Abstract
The order Piroplasmida contains a diverse group of intracellular parasites, many of which can cause significant disease in humans, domestic animals, and wildlife. Two piroplasm species have been reported from raccoons (Procyon lotor), Babesia lotori (Babesia sensu stricto clade) and a species related to Babesia microti (called B. microti-like sp.). The goal of this study was to investigate prevalence, distribution, and diversity of Babesia in raccoons. We tested raccoons from selected regions in the United States and Canada for the presence of Babesia sensu stricto and Babesia microti-like sp. piroplasms. Infections of Babesia microti-like sp. were found in nearly all locations sampled, often with high prevalence, while Babesia sensu stricto infections had higher prevalence in the Southeastern United States (20–45% prevalence). Co-infections with both Babesia sp. were common. Sequencing of the partial 18S rRNA and cytochrome oxidase subunit 1 (cox1) genes led to the discovery of two new Babesia species, both found in several locations in the eastern and western United States. One novel Babesia sensu stricto sp. was most similar to Babesia gibsoni while the other Babesia species was present in the ‘western piroplasm’ group and was related to Babesia conradae. Phylogenetic analysis of the cox1 sequences indicated possible eastern and western genetic variants for the three Babesia sensu stricto species. Additional analyses are needed to characterize these novel species; however, this study indicates there are now at least four species of piroplasms infecting raccoons in the United States and Canada (Babesia microti-like sp., Babesia lotori, a novel Babesia sensu stricto sp., a novel western Babesia sp.) and a possible fifth species (Babesia sensu stricto) in raccoons in Japan.
Keywords: Babesia, Cryptic species, Piroplasms, Raccoons, Tick-borne pathogens
Graphical abstract

Highlights
-
•
Raccoons in all locations tested were infected with piroplasms.
-
•
Babesia microti-like sp. was commonly found in raccoons throughout North America.
-
•
Babesia sensu stricto spp. were less common throughout North America.
-
•
Four, possibly five, distinct species of piroplasms in raccoons.
-
•
Possible spatial genetic variation within the two raccoon piroplasm species.
1. Introduction
The order Piroplasmida contains a diverse group of intracellular parasites, many of which can cause significant disease in humans, domestic animals, and wildlife. Historically, these parasites were identified based on their host or morphologic characteristics (i.e. shape or size), but recent molecular studies have revealed a much greater host range and diversity than previously recognized (Gray et al., 2002; Criado-Fornelio et al., 2003; Allsopp and Allsopp, 2006; Hunfeld et al., 2008; Lack et al., 2012; Schnitteger et al., 2012). Piroplasmida currently include several genera, e.g., Cytauxzoon, Theileria, and Babesia, with the latter genus being polyphyletic and comprising three distinct clades (Babesia sensu stricto, Western Babesia group, and Babesia microti/related small Babesia group) (Criado-Fornelio et al., 2003; Allsopp and Allsopp, 2006; Lack et al., 2012; Schnittger et al., 2012; Schreeg et al., 2016).
A high diversity of Babesia spp. and related piroplasms have been reported in wildlife and some populations have a high prevalence in certain regions (e.g., Northern raccoon (Procyon lotor), Florida puma (Puma concolor coryi), and North American river otter (Lontra canadensis)), but relatively little is known about the natural history, geographic distribution, and diversity of Babesia spp. in these hosts (Birkenheuer et al. 2006, 2007a, 2007b; Yabsley et al., 2006). To date, two species of piroplasms have been reported from raccoons: Babesia lotori (in the Babesia sensu stricto [B. s. s.] group) and a Babesia sp. closely related to Babesia microti (called a Babesia microti-like sp.) (Frerichs and Holbrook, 1970; Anderson et al., 1981; Goethert and Telford, 2003; Kawabuchi et al., 2005; Birkenheuer et al., 2007a; Jinnai et al., 2009; Beltran-Beck et al., 2012). Many studies of piroplasms in raccoons relied on morphologic identification of the parasite, which lacks the sensitivity as well as specificity needed to discern the two morphologically similar B. spp. However, a molecular study conducted in raccoons in North Carolina detected both B. spp. at a high prevalence, and coinfections were common (Frerichs and Holbrook, 1970; Anderson et al., 1981; Goethert and Telford, 2003; Birkenheuer et al. 2006, 2007a).
The nomenclature of piroplasms in raccoons is historically complicated. Piroplasms initially detected in raccoons were reported as Babesia procyoni or Babesia procyonis; however, a large B. sp. (>3 μm) of Eurasian raccoon dogs (Nyctereutes procyonoides) was concurrently named Babesia procyoni (Frerichs and Holbrook, 1970; Anderson et al., 1981; Birkenheuer et al., 2006). To address this issue, Anderson et al. (1981) described the smaller parasite found in raccoons as B. lotori. Subsequent molecular studies detected a B. microti-like species, which currently has no official nomenclature and is reported as B. microti-like sp., B. microti, B. cf. microti, or a B. sp. in the “microti-like in carnivores” clade (Goethert and Telford, 2003; Birkenheuer et al., 2007a). In Japan, where raccoons are non-native, molecular testing has revealed a third novel B. sp. that is in the B. s. s. group, although researchers also detected B. microti-like sp. and a Babesia species similar to B. lotori (Jinnai et al., 2009).
For B. spp. with known life cycles, ticks, primarily ixodids, are the vectors; however, other methods of transmission include vertical (mother to young), biting and fighting, and blood transfusions (Uilenberg, 2006; Hunfeld et al., 2008; Yeagley et al., 2009; Schnittger et al., 2012; Binda, 2016; Yabsley and Shock, 2013; Mierzejewska at al. 2014; Bednarska et al., 2015; Tolkacz et al., 2017; Tufts and Diuk-Wasser, 2018). Currently potential tick vector species for any B.spp. from raccoons in the United States and Japan are unknown.
Recently, a captive maned wolf (Chrysocyon brachyurus) in a zoo from Missouri was seen fighting with a wild raccoon and was later diagnosed with B. lotori, a species of Babesia found in raccoons (Schnellbacher unpublished). Another maned wolf in a zoo in Kansas was also recently reported to suffer from babesiosis associated with B. lotori (Wasserkrug Naor et al., 2019). An additional study on piroplasms in young raccoons from Minnesota had evidence of clinical disease (splenomegaly) associated with B.s.s. infections in young raccoons (Garrett et al., 2018). Thus, parasites of raccoons are of particular interest because of this evidence of possible disease in raccoons and other hosts coupled with a large natural geographic range within North America, established introduced populations in numerous European and Asian countries, and their ability to utilize a diversity of habitat types, including both urban and suburban areas. Given the limited data on piroplasms in raccoons, our primary objective in this study was to investigate the prevalence and distribution of piroplasms in raccoons at selected sites in the United States and Canada. These data could be used to identify areas of risk for exotic animals that may become infected with B. lotori, such as the maned wolf. Also, because a vector is not known for either piroplasm species of raccoons, distribution data for these parasites could be compared with vector distributions to suggest candidate vectors. We also amplified and sequenced partial 18S rRNA and cytochrome oxidase subunit I (cox1) genes to evaluate the species diversity of piroplasms in raccoons and document the intra-specific variation of these piroplasms from different geographic regions.
2. Methods
2.1. Sample collection
Samples were opportunistically obtained from raccoons from various locations in the United States (i.e., California, Colorado, Florida, Georgia, Louisiana, Minnesota, Missouri, North Carolina, South Carolina, Pennsylvania, Idaho, Texas, West Virginia) and Canada (i.e., Ontario and Nova Scotia). Samples were obtained from rehabilitation facilities, zoos, veterinary clinics, wildlife biologists, diagnostic laboratories, and others from June 2015–May 2018. Sample type varied, but most samples consisted of either whole blood or spleen. Samples were stored at −20 C until DNA extraction. No animals were euthanized for the purposes of this study but the collection of biological samples for pathogen testing was reviewed and approved by the University of Georgia Institutional Animal Care and Use Committee (A2014 10–018).
2.2. Molecular testing
Genomic DNA was extracted from ∼10 mg of tissue or 100 μl of whole blood using a commercial kit per manufacturer's instructions (DNeasy Blood and Tissue kit, Qiagen, Hilden, Germany). Two different screening polymerase chain reaction (PCR) assays were employed to amplify the V4 region of the 18S rRNA gene of either the B. microti-like spp. group or B. s. s. spp. group (Table 1). The size of amplicons was carefully examined because it has been reported that, due to sequence similarity within the primer-binding region, the B. microti-like spp. and the western Babesia group may be amplified with the B. s. s. primer set if there was a high number of B. microti-like copies, but the amplicon size was 371bp instead of the expected 341bp (Birkenheuer et al., 2003, 2007a). Samples were amplified in a BioRad DNA Engine Peltier Thermal Cycler (Bio-Rad Laboratories Incorporated, Foster City, CA) using the protocols shown in Table 1.
Table 1.
Information on primers used for PCR analyses.
| Species | Region | Primer | Amplicon Size | Sequence (5′-3′) | Reference |
|---|---|---|---|---|---|
| Babesia sensu stricto | V4 of 18S | 455–479 F | 341bp (*371) | GTCTTGTAATTGGAATGATGGTGAC | Birkenheuer et al. (2007a) |
| 793-772 R | ATGCCCCCAACCGTTCCTATTA | ||||
| Babesia microti and Babesia microti-like sp. | V4 of 18S | Bmlike F | 229bp | CTGCCTTATCATTAATTTCGCTTCCGAACG | Birkenheuer et al. (2007a) |
| 793-772 R | ATGCCCCCAACCGTTCCTATTA | ||||
| All Babesia spp. | cox1 | Babcox1 F | 1080bp | GGAAGTGGWACWGGWTGGAC | Aktas and Ozubek (2017) |
| Babcox1 R | TTCGGTATTGCATGCCTTG | ||||
| All Babesia spp. | 18S rRNA | 5–22 F | 1655bp | GTTGATCCTGCCAGTAGT | Birkenheuer et al. (2007a) |
| 1661 R | AACCTTGTTACGACTTCTC |
*, when high concentrations of B. microti-like sp. or western piroplasm group species are present, they may amplify with this primer set but the amplicon is 371 bp instead of the expected 341 bp.
