Abstract
Background
Conflicting evidence exists regarding the effect of hypertension on the prognosis of metastatic renal cell carcinoma (mRCC) patients treated with tyrosine kinase inhibitors (TKIs). This study aimed to assess the predictive value of TKIs-induced hypertension in patients with mRCC.
Methods
This study was registered in PROSPERO (CRD42019129593). PubMed, Embase, Web of Science and the Cochrane Library database were searched with terms: “renal cell carcinoma”, “hypertension”, “blood pressure”, “tyrosine kinase inhibitor”, “sunitinib”, “axitinib”, “sorafenib” and “pazopanib” until March 21, 2019. Hazard Ratios (HR) and 95% confidence intervals (CI) for progression-free survival (PFS) or overall survival (OS) were extracted and analyzed with Stata 15.0 software. Heterogeneity was assessed using the I2 value. Meta-regression, subgroup analysis and sensitivity analysis were also performed to explore heterogeneity. Publication bias was assessed with funnel plots and precisely assessed by Egger’s and Begg’s tests. The quality of evidence of outcomes was generated according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE).
Results
A total of 4661 patients from 22 studies were included in the study. The results showed that the increase of blood pressure was an effective predictor for longer PFS (HR = 0.59, 95% CI: 0.48–0.71, p < 0.001; I2 = 77.3%) and OS (HR = 0.57, 95% CI: 0.45–0.70, p < 0.001; I2 = 77.4%) of patients with mRCC. Subgroup analysis revealed that patients receiving sunitinib and pazopanib could have longer PFS and OS.
Conclusions
This study indicated that TKIs-induced hypertension may be a good predictor for better prognosis of patients with mRCC receiving TKIs treatment, especially using sunitinib or pazopanib.
Electronic supplementary material
The online version of this article (10.1186/s12894-019-0481-5) contains supplementary material, which is available to authorized users.
Keywords: Metastatic renal cell carcinoma, Tyrosine kinase inhibitors, Hypertension, Prognosis, Meta-analysis
Background
Renal cell carcinoma (RCC) is the 9th most common cancer in men and 14th most common cancer in women worldwide [1]. Its incidence (3–6/100,000) and mortality (1.2–2.5/100,000) are increasing rapidly, which has a great negative effect on our society [2, 3]. In addition, about 25–30% of patients have evidence of metastasis upon its diagnosis [4]. Now, vascular endothelial growth factor receptor tyrosine kinase inhibitors (TKIs), like sunitinib, pazopanib, sorafenib or axitinib, are the favored medicine for metastatic RCC (mRCC). Several clinical trials showed that response to TKIs was uncertain (objective response rate was 31–67.4%) [5–8], which indicated the existence of wide inter-individual variation and the lack of reliable factors for predicting the outcomes of mRCC patients. Therefore, a big challenge faced by urologists is how to predict the prognosis of mRCC patients receiving TKIs more precisely.
The occurrence of several adverse events (AEs) during TKIs therapy, such as hypertension, hand-foot syndrome or hypothyroidism, were shown to be correlated with the longer median progression-free survival (PFS) and overall survival (OS) of mRCC patients [9]. Hypertension during TKIs therapy was very common, with incidence ranging from 22 to 81% [9, 10]. Recently, a study found that RCC patients with longer median PFS (>5.3 months) demonstrated a significantly higher incidence of high-grade hypertension (a treatment-associated adverse event) than those with shorter PFS [11]. It indicated that TKIs-induced hypertension may be associated with improved prognosis [12, 13]. However, others reported insignificant results [14, 15]. In 2014, a systematic review and meta-analysis reported that the occurrence of hypertension due to sorafenib therapy may be associated with improved prognosis of patients with cancer. However, this study did not specifically focus on the mRCC patients. Thus, the association between TKIs-induced hypertension and prognosis of mRCC is still controversial. In the present study, we attempt to conduct a systematic review and meta-analysis to assess the predictive value of the TKIs-induced hypertension for PFS and OS in patients with mRCC during TKIs therapy.
Methods
Data sources and literature search strategy
We conducted this meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement. This study was registered in PROSPERO (CRD42019129593). A literature search was performed in the following databases: PubMed, Embase, Web of Science and the Cochrane Library database. The latest search was performed on March 21, 2019. The search keywords were (renal cell carcinoma) AND [(tyrosine kinase inhibitor) OR sunitinib OR axitinib OR sorafenib OR pazopanib] AND [hypertension OR (blood pressure). The resulted literatures were further scanned by Endnote X7 (Thomson Corporation, Canada) to exclude duplications followed by title and abstract screening. In addition, we manually searched the references of the literatures for additional eligible studies.
Inclusion and exclusion criteria
Studies were included in our meta-analysis if the following criteria were met: 1) mRCC patients were treated with TKIs; 2) studies compared Hazard Ratios (HR) between patients with or without TKIs-induced hypertension for PFS or OS.
Studies were excluded based on the following criteria: 1) reviews, meta-analysis, letters, comments, case reports or conference abstracts; 2) duplicated studies and repeated analysis; 3) studies lacking sufficient data for HR and their 95% confidence intervals (CI); 4) studies included mRCC patients received different therapeutic regimen, such as TKIs or radiotherapy.
Quality of original studies
We assessed the quality of the 22 included studies using the Newcastle-Ottawa Scale (NOS). The total score was 9 stars. Studies awarded 7–9 stars were rated as high-quality, 5–6 stars as moderate-quality, and < 5 stars as low-quality.
Data extraction
Eligible studies were read thoroughly and carefully to extract the following information, including first author, year, region, sample size, male/female ratio, mean age, histology, number of disease sites, prior nephrectomy, Memorial Sloan Kettering Cancer Center (MSKCC) score, Eastern Cooperative Oncology Group performance status (ECOG PS) score, definition of hypertension, type of analysis (univariate or multivariate), study design (prospective or retrospective), type of TKIs. The primary outcome was HR and 95% CI between patients with or without hypertension for PFS and secondary one for OS. The definition of PFS was time from initiation of TKIs therapy to disease progression or death. OS was defined as time from initiation of TKIs therapy to death. If a study provided both univariate and multivariate analysis results, the multivariate analysis would be selected to achieve higher accuracy. Any discrepancies were resolved by discussion with the third reviewer.
Data synthesis and statistical analysis
Data were extracted and analyzed with Stata 15.0 software (Stata Corporation, College Station, TX, USA). P value<0.05 was considered statistically significant. A merged HR greater than 1 indicated a poorer prognosis for mRCC patients. Heterogeneity was assessed using the I2. We considered I2 > 50% as an indicator of substantial heterogeneity. A random effects model and a fixed effects model were applied for I2>50% and I2<50%, respectively. Then, to determine which factors may contribute to heterogeneity, univariate and multivariate meta-regression analysis were performed. The possible factors were year, sample size, gender, mean age, country, ECOG PS, MSKCC score, histology, prior nephrectomy, Number of disease sites, type of analysis (univariate, multivariate), study design (retrospective, prospective), type of TKIs. Then, subgroup analysis was performed to investigate whether different sample size could explain the heterogeneity and whether relationship between hypertension and PFS or OS still exist in different TKIs subgroups. Factor with P value < 0.05 meant that it may be the source of heterogeneity. We did sensitivity analysis to find if some original studies may mainly contribute to the heterogeneity. Publication bias was assessed with funnel plots and precisely assessed by Egger’s and Begg’s tests.
