Abstract
Background
The clinical relevance of mismatch repair (MMR) status in patients with nonmetastatic cancer across tumour types remains unclear. Our goal was to investigate the prognostic role of MMR deficiency in patients with stage I-III colorectal and endometrial cancer.
Methods
Patients with nonmetastatic colorectal and endometrial cancer with tumour tissue available for analysis were identified through the Hellenic Cooperative Oncology Group (HeCOG)’s tumour repository. Patients had been referred to Departments of Medical Oncology affiliated with HeCOG. MMR protein expression was evaluated by immunohistochemistry. The primary outcome measure was overall survival (OS).
Results
From May 1990 to September 2012, 1158 patients with nonmetastatic colorectal (N = 991) and endometrial cancer (N = 167) were identified (median age: 64 years, men: 544). All patients with colorectal and 109 (65%) with endometrial cancer had received adjuvant treatment. MMR deficiency was observed in 114 (11.5%) of colorectal and 80 (47.9%) of endometrial tumours. More commonly deficient proteins were PMS2 (69 patients, 7%) and MLH1 (63 patients, 6.5%) in colorectal cancer and MSH2 (58 patients, 34.7%) in endometrial cancer. Colorectal MMR-deficient (dMMR) tumours were more likely to be right sided (65 % dMMR vs 27 % proficient MMR, pMMR; p < 0.001), high grade (31% vs 15%, χ2, p < 0.001) and with a mucinous component (64% vs 42%, p < 0.001). Endometrial dMMR tumours were more often of endometrioid histology (51.4 % endometrioid vs 20 % serous/clear cell, p = 0.020). Compared with MMR proficiency, MMR deficiency was associated with improved OS in patients with endometrial cancer (HR = 0.38, 95% CI 0.20 to 0.76, p = 0.006), but not in patients with colorectal cancer (HR = 0.73, 95% CI 0.49 to 1.09, p = 0.130). After adjusting for age, stage and grade, MMR deficiency maintained its favourable prognostic significance in patients with endometrial cancer (HR = 0.42, 95% CI 0.20 to 0.88, p = 0.021).
Conclusions
DMMR was associated with improved outcomes in patients with nonmetastatic endometrial cancer, but not in patients with nonmetastatic colorectal cancer who received adjuvant chemotherapy.
Keywords: Colorectal, early-stage, endometrial, MMR, prognosis
Key questions.
What is already known about this subject?
Mismatch repair (MMR) status has several clinical implications in cancer.
MMR status has prognostic and predictive implications and can be used as a selection tool for MMR genetic testing.
Common tumour types that are MMR deficient (dMMR) are endometrial and colorectal adenocarcinomas.
What does this study add?
In our study, dMMR endometrial tumours were more likely to be low grade and of endometrioid histology. MMR deficiency was more frequently associated with MSH2 protein loss.
MMR deficiency was associated with improved overall survival in women with endometrial carcinoma.
In patients with colorectal cancer, the most commonly deficient proteins were PMS2 and MLH1.
MMR status was not associated with prognosis in patients with nonmetastatic colorectal cancer who received adjuvant treatment.
How might this impact on clinical practice?
We show the value of MMR status as a prognostic biomarker in patients with endometrial cancer. These data need to be validated in prospective clinical trials in order to be incorporated into clinical practice and aid patient stratification.
MMR status might not maintain its prognostic significance in high-risk patients with colorectal cancer who received adjuvant treatment.
Introduction
Mismatch repair (MMR) proteins are responsible for excising DNA mismatches introduced by DNA polymerase during cell division, commonly occurring in repetitive DNA sequences (known as microsatellites). Impairment of the MMR system leads to microsatellite instability (MSI), which is characterised by accumulation of mismatches in repeated sequences1 leading to hypermutated tumours.2 3 Defects in the MMR system can be assessed using PCR-based assays testing for MSI, immunohistochemical analysis of MMR protein expression, including MLH1, MSH2, MSH6 and PMS2 proteins, or more recently next-generation sequencing data analysis detecting MSI.4 5
Determination of MMR status in cancer has several clinical implications. First, loss of expression of an MMR protein may be associated with inherited germline defects in the respective gene, leading to an inherited disorder, known as Lynch syndrome.6 MMR deficiency can be used as a marker to select individuals to be tested for the presence of a germline defect in MMR genes. Second, MMR deficiency is being currently used as a biomarker, predicting response to checkpoint inhibitors.7 8 Response to these agents has been attributed to the increased mutational load leading to increased neoantigen load in MMR-deficient (dMMR) tumours.9–11 Recently, the Food and Drug Administration (FDA) approved the first site-agnostic treatment for 'MSI-high' or dMMR cancers.12 Additionally, several studies demonstrated that the MMR status is predictive of benefit from chemotherapy.13 14 Finally, MMR status has been shown to provide valuable prognostic information, mainly in patients with colorectal cancer.15–20
MMR deficiency has been observed within several tumour types.21 Tumour types more commonly found to be dMMR are endometrial22 23 and colorectal adenocarcinomas.2 21 24 The high prevalence of MMR deficiency in colorectal and endometrial tumours in combination with the significant clinical impact of MMR status to the patient’s management underscores the importance of universal screening for MMR deficiency.25–27
The aim of our study was to evaluate the proportion of nonmetastatic colorectal and endometrial dMMR cancers in Greek patients. Furthermore, we aimed to evaluate the prognostic significance of MMR deficiency in these patients.
Methods
Patients
Patients with nonmetastatic (stage I-III) colorectal and nonmetastatic (stage I-III) endometrial cancer available for analysis tissue block were identified through the Hellenic Cooperative Oncology Group (HeCOG)’s tumour repository. All patients had been referred to Departments of Medical Oncology affiliated with HeCOG. Patients with colorectal cancer had received adjuvant chemotherapy at HeCOG-affiliated institutions.3 28–32 Patients with endometrial cancer had been treated at a tertiary hospital, with surgery followed by adjuvant treatment where appropriate, as previously described.33 Patient clinical demographics, tumour histopathological, treatment and outcome data were retrieved from the HeCOG electronic clinical database. Signed informed consent was obtained from all patients for the use of their biological material for research purposes. The translational protocol was approved by the Institutional Review Boards of ‘Papageorgiou’ Hospital (1338/12 January 2015) and ‘Thermi’ Clinic (307/2 March 2016) for colorectal cancer and by the Bioethics Committee of School of Health Sciences, Aristotle University of Thessaloniki (5/6 July 2016) for endometrial cancer.
Tissue samples
Formalin-fixed paraffin-embedded (FFPE) tissue samples had been collected from patients with nonmetastatic colorectal and endometrial cancer. Central tumour histology review, tissue processing and immunophenotyping were performed at the Laboratory of Molecular Oncology (Hellenic Foundation for Cancer Research/Aristotle University of Thessaloniki). In-house low-density tissue microarrays (TMA) were constructed with a manual arrayer (Beecher Instruments, Sun Prairie, WI, USA) and included 2–4×1.5 mm cores from different areas per tumour.