To determine phylogenetic relationships, sequencing of near full-length 18S rRNA genes was conducted on a random set of samples within each geographic region (Table 1). To investigate intraspecific variation between regions, amplification of the cytochrome c oxidase subunit 1 (cox1) gene was also conducted (Table 1).
To augment representative cox1 sequences available from the western Babesia group, we obtained new sequences from a B. sp. from a fallow deer (Dama dama) (FD-879) from California, B. spp. from three spotted hyenas (Crocuta crocuta) (RACH6, Rachhym, and Waterhym) and a lion (Panthera leo) (H1 and Hty-3F) from Zambia, and Theileria youngi from a dusky-footed woodrat (Neotoma fuscipes) from California (Williams et al., 2014; Kauffmann et al., 2017).
Standard precautions were taken to prevent or detect contamination; i.e., performance of DNA extraction, PCR reaction setup, and product analysis in distinct and designated laboratory areas. Negative water controls were included in each set of DNA extractions and for each set of PCR reactions. Positive controls included samples with sequence confirmed B. lotori-like and the B. microti-like species. Amplicons were visualized in a GelRed (Biotium Fremont, CA) stained 1.5% agarose gel.
2.3. Sequencing and phylogenetic analysis
Due to large sample sizes and restricted funding available for sequencing costs, a subset of representative amplicons from each geographic region were purified from gels using a commercial gel-purification kit (QIAquick gel extraction kit, Qiagen) and bi-directionally sequenced at the University of Georgia Genomics Facility (Athens, GA). Chromatograms were analyzed using Geneious (Biomatters Limited, Auckland, New Zealand –Version 11.1.5). Sequences were aligned in MEGA (Molecular Evolutionary Genetics Analysis – Version 7.0.21), visually analyzed, and cropped to include as many sequences as possible. Because multiple sequences were identical, only a single unique sequence from each group was included in our analysis. Sequences in GenBank were included as representatives from each group of piroplasms for comparison based on previous studies of piroplasms in raccoons and other carnivores (Supplemental Table 1) (Kjemtrup et al. 2000, 2001, 2006; Yabsley et al., 2006; Jinnai et al., 2009; Baneth et al., 2015; Schreeg et al., 2016). Percent identities for the 18S and cox1 sequences were calculated using the distance estimation analysis in MEGA using the Tamura 3-parameter model and pairwise deletion. Phylogenetic trees were constructed using the maximum likelihood algorithm in MEGA. Plasmodium falciparum was used as an outgroup, similar to that of other phylogenetic studies on Piroplasmida (Criado-Fornelio et al., 2003; Schreeg et al., 2016).
3. Results
3.1. Prevalence and distribution of the two piroplasm groups
Based on the initial screening PCR targeting the 18S rRNA gene, the prevalence of any Babesia infection in raccoons across all sites was 73.2% [512/699] (95% CI: 70.9–75.6) (Table 2). Among these, 70.1% [490/699] (95% CI: 67.3–72.9) of raccoons were positive for B. microti-like sp. infections, 24.3% [170/699] (95% CI: 21.6–27.0) were positive with the B. s. s. species assay, and 21.6% [151/699] (95% CI: 19.0–24.2) were positive with both PCR assays (Table 2).
Table 2.
Comparison of the prevalence of Babesia infections in raccoons by state. The number of infected raccoons and total raccoons sampled for each location are displayed in parentheses and the 95% confidence interval is in brackets.
| Region (Country) | Location | B. microti-like sp. | Babesia species sensu stricto | Coinfection | Total Babesia Infection |
|---|---|---|---|---|---|
| Southeast (USA) | Georgia | 84% (163/194) [78.8–89.2] | 41% (80/194) [34.2–48.2] | 39% (76/194) [32.3–46.1] | 86% (167/194) [81.2–91.0] |
| Southeast (USA) | Florida | 99% (70/71) [95.8–101.4] | 44% (31/71) [32.0–55.3] | 44% (31/71) [32.0–55.3] | 99% (70/71) [95.8–101.4] |
| Southeast (USA) | West Virginia | 60% (21/35) [43.5–76.5] | 23% (8/35) [8.7–37.0] | 14% (5/35) [2.5–26.1] | 69% (24/35) [52.9–84.2] |
| Southeast (USA) | Pennsylvania | 87% (47/54) [78.8–96.1] | 17% (9/54) [6.6–26.7] | 17% (9/54) [6.6–26.7] | 87% (47/54) [78.8–96.1] |
| Midwest (USA) | Minnesota | 67% (14/21) [45.6–87.4] | 5% (1/21) [-4.6-14.1] | 5% (1/21) [-4.6-14.1] | 67% (14/21) [45.6–87.4] |
| Midwest (USA) | Idaho | 0% (0/4) [0-0] | 25% (1/4) [20.3–29.7] | 0% (0/4) [0-0] | 25% (1/4) [20.3–29.7] |
| West (USA) | Texas | 79% (22/28) [89.4–103.4] | 36% (10/28) [20.8–57.8] | 36% (10/28) [20.8–57.8] | 79% (22/28) [89.4–103.4] |
| West (USA) | Colorado | 88% (7/8) [62.9–112.1] | 13% (1/8) [-12.1–37.1] | 13% (1/8) [-12.1–37.1] | 88% (7/8) [62.9–112.1] |
| West (USA) | California | 39% (13/33) [22.4–56.4] | 27% (9/33) [11.8–42.7] | 15% (5/33) [2.7–27.6] | 52% (17/33) [34.1–68.9] |
| Midwest (USA) | Missouri | 87% (20/23) [74.8–101.1] | 22% (5/23) [4.5–39.0] | 13% (3/23) [-1.1-27.2] | 96% (22/23) [90.5–100.8] |
| Southeast (USA) | South Carolina | 86% (24/28) [72.5–98.9] | 4% (1/28) [-3.4-10.6] | 4% (1/28) [-3.4-10.6] | 86% (24/28) [72.5–98.9] |
| Southeast (USA) | North Carolina | 100% (24/24) [100-100] | 38% (9/24) [17.7–57.3] | 17% (4/24) [1.4–31.9] | 100% (24/24) [100-100] |
| Midwest (USA) | Louisiana | 46% (29/63) [33.6–58.5] | 2% (1/63) [-1.5–4.7] | 2% (1/63) [-1.5–4.7] | 46% (29/63) [33.6–58.5] |
| East (Canada) | Ontario | 100% (31/31) [100-100] | 13% (4/31) [0.9–24.9] | 13% (4/31) [0.9–24.9] | 100% (31/31) [100-100] |
| East (Canada) | Nova Scotia | 6% (5/80) [0.9–11.6] | 0% (0/80) [0-0] | 0% (0/80) [0-0] | 6% (5/80) [0.9–11.6] |
| Total | 70% (490/699) | 24% (170/699) | 22% (151/699) | 73% (512/699) |
Generally, the prevalence of the B. s. s. group was high in the southeastern/Mid-Atlantic United States (20–40%), and low in the Midwestern United States/Ontario (Canada) (4–20%) and the western United States (0–30%) (Table 2). Babesia microti-like sp. infections were common across all study sites (40–100%), except in Nova Scotia where only 6% [5/80] were positive and Idaho where there were no B. microti-like sp. infections (although for the latter, sample size was low [n = 4]) (Table 2).
3.2. Genetic characterization
A subset of random positive samples (n = 84) were selected for sequence analysis to investigate species diversity and variation across the geographical range included in this study. Of these, 43 samples had high quality sequences for one or more gene targets (Table 3). Results of the PCR screening assays were not always concordant with sequencing results, and some samples amplified as different species based on the gene targeted (e.g., GA-02 was positive for a B. s. s. based on 18S rRNA screening assay, but instead of a B. s. s. piroplasm, a western group piroplasm was amplified using cox1 target) (Table 3).
Table 3.
Results of the screening PCR compared to results for the 18S and cox1 gene sequencing results.
