Abstract
A single bout of aerobic exercise modulates corticospinal excitability, intracortical circuits, and serum biochemical markers such as brain‐derived neurotrophic factor (BDNF) and insulin‐like growth factor 1 (IGF‐1). These effects have important implications for the use of exercise in neurorehabilitation. Here, we aimed to determine whether increases in cardiorespiratory fitness (CRF) induced by 18 sessions of high‐intensity interval training (HIIT) over 6 weeks were accompanied by changes in corticospinal excitability, intracortical excitatory and inhibitory circuits, serum biochemical markers and working memory (WM) capacity in sedentary, healthy, young males. We assessed motor evoked potential (MEP) recruitment curves for the first dorsal interosseous (FDI) both at rest and during tonic contraction, intracortical facilitation (ICF), and short‐interval intracortical inhibition (SICI) using transcranial magnetic stimulation (TMS). We also examined serum levels of BDNF, IGF‐1, total and precursor (pro) cathepsin B (CTSB), as well as WM capacity. Compared to pretraining, CRF was increased and ICF reduced after the HIIT intervention, but there were no changes in corticospinal excitability, SICI, BDNF, IGF‐1, total and pro‐CTSB, and WM capacity. Further, greater CRF gains were associated with larger decreases in total and pro‐CTSB and, only in Val/Val carriers, with larger increases in SICI. Our findings confirm that HIIT is efficacious in promoting CRF and show that corticospinal excitability, biochemical markers, and WM are unchanged after 18 HIIT bouts in sedentary males. Understanding how aerobic exercise modulates M1 excitability is important in order to be able to use exercise protocols as an intervention, especially in rehabilitation following brain injuries.
Keywords: Aerobic exercise, BDNF, Cathepsin B, ICF, SICI, TMS
Introduction
Aerobic exercise promotes brain health and function. Indeed, exercise has been shown to improve learning and memory, delay cognitive decline, and protect against brain atrophy in healthy aging individuals (Barnes et al. 2003; Colcombe and Kramer 2003; Weuve et al. 2004; Yaffe et al. 2009; Sofi et al. 2011; Gomez‐Pinilla and Hillman 2013; Ludyga et al. 2016). Exercise programs have been also shown to reduce blood pressure, insulin resistance, and brain injury as well as to delay the onset and progression of neurodegenerative diseases such as Alzheimer's and Parkinson's (Bergen et al. 2002; Whelton et al. 2002; Cuff et al. 2003; Teri et al. 2003; Rovio et al. 2005; Crizzle and Newhouse 2006; O'Leary et al. 2006; Stevens and Killeen 2006; Rabadi 2007; van der Heijden et al. 2010; Dimeo et al. 2012; Shu et al. 2014; Chin et al. 2015). Further, exercise plays protective and therapeutic roles in depression (Blumenthal et al. 1999; Strawbridge et al. 2002; Dunn et al. 2005; Singh et al. 2005; Nabkasorn et al. 2006; Rethorst and Trivedi 2013; Kvam et al. 2016; Schuch et al. 2016). Despite the numerous human studies highlighting the importance of exercise in maintaining brain function and health, exercise‐induced functional changes in the brain and the underlying molecular mechanisms largely remain to be elucidated in humans. Changes in M1 excitability have been observed following a single session of exercise. Yamaguchi et al. (2012) have shown that short‐term, low‐intensity pedaling decreased short‐interval intracortical inhibition (SICI) in the motor cortical representation of the tibialis anterior and soleus muscles. Notably, the reduction in SICI is not restricted to the motor cortical representations of the exercising muscles. Indeed, decreased SICI has been demonstrated in M1 areas representing resting upper limb muscles such as the first dorsal interosseous (FDI) after one session of lower‐limb exercise (e.g., biking) (Takahashi et al. 2011; Singh et al. 2014a, 2014b; Lulic et al. 2017). Further, Singh et al. (2014a, 2014b) have observed increased intracortical facilitation (ICF), while Lulic et al. (2017) found decreased ICF in the M1 representations of upper limb muscles not involved in the exercise following a single biking session. Lastly, enhanced corticospinal excitability in the nonexercising FDI has been reported after a single session of moderate‐intensity lower‐limb aerobic exercise in fit but not in low‐to‐moderately fit individuals (Lulic et al. 2017). Taken together, these findings suggest that acute exercise modulates plasticity of motor cortical representations of both exercising and resting muscles. To date, however, no study has examined whether long‐term aerobic exercise training using the lower limbs induces changes in the excitability of resting upper limb muscle representations.
Animal studies have implicated the neurotrophin BDNF and the growth factor IGF‐1 in mediating the beneficial effects of exercise on hippocampal function and structure as well as cognition (Neeper et al. 1996; Oliff et al. 1998; Carro et al. 2000; Trejo et al. 2001; Gómez‐Pinilla et al. 2002; Vaynman et al. 2004; Berchtold et al. 2005; Ding et al. 2006; Huang et al. 2006; Cotman et al. 2007; Stranahan et al. 2009; Bechara and Kelly 2013). In humans, findings are less clear since exercise‐induced increases in peripheral BDNF have been consistently shown only immediately after a single bout of aerobic exercise (Gold et al. 2003; Ferris et al. 2007; Goekint et al. 2008; Tang et al. 2008; Bos et al. 2011; Cho et al. 2012; Heyman et al. 2012; Schmolesky et al. 2013; Mang et al. 2014; Skriver et al. 2014; Saucedo Marquez et al. 2015). Studies involving longer exercise interventions (i.e., 6 weeks up to 1 year) have reported mixed results. While the majority of reports have found no changes in circulating BDNF and IGF‐1 at the end of the training period (Schiffer et al. 2009; Seifert et al. 2010; Erickson et al. 2011; Ruscheweyh et al. 2011; Voss et al. 2013; Maass et al. 2016; Gourgouvelis et al. 2018), Zoladz and colleagues (2008) demonstrated increased plasma BDNF after 5 weeks of endurance training in physically active male adults. Further, Leckie et al. (2014) found that 1 year of moderate‐intensity walking significantly elevated serum BDNF only in individuals older than 65 years of age. Lastly, Heisz et al. (2017) reported that, although no group differences in serum BDNF were found following 6 weeks of high‐intensity interval training in young adults, participants with greater fitness improvements had higher serum BDNF levels than their counterparts with lower fitness gains. One of the goals of the present study was to investigate whether serum BDNF was enhanced after 6 weeks of high‐intensity interval training in sedentary males.
High‐intensity interval training (HIIT) is as efficacious a protocol at improving aerobic fitness as traditional endurance training despite the reduced time commitment (Batacan et al. 2017). Phillips et al. (2017) recently showed that training using a protocol that involved five 1‐min intervals at an intensity of ~125% peak workload (W peak; determined during a peak oxygen uptake, VO2peak, test) improved cardiorespiratory fitness (CRF) by ~10% in both men and women when performed three times per week for 6 weeks. In the present study, we sought to investigate whether gains in aerobic fitness induced by a similar HIIT protocol were accompanied by changes in TMS‐assessed corticospinal excitability and intracortical circuits (SICI, ICF) in sedentary, healthy males. Further, as previous studies have failed to demonstrate whether long‐term aerobic exercise increases serum BDNF and IGF‐1 (Voss et al. 2013; Leckie et al. 2014; Maass et al. 2016; Heisz et al. 2017), we also examined whether serum levels of BDNF, IGF‐1, and cathepsin B, a myokine that has been associated with CRF and memory (Moon et al. 2016), were elevated after the HIIT protocol. Lastly, since evidence suggests that exercise improves memory and executive function (Colcombe and Kramer 2003; Smith et al. 2010), we examined whether working memory (WM), which is defined as the set of cognitive skills involved in the management and manipulation of information drawn from short‐ and long‐term memory (Baddeley 1992, 2010; Engle 2002; Conway et al. 2005; Fenesi et al. 2015), was increased following 6 weeks of HIIT.
Methods
Subjects
Eighteen healthy males (23.1 ± 3.5 years) were recruited to participate in the study. All participants were deemed sedentary, which was defined as engaging in ≤60 min of exercise/week (Little et al. 2011; Heisz et al. 2017). Handedness was determined using a modified Edinburgh inventory questionnaire, which provides a laterality quotient (LQ) (Oldfield 1971). LQ scores range from – 100 (strong left‐hand preference) to + 100 (strong right‐hand preference) with 0 indicating ambidexterity (Oldfield 1971). In the present study, all participants were right handed (LQ: + 90.00 ± 10.99). The experimental protocol conformed to the declaration of Helsinki and was approved by the Hamilton Integrated Research Ethics Board. All participants provided informed written consent before participation.
Pre‐ and post‐training procedures
At baseline and after the 6‐week exercise intervention, participants completed a body composition assessment, a ramp test to volitional fatigue on a cycle ergometer (Lode Excalibur v2.0, Groningen, the Netherlands) and a memory test (Fig. 1).
Figure 1.

Study timeline. Cardiorespiratory fitness (VO2peak), body composition (BodPod), serum levels of brain‐derived neurotrophic factor (BDNF), insulin‐like growth factor 1 (IGF‐1), total cathepsin B (CTSB) and pro‐CTSB, area under the recruitment curve at rest and during tonic contraction (AUCR est and AUCA ctive), intracortical facilitation (ICF), short‐interval intracortical inhibition (SICI), and WM capacity (automated operation span task, OSPAN) were assessed before (baseline) and after 6 weeks of high‐intensity interval training (HIIT).