MMR immunohistochemistry
Expression of MMR proteins MLH1, PMS2, MSH2, and MSH6 was evaluated by immunohistochemistry (IHC). Intensity and percentage were recorded for each protein. MMR protein expression was considered positive, if ≥1% positive nuclei with mild to strong intensity were counted; negative, if internal controls (stromal cells and lymphocytic infiltrates) were positive and tumour cells were completely negative or exhibited any staining <1%; noninformative, if tumour cells were negative and internal controls were negative. The latter may have corresponded to biallelic loss of the respective protein, which could not be addressed in the absence of genomic data, or, in most cases, to assay failure. Tumours were classified as MMR proficient (pMMR) if informative and positive for all four MMR proteins. Tumours were classified as dMMR in the absence of expression of at least one of the four proteins.32
MSI testing
MSI testing was performed at GeneKor Medical S.A., Athens, Greece. We identified 50 patients with dMMR colorectal cancer with available tumour tissue and peripheral blood samples (as per laboratory specifications), which we submitted for MSI testing. No endometrial tumours were available for this analysis. DNA was extracted from blood with standard methods and from FFPE following enrichment in tumour cells with manual macrodissection from four unstained 10 µm thick sections. FFPE DNA was extracted using the QIAsymphony DNA Midi Kit (Qiagen, Antwerp, Belgium). Analysis was carried out using five polymorphic markers ΒΑΤ−25, BAT-26, D5S346, D17S250 and D2S123 according to Amsterdam meeting criteria.34 Briefly, the aforementioned microsatellite sites were amplified in a 25 µL multiplex PCR (QIAGEN Multiplex PCR Kit, Qiagen) using five pairs of previously described primers35 and 100 ng of DNA. After PCR reaction, products were visualised in a 3% agarose gel. For fragment analysis (ABI3130, Applied Biosystems), denaturation was carried out using a mixture of 1 μL of PCR product, 14 µL of formamide (HiDi Formamide, Applied Biosystems) and 0.5 µL of LIZ-labelled dye-labelled DNA (GS500-LIZ standard; Applied Biosystems) and incubated at 95°C for 3 min. The lengths of the microsatellite sites were compared between tumour and normal samples using GeneMapper software V.4.0. MSI was reported when instability was observed in more than two polymorphic markers of the microsatellites analysed. Low MSI tumours were those with instability in only one of the microsatellites analysed, whereas microsatellite stable (MSS) tumours were those where instability was not observed in any of the analysed microsatellites.
Statistical analysis
The primary endpoint was overall survival (OS), defined as the time (in months) from the date of diagnosis to the date of death from any cause or last contact, whichever occurred first. Due to the heterogeneity in the follow-up time, patients with colorectal cancer were classified into three subcohorts (diagnosed in 1998/1999 study cohort 1, in 2005 study cohort 2 and in 2008 study cohort 3). Study cohort was used as stratification variable in order to account for differences in follow-up time. The following IHC markers were examined in relation to OS: MLH1 status (negative and positive), PMS2 status (negative and positive), MSH2 status (negative and positive), MSH6 status (negative and positive) and MMR status (proficient and deficient). In addition, the following combined variables were examined: MLHS1/PMS2 (both negative vs other) and MSH6/MSH2 (both negative vs other). The effect of the aforementioned markers on OS was examined in multivariate Cox regression analyses, after adjusting for clinicopathological parameters with p<0.10 in univariate analyses. All analyses were conducted in the entire cohort and separately in the cohort of patients with colorectal and endometrial cancer. The statistical analyses were performed using the SAS software (SAS for Windows, V.9.4, SAS Institute, Cary, NC, USA). Statistical significance was set at two-sided p=0.05.
Results
Patient characteristics
In total, 1158 patients were included in the study, diagnosed from May 1990 to September 2012; 991 patients were diagnosed with colorectal and 167 patients with endometrial cancer. The median age at diagnosis was 64 years (range 21–87 years) and 544 (47%) were men. Stage distribution was as follows: stage I. 11.2%; stage II, 36.2% and stage III, 52.6%. Detailed clinicopathological data are summarised in table 1.
Table 1.
Patient characteristics
| Entire cohort n=1158 | Colorectal cancer n=991 | Endometrial cancer n=167 | |
| Age | |||
| Mean±SD | 62.6±10.3 | 62.4±10.3 | 63.4±10.5 |
| Median | 64.3 | 64.4 | 64.2 |
| Min-Max | 21–87 | 21–82 | 35–87 |
| Gender | |||
| Female | 614 (53%) | 447 (45.1%) | 167 (100%) |
| Male | 544 (47%) | 544 (54.9%) | |
| Tumour location | – | – | |
| Right | 308 (31.5%) | ||
| Left | 392 (40.1%) | ||
| Rectum | 278 (28.4%) | ||
| Missing | 13 | ||
| Primary tumour (T) | |||
| T1 | 133 (11.9%) | 4 (0.4%) | 129 (80.1%) |
| T2 | 91 (8.1%) | 79 (8.3%) | 12 (7.5%) |
| T3 | 826 (73.9%) | 806 (84.2%) | 20 (12.4%) |
| T4 | 68 (6.1%) | 68 (7.1%) | 0 (0%) |
| Missing | 40 | 34 | 6 |
| Regional lymph nodes (N) | |||
| N0 | 533 (48.2%) | 408 (42.3%) | 125 (88.7%) |
| N1 | 382 (34.6%) | 370 (38.4%) | 12 (8.5%) |
| N2 | 190 (17.2%) | 186 (19.3%) | 4 (2.8%) |
| Missing | 53 | 27 | 26 |
| Stage | |||
| I | 128 (11.2%) | 8 (0.8%) | 120 (74.5%) |
| II | 415 (36.2%) | 405 (41.2%) | 10 (6.2%) |
| III | 602 (52.6%) | 571 (58%) | 31 (19.3%) |
| Missing | 13 | 7 | 6 |
| Grade | |||
| 1 | 125 (11.1%) | 77 (8%) | 48 (29.1%) |
| 2 | 800 (70.8%) | 725 (75.1%) | 75 (45.5%) |
| 3 | 205 (18.1%) | 163 (16.9%) | 42 (25.4%) |
| Missing | 28 | 26 | 2 |
| Mucinous component | – | – | |
| No | 453 (55.8%) | ||
| Yes | 360 (44.2%) | ||
| Missing | 178 | ||
| Perineural invasion | – | – | |
| No | 716 (85.6%) | ||
| Yes | 121 (14.4%) | ||
| Missing | 154 | ||
| Lymphatic vessel invasion | – | – | |
| No | 637 (75.7%) | ||
| Yes | 205 (24.3%) | ||
| Missing | 149 | ||
| Blood vessel invasion | – | – | |
| No | 721 (85%) | ||
| Yes | 128 (15%) | ||
| Missing | 142 | ||
| PNI/LVI | – | – | |
| No | 590 (69.4%) | ||
| Yes | 260 (30.6%) | ||
| Missing | 141 | ||
| Chemotherapy | – | – | |
| Yes | 991 (100%) | 20 (12%) | |
| No | 147 (88%) | ||
| Chemotherapy regimen | |||
| 5FU/capecitabine | 158 (16.6%) | ||
| FOLFOX | 271 (28.5%) | ||
| CapeOX | 372 (39.1%) | ||
| FOLFIRI | 118 (12.4%) | ||
| Other | 32 (3.4%) | ||
| Missing | 40 | ||
| Radiation therapy | |||
| No | 721 (67.3%) | 657 (72.7%) | 64 (38.3%) |
| Yes | 350 (32.7%) | 247 (27.3%) | 103 (61.7%) |
| Missing | 87 | 87 | 0 |
CapeOX, capecitabine/oxaliplatin; FOLFIRI, 5-FU/ leucovorin/irinotecan; FOLFOX, 5-FU/leucovorin/oxaliplatin; 5FU, 5-fluoruracil; LVI, lymphovascular invasion; N, number; PNI, perineural invasion.
