Abstract
Background and Purpose
The NO/cGMP pathway represents a major physiological signalling controlling tone in pulmonary arteries (PA), and drugs activating this pathway are used to treat pulmonary arterial hypertension. Kv channels expressed in PA smooth muscle cells (PASMCs) are key determinants of vascular tone. We aimed to analyse the contribution of Kv1.5 and Kv7 channels in the electrophysiological and vasodilating effects evoked by NO donors and the GC stimulator riociguat in PA.
Experimental Approach
Kv currents were recorded in isolated rat PASMCs using the patch‐clamp technique. Vascular reactivity was assessed in a wire myograph.
Key Results
The NO donors diethylamine NONOate diethylammonium (DEA‐NO) and sodium nitroprusside hyperpolarized the membrane potential and induced a bimodal effect on Kv currents (augmenting the current between −40 and −10 mV and decreasing it at more depolarized potentials). The hyperpolarization and the enhancement of the current were suppressed by Kv7 channel inhibitors and by the GC inhibitor ODQ but preserved when Kv1.5 channels were inhibited. Additionally, DEA‐NO enhanced Kv7.5 currents in COS7 cells expressing the KCNQ5 gene. Riociguat increased Kv currents at all potentials ≥−40 mV and induced membrane hyperpolarization. Both effects were prevented by Kv7 inhibition. Likewise, PA relaxation induced by NO donors and riociguat was attenuated by Kv7 inhibitors.
Conclusions and Implications
NO donors and riociguat enhance Kv7 currents, leading to PASMC hyperpolarization. This mechanism contributes to NO/cGMP‐induced PA vasodilation. Our study identifies Kv7 channels as a novel mechanism of action of vasodilator drugs used in the treatment of pulmonary arterial hypertension.
Abbreviations
- DEA‐NO
diethylamine NONOate diethylammonium
- Kv
voltage‐gated potassium channels
- PA
pulmonary arteries
- PASMC
PA smooth muscle cell
- Phe
phenylephrine
- sGC
soluble GC
- SNP
sodium nitroprusside
What is already known
Kv channels and the NO/cGMP pathway are key determinants of pulmonary vascular tone.
What this study adds
Kv7 channel activation contributes to the electrophysiological and relaxant effects of NO donors and riociguat.
What is the clinical significance
Kv7 channels represent a new target for drugs activating the NO/cGMP pathway in pulmonary vasculature.
1. INTRODUCTION
NO is a key endogenous vasodilator playing a pivotal role in maintaining pulmonary vascular tone (Barnes & Liu, 1995; Coggins & Bloch, 2007). The classical NO signalling pathway involves the activation of soluble GC (sGC) and the subsequent generation of cGMP (Barnes & Liu, 1995; Kraehling & Sessa, 2017), while alternative mechanisms (such as S‐nitrosylation) may also take place (Coggins & Bloch, 2007; Hess, Matsumoto, Kim, Marshall, & Stamler, 2005). This second messenger activates PKG which, in turn, targets a number of downstream mechanisms to induce vasodilation (Cogolludo et al., 2001; Sausbier et al., 2000).
Activation of K+ channels is considered an important mechanism mediating NO/cGMP‐induced vasodilation by promoting membrane hyperpolarization and, subsequently, closure of L‐type calcium channels (Cogolludo, Moreno, & Villamor, 2007; Cogolludo et al., 2001; Jackson, 2018; Sausbier et al., 2000). Thus, NO‐induced relaxation of systemic and pulmonary arteries (PA) has been related to both cGMP‐dependent and ‐independent activation of large‐conductance calcium‐activated potassium (BKCa) channels (Bolotina, Najibi, Palacino, Pagano, & Cohen, 1994; Robertson, Schubert, Hescheler, & Nelson, 1993) and voltage‐gated K+ (Kv) channels (Cogolludo et al., 2001; Plane, Sampson, Smith, & Garland, 2001; Yuan, Tod, Rubin, & Blaustein, 1996). More than 20 years ago, Yuan et al. (1996) showed that activation of Kv channels contributed to NO‐induced hyperpolarization of PA smooth muscle cells (PASMCs) and PA relaxation. Later, activation of these channels was also reported to contribute to NO‐induced apoptosis of PASMCs (Krick et al., 2002). Among the different members of the Kv channel family expressed in PASMCs, including Kv1.5, Kv2.1, and Kv1.2 channels, the former are considered major contributors to the total Kv current, especially in resistance PASMCs (Archer et al., 1998; Moral‐Sanz et al., 2011; Smirnov, Beck, Tammaro, Ishii, & Aaronson, 2002). Intriguingly, NO donors have been shown to either activate (Remillard et al., 2007) or inhibit (Núñez et al., 2006) Kv1.5 channels when expressed in heterologous systems. Thus, the identity of the Kv channel activated by NO in PASMCs remains unclear.
During the last decade, Kv channels encoded by KCNQ1–5 genes (Kv7.1–Kv7.5) have been reported to regulate resting membrane potential and contractility in various blood vessels (Barrese, Stott, & Greenwood, 2018; Chadha, Zunke, Davis, et al., 2012; Mackie & Byron, 2008; Yeung et al., 2007). Likewise, a number of studies have shown that activation of Kv7 channels contributes to the relaxation induced by agents stimulating the production of cAMP (Chadha, Zunke, Zhu, et al., 2012; Khanamiri et al., 2013; Mani et al., 2016; Morales‐Cano et al., 2015, 2016). However, the possible contribution of these channels in the relaxation induced by the NO/cGMP pathway remains poorly studied.
Impairment of the NO/cGMP pathway has been implicated in the pathogenesis of cardiovascular diseases, including pulmonary hypertension (PH; Klinger & Kadowitz, 2017; Schermuly, Ghofrani, Wilkins, & Grimminger, 2011). Moreover, pharmacological stimulation of this pathway at different levels using inhaled NO, PDE5 inhibitors (eg, sildenafil), or sGC stimulators (eg, riociguat) represents a key strategy in the treatment of pulmonary vascular diseases such as PH (Galiè et al., 2015; Kraehling & Sessa, 2017; Lau, Giannoulatou, Celermajer, & Humbert, 2017; Stasch, Pacher, & Evgenov, 2011). Thus, the elucidation of novel mechanisms underlying the effects of these drugs would have great therapeutic relevance.
In the present study, we aimed to shed light on the functional modulation of Kv currents by the NO/cGMP pathway in freshly isolated PASMCs. We examined the dependence of Kv1.5 and Kv7 channels on NO/cGMP‐mediated responses in PA. The findings of our study provide evidence that Kv7 channels contribute to the pulmonary vasodilation induced by NO donors and riociguat.
2. METHODS
2.1. Animal preparation
All experimental procedures utilizing animals were carried out according to the Care and Use of Laboratory Animals and approved by the institutional Ethical Committee of the Universidad Complutense de Madrid (Madrid, Spain) and the regional Committee for Laboratory Animals Welfare (Comunidad de Madrid, ref. number PROEX‐301/16). Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010). Male Wistar rats (250–300 g) were obtained from Envigo (RRID:RGD_5508396, Barcelona, Spain). All animals were kept with free access to standard rat chow and water in an enriched environment and maintained at 24°C under a 12‐hr light/12‐hr dark cycle. The rats were killed using the CO2 method, which provided a rapid, painless, stress‐free death. The CO2 flow rate was displaced from 10% to 30% of the cage volume per minute following 2013 AVMA guidelines (Leary & Golab, 2013). CO2 flow was maintained for at least 1 min after respiratory arrest. Death was verified by observation for vital signs.
2.2. Tissue and cell isolation
Rat resistance intrapulmonary arteries (250–500 μm) were isolated from the lungs and cut into rings. Rings were randomly assigned to different experimental groups. For isolation of PASMCs, PA were placed into a nominally Ca2+‐free physiological salt solution of the following composition (in mM): NaCl 130, KCl 5, MgCl2 1.2, glucose 10, and HEPES 10 (pH 7.3 with NaOH) containing (in mg·ml−1) papain 1, DTT 0.8, and albumin 0.7 for 8–12 min as previously described (Cogolludo et al., 2006). PASMCs were dissociated using a wide bore, smooth‐tipped pipette, stored in Ca2+‐free physiological salt solution (4°C) and used within 8 hr of isolation.
2.3. Cell culture
COS7 (ATCC Cat# CRL‐1651, RRID:CVCL_0224) cells were grown in DMEM containing 10% FBS and supplemented with 100‐U·ml−1 penicillin/streptomycin. Human wild‐type KCNQ5 cDNA was transfected in COS7 cells at nearly 80% confluence. COS7 were transfected with 1‐μg pEYFP‐N1‐KCNQ5 plasmid using the FUGENE6 transfection method (Promega). The ratio μg DNA:μl Fugene was 1:3. Twenty‐four hours after transfection, cells were washed in PBS and used.