Quality of evidence
The quality of evidence of the predictive effect of TKIs-induced hypertension for the outcomes in mRCC patients was assessed according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) [16].
Results
Study selection
The searching process is shown in Additional file 1: Figure S1. A total of 982 studies were searched in the database. We excluded 345 duplicated articles. After screening title and abstract, 26 relevant studies were identified. In addition, three relevant studies were obtained from the references and seven articles were excluded due to lack of HR and 95% CI for PFS or OS. Finally, 22 studies were selected for the meta-analysis.
Study characteristics and quality
The baseline characteristics of these studies were demonstrated in Table 1. All the studies were published between 2011 and 2017. Of them, 3 were prospective and 19were retrospective. The sample size ranged from 28 to 770 patients. The total number of included patients was 4661 and hypertension occurred in 2932 (62.9%). The male/female ratio included in each study ranged from 1.4 to 3.5%, and the median age of the study patients was between 54 years and 66 years. The histology of most RCC is clear cell (61–100%). Most patients had received nephrectomy, cytokine therapy, targeted therapy or radiation therapy. Among the 22 studies providing HR, four reported univariate HR, which did not adjust for the potential confounding factors. The standard of hypertension or BP increasement during TKIs therapy varied between studies, including systolic blood pressure (SBP) ≥140/135 mmHg, diastolic blood pressure (DBP) ≥90/85 mmHg, mean arterial blood pressure (MAP) > 110 mmHg, an increase in BP (SBP, DBP, MAP) > 10/15 mmHg from baseline, or grades of hypertension according to National Cancer Institute Common Terminology Criteria for Adverse Events Version 3.0 or 4.0 [34, 35]. The quality of the studies varied from a NOS score of 6 to 9, most of which were high-quality (Table 2).
Table 1.
Baseline characteristics of eligible studies in the meta-analyses
| Study | Year | Country | Study design | Sample size | Male/ Female ratio | Mean age | Histology (clear cell%) | Survival analysis | Definition of hypertension | Type of analysis | TKIs | Quality Assessment (NOS Score = 9) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rini (a) [10] | 2011 | the USA | R | 534 | 2.3 | 60.6 | 98% | PFS, OS | SBP ≥ 140 mmHg, DBP ≥ 90 mmHg | multivariate | SUN | 7 |
| Szmit [12] | 2011 | Poland | R | 111 | 3.0 | 55.9 | 100% | PFS, OS | BP ≥ 140/90 mmHg | univariate | SUN | 9 |
| Bono [17] | 2011 | Finland | R | 64 | 1.7 | 64 | 92% | PFS | BP > 150/100 mmHg OR blood pressure requiring intensifi cation of pre-existing anti-hypertensive medication. | multivariate | SUN | 7 |
| Fujita [18] | 2012 | Japan | R | 41 | 2.7 | 64 | 100% | PFS | – | univariate | SUN | 7 |
| Eechoute [19] | 2012 | Netherlands | R | 158 | 1.7 | 60 | 87% | PFS, OS | SBP > 140 mmHg, DBP > 90 mmHg, MAP > 110 mmHg | multivariate | SUN | 7 |
| Rini (b) [13] | 2013 | the USA | R | 168 | 2.5 | 60 | – | PFS, OS | DBP ≥90 mmHg | multivariate | AXI | 7 |
| Motzer (a) [20] | 2013 | the USA | P | 350 | 2.8 | 61 | 100% | PFS, OS | SBP > 140 mmHg, DBP > 90 mmHg | multivariate | AXI | 6 |
| Motzer (b) [20] | 2013 | the USA | P | 336 | 2.5 | 61 | 100% | PFS, OS | SBP > 140 mmHg, DBP > 90 mmHg | multivariate | SOR | 6 |
| Hong [21] | 2013 | China | R | 136 | 2.0 | 56 | 93% | OS | Hypertension class III/IV | multivariate | SUN | 7 |
| Nakano [22] | 2013 | Japan | R | 36 | 3.5 | 65.8 | 61% | PFS | grade 1–3 (NCI-CTCAE, version 3.0) | multivariate | SOR | 7 |
| Fujita [23] | 2014 | Japan | R | 44 | 2.7 | 63.5 | 95% | PFS | – | multivariate | SUN | 7 |
| Eto [24] | 2014 | Japan | R | 64 | 2.2 | 63 | 97% | OS | DBP ≥90 mmHg | – | AXI | 7 |
| Rini (c) [25] | 2015 | the USA | P | 203 | 2.0 | 61.9 | – | PFS | DBP change from baseline ≥10/15 mmHg | – | AXI | 7 |
| Zhang (a) [26] | 2015 | China | R | 256 | 2.5 | 58 | 79% | OS | – | multivariate | SOR | 7 |
| Kucharz [27] | 2015 | Poland | R | 28 | 2.1 | 65 | – | PFS | office SBP ≥140 and/or DBP ≥90 mmHg; home SBP ≥135 and/or DPB ≥85 mmHg; pre-existing medication-controlled arterial hypertension and required additional antihypertensive medication during treatment | multivariate | SUN | 7 |
| Izzedine [28] | 2015 | France | R | 212 | 3.4 | 57.7 | 86% | PFS, OS | – | multivariate | SUN | 8 |
| Donskov [29] | 2015 | the USA | R | 770 | 2.6 | 60 | 98% | PFS | SBP ≥ 140 mmHg | multivariate | SUN | 7 |
| Zhang (b) [30] | 2016 | China | R | 134 | 2.4 | 59.8 | 77% | OS | – | multivariate | SOR | 7 |
| Goldstein (a) [14] | 2016 | Australia | R | 479 | 2.2 | 59.5 | – | PFS, OS | MAP change from baseline>10 mmHg | univariate | PAZ | 9 |
| Goldstein (b) [14] | 2016 | Australia | R | 506 | 2.6187 | 61 | – | PFS, OS | SBP > 140 mm H, DBP > 90 mmHg, MAP change from baseline>10 mmHg, SBP change from baseline>10 mmHg | univariate | PAZ | 9 |
| Goldstein (c) [14] | 2016 | Australia | R | 475 | 3.3394 | 60.9 | – | PFS, OS | SBP > 140 mm H, DBP > 90 mmHg, MAP change from baseline>10 mmHg, SBP change from baseline>10 mmHg | univariate | SUN | 9 |
| Cecere [31] | 2016 | Italy | R | 38 | 1.375 | 61 | 84.2% | OS | grade ≥ 3 (NCI-CTCAE, version 4.0) | multivariate | PAZ | 7 |
| Miyake [32] | 2016 | Japan | R | 50 | 4.0000 | 64 | 80% | PFS | SBP ≥ 140 or DBP ≥ 90 mmHg | multivariate | SUN | 7 |
| Fukuda [15] | 2016 | Japan | R | 62 | 2.4444 | 66 | 92% | PFS, OS | – | univariate | SUN | 7 |
| Matias [33] | 2017 | France | P | 106 | 2.3125 | 54 | 90% | PFS, OS | grade ≥ 3 (NCI-CTCAE, version 4.0) | univariate | AXI | 7 |
R Retrospective, P Prospective, PFS Progression-free survival, OS Overall survival, SBP Systolic blood pressure, DBP Diastolic blood pressure, MAP ≈ 2/3 DBP + 1/3 SBP; SUN Sunitinib, AXI Axitinib, SOR Sorafenib, PAZ Pazopanib, NCI-CTCAE National Cancer Institute Common Terminology Criteria for Adverse Events
—: The data were not available in this study
Table 2.