Colorectal tumours were located at the right colon (308 tumours, 32%), left colon (392, 40%) or rectum (278, 28%). All patients with colorectal had received adjuvant treatment with different chemotherapeutic regimens (table 1). Patients with stage I disease received chemotherapy based on the physician’s discretion.
Patients with endometrial cancer had been diagnosed with endometrioid (144 patients, 86.2%), clear cell/serous (15 patients, 9%), mixed (six patients, 3.6%) or other (two patients, 1.2%) histological type. Myometrial invasion was <50% in 50.6% (81 of 160) patients. Overall, 109 (65%) patients with endometrial cancer had received adjuvant treatment (radiation treatment±chemotherapy, table 1).
Expression of MMR proteins
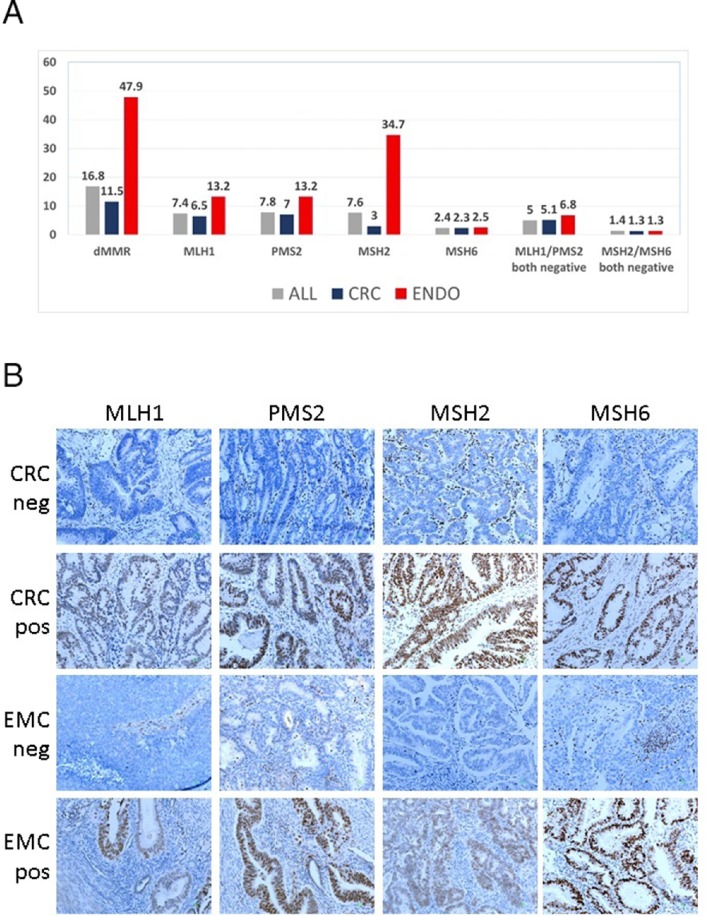
Among 1158 tumours, 194 (16.8%) were classified as dMMR with IHC; 114 (11.5%) colorectal and 80 (47.9%) endometrial tumours. The distribution of dMMR proteins in the entire cohort and per tumour type is shown in figure 1A. In patients with colorectal cancer, the most commonly deficient proteins were PMS2 (69 patients, 7%) and MLH1 (63 patients, 6.5%). In patients with endometrial cancer, MMR deficiency was more frequently associated with MSH2 protein loss (58 patients, 34.7%). However, the rates of combined MLH1/PMS2 or MSH2/MSH6 deficiency were similar in the two tumour types.
Figure 1.
(A) Distribution of dMMR proteins in the entire cohort and per tumour type. (B) Representative examples of MMR immunohistochemistry in CRC and EMC. Indicated are negative and positive tumours in rows for each MMR protein in columns. In negative tumours, note positive stromal cells and lymphocytic infiltrates (positive endogenous control). For all pictures, original magnification ×200. CRC, colorectal carcinoma; dMMR, mismatch repair deficient; EMC, endometrial carcinoma.
Representative examples of IHC staining for MMR proteins are shown in figure 1B. Positivity of the MMR proteins was occasionally not homogeneous, that is, negative and positive cores were recorded for the same tumour while the phenomenon was noticed even within the same core in a few tumours. We noted that even within the same core in a tumour, positivity of the MMR proteins was occasionally not homogeneous. This intratumour heterogeneity would affect the MMR status of 9.8% of colorectal and 3.5% of endometrial carcinomas in our series of TMA-embedded tumours. However, the observed rates were considered underwhelmed since we did not examine whole tumour sections; therefore, we did not further pursue intratumoural heterogeneity for analysis. Heterogeneous tumours were analysed as pMMR in the present series, based on the cut-off used for IHC.
Out of 50 colorectal tumours with dMMR IHC status tested for MSI, 7 (14%) were found with MSS status corresponding to discordant dMMR/MSS, 42 were MSI-high corresponding to concordant dMMR/MSI, while in one tumour the MMR IHC status had been mislabelled and the tumour was MSS. It was possible to revisit six of seven discordant dMMR/MSS tumours. All six tumours had extensive preanalytical (poor fixation, autolysis, extensive necroses or mucin oceans) and therefore analytical problems that hampered reliable IHC interpretation on TMA cores. Notably though, in three cases with repeat stains for the missing protein on whole sections, faint patched (MLH1) or single-cell staining (MLH1 and MSH6), indicative of heterogeneous MMR protein expression, could be identified.
Clinical correlations
In patients with colorectal cancer, dMMR tumours were more likely to be mucinous (64% dMMR vs 42% pMMR, χ2, p<0.001), high grade (31% dMMR vs 15% pMMR, χ2, p<0.001), located at the right colon (65% dMMR vs 27% pMMR, p<0.001). Additionally, MMR deficiency was associated with lower stage (stage I/II) disease in 63% of dMMR vs 39% of pMMR, χ2, p<0.001.
In patients with endometrial cancer, dMMR tumours were more likely to be low grade (80% of dMMR vs 69% of pMMR, χ2, p=0.025) and of endometrioid (74/144, 51.4%) than serous/clear cell (3/15, 20%) histology (χ2, p=0.020). We did not identify any other significant association between MMR status and known clinicopathological parameters in our patient cohort. The rate of dMMR was numerically higher in stage I/II (84%) compared with stage III (77%) disease; however, this association did not yield statistical significance (p=0.425). Detailed clinical correlations with MMR status are shown in table 2.
Table 2.