2.4. Electrophysiological studies
Membrane currents were recorded with an Axopatch 200B and a Digidata 1322A (Axon Instruments, Burlingame, CA, USA) using the whole‐cell configuration of the patch‐clamp technique. Freshly isolated PASMCs were superfused with an external Ca2+‐free HEPES solution (see above) and a Ca2+‐free pipette (internal) solution containing (in mM) KCl 130, MgCl2 1.2, Na2ATP 5, HEPES 10, and EGTA 10 (pH adjusted to 7.3 with KOH). Kv currents were evoked following the application of depolarizing ramps from −60 to +20 mV. In some experiments, long (4 s) depolarizing steps from −60 to +20 mV were applied. Kv currents were initially monitored for 3–5 min, and only cells seen to have stable currents were then used for experiments. Under these conditions, currents remained stable and were usually reproducible for at least 20 min. The electrophysiological effects induced by the NO donors diethylamine NONOate diethylammonium (DEA‐NO) and sodium nitroprusside (SNP) and the sGC stimulator riociguat were tested until they reached steady state. In some experiments, cells were exposed for 10 min to selective inhibitors of Kv1.5 (DPO‐1, 1 μM), Kv7 (XE991, 10 μM or linopirdine, 10 μM), or BKCa channels (iberiotoxin, 0.1 μM), or sGC (ODQ, 10 μM) and then challenged with NO donors or riociguat in the continuous presence of these inhibitors. Currents were normalized for cell capacitance and expressed in pA·pF−1. Membrane potential was recorded under the current‐clamp mode.
Kv7.5 currents expressed in COS7 were recorded using the perforated‐patch configuration of the patch‐clamp technique (amphotericin B, 125 μg·ml−1 in the internal solution) to prevent rundown using an Axopatch 200B amplifier (Axon Instruments) as previously described (Moreno et al., 2017; Oliveras et al., 2014). The intracellular pipette filling solution contained (in mM) K‐aspartate 80, KCl 50, phosphocreatine 3, KH2 PO4 10, MgATP 3, HEPES‐K 10, and EGTA 5 and was adjusted to pH 7.25 with KOH. The bath solution contained (in mM) NaCl 130, KCl 4, CaCl2 1.8, MgCl2 1, HEPES‐Na 10, and glucose 10 and was adjusted to pH 7.40 with NaOH. All experiments were performed at room temperature (22–24°C).
2.5. Recording of arterial reactivity
For contractile tension recording, PA rings were mounted in a wire myograph with Krebs buffer solution maintained at 37°C and bubbled with 21% O2, 74% N2, and 5% CO2. Vessels were stretched to give an equivalent transmural pressure of 30 mmHg. Preparations were firstly stimulated by raising the K+ concentration of the buffer (to 80 mM) in exchange for Na+. Vessels were washed three times and allowed to recover before a new stimulation. The relaxant effects induced by the NO donors DEA‐NO and SNP and riociguat were examined in PA stimulated with phenylephrine (Phe, 1 μM). Relaxation was expressed as a percentage of the reduction in Phe‐induced contraction. Some experiments were performed in the presence of XE991, iberiotoxin, DPO‐1, or ODQ.
2.6. Data and statistical analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2018). The data analyst was blinded, whereas blinding for the operator was not possible since some drugs used (i.e., DEA‐NO) had to be prepared immediately before their use due to their fast decomposition. All data were analysed using GraphPad Prism 5.01 (RRID:SCR_002798). Data are expressed as mean ± SEM; n indicates the number of independent experiments performed at different preparations (PA or PASMC derived from different PA) from at least five animals. Statistical analysis was performed using Student's t test for paired or unpaired observations or one‐way ANOVA followed by a Bonferroni test for multiple comparisons. Current–voltage relations were analysed by two‐way repeated‐measures ANOVA with the Bonferroni post hoc test. Differences were considered statistically significant when P was less than 0.05.
2.7. Materials
Drugs and reagents were obtained from Sigma‐Aldrich Quimica (Madrid, Spain), except riociguat (MedChem Express Europe, Sollentuna, Sweden). Drugs were dissolved in DMSO, and final vehicle concentrations were always ≤0.1%.
2.8. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Fabbro et al., 2017; Alexander, Striessnig et al., 2017).
3. RESULTS
3.1. Bimodal effects of NO donors on total Kv current
Figure 1 shows original traces (panels a and b) and mean data (panels c and d) of the K+ currents recorded following the application of a depolarizing ramp from −60 to +20 mV. The NO donors DEA‐NO (10 μM) and SNP (10 μM) induced a bimodal effect on K+ currents, augmenting the current between ≈−40 and −10 mV and decreasing it at more depolarized potentials. The onset of the responses to NO donors was fast (≈30 s for the inhibitory effects and ≈60–90 s for the enhancing effect), and stable responses were reached within 4–5 min. The increase of the current at −30 mV and the decrease of the current at +20 mV induced by both NO donors are depicted in inset panels. Figure 1e,f illustrates the current sensitive to DEA‐NO and SNP, respectively, obtained by subtracting the current in the absence and in the presence of the drug. Both NO donors enhanced the Kv current at similar membrane potentials, within a physiological range. Likewise, this was associated with PASMC hyperpolarization (Figure 1g,h). We also studied the concentration dependence of DEA‐NO‐induced effects. Both the increase (Figure 2a, top) and the decrease (Figure 2a, bottom) of the current were evident at concentrations ≥0.1 μM and reached a maximal response at 10 and 30 μM, respectively.
Figure 1.
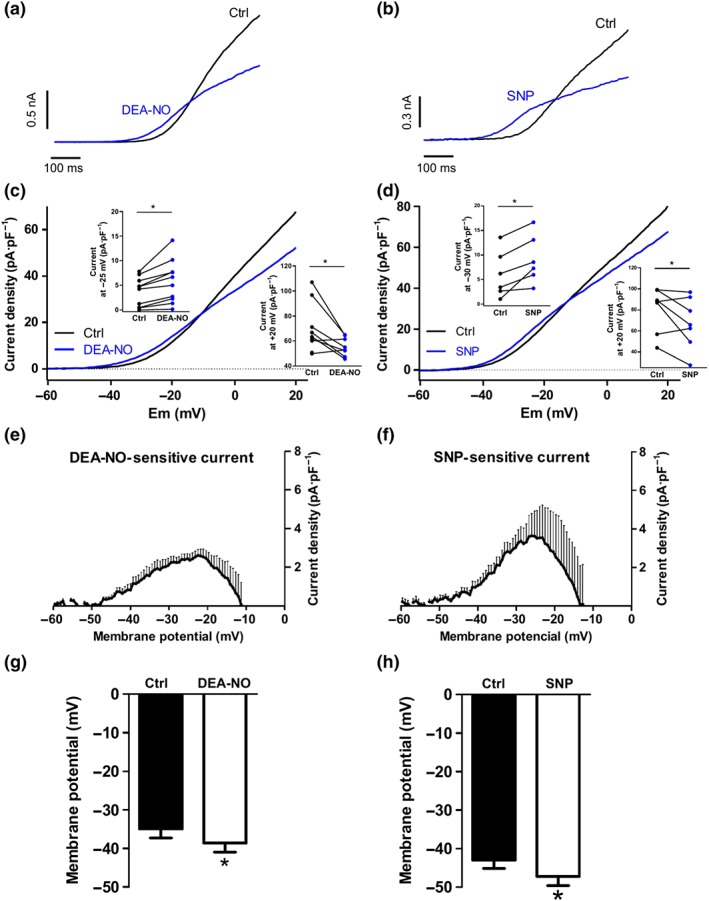
Bimodal modulation of Kv currents by NO donors in PASMCs. (a and b) Representative traces and (c and d) mean values of Kv currents recorded after the application of depolarizing ramps from −60 to +20 mV in the absence (black lines) or the presence (blue lines) of DEA‐NO (10 μM, n = 10 from seven animals) or SNP (10 μM, n = 7 from six animals), respectively. Inset shows the current density at −30 and +20 mV before and after the addition of DEA‐NO or SNP. (e and f) Average values of DEA‐NO‐ and SNP‐sensitive currents obtained by subtracting the current in the presence and in the absence of the drug. (g and h) Mean values of the membrane potential in PASMCs in the absence (Ctrl) and in the presence of DEA‐NO (n = 9 from seven animals) and SNP (n = 7 from six animals). *P < 0.05 versus Ctrl (Student's paired t test). Results are means ± SEM
Figure 2.
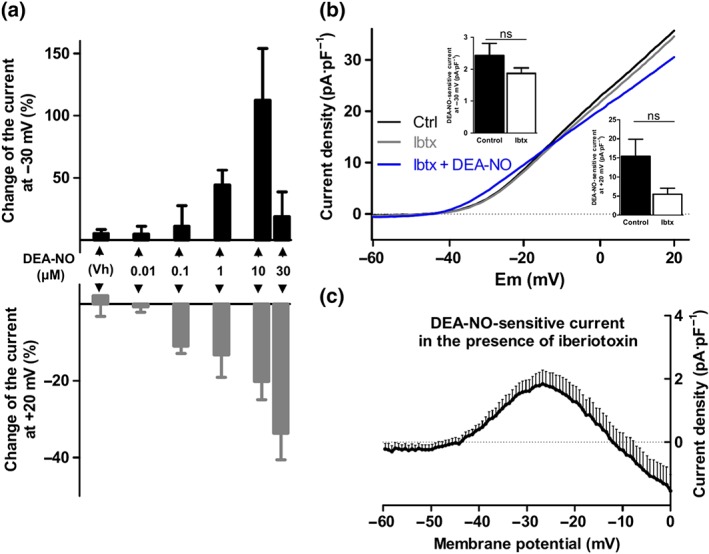
Concentration‐dependence and lack of BKCa channels involvement, in the electrophysiological effects of NO donors. (a) Changes in the current at −30 mV (black, top) and at +20 mV (grey, bottom) induced by vehicle (DMSO, 0.1%) or different concentrations of DEA‐NO (0.01–30 μM, n = 7–10 for each concentration from three to eight animals). (b) Mean values of Kv currents in PASMCs recorded after the application of depolarizing ramps from −60 to +20 mV in the absence and in the presence of the BKCa channel inhibitor iberiotoxin (Ibtx, 0.1 μM, n = 9 from five animals) before (grey lines) and after (blue lines) the addition of DEA‐NO (10 μM). Inset shows the DEA‐NO‐sensitive current at −30 and +20 mV under control conditions or in the presence of Ibtx. (c) DEA‐NO‐sensitive currents in the presence of Ibtx, obtained by subtracting the current in the presence and in the absence of the NO donor. Results are means ± SEM
3.2. Kv7 channel inhibitors prevent the increase in Kv current induced by NO donors
In the next set of experiments, we tested the electrophysiological effects of DEA‐NO in the presence of potassium channel inhibitors. In the presence of the BKCa channel inhibitor iberiotoxin, the bimodal effects of DEA‐NO were still observed (Figure 2b,c), and the changes at −30 and +20 mV were similar to those recorded under control conditions (inset). On the other hand, in the presence of the Kv7 channel inhibitors XE991 (10 μmol·L−1; Figure 3a,c) or linopirdine (10 μmol·L−1; Figure 3b,d), only the inhibitory, but not the enhancing, component was evident. Thus, DEA‐NO‐sensitive current at −30 mV was markedly reduced in the presence of these inhibitors (top insets), while at +20 mV, no statistical differences were found (insets bottom). Under these conditions, DEA‐NO behaved as a Kv channel inhibitor at potentials positive to ≈−25 mV (Figure 3c,d). XE991 and linopirdine also prevented the hyperpolarization induced by DEA‐NO (Figure 3e,f).
