Abstract
Bat trypanosomes consist of more than 30 trypanosome species from over 70 species of bats. Recent studies suggest that bats play a role in disseminating trypanosomes from African continent to the terrestrial mammals both in the Afrotropic-Palearctic Ecozones and Nearctic Ecozone. However, the diversity, distribution, and evolution of bat trypanosomes are still unclear. To better understand their evolution, more genetic data of bat trypanosomes from a variety of locations are required. During a survey of Borrelia spp. of bats inhabiting a cave in Zambia, we observed flagellate parasites from 5 of 43 hemocultures. Sequence and phylogenetic analyses of the glycosomal glyceraldehyde 3-phosphate dehydrogenase gene (gGAPDH; 572 bp) and the 18S ribosomal RNA gene (18S rRNA gene; 1,079–1,091 bp) revealed that all were Trypanosoma spp. belonged to the Trypanosoma cruzi clade. Three and two of them exhibited the similarity with T. conorhini and T. dionisii, respectively. The present study provides the first genetic data on Trypanosoma spp. of bats inhabiting Zambia.
Keywords: Zambia, Bat, Trypanosoma cruzi clade, Trypanosoma conorhini, Trypanosoma dionisii
Graphical abstract
Highlights
-
•
Two putative species of trypanosomes were detected from bats.
-
•
First molecular characterization of bat trypanosome in Zambia.
-
•
First record of trypanosome in Hipposideros vittatus.
1. Introduction
Approximately 1,240 bat species are recognized in the world, representing about 20% of all classified mammalian species worldwide. They play a role as a natural reservoir of wide variety of pathogens including virus, bacteria, and protozoa (Calisher et al., 2006). Trypanosomes, which are blood parasites widespread in all continents and commonly transmitted by blood sucking arthropods and leeches (Hoare, 1972; Hamilton et al., 2007), have been reported in over 70 bat species in the world. These bat trypanosome species belong to three subgenera, namely Herpetosoa, Megatrypanum, and Schizotrypanum. T. cruzi, which is a causative agent of human Chagas disease in South America (Bern, 2015), has been detected in a variety of terrestrial animals including bats. Many species in the subgenus Schizotrypanum constitute a large monophyletic assemblage that has been designated as T. cruzi clade. The members of bat trypanosomes in the T. cruizi clade include T. vespertilionis in European bats and T. vespertilionis-like Trypanosoma spp. in West African bats, T. cruzi marinkellei in South American bats, and T. dionisii in European, Asian, and South American bats (Hoare, 1972; Mafie et al., 2018). Recent molecular evidence from studies on bat trypanosomes suggest that the T. cruzi clade evolved from a broader clade of bat trypanosomes, and that bat trypanosomes had successfully made the host switch to other terrestrial mammalian species in both the Nearctic Ecozone and Afrotropic-Palearctic Ecozones (Hamilton et al., 2012a). Therefore, molecular investigation of the genus Trypanosoma in bats all over the world is important for better understanding of evolution and diversity of pathogenic trypanosomes.
In Sub-Sahara Africa, tsetse-transmitted trypanosomes, including T. brucei sensu lato, T. congolense, and T. vivax, threaten human and animal health (Büscher et al., 2017; Morrison et al., 2016). Non-tsetse-transmitted trypanosomes such as T. lewisi are also known to be prevalent in rodents in the region (Hoare, 1972; Keymer, 1971). In bats, T. livingstonei and T. erneyi belonging to the T. cruzi clade were detected in Mozambique (Lima et al., 2012, 2013). The ancestral species of the parasites in the T. cruzi clade in bats is considered as the origin of trypanosomes in land mammalians. The phylogenetic position of T. livingstonei is peripheral to T. cruzi clade; this fact supports the hypothesis that T. cruzi originated from bats (Lima et al., 2013). Thus, genetic investigation of trypanosomes in bats is important to understand whole picture of evolution and distribution of trypanosomes and their genetic diversity.
