Abstract
Background and Purpose
Propionibacterium acnes is a Gram‐positive bacterium associated with the skin disorder acne. In this study, as fatty acids are considered to be important in the life habitat of P. acnes, we tested our lipopeptide library in an attempt to create potent P. acnes‐specific antimicrobial agents.
Experimental Approach
The antimicrobial activity of various lipopeptides was determined by measuring their minimal inhibitory concentration (MIC). Lipids from P. acnes were used to explore their mode of action. RAW264.7 cells stimulated with LPS and P. acnes respectively were used to measure their anti‐inflammatory activity. Mice ears injected with P. acnes were used to assess the antimicrobial and anti‐inflammatory effects of the peptides tested in vivo.
Key Results
The most potent candidate, C16‐KWKW, was observed to be more active against P. acnes than against other non‐targeted bacterial strains, such as Streptococcus mutans, Staphylococcus aureus, and Escherichia coli. The mode of action of C16‐KWKW was observed to be through interference with the integrity of the bacterial membrane, thereby impairing membrane permeability and causing leakage of inner contents of bacterial cells. Furthermore, C16‐KWKW inhibited the expression of pro‐inflammatory cytokines, such as IL‐1β, TNF‐α, and inducible NOS stimulated by both LPS and P. acnes, thus showing potential anti‐inflammatory activity, which was further verified in the in vivo animal studies.
Conclusions and Implications
C16‐KWKW is a lipopeptide displaying both anti‐P. acnes and anti‐inflammatory effects in vitro and in vivo and shows potential as a treatment for acne vulgaris induced by P. acnes.
Abbreviations
- AMPs
antimicrobial peptides
- iNOS
inducible NOS
- MIC
minimal inhibitory concentration
- PI
propidium iodide
- TEM
transmission electron microscopy
What is already known
Acne vulgaris is a chronic skin disorder often developed on the face and upper trunk.
Propionibacterium acnes (P. acnes) is a Gram‐positive bacterium associated with the skin disorder acne.
What this study adds
We employed peptides as a tool to conjugate with fatty acids to create a P. acnes “nutrient‐like” peptide: C16‐KWKW.
What is the clinical significance
C16‐KWKW exhibited not only good P. acnes‐killing activity but also good anti‐inflammatory activity, representing a promising potential for development as an effective anti‐P. acnes agent.
1. INTRODUCTION
Acne vulgaris is a chronic skin disorder, which often develops on the face and upper trunk and is characterized by the formation of comedones, papules, nodules, pustules, and cysts (Aslam, Fleischer, & Feldman, 2015). In addition, the disease is always associated with an emotional and psychological impact, which can result in anxiety, reduced self‐esteem, and depression or even thoughts of suicide (Tasoula et al., 2012). In 2015, it affected more than 630 million people globally, thus being ranked as the eighth most common disease in the world (Disease, Injury, & Prevalence, 2016). Current research shows that several factors are involved in development of the disease including overgrowth of the skin commensal bacteria, obstruction of the follicle, excess production of sebum, and inflammation of the pilosebaceous unit (Thiboutot et al., 2009), of which an overgrowth of Propionibacterium acnes is widely recognized as a key factor in the formation of acne vulgaris (Kumar et al., 2016).
Propionibacterium acnes is a common skin microorganism predominantly residing in the pilosebaceous follicles of the human skin (Kumar et al., 2016). It is a rod‐shaped Gram‐positive anaerobic bacterium and is known to produce a number of virulent factors, and to induce the release of pro‐inflammatory cytokines such as IL‐1β, IL‐8, and TNF‐α from mononuclear cells and keratinocytes, which is the major cause of acne formation (Jugeau et al., 2005; Kim et al., 2002; Vowels, Yang, & Leyden, 1995). Thus, a molecule possessing dual anti‐P. acnes and anti‐inflammatory effects could be a potential candidate for treating the disease.
So far, a variety of therapeutic agents have been demonstrated to reduce sebum production, inflammation, and bacterial counts (Becerro de Bengoa Vallejo, Losa Iglesias, Alou Cervera, Sevillano Fernandez, & Prieto Prieto, 2009; Inui, Aoshima, Ito, Kobuko, & Itami, 2012; Uppal, Singhi, Singhi, & Aggarwal, 2017). These chemicals are used either topically or systemically based on the severity of the acne lesion. Among them, treatment with antimicrobial peptides (AMPs) is a new approach that possesses several advantages over conventional antibiotic therapy due to their rapid action against a broad array of invading pathogens and low tendency to develop bacterial resistance (Dean, Bishop, & van Hoek, 2011; McInturff et al., 2005). Furthermore, the ability of several AMPs to boost the host's immunity would provide additional protection against development of the disease (Sonenshine & Macaluso, 2017). Based on these findngs, in this study we have investigated the design and development of potent anti‐P. acnes peptides possessing dual functions, including antimicrobial and anti‐inflammatory activities, thereby providing an effective option for the treatment of skin disorders caused by P. acnes.
With respect to antimicrobials, it should be pointed out that there are two types of antimicrobials that are used in the clinic: broad‐spectrum and narrow‐spectrum antimicrobials. As is well‐known, the healthy skin microflora is a well‐organized ecosystem in which beneficial and stable interactions exist among microbes and between microbes and their host. In contrast to a broad‐spectrum antimicrobial, a narrow‐spectrum antimicrobial will only focus on a targeted pathogen, thereby minimizing the impact on the entire ecosystem. Thus, it should produce more benefit than harm (Shu et al., 2013).
Propionibacterium acnes live on fatty acids in sebum secreted by sebaceous glands in the follicles (Perry & Lambert, 2011). This suggests that it might be possible to develop a P. acnes‐targeting AMP by using fatty acid as an “inducer” or a targeting domain, thereby constructing a narrow‐spectrum AMP (Ryu, Han, Song, Armstrong, & Park, 2015). Using this approach in this study, we employed peptides as a tool to conjugate with various fatty acids to create a P. acnes “nutrient‐like” peptide library (Fang et al., 2014). Subsequently, selected candidates were evaluated for their anti‐P. acnes activity, structural modification, modes of action, and in vivo effect on infected mice. The preliminary in vitro and in vivo studies showed that one particular peptide C16‐KWKW exhibited not only good P. acnes‐killing activity but also good anti‐inflammatory activity, and thus, represents a promising candidate for development as an effective anti‐P. acnes agent.
2. METHODS
2.1. Peptide synthesis
Peptides were synthesized by solid‐phase synthesis using amide MHBA resin and standard 9‐fluorenylmethoxy carbonyl (Fmoc) amino acids. Peptide extension reaction conditions were as follows: fivefold excess of HBTU/HOBt as the coupling reagents, N‐dimethylformamide as a solvent, 10‐fold excess of diisopropylethylamine, and threefold excess of Fmoc protecting group amino acids. After assembly of the amino acids, fivefold excess of fatty acid was added into the N‐terminus of the peptide by using the same reaction conditions as that of peptide synthesis. The cleavage of the peptide from the resin was carried out with a reagent composed of 87.5% trifluoroacetic acid, 2.5% ethanedithiol, 5% thioanisole, and 5% deionized water (3 hr, room temperature). The peptide was precipitated in methyl tert‐butyl ether‐petroleum ether (1:1, volume ratio), and the MW was confirmed by electrospray ionization MS (Waters, USA). The purity of the peptide was analysed with a Shimadazu 10A HPLC instrument on a C18 column (250 × 4.6 mm; Shimadzu, Kyoto, Japan); the mobile phase consisted of solvent A: water containing 0.075% trifluoroacetic acid and solvent B: 50% acetonitrile and methanol containing 0.075% trifluoroacetic acid and gradient: 15% to 20% B for 2 min, 20% to 60% B for 6 min, 60% to 80% B for 4 min, and 80% to 90% B for 4 min. The purity of all lipopeptides was above 95%.
2.2. Strain culture
Propionibacterium acnes (ATCC6919 and ATCC11827) were grown under anaerobic conditions in Brucella Broth medium; Staphylococcus epidermidis (ATCC12228), Pseudomonas aeruginosa (ATCC25853), and Escherichia coli (ATCC25922) were cultured under aerobic conditions in Lysogeny Broth medium; Streptococcus mutans (ATCC25175 and UA159) were grown under anaerobic conditions in Brain Heart Infusion Broth medium; and Staphylococcus aureus (ATCC12600 and clinical isolation strain) were grown under aerobic conditions in Mueller–Hinton Broth medium. All strains were cultured at 37°C. For the heat‐killing reaction, the P. acnes suspension was incubated at 80°C for 30 min.
2.3. Cell culture
HaCaT and RAW264.7 cells were cultured in DMEM (Gibco, USA) supplemented with 10% FBS (Gibco, USA). Cells were maintained at 37°C in 5% CO2.
2.4. Minimal inhibitory concentration determinations
In 96‐well microplates, the final test concentration of fatty acid was 125–3.9 μg·ml−1, and the peptide concentration was 31.2–0.5 μg·ml−1. The bacterial suspensions were taken at logarithmic growth phase; P. acnes were diluted to a final concentration of 1 × 106 CFU·ml−1, and other strains were diluted to 1 × 105 CFU·ml−1. One hundred microlitres of bacterial suspensions were added to each well. Results were read after 96‐hr static culture for P. acnes and 17‐ to 20‐hr static culture for other strains; the minimal inhibitory concentration (MIC) values were the lowest drug concentrations observed without any evidence of bacterial growth. The test results were repeated in four to six independent experiments.
2.5. Time‐killing assays and dose–effect relationship tests
Propionibacterium acnes ATCC11827 suspension was diluted to 1 × 106 CFU·ml−1. Next, the cells were treated with a final concentration of 20‐μg·ml−1 C16‐KWKW or 0.5‐μg·ml−1 clindamycin for 0, 1, 2, 4, 6, 8, and 10 hr at 25°C or 37°C anaerobically for time‐killing assays and at a final concentration of 1‐, 10‐, and 100‐μg·ml−1 C16‐KWKW or 0.01‐, 0.1‐, and 1‐μg·ml−1 clindamycin for 4 hr at 25°C anaerobically for the dose–effect relationship tests. The mixture was respectively diluted to 1:10, 1:100, and 1:1,000, and 20 μl of each dilution was placed on Bouillon Broth agar medium plate to culture for 5–7 days at 37°C under anaerobic conditions, following which the number of colonies was recorded.
