Abstract
Background and purpose
PDE inhibitors such as sildenafil alleviate lower urinary tract symptoms; however, a complete understanding of their action on the bladder remains unclear. We are investigating the effects of sildenafil, on post and preganglionic nerve‐mediated contractions of the mouse bladder, and neuronal and urothelial ATP release.
Experimental approach
Bladders were used from young (12 weeks), aged (24 months), and spinal cord transected (SCT), mice, for in vitro contractility experiments. An arterially perfused in situ whole mouse model was used to record bladder pressure. Nerve‐mediated contractions were generated by electrical field stimulation (EFS) of postganglionic nerve terminals or the pelvic nerve. ATP release during EFS in intact detrusor strips, and during stretch of isolated mucosa strips, was measured using a luciferin‐luciferase assay.
Key results
Sildenafil (20 μM) inhibited nerve‐mediated contractions in young mice, with an increase in f 1/2 values in force–frequency relationships, demonstrating a greater effect at low frequencies. Sildenafil reduced the atropine‐resistant, purinergic component of nerve‐mediated contractions, and suppressed neuronal ATP release upon EFS in vitro. Sildenafil reduced the preganglionic pelvic nerve stimulated bladder pressure recordings in situ; comparable to in vitro experiments. Sildenafil reduced stretch‐induced urothelial ATP release. Sildenafil also relaxed nerve‐mediated contractions in aged and SCT mice.
Conclusion and implications
Sildenafil has a greater effect on the low‐frequency, purinergic‐mediated contractions and suppresses neuronal ATP release. In addition, sildenafil reduces stretch‐induced urothelial ATP release. These results demonstrate a novel action of sildenafil to selectively inhibit ATP release from nerve terminals innervating detrusor smooth muscle and the urothelium.
Abbreviations
- ABMA
α,β‐methylene ATP
- CCh
carbachol
- EFS
electrical field stimulation
- IDO
idiopathic bladder overactivity
- LUT
lower urinary tract
- LUTS
lower urinary tract symptoms
- NDO
neurogenic bladder overactivity
- OAB
overactive bladder
- SCT
spinal cord transection
- TTX
tetrodotoxin.
What is already known
Sildenafil alleviates lower urinary tract symptoms; however, its action on the bladder is unclear.
What this study adds
Sildenafil reduces purinergic‐mediated contractions and selectively and completely inhibits neuronal and urothelial ATP release.
What is the clinical significance
Sildenafil may target pathological pathways associated with enhanced purinergic motor and sensory pathways.
1. INTRODUCTION
Lower urinary tract symptoms (LUTS) associated with bladder pathologies, such as overactive bladder (OAB) and neurogenic bladder dysfunction, and also with ageing are highly prevalent and can have a severe effect on the quality of life of patients. Current treatments for LUTS have recognized limitations, including uncertain efficacy and adverse effects. PDE5 inhibitors such as sildenafil (Viagra®), which are used to treat erectile dysfunction (Hatzimouratidis et al., 2010), also alleviate LUTS (McVary et al., 2007). The entire lower urinary tract (LUT) expresses PDE5 (Fibbi et al., 2010; Filippi et al., 2007), and studies support the use of PDE5 inhibitors to reduce LUTS, utilizing the NO/cGMP pathway on several potential targets. These include increasing LUT oxygenation, decreasing afferent nerve activity, negatively regulating proliferation of LUT stroma and LUTS‐related inflammation (Gacci et al., 2016). The ability of sildenafil to relax smooth muscle directly has also been demonstrated, where sildenafil‐induced relaxation of human detrusor strips is significantly decreased using a soluble GC inhibitor to block the formation of cGMP (Oger et al., 2010). However, a complete understanding of their actions on the peripheral control of bladder contractile function remains unclear. In this study, the effects of sildenafil on nerve‐mediated contractions of isolated mouse detrusor strips, as well as on in situ bladder function, were characterized in young adult, aged, and spinal cord injured mice. In particular, the action of sildenafil on ATP‐mediated processes was investigated. ATP is co‐released along with ACh in parasympathetic endings to detrusor. With human detrusor, ATP is a functional transmitter only in overactive pathologies (Bayliss, Wu, Newgreen, Mundy, & Fry, 1999) and so regulation of its release would be a therapeutic target. Moreover, stretch‐activated ATP release from the urothelium, proposed to finally activate bladder afferents, is also enhanced in OAB pathologies (Cheng et al., 2014), thus providing a possible cause for urinary urgency. The effect on neuronal and urothelial neurotransmitter release was also investigated. A comprehensive description of how sildenafil reduces contractile function will give insight into the pathology of bladder functional disorders and should suggest potential therapeutic mechanisms of PDE5 inhibitors in their treatment.
2. METHODS
2.1. Tissue samples and ethics approval
All animal care and experimental procedures were in compliance with the University of Bristol Ethics Committee and in accordance with UK legislation under the Animals (Scientific Procedures) Act 1986 Amendment Regulations (SI 2012/3039). Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010) and with the recommendations made by the British Journal of Pharmacology. Young (12 weeks) and aged (24 months) male C57BL/6 mice (Harlan UK Ltd) were used for experiments.
2.2. Spinal cord transection
Twelve‐week old male mice underwent complete spinal cord transection (SCT) at the T8–T9 level as previously described (Ikeda et al., 2012; Kadekawa et al., 2017; McCarthy et al., 2009). In brief, a laminectomy was performed at the T8–T9 spinal level under sterile surgical conditions. The spinal cord was transected and the cavity cleared to ensure complete transection, the space between cut ends was filled with a haemostatic sponge, and muscle layers and overlying skin were closed with sutures. The surgery was performed under ketamine (70 mg·kg−1, i.p.) and medetomidine (0.5 mg·kg−1, i.p.) and upon recovery the mice were injected with atipamezole (1.0 mg·kg−1, i.p.) and buprenorphine (0.03 mg·kg−1, s.c.). Mice were also treated with ampicillin (0.10 mg·kg−1, s.c.) twice daily for 5 days post‐SCT to prevent urinary tract infections. Bladders of SCT mice were emptied by gentle abdominal compression and perineal stimulation twice a day for 5 days post‐SCT. SCT mice were then killed with pentobarbital sodium (20–40 mg per mouse) and used for experiments.