Associations between MMR status and selected clinicopathological characteristics, in the entire cohort.
| Colorectal cancer (N=991) | Endometrial cancer (N=167) | |||||
| dMMR | pMMR | P value | dMMR | pMMR | P value | |
| Age | < 0.001 | 0.539 | ||||
| N | 114 | 874 | 80 | 87 | ||
| Median | 61.1 | 64.9 | 64.6 | 63.8 | ||
| Range | (23.7–81.5) | (20.5–79.5) | (35.4–87.2) | (35.1–85.1) | ||
| Grade | < 0.001 | 0.025 | ||||
| 1 | 7 (6.4) | 70 (8.2) | 31 (38.8) | 17 (20.0) | ||
| 2 | 69 (62.7) | 656 (76.7) | 33 (41.3) | 42 (49.4) | ||
| 3 | 34 (30.9) | 129 (15.1) | 16 (20.0) | 26 (30.6) | ||
| Primary tumour (T) | 0.369 | 0.234 | ||||
| T1 | 0 (0) | 4 (0.5) | 65 (84.4) | 64 (76.2) | ||
| T2 | 6 (5.4) | 73 (8.6) | 6 (7.8) | 6 (7.1) | ||
| T3 | 94 (84.7) | 712 (84.2) | 6 (7.8) | 14 (16.7) | ||
| T4 | 11 (9.9) | 57 (6.7) | ||||
| Regional lymph nodes (N) | < 0.001 | 0.507 | ||||
| N0 | 71 (63.4) | 337 (39.6) | 65 (89.0) | 60 (88.2) | ||
| N1 | 27 (24.1) | 343 (40.3) | 5 (6.8) | 7 (10.3) | ||
| N2 | 14 (12.5) | 172 (20.2) | 3 (4.1) | 1 (1.5) | ||
| Stage | < 0.001 | 0.425 | ||||
| I | 3 (2.7) | 5 (0.6) | 61 (79.2) | 59 (70.2) | ||
| II | 68 (60.7) | 337 (38.7) | 4 (5.2) | 6 (7.1) | ||
| III | 41 (36.6) | 530 (60.8) | 12 (15.6) | 19 (22.6) | ||
| Radiation therapy | < 0.001 | 0.244 | ||||
| No | 94 (87.9) | 563 (70.6) | 27 (33.8) | 37 (42.5) | ||
| Yes | 13 (12.1) | 234 (29.4) | 53 (66.3) | 50 (57.5) | ||
.N, number; dMMR, mismatch repair deficient; pMMR, mismatch repair proficient.
MMR status and clinical outcome
In the entire cohort, during a median follow-up of 94.8 months (range 0.7–300.9), 321 deaths (27.7%) occurred. Median OS was 225 months (95% CI 225 to not reached). In the colorectal and endometrial cohorts, the median follow-up was 97 (range 0.7–300.9) and 72 (range 1.6–225.0) months, respectively. Among patients with colorectal cancer, 279 deaths (28.2%) occurred, whereas 42 deaths (25.1%) occurred in the cohort of patients with endometrial cancer.
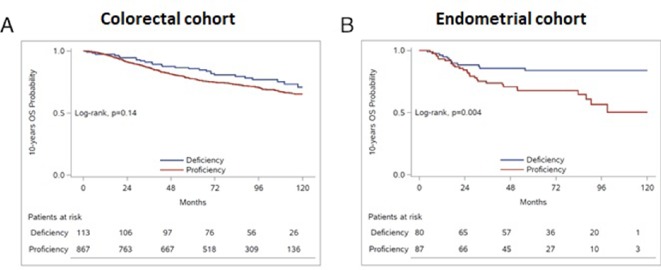
Detailed results of the univariate analysis of all study clinical variables per tumour type are shown in Supplementary Table S1. In patients with colorectal cancer, we did not identify any significant association between MMR status and OS (HR=0.73, 95% CI 0.49 to 1.09, p=0.130, Supplementary Table S2, figure 2A). MMR deficiency did not appear to be prognostic either in patients with stage II or stage III (HR=0.94, 95% CI 0.52 to 1.72, p=0.850 and HR=0.72, 95% CI 0.40 to 1.29, p=0.274, respectively).
Figure 2.
Prognostic significance of MMR status in patients with (A) colorectal and (B) endometrial cancer. MMR, mismatch repair.
esmoopen-2018-000474supp001.pdf (242.6KB, pdf)
esmoopen-2018-000474supp002.pdf (72.2KB, pdf)
In patients with endometrial cancer, MMR deficiency was favourably associated with OS (HR=0.38, 95% CI 0.20 to 0.76, p=0.006, Supplementary Table S2, figure 2B) and maintained its prognostic significance in multivariate analyses on adjusting for age, stage and grade (HR=0.42, 95% CI 0.20 to 0.88, p=0.021). Kaplan-Meier curves for OS according to the status of each MMR protein are presented in Supplementary Figure S1. In this patient group, MSH2 deficiency as a single marker among the four MMR proteins was strongly associated with better outcome and but did not retain its prognostic significance in a multivariate model adjusting for the above clinical parameters and individually for each MMR protein (HR=0.45, 95% CI 0.13 to 1.53, p=0.202).
esmoopen-2018-000474supp003.pdf (144.2KB, pdf)
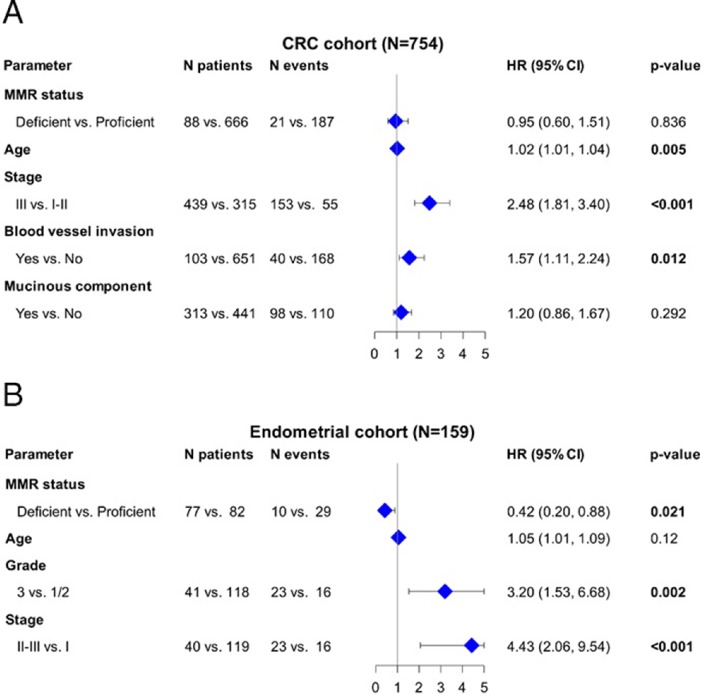
The adjusted HRs for all relevant study parameters are depicted as a forest plot in patients with colorectal (figure 3A) and endometrial (figure 3B) cancer.
Figure 3.
Associations between OS, relevant clinicopathological parameters and MMR status in patients with (A) CRC and (B) endometrial cancer. CRC, colorectal cancer; MMR, mismatch repair; OS, overall survival.