Newcastle-Ottawa scale score of the reviewed studies
| Study | Selection (4 stars) | Comparability (2 stars) | Outcome (3 stars) | Total score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the hypertensive cohort | Selection of the non-hypertensive cohort | Ascertainment of hypertension | Demonstration that outcome of interest was not present at start of study | Assessment of outcome | Was follow up long enough for outcomes to occur? | Adequacy of follow up of cohort | |||
| Rini (a) | ★ | ★ | ★ | ★ | – | ★ | ★ | ★ | 7 |
| Szmit | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Bono | ★ | ★ | ★ | ★ | – | ★ | ★ | ★ | 7 |
| Fujita | ★ | ★ | ★ | ★ | – | ★ | ★ | ★ | 7 |
| Eechoute | ★ | ★ | ★ | ★ | – | ★ | ★ | ★ | 7 |
| Rini (b) | ★ | ★ | ★ | ★ | – | ★ | ★ | ★ | 7 |
| Motzer | – | ★ | ★ | ★ | – | ★ | ★ | ★ | 6 |
| Hong | ★ | ★ | ★ | ★ | – | ★ | ★ | ★ | 7 |
| Nakano | ★ | ★ | ★ | ★ | – | ★ | ★ | ★ | 7 |
| Fujita | ★ | ★ | ★ | ★ | – | ★ | ★ | ★ | 7 |
| Eto | ★ | ★ | ★ | ★ | – | ★ | ★ | ★ | 7 |
| Rini (c) | ★ | ★ | ★ | ★ | – | ★ | ★ | ★ | 7 |
| Zhang (a) | ★ | ★ | ★ | ★ | – | ★ | ★ | ★ | 7 |
| Kucharz | ★ | ★ | ★ | ★ | – | ★ | ★ | ★ | 7 |
| Izzedine | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 |
| Donskov | ★ | ★ | ★ | ★ | – | ★ | ★ | ★ | 7 |
| Zhang (b) | ★ | ★ | ★ | ★ | – | ★ | ★ | ★ | 7 |
| Goldstein | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Miyake | ★ | ★ | ★ | ★ | – | ★ | ★ | ★ | 7 |
| Fukuda | ★ | ★ | ★ | ★ | – | ★ | ★ | ★ | 7 |
| Matias | ★ | ★ | ★ | ★ | – | ★ | ★ | ★ | 7 |
—: The data were not available in this study
Relationship between TKIs-induced hypertension and PFS or OS of mRCC patients
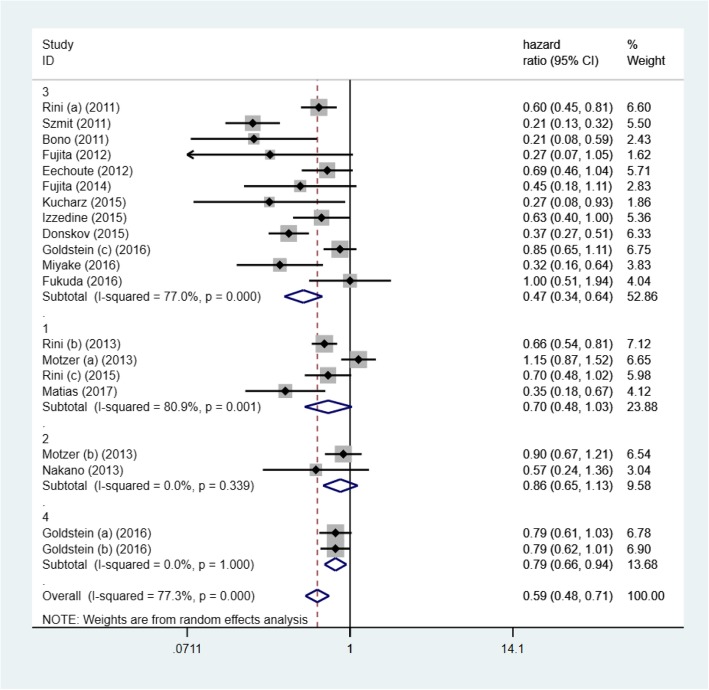
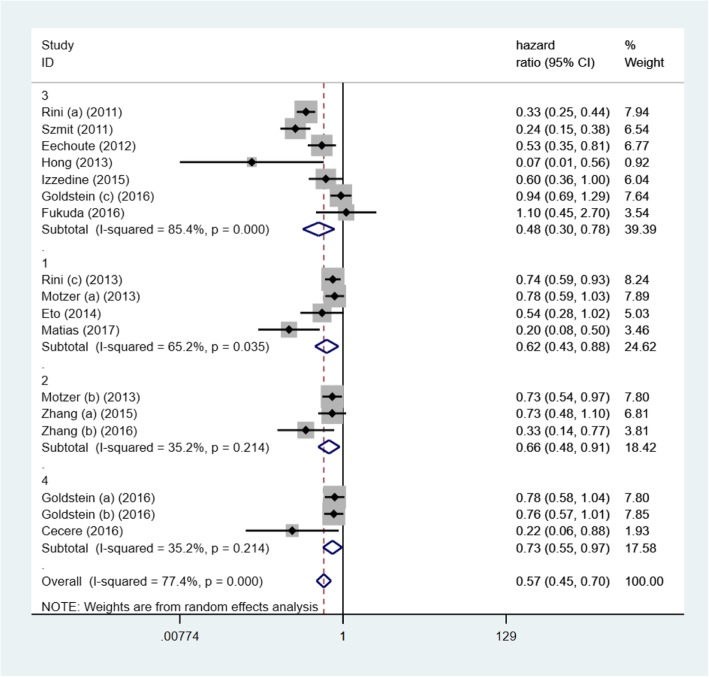
HR of PFS and OS were quantitatively synthesized and data were shown in Figs. 1 and 2. Elevated blood pressure was positively correlated with better PFS (HR = 0.59, 95% CI: 0.48–0.71, p < 0.001; I2 = 77.3%) and OS (HR = 0.57, 95% CI: 0.45–0.70, p < 0.001; I2 = 77.4%). It meant that patients developing hypertension may have a lower mortality risk and live longer without progression of mRCC. The heterogeneity was obvious between studies.
Fig. 1.
Forest plot reflects the association between TKIs -induced hypertension and oncologic outcomes (progression free survival) in different TKIs subgroups (1: axitinib; 2: sorafenib; 3: sunitinib; 4: pazopanib)
Fig. 2.
Forest plot reflects the association between TKIs-induced hypertension and oncologic outcomes (overall survival) in different TKIs subgroups (1: axitinib; 2: sorafenib; 3: sunitinib; 4: pazopanib)
Meta-regression analysis
Univariate meta-regression analysis was performed for PFS and results were showed in Table 3. Among the variables above mentioned, only sample size (Adjusted R2 = 27.34%, P = 0.019) might contribute to the inter-study heterogeneity, while others did not (P = 0.052–0.942).
Table 3.