2.3. Measurement of contractile function in vitro
Young (normal and SCT) and aged mice were killed by cervical dislocation, and the bladder was removed through a midline laparotomy. The whole bladder was bisected and bladder strips from the bladder dome (detrusor with mucosa intact, 4‐ to 5‐mm length, 1‐ to 2‐mm width) were tied to a hook and an isometric force transducer in a horizontal trough. Preparations were superfused with Tyrode's solution at 37°C. Contractures were generated by exposure to carbachol (CCh, 1 μM) or high‐K+ (80 mM) Tyrode's. Contractions generated by electrical field stimulation (EFS; 0.1‐ms pulses, 1–40 Hz, 3‐s train every 90 s) that were inhibited by tetrodotoxin (TTX, 1 μM). With some preparations, TTX‐resistant contractions were present, generated by direct muscle stimulation, and were insensitive to atropine and α,β‐methylene ATP (ABMA). Sildenafil (20 μM, see Section 3) was added to the superfusate and the effect on agonist‐ and nerve‐mediated contractions measured. These were subtracted from the EFS contraction, leaving a nerve‐mediated contraction. Tension amplitude (mN) was normalized to preparation weight (mN·mg−1). Nerve‐mediated amplitude, T(f), plotted as a function of stimulation frequency, f, was fitted to Equation (1a). The frequency‐dependent percentage reduction of force, R(f), by an intervention was fitted to Equation (1b). For frequency‐dependent ATP release, ATP(f), data were fitted to Equation (1c).
| (1a) |
| (1b) |
| (1c) |
where T max is maximum tension at high frequencies, f 1/2 is the frequency to attain T max/2, and n is a constant (Pakzad et al., 2016): R Lf and R Hf are the maximum and minimum force reductions respectively at low and high frequencies, m and k are constants. ATP b is a frequency‐independent component of ATP release from detrusor preparations. Data fits were performed with an iterative, least‐squares algorithm (KaleidaGraph, v4.5, Synergy software, CA, USA). Drug interventions were delivered via the superfusate, with appropriate vehicle and time controls.
2.4. Measurement of nerve‐mediated ATP release in 12‐week normal and SCT mice
Superfusate samples (100 μl) were taken from a fixed point near the preparation, (two‐thirds downstream along the tissue length and 1‐mm lateral to the horizontally mounted preparation), and with minimal mechanical disturbance. Samples were taken before EFS, and 2 s after the initiation of EFS, the nerve‐mediated release was taken as the difference in these two values. Samples were stored on ice before assay of released ATP using a luciferin‐luciferase assay where the emitted light was proportional to the concentrations of ATP. The complete Sigma ATP assay mix (FLAAM, Sigma‐Aldrich, Dorset, UK) was diluted with the assay buffer supplied as per the manufacturer's instructions. Luminescence intensity was read using a luminometer (Glomax 20/20, Promega) and calibrated with an ATP standard on the day of each experiment, with luminescence as a linear function of concentration on a log–log plot over the range of 100 fM to 1 μM. Neuronal ATP release was inhibited by the application of 1‐μM TTX. Appropriate controls were carried out in background solutions using the solvents and chemicals tested. The detection limit of the system was 100‐fM ATP.
2.5. Measurement of mucosa ATP release in 12‐week‐old normal mice
The mucosa was separated from underlying detrusor by blunt dissection, mounted in a similar way to detrusor preparations to an isometric force transducer in the horizontal trough and superfused with Tyrode's solution, with or without sildenafil (20 μM). The hook was connected to a voltage‐activated solenoid that allowed rapid extension (within 1 s) of the tissue by 20% of the original length for 50 s before restoration (within 1 s) to the original length. Samples were taken and assayed, as above, before extension and at various time points upon extension, before and after drug interventions.
2.6. Measurement of nerve‐mediated contractile function in situ
Young mice were administered heparin (50 IU, i.p.) and anaesthetized with isoflurane (2%) until loss of paw withdrawal reflex, following the non‐recovery procedure modified from previous studies with an in situ arterially perfused rat (Sadananda, Drake, Paton, & Pickering, 2011) and mouse (Ito et al., 2019; Ito, Drake, Fry, Kanai, & Pickering, 2018). The brain was removed, and the spinal cord pithed with a blunt wire before arterial perfusion of the preparation. Both ureters were ligated to prevent natural bladder filling, the urethra was clamped, and a catheter was inserted into the bladder lumen to record intravesical pressure and maintain isovolumetric conditions. A glass suction electrode (tip diameter ~100 μm) was used to stimulate the left pelvic nerve once it had been dissected clear of underlying tissue. Nerve‐mediated bladder contractions were generated using an isolated stimulator (Digitimer Ltd. UK; 0.1‐ms pulses, 1–24 Hz, 3‐s train every 90 s) and the pressure amplitude (mmHg) was analysed. Female mice were used due to difficulties in successfully dissecting and stimulating the pelvic nerve in male mice. Nerve‐mediated whole bladder pressure amplitude, P, as a function of frequency, f, was fitted to Equation (1a), with peak pressure, P, values substituting for tension, T, values. Drug interventions were delivered arterially.
2.7. Data and statistical analysis
Data are mean ± SEM and differences between data sets were tested with repeated measures two‐way ANOVA followed by parametric post hoc tests; the null hypothesis was rejected at P < 0.05. n values refer to the number of preparations, one each from separate animals. The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018). All statistical analyses were undertaken using GraphPad® Prism 7 (GraphPad Software Inc., CA, USA; GraphPad Prism, RRID:SCR_002798). The number of repeats in each control and intervention set was based on a power calculation to reject the null hypothesis at P < 0.05 and a power of 80%, with variance of data based on previous experience data with these methods.
2.8. Materials
Tyrode's solution was composed of (mM): NaCl, 118; NaHCO3, 24; KCl, 4.0; NaH2PO4, 0.4; MgCl2, 1.0; CaCl2, 1.8; glucose, 6.1; Na pyruvate, 5.0; 5% CO2, 95% O2, pH 7.4.
The concentration of all stock solutions was between 1.0 and 10 mM. Sildenafil was dissolved in DMSO. CCh, atropine, and ABMA were dissolved in distilled water. Stock solutions were diluted with Tyrode's solution to the final concentration as indicated. All chemicals were from Sigma‐Aldrich (Dorset, UK), except ABMA from Merck Millipore (Watford, UK).
2.9. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Christopoulos, et al., 2017, Alexander, Fabbro, et al., 2017, Alexander, Peters, et al., 2017).
3. RESULTS
3.1. Agonist‐induced contractions in isolated detrusor: Effect of sildenafil
The peak force of the CCh (1 μM) contracture was significantly reduced by sildenafil at concentrations 1–100 μM. An IC50 value was determined for each preparation, with a mean IC50 value of 5.4 ± 2.2 μM and a residual force of 49.5 ± 4.7% (n = 8: Figure 1a). Sildenafil also reduced high‐K+ contracture, with a mean IC50 value of 16.0 ± 9.6 μM and a residual force of 42.3 ± 13.6% (n = 8: Figure 1b). The vehicle control, DMSO, had no effect on CCh and high‐K+ contractures, except at concentrations used for 30‐ to 100‐μM test solutions when force was reduced by 9.6 ± 2.1%. A test dose of 20‐μM sildenafil was used for subsequent experiments with nerve‐mediated contractions.
