment. XPS was performed using a PerkinElmer Phi 5500 ESCA spectrometer in an ultrahigh vacuum chamber with base pressure of 1 × 10−9 Torr. The spectrometer uses an Al Ka X-ray source operating at 15 kV and 27 A. The samples were coated onto carbon tape, and all results were calibrated to C1s 284.5 eV. The room temperature photoluminescence (PL) spectrum was measured on a FL/FS 920 (Edinburgh Instruments) equipped with a 450 W Xe arc lamp as the excitation source and a red sensitive Peltier element cooled Hamamatsu R2658 PMT as the detector. Time-resolved fluorescence decay spectra were recorded on the Delta Pro (HORIBA instruments) using a 357 nm laser as the excitation source. Carbon dioxide temperature-programmed desorption (CO2-TPD) measurements were performed on a Micromeritics AutoChem II 2920 chemisorption analyzer.
Gas-phase photocatalytic measurements
The gas-phase CO2 hydrogenation experiments were conducted in an inner diameter of 2 mm tubular quartz reactor, in which ∼ 20 mg of catalyst sample was packed into and fully irradiated with an unfiltered 130 W Xe lamp. The diameter of the light spot was ~ 2 cm, with an area of about 3.14 cm2, which could fully cover the sample. An OMEGA temperature controller was attached to a heating cartridge inserted into the copper block along with a thermocouple inserted into the quartz tube in contact with the catalyst bed for control of the catalyst temperature. In a typical run, CO2 or 13C isotope-labeled CO2 (99 atom% 13 C; Sigma) and H2 with a ratio of 1: 3 (2 mL min−1 and 6 mL min−1) were introduced into the reactor by Alicat Scientific digital flow controllers. The amounts of CO and CH3OH produced were analyzed by an on-line gas chromatograph (Agilent 7820 A), equipped with a thermal conductivity detector (TCD) and a flame ionization detector (FID).
In situ DRIFTS measurements
In situ DRIFTS measurements were performed to detect and characterize the possible surface intermediates over rhombohedral phase of In2O3-x(OH)y nanocrystals under reaction conditions. The spectra were collected using a Fourier-transform infrared spectroscopy spectrometer (Thermo, Nicolet 6700) equipped with an MCT detector. Before measurement, the catalyst was purged with He at 350 °C for 2 h. The catalyst was subsequently cooled down to 230 °C. The background spectrum with a resolution of 4 cm−1 was obtained at 230 °C in He flow. Then the catalyst was exposed to a CO2/H2/He mixture (1 mL min−1 CO2, 3 mL min−1 H2 and 16 mL min−1 He) for 30 min. The in situ DRIFT spectra were recorded by collecting 32 scans at 4 cm−1 resolution.
DFT calculations
Theoretical calculations are carried out with the context of DFT, as implemented in the Vienna ab initio simulation package. The exchange–correlation interactions were treated within the generalized gradient approximation in the form of the Perdew−Burke−Ernzerhof functional. The projector augmented wave approach with plane wave cutoff energy of 400 eV is used, and the convergence criteria are set to be 10−4 in energy and 0.02 eV Å−1 in force. To model the rhombohedral In2O3 surfaces, four-layer slabs within vacuum spacing larger than 20 Å with the bottom two layers keep fixed are adopted. Brillouin zone integrations are performed over the Gamma point owing to the large supercell. Nudged elastic band method is used to determine the H2 dissociation path and barrier.
Supplementary information
Acknowledgements
T.Y. is thankful for financial support from the National Natural Science Foundation of China (21872081), Natural Science Foundation of Shandong Province (ZR2016BM04), China Postdoctoral Science Foundation (2015M572011, 2017T100494). B.H. and Y.Dai. acknowledge support from the Taishan Scholars Program of Shandong Province. G.A.O. acknowledges the financial support of the Ontario Ministry of Research and Innovation (MRI), the Ministry of Economic Development, Employment and Infrastructure (MEDI), the Ministry of the Environment and Climate Change’s (MOECC) Best in Science (BIS) Award, Ontario Center of Excellence Solutions 2030 Challenge Fund, Ministry of Research Innovation and Science (MRIS) Low Carbon Innovation Fund (LCIF), Imperial Oil, the University of Toronto’s Connaught Innovation Fund (CIF), Connaught Global Challenge (CGC) Fund, and the Natural Sciences and Engineering Research Council of Canada (NSERC). Special thanks to Athan Tountas of the Chemical Engineering and Applied Chemistry and Keshav Raina of the Faculty of Forestry from University of Toronto for proofreading the manuscript.