Discussion
In our patient population, MMR deficiency was noted in 11.5% of patients with nonmetastatic colorectal cancer and in 47.9% of patients with nonmetastatic endometrial cancer. MMR-deficiency rates of each of the four proteins differed between the two tumour types. MSH2 deficiency was noted in the majority of endometrial dMMR tumours. On the contrary, colorectal tumours exhibited more commonly PMS2 and MLH1 deficiency. MMR deficiency was independently associated with improved OS in patients with nonmetastatic endometrial cancer. However, MMR status did not appear to be associated with prognosis in patients with nonmetastatic colorectal tumours, who had received adjuvant treatment.
Reported rates of defective MMR in endometrial tumours vary between 19% and 45%.18–20 23 36–42 In fact, the rate of MMR deficiency in endometrial tumours appears to be nearly twice as high compared with colorectal tumours. High frequency of MMR deficiency has been reported predominantly in endometrioid endometrial tumours.19 20 23 37 43 The high rate of dMMR tumours in our patient cohort could be attributed to the high proportion (86%) of endometrioid endometrial tumours. Interestingly, we found that the MSH2 protein was more frequently deficient compared with the rest of the three proteins. If further validated, this finding suggests that the dual MMR protein test (PMS2 and MSH6) proposed for screening44 45 may miss the majority dMMR endometrial cancers, where staining patterns have not yet been characterised.46 Similar to our study, high rates of MSH2 deficiency have been previously reported in patients with endometrial tumours.40 47 In addition, high rates of MSH2-deficient tumours have been identified in patients with Lynch-associated endometrial cancer, who often carry MSH2 germline mutations.48 Even though our patient population was not selected for age or family history of cancer, it may have been enriched for patients with Lynch syndrome carrying MSH2 mutations. No germline data were available for our patients to confirm this hypothesis. Other investigators reported PMS2 to be more commonly absent in a cohort of 235 (of 1049) dMMR tumours, followed by the MLH1 protein.42 Additionally, PMS2 and MLH1 were found to be absent in the majority of dMMR tumours (24 of 30 tumours) in a study of 140 prospectively examined endometrial tumours. Finally, another study reported dMMR proteins per ethnicity and demonstrated similar numbers of absent MLH1/PMS2 and MSH2/MSH6 proteins in Caucasian patients.49 However, since the number of dMMR tumours was small in most of the aforementioned studies, these results need to be interpreted with caution.
In our study, MMR deficiency was associated with lower-stage colorectal cancer. This association has been well described in previous studies.50 Similarly, compared with MMR proficiency, MMR deficiency was observed in a higher number of stage I/II endometrial tumours. Even though the number of dMMR tumours is small, there seems to be an association with MMR status and disease stage. This might be related to the de-selection for dMMR clones during disease progression, since persistent instability is not beneficial for tumour growth.51 In addition, hypermutation of dMMR tumours leads to high neoantigen rate that can be recognised by the immune system.52 Prospective trials should address this association across tumour types.
The prognostic significance of MMR deficiency in patients with endometrial cancer has not been fully established. Studies have demonstrated that women with dMMR tumours have improved clinical outcomes19 20 while others have reported similar18 36–38 41 or worse clinical outcomes40 53–55 compared with women with pMMR tumours. A meta-analysis of 23 studies evaluating MMR status demonstrated significant associations between MMR and clinical outcomes in 32% of those studies.56 Pooled analysis of six studies did not show any significant association between MMR deficiency and decreased OS. However, 74% were retrospective studies, 6 out of 18 evaluated MSI using the panel of five microsatellite markers recommended by the National Cancer Institute and only two out of seven determined the expression of all four MMR proteins.56 In our cohort, MMR deficiency was independently associated with increased OS in patients with nonmetastatic endometrial cancer. This association might be attributed to the high number of dMMR tumours that were stage I. Specifically, improved prognosis was univariately associated with MSH2 deficiency, in contrast to other studies where there was no association between clinical outcomes and MSH2 expression status.47
MMR status has been previously associated with prognosis in patients with stage II57 and III colorectal tumours.17 57 58 In our patient population, MMR deficiency was not associated with OS in patients with nonmetastatic colorectal tumours who received adjuvant treatment. Lack of statistical power might be one of the reasons for the nonsignificant difference in OS between patients with dMMR and pMMR tumours. This lack of association has been previously shown in another cohort of patients with stage II colon cancer, where 86% of the patient population had received adjuvant chemotherapy.59 In our study, patients with stage II disease received adjuvant chemotherapy based on risk factors assessed by the treating physician. Studies have shown that 5-fluorouracil-based adjuvant chemotherapy not only is ineffective in patients with stage II dMMR tumours but it may also have a detrimental effect on survival.60 In addition, some patients received irinotecan-based adjuvant chemotherapy, as part of a clinical study.29 31 Preclinical data have suggested a potential benefit from the addition of irinotecan to the treatment of patients with dMMR colorectal tumours.61 62 However, studies have shown conflicting data on the clinical benefit from the use of irinotecan in patients with dMMR tumours.57 63–67 In addition, the prognostic significance of MMR deficiency has been shown to be affected by other factors, as the presence of tumour-infiltrating lymphocytes68 or BRAF mutation status.69–71 Our results cannot be compared with these studies because herein we did not examine immune response-related markers or BRAF genotypes. Overall, it appears that the prognostic significance of MMR deficiency in colorectal cancer needs to be prospectively evaluated in combination with additional prognostic markers.
The accurate dMMR status characterisation has always been puzzling and regained interest after the FDA approval of this phenotype as the first tumour-agnostic marker for the selection of patients to receive immunotherapy.72 However, whether tumours should be tested in the routine setting with IHC only, MSI-PCR only or with both methods, has not reached a consensus yet. Evaluation of the most accurate method for dMMR classification was not an objective in the present study. In large series of interlaboratory quality assurance rounds, a high concordance for MMR IHC, when appropriately and vigorously standardised,73 74 and a substantially lower one for microsatellite PCR testing73 have been demonstrated. However, even in expert laboratories, tumours with discrepant dMMR/MSS or pMMR/MSI phenotypes do exist.75 76 Our results with double IHC and PCR testing demonstrated a dMMR/MSS discordance of 14%, similar to the previously published comparison between MMR IHC on TMA slides and MSI-PCR.77 As we show, the reasons for dMMR/MSS discordance may be attributed to preanalytical (tissue) and analytical problems of IHC but also to the heterogeneous expression of MMR proteins. One of the discordant tumours exhibited heterogeneous MSH6 loss, where PCR may fail to detect instability with the applied method.78 Heterogeneous intratumour MMR protein expression is reported in colorectal cancer45 79 but its true incidence, biological background and clinical implications are unclear. Notably, this feature can cause MMR status classification problems, since it is definitely a source for discordant IHC/PCR test results and for true discrepant MMR/MSI phenotypes,75 79 as shown here as well. Recently, it was shown that patients with discrepant MMR/MSI phenotypes may respond to immunotherapy,75 and should hence be regarded as dMMR.76 Here, we highlight the issue of intratumour MMR protein heterogeneity and the chance of missing this feature when IHC is applied on TMA cores. Whether tumours with heterogeneous MMR protein expression patterns should also be classified as dMMR needs evaluation in large series by assessing specific staining patterns (patchy, dispersed, differences in staining intensity) on whole sections with appropriately standardised MMR IHC, along with MSI PCR comparison for matched tumour areas.