Fat and fat‐free mass were determined using air‐displacement plethysmography (BodPod, COSMED, Concord, California). Peak oxygen uptake (VO2peak) was measured using an online gas collection system (Moxus modular oxygen uptake system, AEI technologies, Pittsburgh, Pennsylvania). After a 2‐min warm‐up at 50 W, the workload was increased by 1 W every 2 sec until volitional fatigue (Little et al. 2011; Percival et al. 2015). For each participant, VO2peak was calculated based on the highest value averaged over 30 sec. To assess WM capacity, participants were administered an automated operation span task (OSPAN) (Unsworth et al. 2005) using Inquisit software (millisecond, http://www.millisecond.com/download/library/OSPAN/). One math equation followed by one letter was presented, and participants were required to solve the math equations while remembering the sequence of letters. A practice session, which was comprised of three sections, preceded the actual experimental trial. During the first section of the practice session, a sequence of letters appeared on the screen and participants was required to recall the letters in the same order in which they were presented. In the second practice section, participants were presented with math equations and asked to indicate whether solutions were correct or incorrect. The third practice section included a series of math equations and letter sequences. After each math equation‐letter string, participants were required to recall the letters in the order in which they were presented. The experimental trial consisted of three to seven math equation‐letter sequence sets presented in random order. Each set was repeated three times for a total of 75 letters and 75 math problems. For each participant, the number of correct words recalled in the correct order was summed to obtain the absolute OSPAN score (Unsworth et al. 2005).
High‐intensity interval training (HIIT)
All participants completed a supervised HIIT intervention that involved 3 sessions per week for 6 weeks. The exercise protocol was performed on an electronically braked cycle ergometer and consisted of a 3‐min warm‐up at 50 W, five 1‐min high‐intensity cycling intervals at ~105–135% of the participant's peak power output (W peak; determined during the VO2peak test) interspersed with 1.5 min of active recovery at 30% W peak, and a 2‐min cooldown at 50 W (Fig. 2), for a total duration of 17.5 min (Phillips et al. 2017). Individualized workloads were determined on Visit 2, where participants were asked, after a 2‐min warm‐up at 50 W, to perform 1‐min bouts of exercise starting at 85% W peak interspersed with 90‐sec recovery intervals at 30% W peak. Wattage was increased by 10% (e.g., 95%, 105%, etc.) until participants were unable to complete a full 1‐min interval. The workload of the last exercise interval that participants were able to complete was used as the target workload for the high‐intensity intervals in the subsequent training sessions (Phillips et al. 2017). During these sessions, if a participant was unable to complete a high‐intensity interval at the target workload, the intensity was decreased by 10% W peak to ensure the completion of all 5 intervals. When a participant was able to complete all five high‐intensity intervals at the target workload, the target intensity was increased by 10% W peak on the following training session.
Figure 2.
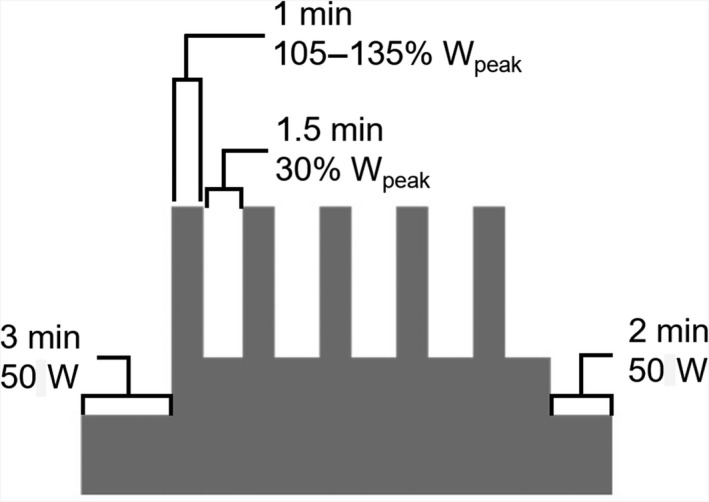
High‐Intensity interval training protocol (HIIT). A 3‐min warm‐up at 50 W was followed by five 1‐min high‐intensity bouts at ~105–135% of participants’ peak power output (W peak) with 1.5‐min recovery at 30% Wpeak between bouts and a 2‐min cool‐down at 50 W to end. Each HIIT session was supervised, lasted 17.5 min, and was carried out on a stationary cycle ergometer.
Electromyography (EMG)
EMG was recorded bilaterally from FDI using surface electrodes (9 mm diameter Ag–AgCl). FDI was chosen because previous studies using this muscle have reported changes in corticospinal excitability and intracortical circuits after an acute bout of lower‐limb exercise (Takahashi et al. 2011; Smith et al. 2014; Lulic et al. 2017). EMG from the FDI muscle was recorded using a monopolar electrode montage whereby one electrode was placed over the muscle belly and referenced to a second electrode positioned over the metacarpal‐phalangeal index joint. Recordings were band‐pass filtered between 20 Hz and 2.5 kHz, amplified by 1000× (Intronix Technologies Corporation Model 2024F, Bolton, Ontario, Canada) and sampled at 5 kHz (Power1401, Cambridge Electronics Design, Cambridge, UK). All participants maintained a supine position of the forearms, as corticospinal excitability depends on limb posture (Forman et al. 2016).
Maximum voluntary contraction (MVC)
To determine MVC, each participant was asked to complete three isometric contractions of the FDI against an immovable structure as described in Lulic et al. (2017). Each contraction lasted 5 sec, and participants were given rest intervals of at least 30 sec in between contraction trials. The greatest maximum EMG activity obtained from the three trials was considered FDI MVC for a given participant. The EMG voltage corresponding to 10% MVC was calculated and displayed on the oscilloscope using a horizontal target line. Participants had to match this line when maintaining a contraction with their FDI muscle during the acquisition of active motor threshold (AMT) and active motor‐evoked potential (MEP) recruitment curve (described below).
Maximum M‐wave (M‐Max)
M‐Max or the peak‐to‐peak amplitude of the maximum M‐wave elicited from the right FDI following stimulation of the ulnar nerve at the wrist was collected using a constant current stimulator (Digitimer, DS7AH; Welwyn Garden City, UK) and a bar electrode (cathode proximal). Square wave pulses with a 200‐μsec pulse width were delivered, and stimulation intensity was increased by 1 mA until the M‐wave ceased to increase for 3 consecutive trials.
Transcranial magnetic stimulation (TMS)
TMS was delivered to M1 using a customized 50‐mm‐diameter figure‐of‐eight branding coil connected to a Magstim Plus stimulator (Magstim, Whitland, UK). The TMS coil was positioned at a 45° angle with respect to the sagittal place to induce a posterior–anterior current. Right FDI motor hotspot was identified within the left hemisphere M1 as the cortical location that elicited the greatest and most consistent MEPs in the muscle at rest. Motor hotspot was then marked using Brainsight Neuronavigation (Rogue Research, Montreal, Canada). Resting motor threshold (RMT) and AMT for FDI were determined at the motor hotspot using the maximum‐likelihood parameter estimation by the sequential testing (ML‐PEST) method (Ah Sen et al. 2017). The freeware for ML‐PEST (TMS Motor Threshold Assessment Tool, MTAT 2.0) was obtained online (http://www.clinicalresearcher.org/software.html), and the assessment without a priori information option was used. The ML‐PEST algorithm was stopped after 20 stimuli (Ah Sen et al. 2017). During AMT acquisition, the horizontal target line on the oscilloscope provided a visual feedback to participants while they maintained a contraction with their right FDI of 10% MVC. MEP recruitment curves (RCs) were obtained in the FDI both at rest and while maintaining a contraction by delivering eight TMS pulses at intensities of 90%, 100%, 110%, 120%, 130%, 140%, 150%, 160%, 170%, 180%, 190%, 200% RMT or AMT in a randomized order (Lulic et al. 2017). For MEP RCs, the mean peak‐to‐peak MEP amplitude at each TMS intensity between 90 and 150% RMT or AMT was calculated by averaging the eight trials. Subsequently, the area under the recruitment curve (AUC) was obtained by trapezoidal integration of the resting and active RCs and normalized to M‐Max (i.e., AUCRest and AUCActive). ICF and SICI were assessed using paired‐pulse TMS paradigms as described in Lulic et al. (2017) with minor modifications. The conditioning stimulus (CS) was set to a TMS intensity of 90% AMT, while the test stimulus (TS) was set to evoke MEPs with peak‐to‐peak amplitudes of 1 mV in the resting FDI. CS and TS were separated by an interstimulus interval (ISI) of 10 msec (ICF) or 2 msec (SICI). For each circuit, 15 unconditioned (MEPTS) and conditioned (MEPCS‐TS) trials were randomly delivered. SICI and ICF were assessed by calculating the peak‐to‐peak amplitude of the unconditioned (MEPTS) and conditioned (MEPCS‐TS) MEP and then by computing the ratio of conditioned over unconditioned MEP (MEPCS‐TS/MEPTS). All single‐ and paired‐pulse TMS measures were collected both before (baseline) and after (6 weeks) the 6‐week HIIT training (Fig. 1).
BDNF, IGF‐1, total Cathepsin B (CTSB), and pro‐CTSB ELISAs
Blood was drawn from 12‐h fasted participants in the morning into BD Vacutainer 10‐mL increased silica act clot activator, silicone‐coated tube (BD, Franklin Lakes, NJ, USA) before (baseline), and after (6 weeks) HIIT training (Fig. 1). Both blood draws (at baseline and after 6 weeks of HIIT) were “resting,” that is, they took place before exercise was performed. Upon collection, blood samples were allowed to clot by leaving them undisturbed at room temperature for ~45 min and then centrifuged at 3488g for 10 min at 4°C. Serum was aliquoted and stored at −80°C prior to use. Serum levels of BDNF, IGF‐1, total CTSB, and pro‐CTSB were measured using human BDNF DuoSet ELISA kit (DY248), human IGF‐I/IGF‐1 Quantikine ELISA kit (DG100), human total Cathepsin B DuoSet ELISA (DY2176) and human pro‐Cathepsin B DuoSet ELISA (DY953) (R&D Systems, Minneapolis, MN) according to manufacturers’ protocols. A standard curve of recombinant BDNF, IGF‐1, total CTSB, and pro‐CTSB was run on each plate. Samples and standards were run in duplicate.
Genotyping
A 2‐mL saliva sample was collected from each participant using a DNA collection kit (Oragene•DNA OG‐500, Genotek, Ottawa, Ontario, Canada). Samples were then sent to GenoFIND Genomic Services (Norcross, Georgia) for processing. Only the region that surrounds the single‐nucleotide polymorphism (SNP) Val66Met (rs6265) on the BDNF gene was examined using a TaqMan® Single Tube Assay. The genotyping results revealed that ten participants were Val/Val carriers, six Val/Met, and 2 Met/Met.
