Abstract
Background
Endometrial mesonephric-like adenocarcinomas exhibit classical histologic features of mesonephric carcinoma; however, it remains unclear whether these tumors represent mesonephric (Wolffian) carcinoma or endometrioid (Müllerian) carcinomas that closely mimic mesonephric carcinoma.
Case presentation
A 32-year-old Japanese primigravida presented with atypical vaginal bleeding. An endometrial biopsy suggested low-grade endometrioid carcinoma, and she was administered medroxyprogesterone acetate. Her tumor recurred 6 years later, and she underwent hysterectomy, salpingo-oophorectomy, and omentectomy, at which point she was diagnosed with mesonephric-like adenocarcinoma of the uterine endometrium. Retrospective pathological review of the initial biopsy confirmed coexisting low-grade endometrioid carcinoma and mesonephric-like adenocarcinoma of the uterine endometrium. On immunohistochemistry, the endometrioid carcinoma component was diffuse positive for estrogen and progesterone receptors but negative for thyroid transcription factor 1. However, the mesonephric-like adenocarcinoma component exhibited a mixture of estrogen receptor- and thyroid transcription factor 1-positive cells within the same glands.
Conclusions
We encountered a patient with coexisting endometrial mesonephric-like adenocarcinoma and low-grade endometrioid carcinoma, which was treated using medroxyprogesterone acetate therapy, resulting in recurrence of mesonephric-like adenocarcinoma alone. These clinicopathological findings support the prevailing notions that mesonephric-like adenocarcinoma is a Müllerian adenocarcinoma exhibiting mesonephric differentiation.
Keywords: Mesonephric-like adenocarcinoma, Endometrioid carcinoma, Endometrial cancer, Medroxyprogesterone acetate therapy, Müllerian duct
Background
Mesonephric carcinoma is a malignant gynecologic tumor most commonly arising in the cervix and is presumed to be derived from normal or hyperplastic mesonephric remnants [1]. Recently McFarland et al. [2] reported a series of 5 ovarian and 7 uterine endometrial neoplasms that were referred to as “mesonephric-like adenocarcinomas (MLAs)”; these tumors exhibited the classical histologic features of mesonephric carcinomas. MLAs are rare, representing approximately 1% of endometrial carcinomas, but appear to be aggressive in nature [3, 4]. Histologically, MLAs are characterized by a variety of morphologies including tubular, ductal, papillary, retiform, and solid. These histologic patterns can easily be mistaken for endometrioid carcinoma, clear cell carcinoma, serous carcinoma, carcinosarcoma, and a variety of other neoplasms [4, 5]. They also have a unique immunohistochemical profiles; they are usually positive for thyroid transcription factor 1 (TTF-1), GATA binding protein-3 (GATA-3), and CD10, and are negative for estrogen receptor (ER) and progesterone receptor (PgR) [2, 6]. MLAs are often confined to the endometrium without deep myometrial involvement, where mesonephric remnants theoretically exist [2]. MLAs harbor recurrent mutations in KRAS and PIK3CA but lack PTEN mutations, demonstrating biological overlap with both mesonephric and endometrioid carcinomas [7]. The pathogenesis of MLAs is unknown, and it remains debated whether they represent mesonephric (Wolffian) carcinomas arising in the endometrium/ovary or endometrioid (Müllerian) carcinomas that closely mimic mesonephric carcinomas. Herein, we report for the first time a case of a patient diagnosed with an endometrial MLA coexisting with a low-grade endometrioid carcinoma.
Case presentation
Clinical history
A 32-year-old Japanese primigravida visited another clinic because of atypical vaginal bleeding, wherein an endometrial mass was detected by transvaginal ultrasonography following which she was referred to our hospital for evaluation and treatment. Her body mass index was 33.8 kg/m2; magnetic resonance imaging (MRI) revealed the presence of a 25 mm mass at the uterine endometrium that was suspected to be endometrial cancer (Fig. 1a). No metastasis was detected using systemic computed tomography. Serum levels of the tumor markers carcinoembryonic antigen, cancer antigen 125, and carbohydrate antigen 19–9 were 1.2 ng/mL, 11.7 U/mL, and 7.9 U/mL, respectively. An endometrial biopsy suggested endometrioid carcinoma G1, which is categorized as low-grade endometrial carcinoma, and she received medroxyprogesterone acetate (MPA) therapy (600 mg/day) for 6 months. Afterwards, routine examination that included transvaginal ultrasonography, pelvic MRI, and endometrial cytology showed no evidence of the tumor. Six years after MPA therapy, an endometrial mass 24 mm in size was detected using MRI, indicating a recurrence (Fig. 1b). The patient underwent a total abdominal hysterectomy, bilateral salpingo-oophorectomy, and partial omentectomy. She has had no recurrence since the surgery (5 months).