| Sample ID | Reference Sample ID | 18S rRNA Screening PCR (n = 43) |
18S rRNA sequence results (n = 17) |
cox1 sequencing results (n-40) |
|
|---|---|---|---|---|---|
| B. microti-like sp. | Babesia sensu stricto | Species (aSF, bND, c-) | Species (aSF, bND, c-) | ||
| GA-01 | Rac 2 | + | + | B. microti-like | – |
| GA-02 | Rac 008 | + | + | SF | Western group |
| GA-03 | Rac 122 | + | – | B. microti-like | Western group |
| GA-04 | Rac 123 | + | + | ND | B. microti-like |
| GA-05 | Rac 126 | + | + | SF | B. microti-like |
| GA-06 | Rac 169 | + | + | SF | B. microti-like |
| FL-01 | FL 1 | + | + | B. microti-like | – |
| FL-02 | FL 11 | + | – | ND | B. microti-like |
| FL-03 | FL 13 | + | + | ND | B. microti-like |
| FL-04 | FL 16 | + | – | ND | B. microti-like |
| FL-05 | FL 31 | + | + | SF | B. microti-like |
| FL-06 | FL 32 | + | – | ND | B. microti-like |
| WV-01 | WV 3 | + | – | ND | B. microti-like |
| WV-02 | WV 10 | – | + | Novel Babesia sp. | B. lotori-like |
| WV-03 | WV 14 | + | – | B. microti-like | B. microti-like |
| WV-04 | WV 16 | + | + | ND | B. lotori-like |
| PA-01 | PA-1 | + | – | B. microti-like | Novel Babesia sp. |
| PA-02 | PAF-6 | + | + | ND | B. lotori-like |
| PA-03 | PAF-9 | + | + | B. lotori-like | B. lotori-like |
| PA-04 | PAF-12 | + | + | ND | B. lotori-like |
| MN-01 | 15–7455 | + | – | B. microti-like | Novel Babesia sp. |
| MN-02 | 15–7986 | + | – | ND | B. microti-like |
| MN-03 | 15–11512 | + | – | SF | B. microti-like |
| MO-01 | MOI | + | + | ND | B. microti-like |
| MO-02 | Rac I | + | + | ND | B. microti-like |
| MO-03 | Rac L | + | – | B. microti-like | B. microti-like |
| MO-04 | Rac P | – | + | B. lotori-like | B. lotori-like |
| MO-05 | Rac Q | + | + | SF | B. lotori-like |
| TX-01 | TX 2 | + | + | ND | B. microti-like |
| ONT-01 | W1110-15 | + | + | ND | B. microti-like |
| ONT-02 | W504-15 | + | + | SF | B. microti-like |
| ONT-03 | W507-15 | + | + | Novel Babesia sp. | Novel Babesia sp |
| ID-01 | ID 2 | – | + | ND | B. lotori-like |
| CO-01 | CO 8 | + | + | Novel Babesia sp. | Novel Babesia spSF |
| CA-01 | SM 1 | + | + | SF | B. lotori-like |
| CA-02 | SM 2 | – | + | ND | B. lotori-like |
| CA-03 | SM 3 | + | – | B. microti-like | B. microti-like |
| CA-04 | Son 4 | – | + | B. lotori-like | B. lotori-like |
| CA-05 | SF 2 | + | – | ND | B. microti-like |
| CA-06 | CC3 | + | + | ND | B. microti-like |
| CA-07 | Mar 1 | + | – | B. microti-like | SF |
| CA-08 | Mar 3 | + | – | B. lotori-like | B. lotori-like |
| CA-09 | Mar 6 | + | + | SF | B. lotori-like |
SF, sequence failed (this sample was PCR positive but the sequence failed).
ND, not done (samples was not run for this PCR).
Sample negative for PCR.
3.3. 18S rRNA sequences
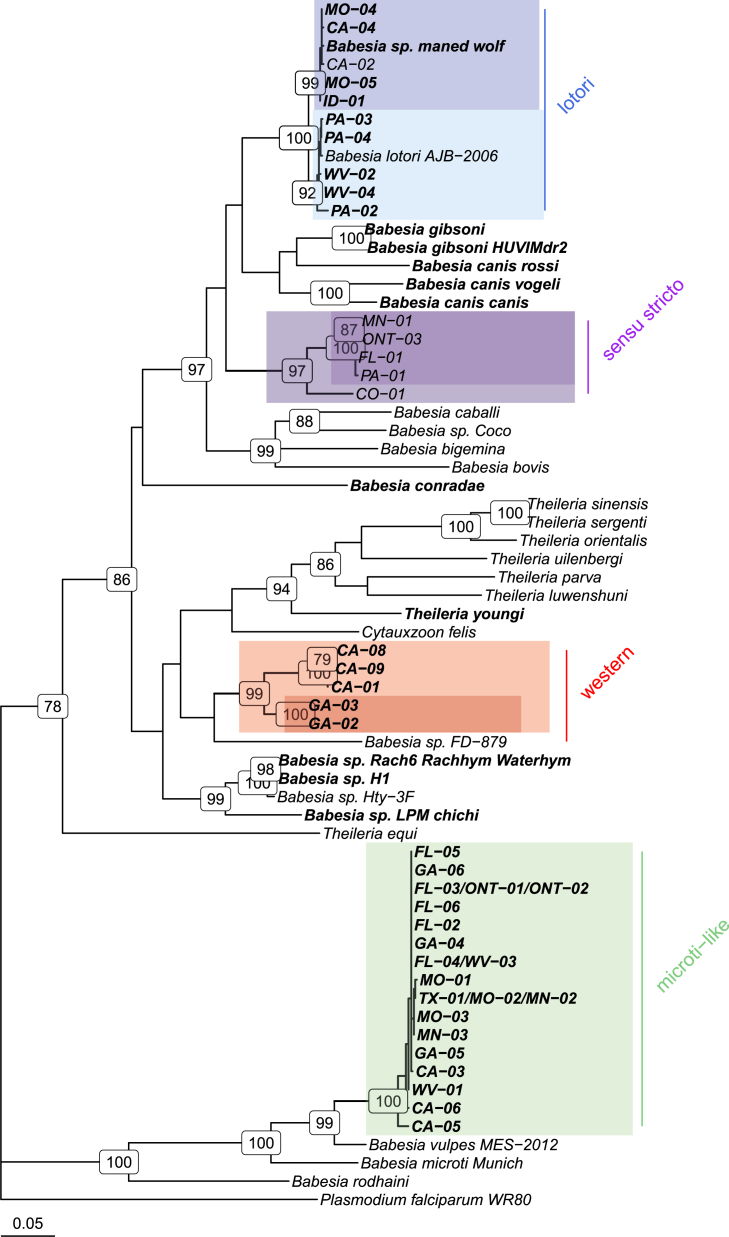
Phylogenetic analysis of 17 samples that had near-full length sequence (minimum of 1655 bp) of 18S rRNA gene revealed four distinct groups of piroplasms in raccoons (Fig. 1). Seven samples grouped with numerous sequences of the B. microti-like sp. from raccoons from the United States and Japan (Fig. 1 [green clade]). This clade was distinct from B. microti-like species from other carnivores (e.g., raccoon dog (Nyctereutes procyonoides), red fox (Vulpes vulpes), river otter (Lontra canadensis), badger (Taxidea taxus)) as well as B. microti sequences from humans and rodents. Intraspecific variation among the B. microti-like sp. from raccoons, regardless of geographic country/region of origin, was low (98.6–100% identity) (Supplemental Table 2). Similarity of this raccoon B. microti-like sp. compared to the B. microti-like sp. from other carnivores was much lower (95–98% identity) (Supplemental Table 2). One sequence from California (CA-08) was included in the western Babesia clade and grouped with Babesia conradae and several Babesia lengau strains (96.7–97.1%) (Fig. 1 [red]). The remaining six sequences were in a large clade with B. lotori that had low bootstrap support (48%) for division into two clades (Fig. 1 [purple and blue]). The purple clade contained sequences from West Virginia (USA), Colorado (USA), and Ontario (Canada) and five previously published sequences from Japan. The blue clade contained B. lotori from a raccoon in Illinois (USA) (DQ028958, Birkenheuer et al., 2006), a B. sp. from a captive maned wolf (KR017880, Wasserkrug Naor et al., 2019), and raccoons from our study from Pennsylvania, Missouri, and California (USA). Sequences within the purple and blue clades were 99% similar to one another. A separate group of previously noted novel Babesia sensu stricto species reported in raccoons in Japan (Jinnai et al., 2009) was also supported in our phylogenetic analysis, but none of our sequences from the USA were contained within that group (Fig. 1 [orange]). This group of Japanese Babesia was 96.5–97.5% similar to the other B. s. s. sequences from raccoons.
Fig. 1.
Phylogenetic analysis of piroplasms based on the 18S rRNA sequences. The purple clade represents the Babesia sensu stricto species in raccoons in Japan and representatives from the United States from our sampling, the blue clade is Babesia lotori in raccoons in the United States, the orange clade represents a separate Babesia sensu stricto species in Japan, the red clade represents our western Babesia sp. in raccoons, and the green clade is the Babesia microti-like sp. in raccoons. Bolded samples are new sequences from this study. Additional geographic data and Genbank accession numbers for each sample is provided in Supplemental Table 1. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.4. cox1 gene sequences
We obtained cox1 sequences (minimum of 1080 bp) from 40 samples; 19 were from the B. s. s. group samples and 21 from B. microti-like sp. samples. Phylogenetic analysis of these sequences resulted in four distinct groups of piroplasms infecting raccoons (Fig. 2). Three of these clades were the same as our 18S rRNA gene analysis but we had one additional well supported clade that was distinct from the B. lotori clade (Fig. 2 [purple]).
Fig. 2.
Phylogenetic analysis of piroplasms based on the cox1 assay. The blue clade is Babesia lotori in raccoons in the United States by geographic variation (light blue and dark blue), the purple clade represents a separate Babesia sensu stricto species in the United States by geographic variation (light purple and dark purple), the red clade represents our western Babesia sp. in raccoons by geographic variation (light red and dark red), and the green clade is the Babesia microti-like sp. in raccoons. Bolded samples are new sequences from this study. Geographic data and Genbank accession numbers for each sample is provided in Supplemental Table 1. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Similar to the 18S rRNA gene analysis, the B. microti-like sp. group was a well-supported clade (100%) that grouped separately from Babesia vulpes in red foxes and B. microti in humans. Although there was more intraspecific variation among the B. microti-like sp. (97–100% identity) compared to our 18S rRNA analysis, there was no phylogenetic grouping by geographic origin; there were no cox1 sequences from B. microti-like sp. available from Japan for comparison. Babesia vulpes, the most similar species to the raccoon B. microti-like sp., was only 90% similar to the cox1 sequences (Fig. 2 [green]; Supplemental Table 3).
