Abstract
Background
The WRKY gene family is one of the most important families in higher plants. As transcription factors, they actively respond to biotic and abiotic stress and are also involved in plant development. Chinese jujube (Ziziphus jujuba Mill.) is the largest type of dried fruit tree in China in terms of production, but its production is largely limited by phytoplasma infection, and the information about the role of WRKY genes under phytoplasma stress was still limited.
Results
We identified 54 ZjWRKYs in the jujube genome and classified them into three subgroups according to conserved WRKY domains and zinc-finger structure. 41 ZjWRKYs were distributed on 11 of 12 pseudo chromosomes in Chinese jujube. The majority of ZjWRKYs were highly expressed in the seven examined tissues, indicating that they play multiple roles in these vegetative and reproductive organs. Transcriptome data showed that most of the characterised ZjWRKYs were highly expressed at later stages of fruit development. RT-qPCR demonstrated that the expression of 23 ZjWRKYs changed following phytoplasma infection, suggesting that they are involved in signalling pathways that respond to phytoplasma stress. Then, STRING analysis and yeast two-hybrid screening proved that some ZjWRKY proteins were interacting with ZjMAPKK proteins, which were also involved in phytoplasma invasion. Moreover, their differential expressions were further confirmed in resistant and susceptible jujube varieties under phytoplasma stress. These results suggest that ZjWRKYs play significant roles in phytoplasma tolerance and should be crucial candidate genes for jujube-phytoplasma interaction.
Conclusions
54 ZjWRKYs in Chinese jujube were identified and classified into three subgroups. 41 ZjWRKYs were unevenly distributed along the chromosomes. The majority of ZjWRKYs were highly expressed in various tissues. Most of the ZjWRKYs were positive responses to phytoplasma invasion, and that provided candidate genes for the future studies of jujube-phytoplasma interaction.
Electronic supplementary material
The online version of this article (10.1186/s12864-019-5789-8) contains supplementary material, which is available to authorized users.
Keywords: WRKYs, Chinese jujube, Bioinformatics analysis, Gene expression, Phytoplasma invasion
Background
The WRKY transcription factors (TFs) bind to a specific promoter sequence in the target gene, known as a W-box, and can positively or negatively regulate target gene expression. The WRKY proteins have one or two DNA binding domains that are 60 amino acids long and contain the conserved heptapeptide WRKYGQK followed by a zinc-finger motif C2H2 (CX4-5CX22–23HXH) or C2HC (CX7CX23–24 HXC) [1]. The WRKY family contains important transcription factors that have multiple functions in processes such as embryogenesis [2], trichome and seed development [3], leaf senescence [4], flowering [5], fruit and pollen development [6], biomass accumulation [7], secondary metabolite biosynthesis [8] and hormone signalling [9]. WRKY transcription factors are also crucial regulatory components of plant responses to pathogen infection. In Arabidopsis, several WRKY genes have been experimentally characterised and associated with responses to fungal or bacterial pathogens [10–12]. AtWRKY70 is required for R gene-mediated pathogen resistance, determining the balance between the SA- and JA-dependent defence systems [13, 14]. Many WRKY genes also act in defence signalling; for example, AtWRKY38 and AtWRKY62 act as negative regulators of basal resistance towards bacterial pathogens [15]. In rice, overexpression of OsWRKY30 enhanced resistance to the rice sheath blight fungus Rhizoctonia solani and the blast fungus Magnaporthe grisea [16, 17]. Owing to their important roles, the WRKY family has been widely studied in many plant species, such as Arabidopsis, rice, grape, apple, pear, and peach [18–22]. However, the information of this gene family in Chinese jujube and their roles under phytoplasma stress was still limited.
Chinese jujube is the largest type of dried fruit tree in China in terms of production [23] and the most important species of family Rhamnaceae. It is cultivated mainly for its fruits, which can be eaten fresh or dried or as raw materials for making Chinese herbal medicine. However, jujube production is threatened by several devastating diseases, such as jujube witches’ broom disease (JWB). The genome of Chinese jujube was recently published [24, 25], paving the way for further investigations. Our transcriptome data indicated that some WRKY genes respond to JWB phytoplasma infection. Since the WRKY family plays a crucial role in biotic stress response, identifying WRKY genes in Chinese jujube and determining their possible functions in response to phytoplasma stress have important significance.
Here, we report on the genome-wide analysis of the WRKY family in Chinese jujube. A non-redundant set of WRKY genes was identified in this species. Subsequently, chromosomal location was determined, phylogenetic and motif analyses were also performed as a base for further comparative genomics studies. Moreover, expression patterns of ZjWRKYs in various tissues and under phytoplasma stress were also investigated. The interacting proteins of ZjWRKYs were also screened. The ZjWRKYs involved in phytoplasma invasion were considered good candidates for subsequent studies of the jujube-phytoplasma interaction.
Results
Identification of ZjWRKYs in Chinese jujube
A total of 54 non-redundant putative WRKY coding sequences (Table 1) were identified in the jujube genome sequence. The sequences were named from ZjWRKY1 to ZjWRKY54 according to their gene structure and motifs. The ORF length for ZjWRKY genes ranged from 522 bp (ZjWRKY26) to 2205 bp (ZjWRKY8), and they encoded proteins ranging from 173 to 734 amino acids (aa) in length, with predicted pIs ranging from 4.65 (ZjWRKY32) to 9.09 (ZjWRKY1) (Table 1).
Table 1.
The information of WRKY gene family in Chinese jujube
| Gene Name | NCBI Reference | ORF (bp) | Size (aa) | MW(D) | PI | Conserved motif | Domain pattern | Zinc finger | Group | Exon number |
|---|---|---|---|---|---|---|---|---|---|---|
| ZjWRKY1 | XM_016044069.1 | 1434 | 477 | 52053.75 | 9.09 | 2 × [WRKYGQK] | C-X4-C-X22–23-HXH | C2H2 | I | 5 |