2.6. Cytotoxicity assays
The cytotoxicity of the peptides on HaCaT (CLS Cat# 300493/p800_HaCaT, RRID:CVCL_0038) and RAW264.7 (CLS Cat# 400319/p462_RAW‐2647, RRID:CVCL_0493) cells was measured using the CCK‐8 assay (Izumi et al., 2001). Cells (2–5 × 104 per well) were seeded in 96‐well plates and grown overnight, then the medium was replaced with fresh medium containing of 2‐ to 125‐μg·ml−1 peptides, and the cells were further incubated at 37°C with 5% CO2 for a period of 48 hr for HaCaT and 8 hr for RAW264.7 cells. After that, 10‐μl CCK‐8 (Dojindo, Japan) was added to each well and incubated at 37°C for 2 hr. The absorbance was determined spectrophotometrically at 450 nm on a microplate reader (Infinite M1000 Pro, Tecan, Swiss). The cytotoxicity of the peptides was estimated by the comparison of the cell inhibition rate of the fusion peptide‐treated cells with that of untreated cells. The inhibition rate of untreated control cells was set as 0%.
2.7. Enzyme‐linked immunostain experiment
RAW264.7 cells (CLS Cat# 400319/p462_RAW‐2647, RRID: CVCL_0493, 2–5 × 104 per well) were seeded in 96‐well plates and grown overnight, the medium was replaced with fresh medium containing of 1‐, 10‐, and 25‐μg·ml−1 C16‐KWKW or 1‐μg·ml−1 melittin, and then 100‐ng·ml−1 LPS or 1 × 107‐CFU·ml−1 heat‐killed P. acnes were added to each well for final concentration after 2 hr. The cells were further incubated at 37°C with 5% CO2 for 6 hr, then the cell culture solution was collected, and TNF‐α, IL‐1β, and IL‐8 levels were determined by use of an enzyme‐linked immunostain kit according to the manufacturer's instructions (Neobioscience, China).
2.8. Quantitative real‐time PCR
PCR experiments were performed according to the reagent specifications, and some modifications were made (Romoser et al., 2011). The total RNA was extracted by using Trizol Reagent (Sigma, USA). RNA yields and purity were assessed by spectrophotometric analysis. Then reverse transcription was conducted and cDNA was synthesized using a PrimeScript RT Master Mix (Takara, Japan) following the instructions provided by the manufacturers. Real‐time qPCR was performed with ABI 7500 Sequence Detection System (PE Applied Biosystems, USA) in the presence of SYBR‐green (Takara, Japan). Briefly, 20‐μl of reaction mix containing 10‐μl Premix Ex Taq, 0.4‐μl ROX reference Dye, 0.4‐μl PCR Forward Primer (10 μM), 0.4‐μl PCR Reverse Primer (10 μM), 2‐μl cDNA, and 6.8‐μl dH2O was premixed before the reaction in 96‐well plates. The reaction protocol was as follows: 95°C for 30 s, 40 cycles of 95°C for 5 s, and 60°C for 34 s, followed by 95°C for 15 s, 60°C for 1 min, and 95°C for 15 s. Each sample was tested three times. Relative gene expression profiles were determined by normalizing to the reference gene (β‐actin) using the 2−ΔΔCt method. The primer sequences are as follows: 5′‐AGAGTTTGATCCTGGCTCAG‐3′ (16‐s rRNA forward); 5′‐GGTTACCTTGTTACGACTT‐3′ (16‐s rRNA reverse); 5′‐AGTCCGGGCAGGTCTACTTT‐3′ (TNF‐α forward); 5′‐GAGTTGGACCCTGAGCCATA‐3′ (TNF‐α reverse); 5′‐ATTGTGGCTGTGGAGAAG‐3′ (IL‐1β forward); 5′‐AAGATGAAGG AAAAGAAGGTG‐3′ (IL‐1β reverse); 5′‐CTTGGAGCGAGTTGTGGATTGTC‐3′ (inducible NOS [iNOS] forward); 5′‐TAGGTGAGGGCTTGGCTGAGTG‐3′ (iNOS reverse); 5′‐TCTGGTGCCTGGTCTGATGATGT‐3′ (COX‐2 [PTGS2] forward); 5′‐AGTCTGCTGGTTTGGAATAGTTGC‐3′ (COX‐2 reverse); 5′‐CTCTCCCTCACGCCATC‐3′ (β‐actin forward); and 5′‐ACGCACGATTTCCCTCTC‐3′ (β‐actin reverse).
2.9. Construction of NF‐κB stably transfected cell line and reporter gene assays
RAW264.7 cells were seeded in 24‐well plates at a density of 5 × 104 per well and grown overnight. Next, 140 μl of polyethylenimine (100 ng·ml−1) was added dropwise into 112 μl of pNF‐κB‐luc DNA (1 μg·ml−1) for each well and then kept at room temperature for 45 min. The old medium in the 24‐well plate was removed, and the DNA–PEI mixture was gently added dropwise and incubated for 4–6 hr. Next, the mixture was aspirated and cultured in DMEM containing 10% FBS. When the cells attained approximately 80% confluency, 1‐mg·ml−1 of the selective antibiotic G418 was added to the medium, and stably transfected resistant cells were screened for 2 weeks (Verma, Stevenson, Schwarz, Van Antwerp, & Miyamoto, 1995).
Stable‐transfected RAW264.7 cells (5 × 104 per well) were seeded in 96‐well plates and grown overnight, and the medium was replaced with fresh medium containing of 1‐, 10‐, and 25‐μg·ml−1 C16‐KWKW or 10‐μg·ml−1 pyrrolidine dithiocarbamate. After 2 hr, LPS or heat‐killed P. acnes were added to each well at a final concentration of 100 ng·ml−1 or 1 × 107 CFU·ml−1, respectively. The cells were further incubated at 37°C with 5% CO2 for 6 hr. Next, the liquid was sucked out; 100‐μl lysed fluid was added to each well. The cells were incubated in the dark for 20 min and then transferred to a white opaque plate. Next, 100‐μl chemical luminescent substrate was added to each well, and chemical luminescence was measured by a microplate reader (Infinite M1000 Pro, Tecan).
2.10. Preparation of bacterial lipids
The bacteria were harvested at the logarithmic phase by centrifugation (4,000 g, 15 min), washed repeatedly, and resuspended in PBS buffer. Next, a mixed solvent of chloroform and methanol (1:2, v/v) was added. After stirring for 90 min, chloroform–water (1:1, v/v) was added, and the mixture was further mixed for 30 min. The chloroform phase was separated using a separation funnel; the solvent was removed by rotary evaporation and then dried in vacuo for 24 hr to obtain the bacterial lipid.
2.11. Isothermal titration calorimetry
The bacterial lipids were prepared and dissolved in 5% DMSO to a concentration of 4 mg·ml−1. After a degassing treatment for 10 min, the change in caloric value of C16‐KWKW (150 μg·ml−1) during its interaction with the lipids was measured by isothermal titration calorimetry (MicroCal PEAQ‐ITC, Malvern, Britain). Melittin (200 μg·ml−1) was used as a control. The ITC reaction conditions are as follows: temp: 25°C; injection numbers: 19; volume: 2 μl; spacing: 100 s; reference power: 10 μcal·s−1; and stir speed: 750 rpm. The experimental data were analysed using MicroCal PEAQ‐ITC Analysis, and the software provided the model to calculate the thermodynamic parameters. The average MW was calculated accordingly to be 3,000 (Jones, Francis, Hutchinson, Handley, & Lyle, 1993).
2.12. Tryptophan fluorescence spectrometry
The lipids were prepared and dissolved to a concentration of 4 mg·ml−1 with 5% DMSO and treated with 150‐μg·ml−1 C16‐KWKW or C10‐KWKW for 2 hr. The tryptophan fluorescence emission spectrum (excitation wavelength: 280 nm and scanning range: 300 to 400 nm) was detected by fluorescence spectroscopy (FLS 980, Edinburgh, Britain). Slit widths were 3 nm for the excitation and emission beams. Melittin at a concentration of 150 μg·ml−1 was used as a control.
2.13. Transmission electron microscope
Propionibacterium acnes ATCC11827 (5 × 106 CFU·ml−1) were treated with 10‐μg·ml−1 C16‐KWKW for 2 hr. The bacteria were harvested by centrifugation (1,000 g, 5 min), washed twice using PBS buffer, and then treated with 2.5% glutaraldehyde overnight. The cells were washed with 0.1‐M phosphate for 15 min at least three times and fixed with 1% osmium tetroxide for 2–3 hr. Dehydration conditions were as follows: 15–20 min of 50% ethanol, 15–20 min of 70% ethanol, 15–20 min of 90% ethanol, 15–20 min of 90% ethanol, and 90% acetone (1:1, v/v). Incubation conditions were as follows: acetone and embedding medium (2:1, v/v) incubated at room temperature for 3–4 hr, acetone and embedding medium (1:2, v/v) incubated overnight at room temperature, and 100% of the embedding medium incubated for 2–3 hr at 37°C. Curing conditions were 37°C overnight, 45°C for 12 hr, and 60°C for 24 hr. After sliced sections had been prepared, they were dyed with 3% uranyl acetate and lead citrate, and the samples were observed by transmission electron microscopy (TEM; Weigel & Glazebrook, 2010).
2.14. UV absorbance detection
The UV absorbance detection was performed as described previously with minor modifications (Marri, Dallai, & Marchini, 1996). P. acnes ATCC11827 was cultured to logarithmic growth phase and centrifuged at 1,000 g for 10 min. The bacteria were harvested and diluted to 1 × 106 CFU·ml−1 with PBS buffer. C16‐KWKW was added at a final concentration of 10 μg·ml−1 for 0, 2, 4, 6, 8, and 10 hr at 25°C. The positive control was 10‐μg·ml−1 melittin, and the negative control was 0.1‐μg·ml−1 clindamycin. The mixture was filtered through a 0.22‐μm cellulose ester microporous membrane, and absorbance of the filtrate was measured at 260 nm using a UV spectrophotometer (UV757CRT, Lengguang Tech., China).