Statistical analysis
Normality was assessed using the Shapiro–Wilks test. Data that were not normally distributed were square root transformed to meet the assumption of normality and then analyzed using parametric statistics. Data that were square root transformed are indicated in Table 1. Dependent measures included VO2peak, AUCRest, AUCActive, ICF, SICI, BDNF, IGF‐1, total CTSB, pro‐CTSB, and OSPAN absolute score and were assessed using two‐tailed paired Student's t‐tests. Effect sizes were calculated using Cohen's d. Hierarchical linear regression analysis was used to determine whether the percent change in CRF (VO2peak %Δ) was associated with the percent change in each dependent measure and whether there was an interaction between VO2peak %Δ and BDNF Vl66Met polymorphism (Brown et al. 2014). The first regression model (model 1) was corrected for age and included only the independent continuous term VO2peak %Δ. The second regression model (model 2) included an interaction term between VO2peak %Δ and the categorical term BDNF genotype (Val/Val, Val/Met). Significant interactions were further analyzed by stratifying participants by BDNF genotype (Val/Val, Val/Met) and by rerunning model 1 on each cohort (Val/Val, Val/Met) separately. Due to their low sample size (n = 2), Met/Met carriers were not included in the hierarchical linear regression analysis. Outliers, defined as values above or below 1.5× the interquartile range (IQR), were removed. Statistical significance was set at P < 0.05.
Table 1.
Group‐averaged means (with SD) of measures
| Measures | Baseline (n = 18) | 6 Weeks (n = 18) | Paired t‐test and effect size |
|---|---|---|---|
| VO2peak | 34.9 ± 4.6 | 39.3 ± 5.0 | P < 0.001, d = 0.894 |
| AUCRest Sqrt Transform | 2.19 ± 0.51 | 2.11 ± 0.60 | P = 0.541, d = 0.144 |
| AUCActive | 8.54 ± 3.46 | 8.59 ± 2.77 | P = 0.933, d = 0.016 |
| ICF | 1.30 ± 0.27 | 1.15 ± 0.19 | P = 0.048, d = 0.656 |
| SICI | 0.52 ± 0.25 | 0.50 ± 0.20 | P = 0.752, d = 0.088 |
| BDNF Sqrt Transform | 4.21 ± 1.06 | 4.37 ± 0.95 | P = 0.549, d = 0.159 |
| IGF‐1 | 160.90 ± 41.15 | 154.50 ± 35.10 | P = 0.198, d = 0.167 |
| Total CTSB | 30.32 ± 12.17 | 31.77 ± 11.85 | P = 0.133, d = 0.121 |
| Pro‐CTSB | 15.39 ± 6.45 | 15.93 ± 6.72 | P = 0.391, d = 0.082 |
| OSPAN | 49.67 ± 13.51 | 53.11 ± 11.57 | P = 0.142, d = 0.274 |
AUC, area under the curve; BDNF, brain‐derived neurotrophic factor; CTSB, cathepsin B; ICF, intracortical facilitation; IGF‐1, insulin‐like growth factor 1; OSPAN, operation span task; SICI, short‐interval intracortical inhibition; VO2peak, peak oxygen uptake. Bold font indicates statistical significance.
Results
All participants successfully completed the experiment. Group means and statistical analyses are reported in Table 1.
The supervised, 6‐week, 5‐by‐1‐min HIIT protocol resulted in an overall robust increase (~12%) in participants’ CRF (VO2peak) (Fig. 3, Table 1). All participants experienced an increase in fitness ranging from ~3% to ~29%.
Figure 3.
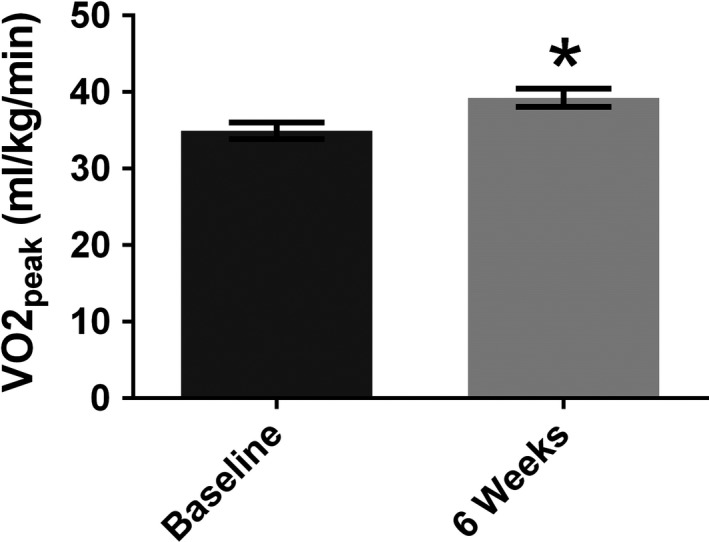
Cardiorespiratory fitness. Group‐averaged VO2peak (with standard error) for all participants (n = 18), showing that low‐volume 5‐by‐1 HIIT significantly increased aerobic capacity in sedentary males after 6 weeks. * indicates significance of P < 0.05.
For rest and active MEP RCs, paired t‐tests revealed no significant differences in AUCs before versus after 6 weeks of HIIT (Table 1), suggesting that the exercise protocol, despite inducing gains in aerobic fitness, did not lead to changes in corticospinal excitability in sedentary males.
ICF was significantly reduced (Fig. 4, Table 1), while SICI showed no change (Table 1) following the 6‐week HIIT intervention.
Figure 4.
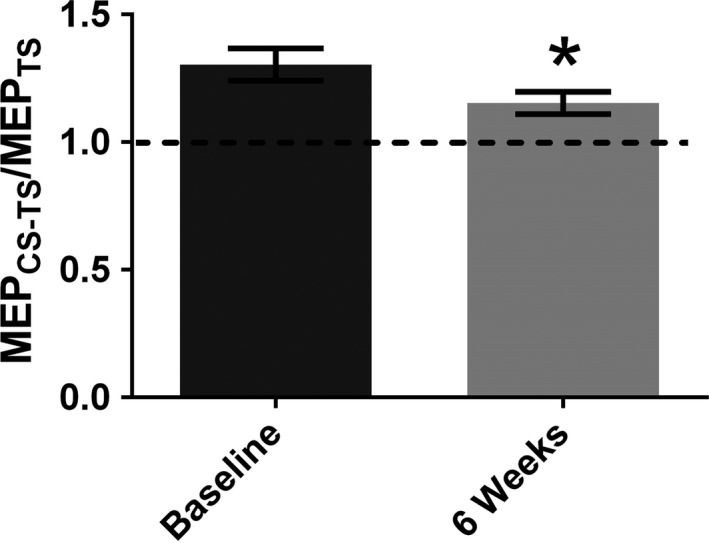
Intracortical facilitation. Group‐averaged intracortical facilitation (ICF) (with standard error) for all participants (n = 18), displaying significantly decreased ICF in sedentary males after 6 weeks of 5‐by‐1‐min HIIT. * indicates significance of P < 0.05.
No significant changes in serum BDNF, IGF‐1, total CTSB, or pro‐CTSB were observed in sedentary males (Table 1).
In summary, these data revealed that 6 weeks of 5‐by‐1‐min HIIT significantly increased aerobic capacity in sedentary males. Further, gains in fitness were accompanied by a reduction in ICF but did not influence corticospinal excitability, SICI or serum levels of BDNF, IGF‐1, total CTSB, and pro‐CTSB.
Model 1 of the hierarchical linear regression analysis showed a relationship between VO2peak %Δ and percent changes in serum levels of total CTSB (β = −0.976, P = 0.051; Fig. 5A, Table 2) and pro‐CTSB (β = −1.136, P = 0.047; Fig. 5B, Table 2) such that larger gains in aerobic capacity were associated with decreases in peripheral total and pro‐CTSB. No relationship between VO2peak %Δ and percent changes in AUCRest %Δ, AUCActive %Δ, ICF %Δ, SICI %Δ, BDNF %Δ, IGF‐1%Δ, and OSPAN %Δ was found (Table 2).
Figure 5.
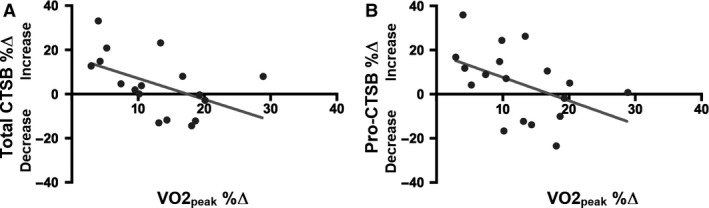
Hierarchical linear regression analysis model 1. A negative relationship between percent change in VO2peak and percent changes in serum levels of total CTSB (A) and pro‐CTSB (B) was found. Results indicate that larger VO2peak gains are associated with decreases in total and pro‐CTSB.
Table 2.