| ZjWRKY2 | XM_016042179.1 | 1629 | 542 | 60186.57 | 7.09 | 2 × [WRKYGQK] | C-X4-C-X22–23-HXH | C2H2 | I | 5 |
| ZjWRKY3 | XM_016047139.1 | 1904 | 583 | 63795.60 | 6 | 2 × [WRKYGQK] | C-X4-C-X22–23-HXH | C2H2 | I | 6 |
| ZjWRKY4 | XM_016025559.1 | 1506 | 501 | 55188.92 | 6.52 | 2 × [WRKYGQK] | C-X4-C-X22–23-HXH | C2H2 | I | 4 |
| ZjWRKY5 | XM_016037165.1 | 1566 | 521 | 57263.14 | 5.12 | 2 × [WRKYGQK] | C-X4-C-X22–23-HXH | C2H2 | I | 5 |
| ZjWRKY6 | XM_016019228.1 | 1077 | 358 | 39860.32 | 8.82 | 2 × [WRKYGQK] | C-X4-C-X22–23-HXH | C2H2 | I | 4 |
| ZjWRKY7 | XM_016024358.1 | 1629 | 542 | 59088.72 | 8.91 | 2 × [WRKYGQK] | C-X4-C-X22–23-HXH | C2H2 | I | 5 |
| ZjWRKY8 | XM_016020284.1 | 2205 | 734 | 80415.39 | 5.9 | 2 × [WRKYGQK] | C-X4-C-X22–23-HXH | C2H2 | I | 1 |
| ZjWRKY9 | XM_016029235.1 | 951 | 316 | 34758.07 | 8.44 | WRKYGQK | C-X5-C-X23-HXH | C2H2 | IIa | 4 |
| ZjWRKY10 | XM_016014490.1 | 951 | 316 | 35076.43 | 8.68 | WRKYGQK | C-X5-C-X23-HXH | C2H2 | IIa | 5 |
| ZjWRKY11 | XM_016028547.1 | 801 | 266 | 29844.33 | 8.99 | WRKYGQK | C-X5-C-X23-HXH | C2H2 | IIa | 4 |
| ZjWRKY12 | XM_016022282.1 | 1863 | 620 | 67027.91 | 6.26 | WRKYGQK | C-X5-C-X23-HXH | C2H2 | IIb | 5 |
| ZjWRKY13 | XM_016029844.1 | 1902 | 633 | 67897.98 | 6.12 | WRKYGQK | C-X5-C-X23-HXH | C2H2 | IIb | 6 |
| ZjWRKY14 | XM_016043879.1 | 1611 | 536 | 58692.78 | 6.48 | WRKYGQK | C-X5-C-X23-HXH | C2H2 | IIb | 6 |
| ZjWRKY15 | XM_016036346.1 | 1125 | 374 | 40424.14 | 8.09 | WRKYGQK | C-X5-C-X23-HXH | C2H2 | IIb | 3 |
| ZjWRKY16 | XM_016036345.1 | 1659 | 552 | 60686 | 7.71 | WRKYGQK | C-X5-C-X23-HXH | C2H2 | IIb | 7 |
| ZjWRKY17 | XM_016037515.1 | 1512 | 503 | 56033.17 | 5.48 | WRKYGQK | C-X5-C-X23-HXH | C2H2 | IIb | 5 |
| ZjWRKY18 | XM_016039435.1 | 1251 | 416 | 44528.06 | 9.13 | WRKYGQK | C-X5-C-X23-HXH | C2H2 | IIb | 3 |
| ZjWRKY19 | XM_016014870.1 | 1878 | 625 | 67519.49 | 7.97 | WRKYGQK | C-X5-C-X23-HXH | C2H2 | IIb | 5 |
| ZjWRKY20 | XM_016014977.1 | 1764 | 587 | 63426.84 | 8.8 | WRKYGQK | C-X5-C-X23-HXH | C2H2 | IIb | 4 |
| ZjWRKY21 | XM_016014513.1 | 1878 | 625 | 67533.52 | 7.97 | WRKYGQK | C-X5-C-X23-HXH | C2H2 | IIb | 5 |
| ZjWRKY22 | XM_016038792.1 | 579 | 192 | 21894.40 | 9.43 | WRKYGQK | C-X4-C-X23-HXH | C2H2 | IIc | 2 |
| ZjWRKY23 | XM_016040974.1 | 636 | 211 | 24030.21 | 8.47 | WRKYGQK | C-X4-C-X23-HXH | C2H2 | IIc | 3 |
| ZjWRKY24 | XM_016026686.1 | 597 | 198 | 22899.66 | 9.23 | WRKYGQK | C-X4-C-X23-HXH | C2H2 | IIc | 2 |
| ZjWRKY25 | XM_016014637.1 | 588 | 195 | 21527.72 | 6.73 | WRKYGKK | C-X4-C-X23-HXH | C2H2 | IIc | 3 |
| ZjWRKY26 | XM_016028953.1 | 522 | 173 | 19750.96 | 5.59 | WRKYGKK | C-X4-C-X23-HXH | C2H2 | IIc | 3 |
| ZjWRKY27 | XM_016011550.1 | 1008 | 335 | 37240.76 | 6.34 | WRKYGQK | C-X4-C-X23-HXH | C2H2 | IIc | 4 |
| ZjWRKY28 | XM_016024581.1 | 1038 | 345 | 39158.92 | 6.76 | WRKYGQK | C-X4-C-X23-HXH | C2H2 | IIc | 3 |
| ZjWRKY29 | XM_016039492.1 | 933 | 310 | 34331.69 | 5.65 | WRKYGQK | C-X4-C-X23-HXH | C2H2 | IIc | 3 |
| ZjWRKY30 | XM_016011683.1 | 1110 | 369 | 40614.00 | 5.16 | WRKYGQK | C-X4-C-X23-HXH | C2H2 | IIc | 3 |
| ZjWRKY31 | XM_016036211.1 | 1008 | 335 | 37135.37 | 6.43 | WRKYGQK | C-X4-C-X23-HXH | C2H2 | IIc | 3 |
| ZjWRKY32 | XM_016041473.1 | 756 | 251 | 28026.78 | 4.65 | WRKYGQK | C-X4-C-X23-HXH | C2H2 | IIc | 2 |
| ZjWRKY33 | XM_016028867.1 | 1116 | 371 | 40189.57 | 9.57 | WRKYGQK | C-X5-C-X23-HXH | C2H2 | IId | 3 |
| ZjWRKY34 | XM_016011768.1 | 1101 | 366 | 39383.33 | 9.64 | WRKYGQK | C-X5-C-X23-HXH | C2H2 | IId | 3 |
| ZjWRKY35 | XM_016036017.1 | 1083 | 360 | 40498.69 | 9.65 | WRKYGQK | C-X5-C-X23-HXH | C2H2 | IId | 4 |
| ZjWRKY36 | XM_016045200.1 | 1497 | 498 | 53218.21 | 5.81 | WRKYGQK | C-X5-C-X23-HXH | C2H2 | IIe | 3 |
| ZjWRKY37 | XM_016020139.1 | 858 | 285 | 31393.44 | 5.62 | WRKYGQK | C-X5-C-X23-HXH | C2H2 | IIe | 3 |
| ZjWRKY38 | XM_016025812.1 | 849 | 282 | 30475.83 | 5.46 | WRKYGQK | C-X5-C-X23-HXH | C2H2 | IIe | 3 |
| ZjWRKY39 | XM_016022820.1 | 1422 | 473 | 51502.28 | 5.19 | WRKYGQK | C-X5-C-X23-HXH | C2H2 | IIe | 3 |
| ZjWRKY40 | XM_016044078.1 | 969 | 322 | 36328.33 | 8.98 | WRKYGQK | C-X5-C-X23-HXH | C2H2 | IIe | 4 |
| ZjWRKY41 | XM_016044080.1 | 1068 | 355 | 38920.61 | 5.92 | WRKYGQK | C-X5-C-X23-HXH | C2H2 | IIe | 3 |
| ZjWRKY42 | XM_016036213.1 | 870 | 289 | 32200.49 | 5.27 | WRKYGQK | C-X5-C-X23-HXH | C2H2 | IIe | 3 |
| ZjWRKY43 | XM_016013400.1 | 1215 | 404 | 45202.30 | 6.64 | WRKYGQK | C-X7-C-X23-HXC | C2HC | III | 4 |
| ZjWRKY44 | XM_016040779.1 | 1113 | 370 | 41825.52 | 5.26 | WRKYGQK | C-X7-C-X23-HXC | C2HC | III | 3 |
| ZjWRKY45 | XM_016022705.1 | 1176 | 391 | 43607.65 | 5.9 | WRKYGQK | C-X7-C-X23-HXC | C2HC | III | 3 |
| ZjWRKY46 | XM_016033893.1 | 1065 | 354 | 40462.32 | 5.16 | WRKYGQK | C-X7-C-X23-HXC | C2HC | III | 4 |
| ZjWRKY47 | XM_016041850.1 | 960 | 319 | 36005.79 | 5.25 | WRKYGQK | C-X7-C-X23-HXC | C2HC | III | 3 |
| ZjWRKY48 | XM_016013401.1 | 1035 | 344 | 38711.93 | 5.39 | WRKYGQK | C-X7-C-X23-HXC | C2HC | III | 3 |
| ZjWRKY49 | XM_016041861.1 | 981 | 326 | 37477.17 | 8.15 | WRKYGQK | C-X7-C-X23-HXC | C2HC | III | 3 |
| ZjWRKY50 | XM_016041806.1 | 930 | 309 | 35609.87 | 5.91 | WRKYGQK | C-X7-C-X23-HXC | C2HC | III | 3 |
| ZjWRKY51 | XM_016047371.1 | 924 | 307 | 35268.77 | 6.71 | WRKYGQK | C-X7-C-X23-HXC | C2HC | III | 3 |
| ZjWRKY52 | XM_016041801.1 | 948 | 315 | 35731.33 | 6.46 | WRKYGQK | C-X7-C-X23-HXC | C2HC | III | 3 |
| ZjWRKY53 | XM_016041802.1 | 972 | 323 | 37104.55 | 8.78 | WRKYGQK | C-X7-C-X23-HXC | C2HC | III | 3 |
| ZjWRKY54 | XM_016041803.1 | 951 | 316 | 36421.82 | 5.76 | WRKYGQK | C-X7-C-X23-HXC | C2HC | III | 3 |
Previous genome evolution studies showed that Chinese jujube is closely related to species of the family Rosaceae [24, 26], so the WRKY genes of three Rosaceae species (apple, pear and peach) and Arabidopsis were compared with that of Chinese jujube (Additional file 1). Compared with Arabidopsis, apple and pear [18, 19, 21], there are fewer WRKY genes in jujube, but the number was similar to that of peach [22]. The smaller number of WRKY genes in Chinese jujube and peach may be due to the occurrence of only one genome duplication event during the evolution of the two species [24, 27]. Based on the above comparison, it was suggested that most of the expected WRKY genes in jujube were identified.