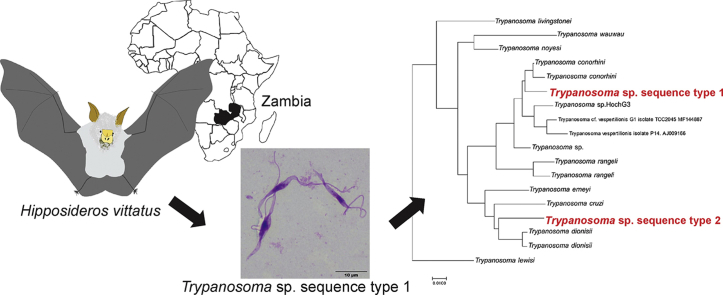
During the survey on Borrelia spp. in bats captured in a cave in Zambia, where a patient suffering from relapsing fever got a tick bite (Qiu et al., in press), we unexpectedly observed flagellate protozoa in the five hemocultures. We found that these parasites were phylogenetically divided into two distinct subclades within the T. cruzi clade. This study provides the first genetic characterization of bat trypanosomes in Zambia.
2. Materials and methods
2.1. Culture
In December 2017, a total of 43 bats (32 Rousettus aegyptiacus and 11 Hipposideros vittatus) were captured at the cave (15.44 S, 28.51 E) in Zambia as part of a surveillance program of filovirus infection and Borrelia spp. in accordance with the ethical standards approved by the Department of National Parks and Wildlife (formerly Zambia Wildlife Authority), Ministry of Tourism and Arts of the Republic of Zambia (Act No. 12 of 1998) (Changula et al., in press). Uncoagulated whole blood samples were collected from bats. For the purpose of Borrelia spp. isolation, 100 μl of the peripheral blood were added into the Barbour-Stoenner-Kelly (BSK)-M medium and incubated for 4 weeks at 34 °C, with 5.0% CO2 as previously described (Takano et al., 2014). The culture was observed microscopically every 2–3 days.
2.2. Morphological investigation
Giemsa staining was carried out for visualizing flagellate protozoa from one of the bat samples in the BSK-M medium (ZB17-105). Microscopy (Shimadzu Motic BA210E) was used for observation and image acquisition of the parasites.
2.3. DNA extraction and polymerase chain reaction (PCR)
The culture media that contained the parasites were harvested at 2 weeks after culture initiation. After centrifugation at 1,600 g for 10 min, DNA was extracted from the pellet using DNAzol (Invitrogen, MA, USA) according to the manufacturer's instructions.
PCR amplification of the glycosomal glyceraldehyde 3-phosphate dehydrogenase (gGAPDH), and 18S ribosomal RNA genes was carried out using previously described primers (Table 1) (Hamilton et al., 2004; da Silva et al., 2004; Lemos et al., 2015). All PCR reactions were conducted in a 20 μl-reaction mixture containing 2 μl of 10 × Ex Taq Buffer (TaKaRa Bio Inc., Shiga, Japan), 0.1 μl of Ex Taq Hot Start Version (TaKaRa Bio Inc.), 1.6 μl of 2.5 mM dNTPs mixture, 200 nM of each primer, and 2 μl of template DNA. The reaction conditions were 98 °C for 1 min and 35 cycles of 98 °C for 10 s, 55 °C for 30 s, and 72 °C for 60 s, followed by a final extension at 72 °C for 5 min. The PCR products were subjected to electrophoresis in a 1.2% agarose gel and stained with ethidium bromide.
Table 1.
Primers used in this study.
| Primer | Sequence 5′-3′ | Target gene | Amplicon size | Reference |
|---|---|---|---|---|
| TRY927F | GAAACAAGAAACACGGGAG | 18S rRNA gene | 900 bp | Hamilton et al. (2004) |
| TRY927R | CTACTGGGCAGCTTGGA | |||
| 609F | CACCCGCGGTAATTCCAGC | 18S rRNA gene | 900 bp | da Silva et al. (2004) |
| 706R | TCTGAGACTGTAACCTCAA | |||
| GAP3F | GTGAAGGCGCAGCGCAAC | gGAPDH gene | 600 bp | Lemos et al. (2015) |
| GAP5R | CCGAGGATGYCCTTCATG |
2.4. Sequencing and phylogenetic analyses
Cycle sequencing was performed using the BigDye Terminator version 3.1 chemistry (Applied Biosystems, MA, USA). Sequencing products were run on a 3130xl Genetic Analyzer (Applied Biosystems). Sanger sequencing data were analyzed using GENETYX version 9.1 (GENETYX Corporation, Tokyo, Japan). In order to obtain a longer sequence of the 18S rRNA gene, sequences of each sample from 2 PCRs targeting 18S rRNA gene were combined. Approximately 1,080-bp fragments of the 18S rRNA gene sequence were obtained.