Hierarchical linear regressions
| Dependent Measures | β | SE | P‐value | 95% CI | R 2 | Adjusted R 2 | ΔR 2 | |
|---|---|---|---|---|---|---|---|---|
| AUCRest | Model 1 | 0.094 | −0.027 | |||||
| Age | 4.026 | 3.349 | 0.248 | 97.5 | ||||
| VO2peak | −0.884 | 1.753 | 0.621 | 2.5 | ||||
| Model 2 | 0.161 | −0.049 | −0.067 | |||||
| Age | 4.075 | 3.858 | 0.312 | 97.5 | ||||
| VO2peak | 0.626 | 2.441 | 0.802 | 2.5 | ||||
| VO2peak × BDNF Val66Met | −2.019 | 1.897 | 0.308 | |||||
| AUCActive | Model 1 | 0.000 | −0.142 | |||||
| Age | −0.142 | 1.834 | 0.940 | 97.5 | ||||
| VO2peak | −0.012 | 0.960 | 0.990 | 2.5 | ||||
| Model 2 | 0.022 | −0.245 | −0.022 | |||||
| Age | 0.705 | 2.340 | 0.769 | 97.5 | ||||
| VO2peak | −0.551 | 1.718 | 0.754 | 2.5 | ||||
| VO2peak × BDNF Val66Met | 0.363 | 1.375 | 0.797 | |||||
| ICF | Model 1 | 0.105 | −0.022 | |||||
| Age | −0.922 | 1.169 | 0.444 | 97.5 | ||||
| VO2peak | −0.735 | 0.744 | 0.340 | 2.5 | ||||
| Model 2 | 0.174 | −0.051 | −0.069 | |||||
| Age | −0.317 | 1.313 | 0.814 | 97.5 | ||||
| VO2peak | 0.475 | 0.862 | 0.592 | 2.5 | ||||
| VO2peak × BDNF Val66Met | −0.608 | 0.671 | 0.385 | |||||
| SICI | Model 1 | 0.012 | −0.129 | |||||
| Age | 1.374 | 3.473 | 0.698 | 97.5 | ||||
| VO2peak | 0.090 | 1.821 | 0.961 | 2.5 | ||||
| Model 2 | 0.535 | 0.408 | −0.523 | |||||
| Age | 3.833 | 2.597 | 0.168 | 97.5 | ||||
| VO2peak | −3.446 | 1.649 | 0.061 | 2.5 | ||||
| VO2peak × BDNF Val66Met | 4.050 | 1.299 | 0.010 | |||||
| BDNF | Model 1 | 0.198 | 0.091 | |||||
| Age | 2.095 | 4.020 | 0.610 | 97.5 | ||||
| VO2peak | −4.015 | 2.104 | 0.076 | 2.5 | ||||
| Model 2 | 0.215 | 0.018 | −0.017 | |||||
| Age | 0.712 | 4.852 | 0.886 | 97.5 | ||||
| VO2peak | −5.339 | 3.070 | 0.108 | 2.5 | ||||
| VO2peak × BDNF Val66Met | 1.910 | 2.386 | 0.439 | |||||
| IGF‐1 | Model 1 | 0.071 | −0.053 | |||||
| Age | 0.485 | 0.742 | 0.524 | 97.5 | ||||
| VO2peak | −0.362 | 0.388 | 0.367 | 2.5 | ||||
| Model 2 | 0.142 | −0.072 | −0.072 | |||||
| Age | 0.851 | 0.894 | 0.360 | 97.5 | ||||
| VO2peak | −0.634 | 0.566 | 0.284 | 2.5 | ||||
| VO2peak × BDNF Val66Met | 0.230 | 0.440 | 0.610 | |||||
| Total CTSB | Model 1 | 0.248 | 0.140 | |||||
| Age | 0.226 | 0.954 | 0.816 | 97.5 | ||||
| VO2peak | −0.976 | 0.457 | 0.051 | 2.5 | ||||
| Model 2 | 0.273 | 0.092 | −0.026 | |||||
| Age | 0.093 | 1.030 | 0.930 | 97.5 | ||||
| VO2peak | −1.210 | 0.652 | 0.088 | 2.5 | ||||
| VO2peak × BDNF Vall66Met | 0.256 | 0.506 | 0.622 | |||||
| Pro‐CTSB | Model 1 | 0.254 | 0.154 | |||||
| Age | 0.963 | 1.003 | 0.352 | 97.5 | ||||
| VO2peak | −1.136 | 0.525 | 0.047 | 2.5 | ||||
| Model 2 | 0.269 | 0.086 | −0.015 | |||||
| Age | 0.237 | 1.130 | 0.837 | 97.5 | ||||
| VO2peak | −0.986 | 0.715 | 0.193 | 2.5 | ||||
| VO2peak × BDNF Val66Met | −0.134 | 0.556 | 0.813 | |||||
| OSPAN | Model 1 | 0.028 | −0.148 | |||||
| Age | −0.483 | 0.901 | 0.603 | 97.5 | ||||
| VO2peak | 0.097 | 0.482 | 0.844 | 2.5 | ||||
| Model 2 | 0.198 | −0.103 | −0.169 | |||||
| Age | −0.598 | 1.106 | 0.604 | 97.5 | ||||
| VO2peak | 0.641 | 0.687 | 0.378 | 2.5 | ||||
| VO2peak × BDNF Val66Met | −0.632 | 0.522 | 0.260 |
AUC, area under the curve; BDNF, brain‐derived neurotrophic factor; CI, confidence interval; CTSB: cathepsin B; ICF, intracortical facilitation; IGF‐1, insulin‐like growth factor 1; OSPAN, operation span task; SE, standard error; SICI, short‐interval intracortical inhibition; VO2peak, peak oxygen uptake.
Model 2 revealed a significant VO2peak %Δ X BDNF Val66Met interaction (β=4.050, P = 0.010; Table 2) for SICI %Δ. Subsequent post hoc analyses indicated that only Val/Val carriers demonstrated a significant relationship between changes in VO2peak and changes in SICI (β = −4.250, P = 0.049; Fig. 6) such that greater gains in CRF were associated with larger increases in SICI (i.e., greater GABAA‐mediated intracortical inhibition). No interaction was found between VO2peak %Δ X BDNF Val66Met for AUCRest %Δ, AUCActive %Δ, ICF %Δ, BDNF %Δ, IGF‐1%Δ, total CTSB %Δ, pro‐CTSB %Δ, and OSPAN %Δ (Table 2).
Figure 6.
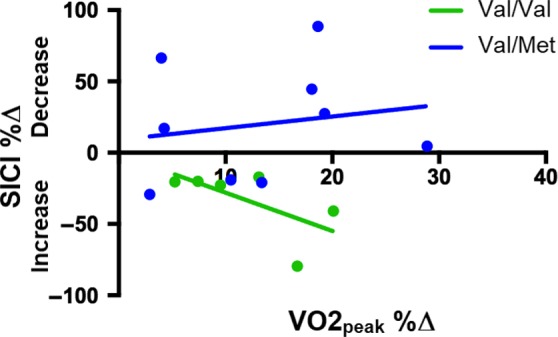
Relationship between percent change in VO2peak and percent change in SICI. A significant interaction between percent change in VO2peak and BDNF Val66Met polymorphism was revealed for SICI. Only Val/Val carriers showed a significant relationship between percent change in VO2peak and percent change in SICI such that greater gains in VO2peak were associated with greater increases in the depth of SICI.
Discussion
While there is evidence that demonstrates changes in M1 excitability following a single bout of aerobic exercise (Takahashi et al. 2011; Singh et al. 2014a, 2014b; Lulic et al. 2017), there are no reports of the effects of long‐term exercise interventions on corticospinal output and intracortical circuitry. In the present study, we examined whether increases in CRF following a 6‐week HIIT protocol at work‐loads of ~105–135% VO2peak were accompanied by changes in corticospinal excitability, TMS‐evoked circuits, and WM in sedentary, healthy males. Further, we investigated whether serum levels of BDNF, IGF‐1, and CTSB, which are thought to contribute to exercise‐induced changes in brain plasticity and memory, were elevated after this exercise protocol. We found that the HIIT intervention resulted in a robust gain in CRF after 6 weeks. We also demonstrated that the increase in fitness was not paralleled by changes in AUCRest, AUCActive, SICI, serum levels of BDNF, IGF‐1, total CTSB, pro‐CTSB, or WM capacity. However, we observed a reduction in ICF following the exercise intervention. Further, we determined an association between fitness gains and total and pro‐CTSB such that greater improvements in aerobic capacity were associated with decreases in total and pro‐CTSB. Lastly, we found that only in Val/Val carriers greater fitness gains were associated with larger increases in SICI.
In line with Phillips et al. (2017), we showed that five high‐intensity, 1‐min bouts of exercise were effective in increasing aerobic capacity in sedentary males after 6 weeks, confirming that this time‐efficient exercise protocol is efficacious in yielding improvements in fitness and could be an alternative to high‐volume training in rehabilitative settings.
Previous studies have demonstrated that a single session of aerobic exercise modulates M1 excitability, suggesting that acute exercise might promote short‐term plasticity within M1 (Takahashi et al. 2011; Singh et al. 2014a, 2014b; Lulic et al. 2017). We report that 6 weeks of HIIT, contrary to a single bout, was not accompanied by changes in corticospinal excitability or the TMS‐evoked circuit SICI, indicating that the propensity for long‐term M1 plasticity was unaltered after the HIIT intervention. Interestingly, we further observed that Val/Val carriers who experienced greater gains in fitness showed larger increases in SICI, suggesting that the BDNF Val/Val genotype might moderate the relationship between CRF and GABAA‐mediated intracortical inhibition. This is consistent with previous studies reporting that physical activity is correlated with greater hippocampal and temporal lobe volumes and better episodic memory only in Val/Val individuals (Brown et al. 2014; Canivet et al. 2015). Further, Nascimento et al. (2015) found that only Val/Val homozygotes displayed increased plasma BDNF after a 16‐week multimodal exercise program, while Keyan and Bryant (2017) showed that only Val/Val carriers had strong emotional memory formation following 10 min of intense exercise. These findings suggest that the Val/Val genotype might be involved in modulating the effects of exercise on brain plasticity, structure, and function and that individualized programs might be important to maximize the beneficial effects of exercise.
The present work also investigated intracortical excitation within M1 and showed that, similar to acute exercise (Lulic et al. 2017), ICF was reduced after 6 weeks of HIIT in sedentary males. Our finding suggests that acute and chronic exercise might have comparable effects on ICF modulation. However, Singh et al. (2014a, 2014b) reported both an increase and no change in ICF following a single bout of moderate‐intensity lower‐limb exercise. Presently, it cannot be ruled out whether long‐term and acute exercise protocols have a similar influence on cortical excitation. ICF is thought to reflect activation of glutamatergic interneurons and N‐methylD‐aspartate (NMDA) receptors (Liepert et al. 1997; Ziemann et al. 1998). Suppression of ICF following long‐term exercise might help maintain excitability within a physiological range and prime the release of GABAergic inhibition (i.e., decrease in SICI) immediately after a single bout of exercise (Singh et al. 2014a, 2014b; Smith et al. 2014; Lulic et al. 2017). The acute reduction in SICI might promote neuroplasticity such as early acquisition and consolidation of motor skills facilitating motor learning. This can lead to improved performance and recovery in rehabilitative settings as supported by poststroke studies (Stinear et al. 2008; Blicher et al. 2009).