Five sequences were included in the western Babesia clade. Our 18S analysis suggested that a raccoon piroplasm sequence from California was related to B. conradae and B. lengau, but there were no cox1 sequences from this group available for comparison. Therefore, we supplemented with sequences from several samples that had previously been included in the western piroplasm clade based on 18S rRNA analysis (Williams et al., 2014; Kauffmann et al., 2017). The Babesia sequences from raccoons were most similar to sequences of B. lengau in spotted hyenas (RACH6, Rachhym and Waterhym) and a lion from Zambia (H1 and Hty-3F) (79–81% identity) followed by Babesia in a fallow deer (FD-897) (79–80% identity) (Supplemental Table 3). Phylogenetically, these raccoon Babesia sequences were in a clade with the deer piroplasm sequence that was a sister clade to the B. lengau strains, although bootstrap support for clades within this group were low (<80%). Interestingly, there was high support (99%) for geographic variation within the raccoon western Babesia group, with the three California raccoon Babesia samples being separate from the two Georgia raccoon Babesia samples (Fig. 2 [light and dark red, respectively]). The intraspecific similarity within the two geographic regions was high (99–100% identity), with lower interspecific similarity between the two geographic regions (90–90.3%) (Supplemental Table 3).
The remaining sequences formed two clades. As shown in Fig. 2 (blue and purple), the two clades are well separated; however, the placement of these clades is poorly supported (46% bootstrap). Within the B. lotori group, there was high support (100%) for geographic variation with eastern samples from Pennsylvania (USA), West Virginia (USA), and Illinois (USA) being a sister group to sequences from Missouri (USA), Idaho (USA), and California (USA) (Fig. 2 [dark blue and light blue, respectively]). However, the percent identity between these two groups was high (97–99%). The other B. s. s. sp. group had high variability within the group (91–100% identity) but this was also due to the apparent geographic variation with one sample from Colorado (USA) being separated from the other Babesia sequences from Pennsylvania (USA), Minnesota (USA), and Ontario (Canada) (Fig. 2 [light purple and dark purple, respectively]; Supplemental Table 3). The Colorado sequence only had 91% identity to other samples within this group.
4. Discussion
We found that raccoons throughout much of the USA and Canada are infected with piroplasms and, in some areas, prevalence is high and coinfections with multiple B. sp. are common. Previously, Babesia infections were reported in raccoons from multiple locations in the United States; however, few of these studies used molecular assays to distinguish piroplasm species (Anderson et al., 1981; Telford and Forrester, 1991; Birkenheuer et al. 2006, 2007a). Prior molecular studies indicated raccoons could be infected with two piroplasms (B. lotori and B. microti-like sp.) and coinfections were common in one state (North Carolina) (Birkenheuer et al., 2007a). Because these two species are morphologically similar, molecular analysis is required to differentiate them; however, our data combined with other molecular-based studies now indicate raccoons may harbor five distinct species, four of which are present in raccoons from the USA and another that occurs in introduced raccoons in Japan. In addition, there is genetic variation within three of these species that resulted in separate clades based on geographic origin. Currently, morphological data are not available for the western Babesia or novel B. s. s. sp. groups. Thus, future efforts to combine morphologic and molecular studies on piroplasms of raccoons are needed to better understand the diversity of this parasitic group in raccoons.
We found the most common and widespread piroplasm in raccoons was the B. microti-like sp. This species was detected in all sampled states and provinces except Idaho, where the sample size was small (n = 4). Previously, this piroplasm species had been reported from a single raccoon in Massachusetts and at a high prevalence (82–83%) in surveys in North Carolina and Florida (Goethert and Telford, 2003; Birkenheuer et al., 2007a; Clark et al., 2012). Similarly, we found a high prevalence (67–100%) in the southeastern United States, although we confirmed infections at a number of locations in the midwestern and western states and the two Canadian provinces sampled.
The B. microti group is diverse and includes parasites of rodents and humans as well as numerous carnivore species. There is mounting evidence that the B. microti group should be included in a distinct genus separate from Babesia (which should be retained for B. s. s. representatives) and that members of this group appear to have a high degree of host-specificity (Criado-Fornelio et al., 2003; Allsopp and Allsopp, 2006; Lack et al., 2012; Schnittger et al., 2012; Yabsley and Shock, 2013; Schreeg et al., 2016). Our 18S rRNA and cox1 gene data indicate the parasites that infect raccoons are clearly distinct from the B. microti-like species that infect other carnivores. Also, the intraspecific variability of the raccoon B. microti-like sp. was low with 98–100% sequence identity for the 18S rRNA gene and 97–100% sequence identity for the cox1 gene. However, at these loci, this group was at least 10% different from the closest available cox1 sequence from B. vulpes from red foxes and dogs in Europe (Baneth et al., 2015). Based on 18S rRNA gene analysis, we found no separation among the sequence from raccoons in the USA or Japan, but analysis of additional gene targets would be ideal to better assess for variation within the group, including the cox1 gene in Japanese samples that were unavailable for comparison (Jinnai et al., 2009). Japanese researchers have sequenced the chaperonin-containing t-complex polypeptide l (CCTη) gene for several B. microti-like samples, including one sample from a Japanese raccoon, but this gene target has not been sequenced from USA or Canadian samples (Nakajima et al., 2008).
In an effort to clarify this group of B. microti-like species, Baneth et al. (2015) recently proposed the name B. vulpes for a B. sp. of dogs and red foxes that had previously been published under numerous names including Babesia Spanish dog isolate, B. microti-like, B. annae, Theileria annae, and B. cf. microti. Although we believe there is sufficient evidence for the raccoon B. microti-like species to be a unique species that appears to be specific to raccoons, we have chosen not to propose a new name until a holotype can be obtained and deposited as required by the International Code of Zoological Nomenclature (Harris, 2016). In addition, in keeping with tradition of this group, the new species would be in the genus Babesia; however, numerous analyses, including our own, indicate this B. microti group, as well as other related small babesids, should be reclassified into a new genus (Criado-Fornelio et al., 2003; Allsopp and Allsopp, 2006; Lack et al., 2012; Schnittger et al., 2012; Yabsley and Shock, 2013; Schreeg et al., 2016).
Our initial screening results based on 18S screening PCR found the B. s. s. group was most common in raccoons in the southeastern US, but infections were noted at all sampled areas except Nova Scotia (Canada). We assumed these positives were B. lotori, the only other Babesia sp. reported from raccoons in the USA, or the novel B. sp. reported from raccoons in Japan (although it is unknown if the Babesia in Japanese raccoons was from the USA or was acquired by raccoons after their introduction to Japan) (Anderson et al., 1981; Birkenheuer et al. 2006, 2007a; Jinnai et al., 2009; Clark et al., 2012). Sequence analysis of selected samples from our study showed there were three species of Babesia being amplified by the B.s.s. screening PCR. A recent study on piroplasms in young raccoons also show similar evidence, with multiple species of B. s. s. being present in young raccoons, and a possible association of disease (splenomegaly). However, due to low sample sizes of the various B. s. s. species in young raccoons there was insufficient data to assess which B. s. s. species was causing splenomegaly in the young raccoons (Garrett et al., 2018). The data from both this study and Garrett et al., (2018) highlight the need to conduct sequence analysis when using genus- or group-wide molecular assays to confirm or identify the species detected.
One of the B. s. s. groups we detected included B. lotori, previously only noted to occur in raccoons in Illinois and North Carolina (Birkenheuer et al. 2006, 2007a). However, recently a genetically similar genotype was also reported in two sick captive maned wolves (Chrysocyon brachyurus), one from Kansas and one from Missouri (KR017880, Wasserkrug Naor et al., 2019; Schnellbacher unpublished). Interestingly, based on cox1 phylogenetic analysis, there was evidence of geographic differences among the B. lotori-like sp. sequences. One group included the original B. lotori sequence from Illinois (Birkenheuer et al., 2006) as well as sequences in our study from Pennsylvania and West Virginia. The other group included the B. sp. from the maned wolf, as well as sequences from the western and central United States, including Missouri, Idaho, and California. The eastern and western groups were closely related to each other (96–99% similar) but the within-group sequence similarity was higher (98.7–99.8%). The mechanisms of this geographic variation are unknown but could be related to different possible tick vectors specific to these regions (although there are some tick vectors such as I. texanus that occur throughout North America), or through variation that developed within separate raccoon populations (Dennis et al., 1994; Pung et al., 1994; Ouellette et al., 1997; Yabsley et al., 2008). Currently there are numerous subspecies of raccoons recognized in North America, but more research is needed to reveal if certain host subspecies may respond differently to these genetic variants of Babesia (Lotze et al., 1979). Although additional data are needed to better understand the genetic diversity within the B. lotori-group, at this time the eastern and western genetic variants appear to be a single species (B. lotori). Of note is that B. lotori appears to lack host specificity for raccoons; thus, some species, such as the maned wolf, may be at risk if they become infected.
Within the B. s. s. group, there was a probable novel species that was closely related to B. lotori. The 18S rRNA analysis showed that these samples from West Virginia (USA), Colorado (USA), and Ontario (Canada) were closely related to several sequences from raccoons in Japan (Jinnai et al., 2009). Although, bootstrap support for separation of this novel B. s. s. group from B. lotori was low for both gene analyses (46%), the groups were only 81–83% similar based on cox1 sequences, suggesting they likely represent a distinct species depsite the unresolved phylogenetic relationships. Unfortunately, we were unable to include the Japanese samples for the cox1 gene analysis, as sequencing of this gene has not been conducted; however, based on our 18S rRNA data, this group of B. spp. that had previously been reported in Japan likely originated from the USA.
Data from Jinnai et al. (2009) indicated there was a novel clade of Babesia in raccoons in Japan. In our 18S analysis, this group remained distinct and none of our USA or Canadian samples occurred in this group. Thus, the origin of this group of Babesia is currently unknown. This species could be endemic to North America and we failed to detect it due to low samples sizes at some of our sampled areas, or we simply did not sample where this parasite was present. However, the four groups of Babesia we did detect were found in the eastern and western US, suggesting these parasites are not geographically isolated or restricted. It is also possible this novel Babesia group is native to Japan and now infects raccoons. Additional surveillance of possible Babesia hosts in Japan may ultimately discover the natural native host.