Figure 1.
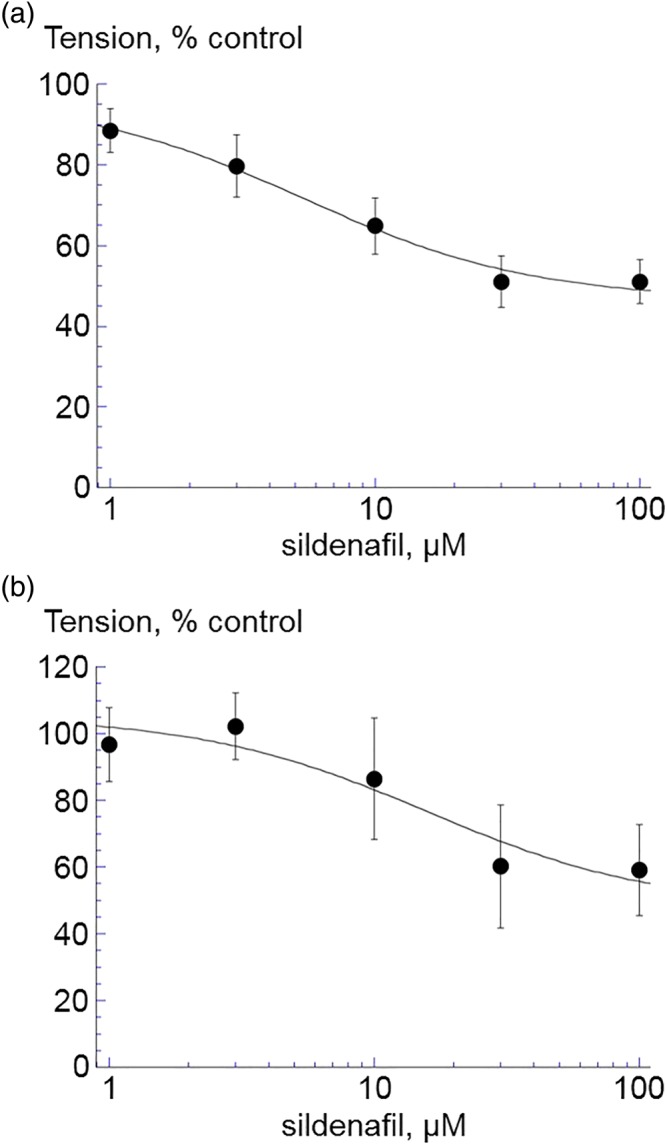
The effect of sildenafil (1–100 μM) on (a) carbachol (CCh, 1 μM, n = 8) and (b) high‐K+ (80 mM, n = 5) induced contractions in isolated detrusor from 12‐week‐old mice. Data are mean ± SEM. *P < 0.05. Data are fitted to Equation (1b), Section 2
3.2. Nerve‐mediated contractions in isolated detrusor: Effect of sildenafil
A combination of ABMA (10 μM, to desensitize ionotropic purinergic receptors) and atropine (1 μM, to block muscarinic receptors) abolished nerve‐mediated contractions in detrusor from 12‐week mice. Thus, nerve‐mediated contractions are mediated by ACh and ATP release. Force–frequency relationships (Figure 2a) show atropine alone reduced contractions more at high frequencies, and ABMA alone had a greater effect at low frequencies, data are shown for 12‐week mice. This was quantified by calculation of f 1/2 values (see Figure 2a control curve) that were reduced by atropine and increased by ABMA (Figure 2b). Thus, ATP as a neurotransmitter is released more at low stimulation frequencies and ACh at higher frequencies. With detrusor from 24‐month mice, atropine and ABMA had similar effects on f 1/2 as in detrusor from young mice (Figure 2bii). In addition, control T max and f 1/2 values were not significantly different with young and aged mice (young vs. aged: T max, control; 1.77 ± 0.35 vs. 0.97 ± 0.37 mN·mm−2; n = 8.5).
Figure 2.
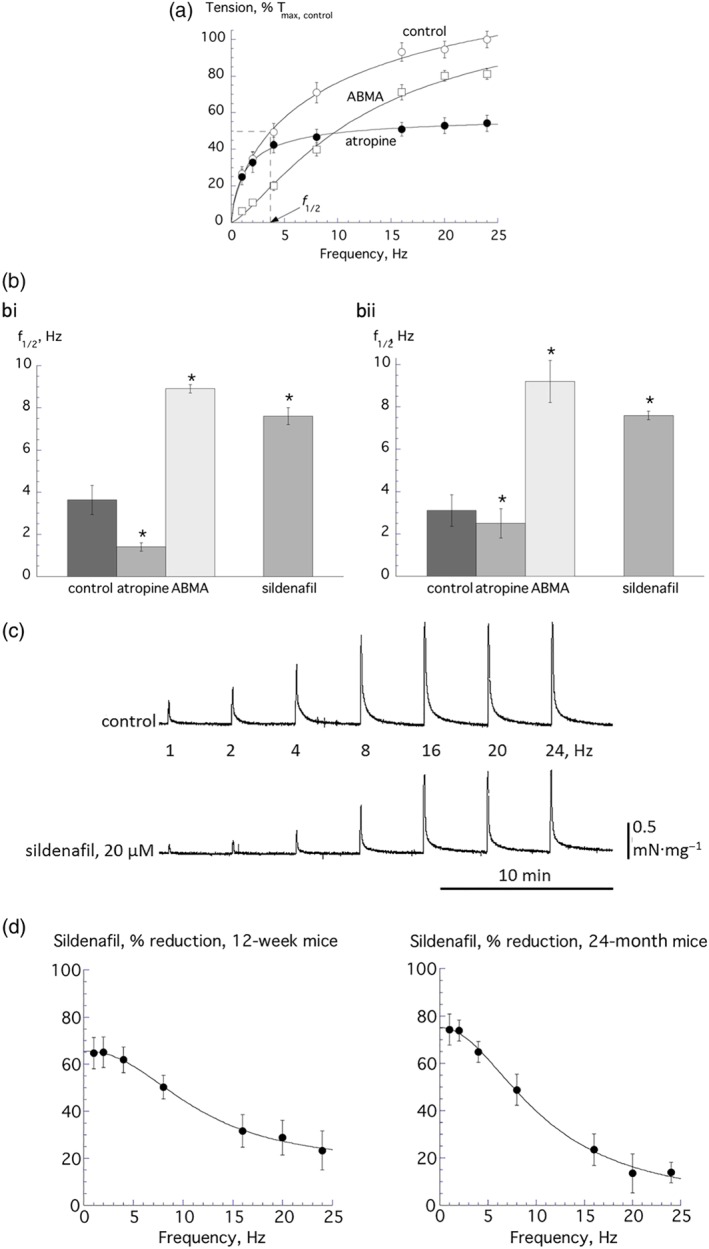
(a) Frequency‐response curves for nerve‐mediated contractions in control (n = 8) and in the presence of α,β‐methylene ATP (ABMA, 1 μM, n = 6) or atropine (1 μM, n = 6). Data are plotted as a percentage of the maximum estimated contraction under control conditions, T max,control. The value of the f 1/2 for the control curve is shown, that is, the frequency to achieve T max/2. The fits are from Equation (1a), Section 2. (b) f 1/2 values for control conditions (n = 8), and in the presence of ABMA (n = 6) and atropine (n = 6), the value in the presence of 20‐μM sildenafil is also shown (n = 8, 5). *P < 0.05; data are for 12‐week‐old mice (bi) and 24‐month‐old mice (bii). (c) Traces of nerve‐mediated contractions under control conditions and with 20‐μM sildenafil. The frequency values are those used to elicit nerve‐mediated responses. (d) Percentage reduction of nerve‐mediated contractions by sildenafil with preparations from 12‐week‐old (left, n = 8) and 24‐month‐old (right, n = 5) mice. Fits are from Equation (1b), Section 2. All group data are mean ± SEM
Sildenafil (20 μM) reduced nerve‐mediated contractions in isolated detrusor preparations from 12‐week (Figure 2c) and 24‐month mice. Figure 2d plots the percentage reduction of force by sildenafil at each frequency in 12‐week and 24‐month mice, with a greater reduction of force at lower stimulation frequencies compared to that at higher frequencies. This was corroborated by an increase of f 1/2 value in both the 12‐week and 24‐month mice data (Figure 2bi and 2bii), where values were increased to those similar to that in ABMA. The difference between the contraction suppression by sildenafil at high and low frequencies (∆R Lf − R Hf, Equation 1b) is a measure of the purinergic