Figure 3.
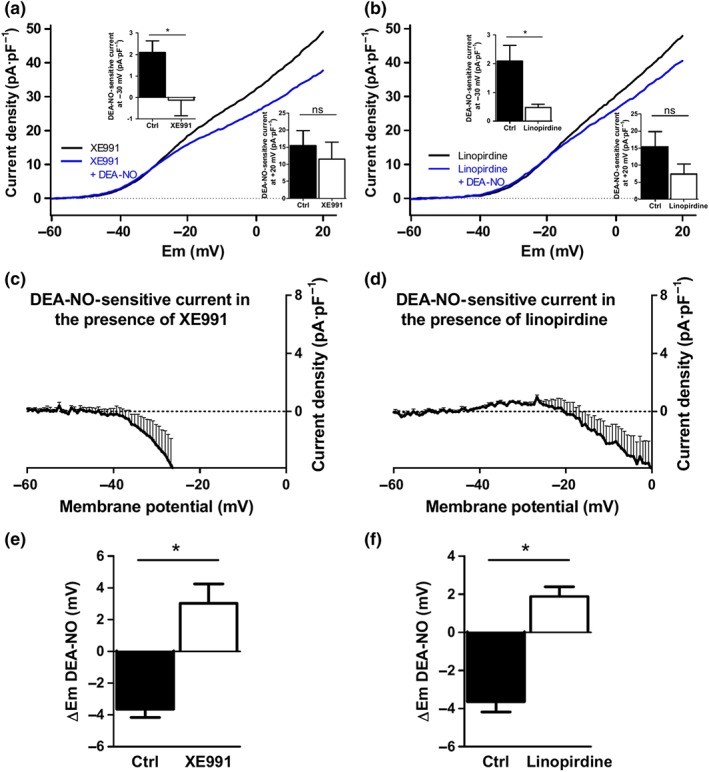
The activation of Kv current and hyperpolarization induced by DEA‐NO is prevented by Kv7 channel inhibitors. (a and b) Mean values of Kv currents in PASMCs recorded after the application of depolarizing ramps from −60 to +20 mV in the presence of the Kv7 channel inhibitors (a) XE991 (10 μM, n = 6 from five animals) or (b) linopirdine (10 μM, n = 6 from five animals) before (black lines) and after (blue lines) the addition of DEA‐NO (10 μM). Inset shows the DEA‐sensitive current at −30 and +20 mV under control conditions (n = 10 from seven animals) and in the presence of XE991 or linopirdine (n = 6 from five animals). (c and d) Average values of DEA‐NO‐sensitive currents under both conditions obtained by subtracting the current in the presence and in the absence of DEA‐NO. (e and f) Mean values of the changes in the membrane potential induced by DEA‐NO in the absence (Ctrl) and in the presence of the Kv7 channel inhibitors (XE991, n = 6 from five animals, or linopirdine, n = 5). *P < 0.05 versus Ctrl (Student's unpaired t test). Results are means ± SEM
In another set of experiments, long (4 s) depolarizing pulses were applied to increase the contribution of the slowly activating channels (such as Kv7) to the total current. Using this protocol, we found that some cells showed a clear time inactivation, while others showed apparently no inactivation (Figure 4a, top and bottom). Addition of SNP led to a similar increase in the current in both cases. Thus, rather than inducing a bimodal effect, SNP exclusively enhanced the Kv current at all potentials tested ≥−40 mV (Figure 4b), resulting in a positive SNP‐sensitive current at all these potentials (Figure 4e). When PASMCs were perfused with XE991, a modest reduction on the Kv currents was observed (Figure 4c,d). Under these conditions, the addition of SNP in the continuous presence of XE991 did not produce any effect. Thus, the SNP‐sensitive current was blunted in the presence of the Kv7 channel blocker (Figure 4e). In addition, XE991 prevented the hyperpolarizing effect (Figure 4f) induced by SNP.
Figure 4.
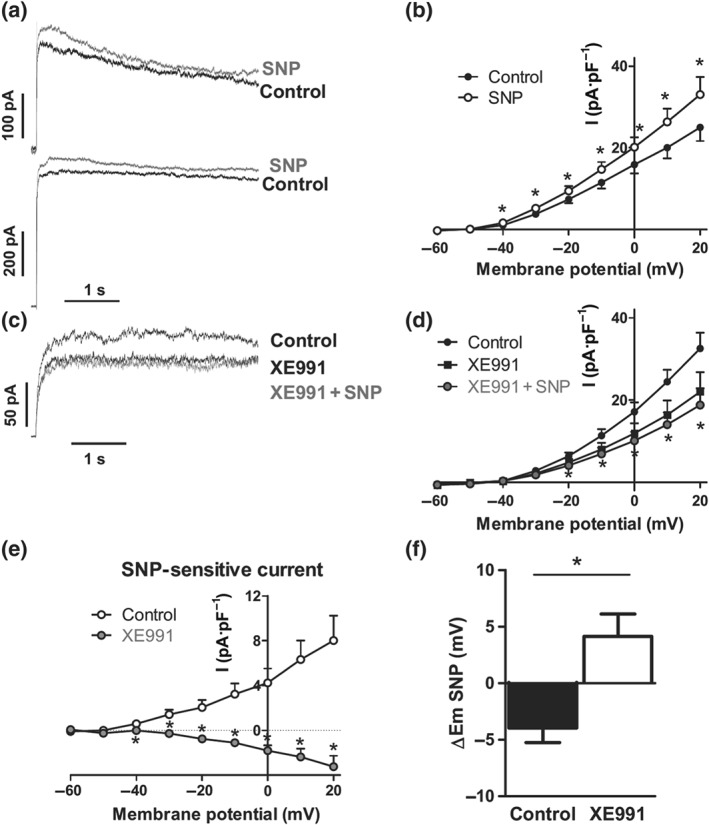
SNP‐induced activation of Kv current and hyperpolarization is prevented by Kv7 channel inhibition. (a) Representative experiments in PASMCs showing inactivating (top) or non‐inactivating (bottom) Kv currents obtained after 4‐s depolarization pulses from −60 to +20 mV in the absence and in the presence of SNP (10 μM, n = 11 from eight animals). (b) Current–voltage relationships in the absence and in the presence of SNP. (c) Representative current traces and (d) average values of the Kv currents under control conditions or following the addition of XE991 (10 μM) and XE991 + SNP (10 μM, n = 7 from five animals). (e) Average values of SNP‐sensitive currents in the absence (control) or the presence of XE991 obtained by subtracting the current in the presence and in the absence of SNP. (f) Mean values of the changes in the membrane potential induced by SNP in the absence (Ctrl, n = 11 from eight animals) and in the presence of XE991 (n = 6 from five animals). *P < 0.05 versus Ctrl (Student's paired or unpaired t test). Results are means ± SEM
3.3. DEA‐NO activates Kv7.5 channels
In order to confirm the regulation of NO on Kv7 channels observed in isolated PASMC, we tested the electrophysiological effects of DEA‐NO in COS7 cells expressing Kv7.5 channels. As previously reported, Kv7.5 currents were evident at membrane potentials more positive than −50 mV (Figure 5a,b). The application of DEA‐NO increased Kv7.5 current amplitudes at potentials positive to −40 mV reaching statistical significance at potentials ranging from −40 to +40 mV.
Figure 5.
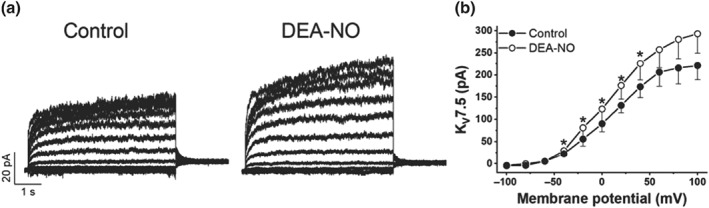
DEA‐NO enhances K+ currents in COS7 cells expressing Kv7.5 channels. (a) Representative current traces and (b) average values of the Kv7.5 currents in COS7 in the presence of DEA‐NO (10 μM, n = 5). Currents were evoked by series of 5.5‐s pulses from −100 to +100 mV in 20‐mV steps from a holding potential of −80 mV. *P < 0.05 versus Ctrl (Student's paired t test). Results are means ± SEM
3.4. Activation of Kv7 channels contributes to the pulmonary vasodilation induced by NO donors
To ascertain if the electrophysiological effects of NO donors contribute to their relaxing effect, we studied the vasodilation induced by DEA‐NO and SNP in isolated PA. Phe‐stimulated PA were relaxed in a concentration‐dependent manner by DEA‐NO (Figure 6a) and SNP (Figure 6b). XE991 (Figure 6a,b), but not iberiotoxin (Figure 6c), attenuated this relaxant response. Moreover, XE991 did not affect the relaxation induced by the selective KATP channel opener pinacidil (Figure 6d). These data indicated that Kv7 channels contributed to NO‐induced pulmonary vasodilation.