Meta-regression and subgroup analyses of pooled hazard ratios for progression-free survival
| Subgroup | Meta-regression | Pooled HR of PFS | Heterogeneity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Coefficient | Standard error | T value | P value | Tau2 | Adjusted R2 | HR (95% CI) | P value | I2 | P value | |
| Year | 20 | 0.060 | 0.228 | 0.26 | 0.795 | 0.182 | −8.86% | ||||
| 2011–2014 | 0.56 (0.40–0.77) | <0.001 | 83.00% | <0.001 | |||||||
| 2015–2017 | 0.61 (0.48–0.77) | <0.001 | 73.60% | <0.001 | |||||||
| Sample size | 20 | 0.507 | 0.197 | 2.57 | 0.019 | 0.122 | 27.34% | ||||
| <200 | 0.43 (0.30–0.61) | <0.001 | 72.10% | <0.001 | |||||||
| ≥200 | 0.73 (0.60–0.89) | 0.002 | 75% | <0.001 | |||||||
| Gender (male/female ratio) | 20 | −0.117 | 0.228 | −0.51 | 0.614 | 0.177 | −5.64% | ||||
| <2.5 | 0.66 (0.53–0.82) | <0.001 | 54.50% | 0.025 | |||||||
| ≥2.5 | 0.55 (0.40–0.74) | <0.001 | 84.70% | <0.001 | |||||||
| Mean age | 20 | 0.320 | 0.264 | 2.21 | 0.241 | 0.156 | 6.69% | ||||
| <60 | 0.44 (0.23–0.86) | 0.015 | 89.40% | <0.001 | |||||||
| ≥60 | 0.64 (0.53–0.78) | <0.001 | 69.70% | <0.001 | |||||||
| Country | 20 | 0.168 | 0.301 | 0.56 | 0.584 | 0.173 | −3.31% | ||||
| the USA, Europe | 0.60 (0.49–0.74) | <0.001 | 81.40% | <0.001 | |||||||
| Asia | 0.51 (0.31–0.83) | 0.006 | 39.50% | 0.158 | |||||||
| ECOG PS (grade 0%) | 20 | 0.036 | 0.133 | 0.27 | 0.79 | 0.182 | −8.59% | ||||
| <0.5 | 0.35 (0.14–0.89) | 0.028 | 87.20% | <0.001 | |||||||
| ≥0.5 | 0.64 (0.52–0.79) | <0.001 | 71.90% | <0.001 | |||||||
| MSKCC score (favorable%) | 20 | 0.231 | 0.111 | 2.08 | 0.052 | 0.123 | 26.74% | ||||
| <0.25 | 0.43 (0.21–0.87) | 0.019 | 76% | 0.002 | |||||||
| ≥0.25 | 0.86 (0.73–1.02) | 0.076 | 40.30% | 0.137 | |||||||
| Histology (clear cell%) | 20 | −0.139 | 0.126 | −1.11 | 0.283 | 0.166 | 1.11% | ||||
| <0.9 | 0.53 (0.39–0.71) | <0.001 | 29.30% | 0.226 | |||||||
| ≥0.9 | 0.51 (0.33–0.79) | 0.002 | 87.90% | <0.001 | |||||||
| Prior nephrectomy (%) | 20 | −0.162 | 0.129 | −1.25 | 0.226 | 0.166 | 1.16% | ||||
| <0.9 | 0.52 (0.40–0.67) | 0.296 | 17.50% | <0.001 | |||||||
| ≥0.9 | 0.52 (0.33–0.81) | <0.001 | 89.30% | 0.005 | |||||||
| No. of disease sites (1%) | 20 | −0.253 | 0.139 | −1.81 | 0.086 | 0.144 | 13.81% | ||||
| <0.2 | 0.41 (0.28–0.60) | <0.001 | 62.40% | 0.047 | |||||||
| ≥0.2 | 0.52 (0.37–0.74) | <0.001 | 0 | 0.594 | |||||||
| Type of analysis | 20 | −0.030 | 0.231 | −0.13 | 0.898 | 0.182 | −8.91% | ||||
| Univariate | 0.59 (0.42–0.82) | 0.002 | 82.70% | <0.001 | |||||||
| Multivariate | 0.58 (0.46–0.75) | <0.001 | 74.50% | <0.001 | |||||||
| Study design | 20 | 0.343 | 0.257 | 1.34 | 0.198 | 0.157 | 6.32% | ||||
| Retrospective | 0.54 (0.44–0.67) | <0.001 | 74.90% | <0.001 | |||||||
| Prospective | 0.77 (0.53–1.12) | 0.175 | 76.20% | 0.006 | |||||||
| Type of TKIs | 20 | −0.073 | 0.114 | −0.64 | 0.942 | 0.178 | −10.49% | ||||
| Axitinib | 0.70 (0.48–1.03) | 0.07 | 80.90% | 0.001 | |||||||
| Sorafenib | 0.86 (0.65–1.13) | 0.277 | 0 | 0.339 | |||||||
| Sunitinib | 0.47 (0.34–0.64) | <0.001 | 77.90% | <0.001 | |||||||
| Pazopanib | 0.79 (0.66–0.94) | 0.010 | 0 | 1.000 | |||||||
No. number, HR hazard ratio, PFS progression-free survival, ECOG PS Eastern Cooperative Oncology Group performance status, MSKCC score Memorial Sloan Kettering Cancer Center score
Multivariate meta-regression analysis revealed that P value of sample size changed to 0.025. The overall adjusted R2 was 74.84% (P = 0.239), which meant that all these factors together could account for 74.84% of heterogeneity.
Subgroup analysis
Subgroup analysis for PFS revealed that sample size could not change the subgroup heterogeneity significantly (I2 = 72.1 and 75%) (Table 3). In addition, for different TKIs, only patients with TKIs-induced hypertension in sunitinib subgroup (HR = 0.47, 95% CI: 0.34–0.64, p<0.001) and pazopanib subgroup (HR = 0.79, 95% CI: 0.66–0.94, p = 0.01) could have longer PFS. However, development of hypertension in four different TKIs subgroups could all predict longer OS, including sunitinib subgroup (HR = 0.48, 95% CI: 0.30–0.78, p = 0.003), pazopanib subgroup (HR = 0.73, 95% CI: 0.55–0.97, p = 0.032), axitinib subgroup (HR = 0.62, 95% CI: 0.43–0.88, p = 0.007) and sorafenib subgroup (HR = 0.66, 95% CI: 0.48–0.91, p = 0.010) (Figs. 1 and 2).
Sensitivity analysis
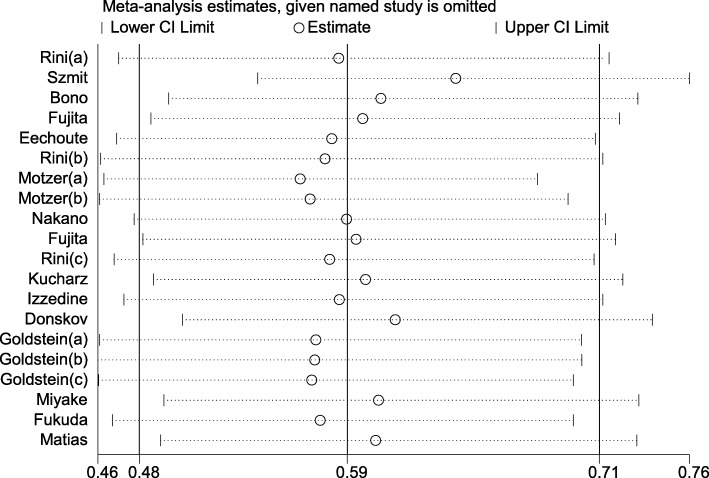
As shown in Fig. 3, study of “Szmit”, “Motzer (a)” and “Donskov” could affect the heterogeneity for PFS. We excluded these studies and found that I2 decreased to 44.6% (P = 0.03), with pooled HR = 0.665 (0.579–0.764, P = 0.025). Then, we reviewed these studies carefully to find something they had in common. Interestingly, most of the mRCC patients in these three studies had failed one previous cytokine immunotherapy. Maybe it was the reason for high heterogeneity.