Author contributions
T.Y. conducted the catalysts preparation and the catalysts testing flow experiments for CO2 hydrogenation. L.W. performed the XPS characterization. Y.L., Y.Dai., B.H., and M.M. conducted and analyzed the DFT calculations. T.Y., L.W., and Y.Dong performed the in situ DRIFTS study. T.W. and F.M.A. performed the GC-MS test using 13CO2 as well as the TEM characterization. T.Y., L.W., and G.A.O. conceived the project and co-wrote the manuscript. T.Y. and L.W. contributed equally to this work, and G.A.O spearheaded the project. The manuscript was written through collective contributions from all authors. All authors approved the final version of the manuscript.
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Journal Peer Review Information: Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Tingjiang Yan and Lu Wang.
Contributor Information
Tingjiang Yan, Email: tingjiangn@163.com.
Ying Dai, Email: daiy60@sdu.edu.cn.
Geoffrey A. Ozin, Email: gozin@chem.utoronto.ca
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-10524-2.
References
- 1.Jia J, et al. Heterogeneous catalytic hydrogenation of CO2 by metal oxides: defect engineering-perfecting imperfection. Chem. Soc. Rev. 2017;46:4631–4644. doi: 10.1039/C7CS00026J. [DOI] [PubMed] [Google Scholar]
- 2.Zhao Y, et al. Two-dimensional-related catalytic materials for solar-driven conversion of COx into valuable chemical feedstocks. Chem. Soc. Rev. 2019;48:1972–2010. doi: 10.1039/C8CS00607E. [DOI] [PubMed] [Google Scholar]
- 3.Manzoli M, Bonelli B. Microwave, ultrasound, and mechanochemistry: unconventional tools that are used to obtain “smart” catalysts for CO2 hydrogenation. Catalysts. 2018;8:262. doi: 10.3390/catal8070262. [DOI] [Google Scholar]
- 4.Varghese OK, et al. High-rate solar photocatalytic conversion of CO2 and water vapor to hydrocarbon fuels. Nano. Lett. 2009;9:731–737. doi: 10.1021/nl803258p. [DOI] [PubMed] [Google Scholar]
- 5.Gao P, et al. Influence of Zr on the performance of Cu/Zn/Al/Zr catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol. J. Catal. 2013;298:51–60. doi: 10.1016/j.jcat.2012.10.030. [DOI] [Google Scholar]
- 6.Dong YC, et al. Tailoring surface frustrated lewis pairs of In2O3-x(OH)y for gas-phase heterogeneous photocatalytic reduction of CO2 by isomorphous substitution of In3+ with Bi3+ Adv. Sci. 2018;5:1700732. doi: 10.1002/advs.201700732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang JJ, et al. A highly selective and stable ZnO-ZrO2 solid solution catalyst for CO2 hydrogenation to methanol. Sci. Adv. 2017;3:1701290–1701300. doi: 10.1126/sciadv.1701290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu C, et al. Carbon dioxide conversion to methanol over size-selected Cu4 clusters at low pressures. J. Am. Chem. Soc. 2015;137:8676–8679. doi: 10.1021/jacs.5b03668. [DOI] [PubMed] [Google Scholar]
- 9.Lablokov V, et al. Size-controlled model Co nanoparticle catalysts for CO2 hydrogenation: synthesis, characterization, and catalytic reactions. Nano. Lett. 2012;12:3091–3096. doi: 10.1021/nl300973b. [DOI] [PubMed] [Google Scholar]