2.15. Propidium iodide uptake assay
Propionibacterium acnes ATCC11827 suspension was diluted to 1 × 106 CFU·ml−1 and treated with C16‐KWKW at a final concentration of 1, 10, and 100 μg·ml−1 for 4 hr at 25°C. The bacteria were harvested by centrifugation (1,000 g, 10 min), washed twice with PBS buffer, and then stained with propidium iodide (PI) at the final concentration of 30 μM for 10 min. After centrifugation (1,000 g, 10 min), the excess PI was removed and washed twice with PBS buffer. Afterwards, the bacteria were resuspended with 1‐ml PBS and detected by flow cytometry (FACSCanto II, BD, USA).
The remaining bacterial suspension was added to a black 96‐well plate (100 μl per well). The fluorescence intensity of PI in bacteria was tested by a fluorescence microplate reader (Infinite M1000 Pro, Tecan) at 535‐nm excitation wavelength and 615‐nm emission wavelength.
2.16. In vivo mice experiments
Six‐week‐old female Kunming mice (22–25 g; n = 5) were purchased from the Experimental Animal Centre of Southern Medical University and randomly subdivided into four groups with five mice in each group. One group was blank control, and the remaining three groups of mice ears were injected i.d. with P. acnes in PBS at a concentration of 3.0 × 107 CFU in 40 μl. After that, C16‐KWKW (100 and 200 μg) and clindamycin (10 μg) mixed with vaseline (50 mg) were respectively applied to the surface of the right ear. At the same time, the equivalent amounts of vaseline were applied to the left ear as a control. Twenty‐four hours later, the animals were killed by cervical dislocation, and the ears were quickly excised. After the thickness of the ear had been measured using a micro caliper, the ear was added into liquid nitrogen for grinding, and then 0.1 mg of tissue powder was weighed and homogenized in 500‐μl PBS buffer. After centrifugation at 3,000 g for 15 min, the supernatant was collected to determine the number of colonies of P. acnes g‐1of ear tissue powder on Brucella Broth Ager plate after 96‐hr incubation under anaerobic conditions at 37°C, and the expression of TNF‐α, IL‐1β, and iNOS was measured by using qRT‐PCR (7500, PE Applied Biosystems). The remaining ear was made into paraffin‐embedded sections and stained with haematoxylin and eosin for histological examination. The protocol of care and use of animals was approved by the Animal Review Board of Southern Medical University. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010) and with the recommendations made by the British Journal of Pharmacology.
2.17. Data and statistical analysis
Each experiment was repeated independently at least five times and results are expressed as the means ± SD. All the data were determined by one‐way ANOVA followed by Dunnett's post hoc t test or paired‐samples t test (ear thickness comparison) using SPSS 13.0 software (SPSS, Ver# 13.0, RRID:SCR_002865). Statistical significance was defined as * P < .05. The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology.
2.18. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and the permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Fabbro, et al., 2017; Alexander, Kelly, et al., 2017).
3. RESULTS
3.1. Anti‐Propionibacterium acnes peptide library was constructed and modified by conjugating a peptide KWKW with various lengths of fatty acids
Our previous structural analyses on several naturally occurring AMPs concluded that the amino acids R, W, and K are key residues in the peptidyl sequence for retaining antimicrobial activity. Thus, a peptide library was designed by adopting the amino acids K, W, and R as the peptide head to conjugate with various lengths of fatty acids (Fang et al., 2014). In the current study, taking into account the significance of fatty acids in the habitat of P. acnes (Perry & Lambert, 2011), we screened this library by using P. acnes as a targeting bacterium. Consequently, a peptide C10‐KWKW was identified as showing good activity against P. acnes, with a MIC of 7.8 μg·ml−1. C10‐KWKW was then modified by varying the lengths of fatty acids ranging from C6 to C20, which was subsequently assembled via a solid‐phase synthetic approach on rink amide resin, as listed in Table 1.
Table 1.
Antimicrobial activity of the designated lipopeptides
| Sequence | MW | RT | MIC (μg·ml−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Propionibacterium acnes ATCC6919 | P. acnes ATCC11827 | Staphylococcus epidermidis ATCC12228 | Staphylococcus aureus ATCC12600 | S. aureus CI | Streptococcus mutans ATCC25175 | S. mutans UA159 | Pseudomonas aeruginosa ATCC25853 | Escherichia coli ATCC25922 | |||
| C6‐KWKW | 744.96 | NT | 31.25 | 31.25 | NT | NT | NT | >31.2 | NT | >31.2 | >31.2 |
| C8‐KWKW | 773.01 | NT | 7.8–15.6 | 15.6 | NT | NT | NT | 31.2 | NT | 31.2 | >31.2 |
| C10‐KWKW | 801.07 | 19.040 | 3.9–7.8 | 3.9–7.8 | 7.8 | 15.6 | 31.2 | 15.6 | 15.6 | 15.6 | 15.6 |
| C12‐KWKW | 829.12 | 20.986 | 3.9 | 3.9 | 3.9 | 15.6 | 31.2 | 7.8 | 15.6 | 7.8 | 15.6 |
| C14‐KWKW | 857.17 | 22.753 | 3.9 | 3.9 | 3.9 | 15.6 | 15.6 | 7.8 | 15.6 | 15.6 | 15.6 |
| C16‐KWKW | 885.22 | 24.581 | 2.0 | 2.0 | 3.9 | 31.2 | >31.2 | 15.6 | 31.2 | >31.2 | 15.6 |
| C18‐KWKW | 913.28 | 26.542 | 7.8 | 3.9–7.8 | 7.8–15.6 | 31.2 | >31.2 | >31.2 | >31.2 | >31.2 | 31.2 |
| C20‐KWKW | 941.33 | 27.232 | 31.2 | 15.6 | 31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 | >31.2 |
| Clindamycin | 424.98 | NT | 0.02 | 0.02 | 0.05 | NT | NT | NT | NT | NT | NT |
Abbreviations: MIC, minimal inhibitory concentration; NT, not tested; RT, retention time.
3.2. Peptide C16‐KWKW shows potent and specific anti‐Propionibacterium acnes activity
The antimicrobial activity of the designated peptides against P. acnes was examined by employing the MIC. The data in Table 1 show that the antimicrobial activity of these peptides was strongly dependent on the lengths of lipids, of which C16‐KWKW was identified as the most potent against P. acnes with an MIC value of 2.0 μg·ml−1, lower than its congeners of C14‐KWKW (3.9 μg·ml−1) and C18‐KWKW (7.8 μg·ml−1). In addition, the MIC values of C16‐KWKW towards P. acnes were the lowest compared to the other bacterial strains tested, such as S. mutans and E. coli. This indicates the specificity of C16‐KWKW against P. acnes, which was further confirmed by quantitative real‐time PCR. As shown in Figure 1a, after treatment of bacterial strains with 0.5‐, 1‐, and 2‐μg·ml−1 C16‐KWKW respectively for 4 hr, we then inspected the 16S rRNA expression of P. acnes, S. mutans, and E. coli compared with the same gene without C16‐KWKW treatment.
Figure 1.
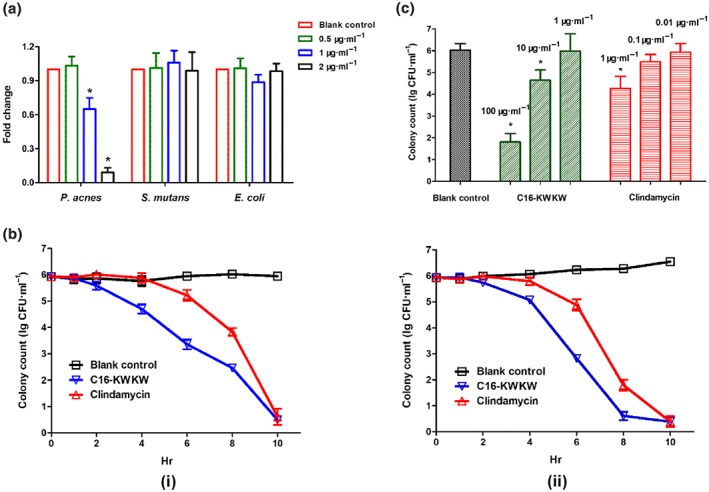
The specific and rapid anti‐Propionibacterium acnes activity of C16‐KWKW. (a) The different levels of 16S rRNA expression in P. acnes, Streptococcus mutans, and Escherichia coli after treatment with various doses of C16‐KWKW. C16‐KWKW exhibited a higher potency against P. acnes than against the other two bacterial strains. (b) The time‐killing effects of 20‐μg·ml−1 C16‐KWKW against P. acnes ATCC11827 for 10 hr at 25°C (i) or 37°C (ii). Clindamycin at 0.5 μg·ml−1 was used as a control. At 6 hr after treatment, only 103 colonies were observed for the group treated with C16‐KWKW, in contrast to 105 colonies remaining in the clindamycin treatment group. (c) The killing effect of C16‐KWKW against P. acnes ATCC11827 treated with different doses for 4 hr. Clindamycin was used as a positive control. The concentrations of C16‐KWKW and clindamycin were 0.5×, 5×, and 50× MIC, respectively; n = 5. Each experiment was repeated independently five times. *P< .05, compared to control
These initial data prompted us to speculate whether the fatty acids alone also possess such high anti‐P. acnes activity. The anti‐P. acnes activity was thus tested with the fatty acids alone. As indicated in Table S1, only C12 and C14 fatty acids were moderately active towards P. acnes with MICs around 62.5 μg·ml−1 under the same conditions, while the others were not active in our test range.
It is noted that the MIC test was performed in different culture media, which may result in different MIC values. To further address the anti‐P. acnes activity of these peptides, we then tested the antimicrobial activity of C14‐KWKW and C16‐KWKW in the same medium (Mueller–Hinton medium). As shown in Table S3, for these two peptides, no significant differences were observed as compared with the tests in other different media.
A significant concern in the development of AMPs is their reduced susceptibility to salts, especially divalent cations (Ahn et al., 2013). We evaluated the anti‐P. acnes activity of C16‐KWKW in different salts including mono and divalent cations. As shown in Table S2, no significant decrease in the killing effect was observed in the presence of 10‐mM saline solutions of CaCl2, MgCl2, or 150 mM of NaCl.