Figure 6.
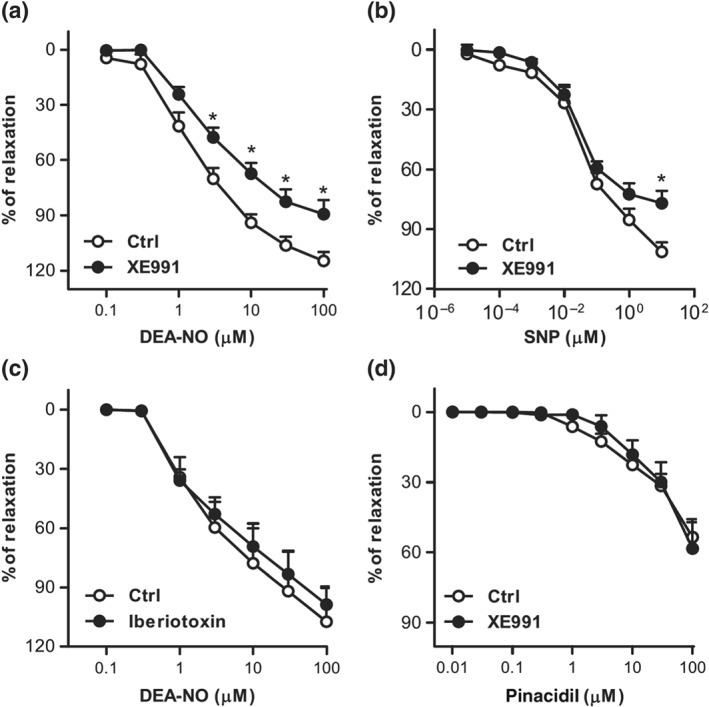
Pulmonary vasodilation induced by NO donors is attenuated by Kv7 channel inhibition. (a and b) Averaged data showing the concentration‐dependent relaxant responses induced by DEA‐NO (0.1–30 μM, n = 8–11) or SNP (0.00001–10 μM, n = 5–6) in the absence or the presence of XE991 (10 μM). (c) Averaged data showing the concentration‐dependent relaxant responses induced by DEA‐NO in the absence or the presence of iberiotoxin (0.1 μM, n = 6). (d) Averaged data showing the concentration‐dependent relaxant responses induced by pinacidil in the absence or the presence of XE991 (10 μM, n = 6). *P < 0.05 versus Ctrl. Results are means ± SEM
3.5. The effects of DEA‐NO are not affected by Kv1.5 channel inhibition
So far, our data suggested that activation of Kv1.5 channels did not contribute to the activation of Kv current and the hyperpolarization induced by NO donors. To confirm this, we analysed the effects of DEA‐NO in the presence of the Kv1.5 channel blocker DPO‐1. Current amplitude in the presence of DPO‐1 was reduced in relation to control conditions (cf. Figure 7a vs. Figure 4b). We observed that the enhancing effect of this NO donor on Kv currents was preserved in the presence of DPO‐1 (Figure 7a). Moreover, under these conditions, DEA‐NO caused an increase in the outward current amplitude at all potentials, rather inhibiting the current at positive potentials (Figure 7b). In line with these data, the hyperpolarizing (Figure 7c) and the vasodilating (Figure 7d) effects of DEA‐NO were unaffected by the Kv1.5 channel inhibitor.
Figure 7.
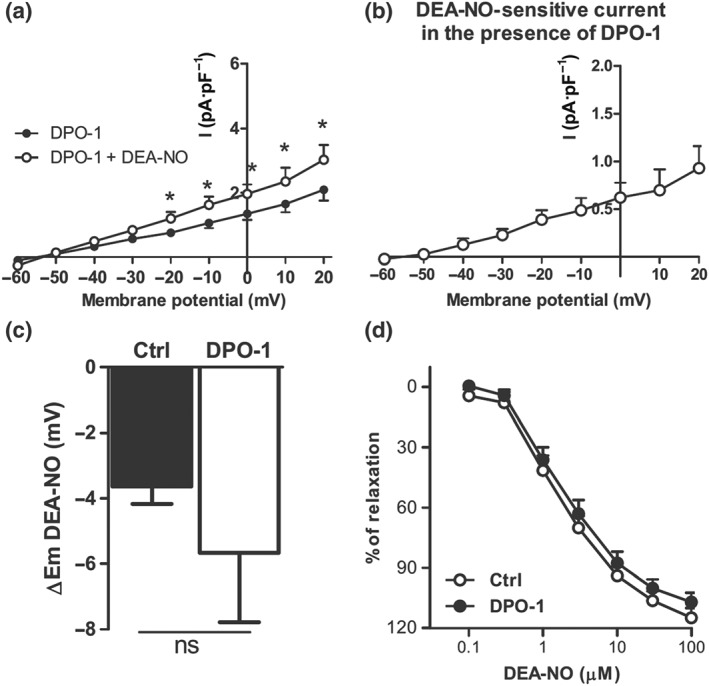
DEA‐NO‐induced activation of Kv current, hyperpolarization, and relaxation is not affected by Kv1.5 channel inhibition. (a) Average values of the Kv currents obtained after 4‐s depolarization pulses from −60 to +20 mV in PASMCs perfused with the Kv1.5 channel inhibitor DPO‐1 (1 μM, n = 10 from seven animals) in the absence and in the presence of DEA‐NO (10 μM). (b) Average values of DEA‐NO‐sensitive currents in the presence of DPO‐1 obtained by subtracting the current in the presence and in the absence of DEA‐NO. (c) Mean values of the changes in the membrane potential induced by DEA‐NO in the absence and in the presence of DPO‐1 (n = 8–9 from five to seven animals). (d) Averaged data of the concentration‐dependent relaxant responses induced by DEA‐NO (0.1–30 μM) in the absence (n = 11) or the presence of DPO‐1 (n = 6). *P < 0.05 versus DPO‐1 (Student's paired t test). Results are means ± SEM
3.6. Role of the sGC
We studied the involvement of sGC in the relaxation induced by NO donors. We found that the sGC inhibitor ODQ, at 10 μM, induced a marked inhibition of DEA‐NO‐induced vasodilation (Figure 8a). Interestingly, in the presence of the ODQ, the Kv7 channel inhibitor XE991 did not significantly affect DEA‐NO‐induced relaxation. Moreover, ODQ suppressed the relaxations elicited by concentrations of DEA‐NO ≤3 μM, indicating that at concentrations up to 3 μM, the relaxation to DEA‐NO was due to an increase in cGMP levels. Thus, we tested the electrophysiological effects of 1‐μM DEA‐NO in the presence of ODQ. Notably, ODQ markedly reduced the increase in Kv current (Figures 8c) and the hyperpolarization (Figure 8d) induced by the NO donor in PASMC. Likewise, ODQ prevented the enhancement of Kv7.5 currents expressed in COS7 cells which was induced by DEA‐NO (Figure 8e,f).
Figure 8.