The final group of Babesia we detected belonged to the group known as the ‘western piroplasms’ or ‘western Babesia’, named because members of this group were first detected in various host species in California (Kjemtrup et al., 2006). The western Babesia group is a relatively new group that includes species of medical and veterinary importance, but these species are poorly understood relative to their natural history. Thus far, only one vector, Dermacentor albipictus, has been identified as a likely candidate for Babesia duncani, but no other known vectors have been identified for other members of this group (Swei et al., 2019). Phylogenetically, this group is most similar to Theileria and Cytauxzoon spp.; however, the members of this group that have been named were included in the genus Babesia, e.g., B. duncani, B. conradae, B. lengau, B. poelea, and others (Criado-Fornelio et al., 2003; Kjemtrup et al., 2006; Yabsley et al., 2006; Schreeg et al., 2016). Not only do phylogenetic data on numerous gene targets suggest that this group is not within the Babesia genus, but the mitochondrial genome structure of B. conradae supports this group as a novel genus (Schreeg et al., 2016). Our data show that raccoons in Georgia and California are infected with a parasite within this group, but their phylogenetic relationships to other species remain unresolved in both 18S rRNA and cox1 gene analyses. The cox1 sequence data indicated there is geographic separation of the Georgia and California sequences, consistent with the other two B. s. s. groups. There was 9.7–10% difference in the cox1 sequences of these two geographic groups as well as 100% bootstrap support for separation. In recent years, numerous new species have been identified from this group, but hopefully as more sequences are included in future analyses, the relationships within this diverse group will clarify (Kjemtrup et al., 2000; Yabsley et al., 2006; Schreeg et al., 2016). Further analysis of multiple gene targets and morphologic features are needed before a definitive new species can be named within this group.
Currently there are no known transmission routes for any piroplasm species in raccoons; however, all Babesia spp. with known life cycles are transmitted by tick vectors, typically ixodid ticks, although several Babesia species can be transmitted by fighting (Yeagley et al., 2009). If the B. microti-like species is transmitted by ticks, either the vector(s) must be widespread or the parasite must be able to use multiple species of ticks due to the large geographic range that we noted for the B. microti-like sp. The other three B. spp. detected in raccoons from the USA. and Canada occurred in much lower prevalences but they were all also geographically widespread. Most work on tick communities in raccoons has been conducted in the eastern USA, where multiple species have been found. These include Ixodes texanus, I. scapularis, I. affinis, Dermacentor variabilis, Amblyomma americanum, and A. maculatum, with I. texanus being of interest as it is not only a common nest species of raccoons, but it also has a wide distribution, ranging from the eastern USA. to the west coast and Alaska (Anderson and Magnarelli, 1980; Dennis et al., 1994; Pung et al., 1994; Ouellette et al., 1997; Yabsley et al., 2008; Gabriel et al., 2009; Durden et al., 2016; Ondrejicka et al., 2017). Another species of interest is Ixodes scapularis which has a large geographic range, is a competent reservoir for Babesia microti, and has been infected with B. microti-like strains (Hersh et al., 2012). Although fewer studies have investigated ticks on raccoons in the western USA, several tick species have been reported, including I. texanus, I. rugosus, I. pacificus, and D. variablis (Gregson, 1956; Furman and Loomis, 1984).
In conclusion, this study provides data on the prevalence and distribution of piroplasms in raccoons across a large geographic scale. These data have greatly expanded our knowledge of piroplasms in raccoons and led to the discovery of two new clades of parasites, bringing the total putative piroplasm species in raccoons to four in the USA. with an additional fifth present, to date, only in raccoons from Japan. Our data support the use of cox1 for the classification of the piroplasms as we obtained the same well supported clades of piroplasms as previous studies and also identified potential geographic separation of eastern and western parasites in three of the Babesia clades from raccoons (Chae et al., 1999; Allsopp and Allsopp, 2006). For several clades, we identified spatial genetic variation, which raises interesting questions about parasite transmission and/or raccoon population structure that has led to these variants. Finally, we obtained data that further confirm raccoons are the natural host of the B. sp. detected in a sick maned wolf (Wasserkrug Naor et al., 2019), thereby highlighting the need for tick preventive product use and raccoon control around this species and possibly other species of concern.
Acknowledgements
Financial support for KBG was provided by the Warnell School of Forestry and Natural Resources. Additional support was obtained through sponsorship of the Southeastern Cooperative Wildlife Disease Study by the fish and wildlife agencies of Alabama, Arkansas, Florida, Georgia, Kentucky, Kansas, Louisiana, Maryland, Mississippi, Missouri, Nebraska, North Carolina, Ohio, Oklahoma, Pennsylvania, South Carolina, Tennessee, Virginia, and West Virginia, USA. Support from the states to SCWDS was provided in part by the Federal Aid to Wildlife Restoration Act (50 Stat. 917). Financial support for ML was provided by the Young Scholars Program at the University of Georgia. Contributions of JCB were partially supported through funding from the US Department of Energy under Award Number DE-EM0004391 to the University of Georgia Research Foundation. We thank the various organizations and their staff that assisted with the collection of samples for this project including Rachel Curtis-Robles (Department of Veterinary Integrative Biosciences at Texas A&M University) and numerous field biologists and wildlife rehabilitators. Thank you to Anne Kjemtrup at the California Department of Public Health for reviewing the paper and for samples of western piroplasms from a fallow deer and a woodrat.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2019.05.007.
Contributor Information
Kayla B. Garrett, Email: Kaylab92@uga.edu.
Gary Balsamo, Email: gary.balsamo@la.gov.
Heather Barron, Email: hbarron@crowclinic.org.
James C. Beasley, Email: beasley@srel.uga.edu.
Justin D. Brown, Email: judbrow@pa.gov.
Erin Cloherty, Email: ercloherty@nola.gov.
Hossain Farid, Email: ah.farid@dal.ca.
Mourad Gabriel, Email: mgabriel@iercecology.org.
Bethany Groves, Email: bgroves@paws.org.
Sarah Hamer, Email: shamer@cvm.tamu.edu.
Meghan Lewis, Email: Meghan.lewis25@uga.edu.
Nicole Nemeth, Email: nnemeth@uoguelph.ca.
Paul Oesterle, Email: oesterle@uoguelph.ca.
Lea Peshock, Email: Lea@greenwoodwildlife.org.
Rodney Schnellbacher, Email: rschnellbacher@springfieldmo.gov.
Renee Schott, Email: Renee@wrcmn.org.
Susanne Straif-Bourgeois, Email: Sstra1@lsuhsc.edu.
Michael J. Yabsley, Email: myabsley@uga.edu.
Conflict of Interest
There are no conflicts of interest associated with this manuscript.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Aktas M., Ozubek S. A survey of canine haemoprotozoan parasites from Turkey, including molecular evidence of an unnamed Babesia. Comp. Immunol. Microbiol. Infect. Dis. 2017;52:36–42. doi: 10.1016/j.cimid.2017.05.007. [DOI] [PubMed] [Google Scholar]; Aktas, M., Ozubek, S., 2017. A survey of canine haemoprotozoan parasites from Turkey, including molecular evidence of an unnamed Babesia. Comp Immunol Microbiol Infect Dis. 52, 36-42. [DOI] [PubMed]
- Allsopp M.T., Allsopp B.A. Molecular sequence evidence for the reclassification of some Babesia species. Ann. NY. Acad. Sci. 2006;1081:509–517. doi: 10.1196/annals.1373.076. [DOI] [PubMed] [Google Scholar]; Allsopp, M.T., Allsopp, B.A., 2006. Molecular sequence evidence for the reclassification of some Babesia species. Ann. NY. Acad. Sci. 1081, 509-517. [DOI] [PubMed]
- Anderson J.F., Magnarelli L.A. Vetebrate host relationships and distribution of ixodid ticks (Acari: ixodidae) in Connecticut, USA. J. Med. Entomol. 1980;17(4):314–323. doi: 10.1093/jmedent/17.4.314. [DOI] [PubMed] [Google Scholar]; Anderson, J.F., Magnarelli, L.A., 1980. Vetebrate host relationships and distribution of ixodid ticks (Acari: Ixodidae) in Connecticut, USA. J. Med. Entomol. 17(4), 314-323. [DOI] [PubMed]
- Anderson J.F., Magnarelli L.A., Sulze A.J. Raccoon babesiosis in Connecticut, USA - Babesia-lotori sp-N. J. Parasitol. 1981;67:417–425. [PubMed] [Google Scholar]; Anderson, J.F., Magnarelli, L.A., Sulze, A. J., 1981. Raccoon Babesiosis in Connecticut, USA - Babesia-Lotori Sp-N. J. Parasitol. 67, 417-425. [PubMed]
- Baneth G., Florin-Christensen M., Cardoso L., Schnittger L. Reclassification of Theileria annae as Babesia vulpes sp. nov. Parasites Vectors. 2015;8:207. doi: 10.1186/s13071-015-0830-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; Baneth, G., Florin-Christensen, M., Cardoso, L., Schnittger, L., 2015. Reclassification of Theileria annae as Babesia vulpes sp. nov. Parasit Vectors. 8, 207. https://doi.org/10.1186/s13071-015-0830-5. [DOI] [PMC free article] [PubMed]
- Bednarska M., Bajer A., Drozdowska A., Mierzejewska E.J., Tolkacz K., Welc-Faleciak R. Vertical transmission of Babesia microti in BALB/c mice: preliminary report. PLoS One. 2015;10(9) doi: 10.1371/journal.pone.0137731. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bednarska, M., Bajer, A., Drozdowska, A., Mierzejewska, E. J., Tolkacz, K., Welc-Faleciak, R., 2015. Vertical transmission of Babesia microti in BALB/c mice: preliminary report. PLoS ONE. 10(9). https://doi.org/10.1371/journal.pone.0137731. [DOI] [PMC free article] [PubMed]
- Beltran-Beck B., Garcia F.J., Gortázar C. Raccoons in Europe, disease hazards due to the establishment of an invasive species. Eur. J. Wildl. Res. 2012;58(1):5–15. [Google Scholar]; Beltran-Beck, B., Garcia, F.J., Gortazar, C., 2012. Raccoons in Europe, disease hazards due to the establishment of an invasive species. Eur. J. Wildl. Res. 58(1), 5-15. 10.1007/s10344-011-0600-4.