Conserved motifs and phylogenetic tree construction of ZjWRKYs
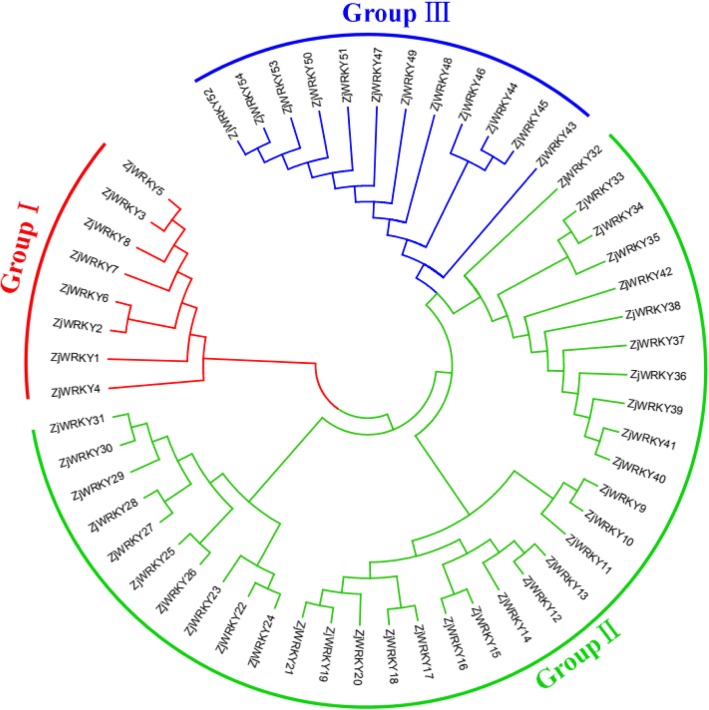
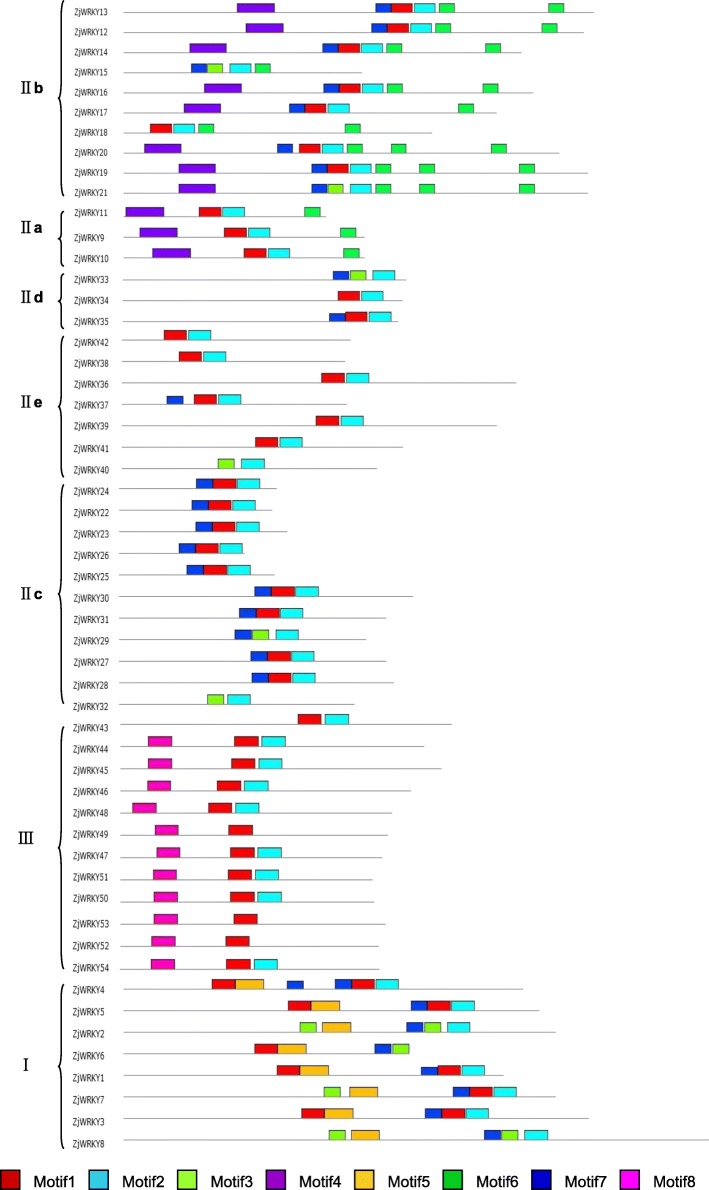
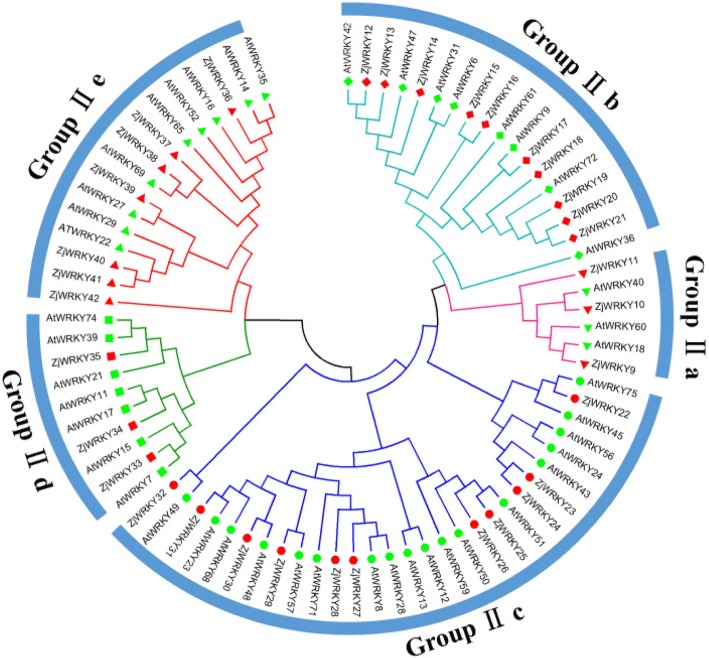
The phylogenetic tree of the ZjWRKY proteins was constructed by aligning multiple domain sequences (Fig. 1). The ZjWRKY proteins were classified into three groups (Group I, II and III) (Table 1) according to their WRKY and zinc-finger motifs. The domain sequences in the ZjWRKY gene family were highly conserved. There were 8 motifs among ZjWRKYs and proteins in the same group had similar numbers and types of motifs (Fig. 2, Additional file 2). The WRKY domain (WRKYGQK, Motif 1) was highly conserved among the 54 proteins (Additional file 2) and only two of them contained variations. The group II proteins ZjWRKY25 and ZjWRKY26 showed a WRKY motif with one amino acid modifications (WRKYGKK) (Table 1, Fig. 2). Motif 2 was also highly conserved except in the two Group III proteins ZjWRKY52 and ZjWRKY53. Motif 5 and Motif 8 were specific to groups I and III respectively.
Fig. 1.
The phylogenetic tree of the ZjWRKY proteins. The NJ tree was constructed from the amino acid sequences of ZjWRKYs using MEGA5.2 with 1000 bootstrap replicates
Fig. 2.
Conserved motifs of the ZjWRKY proteins arranged according to their phylogenetic relationships. The motifs in the ZjWRKYs were identified using Multiple Em for Motif Elicitation (MEME). In ZjWRKY proteins, 8 conserved motifs were identified and shown in different colors
Group I had 8 proteins (Table 1), that contained two WRKY motifs, and two C2H2 zinc-finger motifs. Group II was the biggest group and included 34 proteins that contained a WRKY motif and a C2H2 zinc-finger motif. According to the phylogenetic analysis, the 34 genes could be further divided into five subgroups (IIa to e) that included 3, 10, 11, 3 and 7 genes, respectively (Table 1). The members of subgroups IIa, IIb, IId and IIe had a CX5CX23HX1H zinc-finger motif, while that of subgroup IIc had a CX4CX23HX1H structure (Table 1). Group III contained 12 proteins, and they had one WRKY motif and a C2HC zinc-finger motif (CX7CX23HX1C, Table 1).