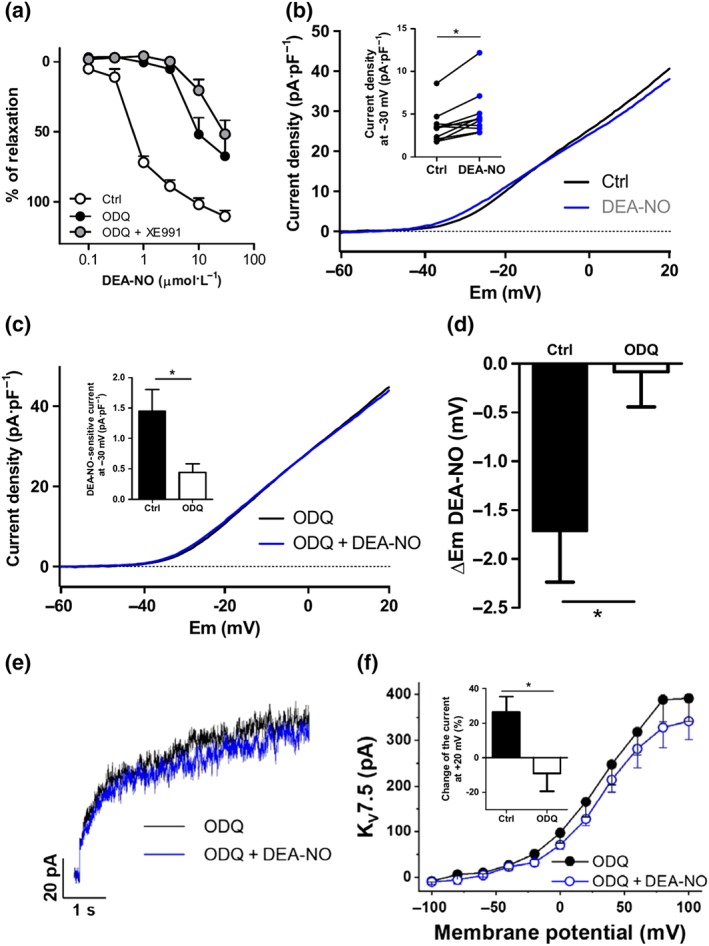
Role of sGC in DEA‐NO‐induced effects. (a) Averaged data of the concentration‐dependent relaxant responses induced by DEA‐NO (0.1–30 μM) in the absence (Ctrl, n = 15 from 12 animals) or the presence of the sGC inhibitor ODQ (10 μM, n = 9 from eight animals) or ODQ + XE991 (10 μM, n = 7 from five animals). (b) Mean values of the Kv currents recorded after the application of depolarizing ramps from −60 to +20 mV in the absence or the presence of DEA‐NO (1 μM, n = 11 from six animals). Inset shows the current density at −30 mV before and after the addition of DEA‐NO. (c) Mean values of Kv currents recorded after the application of depolarizing ramps from −60 to +20 mV in PASMCs perfused with ODQ in the absence or the presence of DEA‐NO (1 μM, n = 11 from five animals). Inset shows the changes in current density at −30 mV induced by DEA‐NO in the absence and in the presence of ODQ. (d) Mean values of the changes in the membrane potential induced by DEA‐NO in the absence and in the presence of ODQ (n = 7–12 from five animals). (e) Representative current traces at +20 mV and (f) average values of Kv7.5 currents in COS7 perfused with ODQ (10 μM) before and after the addition of DEA‐NO (10 μM, n = 6). Inset shows the changes in the current at +20 mV (expressed as a percentage) induced by DEA‐NO in the absence and in the presence of ODQ. *P < 0.05 versus Ctrl (Student's paired or unpaired t test). Results are means ± SEM
3.7. Activation of Kv7 channels contributes to the relaxation induced by riociguat
We then tested the electrophysiological effects of the sGC stimulator riociguat. This drug induced a substantial increase in K+ currents at all potentials ≥−40 mV within the first 1–2 min and reached a stable effect at 5–7 min (Figure 9a,b). In addition, riociguat induced hyperpolarization of the PASMC (Figure 9c). Both effects were inhibited in the presence of XE991 (Figure 9d–f). Thus, riociguat‐sensitive K+ current was essentially abolished in the presence of the Kv7 channel inhibitor (Figure 9g). Notably, the hyperpolarizing effect induced by riociguat persisted in the presence of the Kv1.5 channel inhibitor DPO‐1 (Figure 9h,i) but was blunted under the combination of DPO‐1 plus XE991 (Figure 9h). Remarkably, the pulmonary vasodilation induced by riociguat was attenuated by XE991 (Figure 9j), indicating that activation of Kv7 channels contributes to its main pharmacological effect.
Figure 9.
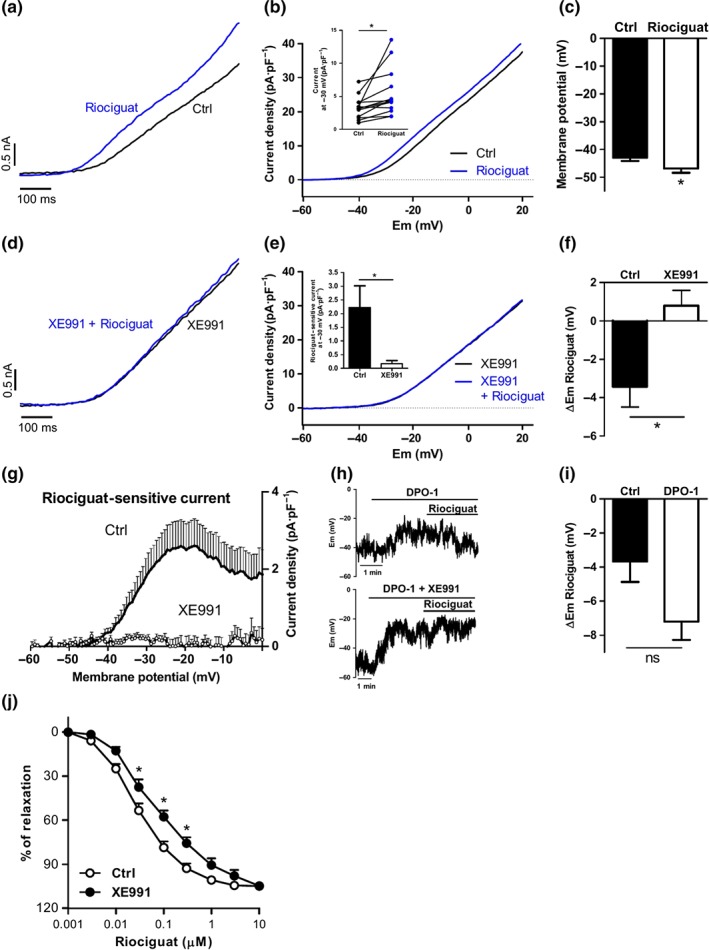
Role of Kv7 channels in riociguat‐induced electrophysiological and vascular responses in PA. (a) Representative traces and (b) mean values of Kv currents recorded after the application of depolarizing ramps from −60 to +20 mV in the absence or the presence of riociguat (0.3 μM, n = 15 from eight animals). Inset shows the current density at −30 mV before and after the addition of riociguat. (c) Mean values of the membrane potential in PASMC before and after the addition of riociguat (n = 14 from eight animals). (d) Representative traces and (e) mean values of Kv currents recorded after the application of depolarizing ramps from −60 to +20 mV in PASMCs perfused with XE991 before and after the addition of riociguat (0.3 μM, n = 7 from five animals). Inset shows the changes in the current at −30 mV induced by DEA‐NO riociguat. * P < 0.05 versus Ctrl (Student's unpaired t test). (f) Mean values of the changes in the membrane potential induced by riociguat in the absence and in the presence of XE991 (n = 5–14 from five to eight animals). (g) Averaged values of riociguat‐sensitive currents obtained by subtracting the current in the presence and in the absence of the drug. (h) Representative traces of the effects of riociguat on the membrane potential in the presence of DPO‐1 (1 μM) or DPO‐1 + XE991 (10 μM). (i) Mean values of the changes in the membrane potential induced by riociguat in the absence and in the presence of DPO‐1 (n = 5–14 from five to eight animals). (j) Averaged data of the concentration‐dependent relaxation induced by riociguat (0.001–10 μM) in PA in the absence (n = 6) or the presence of XE991 (10 μM, n = 6). *P < 0.05 versus Ctrl (Student's paired or unpaired t test). Results are means ± SEM
4. DISCUSSION
Herein, we demonstrate that NO donors induce a bimodal effect on Kv currents in PASMC, with an enhancement at potentials ranging between −40 and −10 mV and a reduction at more depolarized potentials. The increased Kv currents and the hyperpolarization of PASMC induced by NO donors are triggered by the activation of Kv7, but not Kv1.5, channels. We also identify Kv7.5 channels as targets of NO donors. The increase of Kv7 channel activity involves the activation of sGC. Accordingly, the sGC stimulator riociguat increases Kv7 currents leading to membrane hyperpolarization. Remarkably, Kv7 channel activation contributes to the pulmonary vasodilating effect induced by drugs stimulating the NO/cGMP pathway.
More than two decades years ago, Yuan et al. (1996) showed that NO activated Kv channels in cultured PASMC and proposed that this effect contributed to NO‐induced hyperpolarization of PASMC and PA relaxation. Intriguingly, controversial effects of NO donors on Kv1.5 channels, which represent the major component (around 80%) of the total Kv current present in PASMC (Archer et al., 1998; Moral‐Sanz et al., 2011), have been reported. Thus, NO donors such as SNAP or SNP have been shown to activate (Remillard et al., 2007) and inhibit (Núñez et al., 2006) Kv1.5 currents in KCNA5‐transfected HEK 293 and Ltk − cells, respectively. In the present study, we aimed to gain more insight into the modulation of Kv channels by NO in pulmonary arteries. Since culture conditions may lead to a dramatic down‐regulation of several potassium channels including Kv1.5 channels (Manoury, Etheridge, Reid, & Gurney, 2009), we used freshly isolated PASMCs which represent a more reliable model for characterizing the impact of ion channels in the regulation of vascular reactivity. We found that the NO donors DEA‐NO and SNP hyperpolarized PASMC and exerted a bimodal effect on the total Kv current recorded following the application of a depolarizing ramp. Thus, these drugs increased the current activity at negative potentials (from −40 to −10 mV) and decreased the current at more positive potentials in a concentration‐dependent manner. The enhancement of the current at −30 mV induced by DEA‐NO reached a maximal value at 10 μM and decreased at a higher concentration, probably counterbalanced by the inhibitory component. Upon the application of long depolarizing pulses (to enhance the contribution of the slowly activating Kv7 channels), we only observed the activating component, with no evidence of the inhibitory component. Both the increase in Kv current and the hyperpolarization of PASMC induced by DEA‐NO still occurred in the presence of the selective Kv1.5 channel inhibitor DPO‐1. These data strongly suggested that Kv1.5 channels present in freshly isolated PASMC were not activated by NO donors but rather might contribute to the inhibitory component of NO donors in line with previous observations in Ltk − cells (Núñez et al., 2006).