Our study has certain limitations. First, its retrospective nature. Second, there were no chemotherapy-naïve patients with stage II colorectal cancer to evaluate the prognostic significance of MMR deficiency. In addition, due to lack of associated blood samples, we were not able to perform germline testing in patients with dMMR tumours, to identify inherited mutations. Finally, the sample size of our study cohorts did not allow for subgroup analysis to assess the prognostic significance of MMR status per stage and per tumour location in patients with colorectal tumours.
In conclusion, our study demonstrates that MMR deficiency was associated with improved outcomes in women with endometrial carcinoma. MMR status was not associated with prognosis in patients with nonmetastatic colorectal cancer who received adjuvant treatment. Future prospective studies are needed to assess the prognostic significance of each MMR protein deficiency, in combination with relevant clinical and pathological parameters to improve risk stratification.
Acknowledgments
The authors are indebted to all patients and their families for the provision of biological material for research purposes. The authors would also like to thank Eneida Jaupaj for sample collection and Maria Moschoni for coordinating the data management.
Footnotes
Funding: This study was supported by a research grant from Astra-Zeneca (ESR-15-10766) and by two internal Hellenic Cooperative Oncology Group (HeCOG) translational research grants (HE TRANS_E and HE TRANS_CRC). Dr. E. Fountzilas has received a scholarship from the Hellenic Society of Medical Oncology (10/2017-09/2018).
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Lynch HT, Snyder CL, Shaw TG, et al. Milestones of Lynch syndrome: 1895-2015. Nat Rev Cancer 2015;15:181–94. 10.1038/nrc3878 [DOI] [PubMed] [Google Scholar]
- 2. Cancer Genome Atlas Network Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487:330–7. 10.1038/nature11252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fountzilas E, Kotoula V, Tikas I, et al. Prognostic significance of tumor genotypes and CD8+ infiltrates in stage I-III colorectal cancer. Oncotarget 2018;9:35623–38. 10.18632/oncotarget.26256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Middha S, Zhang L, Nafa K, et al. Reliable pan-cancer microsatellite instability assessment by using targeted next-generation sequencing data. JCO Precis Oncol 2017. 10.1200/PO.17.00084. [Epub ahead of print: 03 Oct 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kautto EA, Bonneville R, Miya J, et al. Performance evaluation for rapid detection of pan-cancer microsatellite instability with MANTIS. Oncotarget 2017;8:7452–63. 10.18632/oncotarget.13918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med 2003;348:919–32. 10.1056/NEJMra012242 [DOI] [PubMed] [Google Scholar]
- 7. Overman MJ, Lonardi S, Wong KYM, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch Repair-Deficient/Microsatellite Instability-High metastatic colorectal cancer. J Clin Oncol 2018;36:773–9. 10.1200/JCO.2017.76.9901 [DOI] [PubMed] [Google Scholar]
- 8. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409–13. 10.1126/science.aan6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015;348:69–74. 10.1126/science.aaa4971 [DOI] [PubMed] [Google Scholar]
- 10. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509–20. 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124–8. 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. FDA Pembrolizumab for first tissue/site agnostic indication, 2017. [Google Scholar]
- 13. Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003;349:247–57. 10.1056/NEJMoa022289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jover R, Zapater P, Castells A, et al. Mismatch repair status in the prediction of benefit from adjuvant fluorouracil chemotherapy in colorectal cancer. Gut 2006;55:848–55. 10.1136/gut.2005.073015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zaanan A, Shi Q, Taieb J, et al. Role of deficient DNA mismatch repair status in patients with stage III colon cancer treated with FOLFOX adjuvant chemotherapy: a pooled analysis from 2 randomized clinical trials. JAMA Oncol 2018;4:379–83. 10.1001/jamaoncol.2017.2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kawakami H, Zaanan A, Sinicrope FA. Implications of mismatch repair-deficient status on management of early stage colorectal cancer. J Gastrointest Oncol 2015;6:676–84. 10.3978/j.issn.2078-6891.2015.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sinicrope FA, Mahoney MR, Smyrk TC, et al. Prognostic impact of deficient DNA mismatch repair in patients with stage III colon cancer from a randomized trial of FOLFOX-based adjuvant chemotherapy. J Clin Oncol 2013;31:3664–72. 10.1200/JCO.2013.48.9591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shikama A, Minaguchi T, Matsumoto K, et al. Clinicopathologic implications of DNA mismatch repair status in endometrial carcinomas. Gynecol Oncol 2016;140:226–33. 10.1016/j.ygyno.2015.11.032 [DOI] [PubMed] [Google Scholar]
- 19. Mackay HJ, Gallinger S, Tsao MS, et al. Prognostic value of microsatellite instability (MSI) and PTEN expression in women with endometrial cancer: results from studies of the NCIC Clinical Trials Group (NCIC CTG). Eur J Cancer 2010;46:1365–73. 10.1016/j.ejca.2010.02.031 [DOI] [PubMed] [Google Scholar]
- 20. Black D, Soslow RA, Levine DA, et al. Clinicopathologic significance of defective DNA mismatch repair in endometrial carcinoma. J Clin Oncol 2006;24:1745–53. 10.1200/JCO.2005.04.1574 [DOI] [PubMed] [Google Scholar]
- 21. Bonneville R, Krook MA, Kautto EA, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol 2017. 10.1200/PO.17.00073. [Epub ahead of print: 03 Oct 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goodfellow PJ, Buttin BM, Herzog TJ, et al. Prevalence of defective DNA mismatch repair and MSH6 mutation in an unselected series of endometrial cancers. Proc Natl Acad Sci U S A 2003;100:5908–13. 10.1073/pnas.1030231100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kandoth C, Schultz N, Cherniack AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013;497:67–73. 10.1038/nature12113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cortes-Ciriano I, Lee S, Park W-Y, et al. A molecular portrait of microsatellite instability across multiple cancers. Nat Commun 2017;8 10.1038/ncomms15180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Adar T, Rodgers LH, Shannon KM, et al. Universal screening of both endometrial and colon cancers increases the detection of Lynch syndrome. Cancer 2018;124:3145–53. 10.1002/cncr.31534 [DOI] [PubMed] [Google Scholar]
- 26. Dinjens WNM, Dubbink HJ, Wagner A. Guidelines on genetic evaluation and management of Lynch syndrome. Am J Gastroenterol 2015;110:192–3. 10.1038/ajg.2014.329 [DOI] [PubMed] [Google Scholar]