3.3. C16‐KWKW shows a faster killing kinetics than clindamycin
After the anti‐P. acnes activity of C16‐KWKW had been established, we next measured its killing kinetics at different time points (Figure 1b) and the dose–response relationship during the same period of time (Figure 1c). As shown in Figure 1b, after treatment of P. acnes (1 × 106 CFU·ml−1) with C16‐KWKW at a concentration of 20 μg·ml−1 for 4 hr, approximately 5 × 104 bacterial colonies remained, compared with 9 × 105 colonies following treatment with clindamycin under the same conditions. At 6 hr after treatment, only 3 × 103 colonies were observed for the group treated with C16‐KWKW, in contrast to 2 × 105 colonies remaining in the clindamycin‐treated group.
The faster killing effect of C16‐KWKW was further assessed by treating P. acnes with C16‐KWKW and clindamycin separately at concentrations of 0.5×, 5×, and 50× MIC for 4 hr. The data in Figure 1c show that the killing effect of both C16‐KWKW and clindamycin was dose‐dependent, and that the killing effect of C16‐KWKW was apparently faster than that of clindamycin at 5× and 50× MIC, while no significant killing effects were observed at concentrations of 0.5× MIC for both C16‐KWKW and clindamycin, consistent with the MIC results (Figure 1c).
3.4. Toxicity of C16‐KWKW is moderate
The cytotoxicity of C16‐KWKW towards HaCaT and RAW264.7 cells was evaluated by employing the CCK‐8 assay (Oyadomari et al., 2001). As indicated in Figure 2a, no significant toxicity was observed for C16‐KWKW and its congeners at a concentration of 31.25 μg·ml−1, compared with 3.9 μg·ml−1 of melittin, the positive control. As the minimum inhibitory concentration of C16‐KWKW against P. acnes is only 2 μg·ml−1, far below its cytotoxicity range, it can be deduced that C16‐KWKW should be safe within the range needed for effective treatment of P. acnes.
Figure 2.
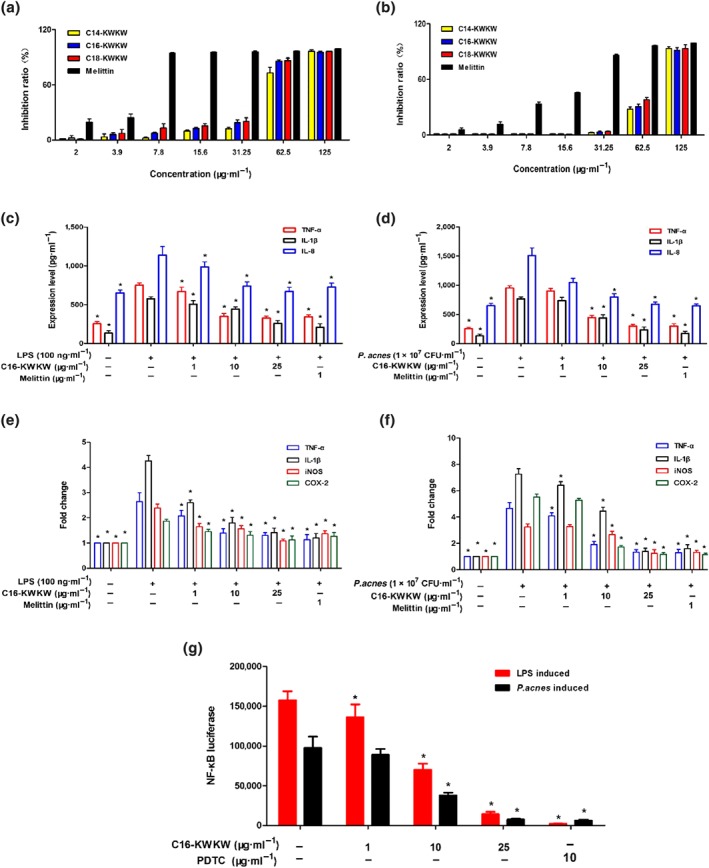
The cytotoxic and anti‐inflammatory activities of C16‐KWKW in vitro. (a, b) Cytotoxicity of the designated peptides evaluated with CCK‐8 assays in HaCaT cells (a) for 48 hr or RAW264.7 cells (b) for 8 hr. (c, d) Inhibitory effects of C16‐KWKW on the secretion of TNF‐α, IL‐1β, and IL‐8 in RAW264.7 cells induced with LPS or heat‐killed Propionibacterium acnes and measured by ELISA. (e, f) C16‐KWKW suppressed the secretion of TNF‐α, IL‐1β, iNOS, and COX‐2 in RAW264.7 cells induced with LPS or heat‐killed P. acnes, assessed by qPCR. (g) C16‐KWKW suppressed the expression of NF‐κB in RAW264.7 cells stimulated with LPS or heat‐killed P. acnes, determined by luciferase reporter assay. Pyrrolidine dithiocarbamate (PDTC) was used as a positive control. n = 5. Each experiment was repeated independently five times. *P< .05, compared to control
3.5. C16‐KWKW inhibits the expression of pro‐inflammatory cytokines stimulated by LPS and Propionibacterium acnes
Given the significant role of inflammation in the formation of acne vulgaris, we next tested the anti‐inflammatory activity of C16‐KWKW by measuring its inhibition of the expression of pro‐inflammatory cytokines produced by mouse macrophage RAW264.7 cells (Park et al., 2014). As observed previously, the inflammation associated with acne is possibly triggered by sebaceous lipids (Zouboulis et al., 2005; Zouboulis, Jourdan, & Picardo, 2014) or P. acnes (Nagy et al., 2006); thus, in this study, the production of cytokine was induced respectively by LPS and heat‐killed P. acnes. Based on the cytotoxicity data (Figure 2b), the peptide concentration was set at 1, 10, and 25 μg·ml−1. Treatment with C16‐KWKW significantly suppressed both LPS (Figure 2c) and P. acnes‐induced (Figure 2d) TNF‐α, IL‐8, and IL‐1β production, as assessed by ELISA, which was further supported by qRT‐PCR. As indicated in Figure 2e,f, C16‐KWKW also suppressed the gene expression of IL‐1β and TNF‐α. Next, the suppression of other key enzymes involved in the inflammatory response (Cheng et al., 2016) such as iNOS and COX‐2 was determined in both LPS and P. acnes‐stimulated RAW264.7 cells, as shown in Figure 2e,f.
Furthermore, considering the significance of nuclear NF‐κB in the development of inflammation, as well as in the signal transduction of multiple downstream cytokines (Dev, Iyer, Razani, & Cheng, 2011), we measured the inhibitory effect of C16‐KWKW on the expression of NF‐κB under the same conditions as utilized in the measurement of cytokines (Figure 2g). The RAW264.7 cells stably transfected with the plasmids encoding NF‐κB and luciferase gene were pretreated with C16‐KWKW for 2 hr, followed by stimulation with LPS and heat‐killed P. acnes, respectively, for an additional 6 hr. The inhibition of NF‐κB was then evaluated by measuring the inhibition of luciferase activity on RAW264.7 cells. An apparent inhibition of NF‐κB expression was observed at the concentration of C16‐KWKW higher than 10 μg·ml−1 (Figure 2g).
3.6. Antimicrobial action of C16‐KWKW involves an interaction with the bacterial membrane of Propionibacterium acnes
Generally, the modes of action of several AMPs involve an interaction with cell membranes, which induces leakage of internal contents of cells (Malmsten, 2016). To determine the mechanism of action and evaluate the interaction of C16‐KWKW with the bacterial lipid membrane, ITC was used to compare the thermodynamic parameters of the interaction between C16‐KWKW and bacterial membrane lipids. The lipids were extracted from P. acnes, S. aureus, and E. coli, dried in vacuo, and resuspended in 5% DMSO at 4 mg·ml−1. The thermal changes due to the interaction between the lipids and C16‐KWKW at 150 μg·ml−1 were measured (Figure S1). The ITC isotherms showed that C16‐KWKW binds more efficiently to the lipid from P. acnes than those from S. aureus and E. coli. ITC is a thermodynamic technique for monitoring the quantitative heat curve of a changing process to identify the interactions between biomolecules. The binding affinities K d calculated between C16‐KWKW and P. acnes were 0.287 μM, while no K d values were able to be calculated for S. aureus and E. coli under the conditions tested. Furthermore, the thermogram showed that the ITC peaks were negative, indicating that the predominant interaction was exothermic, because both ΔH and TΔS values were negative (Table S4). In contrast, ITC isotherms for the interaction between the positive control melittin (200 μg·ml−1) and multiple lipids did not exhibit such dramatic differences (Figure S1 and Table S4).
To confirm this observation, the interaction between the bacterial membrane and C16‐KWKW was studied further by employing tryptophan fluorescence spectroscopic analysis (Caesar, Esbjorner, Lincoln, & Norden, 2006; Lin et al., 2016). In the fluorescence spectra, the amino acid tryptophan has a wavelength of maximum absorption of 280 nm and an emission peak ranging from approximately 300 to 350 nm depending on changes in the local environment. Therefore, the tryptophan residue always serves as an important intrinsic fluorescent probe to detect the change in the micro‐environment of the tryptophan. As shown in Table S5 and Figure S2, with a similar approach, a 23‐nm blueshift was observed for the interaction between C16‐KWKW and the lipid from P. acnes. There was a difference of one order of magnitude between the interaction with the lipid from P. acnes and that with the lipids from S. aureus and E. coli. However, this blueshift difference was not observed for the interaction between the peptide melittin and the lipids from P. acnes, S. aureus, and E. coli.