The obtained gGAPDH and 18S rRNA gene sequences of trypanosomes from bats were aligned with closely related parasite sequences deposited in the database (DDBJ/EMBL/GenBank) using ClustalW multiple alignment program. Phylogenetic trees were constructed by using three methods; the maximum likelihood, neighbor joining, and minimum evolution methods embedded in the MEGA version 6.06 (Tamura et al., 2013). Phylograms were constructed with concatenated gGAPDH and 18S rRNA gene sequences, as previously described (Cottonatil et al., 2014; Espinosa-Álvarez et al., 2018). The DDBJ/EMBL/GenBank accession numbers obtained in this study are as follows: gGAPDH: LC415422 and LC415423, 18S rRNA gene: LC415424 and LC415425.
2.5. Species delimitation
Poisson tree processes (PTP) model for species delimitation was used for inferring the relationship between Trypanosoma spp. detected in this study and their closely related species. PTP species delimitation analysis was carried out via the bPTP webserver (Zhang et al., 2013). We employed a maximum likelihood phylogeny of the concatenated sequences of 18S rDNA and gGAPDH with default parameters as reported elsewhere (Cottontail et al., 2014).
3. Results
3.1. Detection of flagellate protozoa
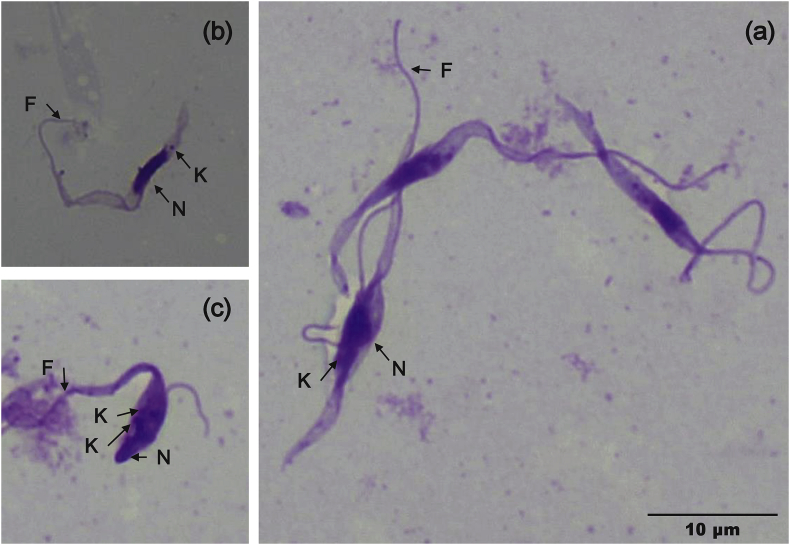
Of the 43 bat blood samples that were added to the BSK-M medium, flagellate protozoa were observed at about 1 week after culture initiation in 5 samples from H. vittatus. During the 4 weeks of observation period, the number of parasites decreased gradually. Though the parasites were co-cultured with primary cells derived from H. vittatus, the number of parasites did not increase (data not shown). Microscopic observation of the slides prepared from one culture (ZB17-105) clearly showed flagellate protozoa with stumpy and slender forms of variable sizes (13.0–36.0 μm from the anterior to the posterior) (Fig. 1). Unfortunately, the morphological data from other cultures were not recorded.
Fig. 1.
Giemsa staining of Trypanosoma sp. from ZB17–105 in BSK-M medium
Representative images of ZB17-105 in the BSK-M medium are displayed at the same magnification (x1000). (a,b) flagellates resembling promastigote forms. (c) possibly epimastigote forms under division. K: kinetoplast, N: nucleus, F: flagellum.