component of the contraction if it blocks ATP release during nerve stimulation (see Figure 3a below). With detrusor from 24‐month mice, this was significantly greater than with 12‐week mice (64.9 ± 7.6 vs. 41.3 ± 7.6%, P = 0.05, n = 5.8 respectively), suggesting a more significant contribution from purinergic transmission in 24‐month mice. Thus, the action of sildenafil may be due either to a reduction of ATP release during nerve stimulation or a selective inhibition of the action of ATP on detrusor. However, the latter is unlikely as sildenafil also attenuated contractures mediated by CCh (Figure 1a). Accordingly, the first hypothesis was tested by investigating the mode of action of sildenafil with detrusor from 12‐week mice.
Figure 3.
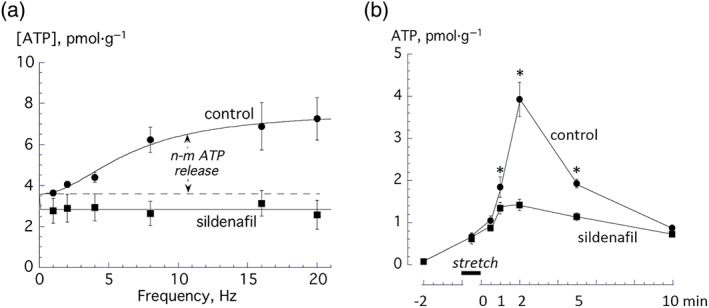
(a) Frequency‐dependent nerve‐mediated ATP release from 12‐week‐old mice under control conditions (n = 6) and in the presence of 20‐μM sildenafil (n = 6). Data were fitted to Equation (1b), Section 2. (b) Stretch‐activated ATP from the mucosa of from 12‐week‐old mice under control conditions (n = 6) and in the presence of 20‐μM sildenafil (n = 6). Samples were obtained 2 min prior, during, and 0.5, 1.0, 2.0, 5.0, and 10.0 min after a 50‐s stretch. All group data are mean ± SEM. *P < 0.05
3.3. Nerve‐mediated and stretch‐induced ATP release: Effect of sildenafil
Nerve‐mediated ATP release was measured directly by measuring superfusate [ATP] near the preparation. In control conditions, a frequency‐dependent release of [ATP] was superimposed on a background fraction (ATP b, Figure 3a); data were analysed between 1 and 16 Hz. With subsequent addition of 20‐μM sildenafil, ATP b was not significantly different (0.54 vs. 0.43 pmol·μl−1, n = 7); however, the frequency‐dependent component was completely abolished.
Previous studies have shown that the mucosa is also a source of ATP, especially when stretched and it was of interest to determine if this also is affected by sildenafil. Rapid stretch by 20% of the resting length for 50 s was followed by restoration of the original length. Superfusate samples were collected prior to stretch, during the stretch, and at 0.5, 1.0, 2.0, 5.0, and 10.0 min after restoration. In control conditions, ATP release peaked at 2 min after restoration to nearly 60‐fold increased over pre‐stretch levels and remained elevated after 10 min. Sildenafil (20 μM) did not affect pre‐stretch levels but significantly attenuated the post‐stretch increase (Figure 3b).
3.4. Bladder contractions evoked by preganglionic pelvic nerve stimulation: Effect of sildenafil
The preganglionic pelvic nerve was dissected as much as possible from adhering tissue and stimulated in situ in 12‐week mice (Figure 4a) to generate intravesical bladder pressure transients (Figure 4b). The frequency dependence was identical to in vitro contractions through postganglionic nerve stimulation (Figure 4c). Sildenafil (20 μM) also reduced the amplitude of the pressure transients with an action greater at lower frequencies (Figure 4d), as also observed in vitro, Figure 2d. Thus, as above, the f 1/2 value was increased by sildenafil (3.7 ± 0.4 vs. 6.0 ± 0.6 Hz, respectively, n = 5, P < 0.05).
Figure 4.