- 10.He L, et al. Spatial separation of charge carriers in In2O3-x(OH)y nanocrystal superstructures for enhanced gas-phase photocatalytic activity. Acs. Nano. 2016;10:5578–5586. doi: 10.1021/acsnano.6b02346. [DOI] [PubMed] [Google Scholar]
- 11.Qu J, et al. Shape effect of Pd-promoted Ga2O3 nanocatalysts for methanol synthesis by CO2 hydrogenation. J. Phys. Chem. C. 2014;118:24452–24466. doi: 10.1021/jp5063379. [DOI] [Google Scholar]
- 12.Jia J, et al. Visible and near-infrared photothermal catalyzed hydrogenation of gaseous CO2 over nanostructured Pd@Nb2O5. Adv. Sci. 2016;3:1600189. doi: 10.1002/advs.201600189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia J, et al. Photothermal catalyst engineering: hydrogenation of gaseous CO2 with high activity and tailored selectivity. Adv. Sci. 2017;4:1700252. doi: 10.1002/advs.201700252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoch LB, et al. Nanostructured indium oxide coated silicon nanowire arrays: a hybrid photothermal/photochemical approach to solar fuels. Acs. Nano. 2016;10:9017–9025. doi: 10.1021/acsnano.6b05416. [DOI] [PubMed] [Google Scholar]
- 15.Wu HC, et al. The effect of an Fe promoter on Cu/SiO2 catalysts for improving their catalytic activity and stability in the water-gas shift reaction. Catal. Sci. Technol. 2016;6:6087–6096. doi: 10.1039/C6CY00542J. [DOI] [Google Scholar]
- 16.Wei J, et al. Directly converting CO2 into a gasoline fuel. Nat. Commun. 2017;8:15174. doi: 10.1038/ncomms15174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin O, et al. Indium oxide as a superior catalyst for methanol synthesis by CO2 hydrogenation. Angew. Chem. Int. Ed. 2016;55:6261–6265. doi: 10.1002/anie.201600943. [DOI] [PubMed] [Google Scholar]
- 18.Tang JW, et al. Effects of substituting Sr2+ and Ba2+ for Ca2+ on the structural properties and photocatalytic behaviors of CaIn2O4. Chem. Mater. 2004;16:1644–1649. doi: 10.1021/cm0353815. [DOI] [Google Scholar]
- 19.Zou ZG, et al. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Nature. 2001;414:625–627. doi: 10.1038/414625a. [DOI] [PubMed] [Google Scholar]
- 20.Tang JW, et al. Photophysical and photocatalytic properties of AgInW2O8. J. Phys. Chem. B. 2003;107:14265–14269. doi: 10.1021/jp0359891. [DOI] [Google Scholar]
- 21.Hoch LB, et al. Effect of precursor selection on the photocatalytic performance of indium oxide nanomaterials for gas-phase CO2 reduction. Chem. Mater. 2016;28:4160–4168. doi: 10.1021/acs.chemmater.6b00301. [DOI] [Google Scholar]
- 22.He YH, et al. A new application of nanocrystal In2S3 in efficient degradation of organic pollutants under visible light irradiation. J. Phys. Chem. C. 2009;113:5254–5262. doi: 10.1021/jp809028y. [DOI] [Google Scholar]
- 23.Lei, Z. B. et al. Photocatalytic water reduction under visible light on a novel ZnIn2S4 catalyst synthesized by hydrothermal method. Chem. Commun. 7, 2142–2143 (2003). [DOI] [PubMed]
- 24.Yan TJ, et al. Efficient photocatalytic degradation of volatile organic compounds by porous indium hydroxide nanocrystals. Environ. Sci. Technol. 2010;44:1380–1385. doi: 10.1021/es902702v. [DOI] [PubMed] [Google Scholar]
- 25.Li ZH, et al. Wide band gap p-block metal oxyhydroxide InOOH: a new durable photocatalyst for benzene degradation. J. Phys. Chem. C. 2007;111:18348–18352. doi: 10.1021/jp076107r. [DOI] [Google Scholar]