3.2. Sequence analysis of Trypanosoma spp.
Sequence analysis of gGAPDH revealed the presence of two distinct sequence types. The gGAPDH sequence type 1 from the samples ZB17-105, −111, and −115 (GenBank no. LC415422) showed 97.0% (555/572 bp) identity with the T. conorhini isolate TCC2156, which was detected from a triatomine (Triatomia rubrofasciata) of Hawaii (GenBank no. MF144731) (Espinosa-Álvarez et al., 2018). The sequence type 2 from the samples ZB17-108 and −109 (GenBank no. LC415423) showed 94.2% (539/572 bp) identity with the T. dionisii isolate TryCC495 from a bat (Carollia perspicillata) in Brazil (GenBank no. GQ140363) (Cavazzana et al., 2010).
The same grouping was obtained by the sequence analysis of 18S rRNA. The 18S rRNA gene of the sequence type 1 from the samples ZB17-105, −111, and −115 (GenBank no. LC415424) showed 97.7% (1074/1099 bp) identity with Trypanosoma sp. isolate NanDoum1 from the African palm civet (N. binotata) in Cameroon (GenBank no. FM202492) (Hamilton et al., 2009). The sequence type 2 from the samples ZB17-108 and −109 (GenBank no. LC415425) showed 95.2% (1033/1084 bp) identity with the T. dionisii isolate P3 from a bat (Pipistrellus pipistrellus) captured in the United Kingdom (GenBank no. AJ009151) (Stevens et al., 1998).
3.3. Phylogenetic analysis of Trypanosoma spp.
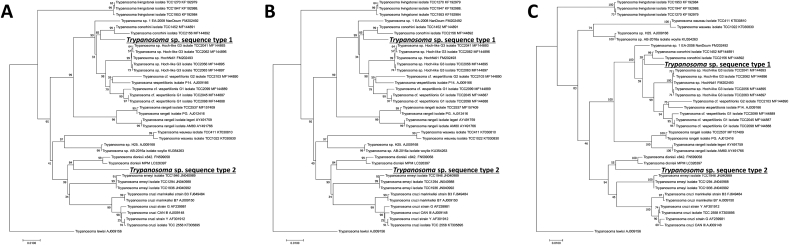
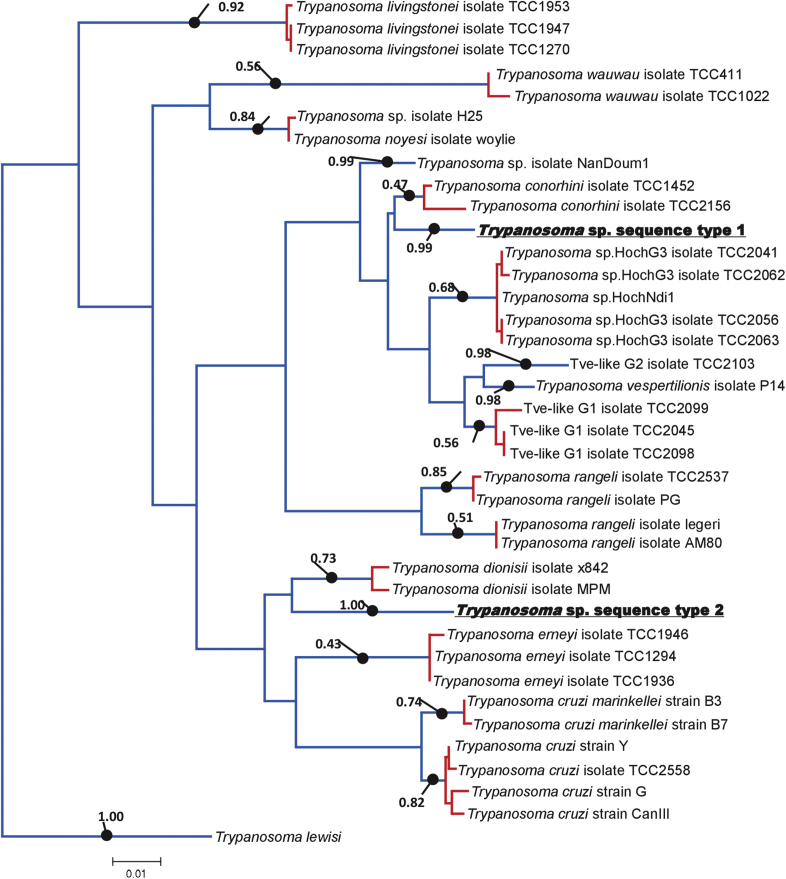
In the phylogenetic trees based on the concatenated 18S rRNA and gGAPDH gene sequences, the sequence type 1 (ZB17-105, −111, and −115) and type 2 (ZB17-108 and −109) belonged to the clades clustering respectively with T. conorhini and T. dionisii (Fig. 2). The topologies of the trees generated with three different methods are in good agreement, except for the position of parasites in the Australian clade (T. noyesi and Trypanosoma sp. isolate H25) and the Neobats clade (T. wauwau) (Fig. 2). The PTP analysis inferred two types of Trypanosoma spp. detected in this study as putative species (Fig. 3). Trypanosoma sp. sequence type 1 and T. conorhini were clustered together, while Trypanosoma sp. sequence type 2 and T. dionisii were clustered together, both of which formed a distinct monophyletic group (Fig. 3).