The chromosomal location and gene structure of ZjWRKYs
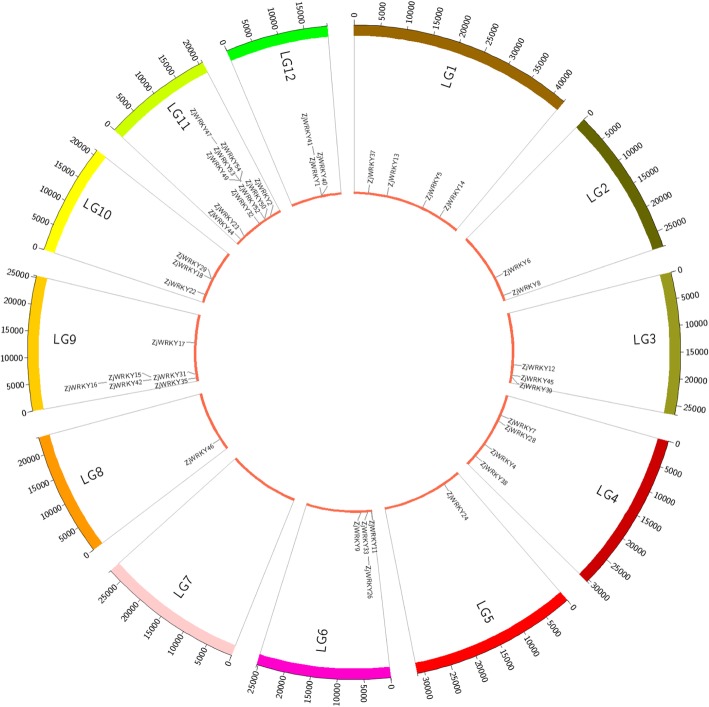
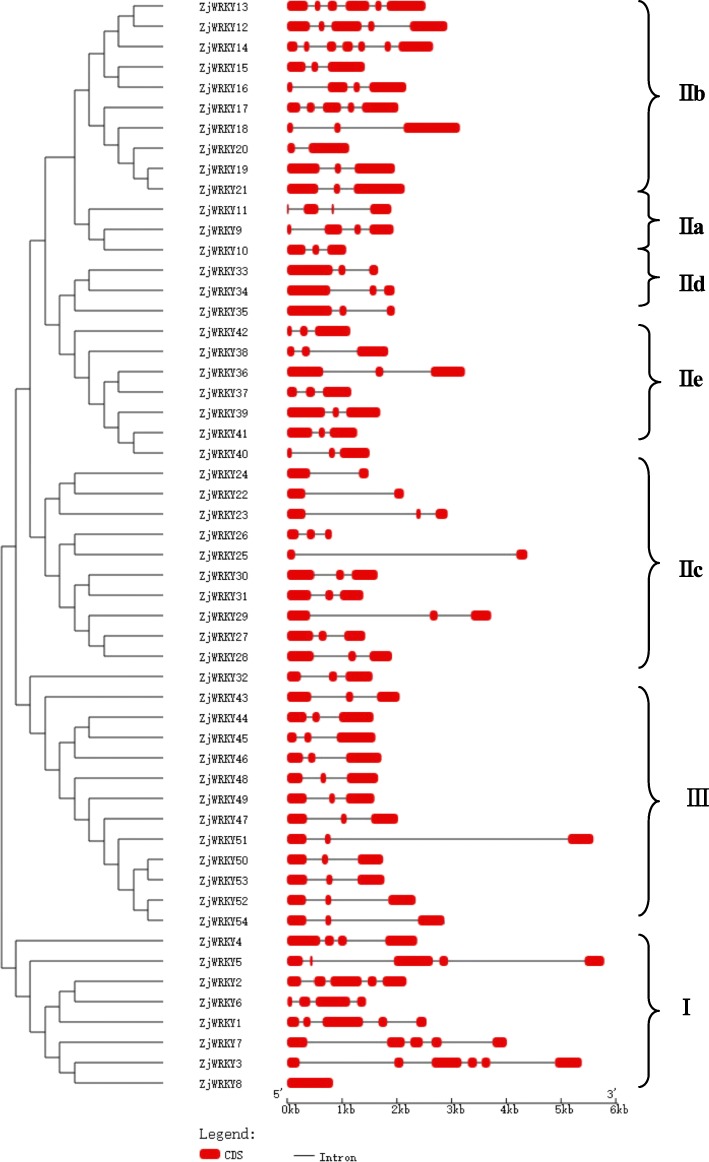
Of the 54 ZjWRKY genes, 41 were mapped to 11 of 12 pseudo chromosomes in the jujube genome (Fig. 3), and 13 genes were located on 12 scaffolds (Table 1, Additional file 3). ZjWRKYs were not evenly distributed across the 11 pseudo chromosomes (Fig. 3). Ten ZjWRKYs (18.5%) were located on Chr. 11, whereas only one ZjWRKY gene was on Chr. 5 and 8 each. No ZjWRKY gene was found on Chr. 7. Additionally, the gene structure was highly conserved within each group, especially in groups IId, IIe, and III. We found that Group I genes contained more introns and were more complicated than genes in the other two groups (Fig. 4). Tandem duplications were present in 40.7% of ZjWRKY genes (ZjWRKY1, 15, 16, 18, 26, 29, 31, 33, 35, 39, 40, 41, 42, 45, 47, 49, 50, 52, 53, and 54), which contributed to the expansion of the ZjWRKY gene family. This dynamic was particularly evident in Group III in which 8 out of the 12 genes (66.7%) mapped to duplicated chromosome or scaffold regions.
Fig. 3.
Positions of 41 ZjWRKY genes on the jujube chromosomes. Genes were mapped to the jujube chromosomes via the Circos tool. The jujube chromosomes were arranged in a circle
Fig. 4.
The exon/intron structure of 54 ZjWRKY genes in Chinese jujube. Introns and exons are represented by black lines and red boxes respectively
Expression profiles of ZjWRKYs in various tissues/organs
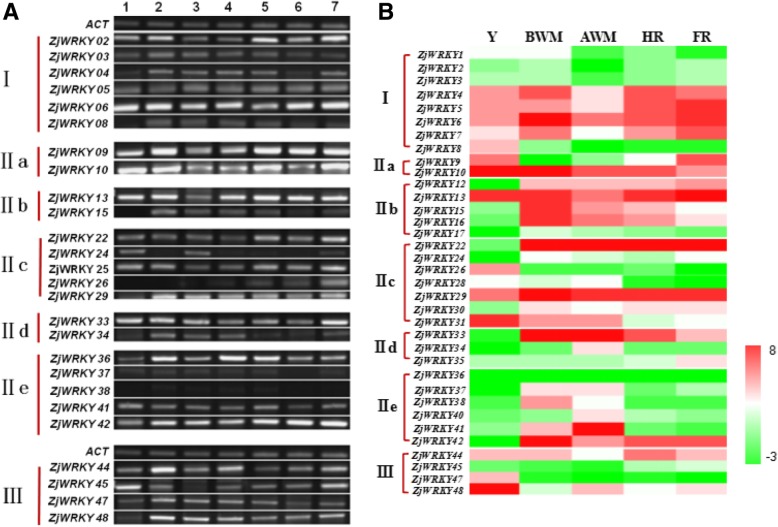
To investigate the tissue-specific expression of the jujube WRKY genes, RT-PCR was used to determine their expression patterns in seven tissues. The expression patterns of 26 ZjWRKY genes were analysed and are shown in Fig. 5a. Of the 26 ZjWRKY genes, six genes were actively expressed in at least five tissues, including ZjWRKY6, 9, 10, 13, 29, and 42. A total of 8 ZjWRKY genes (ZjWRKY2, 22, 25, 33, 36, 44, 45 and 48) were found to be upregulated in only one or two tissues, indicative of the tissue-specific expression of these genes. ZjWRKY24 expression could only be detected in roots and old branches. The expression of the remaining genes was comparatively low in the different organs, suggesting that genes in the same group might have different functions. These results showed that most of the ZjWRKY genes had diverse tissue-specific expression patterns, indicating that ZjWRKYs play multiple roles in various organs.
Fig. 5.
a Expression pattern of ZjWRKY genes in seven tissues/organs by RT-PCR. ZjACT was used as an internal control. From left to right: root, bearing shoot, secondary shoot, leaf, flower bud, flower, and fruit. b Heat map of RNA-Seq data for WRKY genes during jujube fruit ripening. Y, young fruit; BWM, before white mature fruit; WM, white mature fruit; HR, half-red fruit; FR, full red fruit. Scaled log2 expression values are shown from green to red, indicating low to high expression
Moreover, a heat map of our RNA-Seq data highlighted differential expression of ZjWRKYs during jujube fruit development (Fig. 5b), and most of the genes were expressed at different levels. The genes of group IIe were mainly expressed at before white mature period (BWM) and white mature period (WM), and the expression of group IIb genes was lower at young fruit period (Y) except for ZjWRKY13. ZjWRKY8, 26, 47, and 48 were only involved in the development of young fruit, suggesting the role for these WRKY genes in jujube fruit development.