- Binda A.P.D. 2016. Babesiosis. Salem Press: Magill's Medical Guide; p. 1. Online Edition. [Google Scholar]; Binda, A.P.D., 2016. Babesiosis. Salem Press: Magill’s Medical Guide (Online Edition). 1.
- Birkenheuer A.J., Levy M.G., Breitschwerdt E.B. Development and evaluation of a seminested PCR for detection and differentiation of Babesia gibsoni (Asian Genotype) and B. canis DNA in canine blood samples. J. Clin. Microbiol. 2003;41(9):4172–4177. doi: 10.1128/JCM.41.9.4172-4177.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]; Birkenheuer, A.J., Levy, M.G., Breitschwerdt, E.B., 2003. Development and evaluation of a seminested PCR for detection and differentiation of Babesia gibsoni (Asian Genotype) and B. canis DNA in canine blood samples. J. Clin. Microbiol. 41(9), 4172-4177. 10.1128/JCM.41.9.4172-4177.2003. [DOI] [PMC free article] [PubMed]
- Birkenheuer A.J., Whittington J., Neel J., Large E., Barger A., Levy M.G., Breitschwerdt E.B. Molecular characterization of a Babesia species identified in a North American raccoon. J. Wildl. Dis. 2006;42(2):375–380. doi: 10.7589/0090-3558-42.2.375. [DOI] [PubMed] [Google Scholar]; Birkenheuer, A.J., Whittington, J., Neel, J., Large, E., Barger, A., Levy, M.G., Breitschwerdt, E.B., 2006. Molecular characterization of a Babesia species identified in a North American raccoon. J. Wildl. Dis. 42(2), 375-380. https://doi.org/10.7589/0090-3558-42.2.375. [DOI] [PubMed]
- Birkenheuer A.J., Marr H.S., Hladio N., Acton A.E. Molecular evidence of prevalent dual piroplasma infections in North American raccoons (Procyon lotor) Parasitology. 2007;135:33–37. doi: 10.1017/S0031182007003538. [DOI] [PubMed] [Google Scholar]; Birkenheuer, A.J., Marr, H.S., Hladio N., Acton, A.E., 2007a. Molecular evidence of prevalent dual piroplasma infections in North American raccoons (Procyon lotor). Parasitol. 135, 33-37. [DOI] [PubMed]
- Birkenheuer A.J., Harms C.A., Neel J., Marr H.S., Tucker M.D., Acton A.E., Tuttle A.D., Stoskopf M.K. The identification of a genetically unique piroplasma in north amrican river otters (Lontra canadensis) Parasitology. 2007;134:631–635. doi: 10.1017/S0031182006002095. [DOI] [PubMed] [Google Scholar]; Birkenheuer, A.J., Harms, C.A., Neel, J., Marr, H.S., Tucker, M.D., Acton, A.E., Tuttle, A.D., Stoskopf, M.K., 2007b. The identification of a genetically unique piroplasma in north amrican river otters (Lontra canadensis). Parasitology. 134, 631-635. https://doi.org/10.1017/S0031182006002095 [DOI] [PubMed]
- Chae J.S., Allsopp B.A., Waghela S.D. A study of the systematics of Theileria spp. based upon small-subunit ribosomal RNA sequences. Parasitol. Res. 1999;85:877–883. doi: 10.1007/s004360050651. [DOI] [PubMed] [Google Scholar]; Chae, J.S., Allsopp, B.A., Waghela, S.D., 1999. A study of the systematics of Theileria spp. based upon small-subunit ribosomal RNA sequences. Parasitol Res. 85, 877-883. [DOI] [PubMed]
- Clark K., Savick K., Butler J. Babesia microti in rodents and raccoons from northeast Florida. J. Parasitol. 2012;98(6):1117–1121. doi: 10.1645/GE-3083.1. [DOI] [PubMed] [Google Scholar]; Clark, K., Savick, K., Butler, J., 2012. Babesia microti in rodents and raccoons from northeast Florida. The J. Parasitol. 98(6), 1117-1121. [DOI] [PubMed]
- Criado-Fornelio A., Martinez-Marcos A., Buling-Saraña A., Barba-Carretero J.C. Molecular studies on Babesia, Theileria and Hepatozoon in southern Europe. Part II. Phylogenetic analysis and evolutionary history. Vet. Parasitol. 2003;114(3):173–194. doi: 10.1016/s0304-4017(03)00141-9. [DOI] [PubMed] [Google Scholar]; Criado-Fornelio, A., Martinez-Marcos, A., Buling-Saraña, A., Barba-Carretero, J.C., 2003. Molecular studies on Babesia, Theileria and Hepatozoon in southern Europe. Part II. Phylogenetic analysis and evolutionary history. Vet. Parasitol. 114(3), 173-194. [DOI] [PubMed]
- Dennis J.R., Durden L.A., Snyder D.E. Ectoparasites of the raccoon (Procyon lotor) from north-central Arkansas. J. Kans. Entomol. Soc. 1994;67(2):208–212. [Google Scholar]; Dennis, J.R., Durden, L.A., Snyder, D.E., 1994. Ectoparasites of the raccoon (Procyon lotor) from North-Central Arkansas. J. Kans. Entomol. Soc. 67(2), 208-212.
- Durden L.A., Beckmen K.B., Gerlach R.F. New records of ticks (Acari: ixodidae) from dogs, cats, humans, and some wild vertebrates in Alaska: invasion potential. J. Med. Entomol. 2016;53(6):1391–1395. doi: 10.1093/jme/tjw128. [DOI] [PubMed] [Google Scholar]; Durden, L.A., Beckmen, K.B., Gerlach, R.F., 2016. New records of ticks (Acari: Ixodidae) from dogs, cats, humans, and some wild vertebrates in Alaska: invasion potential. J. Med. Entomol. 53(6), 1391-1395. [DOI] [PubMed]
- Frerichs W.M., Holbrook A.A. Babesia spp. and Haemobartonella sp. in wild mammals trapped at the agricultural research center, Beltsville, Maryland. J. Parasitol. 1970;56(1):130. [PubMed] [Google Scholar]; Frerichs, W.M., Holbrook, A.A., 1970. Babesia spp. and Haemobartonella sp. in wild mammals trapped at the Agricultural Research Center, Beltsville, Maryland. J. Parasitol. 56(1) 130. [PubMed]
- Furman D.P., Loomis E.C. ume 25. University of California Press Berkeley; Los Angeles, CA: 1984. (The Ticks of California (Acari:Ixodida). Bulletin of the California Insect Survey). [Google Scholar]; Furman, D.P., Loomis, E.C., 1984. The ticks of California (Acari:Ixodida). Bulletin of the California Insect Survey (Volume 25). University of California Press Berkeley, Los Angeles, CA.
- Gabriel M.W., Brown R.N., Foley J.E., Higley J.M., Botzler R.G. Ecology of Anaplasma phagocytophilum infection in gray foxes (Urocyon cineroargenteus) in northwestern California. J. Wildl. Dis. 2009;45(2):344–354. doi: 10.7589/0090-3558-45.2.344. [DOI] [PubMed] [Google Scholar]; Gabriel, M.W., Brown, R.N., Foley, J.E., Higley, J.M., Botzler, R.G., 2009. Ecology of Anaplasma phagocytophilum infection in gray foxes (Urocyon cineroargenteus) in northwestern California. J. Wildl. Dis. 45(2), 344-354. [DOI] [PubMed]
- Garrett K.B., Schott R., Peshock L., Yabsley M.J. Prevalence and diversity of piroplasms and ticks in young raccoons and an association of Babesia sensu stricto infections with splenomegaly. Parasitol. Open. 2018;4(12) [Google Scholar]; Garrett, K. B., Schott, R., Peshock, L., Yabsley, M.J., 2018. Prevalence and diversity of piroplasms and ticks in young raccoons and an association of Babesia sensu stricto infections with splenomegaly. Parasitology Open. 4(12). doi:10.1017/pao.2018.7
- Goethert H.K., Telford S.R. What is Babesia microti? Parasitology. 2003;127:301–309. doi: 10.1017/s0031182003003822. [DOI] [PubMed] [Google Scholar]; Goethert, H.K., Telford, S.R., 2003. What is Babesia microti? Parasitology. 127, 301-309. [DOI] [PubMed]
- Gray J., von Stedingk L.V., Gürtelschmid M., Granström M. Transmission studies of Babesia microti in Ixodes ricinus ticks and gerbils. J. Clin. Microbiol. 2002;40(4):1259–1263. doi: 10.1128/JCM.40.4.1259-1263.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gray, J., von Stedingk, L.V., Gurtelschmid, M., Granstrom, M., 2002. Transmission studies of Babesia microti in Ixodes ricinus ticks and gerbils. J. Clin. Microbiol. 40(4), 1259-1263. 10.1128/JCM.40.4.1259-1263.2002. [DOI] [PMC free article] [PubMed]
- Gregson J.D. Canada Department of Agriculture; Ottawa, Ont: 1956. The Ixodoides of Canada. Veterinary and Medical Entomology Section. [Google Scholar]; Gregson, J.D., 1956. The Ixodoides of Canada. Veterinary and Medical Entomology Section, Canada Department of Agriculture, Ottawa, Ont.