Fig. 2.
Phylogeny of Trypanosoma cruzi clade. Phylogenetic trees of Trypanosomacruzi clade inferred using combined gGAPDH and 18S rRNA gene sequences (1,363 bp). The phylogenetic trees with outgroup (Trypanosoma lewisi) were generated using a maximum likelihood method (A), neighbor joining method (B), and minimum evolution method (C). All bootstrap values from 1,000 replications are shown on the interior branch nodes. Our isolated Trypanosoma spp. are highlighted in bold and under line. GenBank accession numbers used in this phylogeny are listed in Table S1.
Fig. 3.
Species delimitation of Trypanosoma cruzi clade. Maximum likelihood phylogeny with outgroup (Trypanosoma lewisi) and with Baysian support values presented 17 linages recognized as species for the PTP analysis. Monophyletic groups in red indicated single putative species as well as terminal branches in blue. . (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
We observed two distinct sequence types of Trypanosoma spp. in BSK-M medium inoculated with blood samples of striped leaf-nosed bats (H. vittatus). BSK medium and its derivatives are generally used for the isolation of Borrelia spp., however, Mafie and colleagues also incidentally isolated T. dionisii from an Eastern bent-winged bat (Miniopterus fuliginosus) using the BSK medium (Mafie et al., 2018). To the best of our knowledge, this study is the first record of trypanosome infection in H. vittatus bats and the first molecular study of bat trypanosomes in Zambia.
The nucleotide sequences of both gGAPDH and 18S rRNA genes of the samples ZB17-105, −111, and −115 (sequence type 1) showed high similarity with that of a trypanosome member of the T. conorhini clade. In the phylogenetic trees and PTP analysis based on the concatenated 18S rRNA and gGAPDH gene sequences, the detected trypanosome was a putative species forming a monophyletic group with T. conorhini. T. conorhini, a non-pathogenic rat trypanosome, is distributed all over the world (Hoare, 1972). The origin of T. conorhini is still unclear. Previously, T. conrhini was considered to originate in the Nearctic Ecozone and disseminated to the Afrotropic-Palearctic Ecozones through rats in ships (Stevens et al., 1999). On the contrary, recent studies with the phylogenetic analysis of the vector bug Triatoma rubrofasciata suggested that T. conorhini might have originated in the Afrotropic-Palearctic Ecozones (Hypsa et al., 2002; Patterson et al., 2010). Interestingly, the Trypanosoma sp. isolate NanDoum1, a member of the T. conorhini clade, was detected from civet in Cameroon, the Afrotropic Ecozones (Hamilton et al., 2009). The fact that Trypanosoma sp. sequence type 1 belonging to the T. conorhini clade was detected from H. vittatus bats in Zambia in this study supports the hypothesis that the ancestor species of the T. conorhini clade might have originated in the Afrotropic Ecozones.
Both gGAPDH and 18S rRNA gene sequences of Trypanosoma sp. sequence type 2 (ZB17-108 and −109) showed 95.2% and 94.2% identities with those of T. dionisii, respectively. In the phylogenetic trees based on the concatenated gGAPDH and 18S rRNA gene sequences, the detected trypanosome formed a monophyletic group with T. dionisii distributed in the European and American continents (Hoare, 1972; Hamilton et al., 2012b). In addition, the PTP analysis suggested that Trypanosoma sp. sequence type 2 is a new putative trypanosome species in the T. dionisii clade. Recently, T. dionisii was isolated from Eastern bent-winged bats in Japan (Mafie et al., 2018), indicating that T. dionisii has a wide geographical distribution. Importantly, a human case of T. dionisii infection was reported in Brazil (Dario et al., 2016). Hence, it is particularly of interest to evaluate the pathogenicity and zoonotic potential of the Trypanosoma sp. sequence type 2.