- 27. Di Marco M, DAndrea E, Panic N, et al. Which Lynch syndrome screening programs could be implemented in the "real world"? A systematic review of economic evaluations. Genet Med 2018;20:1131–44. 10.1038/gim.2017.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fountzilas G, Zisiadis A, Dafni U, et al. Postoperative radiation and concomitant bolus fluorouracil with or without additional chemotherapy with fluorouracil and high-dose leucovorin in patients with high-risk rectal cancer: a randomized phase III study conducted by the Hellenic Cooperative Oncology Group. Ann Oncol 1999;10:671–6. 10.1023/A:1008357609434 [DOI] [PubMed] [Google Scholar]
- 29. Kalofonos HP, Kardamakis D, Bamias A, et al. Adjuvant chemotherapy using CPT-11, leucovorin plus bolus 5-fluorouracil and radiotherapy in patients with rectal cancer. A feasibility study. Anticancer Res 2003;23:1687–91. [PubMed] [Google Scholar]
- 30. Fountzilas G, Zisiadis A, Dafni U, et al. Fluorouracil and leucovorin with or without interferon alfa-2a as adjuvant treatment, in patients with high-risk colon cancer: a randomized phase III study conducted by the Hellenic Cooperative Oncology Group. Oncology 2000;58:227–36. 10.1159/000012105 [DOI] [PubMed] [Google Scholar]
- 31. Kalofonos HP, Bamias A, Koutras A, et al. A randomised phase III trial of adjuvant radio-chemotherapy comparing irinotecan, 5FU and leucovorin to 5FU and leucovorin in patients with rectal cancer: a Hellenic Cooperative Oncology Group study. Eur J Cancer 2008;44:1693–700. 10.1016/j.ejca.2008.05.025 [DOI] [PubMed] [Google Scholar]
- 32. Pectasides D, Karavasilis V, Papaxoinis G, et al. Randomized phase III clinical trial comparing the combination of capecitabine and oxaliplatin (CAPOX) with the combination of 5-fluorouracil, leucovorin and oxaliplatin (modified FOLFOX6) as adjuvant therapy in patients with operated high-risk stage II or stage III colorectal cancer. BMC Cancer 2015;15 10.1186/s12885-015-1406-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Polychronidou G, Kotoula V, Manousou K, et al. Mismatch repair deficiency and aberrations in the Notch and hedgehog pathways are of prognostic value in patients with endometrial cancer. PLoS One 2018;13 10.1371/journal.pone.0208221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Boland CR, Thibodeau SN, Hamilton SR, et al. A national Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 1998;58:5248–57. [PubMed] [Google Scholar]
- 35. Bocker T, Diermann J, Friedl W, et al. Microsatellite instability analysis: a multicenter study for reliability and quality control. Cancer Res 1997;57:4739–43. [PubMed] [Google Scholar]
- 36. MacDonald ND, Salvesen HB, Ryan A, et al. Frequency and prognostic impact of microsatellite instability in a large population-based study of endometrial carcinomas. Cancer Res 2000;60:1750–2. [PubMed] [Google Scholar]
- 37. Zighelboim I, Goodfellow PJ, Gao F, et al. Microsatellite instability and epigenetic inactivation of MLH1 and outcome of patients with endometrial carcinomas of the endometrioid type. J Clin Oncol 2007;25:2042–8. 10.1200/JCO.2006.08.2107 [DOI] [PubMed] [Google Scholar]
- 38. Baldinu P, Cossu A, Manca A, et al. Microsatellite instability and mutation analysis of candidate genes in unselected Sardinian patients with endometrial carcinoma. Cancer 2002;94:3157–68. 10.1002/cncr.10606 [DOI] [PubMed] [Google Scholar]
- 39. Muresu R, Sini MC, Cossu A, et al. Chromosomal abnormalities and microsatellite instability in sporadic endometrial cancer. Eur J Cancer 2002;38:1802–9. 10.1016/S0959-8049(02)00152-1 [DOI] [PubMed] [Google Scholar]
- 40. Cohn DE, Frankel WL, Resnick KE, et al. Improved survival with an intact DNA mismatch repair system in endometrial cancer. Obstet Gynecol 2006;108:1208–15. 10.1097/01.AOG.0000239097.42987.0c [DOI] [PubMed] [Google Scholar]
- 41. Ruiz I, Martín-Arruti M, Lopez-Lopez E, et al. Lack of association between deficient mismatch repair expression and outcome in endometrial carcinomas of the endometrioid type. Gynecol Oncol 2014;134:20–3. 10.1016/j.ygyno.2014.04.053 [DOI] [PubMed] [Google Scholar]
- 42. Joehlin-Price AS, Perrino CM, Stephens J, et al. Mismatch repair protein expression in 1049 endometrial carcinomas, associations with body mass index, and other clinicopathologic variables. Gynecol Oncol 2014;133:43–7. 10.1016/j.ygyno.2014.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Llobet D, Pallares J, Yeramian A, et al. Molecular pathology of endometrial carcinoma: practical aspects from the diagnostic and therapeutic viewpoints. J Clin Pathol 2009;62:777–85. 10.1136/jcp.2008.056101 [DOI] [PubMed] [Google Scholar]
- 44. Hall G, Clarkson A, Shi A, et al. Immunohistochemistry for PMS2 and MSH6 alone can replace a four antibody panel for mismatch repair deficiency screening in colorectal adenocarcinoma. Pathology 2010;42:409–13. 10.3109/00313025.2010.493871 [DOI] [PubMed] [Google Scholar]
- 45. Pearlman R, Markow M, Knight D, et al. Two-stain immunohistochemical screening for Lynch syndrome in colorectal cancer may fail to detect mismatch repair deficiency. Mod Pathol 2018;31:1891–900. 10.1038/s41379-018-0058-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang Y, Wang Y, Li J, et al. Lynch syndrome related endometrial cancer: clinical significance beyond the endometrium. J Hematol Oncol 2013;6 10.1186/1756-8722-6-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schröer A, Köster F, Fischer D, et al. Immunohistochemistry of DNA mismatch repair enzyme MSH2 is not correlated with prognostic data from endometrial carcinomas. Anticancer Res 2009;29:4833–7. [PubMed] [Google Scholar]
- 48. Bonadona V, Bonaïti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA 2011;305:2304–10. 10.1001/jama.2011.743 [DOI] [PubMed] [Google Scholar]
- 49. Grzankowski KS, Shimizu DM, Kimata C, et al. Clinical and pathologic features of young endometrial cancer patients with loss of mismatch repair expression. Gynecol Oncol 2012;126:408–12. 10.1016/j.ygyno.2012.05.019 [DOI] [PubMed] [Google Scholar]
- 50. Greenson JK, Huang S-C, Herron C, et al. Pathologic predictors of microsatellite instability in colorectal cancer. Am J Surg Pathol 2009;33:126–33. 10.1097/PAS.0b013e31817ec2b1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lengauer C, Kinzler KW, Vogelstein B. Genetic instability in colorectal cancers. Nature 1997;386:623–7. 10.1038/386623a0 [DOI] [PubMed] [Google Scholar]
- 52. Ray K. Biomarkers: matching mutations to immunotherapy response. Nat Rev Gastroenterol Hepatol 2017;14:566–7. 10.1038/nrgastro.2017.115 [DOI] [PubMed] [Google Scholar]