3.7. C16‐KWKW damages the bacterial membrane and causes the leakage of its inner components
Next, we investigated whether the interaction of C16‐KWKW with the bacterial membrane causes the interior components of cells to be released. To this end, we used TEM (Figure 3a), nucleic acid leakage measurement (Figure 3b), and PI uptake assay (Figure 3c,d) to unravel the possible mechanism of C16‐KWKW. The nucleic acid has a maximum absorption peak at 260 nm, and the intensity of the peak is proportional to the concentration of the nucleic acid. Therefore, the change in the amount of nucleic acid in the extracellular fluid can be monitored by the UV absorption method, from which the extent of the damage to the cell membrane can be determined. As shown in Figure 3b, after treatment with C16‐KWKW or melittin at 10 μg·ml−1, both compounds displayed potential for damaging the bacterial cell membrane and inducing leakage of nucleic acid, with melittin demonstrating a considerably faster action than C16‐KWKW. In contrast, clindamycin did not affect the integrity of the cell membrane under the same treatment conditions. This phenomenon was again assessed through the PI uptake assay and detected by both flow cytometry analysis and fluorescence microplate. In Figure 3c, the shifts of data in flow cytometry reflect the fluorescence intensity of PI, which are associated with the degree of damage to the bacterial cell membrane induced by C16‐KWKW. The treatment of the bacterial cells revealed a dose‐dependent response (Figure 3d); the uptake of PI was the highest at a concentration of 10 μg·ml−1 of peptide.
Figure 3.
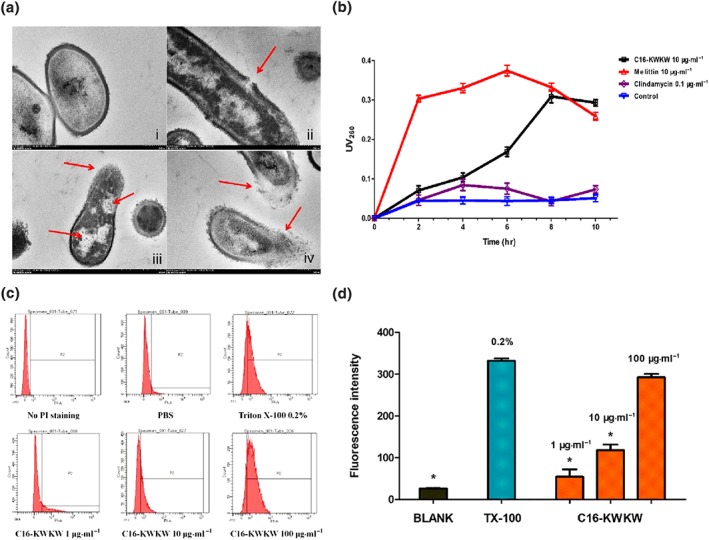
C16‐KWKW affects membrane permeability by interfering with bacterial membrane integrity. (a) Transmission electron micrograph of Propionibacterium acnes treated with C16‐KWKW. (i) Blank control. The internal electron microscope form of normal P. acnes, the cell surface was smooth, the edge was neat, the cell wall structure was close, there was no gap between the inner edge and the cell membrane, and the cytoplasm was evenly distributed; (ii, iii, iv) P. acnes treated with 10‐μg·ml−1 C16‐KWKW for 2 hr, the cell wall was dissolved, the cell membrane was damaged, the cytoplasm was lost, and a clear vacuolar structure was formed inside the cell. (b) Total nucleotide leakage from P. acnes treated with 10‐μg·ml−1 C16‐KWKW and monitored with UV absorption band at 260 nm, 10‐μg·ml−1 melittin was used as a positive control and 0.1‐μg·ml−1 clindamycin as a negative control. C16‐KWKW caused pathological damage and damage to the cell membrane, resulting in the leakage of nucleic acids and other substances with UV absorption characteristics. Compared with the control group, the C16‐KWKW group showed a clear absorption peak at 260 nm that continued to increase within 8 hr. (c, d) The damaging effect of C16‐KWKW on the cytomembrane determined by PI uptake assay with flow cytometry (c) and fluorescence microplate (d), 0.2% Triton X‐100 was the positive control. After C16‐KWKW disrupts the cell membrane, PI enters the cell and intercalates into the DNA double‐strand to generate fluorescence, and the intensity is dependent on the concentration of C16‐KWKW. n = 5. Each experiment was repeated independently five times. *P< .05, compared to control
3.8. C16‐KWKW inhibits the colonization of Propionibacterium acnes in vivo
We next performed a preliminary experiment to test the anti‐P. acnes effect of C16‐KWKW in vivo. The 6‐week‐old Kunming mice were divided into three groups treated with C16‐KWKW, clindamycin as control, and PBS. P. acnes was injected at 3.0 × 107 CFU in 40‐μl PBS into each ear of the mice, followed by challenge with the peptide, clindamycin, and PBS, respectively. Before the experiment, a preliminary toxicity test was performed with a 10‐fold higher concentration on healthy or scratched mice skin, and no side effect was observed (data not shown). As shown in Figure 4a, after 24 hr, the number of colonies of P. acnes in the ear for the PBS treatment group was 1.36 × 106 CFU·ml−1, while the number of colonies of P. acnes in the C16‐KWKW group was reduced to 67% and 45% at concentrations of 100‐ and 200‐μg C16‐KWKW in 50‐mg vehicle (vaseline), respectively. In the control group, treatment with 10‐μg clindamycin in 50‐mg vaseline resulted in only 23% of P. acnes remaining.
Figure 4.
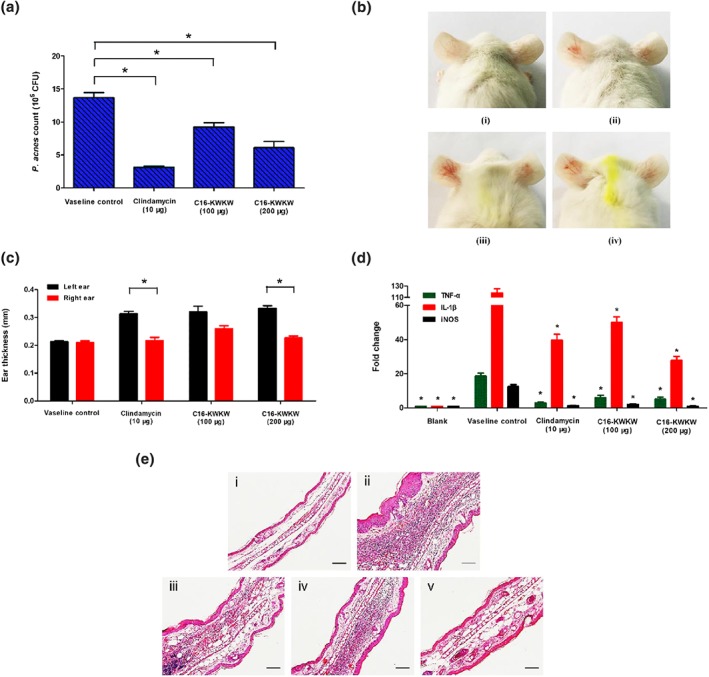
C16‐KWKW inhibits the colonization of Propionibacterium acnes and reduces P. acnes‐induced inflammation in vivo (clindamycin acts as a positive control). (a) In vivo anti‐P. acnes activity of C16‐KWKW in P. acnes‐injected mice ears. The inhibition ratio of P. acnes in the mouse ear was increased by 100‐ and 200‐μg C16‐KWKW in 50‐mg vaseline. (b) The ear redness and swelling in P. acnes‐injected inflammatory skin were reduced after treatment with C16‐KWKW mixed with 50 mg of vaseline. (i) Blank control mice ear (not treated drugs and bacterium). Ears of Kunming mice were injected i.d. with 3.0 × 107 CFU, in 40 μl PBS, of P. acnes, and then 10‐μg clindamycin (ii), 100‐μg C16‐KWKW (iii), or 200‐μg C16‐KWKW (iv) mixed with 50 mg of vaseline were respectively applied to the surface of the right ear while the corresponding left ear was not treated with drugs. (c) The changes in the ear thickness in P. acnes‐injected inflammatory skin after application of C16‐KWKW on the right ear. (d) C16‐KWKW suppressed the secretion of TNF‐α, IL‐1β, and iNOS in P. acnes‐injected inflammatory skin as detected by qRT‐PCR. (e) The histological examination results of mice ear. The samples were from the in vivo mice tested. It can be seen from the picture that after the injection of P. acnes, swelling and an infiltration of inflammatory cells were clearly observed in the left ear tissue section, and no improvement was found after treatment with vaseline (ii). The degree of swelling and number of inflammatory factors infiltrating the ear were significantly reduced in the corresponding right ear tissue, which were treated with 100‐μg C16‐KWKW (iii), 200‐μg C16‐KWKW (iv), or 10‐μg clindamycin (v), respectively; the effects are similar to the untreated blank control group (i). n = 5. Each experiment was repeated independently five times. *P< .05, compared to control
3.9. C16‐KWKW reduces Propionibacterium acnes‐induced inflammation in vivo
To investigate the anti‐inflammatory effects of C16‐KWKW on skin diseases induced by live P. acnes, we monitored the changes in ear thickness and cytokines associated with the inflammation by employing a P. acnes‐treated inflammatory animal model. As shown in Figure 4b, ear cutaneous erythema and swelling were observed 24 hr after injection of P. acnes. The thickness of the vehicle‐treated ear was 1.5 times that of the blank ear, which was not injected with P. acnes. No swelling was observed in the ear of the control mice injected with only PBS. The thickness of the ears treated with C16‐KWKW at 100‐ and 200‐μg concentrations was 124% and 109% that of the control, respectively, and the thickness of the ear treated with clindamycin was 103% compared with that for the control (Figure 4c).
The anti‐inflammatory effect of C16‐KWKW was further evaluated by qRT‐PCR to measure the level of cytokines after treatment with the test drugs on the animal model. As indicated in Figure 4d, after 24 hr of P. acnes challenge, the levels of TNF‐α, IL‐1β, and iNOS in the PBS‐treated group (negative control) were all dramatically increased. In contrast, the secretion levels of TNF‐α, IL‐1β, and iNOS in the C16‐KWKW‐ and clindamycin‐treated groups were significantly inhibited. Compared with the PBS‐treated group, the levels of TNF‐α, IL‐1β, and iNOS were 32%, 42%, and 16%, respectively, in the group treated with 100‐μg C16‐KWKW and 27%, 24%, and 8%, respectively, in the group treated with 200‐μg C16‐KWKW. In addition, TNF‐α, IL‐1β, and iNOS levels were reduced in the clindamycin‐treated group, where the secretion levels of TNF‐α, IL‐1β, and iNOS were 15%, 33%, and 10% after 24 hr of P. acnes challenge.