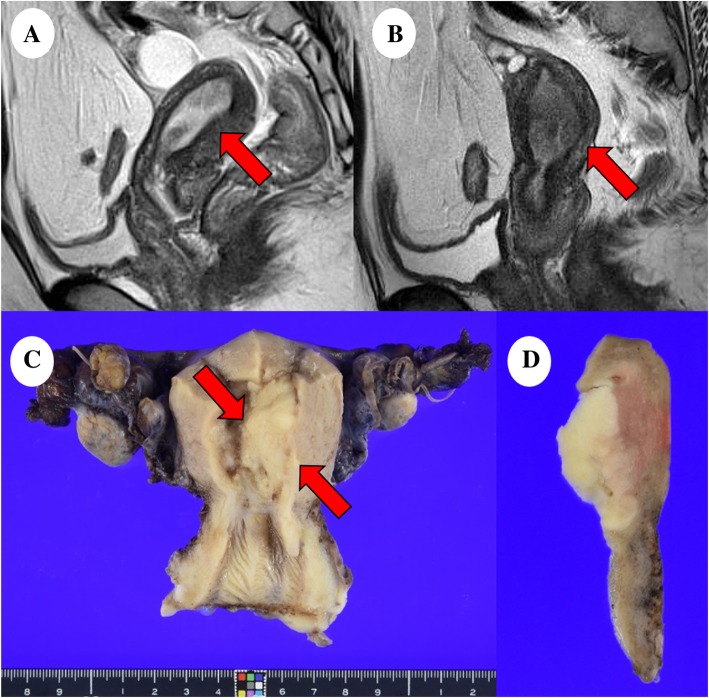
Fig. 1.
Magnetic resonance imaging and macroscopic analysis: (a) T2-weighted image of the initially diagnosed tumor (red arrow). (b) T2-weighted image of the recurred tumor (red arrow). (c) The endometrial mass (red arrows) was 40 × 23 mm-sized in the left wall of the uterine body and (d) had yellow-whitish cut surface
Pathological findings
The uterine body showed a tumor of the size 40 × 23 mm in the left wall with a yellow-whitish cut surface (Fig. 1c). The tumor was histologically found to be endometrial carcinoma with an unusual epithelial component exhibiting variable patterns such as tubular (30%), glandular (30%), papillary (5%), and solid (30%) structure, mimicking mesonephric carcinoma of the cervix (Fig. 2a, b). Additionally, the tumor had a heterologous element of cartilaginous cells (5%) with no atypia (Fig. 2c). The tumor was confined to the uterine body where the infiltration reached the inner half of the myometrium, but with no extension into the cervix, adnexae, and omentum (International Federation of Gynecology and Obstetrics stage IA [pT1a pNX cM0]). No associated mesonephric remnants or lesions were seen. No other neoplasias were present in the ovaries, fallopian tubes, cervix, or omentum. Immunohistochemical analysis of the surgical sample showed that the tumor cells were positive for TTF-1 (focal, strong), GATA-3 (focal, strong), CD10 (focal, strong), p53 (focal, weak), CA125 (focal, strong), CK7 (diffuse, strong), and p16 (focal, strong). Moreover, they were negative for ER, PgR, calretinin, hepatocyte nuclear factor-1-β, napsin A, androgen receptor, and Wilms’ tumor 1 (WT-1) protein (Fig. 2d–f) (Table 1). On molecular testing, KRAS mutation (c.35G > C, p.G12A; Gly12Ala) was detected in this endometrial cancer.
Fig. 2.