- 53. Bilbao C, Lara PC, Ramírez R, et al. Microsatellite instability predicts clinical outcome in radiation-treated endometrioid endometrial cancer. Int J Radiat Oncol Biol Phys 2010;76:9–13. 10.1016/j.ijrobp.2009.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shih KK, Garg K, Levine DA, et al. Clinicopathologic significance of DNA mismatch repair protein defects and endometrial cancer in women 40years of age and younger. Gynecol Oncol 2011;123:88–94. 10.1016/j.ygyno.2011.06.005 [DOI] [PubMed] [Google Scholar]
- 55. Garg K, Shih K, Barakat R, et al. Endometrial carcinomas in women aged 40 years and younger: tumors associated with loss of DNA mismatch repair proteins comprise a distinct clinicopathologic subset. Am J Surg Pathol 2009;33:1869–77. 10.1097/PAS.0b013e3181bc9866 [DOI] [PubMed] [Google Scholar]
- 56. Diaz-Padilla I, Romero N, Amir E, et al. Mismatch repair status and clinical outcome in endometrial cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol 2013;88:154–67. 10.1016/j.critrevonc.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 57. Klingbiel D, Saridaki Z, Roth AD, et al. Prognosis of stage II and III colon cancer treated with adjuvant 5-fluorouracil or FOLFIRI in relation to microsatellite status: results of the PETACC-3 trial. Ann Oncol 2015;26:126–32. 10.1093/annonc/mdu499 [DOI] [PubMed] [Google Scholar]
- 58. Samowitz WS, Curtin K, Ma KN, et al. Microsatellite instability in sporadic colon cancer is associated with an improved prognosis at the population level. Cancer Epidemiol Biomarkers Prev 2001;10:917–23. [PubMed] [Google Scholar]
- 59. Kim JE, Hong YS, Kim HJ, et al. Defective mismatch repair status was not associated with DFS and os in stage II colon cancer treated with adjuvant chemotherapy. Ann Surg Oncol 2015;22(Suppl 3):630–7. 10.1245/s10434-015-4807-6 [DOI] [PubMed] [Google Scholar]
- 60. Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol 2010;28:3219–26. 10.1200/JCO.2009.27.1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Magrini R, Bhonde MR, Hanski M-L, et al. Cellular effects of CPT-11 on colon carcinoma cells: dependence on p53 and hMLH1 status. Int J Cancer 2002;101:23–31. 10.1002/ijc.10565 [DOI] [PubMed] [Google Scholar]
- 62. Bras-Gonçalves RA, Rosty C, Laurent-Puig P, et al. Sensitivity to CPT-11 of xenografted human colorectal cancers as a function of microsatellite instability and p53 status. Br J Cancer 2000;82:913–23. 10.1054/bjoc.1999.1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bertagnolli MM, Niedzwiecki D, Compton CC, et al. Microsatellite instability predicts improved response to adjuvant therapy with irinotecan, fluorouracil, and leucovorin in stage III colon cancer: Cancer and leukemia group B protocol 89803. J Clin Oncol 2009;27:1814–21. 10.1200/JCO.2008.18.2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kim JE, Hong YS, Ryu M-H, et al. Association between deficient mismatch repair system and efficacy to irinotecan-containing chemotherapy in metastatic colon cancer. Cancer Sci 2011;102:1706–11. 10.1111/j.1349-7006.2011.02009.x [DOI] [PubMed] [Google Scholar]
- 65. Ma J, Zhang Y, Shen H, et al. Association between mismatch repair gene and irinotecan-based chemotherapy in metastatic colon cancer. Tumour Biol 2015;36:9599–609. 10.1007/s13277-015-3723-5 [DOI] [PubMed] [Google Scholar]
- 66. Braun MS, Richman SD, Quirke P, et al. Predictive biomarkers of chemotherapy efficacy in colorectal cancer: results from the UK MRC FOCUS trial. J Clin Oncol 2008;26:2690–8. 10.1200/JCO.2007.15.5580 [DOI] [PubMed] [Google Scholar]
- 67. Fallik D, Borrini F, Boige V, et al. Microsatellite instability is a predictive factor of the tumor response to irinotecan in patients with advanced colorectal cancer. Cancer Res 2003;63:5738–44. [PubMed] [Google Scholar]
- 68. Williams DS, Mouradov D, Jorissen RN, et al. Lymphocytic response to tumour and deficient DNA mismatch repair identify subtypes of stage II/III colorectal cancer associated with patient outcomes. Gut 2018. 10.1136/gutjnl-2017-315664. [Epub ahead of print: 30 Jan 2018]. [DOI] [PubMed] [Google Scholar]
- 69. Lochhead P, Kuchiba A, Imamura Y, et al. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J Natl Cancer Inst 2013;105:1151–6. 10.1093/jnci/djt173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. French AJ, Sargent DJ, Burgart LJ, et al. Prognostic significance of defective mismatch repair and BRAF V600E in patients with colon cancer. Clin Cancer Res 2008;14:3408–15. 10.1158/1078-0432.CCR-07-1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ogino S, Shima K, Meyerhardt JA, et al. Predictive and prognostic roles of BRAF mutation in stage III colon cancer: results from intergroup trial CALGB 89803. Clin Cancer Res 2012;18:890–900. 10.1158/1078-0432.CCR-11-2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lemery S, Keegan P, Pazdur R. First FDA approval agnostic of cancer site - when a biomarker defines the indication. N Engl J Med 2017;377:1409–12. 10.1056/NEJMp1709968 [DOI] [PubMed] [Google Scholar]
- 73. Boyle TA, Bridge JA, Sabatini LM, et al. Summary of microsatellite instability test results from laboratories participating in proficiency surveys: proficiency survey results from 2005 to 2012. Arch Pathol Lab Med 2014;138:363–70. 10.5858/arpa.2013-0159-CP [DOI] [PubMed] [Google Scholar]
- 74. Richman SD, Adams R, Quirke P, et al. Pre-trial inter-laboratory analytical validation of the FOCUS4 personalised therapy trial. J Clin Pathol 2016;69:35–41. 10.1136/jclinpath-2015-203097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cohen R, Hain E, Buhard O, et al. Association of primary resistance to immune checkpoint inhibitors in metastatic colorectal cancer with misdiagnosis of microsatellite instability or mismatch repair deficiency status. JAMA Oncol 2018. 10.1001/jamaoncol.2018.4942. [Epub ahead of print: 15 Nov 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. McCracken J, Neff J, Cap Today . AMP case report: discordant IHC/PCR test results for mismatch repair status in colorectal adenocarcinoma 2018.
- 77. Lee J-H, Cragun D, Thompson Z, et al. Association between IHC and MSI testing to identify mismatch repair-deficient patients with ovarian cancer. Genet Test Mol Biomarkers 2014;18:229–35. 10.1089/gtmb.2013.0393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. You J-F, Buhard O, Ligtenberg MJL, et al. Tumours with loss of MSH6 expression are MSI-H when screened with a pentaplex of five mononucleotide repeats. Br J Cancer 2010;103:1840–5. 10.1038/sj.bjc.6605988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Joost P, Veurink N, Holck S, et al. Heterogenous mismatch-repair status in colorectal cancer. Diagn Pathol 2014;9 10.1186/1746-1596-9-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2018-000474supp001.pdf (242.6KB, pdf)
esmoopen-2018-000474supp002.pdf (72.2KB, pdf)
esmoopen-2018-000474supp003.pdf (144.2KB, pdf)