Next, a histological test was employed to confirm the anti‐inflammatory effect of C16‐KWKW in a mouse model. As shown in Figure 4e, after 24 hr of challenge with P. acnes, ear swelling was observed, and histological analysis revealed a considerable increase in the number of infiltrated inflammatory cells, especially in the injection site of P. acnes (Figure 4e, ii). The epicutaneous application of C16‐KWKW on the ear of the mice resulted in a decrease in the infiltrated inflammatory cells and inflammation induced by P. acnes (Figure 4e, iii and iv). In addition, the clindamycin‐treated group demonstrated a decrease in ear inflammation and infiltrated inflammatory cells (Figure 4e, v).
4. DISCUSSION
Propionibacterium acnes is a Gram‐positive bacterium known for its critical role in inducing acne vulgaris, which is partially due to its ability to produce a number of virulence factors including the release of pro‐inflammatory cytokines (Li et al., 2015). Several peptides possess a dual antimicrobial and immunomodulatory function (Lima et al., 2017), with modes of action different from currently used antibiotics, a low tendency for bacteria to develop resistance, and, in some cases, fast killing kinetics. We therefore chose AMPs as a tool to develop potent anti‐P. acnes agents. Additionally, the fact that P. acnes lives primarily on fatty acids in sebum secreted by sebaceous glands in the follicles (Perry & Lambert, 2011) suggests that it might be possible to create a narrow spectrum anti‐P. acnes agent by using a fatty acid as a targeting domain. Thus, we screened our combinatorial lipopeptide library, from which a peptide C16‐KWKW with potent anti‐P. acnes activities was identified. C16‐KWKW is a lipopeptide generated by conjugating palmitic acid to the N‐terminus of KWKW. The structural modification study indicated that KWKW substituted with C16 fatty acid was the most active lipopeptide among the congeners and possessed faster killing kinetics than the positive control clindamycin. Therefore, this peptide was selected for further extensive investigation in vitro and in vivo.
Compared with a narrow‐spectrum antimicrobial therapy, a number of clinical practices including acute respiratory infections (Messacar, 2018), children hospitalized with pneumonia (Selby, Pettit, & Brown, 2015; Williams et al., 2013), and skin infection showed that elimination of the entire bacteria from microflora by a broad‐spectrum antimicrobial does more harm than good(Shu et al., 2013). As is well‐known, the healthy skin microflora is a well‐organized and peaceful ecosystem, in which mutually beneficial and stable interactions exist among commensal microbes and between microbes and the host (Chen et al., 2018). However, this balance will be broken when some pathogens like P. acnes overgrow skin, thereby leading to alterations in the composition or function of the skin microbiota. However, in contrast to broad‐spectrum antimicrobials, a narrow‐spectrum antimicrobial therapy will only focus on the pathogen itself, minimize the impact on the entire ecosystem, maintain homeostasis, and as a result, reduce the development of bacterial resistance.
The MIC test (Table 1) indicated that in addition to high antibacterial potency against P. acnes, C16‐KWKW exhibited a higher potency against P. acnes than other non‐targeting bacterial strains such as S. mutans, S. aureus, and E. coli, which was additionally supported by the results of the qRT‐PCR studies (Figure 1a). After treatment with C16‐KWKW, the 16S rRNA expression of P. acnes, S. mutans, and E. coli clearly indicated that C16‐KWKW possessed a higher activity against P. acnes than other bacterial strains.
In this study, it is deduced that the anti‐P. acnes or anti‐inflammatory activity of these lipopeptides is strongly related to the length of their fatty acid tail, of which the optimized length can be reached by a structure and activity relationship study.
As for the Cn‐KWKW peptides activity against P. acnes, our data indicated that the most active one should be the peptide conjugated with C16 fatty acid (C16‐KWKW). Whereas in another study, with respect to the peptide RKWWK, the most potent one towards S. aureus is the peptide conjugated with C10 fatty acid (C10‐RKWWK; Fang et al., 2014).
This phenomenon might be attributed to two aspects: peptides versus bacterial strains tested. We know that the molecular target of these peptides is the bacterial membrane, of which the major lipid components may vary from bacteria to bacteria (Epand & Epand, 2011). It is, therefore, deduced that the optimal length of the lipid tail in an antimicrobial lipopeptide may vary among bacterial strains. A similar conclusion was also drawn from our earlier work, which showed that by designing matrix peptide library based on different charge and hydrophobicity, peptides targeting different bacterial strains were able to be identified (Yarbrough et al., 2011).
Nevertheless, from the peptide point of view, the antimicrobial activities are strongly associated with the physicochemical properties of a peptide, especially with the properties of hydrophobicity, charge, and conformation. Therefore, the antimicrobial potency may vary from peptide to peptide, as indicated in our previous work (Fang et al., 2014).
To investigate the possible target of C16‐KWKW, ITC and tryptophan fluorescence spectrometry were employed as tools to study the interactions between bacterial membrane lipids and C16‐KWKW. ITC is a physical technique most often used to determine the thermodynamic parameters of interactions between small molecules and larger macromolecules (e.g., proteins and DNA; Pierce, Raman, & Nall, 1999). The lipid was directly extracted from each bacterium. As indicated in Figure S1 and Table S4, the reaction was exothermic, with negative ITC peaks occurring at the beginning of the titrations. The association constant between C16‐KWKW and P. acnes membrane lipid was 3.484 μM−1, indicating the presence of a strong interaction between them (Arouri, Dathe, & Blume, 2013). Furthermore, a larger blueshift of C16‐KWKW observed in tryptophan fluorescence spectroscopic analysis (Figure S2 and Table S5) suggested a deeper insertion of KWKW residues into the P. acnes lipid membrane than interactions with other bacterial membrane, further confirming the conclusion of the ITC experiment.
The detailed interaction between C16‐KWKW and P. acnes membrane was further studied by TEM, nucleic acid leakage measurement, and PI uptake assay (Figure 3a–d), all of which indicated that C16‐KWKW was able to affect the integrity of the cell membrane and therefore to induce leakage of nucleic acids, leading to the death of bacterial cells (Le, Fang, & Sekaran, 2017).
On the basis of these experiments, and as inflammation significantly contributes to the development of acne vulgaris, we further evaluated whether C16‐KWKW was able to modulate the host immune system and consequently play a critical role in anti‐inflammatory activity. Inflammatory acne is regulated by inflammatory factors that include TNF‐α and IL‐1β, which are secreted by monocytes (Kim et al., 2002). Therefore, the levels of these factors indicate the degree of inflammation. The determination of the levels of these cytokines induced by LPS and heat‐killed P. acnes and assayed by ELISA and qRT‐PCR, respectively, proved that treatment with C16‐KWKW significantly suppressed both LPS‐ (Figure 2c) and P. acnes‐induced (Figure 2d) TNF‐α, IL‐8, and IL‐1β secretion, thus displaying a potential for application against inflammation.
Nevertheless, to understand the mode of action of C16‐KWKW, it was important to determine the mechanism of its anti‐inflammatory action. It is known that the NF‐κB transcription factor plays a critical role in the regulation of immediate transcriptional responses in inflammatory situations (Denk, Wirth, & Baumann, 2000). This factor is strongly induced downstream of most pathogen recognition receptors, and a number of NF‐κB pathway components are involved in the development of the innate immune response (Dominguez‐Lopez, Bautista‐de Lucio, Serafin‐Lopez, Robles‐Sanchez, & Garfias, 2014). Among the genes regulated by NF‐κB, there are a variety of pro‐inflammatory cytokines including TNF‐α, IL‐8, and IL‐1β (Katanov et al., 2015). Therefore, the expression of NF‐κB was compared with and without treatment with the peptide (Figure 2g). Consequently, the apparently inhibitory effect of C16‐KWKW on NF‐κB expression indicated a possible mechanism of its anti‐inflammatory action. However, further studies need to be conducted in this regard, including investigations of critical proteins involved in the control of NF‐κB activity, such as TLR2/4, p65, P‐p65, and IκB proteins, which could play a significant role in the anti‐inflammatory mechanism of these peptides.
To evaluate the therapeutic effect of C16‐KWKW, in vivo studies were conducted to determine the anti‐P. acnes and anti‐inflammatory activity of the peptide by using P. acnes‐treated mouse. The in vivo animal study showed that C16‐KWKW was able to effectively inhibit the growth of P. acnes, decrease the swelling of ear, and reduce the level of cytokines secreted, thus displaying promising antibacterial and anti‐inflammatory effects in vivo.
Overall, our findings from this study demonstrate that C16‐KWKW is a lipopeptide that exhibits potent anti‐P. acnes and anti‐inflammatory activities in vitro and in vivo. It is more active against P. acnes than other bacteria and shows faster killing kinetics than the positive control, clindamycin. As compared with a panel of other AMPs that have the potential to be used for a treatment of acne vulgaris (Lee et al., 2014; Zhang et al., 2013), C16‐KWKW consists of only four amino acids and has higher specificity and lower mammalian toxicity, indicating that it is a promising candidate peptide for development as part of a new generation of anti‐P. acnes agents.
4.1. NOTE
Since November 2016, a huge taxonomic modification has been reported by Scholz and Kilian (2016) and the name Propionibacterium acnes has been changed to Cutibacterium acnes. However in this article, in order to facilitate readers' understanding, we decided to still use the name “Propionibacterium acnes.”
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
G.Y. performed the experiments and data analysis. J.W. did the peptides screening and modification. S.L. participated in peptides purification. Z.C. and S.F. participated in data statistical analysis. D.C. and H.X. participated in animal studies. W.S. did the manuscript reading and correction. J.H. conceived, supervised, and funded the work. J.H. and G.Y. wrote the paper. All authors reviewed the manuscript.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design & Analysis, and Animal Experimentation and as recommended by funding agencies, publishers, and other organizations engaged with supporting research.