Since the net effect of NO donors was PASMC hyperpolarization, according with an enhancement of the Kv currents at physiological membrane potentials, we focused in characterizing this activating component of the Kv current. Although activation of BKCa channels has been shown to contribute to NO‐induced relaxation (Bolotina et al., 1994; Robertson et al., 1993), we found that the selective inhibitor iberiotoxin did not affect the enhanced Kv current or the relaxation induced by DEA‐NO. Kv7 channels have recently emerged as key players regulating systemic (Chadha, Zunke, Zhu, et al., 2012; Khanamiri et al., 2013; Mani et al., 2016; Morales‐Cano et al., 2015, 2016) and pulmonary (Joshi, Sedivy, Hodyc, Herget, & Gurney, 2009; Ng et al., 2011; Sedivy et al., 2015) vascular tone. Accordingly, we observed that the Kv7 inhibitor XE991 consistently depolarized the membrane potential but to a lower extent (3 mV) than previously reported (6 mV, Eid & Gurney, 2018, and 15 mV, Joshi et al., 2009). One possible explanation for these discrepancies is that, in comparison with ours, these studies were performed in the presence of other K+ channel inhibitors (glibenclamide and glibenclamide plus TEA, respectively) which could potentiate XE991‐induced depolarization. Whereas many studies have highlighted their role in cAMP‐induced relaxation, information on the modulation of Kv7 channels by the NO/cGMP vasodilation pathway is very scarce (Jepps, Olesen, Greenwood, & Dalsgaard, 2016; Stott, Barrese, Jepps, Leighton, & Greenwood, 2015). Thus, we tested this possibility and found that NO donors failed to increase Kv currents in the presence of XE991 or linopirdine indicating that Kv7 channels mediated the enhanced NO‐sensitive current in PASMC. Likewise, these Kv7 channel inhibitors prevented the hyperpolarizing effects induced by DEA‐NO or SNP. Our study is, to the best of our knowledge, the first to show the activation of Kv7 channels by NO in isolated vascular myocytes. Importantly, these electrophysiological effects had functional significance, since Kv7 channel inhibitors also attenuated their relaxant responses. These data indicated that pulmonary vasodilation induced by NO donors is driven, at least partly, through the activation of vascular Kv7 channels.
While the main mechanism of NO‐induced pulmonary vasodilation involves the activation of sGC and the subsequent increase in cGMP levels (Barnes & Liu, 1995; Cogolludo et al., 2001; Sausbier et al., 2000), alternative mechanisms (i.e., S‐nitrosylation) have been reported (Hess et al., 2005). In order to gain more insight into the mechanism of NO‐mediated Kv7 channel activation, we performed experiments with the sGC inhibitor ODQ. This compound prevented DEA‐NO‐induced increase in Kv7.5 currents, enhancement of Kv currents and hyperpolarization in PASMC, and relaxation, consistent with an involvement of the canonical NO/cGMP pathway in the activation of Kv7 channel by NO. This idea was also supported by the lack of inhibitory effects of XE991 on DEA‐NO‐induced relaxation in the presence of ODQ. To further confirm this, we analysed the electrophysiological effects of the sGC stimulator riociguat. In contrast to the NO donors, riociguat did not inhibit but only enhanced Kv currents at all membrane potentials, and this effect was associated with PASMC hyperpolarization. Both the increase in Kv current and the hyperpolarization induced by riociguat were suppressed by the Kv7 channel inhibitor XE991, while the inhibition of Kv1.5 channels failed to prevent riociguat‐induced hyperpolarization. Conversely, the reduction of Kv currents induced by the NO donors was unaffected by Kv7 channel inhibition, according with a plausible involvement of Kv1.5 channels as discussed above. Altogether, our data support the notion that the bimodal effects of NO donors on total Kv current may reflect an opposite modulation of different Kv channels by NO. Thus, while NO activates Kv7 channels by a cGMP‐dependent mechanism, it inhibits other Kv channels (most likely Kv1.5 channels) through a cGMP‐independent mechanism, which may involve S‐nitrosylation as reported (Núñez et al., 2006; Zhao, Wang, Rubin, & Yuan, 1997).
Interestingly, Kv7 channels have been reported to be negatively regulated by NO in neurons (Ooi, Gigout, Pettinger, & Gamper, 2013). The higher concentrations reported to induce this effect, the mechanism involved (S‐nitrosylation) and the possible Kv7 channel subtypes targeted may explain the differences with respect to our study. Among the different Kv7 channels expressed in vascular smooth muscle (Morales‐Cano et al., 2015, 2016; Yeung et al., 2007) including the pulmonary circulation (Joshi et al., 2009; Morales‐Cano et al., 2014), Kv7.4 and Kv7.5 channels are considered to play a predominant role in regulating vascular tone (Barrese et al., 2018). A previous study (Stott et al., 2015) showed that Kv7.4 currents are increased following the application of cGMP in HEK‐293 cells. Our study extends this observation identifying Kv7.5 channels as another NO target. Thus, it is possible that both Kv7.4 and Kv7.5 channels may contribute to the enhanced Kv7 current induced by the NO/cGMP pathway in PASMC. The mechanisms leading to Kv7.5 channel activation observed herein remain unknown, but a direct phosphorylation could play a role since the analysis of the peptide sequence of these channels reveals the presence of several sites for phosphorylation by PKG. Alternatively, activation of PKA by cGMP has been shown to contribute to the relaxation induced by high concentrations of NO (Sausbier et al., 2000). Thus, it is plausible that cGMP may activate Kv7 channels through a PKA‐dependent activation of Kv7 channels (Chambard & Ashmore, 2005; Mani et al., 2016). Interestingly, we and other authors have shown that Kv7.5 may form functional heterotetrameric channels with Kv7.1 (Oliveras et al., 2014) and Kv7.4 (Brueggemann et al., 2014) in vascular tissues. Thus, it is conceivable that activation of Kv7.1/Kv7.5 or Kv7.4/Kv7.5 heterotetramers could contribute to cGMP‐mediated responses as previously proposed (Stott et al., 2015).
The results from the present study are of great interest from a physiological, pathological, and pharmacological point of view. The NO/cGMP vasodilator pathway is a key player determining pulmonary vascular tone (Barnes & Liu, 1995). In fact, impairment of this classic pathway at any level is known to importantly contribute to the pathophysiology of PH (Klinger & Kadowitz, 2017; Schermuly et al., 2011). Detrimental effects of vascular Kv7 loss have been reported in experimental models of essential hypertension (Barrese et al., 2018; Carr et al., 2016; Jepps et al., 2011) and diabetes (Morales‐Cano et al., 2015, 2016). Likewise, several studies suggest that Kv7 channels may also be impaired in murine models of PH (Morales‐Cano et al., 2014; Morecroft, Murray, Nilsen, Gurney, & MacLean, 2009; Sedivy et al., 2015). It is therefore likely that the impairment of Kv7 channels in the pulmonary circulation could have detrimental consequences associated with an altered response to the NO/cGMP pathway. Interestingly, KCNQ5, which encodes for Kv7.5 channels, has been shown to be significantly down‐regulated in a model of congenital diaphragmatic hernia (Zimmer, Takahashi, Hofmann, & Puri, 2017), a cause of PH in which the therapeutic response to inhaled NO is very poor (Neonatal Inhaled Nitric Oxide Study Group, 1997; Putnam et al., 2016). Finally, our study is of great pharmacological significance since it identifies a novel mechanism by which riociguat, a drug approved for the treatment of pulmonary arterial hypertension and chronic thromboembolic PH, may exert, at least partly, its therapeutic effect. Likewise, this mechanism is expected to contribute to the pulmonary vascular effects of other drugs activating this pathway such as PDE5 inhibitors (sildenafil or tadalafil).
In summary, our study is the first to show that the stimulation of the NO/cGMP pathway enhances Kv7 currents and induces hyperpolarization in PASMC. Importantly, activation of these channels partly mediates the relaxation induced by NO donors and riociguat. Our study identifies a novel mechanism that may contribute to the therapeutic effect of drugs activating the NO/cGMP pathway indicated for the treatment of PH.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
A.C. and F.P.‐V. conceived and designed the research. G.M.‐P., J.M.‐S., B.B., T.G., A.D.C., and M.C. performed the experiments. G.M.‐P., J.M.‐S., B.B., T.G, A.D.C., D.M.‐C., M.C., S.E.‐R., and A.C. analysed the data. A.C., F.P.‐V., G.M.‐P., T.G, L.M., and C.V. interpreted the results of the experiments. A.C., G.M.‐P, A.D.C., T.G., and B.B. prepared the figures. A.C. drafted the manuscript. A.C., G.M.‐P., T.G., L.M., C.V., and F.P.‐V. edited and revised the manuscript. All authors approved the final version of the manuscript.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design & Analysis, and Animal Experimentation, and as recommended by funding agencies, publishers, and other organizations engaged with supporting research.
ACKNOWLEDGEMENTS
This work was funded by Ministerio de Economía y Competitividad grants (SAF2016‐77222R to F.P.‐V. and A.C. and SAF2016‐75021‐R to C.V. and T.G.), the Cardiovascular Medical Research and Education Fund (A.C.), Fundación Contra la Hipertensión Pulmonar‐EMPATHY, Comunidad de Madrid (B2017/BMD‐3727 to A.C. and L.M.), and Instituto de Salud Carlos III (PI15/01100 to L.M. and CB/11/00222 to C.V.) with funds from the European Union (Fondo Europeo de Desarrollo Regional FEDER). G.M.‐P., M.C., and S.E.‐R. are supported by CIBERES, Universidad Complutense and FPU grants. We thank Alba Vera‐Zambrano and Maria Baena‐Nuevo for their excellent technical assistance in electrophysiological experiments involving Kv7.5 channels.