Vectors of trypanosomes detected in the current study are not yet known. T. dionisii is mainly transmitted by bat bugs (cimicids) (Molyneux, 1991), although the contribution of other vectors such as triatomines cannot be ruled out (Dario et al., 2017). Furthermore, Trypanosoma spp. in Schizotrypanum clade were detected from a bat bug (Stricticimex brecispinosus) in Burundi, Africa (Van den Berghe et al., 1963). Thus, investigation of bat bugs and other blood-sucking insects in the cave is required to determine particular vector arthropods for the Trypanosoma spp. found in this study.
Our findings expanded the knowledge on genetic diversity of trypanosomes infesting African bats. Particularly, the first detection of the parasite in the T. conorhini clade in African bats provided important information to estimate the origin, dispersion, and speciation of the trypanosomes in the clade. The presence of the parasite in the Schizotrypanum clade is also of importance to study zoonotic potential of non-tsetse transmitted trypanosomes in the tested region. To better understanding of geographical distribution and individual speciation of trypanosomes in the T. cruzi clade, accumulation of the genetic information on trypanosomes in different locations is essential.
Conflicts of interest
The authors declare that they have no conflict of interest.
Acknowledgments
We appreciate Mr. Kenji Yokoi (Japan International Cooperation Agency) for his arrangement of the bat sampling activity. It was very kind of Dr. Keisuke Suganuma to advise us on the storage method of Trypanosoma spp. This study funded in part by the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID) [15FM0108008H0001], Japan Society for the Promotion of Science (JSPS) KAKENHI [16K19112], and the Agency for Medical Research and Development (AMED) and Japan International Cooperation Agency (JICA) within the framework of the Science and Technology Research Partnership for Sustainable Development (SATREPS) [15JM0110005H0004].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2019.04.009.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- Bern C. Chagas' disease. N. Engl. J. Med. 2015;373:456–466. doi: 10.1056/NEJMra1410150. [DOI] [PubMed] [Google Scholar]
- Büscher P., Cecchi G., Jamonneau V., Priotto G. Human African trypanosomiasis. Lancet. 2017;390:2397–2409. doi: 10.1016/S0140-6736(17)31510-6. [DOI] [PubMed] [Google Scholar]
- Calisher C.H., Childs J.E., Field H.E., Holmes K.V., Schountz T. Bats: important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 2006;19:531–545. doi: 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazzana M., Marcili A., Lima L., da Silva F.M., Junqueira A.C., Veludo H.H., Viola L.B., Campaner M., Nunes V.L., Paiva F., Coura J.R., Camargo E.P., Teixeira M.M. Phylogeographical, ecological and biological patterns shown by nuclear (ssrRNA and gGAPDH) and mitochondrial (Cyt b) genes of trypanosomes of the subgenus Schizotrypanum parasitic in Brazilian bats. Int. J. Parasitol. 2010;40:345–355. doi: 10.1016/j.ijpara.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Changula, K., Kajihara, M., Mori-Kajihara, A., Eto, Y., Miyamoto, H., Yoshida, R., Shigeno, A., Hang'ombe, B.M., Qiu, Y., Mwizabi, D., Squarre, D., Ndebe, J., Ogawa, H., Harima, H., Simulundu, E., Moonga, L., Kapila, P., Furuyama, W., Kondoh, T., Sato, M., Takadate, Y., Kaneko, C., Nakao, R., Mukonka, V., Mweene, A., Takada, A., (in press). Seroprevalence of filovirus infection of Rousettus aegyptiacus bats in Zambia. J. Infect. Dis. [DOI] [PubMed]