- Harris D.J. Naming no names: comments on the taxonomy of small piroplasmids in canids. Parasites Vectors. 2016;9(289) doi: 10.1186/s13071-016-1567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; Harris, D.J., 2016. Naming no names: comments on the taxonomy of small piroplasmids in canids. Parasit Vectors. 9(289). https://doi.org/10.1186/s13071-016-1567-5. [DOI] [PMC free article] [PubMed]
- Hersh M.H., Tibbetts M., Strauss M., Ostfeld R.S., Keesing F. Resevoir competence of wildlfie host species for Babesia microti. Emerg. Infect. Dis. 2012;18(12) doi: 10.3201/eid1812.111392. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hersh, M.H., Tibbetts, M., Strauss, M., Ostfeld, R.S., Keesing, F., 2012. Resevoir competence of wildlfie host species for Babesia microti. Emerg. Infect. Dis. 18(12). [DOI] [PMC free article] [PubMed]
- Hunfeld K.P., Hildebrant A., Gray J.S. Babesiosis: recent insights into an ancient disease. Int. J. Parasitol. 2008;38:1219–1237. doi: 10.1016/j.ijpara.2008.03.001. [DOI] [PubMed] [Google Scholar]; Hunfeld, K.P., Hildebrant, A., Gray, J.S., 2008. Babesiosis: Recent insights into an ancient disease. Int. J. Parasitol.. 38, 1219-1237. 10.1016/j.ijpara.2008.03.001. [DOI] [PubMed]
- Jinnai M., Kawabuchi-Kurata T., Tsuji M., Nakajima R., Fujisawa K., Nagata S., Koide H., Matoba Y., Asakawa M., Takahashi K., Ishihara C. Molecular evidence for the presence of new Babesia species in feral raccoons (Procyon lotor) in Hokkaido, Japan. Vet. Parasitol. 2009;162:241–247. doi: 10.1016/j.vetpar.2009.03.016. [DOI] [PubMed] [Google Scholar]; Jinnai, M., Kawabuchi-Kurata, T., Tsuji, M., Nakajima, R., Fujisawa, K., Nagata, S., Koide, H., Matoba, Y., Asakawa, M., Takahashi K., Ishihara, C., 2009. Molecular evidence for the presence of new Babesia species in feral raccoons (Procyon lotor) in Hokkaido, Japan. Vet. Parasitol. 162, 241-247. [DOI] [PubMed]
- Kauffmann M., Rehbein S., Hamel D., Lutz W., Heddergott M., Pfister K., Silaghi C. Anaplasma phagocytophilum and Babesia spp. in roe deer (Capreolus capreolus), fallow deer (Dama dama) and mouflon (Ovis musimon) in Germany. Mol. Cell. Probes. 2017;31:46–54. doi: 10.1016/j.mcp.2016.08.008. [DOI] [PubMed] [Google Scholar]; Kauffmann, M., Rehbein, S., Hamel, D., Lutz, W., Heddergott, M., Pfister, K., Silaghi, C., 2017. Anaplasma phagocytophilum and Babesia spp. in roe deer (Capreolus capreolus), fallow deer (Dama dama) and mouflon (Ovis musimon) in Germany. Mol. Cell. Probes. 31, 46-54. 10.1016/j.mcp.2016.08. [DOI] [PubMed]
- Kawabuchi T., Tsuji M., Sado A., Matoba Y., Asakawa M., Ishihara C. Babesia microti- like parasites detected in feral raccooons (Procyon lotor) captured in Hokkaido. J. Vet. Med. Sci. 2005;67:825–827. doi: 10.1292/jvms.67.825. [DOI] [PubMed] [Google Scholar]; Kawabuchi, T., Tsuji, M., Sado, A., Matoba, Y., Asakawa, M., Ishihara, C., 2005. Babesia microti- like parasites detected in feral raccooons (Procyon lotor) captured in Hokkaido. J. Vet. Med. Sci. 67, 825-827. [DOI] [PubMed]
- Kjemtrup A.M., Kocan A.A., Whitworth L., Meinkoth J., Birkenheuer A.J., Cummings J., Boudreaux M.K., Stockham S.L., Irizarry-Rovira A., Conrad P.A. There are at least three genetically distinct small pirplasms from dogs. Int. J. Parasitol. 2000;30:1501–1505. doi: 10.1016/s0020-7519(00)00120-x. [DOI] [PubMed] [Google Scholar]; Kjemtrup, A.M., Kocan, A.A., Whitworth, L., Meinkoth, J., Birkenheuer, A.J., Cummings, J., Boudreaux, M.K., Stockham,S.L., Irizarry-Rovira, A., Conrad, P.A., 2000. There are at least three genetically distinct small pirplasms from dogs.Int. J. Parasitol.. 30, 1501-1505. [DOI] [PubMed]
- Kjemtrup A.M., Robinson T., Conrad P.A. Description and epidemiology of Theileria youngi n. sp. from a northern California dusky-footed woodrat (Neotoma fuscipes) population. J. Parasitol. 2001;87(2):373–378. doi: 10.1645/0022-3395(2001)087[0373:DAEOTY]2.0.CO;2. [DOI] [PubMed] [Google Scholar]; Kjemtrup, A.M., Robinson, T., Conrad, P.A., 2001. Description and epidemiology of Theileria youngi n. sp. from a northern California dusky-footed woodrat (Neotoma fuscipes) population. J. Parasitol. 87(2), 373-378. 10.2307/3285054. [DOI] [PubMed]
- Kjemtrup A.M., Wainwright K., Miller M., Penzhorn B.L., Carreno R.A. Babesia conradae, sp. Nov., a small canine Babesia identified in California. Vet. Parasitol. 2006;138:103–111. doi: 10.1016/j.vetpar.2006.01.044. [DOI] [PubMed] [Google Scholar]; Kjemtrup, A.M., Wainwright, K., Miller, M., Penzhorn, B.L., Carreno, R.A., 2006. Babesia conradae, sp. Nov., a small canine Babesia identified in California. Vet. Parasitol. 138, 103-111. 10.1016/j.vetpar.2006.01.044. [DOI] [PubMed]
- Lack J.B., Reichard M.V., Van Den Bussche R.A. Phylogeny and evolution of the piroplasmida as inferred from 18S rRNA sequences. Int. J. Parasitol. 2012;42:353–363. doi: 10.1016/j.ijpara.2012.02.005. [DOI] [PubMed] [Google Scholar]; Lack, J.B., Reichard, M.V., Van Den Bussche, R.A., 2012. Phylogeny and evolution of the piroplasmida as inferred from 18S rRNA sequences.Int. J. Parasitol.. 42, 353-363. [DOI] [PubMed]
- Lotze J.H., Anderson S. Procyon lotor. Mamm. Species. 1979;119:1–8. [Google Scholar]; Lotze, J.H., Anderson, S., 1979. Procyon lotor. Mamm. Species. 119, 1-8.