Supporting information
Table S1 Antimicrobial activity of fatty acids
Table S2 Antibacterial activity of lipopeptides against P. acnes ATCC11827 in saline solution
Table S3 Antimicrobial activity of designed lipopeptides in MH media
Table S4 Thermodynamic parameters of interactions between lipopeptides with lipids
Table S5 Fluorescence spectroscopy parameters measured for blueshift of tryptophan in different lipids
Table S6 The design of in vivo experiment
Figure S1 Binding of C16‐KWKW with lipids from P. acnes, S. aureus and E. coli, melittin was used as control. A significant binding can be observed between 150 μg/mL C16‐KWKW and 4 mg/mL lipid extracted from P. acnes, whereas C16‐KWKW bound very weakly to other lipids at the same concentration. In contrast, 200 μg/mL melittin shows binding with lipids from all the three strains at 4 mg/mL, and the binding difference was not significant. N=5. Each experiment was repeated independently 5 times.
Figure S2 Tryptophan fluorescence emission spectra of the lipopeptides using various liposome models. The lipid extracted from P. acnes treated with C16‐KWKW or C10‐KWKW at 150 μg/mL showed a significant blueshift in the maximum emission spectrum, while the blueshift in other lipids was not obvious. In contrast, melittin produced a significant blueshift with all three lipids at the same concentration. N=5. Each experiment was repeated independently 5 times.
ACKNOWLEDGEMENTS
This work was financially supported by the funding from Southern Medical University (B1040903), Science and Technology Department of Guangdong Province of China (2014A020210014 and 2015A020211010), and the National Natural Science Foundation of China (81773556).
Yang G, Wang J, Lu S, et al. Short lipopeptides specifically inhibit the growth of Propionibacterium acnes with a dual antibacterial and anti‐inflammatory action. Br J Pharmacol. 2019;176:2321–2335. 10.1111/bph.14680
REFERENCES
- Ahn, M. , Murugan, R. N. , Jacob, B. , Hyun, J. K. , Cheong, C. , Hwang, E. , … Bang, J. K. (2013). Discovery of novel histidine‐derived lipo‐amino acids: Applied in the synthesis of ultra‐short antimicrobial peptidomimetics having potent antimicrobial activity, salt resistance and protease stability. European Journal of Medicinal Chemistry, 68, 10–18. 10.1016/j.ejmech.2013.07.008 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174(Suppl 1), S272–s359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: Overview. British Journal of Pharmacology, 174(Suppl 1), S1–s16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arouri, A. , Dathe, M. , & Blume, A. (2013). The helical propensity of KLA amphipathic peptides enhances their binding to gel‐state lipid membranes. Biophysical Chemistry, 180‐181, 10–21. 10.1016/j.bpc.2013.05.003 [DOI] [PubMed] [Google Scholar]
- Aslam, I. , Fleischer, A. , & Feldman, S. (2015). Emerging drugs for the treatment of acne. Expert Opinion on Emerging Drugs, 20(1), 91–101. 10.1517/14728214.2015.990373 [DOI] [PubMed] [Google Scholar]
- Becerro de Bengoa Vallejo, R. , Losa Iglesias, M. E. , Alou Cervera, L. , Sevillano Fernandez, D. , & Prieto Prieto, J. (2009). Preoperative skin and nail preparation of the foot: Comparison of the efficacy of 4 different methods in reducing bacterial load. Journal of the American Academy of Dermatology, 61(6), 986–992. 10.1016/j.jaad.2009.04.045 [DOI] [PubMed] [Google Scholar]
- Caesar, C. E. , Esbjorner, E. K. , Lincoln, P. , & Norden, B. (2006). Membrane interactions of cell‐penetrating peptides probed by tryptophan fluorescence and dichroism techniques: Correlations of structure to cellular uptake. Biochemistry, 45(24), 7682–7692. 10.1021/bi052095t [DOI] [PubMed] [Google Scholar]
- Cheng, A. , Han, C. , Fang, X. , Sun, J. , Chen, X. , & Wan, F. (2016). Extractable and non‐extractable polyphenols from blueberries modulate LPS‐induced expression of iNOS and COX‐2 in RAW264.7 macrophages via the NF‐κB signalling pathway. Journal of the Science of Food and Agriculture, 96(10), 3393–3400. 10.1002/jsfa.7519 [DOI] [PubMed] [Google Scholar]
- Dean, S. N. , Bishop, B. M. , & van Hoek, M. L. (2011). Natural and synthetic cathelicidin peptides with anti‐microbial and anti‐biofilm activity against Staphylococcus aureus . BMC Microbiology, 11, 114 10.1186/1471-2180-11-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk, A. , Wirth, T. , & Baumann, B. (2000). NF‐κB transcription factors: Critical regulators of hematopoiesis and neuronal survival. Cytokine & Growth Factor Reviews, 11(4), 303–320. 10.1016/S1359-6101(00)00009-5 [DOI] [PubMed] [Google Scholar]
- Dev, A. , Iyer, S. , Razani, B. , & Cheng, G. (2011). NF‐kappaB and innate immunity. Current Topics in Microbiology and Immunology, 349, 115–143. 10.1007/82_2010_102 [DOI] [PubMed] [Google Scholar]
- Disease, G. B. D. , Injury, I. , & Prevalence, C. (2016). Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990‐2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet, 388(10053), 1545–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez‐Lopez, A. , Bautista‐de Lucio, V. M. , Serafin‐Lopez, J. , Robles‐Sanchez, E. , & Garfias, Y. (2014). Amniotic membrane modulates innate immune response inhibiting PRRs expression and NF‐κB nuclear translocation on limbal myofibroblasts. Experimental Eye Research, 127, 215–223. 10.1016/j.exer.2014.08.002 [DOI] [PubMed] [Google Scholar]
- Epand, R. M. , & Epand, R. F. (2011). Bacterial membrane lipids in the action of antimicrobial agents. Journal of Peptide Science: An Official Publication of the European Peptide Society, 17(5), 298–305. 10.1002/psc.1319 [DOI] [PubMed] [Google Scholar]
- Fang, Y. , Zhong, W. , Wang, Y. , Xun, T. , Lin, D. , Liu, W. , … He, J. (2014). Tuning the antimicrobial pharmacophore to enable discovery of short lipopeptides with multiple modes of action. European Journal of Medicinal Chemistry, 83, 36–44. 10.1016/j.ejmech.2014.06.003 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR . (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46(D1), D1091–d1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui, S. , Aoshima, H. , Ito, M. , Kobuko, K. , & Itami, S. (2012). Inhibition of sebum production and Propionibacterium acnes lipase activity by fullerenol, a novel polyhydroxylated fullerene: Potential as a therapeutic reagent for acne. Journal of Cosmetic Science, 63(4), 259–265. [PubMed] [Google Scholar]
- Izumi, T. , Saito, Y. , Kishimoto, I. , Harada, M. , Kuwahara, K. , Hamanaka, I. , … Nakao, K. (2001). Blockade of the natriuretic peptide receptor guanylyl cyclase‐A inhibits NF‐κB activation and alleviates myocardial ischemia/reperfusion injury. The Journal of Clinical Investigation, 108(2), 203–213. 10.1172/JCI12088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, M. N. , Francis, S. E. , Hutchinson, F. J. , Handley, P. S. , & Lyle, I. G. (1993). Targeting and delivery of bactericide to adsorbed oral bacteria by use of proteoliposomes. Biochimica et Biophysica Acta, 1147(2), 251–261. 10.1016/0005-2736(93)90010-W [DOI] [PubMed] [Google Scholar]
- Jugeau, S. , Tenaud, I. , Knol, A. C. , Jarrousse, V. , Quereux, G. , Khammari, A. , & Dreno, B. (2005). Induction of toll‐like receptors by Propionibacterium acnes . The British Journal of Dermatology, 153(6), 1105–1113. 10.1111/j.1365-2133.2005.06933.x [DOI] [PubMed] [Google Scholar]
- Katanov, C. , Lerrer, S. , Liubomirski, Y. , Leider‐Trejo, L. , Meshel, T. , Bar, J. , … Ben‐Baruch, A. (2015). Regulation of the inflammatory profile of stromal cells in human breast cancer: Prominent roles for TNF‐α and the NF‐κB pathway. Stem Cell Research & Therapy, 6, 87 10.1186/s13287-015-0080-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. , Ochoa, M. T. , Krutzik, S. R. , Takeuchi, O. , Uematsu, S. , Legaspi, A. J. , … Modlin, R. L. (2002). Activation of toll‐like receptor 2 in acne triggers inflammatory cytokine responses. Journal of Immunology, 169(3), 1535–1541. 10.4049/jimmunol.169.3.1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, B. , Pathak, R. , Bertin Mary, P. , Jha, D. , Sardana, K. , & Gautam, H. K. (2016). New insights into acne pathogenesis: Exploring the role of acne‐associated microbial populations. Dermatologica Sinica, 34, 67–73. 10.1016/j.dsi.2015.12.004 [DOI] [Google Scholar]
- Le, C. F. , Fang, C. M. , & Sekaran, S. D. (2017). Intracellular targeting mechanisms by antimicrobial peptides. Antimicrobial Agents and Chemotherapy, 61(4). 10.1128/AAC.02340-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, W. R. , Kim, K. H. , An, H. J. , Kim, J. Y. , Chang, Y. C. , Chung, H. , … Park, K. K. (2014). The protective effects of melittin on Propionibacterium acnes‐induced inflammatory responses in vitro and in vivo. The Journal of Investigative Dermatology, 134(7), 1922–1930. 10.1038/jid.2014.75 [DOI] [PubMed] [Google Scholar]
- Li, W. H. , Zhang, L. , Lyte, P. , Rodriguez, K. , Cavender, D. , & Southall, M. D. (2015). p38 MAP kinase inhibition reduces Propionibacterium acnes‐induced inflammation in vitro. Dermatology and Therapy, 5(1), 53–66. 10.1007/s13555-015-0072-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima, S. M. F. , Freire, M. S. , Gomes, A. L. O. , Cantuaria, A. P. C. , Dutra, F. R. P. , Magalhaes, B. S. , … Rezende, T. M. B. (2017). Antimicrobial and immunomodulatory activity of host defense peptides, clavanins and LL‐37, in vitro: An endodontic perspective. Peptides, 95, 16–24. 10.1016/j.peptides.2017.07.005 [DOI] [PubMed] [Google Scholar]