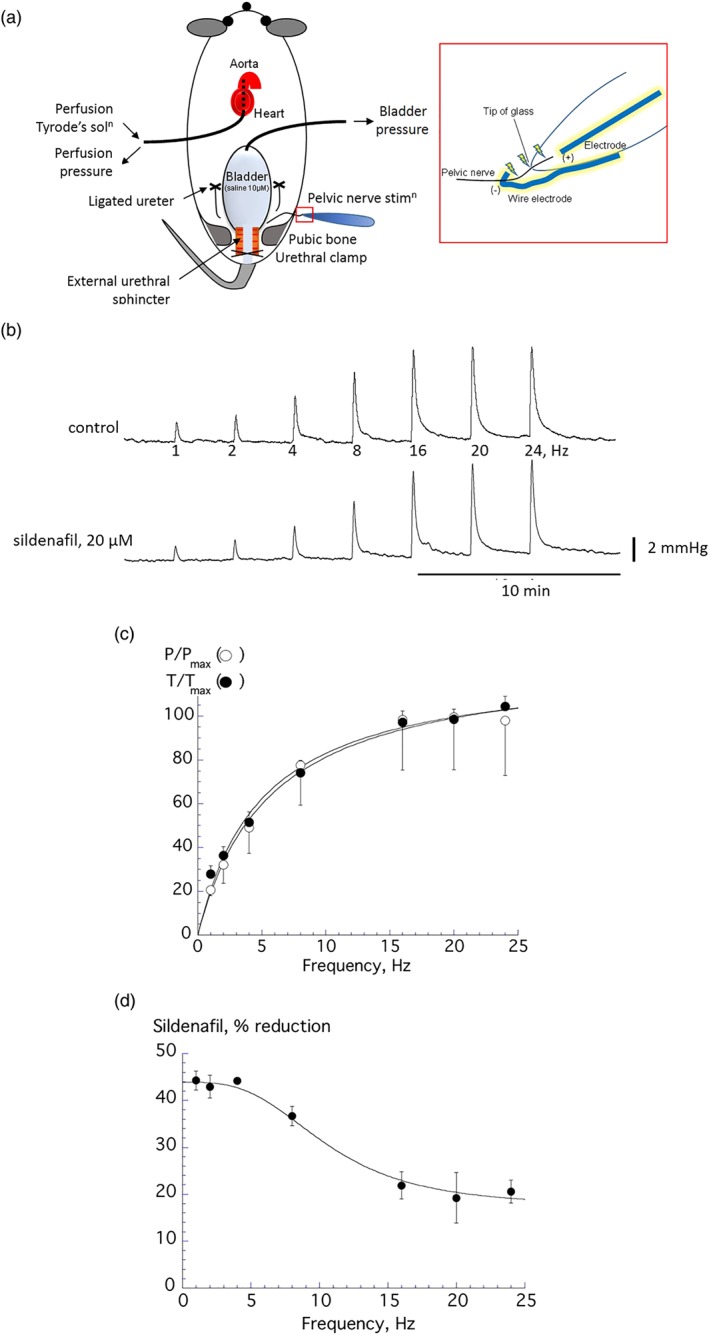
(a) Schematic diagram of the arterially perfused in situ mouse model used for preganglionic pelvic nerve stimulation (n = 6). The pelvic nerve was dissected and stimulated. (b) Traces of nerve‐mediated whole bladder pressure contractions during control conditions and in the presence of 20‐μM sildenafil. (c) Force– and pressure–frequency relationships with data from the same 12‐week‐old mice obtained in situ (P/P max) and in vitro (T/T max). Data fitted to Equation (1a), Section 2. (d) Percentage reduction of nerve‐mediated pressure transients by sildenafil with preparations from 12‐week‐old mice (n = 6), data fitted to Equation (1b), Section 2. All group data are mean ± SEM
3.5. Nerve‐mediated contractions and ATP release in spinal cord‐transected mice: Effect of sildenafil
Sildenafil (20 μM) reduced nerve‐mediated contractions in SCT 12‐week mice, with a greater effect at low stimulation frequencies as observed in young and aged mice with an intact spinal cord (Figure 5a). The inset shows that f 1/2 values were significantly increased with sildenafil. Nerve‐mediated ATP release was also measured that was also abolished by sildenafil and superimposed on a background release (ATP b, Figure 5b). When compared with 12‐week intact mice, the frequency‐dependent release of ATP was however significantly smaller: for example, at 8 Hz stimulation, the frequency‐dependent ATP release was 2.63 ± 0.62 versus 0.54 ± 0.12 pmol·g−1 (n = 8.5; P < 0.05) in normal and SCT 12‐week mice.
Figure 5.
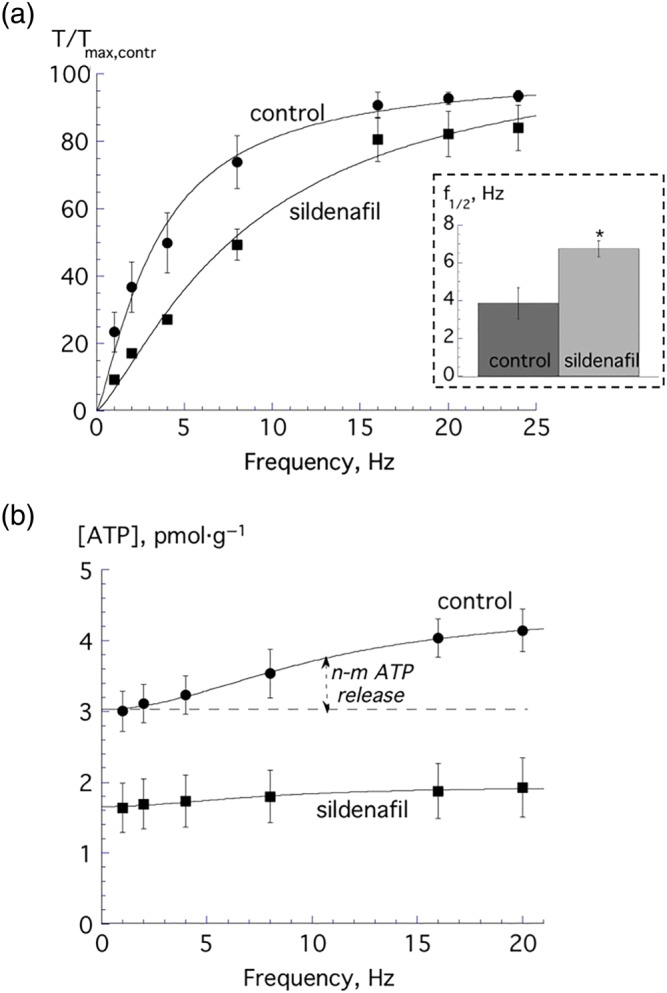
(a) Nerve‐mediated contractions from spinal cord transected (SCT) 12‐week‐old mice under control conditions and in the presence of 20‐μM sildenafil (n = 5). Data were fitted to Equation (1a), Section 2. The inset shows the respective f 1/2 values, *P < 0.05. (b) Frequency‐dependent nerve‐mediated ATP release in 12‐week‐old SCT mice under control conditions (n = 5) and in the presence of 20‐μM sildenafil (n = 5). Data were fitted to Equation (1c), Section 2. All data are mean ± SEM
4. DISCUSSION
4.1. Actions of sildenafil on detrusor and efferent nerve activity
PDE5 inhibitors, such as sildenafil and tadalafil, are successfully used to manage erectile dysfunction, and it has also been observed that they relieve LUTS, especially in men with benign prostatic hyperplasia, although there is no consensus about their principal modes of action (Andersson, 2018; Gacci et al., 2016). This study, using mice, confirmed a direct relaxant effect of sildenafil on detrusor smooth muscle, with a similar potency to that with human detrusor (Oger et al., 2010) where it was proposed to act by altering cyclic nucleotide levels and modulating K+ channel activity, such as KATP and BK channels. However, there has been less investigation of their action on nerve‐mediated contractions, and this study reports a substantial and frequency‐dependent action. It cannot be excluded that the concentration chosen for interventions (i.e., 20 μM) had an effect on other PDEs; however, this concentration was that which produced a half‐maximal relaxation in in vitro experiments.