- Cottontail V.M., Kalko E.K.V., Cottonatail I., Wellinghausen N., Tschapka M., Perkins S.L., Pinto C.M. High local diversity of Trypanosoma in common bat species, and lmplications for the biogeography and taxonomy of the T. cruzi clade. PLoS One. 2014;9 doi: 10.1371/journal.pone.0108603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva F.M., Noyes H., Campaner M., Junqueira A.C., Coura J.R., Añez N., Shaw J.J., Stevens J.R., Teixeira M.M. Phylogeny, taxonomy and grouping of Trypanosoma rangeli isolates from man, triatomines and sylvatic mammals from widespread geographical origin based on SSU and ITS ribosomal sequences. Parasitology. 2004;129:549–561. doi: 10.1017/s0031182004005931. [DOI] [PubMed] [Google Scholar]
- Dario M.A., Lisboa C.V., Costa L.M., Moratelli R., Nascimento M.P., Costa L.P., Leite Y.L.R., Llewellyn M.S., Xavier S.C.D.C., Roque A.L.R., Jansen A.M. High Trypanosoma spp. diversity is maintained by bats and triatomines in Espírito Santo state, Brazil. PLoS One. 2017;12 doi: 10.1371/journal.pone.0188412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dario M.A., Rodrigues M.S., Barros J.H., Xavier S.C., D'Andrea P.S., Roque A.L., Jansen A.M. Ecological scenario and Trypanosoma cruzi DTU characterization of a fatal acute Chagas disease case transmitted orally (Espírito Santo state, Brazil) Parasites Vectors. 2016;9:477. doi: 10.1186/s13071-016-1754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa-Álvarez O., Ortiz P.A., Lima L., Costa-Martins A.G., Serrano M.G., Herder S., Buck G.A., Camargo E.P., Hamilton P.B., Stevens J.R., Teixeira M.M.G. Trypanosoma rangeli is phylogenetically closer to Old World trypanosomes than to Trypanosoma cruzi. Int. J. Parasitol. 2018;48:569–584. doi: 10.1016/j.ijpara.2017.12.008. [DOI] [PubMed] [Google Scholar]
- Hamilton P.B., Adams E.R., Njiokou F., Gibson W.C., Cuny G., Herder S. Phylogenetic analysis reveals the presence of the Trypanosoma cruzi clade in African terrestrial mammals. Infect. Genet. Evol. 2009;9:81–86. doi: 10.1016/j.meegid.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Hamilton P.B., Cruickshank C., Stevens J.R., Teixeira M.M., Mathews F. Parasites reveal movement of bats between the new and old worlds. Mol. Phylogenet. Evol. 2012;63:521–526. doi: 10.1016/j.ympev.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton P.B., Gibson W.C., Stevens J.R. Patterns of co-evolution between trypanosomes and their hosts deduced from ribosomal RNA and protein-coding gene phylogenies. Mol. Phylogenet. Evol. 2007;44:15–25. doi: 10.1016/j.ympev.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Hamilton P.B., Stevens J.R., Gaunt M.W., Gidley J., Gibson W.C. Trypanosomes are monophyletic: evidence from genes for glyceraldehyde phosphate dehydrogenase and small subunit ribosomal RNA. Int. J. Parasitol. 2004;34:1393–1404. doi: 10.1016/j.ijpara.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Hamilton P.B., Teixeira M.M., Stevens J.R. The evolution of Trypanosoma cruzi: the 'bat seeding' hypothesis. Trends Parasitol. 2012;28:136–141. doi: 10.1016/j.pt.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Hypsa V., Tietz D.F., Zrzavý J., Rego R.O., Galvao C., Jurberg J. Phylogeny and biogeography of triatominae (Hemiptera: Reduviidae): molecular evidence of a new world origin of the Asiatic clade. Mol. Phylogenet. Evol. 2002;23:447–457. doi: 10.1016/s1055-7903(02)00023-4. [DOI] [PubMed] [Google Scholar]
- Hoare C.A. Blackwell; Oxford: 1972. The Trypanosomes of Mammals. [Google Scholar]
- Keymer I.F. Blood protozoa of insectivores, bats and primates in Central Africa. J. Zool. Lind. 1971;163:421–441. [Google Scholar]