ZjWRKYs involved in the jujube-phytoplasma interaction
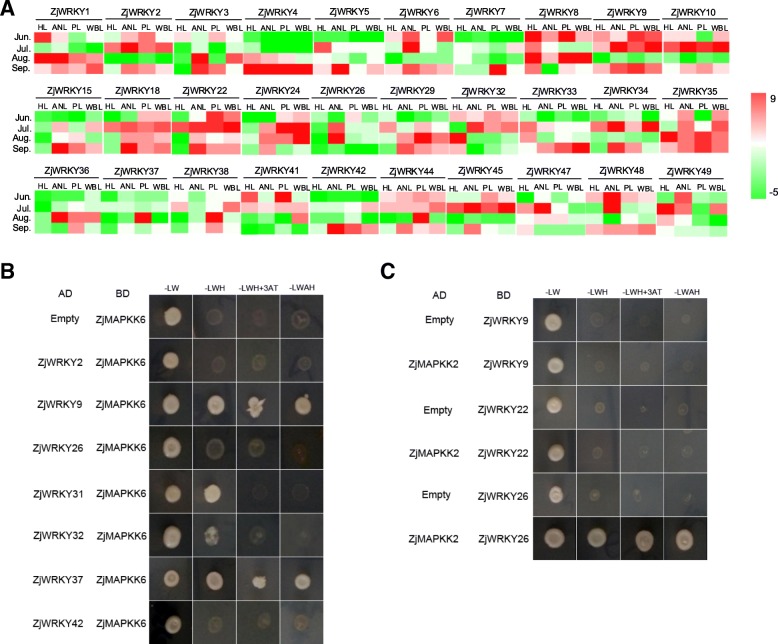
The expression of the phytoplasma TMK gene was not detected in the healthy leaves, however its highly expression was found in other diseased tissues, including the apparently normal leaves (Additional file 4). Among the 30 ZjWRKY genes detected, 17 ZjWRKYs (ZjWRKY2, 3, 6, 9, 10, 15, 18, 22, 24, 26, 34, 36, 37, 38, 42, 44, and 45) were significantly upregulated under phytoplasma stress, while 5 ZjWRKYs (ZjWRKY5, 8, 33, 47, and 49) were downregulated (Fig. 6a). The expression of ZjWRKY32 first increased and then decreased in diseased jujube. Most Group II genes were upregulated under phytoplasma stress. ZjWRKY37, ZjWRKY38, and ZjWRKY44 were significantly upregulated in phyllody leaves. ZjWRKY5 and ZjWRKY49 were significantly downregulated in diseased leaves. These ZjWRKY genes displayed noticeable changes in expression and should play vital roles in jujube-phytoplasma interactions.
Fig. 6.
a Heat map of relative expression of WRKY genes under phytoplasma stress. Scaled log2 expression values are shown from green to red, indicating low to high expression. (b and c) Yeast two-hybrid screening of ZjWRKYs and ZjMAPKK2/ZjMAPKK6
STRING analysis displayed that WRKY proteins could function by interacting with each other, as well as with MPK3 (Additional file 7A). Furtherly, yeast two-hybrid screening proved that ZjWRKY9 and ZjWRKY37 were interacting with ZjMAPKK6 (Fig. 6b), and ZjWRKY26 was interacting with ZjMAPKK2 (Fig. 6c). In previous study, it was found that ZjMAPKs and ZjMAPKKs were also involved in phytoplasma infection [28].
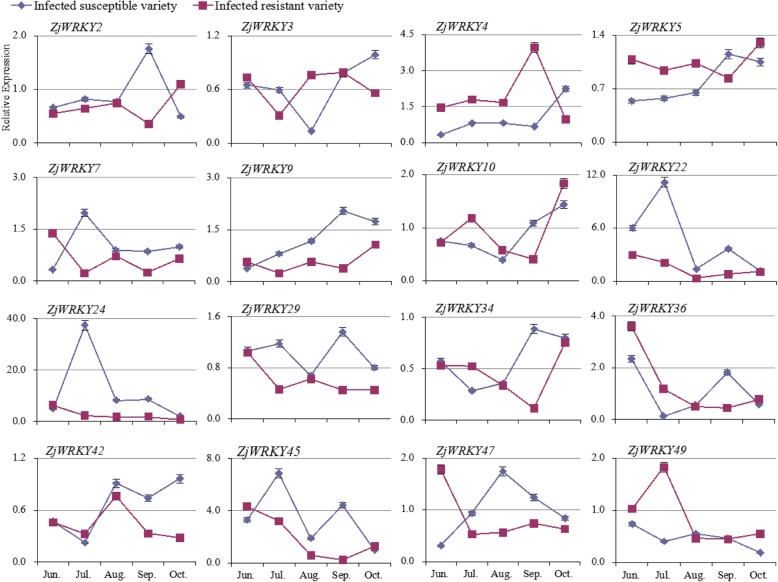
To confirm the identities of these ZjWRKY genes for phytoplasma tolerance, we analysed the transcript profiles of ZjWRKY genes in a JWB-resistant variety and a susceptible variety (Fig. 7). The detection of the phytoplasma in the two varieties was shown in Additional file 4. After phytoplasma infection, the expression of 9 ZjWRKYs (ZjWRKY2, 9, 22, 24, 29, 34, 36, 42, and 45) in the susceptible variety were higher than in the resistant variety, and they were also upregulated in above diseased tissues (Fig. 6). In contrast, the expression of ZjWRKY5 and ZjWRKY49 in the resistant variety was higher than in the susceptible one during the early stages of infection. These two genes were significantly downregulated in diseased tissues. ZjWRKY4 expression in the resistant variety was also higher than in the susceptible one. The above results indicated that some ZjWRKYs might play a role in phytoplasma tolerance.
Fig. 7.
Relative expression of ZjWRKY genes in JWB-resistant and susceptible varieties under phytoplasma stress
Discussion
In this study, a total of 54 WRKY-encoding genes were identified in the jujube genome. These genes can be can be divided into three groups (Group I to III), but this number may increase in the future once problems with the assembly and annotation of the jujube genome are addressed. As in other plants, almost all of the ZjWRKY genes share the WRKYGQK signature motif. However, the WRKYGKK variant was found in two jujube genes (Table 1, Fig. 2). Such slight variations in this region have also been reported in other plants such as Arabidopsis and apple [29].
Gene duplication events are the biggest contributors to the rapid expansion and evolution of gene families. Previous research has demonstrated that the Arabidopsis Group III WRKY gene family expanded rapidly as a result of recent segmental and tandem duplication events [30], and we found that this was also the case in the jujube genome. There are 6 tandemly duplicated ZjWRKY genes (ZjWRKY47, ZjWRKY49, ZjWRKY50, ZjWRKY52, ZjWRKY53, and ZjWRKY54) in Group III. The phylogenetic analysis (Additional file 5) indicated that 6 Group III ZjWRKYs were grouped and then clustered with 6 other genes from Arabidopsis; this also occurred in other subgroups from apple and pear. This finding suggests that the duplications in Group III WRKY genes occurred after the divergence of these plant species and tandem duplication events are the main contributors to the expansion of the Group III genes.
Previous research has demonstrated that Group I WRKY genes are the ancestors of the other WRKY genes in plants and are more likely to be constitutively expressed in different tissues [30]. In our study, the Group I genes and many genes from the other two groups were expressed in various tissues (Fig. 5), indicative of their diverse functions. These results provide some useful clues for additional investigations into the biological functions of these WRKY genes in jujube growth and development.
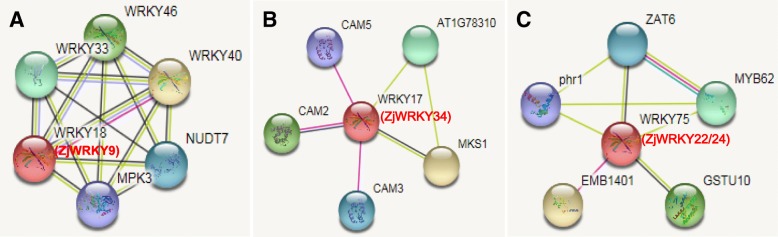
Transgenic apple lines overexpressing MdWRKY9 were significantly shorter and had significantly lower internode lengths than control plants [31], and its two orthologues in Arabidopsis (AtWRKY11 and AtWRKY17) are negative regulators of basal resistance to a bacterial pathogen [32–34]. The Group II phylogenetic tree (Fig. 8) indicates that AtWRKY11, 17, and ZjWRKY34 are closely related. In this study, we found that ZjWRKY34 was expressed at a noticeably higher level in infected jujube (Fig. 6) and in the JWB-resistant variety than in the susceptible variety at later stages of infection. STRING analysis showed that ZjWRKY34 (the orthologous of AtWRKY17) can interact with MSK1 and calmodulin (CAM, Fig. 9b). MKS1 is a regulator of plant defense response and it may contribute to MPK4-regulated defense activation by coupling the kinase to specific WRKY transcription factors. It also indicated that ZjWRKY34 might interact with the calmodulin-Ca2+ complex. Inferring the potential functions of ZjWRKY34 from the known AtWRKYs suggests that ZjWRKY34 might also act as a negative regulator in the defence process during jujube-phytoplasma interactions.