Parasympathetic postganglionic fibres activate detrusor by release of ACh and ATP and abolition of contractions by atropine and ABMA implies that muscarinic and P2X1 receptors mediate this process, in contrast to the situation in guinea pig detrusor (Kennedy, Tasker, Gallacher, & Westfall, 2007). In most animals and human OAB, both transmitters are functionally active, although in normal human bladders, only ACh has a role due to high ectoATPase activity (Bayliss et al., 1999; Harvey, Skennerton, Newgreen, & Fry, 2002). Sildenafil suppressed nerve‐mediated contractions but more at low stimulation frequencies (i.e., 1–4 Hz) so it is unlikely this was due only to a direct effect on smooth muscle. Release of noradrenaline and ATP in the sympathetic nervous system can be separately regulated (Speirs, Donnelly, Lynch, Scholfield, & Johnson, 2006). Differential release of ACh and ATP in detrusor parasympathetic endings also occurs (Pakzad et al., 2016), where ATP release is reduced selectively by A1 receptor agonists. The cellular pathways regulating separate release of ATP and ACh remain to be established; however, it has potential implications for therapeutic management of human OAB, as ATP is a functional transmitter in this condition. The ability of sildenafil to regulate ATP release selectively was more directly shown by its abolition of TTX‐sensitive, frequency‐dependent release of ATP. EFS may possibly result in urothelial ATP release in these experimental conditions, either by direct stimulation (Sadananda, Shang, Liu, Mansfield, & Burcher, 2009) or indirectly by the changes in muscle contraction. However, sildenafil reduced both neuronal and urothelial ATP release in this study.
In vitro experiments with isolated detrusor preparations were mirrored by stimulation of preganglionic fibres in an in situ model. The frequency dependence of pressure transients mirrored that of contractions in vitro and were similarly influenced by sildenafil. This implies that the actions of sildenafil may be explained only by its action at the postganglionic junction and on smooth muscle, with no separate action on preganglionic fibres. The data also imply that under these experimental conditions, the pelvic ganglion does not modulate the gain of pre to postganglionic transmission.
4.2. Sildenafil effects on mucosal ATP release and afferent signalling
The mucosa exerts considerable effects on detrusor contractile performance; it reduces contractility (Hawthorn, Chapple, Cock, & Chess‐Williams, 2000) but enhances spontaneous activity (Ikeda & Kanai, 2008). Mucosal influence over spontaneous activity is in part due to stretch‐mediated ATP release (Kushida & Fry, 2016), and the observation that sildenafil greatly reduced such ATP release offers another route for the PDE5 inhibitor to reduce detrusor contractile function. Of note, A1 receptor agonists and sildenafil both reduce stretch‐activated mucosal ATP release (Dunning‐Davies, Fry, Mansour, & Ferguson, 2013), as well as nerve‐mediated release, suggesting a common regulator of ATP release in these two tissues. Another proposed role for mucosal ATP release is suburothelial afferent nerve activation to provide a transduction pathway between bladder wall stretch and sensation (Vlaskovska et al., 2001). Sildenafil reduces bladder afferent activity (Behr‐Roussel et al., 2011), but it has to be determined if the action is a direct one on nerves or indirectly through modulation of mucosal ATP release.
4.3. Sildenafil and the ageing bladder
Ageing is associated with fibrosis and either a decline or maintenance of detrusor contractile function in animal and human models (Fry, Bayliss, Young, & Hussain, 2011; Kamei et al., 2018; Lluel et al., 2000). More consistent is the increased significance of purinergic neurotransmission with age (Yoshida, Miyamae, Iwashita, Otani, & Inadome, 2004), greater purinergic receptor expression (Daly et al., 2014), and enhanced stretch‐mediated mucosal ATP release (Sui et al., 2014). This suggests a potential role for sildenafil to suppress neural and mucosal release of ATP that is associated with OAB in humans. Data here (Figure 2d) showed the frequency‐dependent reduction of nerve‐mediated contractions by sildenafil was greater in aged animals, suggesting an increased purinergic component in neuromuscular transmission.
4.4. Sildenafil and bladder function with spinal cord injury
A greater role for purinergic transmission and mucosal release is also suggested in idiopathic and neurogenic bladder overactivity (IDO, NDO). Atropine resistance, suggesting functional purinergic transmission, is a feature of IDO and NDO (Bayliss et al., 1999) but the data here showed nerve‐mediated ATP release was actually smaller in SCT mice. If this is extrapolated to the human condition, purinergic contractions can only be explained by reduced hydrolysis of ATP in the neuromuscular junction, as indeed observed in detrusor from patients with IDO and NDO (Fry et al., 2018). Mucosal ATP release is also reported in tissue isolated from OAB (Khera, Somogyi, Kiss, Boone, & Smith, 2004; Salas, Somogyi, Gangitano, Boone, & Smith, 2007; Smith et al., 2008) which will potentially increase afferent firing for a given stretch of the bladder wall. Both targets are therefore useful for PDE5 inhibitors to suppress, potentially to alleviate symptoms of OAB. This approach offers an advantage over current therapeutic pathways to suppress OAB that use muscarinic receptor antagonists, receptors that mediate the normal mode of bladder contraction.
5. CONCLUSIONS
The PDE5 inhibitor sildenafil exerts a direct detrusor relaxant effect as previously reported. The novel finding of this study is that sildenafil also selectively and completely inhibits ATP release from motor nerves to detrusor, as well as stretch‐activated release from the mucosa. The particular pathways whereby this effect is mediated is yet to be determined—for example, is this a NO/cGMP dependent effect. This blockade was observed not just in young animal animals but also in aged animals and those subjected to spinal cord injury. The significance of this observation in aged and SCT animals is that in humans, these conditions are especially associated with OAB syndromes and enhanced purinergic motor and sensory pathways. This offers the possibility that PDE5 inhibitors will be useful to suppress OAB symptoms by suppressing pathological pathways in humans, rather than suppressing normal physiological pathways, as occurs at present with current therapeutic interventions.
AUTHOR CONTRIBUTIONS
B.C., H.I., B.V., A.K., A.E.P., M.J.D., and C.H.F. contributed to the conception or design of the work. B.C., H.I., M.X., and N.N. acquired the data, B.C., H.I., and C.H.F. analysed the data, and B.C., M.J.D., and C.H.F. interpreted the data. B.C., B.V., A.J.K., M.J.D., and C.H.F. drafted or critically revised the work.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design & Analysis, and Animal Experimentation, as recommended by funding agencies, publishers and other organizations engaged with supporting research.