Fig. 3.
Sensitivity analysis of progression free survival for the evaluation of potential heterogeneity
Publication bias
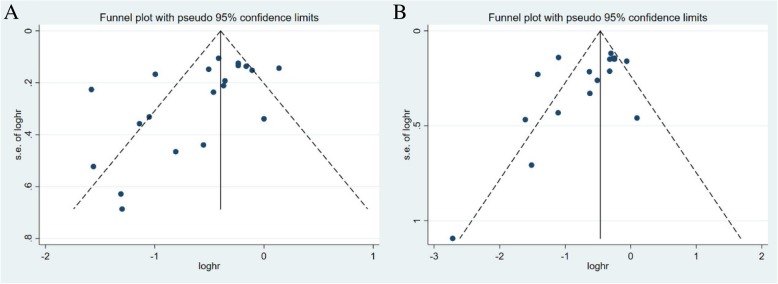
We assessed the publication bias with funnel plot and Egger’s and Begg’s tests (Fig. 4). The shape of funnel plots was not symmetric. The Egger’s and Begg’s tests were further performed. The results indicated significant publication bias for studies, with merged PFS (Begg’s test, P = 0.015; Egger’s test, P = 0.028) and merged OS (Begg’s test, P = 0.026; Egger’s test, P = 0.085).
Fig. 4.
Funnel plot for publication bias. a progression free survival, b overall survival
Evaluation of the quality of evidence according to GRADE system
The assessment of the quality of evidence was performed for PFS and OS which were critical in evaluating the outcome of mRCC patients. The quality of evidence of PFS and OS was both “very low” due to retrospective studies, publication bias and high heterogeneity (Table 4).
Table 4.
Evaluation of the quality of evidence according to GRADE system
| Quality assessment | No. of patients | Hazard Ratios (95% CI) | Quality | Importance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | TKI-induced hypertension | Control | |||
| Progression-free survival (follow-up median 5.6–43.2 years; measured with: follow-up) | |||||||||||
| 20 | observational studies | no serious risk of bias | serious1 | no serious indirectness | no serious imprecision | reporting bias2 | 3021 | 1327 | 0.59 (0.48–0.71) | Very low | Critical |
| Overall survival (follow-up median 5.2–61.8 months; measured with: follow-up) | |||||||||||
| 17 | observational studies | no serious risk of bias | serious1 | no serious indirectness | no serious imprecision | reporting bias3 | 2313 | 1804 | 0.57 (0.45–0.70) | Very low | Critical |
1The heterogeneity of this outcome was obvious between studies
2The shape of funnel plots was not symmetric. The Egger’s and Begg’s tests were further performed. The results indicated significant publication bias for studies, with merged PFS (Begg’s test, P = 0.015; Egger’s test, P = 0.028).
3The shape of funnel plots was not symmetric. The Egger’s and Begg’s tests were further performed. The results indicated significant publication bias for studies, with merged OS (Begg’s test, P = 0.026; Egger’s test, P = 0.085)
Discussion
This meta-analysis and systematic review investigated whether TKIs-induced hypertension can predict the prognosis of patients suffering from mRCC. AEs, like hypothyroidism, though shown to be a good predictor of PFS or OS, was usually diagnosed later than hypertension [17, 36]. Thus, it would be better if we can predict the prognosis of mRCC patients based on the TKIs-induced hypertension. It will help urologists decide whether patients should continue this TKIs therapy or not, which may help patients get more suitable treatments as soon as possible. Based on 22 original studies, our results showed that the occurrence of hypertension during treatment may predict better survival for mRCC, with longer PFS and OS. Additionally, when it comes to different TKIs, sunitinib or pazopanib therapy were both associated with longer PFS and OS.
The mechanisms of hypertension induced by TKIs are complicated. First, activation of VEGF receptor-2 via phosphoinositide 3-kinase and its downstream serine protein kinase Akt can stimulate endothelium-derived nitric oxide synthase, leading to the production of nitric oxide (NO). Therefore, the inhibition of VEGF receptor might lead to a decrease in NO bioavailability, followed by vasoconstriction and increased blood pressure (BP) [17, 37–39]. Second, plasma vasoconstrictor endothelin-1, a vasoconstrictor produced by the endothelium, also increased in patients or rats receiving sunitinib [40–42]. Third, NO is also involved in the control of renal and glomerular hemodynamics, tubuloglomerular feedback response, release of renin and sympathetic transmitters, tubular ion transport. As a result, reduction of NO can also result in renal water and sodium retention, leading to hypertension [43]. TKIs may also directly cause renal injury and proteinuria, which could play a role in long-lasting hypertension [44]. Finally, hypertension may also result from structural or functional vascular rarefaction [45–47]. VEGF is also crucial in the maintenance of endothelial viability [48, 49]. Therefore, the inhibition of VEGF and PDGF receptors can induce endothelial cell apoptosis, reduction in capillary density, vascular diameter and microvascular flow, and thus increase the blood pressure.
The reason why TKIs-induced hypertension might be a prognostic factor in patients with mRCC is still not quite clear. The anticarcinogenic effect of TKIs relies on the block of VEGF receptor, which may also lead to hypertension as above mentioned. Thus, when TKIs inhibit the progress of mRCC and prolong the PFS or OS of patients, hypertension may occur at the same time. It may be the reason why the rise of blood pressure could indicate a better prognosis in mRCC patients.
Of note, the intention of therapy is not to drive all patients into a hypertensive state. On the contrary, when hypertension occurs, patients should receive antihypertensive therapy as soon as possible. Additionally, despite the PFS and OS of mRCC patients have prolonged because of TKIs therapy, few comparable benefits have been described in terms of the quality of life [50, 51]. An innovative study that compared sunitinib with pazopanib to evaluate patient preferences suggested that the toxicity profile may have an impact on quality of life and the choice of treatment when two comparable options are on offer [52]. In addition, sunitinib-induced hypertension may be associated with cardiotoxicity or reversible posterior leukoencephalopathy [53–55]. Thus, the panel of National Cancer Institute of the USA had several recommendations for mRCC patients received TKIs [56]. First, urologists should recognize the preexisting hypertension prior to therapy or actively monitor BP throughout treatment. Early use of antihypertensive medication is necessary when high blood pressure occurs, and it is critical for maintaining dose intensity and improving a patient’s quality of life simultaneously [56]. If possible, the goal of management is to reduce BP below 140/90 mmHg. It was also suggested that there was no need to reduce the dose of antihypertensive medication because it did not compromise efficacy of TKIs [57]. Some evidence indicated that using Angiotensin receptor blockers (ARBs) or angiotensin-I-converting enzyme inhibitors may even protect against cancer [58–60]. ARBs were shown to induce apoptosis and inhibit the proliferation of RCC cells in vitro [28]. Thus, some randomized prospective studies could be carried out to see if it is better to use more than one drugs with different antihypertensive mechanisms, which may improve the prognosis [12]. Second, doctors should assess the risk of potential cardiovascular complications.
Several underlying limitations of the study should be presented. First, most eligible studies were retrospective, though with high NOS scores. Second, the reciprocal correlation between the hypertension and other AEs should be noted. Patients with more than one adverse event of any grade had longer PFS and OS [61]. Thus, further studies are needed to analyze the relationship between several adverse events and mRCC. Third, obvious inter-study heterogeneity was observed in this meta analysis, which may be due to different therapies mRCC patients had received before, such as cytokine immunotherapy. In addition, a publication bias was detected in this study. The potential reason may be that studies with non-significant results were not published. High heterogeneity and publication bias weakened the quality of evidence, which may be improved by more randomized prospective trials.