Fig. 8.
Phylogenetic relationships of GroupII WRKY genes from Chinese jujube and Arabidopsis
Fig. 9.
The protein-protein interaction analysis of ZjWRKY9, ZjWRKY34, ZjWRKY22 and ZjWRKY24 by STRING database. a ZjWRKY9 is the orthologous of AtWRKY18; b ZjWRKY34 is the orthologous of AtWRKY17; c ZjWRKY22 and ZjWRKY24 were the orthologous of AtWRKY75
ZjWRKY9 was actively expressed in JWB-diseased tissues. The phylogenetic tree (Fig. 8) and sequence alignment showed that ZjWRKY9 was the orthologous gene of AtWRKY18. Transgenic AtWRKY18 plants had increased expression of pathogenesis-related genes and resistance to the bacterial pathogen Pseudomonas syringae, indicating that AtWRKY18 can positively modulate defence-related gene expression and disease resistance [12]. AtWRKY18/40 act in a feedback repression system controlling basal defences [10]. In the other side, high AtWRKY18 expression can cause severely abnormal plant growth [12]. These results suggest that proper expression of ZjWRKY9 is critical for enhancing jujube’s defence response without negatively impacting plant growth. ZjWRKY9’s higher expression might be related to the abnormal growth of diseased jujube trees, such as witches’ broom and tiny leaves.
Overexpression of AtWRKY28 and AtWRKY75 induced an oxidative burst in host plants, which suppressed the hyphal growth of Sclerotinia sclerotiorum and consequently inhibited fungal infection [35]. STRING analysis predicted that AtWRKY75 could interact with GSTU10 (Fig. 9), which can eliminate the toxicity of oxygen bursts on plant cells and increase plant tolerance [36]. In this study, ZjWRKY22 and ZjWRKY24 are orthologous of AtWRKY75 (Fig. 8), which behaved higher expression in the susceptible variety than in the resistant one (Fig. 7). That means that the two genes might eliminate the toxicity of oxygen bursts caused by phytoplasma infection. Previous study also indicated that the expression of WRKY gene was responsive to phytoplasma infection [37]. A divergent behaviour was previously observed for OsWRKY28. In rice, overexpression of OsWRKY28 enhanced susceptibility to the rice blast fungus Magnaporthe oryzae and decreased accumulation of PR5 [38]. The knock-out of OsWRKY28 led to a two-fold increase in resistance to a compatible rice blast fungus and this phenotype is accompanied by the increased expression of several defence-related genes [39]. Hence, OsWRKY28 acts as a negative regulator of basal defence responses. Similarly, some WRKY genes might act as negative regulators of the basal resistance of jujube under phytoplasma stress, but further study is necessary to verify their specific functions.
Conclusions
This paper described the WRKY gene family of Chinese jujube at the genome level. Their gene structure, chromosomal distribution, phylogenetic relationship, and tissue-specific expression patterns were presented in this study. Most of the ZjWRKYs were positive responses to phytoplasma invasion, and that provided meaningful candidates for the future studies of ZjWRKYs involved in jujube-phytoplasma interaction.
Methods
Plant material
The seven tissues including roots, young branches, old branches, leaves, flower buds, flowers and young fruits were collected from three jujube trees and used for organ-specific expression analysis.
Four kinds of tissues representing different degrees of JWB disease (apparently normal leaves (ANL), phyllody leaves (PL), and witches’-broom leaves (WBL)) from diseased trees, and healthy leaves (HL) from healthy trees were collected at four growth periods (June, July, August, and September). The All treatments were conducted with three biological replicates.
Phytoplasma cannot be cultured in vitro, and thus, JWB phytoplasma infection was transmitted by grafting. A JWB-resistant variety and a susceptible variety were used as scions for grafting onto JWB-diseased and healthy trees. All grafting treatments were conducted with three replicates. The samples were collected from sprouted scions at five growth periods (June, July, August, September and October). The samples were stored at − 80 °C until RNA extraction and expression analysis.
The JWB phytoplasma presence of the samples was detected by quantitative real-time PCR (qRT-PCR) [40]. The expression of phytoplasma TMK gene in jujube samples was analysed and ZjACT was used as an internal control.
Identification and protein structure analysis of ZjWRKYs in Chinese jujube
First, WRKY genes from Arabidopsis were used as queries to search the jujube genome database. Next, the Pfam (http://pfam.xfam.org/) and SMART (http://smart.embl-heidelberg.de/) databases were used to confirm the predicted jujube WRKY proteins. To further confirm that the amino acid sequences in our data set were WRKYs, we manually examined the conserved WRKYGQK amino acid motif at the N-terminus and the zinc-finger-like motif at the C-terminus of the predicted WRKY domain. Truncated and false genes were excluded from our analysis. The number of amino acids, molecular weight, and theoretical pI of ZjWRKY genes were predicted by Protparam (https://web.expasy.org/compute_pi/). The conserved motifs of ZjWRKY proteins were detected by MEME (http://meme-suite.org/), using the following parameters: number of repetitions, any; maximum number of motifs, 20; and the optimum motif widths, 6–60 amino acid resides [41].
The chromosomal location and gene structure of ZjWRKYs
To determine the chromosomal location of the ZjWRKY genes, their gene sequences were used as query sequences in BLASTN searches against the jujube genome. Each ZjWRKY gene was mapped to the jujube genome according to their genome coordinates. Tandem duplications were identified as previously described [42].
The website GSDS (http://gsds.cbi.pku.edu.cn/) was used to predict the number of exons from the coding domain sequences (CDS) and DNA sequences of the WRKY genes [43].
Multiple sequence alignment and phylogenetic tree construction
The jujube WRKY proteins were classified into different groups based on their conserved domains. A phylogenetic tree was constructed from the amino acid sequences of WRKY conserved domains from jujube (54 sequences). The Arabidopsis thaliana WRKY proteins were retrieved from the TAIR database (http://www.arabidopsis.org/) as reported previously. Additionally, WRKY proteins of three other species (Persica prunus [22], Pyres bretschneideri [21], and Malus domestica [29]) were downloaded from NCBI. The classification of jujube Group II WRKY genes using the phylogenetic tree was dependent on the putative Arabidopsis thaliana orthologs. The MEGA 5.2 software and the neighbour-joining statistical method were used to construct a rooted phylogenetic tree [44–46]. The evolutionary distances were obtained using the p-distances method, and these distances were used to estimate the number of amino acid substitutions per site. The reliability of each phylogenetic tree was established by conducting 1000 bootstrap sampling iterations.
RNA isolation and expression and statistical analysis
Total RNA was extracted using an RNAprep Pure Plant Kit (TIANGEN) according to the manufacturer’s protocol. After genomic DNA was removed by RNase-free DNase I (TIANGEN), RNA concentration and purity were checked on a NanoDrop2000 spectrophotometer. First-strand cDNA was synthesized by reverse transcribing 500 ng of total RNA with FastQuant RT Super Mix Kit (TIANGEN). The cDNA was used as the template for qRT-PCR.
Gene expression was detected by qRT-PCR. The primers used in this study are listed in Additional file 6. PCR products were amplified in triplicate using the Bio-Rad iQ™5 with TransStart Top Green qPCR SuperMix AQ131 (TransGen Biotech, China) in 20 μL reactions. Each reaction contained 10 μL of 2 × TransStart® Top Green qPCR SuperMix, 0.4 μL each of 10 μM primers, 8.2 μL of ddH2O and 1 μL of cDNA. The thermal profile for RT-qPCR was as follows: preincubation for 30 s at 95 °C, followed by 40 cycles of 5 s at 95 °C, 10 s at 53–58 °C, and 10 s at 72 °C. Three biological replicates were performed for each treatment. Threshold cycle values were calculated using iCycler software, and ZjACT was used as an internal control [47]. Relative transcript levels were calculated according to the 2–ΔΔCT method [48].