BDNF and IGF‐1 are among the factors that have been shown to modulate exercise‐induced brain plasticity, resulting in improvements in cognition and memory (Carro et al. 2000; Trejo et al. 2001; Gómez‐Pinilla et al. 2002; Ding et al. 2006; Stranahan et al. 2009; Bechara and Kelly 2013). Increases in peripheral BDNF have been repeatedly reported after a single bout of aerobic exercise (Gold et al. 2003; Ferris et al. 2007; Tang et al. 2008; Bos et al. 2011; Griffin et al. 2011; Cho et al. 2012; Nofuji et al. 2012; Schmolesky et al. 2013; Mang et al. 2014; Skriver et al. 2014). However, no changes in serum BDNF or IGF‐1 have been observed following long‐term aerobic exercise (Voss et al. 2013; Maass et al. 2016; Heisz et al. 2017; Gourgouvelis et al. 2018). Consistently, we demonstrated that 6 weeks of HIIT induced gains in aerobic capacity but left serum levels of BDNF and IGF‐1 in sedentary males unaltered. As shown by Leckie et al. (2014), it is possible that long‐term exercise protocols induce significant changes in peripheral BDNF only in older individuals (≥65 years). Further, although 6 weeks of HIIT did not change serum levels of BDNF and IGF‐1, it might facilitate increases in peripheral BDNF and IGF‐1 immediately after a single exercise bout.
In the present study, we also assessed serum levels of the lysosomal cysteine protease, cathepsin B. Moon et al. (2016) reported that plasma levels of CTSB were elevated in young adults after 4 months of aerobic exercise and that CTSB increases were associated with gains in CRF and hippocampal‐dependent memory. Contrary to Moon et al. (2016) but similar to Gourgouvelis et al. (2018), who assessed plasma CTSB in sedentary young adults after 8 weeks of moderate to vigorous aerobic exercise, we found no changes in serum total or pro‐CTSB levels following 6 weeks of HIIT. However, we observed that larger gains in aerobic capacity were associated with decreases in serum levels of total and pro‐CTSB. It is possible that the decreases in serum CTSB associated with the greatest fitness gains might reflect a higher demand of enzymatically active CTSB (mature form) in response to exercise requiring increased cleavage of the inactive CTSB precursor (pro‐CTSB) into the active, mature CTSB (Mach et al. 1994; Mort and Buttle 1997; Hook et al. 2015). Interestingly, Moon et al. (2016) proposed that, following exercise, CTSB is secreted by skeletal muscles and, being able to cross the blood–brain barrier, increases BDNF which in turn promotes brain plasticity, ultimately improving cognition and memory function.
Exercise has beneficial effects on memory and executive function (Cotman and Berchtold 2002; Colcombe and Kramer 2003; Hillman et al. 2003; Chang et al. 2014). WM is an important aspect of executive function involved in temporarily storing, maintaining, and updating information for the execution of high‐order cognitive processes such as learning and reasoning (Engle 2002; Baddeley 2003). Few studies have investigated whether exercise influences WM, and results are inconsistent. Coles and Tomporowski (2008) as well as Li et al. (2014) observed no effect of aerobic exercise on WM. Conversely, McMorris et al. (2011) found that acute, moderate‐intensity exercise improves speed but not accuracy in WM, while Sibley and Beilock (2007) showed that acute exercise benefits WM only in individuals with poor WM. Further, both Pontifex et al. (2009) and Martins et al. (2013) reported that acute aerobic exercise, but not resistance exercise (Pontifex et al. 2009), positively influences WM. Lastly, Gourgouvelis et al. (2018), who assessed executive function, learning and memory using the Cambridge Neuropsychological Test Automated Battery (CANTAB), showed that 8 weeks of moderate‐to‐vigorous aerobic exercise did not change cognitive performance in sedentary, young adults. In the current work, we showed that WM capacity was unaltered after 6 weeks of HIIT in sedentary males. It is possible, as suggested by Li et al. (2014), that, even though the HIIT intervention did not have a measurable effect on OSPAN performance, it might have nonetheless had an impact on the activity of the brain regions involved in modulating WM.
It is interesting to note the similarities in outcome between Gourgouvelis et al. (2018), who also tested sedentary individuals, and the present work. Indeed, neither study demonstrated an effect of exercise training on BDNF, CTSB, or WM. Gourgouvelis et al. (2018) used a combination of resistance and moderate‐to‐vigorous‐intensity aerobic training for 8 weeks, while we used a 5‐by‐1‐min HIIT protocol for 6 weeks. Taken together, these studies suggest a resistance to change in response to exercise in sedentary individuals. It remains unknown whether this is a characteristic of sedentary individuals or whether longer and more intense training interventions are needed to alter neurophysiological and biochemical measures in sedentary individuals.
Limitations
Despite our finding of reduced ICF following the HIIT intervention, we cannot conclude that ICF is a mediator of exercise‐induced neuroplasticity. This should be investigated in future research using a two‐armed design with a control group not experiencing HIIT. Since peripheral BDNF levels fluctuate during the menstrual cycle (e.g., higher in the luteal vs. the follicular phase) (Begliuomini et al. 2007; Pluchino et al. 2009; Cubeddu et al. 2011), we did not examine females in this study. There is evidence that females and males respond differently to exercise, for example, females show lower total carbohydrate oxidation and serum leptin than males after the same training protocol (Tarnopolsky et al. 1990, 1995; Hickey et al. 1997; Friedlander et al. 1998). Thus, it should be addressed in future research whether 6 weeks of HIIT influence TMS and serum measures in sedentary females. Further, we did not examine whether short‐term (i.e., immediately after a single exercise session) neuroplasticity is increased following six consecutive weeks of HIIT. It is also possible that extending the duration of the exercise protocol (e.g., 1 year) might lead to significant changes in corticospinal excitability, SICI and serum factors as well as increases in WM capacity. In addition, only young adults were tested here, and thus, it remains to be established whether 6 weeks of HIIT have a higher impact on facilitating motor cortex excitability and on priming neuroplasticity in older adults than in younger adults. Lastly, due to our limited sample size (n = 2, Met/Met), we were not able to determine whether the Met allele constitutes a disadvantage for achieving exercise‐induced benefits.
Conclusions
Our findings confirm that the time‐efficient HIIT protocol robustly increases CRF and demonstrate that this increase is not accompanied by changes in corticospinal excitability, serum biochemical markers, and WM. Understanding how aerobic exercise modulates neural activity within M1 has implications for the use of exercise as an intervention to modify M1 neural activity, for example, following neurological injury. The literature thus far suggests that exercise does not influence BDNF, CTSB, or WM in sedentary individuals.
Conflict of Interest
All authors have no potential sources of conflict of interest to declare.
Nicolini C., Toepp S., Harasym D., Michalski B., Fahnestock M., Gibala M. J., Nelson A. J.. No changes in corticospinal excitability, biochemical markers, and working memory after six weeks of high‐intensity interval training in sedentary males. Physiol Rep, 7 (11), 2019, e14140, 10.14814/phy2.14140
Funding Information
This work was supported by a Natural Sciences and Engineering Research Council of Canada grant to AJN.