Mondéjar‐Parreño G, Moral‐Sanz J, Barreira B, et al. Activation of Kv7 channels as a novel mechanism for NO/cGMP‐induced pulmonary vasodilation. Br J Pharmacol. 2019;176:2131–2145. 10.1111/bph.14662
REFERENCES
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174(S1), S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Striessnig, J. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: Voltage‐gated ion channels. British Journal of Pharmacology, 174, S160–S194. 10.1111/bph.13884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer, S. L. , Souil, E. , Dinh‐Xuan, A. T. , Schremmer, B. , Mercier, J. C. , El Yaagoubi, A. , … Hampl, V. (1998). Molecular identification of the role of voltage‐gated K+ channels, Kv1.5 and Kv2.1, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. The Journal of Clinical Investigation, 101, 2319–2330. 10.1172/JCI333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes, P. J. , & Liu, S. F. (1995). Regulation of pulmonary vascular tone. Pharmacological Reviews, 47, 87–131. [PubMed] [Google Scholar]
- Barrese, V. , Stott, J. B. , & Greenwood, I. A. (2018). KCNQ‐encoded potassium channels as therapeutic targets. Annual Review of Pharmacology and Toxicology, 58, 625–648. 10.1146/annurev-pharmtox-010617-052912 [DOI] [PubMed] [Google Scholar]
- Bolotina, V. M. , Najibi, S. , Palacino, J. J. , Pagano, P. J. , & Cohen, R. A. (1994). Nitric oxide directly activates calcium‐dependent potassium channels in vascular smooth muscle. Nature, 368, 850–853. 10.1038/368850a0 [DOI] [PubMed] [Google Scholar]
- Brueggemann, L. I. , Mackie, A. R. , Cribbs, L. L. , Freda, J. , Tripathi, A. , Majetschak, M. , & Byron, K. L. (2014). Differential protein kinase C‐dependent modulation of Kv7.4 and Kv7.5 subunits of vascular Kv7 channels. The Journal of Biological Chemistry, 289, 2099–2111. 10.1074/jbc.M113.527820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr, G. , Barrese, V. , Stott, J. B. , Povstyan, O. V. , Jepps, T. A. , Figueiredo, H. B. , … Greenwood, I. A. (2016). MicroRNA‐153 targeting of KCNQ4 contributes to vascular dysfunction in hypertension. Cardiovascular Research, 112, 581–589. 10.1093/cvr/cvw177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadha, P. S. , Zunke, F. , Davis, A. J. , Jepps, T. A. , Linders, J. T. M. , Schwake, M. , … Greenwood, I. A. (2012). Pharmacological dissection of Kv7.1 channels in systemic and pulmonary arteries. British Journal of Pharmacology, 166, 1377–1387. 10.1111/j.1476-5381.2012.01863.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadha, P. S. , Zunke, F. , Zhu, H.‐L. , Davis, A. J. , Jepps, T. A. , Olesen, S. P. , … Greenwood, I. A. (2012). Reduced KCNQ4‐encoded voltage‐dependent potassium channel activity underlies impaired β‐adrenoceptor‐mediated relaxation of renal arteries in hypertension. Hypertension, 59, 877–884. 10.1161/HYPERTENSIONAHA.111.187427 [DOI] [PubMed] [Google Scholar]
- Chambard, J.‐M. , & Ashmore, J. F. (2005). Regulation of the voltage‐gated potassium channel KCNQ4 in the auditory pathway. Pflügers Archiv, 450, 34–44. 10.1007/s00424-004-1366-2 [DOI] [PubMed] [Google Scholar]
- Coggins, M. P. , & Bloch, K. D. (2007). Nitric oxide in the pulmonary vasculature. Arteriosclerosis, Thrombosis, and Vascular Biology, 27, 1877–1885. 10.1161/ATVBAHA.107.142943 [DOI] [PubMed] [Google Scholar]
- Cogolludo, A. , Moreno, L. , Lodi, F. , Frazziano, G. , Cobeño, L. , Tamargo, J. , & Perez‐Vizcaino, F. (2006). Serotonin inhibits voltage‐gated K+ currents in pulmonary artery smooth muscle cells: Role of 5‐HT2A receptors, caveolin‐1, and KV1.5 channel internalization. Circulation Research, 98, 931–938. 10.1161/01.RES.0000216858.04599.e1 [DOI] [PubMed] [Google Scholar]
- Cogolludo, A. , Moreno, L. , & Villamor, E. (2007). Mechanisms controlling vascular tone in pulmonary arterial hypertension: Implications for vasodilator therapy. Pharmacology, 79, 65–75. 10.1159/000097754 [DOI] [PubMed] [Google Scholar]
- Cogolludo, A. L. , Pérez‐Vizcaíno, F. , Zaragozá‐Arnáez, F. , Ibarra, M. , López‐López, G. , López‐Miranda, V. , & Tamargo, J. (2001). Mechanisms involved in SNP‐induced relaxation and [Ca2+]i reduction in piglet pulmonary and systemic arteries. British Journal of Pharmacology, 132, 959–967. 10.1038/sj.bjp.0703894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid, B. G. , & Gurney, A. M. (2018). Zinc pyrithione activates K+ channels and hyperpolarizes the membrane of rat pulmonary artery smooth muscle cells. PLoS ONE, 13, e0192699 10.1371/journal.pone.0192699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiè, N. , Humbert, M. , Vachiery, J.‐L. , Gibbs, S. , Lang, I. , Torbicki, A. , … Hoeper, M. (2015). 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). The European Respiratory Journal, 46, 903–975. 10.1183/13993003.01032-2015 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR . (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess, D. T. , Matsumoto, A. , Kim, S.‐O. , Marshall, H. E. , & Stamler, J. S. (2005). Protein S‐nitrosylation: Purview and parameters. Nature Reviews. Molecular Cell Biology, 6, 150–166. 10.1038/nrm1569 [DOI] [PubMed] [Google Scholar]
- Jackson, W. F. (2018). KV channels and the regulation of vascular smooth muscle tone. Microcirculation, 25 10.1111/micc.12421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepps, T. A. , Chadha, P. S. , Davis, A. J. , Harhun, M. I. , Cockerill, G. W. , Olesen, S. P. , … Greenwood, I. A. (2011). Downregulation of Kv7.4 channel activity in primary and secondary hypertension. Circulation, 124, 602–611. 10.1161/CIRCULATIONAHA.111.032136 [DOI] [PubMed] [Google Scholar]
- Jepps, T. A. , Olesen, S. P. , Greenwood, I. A. , & Dalsgaard, T. (2016). Molecular and functional characterization of Kv7 channels in penile arteries and corpus cavernosum of healthy and metabolic syndrome rats. British Journal of Pharmacology, 173, 1478–1490. 10.1111/bph.13444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi, S. , Sedivy, V. , Hodyc, D. , Herget, J. , & Gurney, A. M. (2009). KCNQ modulators reveal a key role for KCNQ potassium channels in regulating the tone of rat pulmonary artery smooth muscle. The Journal of Pharmacology and Experimental Therapeutics, 329, 368–376. 10.1124/jpet.108.147785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanamiri, S. , Soltysinska, E. , Jepps, T. A. , Bentzen, B. H. , Chadha, P. S. , Schmitt, N. , … Olesen, S.‐P. (2013). Contribution of Kv7 channels to basal coronary flow and active response to ischemia. Hypertension, 62, 1090–1097. 10.1161/HYPERTENSIONAHA.113.01244 [DOI] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger, J. R. , & Kadowitz, P. J. (2017). The nitric oxide pathway in pulmonary vascular disease. The American Journal of Cardiology, 120, S71–S79. 10.1016/j.amjcard.2017.06.012 [DOI] [PubMed] [Google Scholar]
- Kraehling, J. R. , & Sessa, W. C. (2017). Contemporary approaches to modulating the nitric oxide–cGMP pathway in cardiovascular disease. Circulation Research, 120, 1174–1182. 10.1161/CIRCRESAHA.117.303776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krick, S. , Platoshyn, O. , Sweeney, M. , McDaniel, S. S. , Zhang, S. , Rubin, L. J. , & Yuan, J. X.‐J. (2002). Nitric oxide induces apoptosis by activating K+ channels in pulmonary vascular smooth muscle cells. American Journal of Physiology. Heart and Circulatory Physiology, 282, H184–H193. 10.1152/ajpheart.2002.282.1.H184 [DOI] [PubMed] [Google Scholar]
- Lau, E. M. T. , Giannoulatou, E. , Celermajer, D. S. , & Humbert, M. (2017). Epidemiology and treatment of pulmonary arterial hypertension. Nature Reviews. Cardiology, 14, 603–614. 10.1038/nrcardio.2017.84 [DOI] [PubMed] [Google Scholar]
- Leary, S. , & Golab, G. C. (2013). AVMA guidelines for the euthanasia of animals: 2013 edition. Schaumburg, IL: American Veterinary Medical Association. [Google Scholar]
- Mackie, A. R. , & Byron, K. L. (2008). Cardiovascular KCNQ (Kv7) potassium channels: Physiological regulators and new targets for therapeutic intervention. Molecular Pharmacology, 74, 1171–1179. 10.1124/mol.108.049825 [DOI] [PubMed] [Google Scholar]