Yeast two-hybrid screening (Y2H)
ZjWRKY protein is fused to the Gal4 DNA-binding domain (BD) and the screening proteins are fused to the Gal4 activation domain (AD). The AD-fused ZjWRKY and BD-fused ZjMAPK were amplified using the primers shown in Supplementary Table S1, and cloned into the pGADT7 vector and pGBKT7 respectively. ZjWRKYs were digested by SmaI and the ZjMAPKs were digested by EcoRI and co-transformed AH109 stain with pairs of appropriate pGADT7 and pGBKT7 vectors. Successful co-transformants were selected on synthetically defined medium lacking tryptophan and leucine (SD/−Trp/−Leu). To examine protein-protein interactions, freshly transformed yeast colonies were resuspended in 10 μL sterile deionized water, and 0.5 μL aliquots were spotted upon medium lacking leucine and tryptophan (−LW) and medium lacking leucine, tryptophan, histidine (−LWH), supplemented with 7 mM 3-Amino-1,2,4-triazole (3-AT; Sigma Aldrich) (−LWH + 3AT) and medium lacking leucine, tryptophan, histidine, adenine (−LWAH). Growth was scored after 3 d of incubation at 28 °C.
Additional files
Number of WRKY gene family from Chinese jujube and other species (DOC 28 kb)
The amino acid sequences of 8 motifs among ZjWRKY proteins. (DOC 160 kb)
Positions of 13 ZjWRKY genes on the jujube scaffolds. The jujube scaffolds were arranged in a circle. (DOC 319 kb)
Expression analysis of phytoplasma TMK in four kinds of leaves (A) and in susceptible and resistant varieties (B). (DOC 31 kb)
The phylogenetic analysis of Group III WRKYs of Ziziphus jujuba, Arabidopsis thaliana, Pyres Bretschneideri, Persica Prunus and Malus domestica. (DOC 89 kb)
The primers of ZjWRKY genes used in this study. (DOC 36 kb)
Acknowledgements
Not applicable.
Funding
Supported by the National Key R&D Program Project Funding (2018YFD1000607), the National Natural Science Foundation of China (31772285), and Hebei Distinguished Young Scholar (C2016204145). These funding bodies had no role in the design of the study, sample collection, analysis or interpretation of data and in writing the manuscript.
Availability of data and materials
All data and materials are presented in the main paper and additional supporting file.
Abbreviations
- ANL
Apparently normal leaves
- CDS
Coding domain sequences
- HL
Healthy leaves
- JWB
Jujube witches’ broom disease
- PL
Phyllody leaves
- qRT-PCR
Quantitative real-time PCR
- TFs
Transcription factors
- WBL
Witches’-broom leaves
Authors’ contributions
JZ and ML designed the research; CX, HL and JZ performed the experiments, analyzed the data and wrote the paper. ZL, LW and HF participated in the data analysis. YZ and XW performed RT-PCR, RT-qPCR experiments. All authors read and approved the final the manuscript.
Ethics approval and consent to participate
Chinese jujube is one of widespread fruit trees in China, and it is not an endangered species. The healthy and diseased jujube trees were from the Experimental Station of Chinese Jujube, Hebei Agricultural University. No specific permits are required for sample collection on Chinese jujube.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chaoling Xue, Email: 18932666571@189.cn.
Hongtai Li, Email: 1061752104@qq.com.
Zhiguo Liu, Email: jujubeliu@163.com.
Lili Wang, Email: lily850908@163.com.
Yitong Zhao, Email: 1016564743@qq.com.
Ximeng Wei, Email: 804613481@qq.com.
Hu Fang, Email: fanghu@bgitechsolutions.com.
Mengjun Liu, Email: lmj1234567@aliyun.com.
Jin Zhao, Email: zhaojinbd@126.com.
References
- 1.Eulgem T, Rushton P, Robatzek S, Somssich I. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000;5:199–206. doi: 10.1016/S1360-1385(00)01600-9. [DOI] [PubMed] [Google Scholar]
- 2.Lagace M, Matton DP. Characterization of a WRKY transcription factor expressed in late torpedo-stage embryos of Solanum chacoense. Planta. 2004;219(1):185–189. doi: 10.1007/s00425-004-1253-2. [DOI] [PubMed] [Google Scholar]
- 3.Johnson CS, Kolevski B, Smyth DR. Transparent TESTA GLABRA2, a trichome and seed coat development gene of Arabidopsis, encodes a WRKY transcription factor. Plant Cell. 2002;14:1359–1375. doi: 10.1105/tpc.001404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miao Y, Laun T, Zimmermann P, Zentgraf U. Targets of the WRKY53 transcription factor and its role during leaf senescence in Arabidopsis. Plant Mol Biol. 2004;55:853–867. doi: 10.1007/s11103-005-2142-1. [DOI] [PubMed] [Google Scholar]
- 5.Li W, Wang H, Yu D. Arabidopsis WRKY transcription factors WRKY12 and WRKY13 oppositely regulate flowering under short-day conditions. Mol Plant. 2016;9:1492–1503. doi: 10.1016/j.molp.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Guan Y, Meng X, Khanna R, LaMontagne E, Liu Y, Zhang S. Phosphorylation of a WRKY transcription factor by MAPKs is required for pollen development and function in Arabidopsis. PLoS Genet. 2014;10(5):e1004384. doi: 10.1371/journal.pgen.1004384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rinerson CI, Scully ED, Palmer NA, Donze-Reiner T, Rabara RC, Tripathi P, et al. The WRKY transcription factor family and senescence in switchgrass. BMC Genomics. 2015;16:1–17. doi: 10.1186/s12864-015-2057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suttipanta N, Pattanaik S, Kulshrestha M, Patra B, Singh SK, Yuan L. The transcription factor CrWRKY1 positively regulates the terpenoid indole alkaloid biosynthesis in Catharanthus roseus. Plant Physiol. 2011;157(4):2081–2093. doi: 10.1104/pp.111.181834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang ZL. A rice WRKY gene encodes a transcriptional repressor of the gibberellin signaling pathway in aleurone cells. Plant Physiol. 2004;134(4):1500–1513. doi: 10.1104/pp.103.034967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandey SP, Roccaro M, Schon M, Logemann E, Somssich IE. Transcriptional reprogramming regulated by WRKY18 and WRKY40 facilitates powdery mildew infection of Arabidopsis. Plant J. 2010;64(6):912–923. doi: 10.1111/j.1365-313X.2010.04387.x. [DOI] [PubMed] [Google Scholar]
- 11.Mukhtar MS, Deslandes L, Auriac MC, Marco Y, Somssich IE. The Arabidopsis transcription factor WRKY27 influences wilt disease symptom development caused by Ralstonia solanacearum. Plant J. 2008;56(6):935–947. doi: 10.1111/j.1365-313X.2008.03651.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen C, Chen Z. Potentiation of developmentally regulated plant defense response by AtWRKY18, a pathogen-induced Arabidopsis transcription factor. Plant Physiol. 2002;129(2):706–716. doi: 10.1104/pp.001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Brader G, Kariola T, Tapio Palva E. WRKY70 modulates the selection of signaling pathways in plant defense. Plant J. 2006;46:477–491. doi: 10.1111/j.1365-313X.2006.02712.x. [DOI] [PubMed] [Google Scholar]
- 14.Knoth C, Ringler J, Dangl JL, Eulgem T. Arabidopsis WRKY70 is required for full RPP4-mediated disease resistance and basal defense against Hyaloperonospora parasitica. Mol Plant-Microbe Interact. 2007;20:120–128. doi: 10.1094/MPMI-20-2-0120. [DOI] [PubMed] [Google Scholar]
- 15.Kim KC, Lai Z, Fan B, Chen Z. Arabidopsis WRKY38 and WRKY62 transcription factors interact with histone deacetylase 19 in basal defense. Plant Cell. 2008;20:2357–2371. doi: 10.1105/tpc.107.055566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng X, Hu Y, Tang X, Zhou P, Deng X, Wang H, Guo Z. Constitutive expression of rice WRKY30 gene increases the endogenous jasmonic acid accumulation, PR gene expression and resistance to fungal pathogens in rice. Planta. 2012;236:1485–1498. doi: 10.1007/s00425-012-1698-7. [DOI] [PubMed] [Google Scholar]
- 17.Viana VE, Busanello C, da Maia LC, Pegoraro C, Costa de Oliveira A. Activation of rice WRKY transcription factors: an army of stress fighting soldiers? Curr Opin Plant Biol. 2018;45:268–275. doi: 10.1016/j.pbi.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Cannon SB, Mitra A, Baumgarten A, Young ND, May G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004;4(1):10. doi: 10.1186/1471-2229-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross CA, Liu Y, Shen QJ. The WRKY gene family in rice (Oryza sativa) J Integr Plant Biol. 2007;49(6):827–842. doi: 10.1111/j.1744-7909.2007.00504.x. [DOI] [Google Scholar]