References
- Ah Sen, C. B. , Fassett H. J., El‐Sayes J., Turco C. V., Hameer M. M., and Nelson A. J.. 2017. Active and resting motor threshold are efficiently obtained with adaptive threshold hunting. PLoS ONE 12:e0186007 10.1371/journal.pone.0186007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley, A. 1992. Working memory. Science 255:556–559. Available at: http://www.ncbi.nlm.nih.gov/pubmed/1736359 (Accessed: 15 March 2019). [DOI] [PubMed] [Google Scholar]
- Baddeley, A. 2003. Working memory: looking back and looking forward. Nat. Rev. Neurosci. 4:829–839. 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Baddeley, A. 2010. Working memory. Curr. Biol. 20:R136–R140. 10.1016/j.cub.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Barnes, D. E. , Yaffe K., Satariano W. A., and Tager I. B.. 2003. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. J. Am. Geriatr. Soc. 51:459–465. 10.1046/j.1532-5415.2003.51153.x. [DOI] [PubMed] [Google Scholar]
- Batacan, R. B. , Duncan M. J., Dalbo V. J., Tucker P. S., and Fenning A. S.. 2017. Effects of high‐intensity interval training on cardiometabolic health: a systematic review and meta‐analysis of intervention studies. Br. J. Sports Med. 51:494–503. 10.1136/bjsports-2015-095841. [DOI] [PubMed] [Google Scholar]
- Bechara, R. G. , and Kelly Á. M.. 2013. Exercise improves object recognition memory and induces BDNF expression and cell proliferation in cognitively enriched rats. Behav. Brain Res. 245:96–100. 10.1016/J.BBR.2013.02.018. [DOI] [PubMed] [Google Scholar]
- Begliuomini, S. , Casarosa E., Pluchino N., Lenzi E., Centofanti M., Freschi L., et al. 2007. Influence of endogenous and exogenous sex hormones on plasma brain‐derived neurotrophic factor. Hum. Reprod. 22:995–1002. 10.1093/humrep/del479. [DOI] [PubMed] [Google Scholar]
- Berchtold, N. C. , Chinn G., Chou M., Kesslak J. P., and Cotman C. W.. 2005. Exercise primes a molecular memory for brain‐derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience 133:853–861. 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Bergen, J. L. , Toole T., Elliott Iii R. G., Wallace B., Robinson K., and Maitland C. G.. 2002. Aerobic exercise intervention improves aerobic capacity and movement initiation in Parkinson's disease patients. NeuroRehabilitation 17:161–168. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12082243 (Accessed: 15 March 2019). [PubMed] [Google Scholar]
- Blicher, J. U. , Jakobsen J., Andersen G., and Nielsen J. F.. 2009. Cortical excitability in chronic stroke and modulation by training: a TMS study. Neurorehabil. Neural Repair. 23:486–493. 10.1177/1545968308328730. [DOI] [PubMed] [Google Scholar]
- Blumenthal, J. A. , Babyak M. A., Moore K. A., Craighead W. E., Herman S., Khatri P., et al. 1999. Effects of exercise training on older patients with major depression. Arch. Intern. Med. 159:2349–2356. 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- Bos, I. , Jacobs L., Nawrot T. S., De Geus B., Torfs R., Panis L. I., et al. 2011. No exercise‐induced increase in serum BDNF after cycling near a major traffic road. Neurosci. Lett. 500:129–132. 10.1016/J.NEULET.2011.06.019. [DOI] [PubMed] [Google Scholar]
- Brown, B. M. , Bourgeat P., Peiffer J. J., Burnham S., Laws S. M., Rainey‐Smith S. R., et al. 2014. Influence of BDNF Val66Met on the relationship between physical activity and brain volume. Neurology 83:1345–1352. 10.1212/WNL.0000000000000867. [DOI] [PubMed] [Google Scholar]
- Canivet, A. , Albinet C. T., André N., Pylouster J., Rodríguez‐Ballesteros M., Kitzis A., et al. 2015. Effects of BDNF polymorphism and physical activity on episodic memory in the elderly: a cross sectional study. Eur. Rev. Aging Phys. A 12:15 10.1186/s11556-015-0159-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carro, E. , Nuñez A., Busiguina S., and Torres‐Aleman I.. 2000. Circulating insulin‐like growth factor I mediates effects of exercise on the brain. J. Neurosci. 20:2926–2933. 10.1523/JNEUROSCI.20-08-02926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, Y. K. , Tsai C. L., Huang C. C., Wang C. C., and Chu I. H.. 2014. Effects of acute resistance exercise on cognition in late middle‐aged adults: general or specific cognitive improvement? J. Sci. Med. Sport 17:51–55. 10.1016/j.jsams.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Chin, L. M. , Keyser R. E., Dsurney J., and Chan L.. 2015. Improved cognitive performance following aerobic exercise training in people with traumatic brain injury. Arch. Phys. Med. Rehabil. 96:754–759. 10.1016/j.apmr.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, H. C. , Kim J., Kim S., Son Y. H., Lee N., and Jung S. H.. 2012. The concentrations of serum, plasma and platelet BDNF are all increased by treadmill VO2max performance in healthy college men. Neurosci. Lett. 519:78–83. 10.1016/J.NEULET.2012.05.025. [DOI] [PubMed] [Google Scholar]
- Colcombe, S. , and Kramer A. F.. 2003. Fitness effects on the cognitive function of older adults. Psychol. Sci. 14:125–130. 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Coles, K. , and Tomporowski P. D.. 2008. Effects of acute exercise on executive processing, short‐term and long‐term memory. J. Sports Sci. 26:333–344. 10.1080/02640410701591417. [DOI] [PubMed] [Google Scholar]
- Conway, A. R. , Kane M. J., Bunting M. F., Hambrick D. Z., Wilhelm O., and Engle R. W.. 2005. Working memory span tasks: a methodological review and user's guide. Psych. Bull. Rev. 12:769–786. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16523997 (Accessed: 15 March 2019). [DOI] [PubMed] [Google Scholar]
- Cotman, C. W. , and Berchtold N. C.. 2002. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 25:295–301. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12086747 (Accessed: 15 March 2019). [DOI] [PubMed] [Google Scholar]
- Cotman, C. W. , Berchtold N. C., and Christie L.‐A.. 2007. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 30:464–472. 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Crizzle, A. M. , and Newhouse I. J.. 2006. Is physical exercise beneficial for persons with Parkinson's disease? Clin. J. Sport Med. 16:422–425. 10.1097/01.jsm.0000244612.55550.7d. [DOI] [PubMed] [Google Scholar]
- Cubeddu, A. , Bucci F., Giannini A., Russo M., Daino D., Russo N., et al. 2011. Brain‐derived neurotrophic factor plasma variation during the different phases of the menstrual cycle in women with premenstrual syndrome. Psychoneuroendocrinology 36:523–530. 10.1016/j.psyneuen.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Cuff, D. J. , Meneilly G. S., Martin A., Ignaszewski A., Tildesley H. D., and Frohlich J. J.. 2003. Effective exercise modality to reduce insulin resistance in women with type 2 diabetes. Diabetes Care 26:2977–2982. Available at: http://www.ncbi.nlm.nih.gov/pubmed/14578226 (Accessed: 15 March 2019). [DOI] [PubMed] [Google Scholar]
- Dimeo, F. , Pagonas N., Seibert F., Arndt R., Zidek W., and Westhoff T. H.. 2012. Aerobic exercise reduces blood pressure in resistant hypertension. Hypertension 60:653–658. 10.1161/HYPERTENSIONAHA.112.197780. [DOI] [PubMed] [Google Scholar]
- Ding, Q. , Vaynman S., Akhavan M., Ying Z., and Gomez‐Pinilla F.. 2006. Insulin‐like growth factor I interfaces with brain‐derived neurotrophic factor‐mediated synaptic plasticity to modulate aspects of exercise‐induced cognitive function. Neuroscience 140:823–833. 10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- Dunn, A. L. , Trivedi M. H., Kampert J. B., Clark C. G., and Chambliss H. O.. 2005. Exercise treatment for depression. Am. J. Prev. Med. 28:1–8. 10.1016/j.amepre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Engle, R. W. 2002. Working memory capacity as executive attention. Curr. Dir. Psychol. Sci. 11:19–23. 10.1111/1467-8721.00160. [DOI] [Google Scholar]
- Erickson, K. I. , Voss M. W., Prakash R. S., Basak C., Szabo A., Chaddock L., et al. 2011. Exercise training increases size of hippocampus and improves memory. Proc. Natl Acad. Sci. USA 108:3017–3022. 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenesi, B. , Sana F., Kim J. A., and Shore D. I.. 2015. Reconceptualizing working memory in educational research. Educ. Psychol. Rev. 27:333–351. 10.1007/s10648-014-9286-y. [DOI] [Google Scholar]
- Ferris, A. T. , Williams J. S., and Shen C.‐L.. 2007. The effect of acute exercise on serum brain‐derived neurotrophic factor Levels and cognitive function. Med. Sci. Sports Exerc. 39:728–734. 10.1249/mss.0b013e31802f04c7. [DOI] [PubMed] [Google Scholar]
- Forman, D. A. , Baarbé J., Daligadu J., Murphy B., and Holmes M. W.. 2016. The effects of upper limb posture and a sub‐maximal gripping task on corticospinal excitability to muscles of the forearm. J. Electromyogr. Kinesiol. 27:95–101. Available at: https://www.sciencedirect.com/science/article/pii/S1050641116000274 (Accessed: 22 October 2018). [DOI] [PubMed] [Google Scholar]
- Friedlander, A. L. , Casazza G. A., Horning M. A., Huie M. J., Piacentini M. F., Trimmer J. K., et al. 1998. Training‐induced alterations of carbohydrate metabolism in women: women respond differently from men. J. Appl. Physiol. 85:1175–1186. 10.1152/jappl.1998.85.3.1175. [DOI] [PubMed] [Google Scholar]
- Goekint, M. , Heyman E., Roelands B., Njemini R., Bautmans I., Mets T., et al. 2008. No influence of noradrenaline manipulation on acute exercise‐induced increase of brain‐derived neurotrophic factor. Med. Sci. Sports Exerc. 40:1990–1996. 10.1249/MSS.0b013e31817eee85. [DOI] [PubMed] [Google Scholar]
- Gold, S. M. , Schulz K. H., Hartmann S., Mladek M., Lang U. E., Hellweg R., et al. 2003. Basal serum levels and reactivity of nerve growth factor and brain‐derived neurotrophic factor to standardized acute exercise in multiple sclerosis and controls. J. Neuroimmunol. 138:99–105. 10.1016/S0165-5728(03)00121-8. [DOI] [PubMed] [Google Scholar]