- 26.Johnson JA, et al. Facile control of the charge density and photocatalytic activity of an anionic indium porphyrin framework via in situ metalation. J. Am. Chem. Soc. 2014;136:15881–15884. doi: 10.1021/ja5092672. [DOI] [PubMed] [Google Scholar]
- 27.Karazhanov SZh, et al. Phase stability, electronic structure, and optical properties of indium oxide polytypes. Phys. Rev. B. 2007;76:075129–075141. doi: 10.1103/PhysRevB.76.075129. [DOI] [Google Scholar]
- 28.Pan YX, et al. Photocatalytic CO2 reduction by carbon-coated indium-oxide nanobelts. J. Am. Chem. Soc. 2017;139:4123–4129. doi: 10.1021/jacs.7b00266. [DOI] [PubMed] [Google Scholar]
- 29.Hoch LB, et al. The rational design of a single-component photocatalyst for gas-phase CO2 reduction using both UV and visible light. Adv. Sci. 2014;1:1400013. doi: 10.1002/advs.201400013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L, et al. Photocatalytic hydrogenation of carbon dioxide with high selectivity to methanol at atmospheric pressure. Joule. 2018;2:1369–1381. doi: 10.1016/j.joule.2018.03.007. [DOI] [Google Scholar]
- 31.Yan TJ, et al. Controlled preparation of In2O3, InOOH and In(OH)3 via a one-pot aqueous solvothermal route. New J. Chem. 2008;32:1843. doi: 10.1039/b809313j. [DOI] [Google Scholar]
- 32.Ghuman KK, et al. Surface analogues of molecular frustrated Lewis pairs in heterogeneous CO2 hydrogenation catalysis. ACS Catal. 2016;6:5764–5770. doi: 10.1021/acscatal.6b01015. [DOI] [Google Scholar]
- 33.Hoch LB, et al. Carrier dynamics and the role of surface defects: Designing a photocatalyst for gas-phase CO2 reduction. Proc. Natl. Acad. Sci. USA. 2016;113:E8011–E8020. doi: 10.1073/pnas.1609374113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghuman KK, et al. Photoexcited surface frustrated Lewis pairs for heterogeneous photocatalytic CO2 reduction. J. Am. Chem. Soc. 2016;138:1206–1214. doi: 10.1021/jacs.5b10179. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed N, et al. Photocatalytic conversion of carbon dioxide into methanol using zinc-copper-M(III) (M = aluminum, gallium) layered double hydroxides. J. Catal. 2011;279:123–135. doi: 10.1016/j.jcat.2011.01.004. [DOI] [Google Scholar]
- 36.Ahmed N, et al. Photocatalytic conversion of carbon dioxide into methanol using optimized layered double hydroxide catalysts. Catal. Today. 2012;185:263–269. doi: 10.1016/j.cattod.2011.08.010. [DOI] [Google Scholar]
- 37.Liang L, et al. Single unit cell bismuth tungstate layers realizing robust solar CO2 reduction to methanol. Angew. Chem. Int. Ed. 2015;54:13971–13974. doi: 10.1002/anie.201506966. [DOI] [PubMed] [Google Scholar]
- 38.Li H, et al. Carbon quantum dots/Cu2O heterostructures for solar-light-driven conversion of CO2 to methanol. Adv. Energy Mater. 2015;5:1401077. doi: 10.1002/aenm.201401077. [DOI] [Google Scholar]
- 39.Ghoussoub M, et al. Principles of photothermal gas-phase heterogeneous CO2 catalysis. Energy Environ. Sci. 2019;12:1122–1142. doi: 10.1039/C8EE02790K. [DOI] [Google Scholar]
- 40.Ghuman KK, et al. llluminating CO2 reduction on frustrated Lewis pair surfaces: Investigating the role of surface hydroxides and oxygen vacancies on nanocrystalline In2O3-x(OH)y. Phys. Chem. Chem. Phys. 2015;17:14623–14635. doi: 10.1039/C5CP02613J. [DOI] [PubMed] [Google Scholar]