ACKNOWLEDGEMENTS
Funding was provided by the United States National Institutes of Health Grant NIH R01 DK098361 (A.J.K., M.J.D., C.H.F., and A.E.P.). We thank Cherrie H. T. Kong, Simon M. Bryant, and Hanne C. Gadeberg (School of Physiology, Pharmacology, and Neuroscience, University of Bristol) for assistance with acquiring animal tissue, and Jonathan J. Crook (School of Physiology, Pharmacology, and Neuroscience, University of Bristol) for technical assistance.
Chakrabarty B, Ito H, Ximenes M, et al. Influence of sildenafil on the purinergic components of nerve‐mediated and urothelial ATP release from the bladder of normal and spinal cord injured mice. Br J Pharmacol. 2019;176:2227–2237. 10.1111/bph.14669
REFERENCES
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … CGTP Collaborators (2017). The concise guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. British Journal of Pharmacology, 174(Suppl 1), S17–S129. 10.1111/bph.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The concise guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174(Suppl 1), S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Peters, J. A. , Kelly, E. , Marrion, N. V. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators (2017). The concise guide to PHARMACOLOGY 2017/18: Ligand‐gated ion channels. British Journal of Pharmacology, 174(Suppl 1), S130–S159. 10.1111/bph.13879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson, K. E. (2018). PDE5 inhibitors—Pharmacology and clinical applications 20 years after sildenafil discovery. British Journal of Pharmacology, 175, 2554–2565. 10.1111/bph.14205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss, M. , Wu, C. , Newgreen, D. , Mundy, A. R. , & Fry, C. H. (1999). A quantitative study of atropine‐resistant contractile responses in human detrusor smooth muscle, from stable, unstable and obstructed bladders. The Journal of Urology, 162, 1833–1839. 10.1016/S0022-5347(05)68247-X [DOI] [PubMed] [Google Scholar]
- Behr‐Roussel, D. , Oger, S. , Caisey, S. , Sandner, P. , Bernabé, J. , Alexandre, L. , & Giuliano, F. (2011). Vardenafil decreases bladder afferent nerve activity in unanesthetized, decerebrate, spinal cord‐injured rats. European Urology, 59, 272–279. 10.1016/j.eururo.2010.10.037 [DOI] [PubMed] [Google Scholar]
- Cheng, Y. , Mansfield, K. J. , Allen, W. , Chess‐Williams, R. , Burcher, E. , & Moore, K. H. (2014). ATP during early bladder stretch is important for urgency in detrusor overactivity patients. BioMed Research International, 2014, 204604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175(7), 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly, D. M. , Nocchi, L. , Liaskos, M. , McKay, N. G. , Chapple, C. , & Grundy, D. (2014). Age‐related changes in afferent pathways and urothelial function in the male mouse bladder. The Journal of Physiology, 592, 537–549. 10.1113/jphysiol.2013.262634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning‐Davies, B. M. , Fry, C. H. , Mansour, D. , & Ferguson, D. R. (2013). The regulation of ATP release from the urothelium by adenosine and transepithelial potential. BJU International, 111, 505–513. 10.1111/j.1464-410X.2012.11421.x [DOI] [PubMed] [Google Scholar]
- Fibbi, B. , Morelli, A. , Vignozzi, L. , Filippi, S. , Chavalmane, A. , De Vita, G. , … Maggi, M. (2010). Characterization of phosphodiesterase type 5 expression and functional activity in the human male lower urinary tract. The Journal of Sexual Medicine, 7, 59–69. 10.1111/j.1743-6109.2009.01511.x [DOI] [PubMed] [Google Scholar]
- Filippi, S. , Morelli, A. , Sandner, P. , Fibbi, B. , Mancina, R. , Marini, M. , … Maggi, M. (2007). Characterization and functional role of androgen‐dependent PDE5 activity in the bladder. Endocrinology, 148, 1019–1029. 10.1210/en.2006-1079 [DOI] [PubMed] [Google Scholar]
- Fry, C. H. , Bayliss, M. , Young, J. S. , & Hussain, M. (2011). Influence of age and bladder dysfunction on the contractile properties of isolated human detrusor smooth muscle. BJU International, 108, E91–E96. 10.1111/j.1464-410X.2010.09845.x [DOI] [PubMed] [Google Scholar]
- Fry, C. H. , McCarthy, C. , Ikeda, Y. , Kanai, A. J. , Nishikawa, N. , & Jabr, R. (2018). Atropine resistance and ATP release in human overactive bladder. Neurourology and Urodynamics, 37, S312–S313. [Google Scholar]
- Gacci, M. , Andersson, K. E. , Chapple, C. , Maggi, M. , Mirone, V. , Oelke, M. , … Giuliano, F. (2016). Latest evidence on the use of phosphodiesterase type 5 inhibitors for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. European Urology, 70, 124–133. 10.1016/j.eururo.2015.12.048 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey, R. A. , Skennerton, D. E. , Newgreen, D. , & Fry, C. H. (2002). The contractile potency of adenosine triphosphate and ecto‐adenosine triphosphatase activity in guinea pig detrusor and detrusor from patients with a stable, unstable or obstructed bladder. The Journal of Urology, 168, 1235–1239. 10.1016/S0022-5347(05)64632-0 [DOI] [PubMed] [Google Scholar]
- Hatzimouratidis, K. , Amar, E. , Eardley, I. , Giuliano, F. , Hatzichristou, D. , Montorsi, F. , … European Association of Urology (2010). Guidelines on male sexual dysfunction: Erectile dysfunction and premature ejaculation. European Urology, 57, 804–814. 10.1016/j.eururo.2010.02.020 [DOI] [PubMed] [Google Scholar]
- Hawthorn, M. H. , Chapple, C. R. , Cock, M. , & Chess‐Williams, R. (2000). Urothelium‐derived inhibitory factor(s) influences on detrusor muscle contractility in vitro. British Journal of Pharmacology, 129, 416–419. 10.1038/sj.bjp.0703068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda, Y. , & Kanai, A. (2008). Urotheliogenic modulation of intrinsic activity in spinal cord‐transected rat bladders: Role of mucosal muscarinic receptors. American Journal of Physiology. Renal Physiology, 295, F454–F461. 10.1152/ajprenal.90315.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda, Y. , Zabbarova, I. V. , Birder, L. A. , de Groat, W. C. , McCarthy, C. J. , Hanna‐Mitchell, A. T. , & Kanai, A. J. (2012). Botulinum neurotoxin serotype A suppresses neurotransmitter release from afferent as well as efferent nerves in the urinary bladder. European Urology, 62, 1157–1164. 10.1016/j.eururo.2012.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, H. , Chakrabarty, B. , Drake, M. J. , Fry, C. H. , Kanai, A. J. , & Pickering, A. E. (2019). Sildenafil, a phosphodiesterase type 5 inhibitor, augments sphincter bursting and bladder afferent activity to enhance storage function and voiding efficiency in mice. BJU International. 