- Gomez‐Pinilla, F. , and Hillman C.. 2013. ‘The Influence of Exercise on Cognitive Abilities’, in Comprehensive Physiology. John Wiley & Sons, Inc., Hoboken, NJ, USA: 10.1002/cphy.c110063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez‐Pinilla, F. , Ying Z., Roy R. R., Molteni R., and Edgerton V. R.. 2002. Voluntary exercise induces a BDNF‐mediated mechanism that promotes neuroplasticity. J. Neurophysiol. 88:2187–2195. 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- Gourgouvelis, J. , Yielder P., Clarke S. T., Behbahani H., and Murphy B.. 2018. You can't fix what isn't broken: eight weeks of exercise do not substantially change cognitive function and biochemical markers in young and healthy adults. PeerJ 6:e4675 10.7717/peerj.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin, É. W. , Mullally S., Foley C., Warmington S. A., O'Mara S. M., and Kelly Á. M.. 2011. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol. Behav. 104:934–941. 10.1016/J.PHYSBEH.2011.06.005. [DOI] [PubMed] [Google Scholar]
- van der Heijden, G. J. , Wang Z. J., Chu Z. D., Sauer P. J., Haymond M. W., Rodriguez L. M., et al. 2010. A 12‐week aerobic exercise program reduces hepatic fat accumulation and insulin resistance in obese, Hispanic adolescents. Obesity 18:384–390. 10.1038/oby.2009.274. [DOI] [PubMed] [Google Scholar]
- Heisz, J. J. , Clark I. B., Bonin K., Paolucci E. M., Michalski B., Becker S., et al. 2017. The effects of physical exercise and cognitive training on memory and neurotrophic factors. J. Cogn. Neurosci. 29:1895–1907. 10.1162/jocn_a_01164. [DOI] [PubMed] [Google Scholar]
- Heyman, E. , Gamelin F. X., Goekint M., Piscitelli F., Roelands B., Leclair E., et al. 2012. Intense exercise increases circulating endocannabinoid and BDNF levels in humans—possible implications for reward and depression. Psychoneuroendocrinology 37:844–851. 10.1016/J.PSYNEUEN.2011.09.017. [DOI] [PubMed] [Google Scholar]
- Hickey, M. S. , Houmard J. A., Considine R. V., Tyndall G. L., Midgette J. B., Gavigan K. E., et al. 1997. Gender‐dependent effects of exercise training on serum leptin levels in humans. Am. J. Physiol. Endocrinol. Metab. 272:E562–E566. 10.1152/ajpendo.1997.272.4.E562. [DOI] [PubMed] [Google Scholar]
- Hillman, C. H. , Snook E. M., and Jerome G. J.. 2003. Acute cardiovascular exercise and executive control function. Int. J. Psychophysiol. 48:307–314. 10.1016/S0167-8760(03)00080-1. [DOI] [PubMed] [Google Scholar]
- Hook, G. , Jacobsen J. S., Grabstein K., Kindy M., and Hook V.. 2015. Cathepsin B is a new drug target for traumatic brain injury therapeutics: evidence for E64d as a promising lead drug candidate. Front. Neurol. 6:178 10.3389/fneur.2015.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, A. M. , Jen C. J., Chen H. F., Yu L., Kuo Y. M., and Chen H. I.. 2006. Compulsive exercise acutely upregulates rat hippocampal brain‐derived neurotrophic factor. J. Neural. Transm. 113:803–811. 10.1007/s00702-005-0359-4. [DOI] [PubMed] [Google Scholar]
- Keyan, D. , and Bryant R. A.. 2017. Role of BDNF val66met polymorphism in modulating exercised‐induced emotional memories. Psychoneuroendocrinology 77:150–157. 10.1016/j.psyneuen.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Kvam, S. , Kleppe C. L., Nordhus I. H., and Hovland A.. 2016. Exercise as a treatment for depression: a meta‐analysis. J. Affect. Disord. 202:67–86. 10.1016/j.jad.2016.03.063. [DOI] [PubMed] [Google Scholar]
- Leckie, R. L. , Oberlin L. E., Voss M. W., Prakash R. S., Szabo‐Reed A., Chaddock‐Heyman L., et al. 2014. BDNF mediates improvements in executive function following a 1‐year exercise intervention. Front. Hum. Neurosci. 8:985 10.3389/fnhum.2014.00985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L. , Men W. W., Chang Y. K., Fan M. X., Ji L., and Wei G. X.. 2014. Acute aerobic exercise increases cortical activity during working memory: a functional MRI study in female college students. PLoS ONE 9:e99222 10.1371/journal.pone.0099222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepert, J. , Schwenkreis P., Tegenthoff M., and Malin J. P.. 1997. The glutamate antagonist riluzole suppresses intracortical facilitation. J. Neural. Transm. 104:1207–1214. 10.1007/BF01294721. [DOI] [PubMed] [Google Scholar]
- Little, J. P. , Gillen J. B., Percival M. E., Safdar A., Tarnopolsky M. A., Punthakee Z., et al. 2011. Low‐volume high‐intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J. Appl. Physiol. 111:1554–1560. 10.1152/japplphysiol.00921.2011. [DOI] [PubMed] [Google Scholar]
- Ludyga, S. , Gerber M., Brand S., Holsboer‐Trachsler E., and Pühse U.. 2016. Acute effects of moderate aerobic exercise on specific aspects of executive function in different age and fitness groups: a meta‐analysis. Psychophysiology 53:1611–1626. 10.1111/psyp.12736. [DOI] [PubMed] [Google Scholar]
- Lulic, T. , El‐Sayes J., Fassett H. J., and Nelson A. J.. 2017. Physical activity levels determine exercise‐induced changes in brain excitability. PLoS ONE 12:e0173672 10.1371/journal.pone.0173672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass, A. , Düzel S., Brigadski T., Goerke M., Becke A., Sobieray U., et al. 2016. Relationships of peripheral IGF‐1, VEGF and BDNF levels to exercise‐related changes in memory, hippocampal perfusion and volumes in older adults. NeuroImage 131:142–154. 10.1016/j.neuroimage.2015.10.084. [DOI] [PubMed] [Google Scholar]
- Mach, L. , Mort J. S., and Glössl J.. 1994. Maturation of human procathepsin B. Proenzyme activation and proteolytic processing of the precursor to the mature proteinase, in vitro, are primarily unimolecular processes. J. Biol. Chem. 269:13030–13035. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8175723 (Accessed: 14 February 2019). [PubMed] [Google Scholar]
- Mang, C. S. , Snow N. J., Campbell K. L., Ross C. J., and Boyd L. A.. 2014. A single bout of high‐intensity aerobic exercise facilitates response to paired associative stimulation and promotes sequence‐specific implicit motor learning. J. Appl. Physiol. 117:1325–1336. 10.1152/japplphysiol.00498.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins, A. Q. , Kavussanu M., Willoughby A., and Ring C.. 2013. Moderate intensity exercise facilitates working memory. Psychol. Sport Exerc. 14:323–328. 10.1016/J.PSYCHSPORT.2012.11.010. [DOI] [Google Scholar]
- McMorris, T. , Sproule J., Turner A., and Hale B. J.. 2011. Acute, intermediate intensity exercise, and speed and accuracy in working memory tasks: a meta‐analytical comparison of effects. Physiol. Behav. 102:421–428. 10.1016/J.PHYSBEH.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Moon, H. Y. , Becke A., Berron D., Becker B., Sah N., Benoni G., et al. 2016. Running‐induced systemic cathepsin B secretion is associated with memory function. Cell Metab. 24:332–340. 10.1016/j.cmet.2016.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort, J. S. , and Buttle D. J.. 1997. Cathepsin B. Int. J. Biochem. Cell Biol. 29:715–720. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9251238 (Accessed: 14 February 2019). [DOI] [PubMed] [Google Scholar]
- Nabkasorn, C. , Miyai N., Sootmongkol A., Junprasert S., Yamamoto H., Arita M., et al. 2006. Effects of physical exercise on depression, neuroendocrine stress hormones and physiological fitness in adolescent females with depressive symptoms. Eur. J. Pub. Health 16:179–184. 10.1093/eurpub/cki159. [DOI] [PubMed] [Google Scholar]
- Nascimento, C. M. C. , Pereira J. R., Pires de Andrade L., Garuffi M., Ayan C., Kerr D. S., et al. 2015. Physical exercise improves peripheral BDNF levels and cognitive functions in mild cognitive impairment elderly with different bdnf Val66Met genotypes. J. Alzheimer's Dis. 43:81–91. 10.3233/JAD-140576. [DOI] [PubMed] [Google Scholar]
- Neeper, S. A. , Gómez‐Pinilla F., Choi J., and Cotman C. W.. 1996. Physical activity increases mRNA for brain‐derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 726:49–56. 10.1016/0006-8993(96)00273-9. [DOI] [PubMed] [Google Scholar]
- Nofuji, Y. , Suwa M., Sasaki H., Ichimiya A., Nishichi R., and Kumagai S.. 2012. Different circulating brain‐derived neurotrophic factor responses to acute exercise between physically active and sedentary subjects. J. Sports Sci. Med. 11:83 Available at: http://www.ncbi.nlm.nih.gov/pubmed/24137066 (Accessed: 16 January 2019). [PMC free article] [PubMed] [Google Scholar]
- Oldfield, R. C. 1971. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113. 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- O'Leary, V. B. , Marchetti C. M., Krishnan R. K., Stetzer B. P., Gonzalez F., and Kirwan J. P.. 2006. Exercise‐induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat. J. Appl. Physiol. 100:1584–1589. 10.1152/japplphysiol.01336.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliff, H. S. , Berchtold N. C., Isackson P., and Cotman C. W.. 1998. Exercise‐induced regulation of brain‐derived neurotrophic factor (BDNF) transcripts in the rat hippocampus. Mol. Brain Res. 61:147–153. 10.1016/S0169-328X(98)00222-8. [DOI] [PubMed] [Google Scholar]
- Percival, M. E. , Martin B. J., Gillen J. B., Skelly L. E., MacInnis M. J., Green A. E., et al. 2015. Sodium bicarbonate ingestion augments the increase in PGC‐1α mRNA expression during recovery from intense interval exercise in human skeletal muscle. J. Appl. Physiol. 119:1303–1312. 10.1152/japplphysiol.00048.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, B. E. , Kelly B. M., Lilja M., Ponce‐González J. G., Brogan R. J., Morris D. L., et al. 2017. A practical and time‐efficient high‐intensity interval training program modifies cardio‐metabolic risk factors in adults with risk factors for type II diabetes. Front. Endocrinol. 8:229 10.3389/fendo.2017.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino, N. , Cubeddu A., Begliuomini S., Merlini S., Giannini A., Bucci F., et al. 2009. Daily variation of brain‐derived neurotrophic factor and cortisol in women with normal menstrual cycles, undergoing oral contraception and in postmenopause. Hum. Reprod. 24:2303–2309. 10.1093/humrep/dep119. [DOI] [PubMed] [Google Scholar]
- Pontifex, M. B. , Hillman C. H., Fernhall B. O., Thompson K. M., and Valentini T. A.. 2009. The effect of acute aerobic and resistance exercise on working memory. Med. Sci. Sports Exerc. 41:927–934. 10.1249/MSS.0b013e3181907d69. [DOI] [PubMed] [Google Scholar]