However, our analysis also has some advantages. First, we conducted this review with enough data available for extraction by a comprehensive and robust search strategy. Second, we applied a rigorous inclusion/exclusion criterion. Additionally, most of the studies operated multivariable analysis, which could eliminate the co-factors affecting the PFS and OS of mRCC patients.
Conclusions
In summary, our analysis of currently available clinical evidence suggests that TKIs-induced hypertension may predict longer PFS and OS in patients with mRCC during TKIs therapy, especially using sunitinib or pazopanib.
Additional file
Figure S1. Flow chart showing literature searching process of meta-analysis. The search keywords are (renal cell carcinoma) AND [(tyrosine kinase inhibitor) OR sunitinib OR axitinib OR sorafenib OR pazopanib] AND [hypertension OR (blood pressure). (DOCX 140 kb)
Acknowledgements
Not applicable.
Abbreviations
- AEs
Adverse events
- BP
Blood pressure
- CI
Confidence intervals
- DBP
Diastolic blood pressure
- ECOG PS
Eastern Cooperative Oncology Group performance status
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- HR
Hazard Ratios
- MAP
Mean arterial blood pressure
- mRCC
Metastatic renal cell carcinoma
- MSKCC
Memorial Sloan Kettering Cancer Center
- NO
Nitric oxide
- NOS
Newcastle-Ottawa Scale
- OS
Overall survival
- PFS
Progression-free survival
- RCC
Renal cell carcinoma
- SBP
Systolic blood pressure
- TKIs
Tyrosine kinase inhibitors
Authors’ contributions
LY was mainly responsible for the design of the work, the acquisition and analysis of data and manuscript writing. ZL carried out the acquisition of data. CYT and YDH participated in the analysis of data. LBH, WKJ and LH revised the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZY2016104), Project of Sichuan Provincial Health Department (ZH2017–101) and The National Natural Science Fund of China (81800667).
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Ethics approval and consent to participate
All analyses were based on previous published studies. Thus, no ethical approval and patient consent are required.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yu Liu, Liang Zhou and Yuntian Chen contributed equally to this work and should be considered as co-first authors
References
- 1.Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol. 2015;67(3):519–530. doi: 10.1016/j.eururo.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Wong MCS, Goggins WB, Yip BHK, Fung FDH, Leung C, Fang Y, Wong SYS, Ng CF. Incidence and mortality of kidney cancer: temporal patterns and global trends in 39 countries. Sci Rep. 2017;7(1):15698. doi: 10.1038/s41598-017-15922-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossi SH, Klatte T. Epidemiology and screening for renal. cancer. 2018;36(9):1341–1353. doi: 10.1007/s00345-018-2286-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev. 2008;34(3):193–205. doi: 10.1016/j.ctrv.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Naito S, Tsukamoto T, Murai M, Fukino K, Akaza H. Overall survival and good tolerability of long-term use of sorafenib after cytokine treatment: final results of a phase II trial of sorafenib in Japanese patients with metastatic renal cell carcinoma. BJU Int. 2011;108(11):1813–1819. doi: 10.1111/j.1464-410X.2011.10281.x. [DOI] [PubMed] [Google Scholar]
- 6.Tomita Y, Shinohara N, Yuasa T, Fujimoto H, Niwakawa M, Mugiya S, Miki T, Uemura H, Nonomura N, Takahashi M, et al. Overall survival and updated results from a phase II study of sunitinib in Japanese patients with metastatic renal cell carcinoma. Jpn J Clin Oncol. 2010;40(12):1166–1172. doi: 10.1093/jjco/hyq146. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 8.Rixe O, Bukowski RM, Michaelson MD, Wilding G, Hudes GR, Bolte O, Motzer RJ, Bycott P, Liau KF, Freddo J, et al. Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: a phase II study. Lancet Oncol. 2007;8(11):975–984. doi: 10.1016/S1470-2045(07)70285-1. [DOI] [PubMed] [Google Scholar]
- 9.Ravaud A, Schmidinger M. Clinical biomarkers of response in advanced renal cell carcinoma. Ann Oncol. 2013;24(12):2935–2942. doi: 10.1093/annonc/mdt288. [DOI] [PubMed] [Google Scholar]
- 10.Rini BI, Cohen DP, Lu DR, Chen I, Hariharan S, Gore ME, Figlin RA, Baum MS, Motzer RJ. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2011;103(9):763–773. doi: 10.1093/jnci/djr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Li S, Zhu Y, Liang X, Meng H, Chen J, Zhang D, Guo H, Shi B. Incidence and risk of sorafenib-induced hypertension: a systematic review and meta-analysis. J Clin Hypertens (Greenwich, Conn) 2014;16(3):177–185. doi: 10.1111/jch.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szmit S, Langiewicz P, Zlnierek J, Nurzynski P, Zaborowska M, Filipiak KJ, Opolski G, Szczylik C. Hypertension as a predictive factor for survival outcomes in patients with metastatic renal cell carcinoma treated with sunitinib after progression on cytokines. Kidney Blood Press Res. 2012;35(1):18–25. doi: 10.1159/000329933. [DOI] [PubMed] [Google Scholar]
- 13.Rini BI, Garrett M, Poland B, Dutcher JP, Rixe O, Wilding G, Stadler WM, Pithavala YK, Kim S, Tarazi J, et al. Axitinib in metastatic renal cell carcinoma: results of a pharmacokinetic and pharmacodynamic analysis. J Clin Pharmacol. 2013;53(5):491–504. doi: 10.1002/jcph.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein D, Rosenberg JE, Figlin RA, Townsend RR, McCann L, Carpenter C, Pandite L. Is change in blood pressure a biomarker of pazopanib and sunitinib efficacy in advanced/metastatic renal cell carcinoma? Eur J Cancer (Oxford, England : 1990) 2016;53:96–104. doi: 10.1016/j.ejca.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Fukuda H, Kondo T, Iida S, Takagi T, Tanabe K. Treatment-related deterioration of renal function is associated with the antitumor efficacy of sunitinib in patients with metastatic renal cell carcinoma. Urol Oncol. 2016;34(8):338.e331–338.e339. doi: 10.1016/j.urolonc.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 17.Bono P, Rautiola J, Utriainen T, Joensuu H. Hypertension as predictor of sunitinib treatment outcome in metastatic renal cell carcinoma. Acta Oncol (Stockholm, Sweden) 2011;50(4):569–573. doi: 10.3109/0284186X.2010.543696. [DOI] [PubMed] [Google Scholar]
- 18.Fujita T, Iwamura M, Ishii D, Tabata K, Matsumoto K, Yoshida K, Baba S. C-reactive protein as a prognostic marker for advanced renal cell carcinoma treated with sunitinib. Int J Urol. 2012;19(10):908–913. doi: 10.1111/j.1442-2042.2012.03071.x. [DOI] [PubMed] [Google Scholar]