10.1111/bju.14664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, H. , Drake, M. J. , Fry, C. H. , Kanai, A. J. , & Pickering, A. E. (2018). Characterization of mouse neuro‐urological dynamics in a novel decerebrate arterially perfused mouse (DAPM) preparation. Neurourology and Urodynamics, 37, 1302–1312. 10.1002/nau.23471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadekawa, K. , Majima, T. , Shimizu, T. , Wada, N. , de Groat, W. C. , Kanai, A. J. , … Yoshimura, N. (2017). The role of capsaicin‐sensitive C‐fiber afferent pathways in the control of micturition in spinal‐intact and spinal cord‐injured mice. American Journal of Physiology. Renal Physiology, 313, F796–F804. 10.1152/ajprenal.00097.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei, J. , Ito, H. , Aizawa, N. , Hotta, H. , Kojima, T. , Fujita, Y. , … Igawa, Y. (2018). Age‐related changes in function and gene expression of the male and female mouse bladder. Scientific Reports, 8(1), 2089 10.1038/s41598-018-20406-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, C. , Tasker, P. N. , Gallacher, G. , & Westfall, T. D. (2007). Identification of atropine‐ and P2X1 receptor antagonist‐resistant, neurogenic contractions of the urinary bladder. The Journal of Neuroscience, 27, 845–851. 10.1523/JNEUROSCI.3115-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khera, M. , Somogyi, G. T. , Kiss, S. , Boone, T. B. , & Smith, C. P. (2004). Botulinum toxin A inhibits ATP release from bladder urothelium after chronic spinal cord injury. Neurochemistry International, 45, 987–993. 10.1016/j.neuint.2004.06.001 [DOI] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , Altman, D. G. , & NC3RS Reporting Guidelines Working Group (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160(7), 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushida, N. , & Fry, C. H. (2016). On the origin of spontaneous activity in the bladder. BJU International, 117, 982–992. 10.1111/bju.13240 [DOI] [PubMed] [Google Scholar]
- Lluel, P. , Palea, S. , Barras, M. , Grandadam, F. , Heudes, D. , Bruneval, P. , … Martin, D. J. (2000). Functional and morphological modifications of the urinary bladder in aging female rats. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 278, R964–R972. 10.1152/ajpregu.2000.278.4.R964 [DOI] [PubMed] [Google Scholar]
- McCarthy, C. J. , Zabbarova, I. V. , Brumovsky, P. R. , Roppolo, J. R. , Gebhart, G. F. , & Kanai, A. J. (2009). Spontaneous contractions evoke afferent nerve firing in mouse bladders with detrusor overactivity. The Journal of Urology, 181, 1459–1466. 10.1016/j.juro.2008.10.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVary, K. T. , Monning, W. , Camps, J. L. , Young, J. M. , Tseng, L. J. , & van den Enge, G. (2007). Sildenafil citrate improves erectile function and urinary symptoms in men with erectile dysfunction and lower urinary tract symptoms associated with benign prostatic hyperplasia: A randomized, double‐blind trial. The Journal of Urology, 177, 1071–1077. 10.1016/j.juro.2006.10.055 [DOI] [PubMed] [Google Scholar]
- Oger, S. , Behr‐Roussel, D. , Gorny, D. , Lebret, T. , Validire, P. , Cathelineau, X. , … Giuliano, F. (2010). Signalling pathways involved in sildenafil‐induced relaxation of human bladder dome smooth muscle. British Journal of Pharmacology, 160, 1135–1143. 10.1111/j.1476-5381.2010.00748.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakzad, M. , Ikeda, Y. , McCarthy, C. , Kitney, D. G. , Jabr, R. I. , & Fry, C. H. (2016). Contractile effects and receptor analysis of adenosine‐receptors in human detrusor muscle from stable and neuropathic bladders. Naunyn‐Schmiedeberg's Archives of Pharmacology, 389, 921–929. 10.1007/s00210-016-1255-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadananda, P. , Drake, M. J. , Paton, J. F. , & Pickering, A. E. (2011). An exploration of the control of micturition using a novel in situ arterially perfused rat preparation. Frontiers in Neuroscience, 5, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadananda, P. , Shang, F. , Liu, L. , Mansfield, K. J. , & Burcher, E. (2009). Release of ATP from rat urinary mucosa: Role of acid, vanilloids and stretch. British Journal of Pharmacology, 158, 1655–1662. 10.1111/j.1476-5381.2009.00431.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas, N. A. , Somogyi, G. T. , Gangitano, S. A. , Boone, T. B. , & Smith, C. P. (2007). Receptor activated bladder and spinal ATP release in neurally intact and chronic spinal cord injured rats. Neurochemistry International, 50(2), 345–350. 10.1016/j.neuint.2006.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, C. P. , Gangitano, D. A. , Munoz, A. , Salas, N. A. , Boone, T. B. , Aoki, K. R. , … Somogyi, G. T. (2008). Botulinum toxin type A normalizes alterations in urothelial ATP and NO release induced by chronic spinal cord injury. Neurochemistry International, 52, 1068–1075. 10.1016/j.neuint.2007.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speirs, L. , Donnelly, A. , Lynch, J. , Scholfield, C. N. , & Johnson, C. (2006). ATP and norepinephrine contributions to sympathetic vasoconstriction of tail artery are altered in streptozotocin‐diabetic rats. American Journal of Physiology. Heart and Circulatory Physiology, 291, H2327–H2333. 10.1152/ajpheart.01298.2005 [DOI] [PubMed] [Google Scholar]
- Sui, G. , Fry, C. H. , Montgomery, B. , Roberts, M. , Wu, R. , & Wu, C. (2014). Purinergic and muscarinic modulation of ATP release from the urothelium and its paracrine actions. American Journal of Physiology. Renal Physiology, 306, F286–F298. 10.1152/ajprenal.00291.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaskovska, M. , Kasakov, L. , Rong, W. , Bodin, P. , Bardini, M. , Cockayne, D. A. , … Burnstock, G. (2001). P2X3 knock‐out mice reveal a major sensory role for urothelially released ATP. The Journal of Neuroscience, 21, 5670–5677. 10.1523/JNEUROSCI.21-15-05670.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida, M. , Miyamae, K. , Iwashita, H. , Otani, M. , & Inadome (2004). Management of detrusor dysfunction in the elderly: Changes in acetylcholine and adenosine triphosphate release during aging. Urology, 63(3 Suppl 1), 17–23. 10.1016/j.urology.2003.11.003 [DOI] [PubMed] [Google Scholar]