- Lemos M., Fermino B.R., Simas-Rodrigues C., Hoffmann L., Silva R., Camargo E.P., Teixeira M.M., Souto-Padrón T. Phylogenetic and morphological characterization of trypanosomes from Brazilian armoured catfishes and leeches reveal high species diversity, mixed infections and a new fish trypanosome species. Parasites Vectors. 2015;8:573. doi: 10.1186/s13071-015-1193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima L., Espinosa-Álvarez O., Hamilton P.B., Neves L., Takata C.S., Campaner M., Attias M., de Souza W., Camargo E.P., Teixeira M.M. Trypanosoma livingstonei: a new species from African bats supports the bat seeding hypothesis for the Trypanosoma cruzi clade. Parasites Vectors. 2013;6:221. doi: 10.1186/1756-3305-6-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima L., Silva F.M., Neves L., Attias M., Takata C.S., Campaner M., de Souza W., Hamilton P.B., Teixeira M.M. Evolutionary insights from bat trypanosomes: morphological, developmental and phylogenetic evidence of a new species, Trypanosoma (Schizotrypanum) erneyi sp. nov., in African bats closely related to Trypanosoma (Schizotrypanum) cruzi and allied species. Protist. 2012;163:856–872. doi: 10.1016/j.protis.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Mafie E., Rupa F.H., Takano A., Suzuki K., Maeda K., Sato H. First record of Trypanosoma dionisii of the T. cruzi clade from the Eastern bent-winged bat (Miniopterus fuliginosus) in the Far East. Parasitol. Res. 2018;117:673–680. doi: 10.1007/s00436-017-5717-2. [DOI] [PubMed] [Google Scholar]
- Molyneux D.H. Trypanosomes of bats. In: Kreier J.P., Baker J.R., editors. Parasitic Protozoa. Academic Press; New York: 1991. pp. 195–223. [Google Scholar]
- Morrison L.J., Vezza L., Rowan T., Hope J.C. Animal African trypanosomiasis: time to increase focus on clinically Relevant parasite and host species. Trends Parasitol. 2016;32:599–607. doi: 10.1016/j.pt.2016.04.012. [DOI] [PubMed] [Google Scholar]
- Patterson J.S., Gaunt M.W. Phylogenetic multi-locus codon models and molecular clocks reveal the monophyly of haematophagous reduviid bugs and their evolution at the formation of South America. Mol. Phylogenet. Evol. 2010;56:608–621. doi: 10.1016/j.ympev.2010.04.038. [DOI] [PubMed] [Google Scholar]
- Qiu, Y., Nakao, R., Hang’ombe, B.M., Sato, K., Kajihara, M., Kanchela, S., Changula, K., Eto, Y., Ndebe, J., Sasaki, M., Thu, M.J., Takada, A., Sawa, H., Sugimoto, C., Kawabata, H., (in press). Human borreliosis caused by a new world relapsing fever Borrelia-like organism in the old world. Clin. Infect. Dis. [DOI] [PubMed]
- Stevens J., Noyes H., Gibson W. The evolution of trypanosomes infecting humans and primates. Mem. Inst. Oswaldo Cruz. 1998;93:669–676. doi: 10.1590/s0074-02761998000500019. [DOI] [PubMed] [Google Scholar]
- Stevens J.R., Teixeira M.M., Bingle L.E., Gibson W.C. The taxonomic position and evolutionary relationships of Trypanosoma rangeli. Int. J. Parasitol. 1999;29:749–757. doi: 10.1016/s0020-7519(99)00016-8. [DOI] [PubMed] [Google Scholar]
- Takano A., Toyomane K., Konnai S., Ohashi K., Nakao M., Ito T., Andoh M., Maeda K., Watarai M., Sato K., Kawabata H. Tick surveillance for relapsing fever spirochete Borrelia miyamotoi in Hokkaido, Japan. PLoS One. 2014;8 doi: 10.1371/journal.pone.0104532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berghe L., Chardome M., Peel E. An African bat trypanosome in Stricticimex brevispinosus Usinger, 1959. J. Protozool. 1963;10:135–138. doi: 10.1111/j.1550-7408.1963.tb01650.x. [DOI] [PubMed] [Google Scholar]
- Zhang J., Kapli P., Pavlidis P., Stamatakis A. A general species delimitation method with applications to phylogenetic placements. Bioinfomatics. 2013;29:2869–2876. doi: 10.1093/bioinformatics/btt499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.