- Lin, D. , Ren, R. , Tan, Q. , Wu, Q. , Li, F. , Li, L. , … He, J. (2016). A facile and dynamic assay for the detection of peptide aggregation. Analytical and Bioanalytical Chemistry, 408(6), 1609–1614. 10.1007/s00216-015-9271-4 [DOI] [PubMed] [Google Scholar]
- Malmsten, M. (2016). Interactions of antimicrobial peptides with bacterial membranes and membsrane components. Current Topics in Medicinal Chemistry, 16(1), 16–24. [DOI] [PubMed] [Google Scholar]
- Marri, L. , Dallai, R. , & Marchini, D. (1996). The novel antibacterial peptide ceratotoxin A alters permeability of the inner and outer membrane of Escherichia coli K‐12. Current Microbiology, 33(1), 40–43. 10.1007/s002849900071 [DOI] [PubMed] [Google Scholar]
- McInturff, J. E. , Wang, S. J. , Machleidt, T. , Lin, T. R. , Oren, A. , Hertz, C. J. , … Kim, J. (2005). Granulysin‐derived peptides demonstrate antimicrobial and anti‐inflammatory effects against Propionibacterium acnes . The Journal of Investigative Dermatology, 125(2), 256–263. 10.1111/j.0022-202X.2005.23805.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messacar, K. (2018). Narrow‐spectrum, compared with broad‐spectrum, antibiotics equally effective with less adverse events. The Journal of Pediatrics, 196, 324–327. 10.1016/j.jpeds.2018.02.054 [DOI] [PubMed] [Google Scholar]
- Nagy, I. , Pivarcsi, A. , Kis, K. , Koreck, A. , Bodai, L. , McDowell, A. , … Kemény, L. (2006). Propionibacterium acnes and lipopolysaccharide induce the expression of antimicrobial peptides and proinflammatory cytokines/chemokines in human sebocytes. Microbes and Infection, 8(8), 2195–2205. 10.1016/j.micinf.2006.04.001 [DOI] [PubMed] [Google Scholar]
- Oyadomari, S. , Takeda, K. , Takiguchi, M. , Gotoh, T. , Matsumoto, M. , Wada, I. , … Mori, M. (2001). Nitric oxide‐induced apoptosis in pancreatic beta cells is mediated by the endoplasmic reticulum stress pathway. Proceedings of the National Academy of Sciences of the United States of America, 98(19), 10845–10850. 10.1073/pnas.191207498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, H. H. , Kim, S. G. , Kim, M. J. , Lee, J. , Choi, B. K. , Jin, M. H. , & Lee, E. (2014). Suppressive effect of tomentosin on the production of inflammatory mediators in RAW264.7 cells. Biological & Pharmaceutical Bulletin, 37(7), 1177–1183. 10.1248/bpb.b14-00050 [DOI] [PubMed] [Google Scholar]
- Perry, A. , & Lambert, P. (2011). Propionibacterium acnes: Infection beyond the skin. Expert Review of Anti‐Infective Therapy, 9(12), 1149–1156. 10.1586/eri.11.137 [DOI] [PubMed] [Google Scholar]
- Pierce, M. M. , Raman, C. S. , & Nall, B. T. (1999). Isothermal titration calorimetry of protein‐protein interactions. Methods, 19(2), 213–221. 10.1006/meth.1999.0852 [DOI] [PubMed] [Google Scholar]
- Romoser, A. A. , Chen, P. L. , Berg, J. M. , Seabury, C. , Ivanov, I. , Criscitiello, M. F. , & Sayes, C. M. (2011). Quantum dots trigger immunomodulation of the NFκB pathway in human skin cells. Molecular Immunology, 48(12–13), 1349–1359. 10.1016/j.molimm.2011.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu, S. , Han, H. M. , Song, P. I. , Armstrong, C. A. , & Park, Y. (2015). Suppression of Propionibacterium acnes infection and the associated inflammatory response by the antimicrobial peptide P5 in mice. PLoS ONE, 10(7), e0132619 10.1371/journal.pone.0132619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz, C. F. , & Kilian, M. (2016). The natural history of cutaneous propionibacteria, and reclassification of selected species within the genus Propionibacterium to the proposed novel genera Acidipropionibacterium gen. nov., Cutibacterium gen. nov. and Pseudopropionibacterium gen. nov. International Journal of Systematic and Evolutionary Microbiology, 66(11), 4422–4432. [DOI] [PubMed] [Google Scholar]
- Selby, A. , Pettit, K. , & Brown, N. (2015). Narrow‐spectrum antibiotics are as effective as broad‐spectrum antibiotics in the treatment of community‐acquired pneumonia. Archives of Disease in Childhood. Education and Practice Edition, 100(4), 223 10.1136/archdischild-2015-308815 [DOI] [PubMed] [Google Scholar]
- Shu, M. , Wang, Y. , Yu, J. , Kuo, S. , Coda, A. , Jiang, Y. , … Huang, C. M. (2013). Fermentation of Propionibacterium acnes, a commensal bacterium in the human skin microbiome, as skin probiotics against methicillin‐resistant Staphylococcus aureus . PLoS ONE, 8(2), e55380 10.1371/journal.pone.0055380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshine, D. E. , & Macaluso, K. R. (2017). Microbial invasion vs. tick immune regulation. Frontiers in Cellular and Infection Microbiology, 7, 390 10.3389/fcimb.2017.00390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasoula, E. , Gregoriou, S. , Chalikias, J. , Lazarou, D. , Danopoulou, I. , Katsambas, A. , & Rigopoulos, D. (2012). The impact of acne vulgaris on quality of life and psychic health in young adolescents in Greece. Results of a population survey. Anais Brasileiros de Dermatologia, 87(6), 862–869. 10.1590/S0365-05962012000600007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiboutot, D. , Gollnick, H. , Bettoli, V. , Dreno, B. , Kang, S. , Leyden, J. J. , … Global Alliance to Improve Outcomes in Acne . (2009). New insights into the management of acne: An update from the Global Alliance to Improve Outcomes in Acne group. Journal of the American Academy of Dermatology, 60(5 Suppl), S1–S50. 10.1016/j.jaad.2009.01.019 [DOI] [PubMed] [Google Scholar]
- Uppal, L. , Singhi, S. , Singhi, P. , & Aggarwal, R. (2017). Role of rifampin in reducing inflammation and neuronal damage in childhood bacterial meningitis: A pilot randomized controlled trial. The Pediatric Infectious Disease Journal, 36(6), 556–559. 10.1097/INF.0000000000001513 [DOI] [PubMed] [Google Scholar]
- Verma, I. M. , Stevenson, J. K. , Schwarz, E. M. , Van Antwerp, D. , & Miyamoto, S. (1995). Rel/NF‐kappa B/I kappa B family: Intimate tales of association and dissociation. Genes & Development, 9(22), 2723–2735. 10.1101/gad.9.22.2723 [DOI] [PubMed] [Google Scholar]
- Vowels, B. R. , Yang, S. , & Leyden, J. J. (1995). Induction of proinflammatory cytokines by a soluble factor of Propionibacterium acnes: Implications for chronic inflammatory acne. Infection and Immunity, 63(8), 3158–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel, D. , & Glazebrook, J. (2010). Transmission electron microscopy (TEM) freeze substitution of plant tissues. Cold Spring Harbor Protocols, 2010(7). pdb.prot4959 [DOI] [PubMed] [Google Scholar]
- Williams, D. J. , Hall, M. , Shah, S. S. , Parikh, K. , Tyler, A. , Neuman, M. I. , … Grijalva, C. G. (2013). Narrow vs broad‐spectrum antimicrobial therapy for children hospitalized with pneumonia. Pediatrics, 132(5), e1141–e1148. 10.1542/peds.2013-1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarbrough, D. K. , Eckert, R. , He, J. , Hagerman, E. , Qi, F. , Lux, R. , … Shi, W. (2011). Rapid probing of biological surfaces with a sparse‐matrix peptide library. PLoS ONE, 6(8), e23551 10.1371/journal.pone.0023551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z. , Mu, L. , Tang, J. , Duan, Z. , Wang, F. , Wei, L. , … Lai, R. (2013). A small peptide with therapeutic potential for inflammatory acne vulgaris. PLoS ONE, 8(8), e72923 10.1371/journal.pone.0072923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouboulis, C. C. , Eady, A. , Philpott, M. , Goldsmith, L. A. , Orfanos, C. , Cunliffe, W. C. , & Rosenfield, R. (2005). What is the pathogenesis of acne? Experimental Dermatology, 14(2), 143–152. 10.1111/j.0906-6705.2005.0285a.x [DOI] [PubMed] [Google Scholar]
- Zouboulis, C. C. , Jourdan, E. , & Picardo, M. (2014). Acne is an inflammatory disease and alterations of sebum composition initiate acne lesions. Journal of the European Academy of Dermatology and Venereology: JEADV, 28(5), 527–532. 10.1111/jdv.12298 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Antimicrobial activity of fatty acids
Table S2 Antibacterial activity of lipopeptides against P. acnes ATCC11827 in saline solution
Table S3 Antimicrobial activity of designed lipopeptides in MH media
Table S4 Thermodynamic parameters of interactions between lipopeptides with lipids
Table S5 Fluorescence spectroscopy parameters measured for blueshift of tryptophan in different lipids
Table S6 The design of in vivo experiment
Figure S1 Binding of C16‐KWKW with lipids from P. acnes, S. aureus and E. coli, melittin was used as control. A significant binding can be observed between 150 μg/mL C16‐KWKW and 4 mg/mL lipid extracted from P. acnes, whereas C16‐KWKW bound very weakly to other lipids at the same concentration. In contrast, 200 μg/mL melittin shows binding with lipids from all the three strains at 4 mg/mL, and the binding difference was not significant. N=5. Each experiment was repeated independently 5 times.
Figure S2 Tryptophan fluorescence emission spectra of the lipopeptides using various liposome models. The lipid extracted from P. acnes treated with C16‐KWKW or C10‐KWKW at 150 μg/mL showed a significant blueshift in the maximum emission spectrum, while the blueshift in other lipids was not obvious. In contrast, melittin produced a significant blueshift with all three lipids at the same concentration. N=5. Each experiment was repeated independently 5 times.