- Rabadi, M. H. 2007. Randomized clinical stroke rehabilitation trials in 2005. Neurochem. Res. 32:807–821. 10.1007/s11064-006-9211-y. [DOI] [PubMed] [Google Scholar]
- Rethorst, C. , and Trivedi M.. 2013. Evidence‐based recommendations for the prescription of exercise for major depressive disorder. J. Psychiatr. Pract. 19:204–212. 10.1097/01.pra.0000430504.16952.3e. [DOI] [PubMed] [Google Scholar]
- Rovio, S. , Kåreholt I., Helkala E. L., Viitanen M., Winblad B., Tuomilehto J., et al. 2005. Leisure‐time physical activity at midlife and the risk of dementia and Alzheimer's disease. Lancet Neurol. 4:705–711. 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- Ruscheweyh, R. , Willemer C., Krüger K., Duning T., Warnecke T., Sommer J., et al. 2011. Physical activity and memory functions: an interventional study. Neurobiol. Aging 32:1304–1319. 10.1016/J.NEUROBIOLAGING.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Saucedo Marquez, C. M. , Vanaudenaerde B., Troosters T., and Wenderoth N.. 2015. High‐intensity interval training evokes larger serum BDNF levels compared with intense continuous exercise. J. Appl. Physiol. 119:1363–1373. 10.1152/japplphysiol.00126.2015. [DOI] [PubMed] [Google Scholar]
- Schiffer, T. , Schulte S., Hollmann W., Bloch W., and Strüder H. K.. 2009. Effects of strength and endurance training on brain‐derived neurotrophic factor and insulin‐like growth factor 1 in humans. Horm. Metab. Res. 41:250–254. Available at: https://www.researchgate.net/profile/Stefanie_Podlog_nee_Schulte/publication/23442813_Effects_of_Strength_and_Endurance_Training_on_Brain-derived_Neurotrophic_Factor_and_Insulin-like_Growth_Factor_1_in_Humans/links/0f31752f98c0a9040b000000/Effects-of-Stre (Accessed: 15 January 2019). [DOI] [PubMed] [Google Scholar]
- Schmolesky, M. T. , Webb D. L., and Hansen R. A.. 2013. The effects of aerobic exercise intensity and duration on levels of brain‐derived neurotrophic factor in healthy men. J. Sports Sci. Med. 12:502–511. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24149158 (Accessed: 16 January 2019). [PMC free article] [PubMed] [Google Scholar]
- Schuch, F. B. , Vancampfort D., Richards J., Rosenbaum S., Ward P. B., and Stubbs B.. 2016. Exercise as a treatment for depression: a meta‐analysis adjusting for publication bias. J. Psychiatr. Res. 77:42–51. 10.1016/j.jpsychires.2016.02.023. [DOI] [PubMed] [Google Scholar]
- Seifert, T. , Brassard P., Wissenberg M., Rasmussen P., Nordby P., Stallknecht B., et al. 2010. Endurance training enhances BDNF release from the human brain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298: R372–R377. 10.1152/ajpregu.00525.2009. [DOI] [PubMed] [Google Scholar]
- Shu, H. F. , Yang T., Yu S. X., Huang H. D., Jiang L. L., Gu J. W., et al. 2014. Aerobic exercise for Parkinson's disease: a systematic review and meta‐analysis of randomized controlled trials. PLoS ONE 9:e100503 10.1371/journal.pone.0100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley, B. A. , and Beilock S. L.. 2007. Exercise and working memory: an individual differences investigation. J. Sport Exerc. Psychol. 29:783–791. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18089904 (Accessed: 15 March 2019). [DOI] [PubMed] [Google Scholar]
- Singh, N. A. , Stavrinos T. M., Scarbek Y., Galambos G., Liber C., Fiatarone Singh M. A., et al. 2005. A randomized controlled trial of high versus low intensity weight training versus general practitioner care for clinical depression in older adults. J. Gerontol. 60:768–776. 10.1093/gerona/60.6.768. [DOI] [PubMed] [Google Scholar]
- Singh, A. M. , Duncan R. E., Neva J. L., and Staines W. R.. 2014a. Aerobic exercise modulates intracortical inhibition and facilitation in a nonexercised upper limb muscle. BMC Sports Sci. Med. Rehabil. 6:23 10.1186/2052-1847-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, A. M. , Neva J. L., and Staines W. R.. 2014b. Acute exercise enhances the response to paired associative stimulation‐induced plasticity in the primary motor cortex. Exp. Brain Res. 232:3675–3685. 10.1007/s00221-014-4049-z. [DOI] [PubMed] [Google Scholar]
- Skriver, K. , Roig M., Lundbye‐Jensen J., Pingel J., Helge J. W., Kiens B., et al. 2014. Acute exercise improves motor memory: exploring potential biomarkers. Neurobiol. Learn. Mem. 116:46–58. 10.1016/J.NLM.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Smith, P. J. , Blumenthal J. A., Hoffman B. M., Cooper H., Strauman T. A., Welsh‐Bohmer K., et al. 2010. Aerobic exercise and neurocognitive performance: a meta‐analytic review of randomized controlled trials. Psychosom. Med. 72:239 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, A. E. , Goldsworthy M. R., Garside T., Wood F. M., and Ridding M. C.. 2014. The influence of a single bout of aerobic exercise on short‐interval intracortical excitability. Exp. Brain Res. 232:1875–1882. 10.1007/s00221-014-3879-z. [DOI] [PubMed] [Google Scholar]
- Sofi, F. , Valecchi D., Bacci D., Abbate R., Gensini G. F., Casini A., et al. 2011. Physical activity and risk of cognitive decline: a meta‐analysis of prospective studies. J. Intern. Med. 269:107–117. 10.1111/j.1365-2796.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- Stevens, J. , and Killeen M.. 2006. A randomised controlled trial testing the impact of exercise on cognitive symptoms and disability of residents with dementia. Contemp. Nurse 21:32–40. 10.5172/conu.2006.21.1.32. [DOI] [PubMed] [Google Scholar]
- Stinear, C. M. , Barber P. A., Coxon J. P., Fleming M. K., and Byblow W. D.. 2008. Priming the motor system enhances the effects of upper limb therapy in chronic stroke. Brain 131:1381–1390. 10.1093/brain/awn051. [DOI] [PubMed] [Google Scholar]
- Stranahan, A. M. , Lee K., Martin B., Maudsley S., Golden E., Cutler R. G., et al. 2009. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus 19:951–961. 10.1002/hipo.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawbridge, W. J. , Deleger S., Roberts R. E., and Kaplan G. A.. 2002. Physical activity reduces the risk of subsequent depression for older adults. Am. J. Epidemiol. 156:328–334. 10.1093/aje/kwf047. [DOI] [PubMed] [Google Scholar]
- Takahashi, K. , Maruyama A., Hirakoba K., Maeda M., Etoh S., Kawahira K., et al. 2011. Fatiguing intermittent lower limb exercise influences corticospinal and corticocortical excitability in the nonexercised upper limb. Brain Stimul. 4:90–96. 10.1016/j.brs.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Tang, S. W. , Chu E., Hui T., Helmeste D., and Law C.. 2008. Influence of exercise on serum brain‐derived neurotrophic factor concentrations in healthy human subjects. Neurosci. Lett. 431:62–65. 10.1016/J.NEULET.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tarnopolsky, L. J. , MacDougall J. D., Atkinson S. A., Tarnopolsky M. A., and Sutton J. R.. 1990. Gender differences in substrate for endurance exercise. J. Appl. Physiol. 68:302–308. 10.1152/jappl.1990.68.1.302. [DOI] [PubMed] [Google Scholar]
- Tarnopolsky, M. A. , Atkinson S. A., Phillips S. M., and MacDougall J. D.. 1995. Carbohydrate loading and metabolism during exercise in men and women. J. Appl. Physiol. 78:1360–1368. 10.1152/jappl.1995.78.4.1360. [DOI] [PubMed] [Google Scholar]
- Teri, L. , Gibbons L. E., McCurry S. M., Logsdon R. G., Buchner D. M., Barlow W. E., et al. 2003. Exercise plus behavioral management in patients with Alzheimer disease: a randomized controlled trial. JAMA 290:2015–2022. 10.1001/jama.290.15.2015. [DOI] [PubMed] [Google Scholar]
- Trejo, J. L. , Carro E., and Torres‐Alemán I.. 2001. Circulating insulin‐like growth factor i mediates exercise‐induced increases in the number of new neurons in the adult hippocampus. J. Neurosci. 21:1628–1634. 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth, N. , Heitz R. P., Schrock J. C., and Engle R. W.. 2005. An automated version of the operation span task. Behav. Res. Methods 37:498–505. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16405146 (Accessed: 15 March 2019). [DOI] [PubMed] [Google Scholar]
- Vaynman, S. , Ying Z., and Gómez‐Pinilla F.. 2004. Exercise induces BDNF and synapsin I to specific hippocampal subfields. J. Neurosci. Res. 76:356–362. 10.1002/jnr.20077. [DOI] [PubMed] [Google Scholar]
- Voss, M. W. , Erickson K. I., Prakash R. S., Chaddock L., Kim J. S., Alves H., et al. 2013. Neurobiological markers of exercise‐related brain plasticity in older adults. Brain Behav. Immun. 28:90–99. 10.1016/J.BBI.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weuve, J. , Kang J. H., Manson J. E., Breteler M. M., Ware J. H., and Grodstein F.. 2004. Physical activity, including walking, and cognitive function in older women. JAMA 292:1454–1461. 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- Whelton, S. P. , Chin A., and He J.. 2002. Effect of aerobic exercise on blood pressure: a meta‐analysis of randomized, controlled trials. Ann. Intern. Med. 136:493–503. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11926784 (Accessed: 15 March 2019). [DOI] [PubMed] [Google Scholar]
- Yaffe, K. , Fiocco A. J., Lindquist K., Vittinghoff E., Simonsick E. M., Newman A. B., et al. 2009. Predictors of maintaining cognitive function in older adults: the Health ABC study. Neurology 72:2029–2035. 10.1212/WNL.0b013e3181a92c36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi, T. , Fujiwara T., Liu W., and Liu M.. 2012. Effects of pedaling exercise on the intracortical inhibition of cortical leg area. Exp. Brain Res. 218:401–406. 10.1007/s00221-012-3026-7. [DOI] [PubMed] [Google Scholar]
- Ziemann, U. , Chen R., Cohen L. G., and Hallett M.. 1998. Dextromethorphan decreases the excitability of the human motor cortex. Neurology 51:1320–1324. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9818853 (Accessed: 22 February 2019). [DOI] [PubMed] [Google Scholar]
- Zoladz, J. A. , Pilc A., Majerczak J., Grandys M., Zapart‐Bukowska J., and Duda K.. 2008. Endurance training increases plasma brain‐derived neurotrophic factor concentration in young healthy men. J. Physiol. Pharmacol. 59(Suppl 7):119–132. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19258661 (Accessed: 15 March 2019). [PubMed] [Google Scholar]