- 41.Frei MS, et al. Mechanism and microkinetics of methanol synthesis via CO2 hydrogenation on indium oxide. J. Catal. 2018;361:313–321. doi: 10.1016/j.jcat.2018.03.014. [DOI] [Google Scholar]
- 42.Liu H, et al. Conversion of carbon dioxide by methane reforming under visible-light irradiation: Surface-plasmon-mediated nonpolar molecule activation. Angew. Chem. Int. Ed. 2015;54:11545–11549. doi: 10.1002/anie.201504933. [DOI] [PubMed] [Google Scholar]
- 43.Walsh A. Surface oxygen vacancy origin of electron accumulation in indium oxide. Appl. Phys. Lett. 2011;98:261910. doi: 10.1063/1.3604811. [DOI] [Google Scholar]
- 44.Tsuneoka H, et al. Adsorbed species of CO2 and H2 on Ga2O3 for the photocatalytic reduction of CO2. J. Phys. Chem. C. 2010;114:8892–8898. doi: 10.1021/jp910835k. [DOI] [Google Scholar]
- 45.Collins SE, et al. Infrared spectroscopic study of the carbon dioxide adsorption on the surface of Ga2O3 polymorphs. J. Phys. Chem. B. 2006;110:5498–5507. doi: 10.1021/jp055594c. [DOI] [PubMed] [Google Scholar]
- 46.Mino L, et al. CO2 capture by TiO2 anatase surfaces: a combined DFT and FTIR study. J. Phys. Chem. C. 2014;118:25016–25026. doi: 10.1021/jp507443k. [DOI] [Google Scholar]
- 47.Wang Y, et al. Selective photocatalytic CO2 reduction to CH4 over Pt/In2O3: Significant role of hydrogen adatom. Appl. Catal. B Environ. 2018;226:544–553. doi: 10.1016/j.apcatb.2018.01.005. [DOI] [Google Scholar]
- 48.Kattel S, et al. Optimizing binding energies of key intermediates for CO2 hydrogenation to methanol over oxide-supported copper. J. Am. Chem. Soc. 2016;138:12440–12450. doi: 10.1021/jacs.6b05791. [DOI] [PubMed] [Google Scholar]
- 49.Bianchi D, et al. Intermediate species on zirconia supported methanol aerogel catalysts. II. Adsorption of carbon monoxide on pure zirconia and on zirconia containing zinc oxide. Appl. Catal. A Gen. 1993;105:223–249. doi: 10.1016/0926-860X(93)80250-T. [DOI] [Google Scholar]
- 50.Wang X, et al. Mechanism of CO2 hydrogenation on Pd/Al2O3 catalysts: Kinetics and transient DRIFTS-MS studies. ACS Catal. 2015;5:6337–6349. doi: 10.1021/acscatal.5b01464. [DOI] [Google Scholar]
- 51.Ouyang F, et al. IR study on migration of 18OCH3 species on ZrO2. Catal. Lett. 1998;50:179–181. doi: 10.1023/A:1019071103433. [DOI] [Google Scholar]
- 52.Liu C, Liu P. Mechanistic study of methanol synthesis from CO2 and H2 on a modified model Mo6S8 cluster. ACS Catal. 2015;5:1004–1012. doi: 10.1021/cs501354b. [DOI] [Google Scholar]
- 53.Schild C, et al. On the mechanism of CO and CO2 hydrogenation reactions on zirconia-supported catalysts: a diffuse reflectance FTIR study: Part II. Surface species on copper/zirconia catalysts: Implications for methanol synthesis selectivity. J. Mol. Catal. 1990;63:243–254. doi: 10.1016/0304-5102(90)85147-A. [DOI] [Google Scholar]
- 54.Senanayake SD, et al. Interaction of CO with OH on Au(111): HCOO, CO3, and HOCO as key intermediates in the water-gas shift reaction. J. Phys. Chem. C. 2009;113:19536–19544. doi: 10.1021/jp908169s. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.