- 19.Eechoute K, van der Veldt AA, Oosting S, Kappers MH, Wessels JA, Gelderblom H, Guchelaar HJ, Reyners AK, van Herpen CM, Haanen JB, et al. Polymorphisms in endothelial nitric oxide synthase (eNOS) and vascular endothelial growth factor (VEGF) predict sunitinib-induced hypertension. Clin Pharmacol Ther. 2012;92(4):503–510. doi: 10.1038/clpt.2012.136. [DOI] [PubMed] [Google Scholar]
- 20.Motzer RJ, Escudier B, Tomczak P, Hutson TE, Michaelson MD, Negrier S, Oudard S, Gore ME, Tarazi J, Hariharan S, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol. 2013;14(6):552–562. doi: 10.1016/S1470-2045(13)70093-7. [DOI] [PubMed] [Google Scholar]
- 21.Hong YP, Yao XD, Zhu Y, Ye DW, Shi GH, Zhang SL, Dai B, Zhang HL, Shen YJ, Zhu YP, et al. Exploratory analysis of the effect of toxicity of sunitinib on the clinical outcome of patients with advanced renal cell carcinoma. Zhonghua Yi Xue Za Zhi. 2013;93(36):2880–2883. [PubMed] [Google Scholar]
- 22.Nakano K, Komatsu K, Kubo T, Natsui S, Nukui A, Kurokawa S, Kobayashi M, Morita T. Hand-foot skin reaction is associated with the clinical outcome in patients with metastatic renal cell carcinoma treated with sorafenib. Jpn J Clin Oncol. 2013;43(10):1023–1029. doi: 10.1093/jjco/hyt110. [DOI] [PubMed] [Google Scholar]
- 23.Fujita T, Wakatabe Y, Matsumoto K, Tabata K, Yoshida K, Iwamura M. Leukopenia as a biomarker of sunitinib outcome in advanced renal cell carcinoma. Anticancer Res. 2014;34(7):3781–3787. [PubMed] [Google Scholar]
- 24.Eto M, Uemura H, Tomita Y, Kanayama H, Shinohara N, Kamei Y, Fujii Y, Umeyama Y, Ozono S, Naito S, et al. Overall survival and final efficacy and safety results from a Japanese phase II study of axitinib in cytokine-refractory metastatic renal cell carcinoma. Cancer Sci. 2014;105(12):1576–1583. doi: 10.1111/cas.12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rini BI, Melichar B, Fishman MN, Oya M, Pithavala YK, Chen Y, Bair AH, Grunwald V. Axitinib dose titration: analyses of exposure, blood pressure and clinical response from a randomized phase II study in metastatic renal cell carcinoma. Ann Oncol. 2015;26(7):1372–1377. doi: 10.1093/annonc/mdv103. [DOI] [PubMed] [Google Scholar]
- 26.Zhang HL, Qin XJ, Wang HK, Gu WJ, Ma CG, Shi GH, Zhou LP, Ye DW. Clinicopathological and prognostic factors for long-term survival in Chinese patients with metastatic renal cell carcinoma treated with sorafenib: a single-center retrospective study. Oncotarget. 2015;6(34):36870–36883. doi: 10.18632/oncotarget.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kucharz J, Dumnicka P, Kuzniewski M, Kusnierz-Cabala B, Herman RM, Krzemieniecki K. Co-occurring adverse events enable early prediction of progression-free survival in metastatic renal cell carcinoma patients treated with sunitinib: a hypothesis-generating study. Tumori. 2015;101(5):555–559. doi: 10.5301/tj.5000342. [DOI] [PubMed] [Google Scholar]
- 28.Izzedine H, Derosa L, Le Teuff G, Albiges L, Escudier B. Hypertension and angiotensin system inhibitors: impact on outcome in sunitinib-treated patients for metastatic renal cell carcinoma. Ann Oncol. 2015;26(6):1128–1133. doi: 10.1093/annonc/mdv147. [DOI] [PubMed] [Google Scholar]
- 29.Donskov F, Michaelson MD, Puzanov I, Davis MP, Bjarnason GA, Motzer RJ, Goldstein D, Lin X, Cohen DP, Wiltshire R, et al. Sunitinib-associated hypertension and neutropenia as efficacy biomarkers in metastatic renal cell carcinoma patients. Br J Cancer. 2015;113(11):1571–1580. doi: 10.1038/bjc.2015.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Li Y, Cai Y, Wang K, Li H. Efficacy of sorafenib correlates with Memorial Sloan-Kettering Cancer Center (MSKCC) risk classification and bone metastasis in Chinese patients with metastatic renal cell carcinoma. Cell Oncol (Dordr) 2016;39(1):15–21. doi: 10.1007/s13402-015-0245-5. [DOI] [PubMed] [Google Scholar]
- 31.Cecere SC, Rossetti S, Cavaliere C, Della Pepa C, Di Napoli M, Crispo A, Iovane G, Piscitelli R, Sorrentino D, Ciliberto G, et al. Pazopanib in metastatic renal Cancer: a “real-world” experience at National Cancer Institute “Fondazione G. Pascale”. Front Pharmacol. 2016;7:287. doi: 10.3389/fphar.2016.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyake M, Kuwada M, Hori S, Morizawa Y, Tatsumi Y, Anai S, Hosokawa Y, Hayashi Y, Tomioka A, Otani T, et al. The best objective response of target lesions and the incidence of treatment-related hypertension are associated with the survival of patients with metastatic renal cell carcinoma treated with sunitinib: a Japanese retrospective study. BMC Res Notes. 2016;9:79. doi: 10.1186/s13104-016-1895-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matias M, Le Teuff G, Albiges L, Guida A, Brard C, Bacciarelo G, Loriot Y, Massard C, Lassau N, Fizazi K, et al. Real world prospective experience of axitinib in metastatic renal cell carcinoma in a large comprehensive cancer Centre. Eur J Cancer (Oxford, England: 1990) 2017;79:185–192. doi: 10.1016/j.ejca.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 34.Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 35.Chen AP, Setser A, Anadkat MJ, Cotliar J, Olsen EA, Garden BC, Lacouture ME. Grading dermatologic adverse events of cancer treatments: the common terminology criteria for adverse events version 4.0. J Am Acad Dermatol. 2012;67(5):1025–1039. doi: 10.1016/j.jaad.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 36.Buda-Nowak A, Kucharz J, Dumnicka P, Kuzniewski M, Herman RM, Zygulska AL, Kusnierz-Cabala B. Sunitinib-induced hypothyroidism predicts progression-free survival in metastatic renal cell carcinoma patients. Med Oncol (Northwood, London, England) 2017;34(4):68. doi: 10.1007/s12032-017-0928-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aparicio-Gallego G, Blanco M, Figueroa A, Garcia-Campelo R, Valladares-Ayerbes M, Grande-Pulido E, Anton-Aparicio L. New insights into molecular mechanisms of sunitinib-associated side effects. Mol Cancer Ther. 2011;10(12):2215–2223. doi: 10.1158/1535-7163.MCT-10-1124. [DOI] [PubMed] [Google Scholar]
- 38.Hayman SR, Leung N, Grande JP, Garovic VD. VEGF inhibition, hypertension, and renal toxicity. Curr Oncol Rep. 2012;14(4):285–294. doi: 10.1007/s11912-012-0242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinson ES, Khankin EV, Karumanchi SA, Humphreys BD. Hypertension induced by vascular endothelial growth factor signaling pathway inhibition: mechanisms and potential use as a biomarker. Semin Nephrol. 2010;30(6):591–601. doi: 10.1016/j.semnephrol.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merkus D, Sorop O, Houweling B, Boomsma F, van den Meiracker AH, Duncker DJ. NO and prostanoids blunt endothelin-mediated coronary vasoconstrictor influence in exercising swine. Am J Phys Heart Circ Phys. 2006;291(5):H2075–H2081. doi: 10.1152/ajpheart.01109.2005. [DOI] [PubMed] [Google Scholar]