- Mani, B. K. , Robakowski, C. , Brueggemann, L. I. , Cribbs, L. L. , Tripathi, A. , Majetschak, M. , & Byron, K. L. (2016). Kv7.5 potassium channel subunits are the primary targets for PKA‐dependent enhancement of vascular smooth muscle Kv7 currents. Molecular Pharmacology, 89, 323–334. 10.1124/mol.115.101758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoury, B. , Etheridge, S. L. , Reid, J. , & Gurney, A. M. (2009). Organ culture mimics the effects of hypoxia on membrane potential, K+ channels and vessel tone in pulmonary artery. British Journal of Pharmacology, 158, 848–861. 10.1111/j.1476-5381.2009.00353.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales‐Cano, D. , Menendez, C. , Moreno, E. , Moral‐Sanz, J. , Barreira, B. , Galindo, P. , … Perez‐Vizcaino, F. (2014). The flavonoid quercetin reverses pulmonary hypertension in rats. PLoS ONE, 9, e114492 10.1371/journal.pone.0114492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales‐Cano, D. , Moreno, L. , Barreira, B. , Briones, A. M. , Pandolfi, R. , Moral‐Sanz, J. , … Cogolludo, A. (2016). Activation of PPARβ/δ prevents hyperglycaemia‐induced impairment of Kv7 channels and cAMP‐mediated relaxation in rat coronary arteries. Clinical Science, 130, 1823–1836. 10.1042/CS20160141 [DOI] [PubMed] [Google Scholar]
- Morales‐Cano, D. , Moreno, L. , Barreira, B. , Pandolfi, R. , Chamorro, V. , Jimenez, R. , … Cogolludo, A. (2015). Kv7 channels critically determine coronary artery reactivity: Left–right differences and down‐regulation by hyperglycaemia. Cardiovascular Research, 106, 98–108. 10.1093/cvr/cvv020 [DOI] [PubMed] [Google Scholar]
- Moral‐Sanz, J. , Gonzalez, T. , Menendez, C. , David, M. , Moreno, L. , Macias, A. , … Cogolludo, A. (2011). Ceramide inhibits Kv currents and contributes to TP‐receptor‐induced vasoconstriction in rat and human pulmonary arteries. American Journal of Physiology‐Cell Physiology, 301, C186–C194. 10.1152/ajpcell.00243.2010 [DOI] [PubMed] [Google Scholar]
- Morecroft, I. , Murray, A. , Nilsen, M. , Gurney, A. M. , & MacLean, M. R. (2009). Treatment with the Kv7 potassium channel activator flupirtine is beneficial in two independent mouse models of pulmonary hypertension. British Journal of Pharmacology, 157, 1241–1249. 10.1111/j.1476-5381.2009.00283.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno, C. , Oliveras, A. , Bartolucci, C. , Muñoz, C. , de la Cruz, A. , Peraza, D. A. , … Valenzuela, C. (2017). D242N, a KV7.1 LQTS mutation uncovers a key residue for IKs voltage dependence. Journal of Molecular and Cellular Cardiology, 110, 61–69. 10.1016/j.yjmcc.2017.07.009 [DOI] [PubMed] [Google Scholar]
- Ng, F. L. , Davis, A. J. , Jepps, T. A. , Harhun, M. I. , Yeung, S. Y. , Wan, A. , … Greenwood, I. A. (2011). Expression and function of the K+ channel KCNQ genes in human arteries. British Journal of Pharmacology, 162, 42–53. 10.1111/j.1476-5381.2010.01027.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez, L. , Vaquero, M. , Gómez, R. , Caballero, R. , Mateos‐Cáceres, P. , Macaya, C. , … Delpón, E. (2006). Nitric oxide blocks hKv1.5 channels by S‐nitrosylation and by a cyclic GMP‐dependent mechanism. Cardiovascular Research, 72, 80–89. 10.1016/j.cardiores.2006.06.021 [DOI] [PubMed] [Google Scholar]
- Oliveras, A. , Roura‐Ferrer, M. , Solé, L. , de la Cruz, A. , Prieto, A. , Etxebarria, A. , … Felipe, A. (2014). Functional assembly of Kv7.1/Kv7.5 channels with emerging properties on vascular muscle physiology. Arteriosclerosis, Thrombosis, and Vascular Biology, 34, 1522–1530. 10.1161/ATVBAHA.114.303801 [DOI] [PubMed] [Google Scholar]
- Ooi, L. , Gigout, S. , Pettinger, L. , & Gamper, N. (2013). Triple cysteine module within M‐type K+ channels mediates reciprocal channel modulation by nitric oxide and reactive oxygen species. The Journal of Neuroscience, 33, 6041–6046. 10.1523/JNEUROSCI.4275-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neonatal Inhaled Nitric Oxide Study Group (NINOS) (1997). Inhaled nitric oxide and hypoxic respiratory failure in infants with congenital diaphragmatic hernia. Pediatrics, 99, 838–845. [DOI] [PubMed] [Google Scholar]
- Plane, F. , Sampson, L. J. , Smith, J. J. , & Garland, C. J. (2001). Relaxation to authentic nitric oxide and SIN‐1 in rat isolated mesenteric arteries: Variable role for smooth muscle hyperpolarization. British Journal of Pharmacology, 133, 665–672. 10.1038/sj.bjp.0704127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam, L. R. , Tsao, K. , Morini, F. , Lally, P. A. , Miller, C. C. , Lally, K. P. , … Congenital Diaphragmatic Hernia Study Group (2016). Evaluation of variability in inhaled nitric oxide use and pulmonary hypertension in patients with congenital diaphragmatic hernia. JAMA Pediatrics, 170, 1188–1194. 10.1001/jamapediatrics.2016.2023 [DOI] [PubMed] [Google Scholar]
- Remillard, C. V. , Tigno, D. D. , Platoshyn, O. , Burg, E. D. , Brevnova, E. E. , Conger, D. , … Yuan, J. X.‐J. (2007). Function of Kv1.5 channels and genetic variations of KCNA5 in patients with idiopathic pulmonary arterial hypertension. American Journal of Physiology‐Cell Physiology, 292, C1837–C1853. 10.1152/ajpcell.00405.2006 [DOI] [PubMed] [Google Scholar]
- Robertson, B. E. , Schubert, R. , Hescheler, J. , & Nelson, M. T. (1993). cGMP‐dependent protein kinase activates Ca‐activated K channels in cerebral artery smooth muscle cells. The American Journal of Physiology, 265, C299–C303. 10.1152/ajpcell.1993.265.1.C299 [DOI] [PubMed] [Google Scholar]
- Sausbier, M. , Schubert, R. , Voigt, V. , Hirneiss, C. , Pfeifer, A. , Korth, M. , … Hofmann, F. (2000). Mechanisms of NO/cGMP‐dependent vasorelaxation. Circulation Research, 87, 825–830. 10.1161/01.RES.87.9.825 [DOI] [PubMed] [Google Scholar]
- Schermuly, R. T. , Ghofrani, H. A. , Wilkins, M. R. , & Grimminger, F. (2011). Mechanisms of disease: Pulmonary arterial hypertension. Nature Reviews. Cardiology, 8, 443–455. 10.1038/nrcardio.2011.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedivy, V. , Joshi, S. , Ghaly, Y. , Mizera, R. , Zaloudikova, M. , Brennan, S. , … Gurney, A. M. (2015). Role of Kv7 channels in responses of the pulmonary circulation to hypoxia. American Journal of Physiology. Lung Cellular and Molecular Physiology, 308, L48–L57. 10.1152/ajplung.00362.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnov, S. V. , Beck, R. , Tammaro, P. , Ishii, T. , & Aaronson, P. I. (2002). Electrophysiologically distinct smooth muscle cell subtypes in rat conduit and resistance pulmonary arteries. Journal of Physiology (London), 538, 867–878. 10.1113/jphysiol.2001.013003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasch, J.‐P. , Pacher, P. , & Evgenov, O. V. (2011). Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation, 123, 2263–2273. 10.1161/CIRCULATIONAHA.110.981738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott, J. B. , Barrese, V. , Jepps, T. A. , Leighton, E. V. , & Greenwood, I. A. (2015). Contribution of Kv7 channels to natriuretic peptide mediated vasodilation in normal and hypertensive rats. Hypertension, 65, 676–682. 10.1161/HYPERTENSIONAHA.114.04373 [DOI] [PubMed] [Google Scholar]
- Yeung, S. Y. M. , Pucovský, V. , Moffatt, J. D. , Saldanha, L. , Schwake, M. , Ohya, S. , & Greenwood, I. A. (2007). Molecular expression and pharmacological identification of a role for Kv7 channels in murine vascular reactivity. British Journal of Pharmacology, 151, 758–770. 10.1038/sj.bjp.0707284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, X. J. , Tod, M. L. , Rubin, L. J. , & Blaustein, M. P. (1996). NO hyperpolarizes pulmonary artery smooth muscle cells and decreases the intracellular Ca2+ concentration by activating voltage‐gated K+ channels. Proceedings of the National Academy of Sciences of the United States of America, 93, 10489–10494. 10.1073/pnas.93.19.10489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y. J. , Wang, J. , Rubin, L. J. , & Yuan, X. J. (1997). Inhibition of K(V) and K(Ca) channels antagonizes NO‐induced relaxation in pulmonary artery. The American Journal of Physiology, 272, H904–H912. 10.1152/ajpheart.1997.272.2.H904 [DOI] [PubMed] [Google Scholar]
- Zimmer, J. , Takahashi, T. , Hofmann, A. D. , & Puri, P. (2017). Downregulation of KCNQ5 expression in the rat pulmonary vasculature of nitrofen‐induced congenital diaphragmatic hernia. Journal of Pediatric Surgery, 52, 702–705. 10.1016/j.jpedsurg.2017.01.016 [DOI] [PubMed] [Google Scholar]