- 20.Wang L, Zhu W, Fang L, Sun X, Su L, Liang Z, Wang N, Londo JP, Li S, Xin H. Genome-wide identification of WRKY family genes and their response to cold stress in Vitis vinifera. BMC Plant Biol. 2014;14(1):103. doi: 10.1186/1471-2229-14-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang X, Li K, Xu X, et al. Genome-wide analysis of WRKY transcription factors in white pear (Pyrus bretschneideri) reveals evolution and patterns under drought stress. BMC Genomics. 2015;16(1):1104. doi: 10.1186/s12864-015-2233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen M, Tan Q, Sun M, Li D, Fu X, Chen X, Xiao W, Li L, Gao D. Genome-wide identification of WRKY family genes in peach and analysis of WRKY expression during bud dormancy. Mol Gen Genomics. 2016;291:1319–1332. doi: 10.1007/s00438-016-1171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu MJ. Horticultural reviews. 2010. Chinese jujube: botany and horticulture; p. 229. [Google Scholar]
- 24.Liu MJ, Zhao J, Cai QL, et al. The complex jujube genome provides insights into fruit tree biology. Nat Commun. 2014;5:5315. doi: 10.1038/ncomms6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang J, Zhang C, Zhao X, Fei Z, Wan K, Zhang Z, Pang X, Yin X, Bai Y, Sun X, Gao L, Li R, Zhang J, Li X. The jujube genome provides insights into genome evolution and the domestication of sweetness/acidity taste in fruit trees. PLoS Genet. 2016;12(12):e1006433. doi: 10.1371/journal.pgen.1006433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu MJ, Zhao J, Wang JR, Liu ZG, Liu GC. Phylogenetic analysis of 25 plant species representing 19 angiosperm families and one gymnosperm family based on 390 orthologous genes. Plant Syst Evol. 2017;303(3):413–417. doi: 10.1007/s00606-016-1380-9. [DOI] [Google Scholar]
- 27.Verde I, Abbott AG, Scalabrin S, et al. The high-quality draft genome of peach (Prunus persica) identifies unique patterns of genetic diversity, domestication and genome evolution. Nat Genet. 2013;45(5):487–494. doi: 10.1038/ng.2586. [DOI] [PubMed] [Google Scholar]
- 28.Liu Z, Zhang L, Xue C, Fang H, Zhao J, Liu M. Genome-wide identification and analysis of MAPK and MAPKK gene family in Chinese jujube (Ziziphus jujuba mill.) BMC Genomics. 2017;18:855. doi: 10.1186/s12864-017-4259-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meng D, Li Y, Bai Y, et al. Genome-wide identification and characterization of WRKY transcriptional factor family in apple and analysis of their responses to waterlogging and drought stress. Plant Physiol Biochem. 2016;103:71–83. doi: 10.1016/j.plaphy.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Wang L. The WRKY transcription factor superfamily: its origin in eukaryotes and expansion in plants. BMC Evol Biol. 2005;5(1):1. doi: 10.1186/1471-2148-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng X, Zhao Y, Shan D, et al. MdWRKY9 overexpression confers intensive dwarfing in the M26 rootstock of apple by directly inhibiting brassinosteroid synthetase MdDWF4 expression. The New phytologist. 2017;217(3):1086–1098. doi: 10.1111/nph.14891. [DOI] [PubMed] [Google Scholar]
- 32.Journot-Catalino N, Somssich IE, Roby D, Kroj T. The transcription factors WRKY11 and WRKY17 act as negative regulators of basal resistance in Arabidopsis thaliana. Plant Cell. 2006;18:3289–3302. doi: 10.1105/tpc.106.044149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ali MA, Wieczorek K, Kreil DP, Bohlmann H. The beet cyst nematode Heterodera schachtii modulates the expression of WRKY transcription factors in syncytia to favour its development in Arabidopsis roots. PLoS One. 2014;9:e102360. doi: 10.1371/journal.pone.0102360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang CH, Huang ZY, Xie P, Gu C, Li K, Wang DC, Yu YY, Fan ZH, Wang CJ, Wang YP, et al. Transcription factors WRKY70 and WRKY11 served as regulators in rhizobacterium Bacillus cereus AR156-induced systemic resistance to Pseudomonas syringae pv. Tomato DC3000 in Arabidopsis. J Exp Bot. 2016;67:157–174. doi: 10.1093/jxb/erv445. [DOI] [PubMed] [Google Scholar]
- 35.Chen X, Liu J, Lin G, et al. Overexpression of AtWRKY28 and AtWRKY75 in Arabidopsis enhances resistance to oxalic acid and Sclerotinia sclerotiorum. Plant Cell Rep. 2013;32(10):1589–1599. doi: 10.1007/s00299-013-1469-3. [DOI] [PubMed] [Google Scholar]
- 36.Edwards R, Dixon DP. Plant glutathione transferases. Method Enzymol. 2005;401:169–186. doi: 10.1016/S0076-6879(05)01011-6. [DOI] [PubMed] [Google Scholar]
- 37.Ye X, Wang H, Chen P, Fu B, Zhang M, Li J, Zheng X, Tan B, Feng J. Combination of iTRAQ proteomics and RNA-seq transcriptomics reveals multiple levels of regulation in phytoplasma-infected Ziziphus jujuba mill. Hortic Res. 2017;4:17080. doi: 10.1038/hortres.2017.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chujo T, Miyamoto K, Shimogawa T, Shimizu T, Otake Y, Yokotani N. OsWRKY28, a PAMP responsive transrepressor, negatively regulates innate immune responses in rice against rice blast fungus. Plant Mol Biol. 2013;82(1):23–37. doi: 10.1007/s11103-013-0032-5. [DOI] [PubMed] [Google Scholar]
- 39.Delteil A, Blein M, Faivre-Rampant O, Guellim A, Estevan J, Hirsch J. Building a mutant resource for the study of disease resistance in rice reveals the pivotal role of several genes involved in defence. Mol Plant Pathol. 2012;13(1):72–82. doi: 10.1111/j.1364-3703.2011.00731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xue C, Liu Z, Dai L, Bu J, Liu M, Zhao Z, Jiang Z, Gao W, Zhao J. Changing host photosynthetic, carbohydrate and energy metabolisms play important roles in phytoplasma infection. Phytopathology. 2018;108(9):1067–1077. doi: 10.1094/PHYTO-02-18-0058-R. [DOI] [PubMed] [Google Scholar]
- 41.Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37(Web Server):W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paterson AH, Bowers JE, Chapman BA. Ancient polyploidization predating divergence of the cereals, and its consequences for comparative genomics. Proc Natl Acad Sci. 2004;101(26):9903–9908. doi: 10.1073/pnas.0307901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo A, Zhu Q, Chen X, Luo JGSDS. A gene structure display server. Yi Chuan. 2007;29:1023–1026. doi: 10.1360/yc-007-1023. [DOI] [PubMed] [Google Scholar]
- 44.Lui S, Luo C, Zhu L, Sha R, Qu S, Cai B, Wang S. Identification and expression analysis of WRKY transcription factor genes in response to fungal pathogen and hormone treatments in apple (Malus domestica) Journal of Plant Biology. 2017;60(2):215–230. doi: 10.1007/s12374-016-0577-3. [DOI] [Google Scholar]
- 45.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 47.Bu Jiaodi, Zhao Jin, Liu Mengjun. Expression Stabilities of Candidate Reference Genes for RT-qPCR in Chinese Jujube (Ziziphus jujuba Mill.) under a Variety of Conditions. PLOS ONE. 2016;11(4):e0154212. doi: 10.1371/journal.pone.0154212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Livak Kenneth J., Schmittgen Thomas D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Number of WRKY gene family from Chinese jujube and other species (DOC 28 kb)
The amino acid sequences of 8 motifs among ZjWRKY proteins. (DOC 160 kb)
Positions of 13 ZjWRKY genes on the jujube scaffolds. The jujube scaffolds were arranged in a circle. (DOC 319 kb)
Expression analysis of phytoplasma TMK in four kinds of leaves (A) and in susceptible and resistant varieties (B). (DOC 31 kb)
The phylogenetic analysis of Group III WRKYs of Ziziphus jujuba, Arabidopsis thaliana, Pyres Bretschneideri, Persica Prunus and Malus domestica. (DOC 89 kb)
The primers of ZjWRKY genes used in this study. (DOC 36 kb)
Data Availability Statement
All data and materials are presented in the main paper and additional supporting file.