- 41.Kappers MH, van Esch JH, Sluiter W, Sleijfer S, Danser AH, van den Meiracker AH. Hypertension induced by the tyrosine kinase inhibitor sunitinib is associated with increased circulating endothelin-1 levels. Hypertension (Dallas, Tex : 1979) 2010;56(4):675–681. doi: 10.1161/HYPERTENSIONAHA.109.149690. [DOI] [PubMed] [Google Scholar]
- 42.Kappers MH, Smedts FM, Horn T, van Esch JH, Sleijfer S, Leijten F, Wesseling S, Strevens H, Jan Danser AH, van den Meiracker AH. The vascular endothelial growth factor receptor inhibitor sunitinib causes a preeclampsia-like syndrome with activation of the endothelin system. Hypertension (Dallas, Tex : 1979) 2011;58(2):295–302. doi: 10.1161/HYPERTENSIONAHA.111.173559. [DOI] [PubMed] [Google Scholar]
- 43.Zou AP, Cowley AW., Jr Role of nitric oxide in the control of renal function and salt sensitivity. Curr Hypertens Rep. 1999;1(2):178–186. doi: 10.1007/s11906-999-0016-7. [DOI] [PubMed] [Google Scholar]
- 44.Khan G, Golshayan A, Elson P, Wood L, Garcia J, Bukowski R, Rini B. Sunitinib and sorafenib in metastatic renal cell carcinoma patients with renal insufficiency. Ann Oncol. 2010;21:1618–1622. doi: 10.1093/annonc/mdp603. [DOI] [PubMed] [Google Scholar]
- 45.Lacouture ME, Reilly LM, Gerami P, Guitart J. Hand foot skin reaction in cancer patients treated with the multikinase inhibitors sorafenib and sunitinib. Ann Oncol. 2008;19(11):1955–1961. doi: 10.1093/annonc/mdn389. [DOI] [PubMed] [Google Scholar]
- 46.Steeghs N, Gelderblom H, Roodt JO, Christensen O, Rajagopalan P, Hovens M, Putter H, Rabelink TJ, de Koning E. Hypertension and rarefaction during treatment with telatinib, a small molecule angiogenesis inhibitor. Clin Cancer Res. 2008;14(11):3470–3476. doi: 10.1158/1078-0432.CCR-07-5050. [DOI] [PubMed] [Google Scholar]
- 47.Hubner N, Yagil C, Yagil Y. Novel integrative approaches to the identification of candidate genes in hypertension. Hypertension (Dallas, Tex: 1979) 2006;47(1):1–5. doi: 10.1161/01.HYP.0000197951.82190.c4. [DOI] [PubMed] [Google Scholar]
- 48.Rees ML, Khakoo AY. Molecular mechanisms of hypertension and heart failure due to antiangiogenic cancer therapies. Heart Fail Clin. 2011;7(3):299–311. doi: 10.1016/j.hfc.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 49.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130(4):691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cella D, Pickard AS, Duh MS, Guerin A, Mishagina N, Antras L, Neary MP, McCann L, Hodge R, Sternberg CN. Health-related quality of life in patients with advanced renal cell carcinoma receiving pazopanib or placebo in a randomised phase III trial. Eur J Cancer (Oxford, England: 1990) 2012;48(3):311–323. doi: 10.1016/j.ejca.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 51.Bukowski R, Cella D, Gondek K, Escudier B. Effects of sorafenib on symptoms and quality of life: results from a large randomized placebo-controlled study in renal cancer. Am J Clin Oncol. 2007;30(3):220–227. doi: 10.1097/01.coc.0000258732.80710.05. [DOI] [PubMed] [Google Scholar]
- 52.Escudier B, Porta C, Bono P, Powles T, Eisen T, Sternberg CN, Gschwend JE, De Giorgi U, Parikh O, Hawkins R, et al. Randomized, controlled, double-blind, cross-over trial assessing treatment preference for pazopanib versus sunitinib in patients with metastatic renal cell carcinoma: PISCES study. J Clin Oncol. 2014;32(14):1412–1418. doi: 10.1200/JCO.2013.50.8267. [DOI] [PubMed] [Google Scholar]
- 53.Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, Woulfe K, Pravda E, Cassiola F, Desai J, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet (London, England) 2007;370(9604):2011–2019. doi: 10.1016/S0140-6736(07)61865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kapiteijn E, Brand A, Kroep J, Gelderblom H. Sunitinib induced hypertension, thrombotic microangiopathy and reversible posterior leukencephalopathy syndrome. Ann Oncol. 2007;18(10):1745–1747. doi: 10.1093/annonc/mdm454. [DOI] [PubMed] [Google Scholar]
- 55.Padhy BM, Shanmugam SP, Gupta YK, Goyal A. Reversible posterior leucoencephalopathy syndrome in an elderly male on sunitinib therapy. Br J Clin Pharmacol. 2011;71(5):777–779. doi: 10.1111/j.1365-2125.2010.03893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maitland ML, Bakris GL, Black HR, Chen HX, Durand JB, Elliott WJ, Ivy SP, Leier CV, Lindenfeld J, Liu G, et al. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst. 2010;102(9):596–604. doi: 10.1093/jnci/djq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Langenberg MH, van Herpen CM, De Bono J, Schellens JH, Unger C, Hoekman K, Blum HE, Fiedler W, Drevs J, Le Maulf F, et al. Effective strategies for management of hypertension after vascular endothelial growth factor signaling inhibition therapy: results from a phase II randomized, factorial, double-blind study of Cediranib in patients with advanced solid tumors. J Clin Oncol. 2009;27(36):6152–6159. doi: 10.1200/JCO.2009.22.2273. [DOI] [PubMed] [Google Scholar]
- 58.Rao GA, Mann JR, Shoaibi A, Pai SG, Bottai M, Sutton SS, Haddock KS, Bennett CL, Hebert JR. Angiotensin receptor blockers: are they related to lung cancer? J Hypertens. 2013;31(8):1669–1675. doi: 10.1097/HJH.0b013e3283621ea3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lever AF, Hole DJ, Gillis CR, McCallum IR, McInnes GT, MacKinnon PL, Meredith PA, Murray LS, Reid JL, Robertson JW. Do inhibitors of angiotensin-I-converting enzyme protect against risk of cancer? Lancet (London, England) 1998;352(9123):179–184. doi: 10.1016/S0140-6736(98)03228-0. [DOI] [PubMed] [Google Scholar]
- 60.Makar GA, Holmes JH, Yang YX. Angiotensin-converting enzyme inhibitor therapy and colorectal cancer risk. J Natl Cancer Inst. 2014;106(2):djt374. doi: 10.1093/jnci/djt374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iacovelli R, Verri E, Cossu Rocca M, Aurilio G, Cullura D, de Cobelli O, Nole F. Prognostic role of the cumulative toxicity in patients affected by metastatic renal cells carcinoma and treated with first-line tyrosine kinase inhibitors. Anti-Cancer Drugs. 2017;28(2):206–212. doi: 10.1097/CAD.0000000000000439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow chart showing literature searching process of meta-analysis. The search keywords are (renal cell carcinoma) AND [(tyrosine kinase inhibitor) OR sunitinib OR axitinib OR sorafenib OR pazopanib] AND [hypertension OR (blood pressure). (DOCX 140 kb)
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.