- Mierzejewska E.J., Welc-Falęciak R., Bednarska M., Rodo A., Bajer A. The first evidence for vertical transmission of Babesia canis in a litter of Centeral Asian Shepherd dogs. Ann. Agric. Environ. Med. 2014;21(3):500–503. doi: 10.5604/12321966.1120590. [DOI] [PubMed] [Google Scholar]; Mierzejewska, E.J., Welc-Falęciak, R., Bednarska, M., Rodo, A., Bajer, A., 2014. The first evidence for vertical transmission of Babesia canis in a litter of Centeral Asian Shepherd dogs. Ann. Agric. Environ. Med. 21(3), 500-503. https://doi.org/10.5604/12321966.1120590 [DOI] [PubMed]
- Nakajima R., Tsuji M., Oda K., Zamoto-Niikura A., Wei Q., Kawabuchi-Kurata T., Nishida A., Ishihara C. Babesia microti-group parasites compared phylogenetically by complete sequencing of the CCTn gene in 36 isolates. J. Vet. Sci. 2008;71(1):55–68. doi: 10.1292/jvms.71.55. [DOI] [PubMed] [Google Scholar]; Nakajima, R., Tsuji, M., Oda, K., Zamoto-Niikura, A., Wei, Q., Kawabuchi-Kurata, T., Nishida, A., Ishihara, C., 2008. Babesia microti-group parasites compared phylogenetically by complete sequencing of the CCTn gene in 36 isolates.J. Vet. Sci.. 71(1), 55-68. https://doi.org/10.1292/jvms.71.55. [DOI] [PubMed]
- Ondrejicka D.A., Morey K.C., Hanner R.H. DNA barcodes identify medically imporant tick species in Canada. Genome. 2017;60:74–84. doi: 10.1139/gen-2015-0179. [DOI] [PubMed] [Google Scholar]; Ondrejicka, D.A., Morey, K.C., Hanner, R.H., 2017. DNA barcodes identify medically imporant tick species in Canada. Genome. 60, 74-84. https://doi.org/10.1139/gen-2015-0179. [DOI] [PubMed]
- Ouellette J., Appoerson C.S., Howar P., Evans T.L., Levine J.F. Tick-raccoon associations and the potential for lyme disease spirochete transmission in the costal plain of North Carolina. J. Wildl. Dis. 1997;33(1):28–29. doi: 10.7589/0090-3558-33.1.28. [DOI] [PubMed] [Google Scholar]; Ouellette, J., Appoerson, C.S., Howar, P., Evans, T.L., Levine, J. F., 1997. Tick-raccoon associations and the potential for lyme disease spirochete transmission in the costal plain of North Carolina. J. Wildl. Dis. 33(1), 28-29. https://doi.org/10.7589/0090-3558-33.1.28. [DOI] [PubMed]
- Pung O.J., Durden L.A., Banks C.W., Jones D.N. Ectoparasites of opossums and raccoons in southeastern Georgia. Ann. Entomol. Soc. Am. 1994;31(6):915–919. doi: 10.1093/jmedent/31.6.915. [DOI] [PubMed] [Google Scholar]; Pung, O.J., Durden, L.A., Banks, C.W., Jones, D.N., 1994. Ectoparasites of opossums and raccoons in southeastern Georgia. Ann. Entomol. Soc. A. 31(6), 915-919. [DOI] [PubMed]
- Schnittger S., Rodriguez A.E., Florin-Christensen M., Morrison D.A. Babesia: a world emerging. Infect. Genet. Evol. 2012;12:1788–1809. doi: 10.1016/j.meegid.2012.07.004. [DOI] [PubMed] [Google Scholar]; Schnittger, S., Rodriguez, A.E., Florin-Christensen, M., Morrison, D.A., 2012. Babesia: A world emerging. Infect. Genet. Evol. 12, 1788-1809. [DOI] [PubMed]
- Schreeg M.E., Marr H.S., Tarigo J.L., Cohn L.A., Birdh D.M., Scholl E.H., Levy M.G., Weigmann B.M., Birkenheuer A.J. Mitochondrial genome sequences and structures aid in the resolution of Piroplasmida phylogeny. PLoS One. 2016 doi: 10.1371/journal.pone.0165702. [DOI] [PMC free article] [PubMed] [Google Scholar]; Schreeg, M.E., Marr, H.S., Tarigo, J.L., Cohn, L.A., Birdh, D.M., Scholl, E.H., Levy, M.G., Weigmann, B.M., Birkenheuer, A.J., 2016. Mitochondrial genome sequences and structures aid in the resolution of Piroplasmida phylogeny. PLoS ONE. https://doi.org/10.1371/journal.pone.0165702. [DOI] [PMC free article] [PubMed]
- Swei A., O'Conner K.E., Couper L.I., Thekkiniath J., Conrad P.A., Padgett K.A., Burns J., Yoshimizu M.H., Gonzales B., Munk B., Shirkey N., Konde L., Mamoun C.B., Lane R.S., Kjemtrup A. Evidence for transmission of the zoonotic apicomplexan parasite Babesia duncani by the tick Dermacentor albipictus. Int. J. Parasitol. 2019 doi: 10.1016/j.ijpara.2018.07.002. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]; Swei, A., O’Conner, K.E., Couper, L.I., Thekkiniath, J., Conrad, P.A., Padgett, K.A., Burns, J., Yoshimizu, M.H., Gonzales, B., Munk, B., Shirkey, N., Konde, L., Mamoun, C.B., Lane, R.S., Kjemtrup, A., 2019. In Press. Evidence for transmission of the zoonotic apicomplexan parasite Babesia duncani by the tick Dermacentor albipictus. Int. J. Parasitol. [DOI] [PMC free article] [PubMed]
- Telford S.R., Jr., Forrester D.J. Haemoparasites of raccoons (Procyon lotor) in Florida. J. Wildl. Dis. 1991;27(3):486–490. doi: 10.7589/0090-3558-27.3.486. [DOI] [PubMed] [Google Scholar]; Telford, S.R. Jr., Forrester, D.J., 1991. Haemoparasites of Raccoons (Procyon lotor)in Florida. J. Wildl. Dis. 27(3), 486-490. [DOI] [PubMed]
- Tolkacz K., Bednarska M., Alsarraf M., Dwużnik D., Grzybek M., Welc-Falęciak R., Behnke J.M., Bajer A. Prevalence, genetic identity and vertical transmission of Babesia microti in three naturally infected species of vole, Microtus spp. (Cricetidae) Parasites Vectors. 2017;10(66) doi: 10.1186/s13071-017-2007-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tolkacz, K., Bednarska, M., Alsarraf, M., Dwużnik, D., Grzybek, M., Welc-Falęciak, R., Behnke, J.M., Bajer, A., 2017. Prevalence, genetic identity and vertical transmission of Babesia microti in three naturally infected species of vole, Microtus spp. (Cricetidae). Parasites Vectors. 10(66). https://doi.org/10.1186/s13071-017-2007-x [DOI] [PMC free article] [PubMed]
- Tufts D.M., Diuk-Wasser M.A. Transplacental transmission of tick-borne Babesia microti in its natural host Peromyscus leucopus. Parasit. Vecotrs. 2018;11(286) doi: 10.1186/s13071-018-2875-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tufts, D.M., Diuk-Wasser, M.A., 2018. Transplacental transmission of tick-borne Babesia microti in its natural host Peromyscus leucopus. Parasit. Vecotrs. 11(286). https://doi.org/10.1186/s13071-018-2875-8 [DOI] [PMC free article] [PubMed]
- Uilenberg G. Babesia-A historical overview. Vet. Parasitol. 2006;138:3–10. doi: 10.1016/j.vetpar.2006.01.035. [DOI] [PubMed] [Google Scholar]; Uilenberg, G., 2006. Babesia-A historical overview. Vet. Parasitol. 138, 3-10. [DOI] [PubMed]
- Wasserkrug Naor A., Lindemann D.M., Schreeg M.E., Marr H.S., Birkenheuer A.J., Carpenter J.W., Ryseff J.K. Clinical, morphological, and molecular characterization of an undetermined Babesia species in a maned wolf (Chrysocyon brachyurus) Ticks Tick Borne Dis. 2019;10:124–126. doi: 10.1016/j.ttbdis.2018.09.005. [DOI] [PubMed] [Google Scholar]; Wasserkrug Naor, A., Lindemann, D.M., Schreeg, M.E., Marr, H.S., Birkenheuer, A.J., Carpenter, J.W., Ryseff, J.K., 2018. in press. Clinical, morphological, and molecular characterization of an undetermined Babesia species in a maned wolf (Chrysocyon brachyurus). Ticks Tick Borne Dis. 10.1016/j.ttbdis.2018.09.005. [DOI] [PubMed]
- Williams B.M., Berensten A., Shock B.C., Teixiera M., Dunbar M.R., Becker M.S., Yabsley M.J. Prevalence and diversity of Babesia, Hepatozoon, Ehrlichia, and Bartonella in wild and domestic carnivores from Zambia, Africa. Parasitol. Res. 2014;113(3):911–918. doi: 10.1007/s00436-013-3722-7. [DOI] [PubMed] [Google Scholar]; Williams, B. M., Berensten, A., Shock, B. C., Teixiera, M., Dunbar, M. R., Becker, M. S., Yabsley, M. J., 2014. Prevalence and diversity of Babesia, Hepatozoon, Ehrlichia, and Bartonella in wild and domestic carnivores from Zambia, Africa. Parasitol Res. 113(3), 911-918. 10.1007/s00436-013-3722-7. [DOI] [PubMed]
- Yabsley M.J., Work T.M., Rameyer R.A. Molecular phylogeny of Babesia poelea from brown boobies (Sula leucogater) from Johnston Atoll, central pacific. J. Parasitol. 2006;92(2):423–425. doi: 10.1645/GE-617R.1. [DOI] [PubMed] [Google Scholar]; Yabsley, M.J., Work, T.M., Rameyer, R.A., 2006. Molecular phylogeny of Babesia poelea from brown boobies (Sula leucogater) from Johnston Atoll, Central Pacific. The J. Parasitol. 92(2), 423-425. [DOI] [PubMed]
- Yabsley M.J., Murphy S.M., Luttrell M.P., Little S.E., Massung R.F., Stallknecht D.E., Conti L.A., Blackmore C.G.M., Durden L.A. Experimental and field studies on the suitability of raccoons (Procyon lotor) as hosts for tick-borne pathogens. Vector Borne Zoonotic Dis. 2008;8(4):491–503. doi: 10.1089/vbz.2007.0240. [DOI] [PubMed] [Google Scholar]; Yabsley, M.J., Murphy, S.M., Luttrell, M.P., Little, S.E., Massung, R.F., Stallknecht, D.E., Conti, L.A., Blackmore, C.G.M., Durden, L.A., 2008. Experimental and field studies on the suitability of raccoons (Procyon lotor) as hosts for tick-borne pathogens. Vector Borne Zoonotic Dis. 8(4), 491-503. https://doi.org/10.1089/vbz.2007.0240. [DOI] [PubMed]
- Yabsley M.J., Shock B.C. Natural history of zoonotic Babesia: role of wildlife reservoirs. Int. J. Parasitol. Parasites Wildl. 2013;2:18–31. doi: 10.1016/j.ijppaw.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yabsley, M.J., Shock, B.C., 2013. Natural history of zoonotic Babesia: Role of wildlife reservoirs. Int. J. Parasitol. Parasites Wildl. 2, 18-31. https://doi.org/10.1016/j.ijppaw.2012.11.003. [DOI] [PMC free article] [PubMed]
- Yeagley T.J., Mason V.R., Hempstead J.E., Allen K.E., Parsons L.M., White M.A., Little S.E., Meinkoth J.H. Detection of Babesia gibsoni and the canine small Babesia ‘Spanish isolate’ in blood samples obtained from fighting dogs confiscated from dogfighting operations. J. Am. Vet. Med. Assoc. 2009;235(5):535–539. doi: 10.2460/javma.235.5.535. [DOI] [PubMed] [Google Scholar]; Yeagley, T.J., Mason, V.R., Hempstead, J.E., Allen, K.E., Parsons, L.M., White, M.A., Little, S.E., Meinkoth, J.H., 2009. Detection of Babesia gibsoni and the canine small Babesia ‘Spanish isolate’ in blood samples obtained from fighting dogs confiscated from dogfighting operations. J. Am. Vet. Med. Assoc. 235(5), 535-539. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.