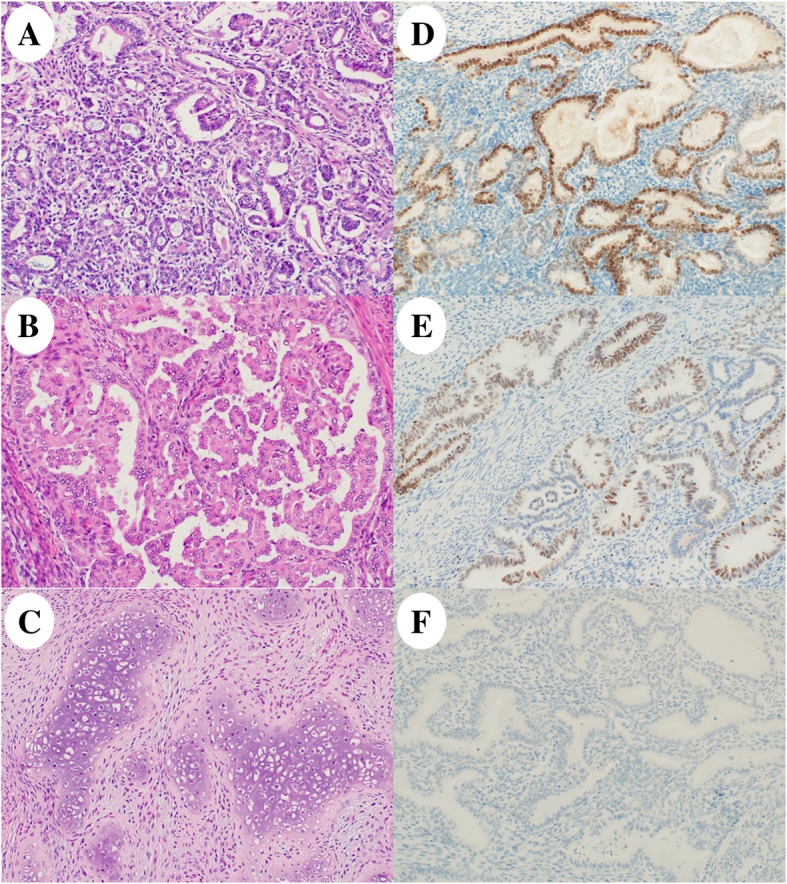
Histology and immunohistochemistry of the recurred tumor. The tumor had a variety of histologic patterns including (a, 20×) tubular and glandular, (b, 20×) papillary, and (c, 40×) spindled and solid with a heterologous element (cartilage without atypia). Immunohistochemical analysis showed positive staining for (d, 2 × 0) thyroid transcription factor 1 and (e, 20×) GATA-3; however, the tumor was negative for (f, 20×) estrogen receptor
Table 1.
List of antibodies
| Antigen | Clone | Dilution | Manufacturer |
|---|---|---|---|
| Estrogen receptor | SP1 | 1:1 | Ventana, AZ, USA |
| Progesterone receptor | 1E2 | 1:1 | Ventana, AZ, USA |
| TTF-1 | 8G7G3/1 | 1:50 | Dako, Kyoto, Japan |
| GATA-3 | L50–823 | 1:1 | Ventana, AZ, USA |
| CD10 | 56C6 | 1:40 | Leica Biosystems, Wetzlar, Germany |
| p53 | DO-7 | 1:50 | Dako, Kyoto, Japan |
| CA125 | M11 | 1:50 | Dako, Kyoto, Japan |
| CK7 | OV-TL 12/30 | 1:100 | Dako, Kyoto, Japan |
| p16 (INK4) | G175–405 | 1:10 | Becton Dickinson, NJ, USA |
| Calretinin | 5A5 | 1:100 | Leica Biosystems, Wetzlar, Germany |
| HNF-1β | Poly | 1:400 | SIGMA, Kanagawa, Japan |
| Napsin A | TMU-Ad02 | 1:50 | IML, Gunma, Japan |
| Androgen receptor | AR441 | 1:50 | Dako, Kyoto, Japan |
| WT-1 | 6F-H2 | 1:50 | Dako, Kyoto, Japan |
TTF-1 thyroid transcription factor 1, GATA-3 GATA binding protein 3, CA125 cancer antigen 125; HNF-1β, hepatocyte nuclear factor-1β
The initial endometrial biopsy showed the following: the tumor was found to have a coexistence of usual and unusual carcinomas in which the former was a low-grade endometrial carcinoma, i.e., endometrioid carcinoma G1 (70%), and the latter was composed of the same components as the surgical sample (30%) (Fig. 3a). On immunohistochemical analysis of the initial biopsy (Table 1), the endometrioid carcinoma component was diffuse positive for ER and PgR, but negative for TTF-1 and GATA-3 (Fig. 3b, c). The unusual epithelial components showed a transitioning pattern with a mixture of ER- and TTF-1-positive cells within the same glands, representing Müllerian and Wolffian duct markers, respectively (Fig. 3d–f). On molecular testing, same KRAS mutation (c.35G > C, p.G12A; Gly12Ala) was detected in the initial biopsy.
Fig. 3.
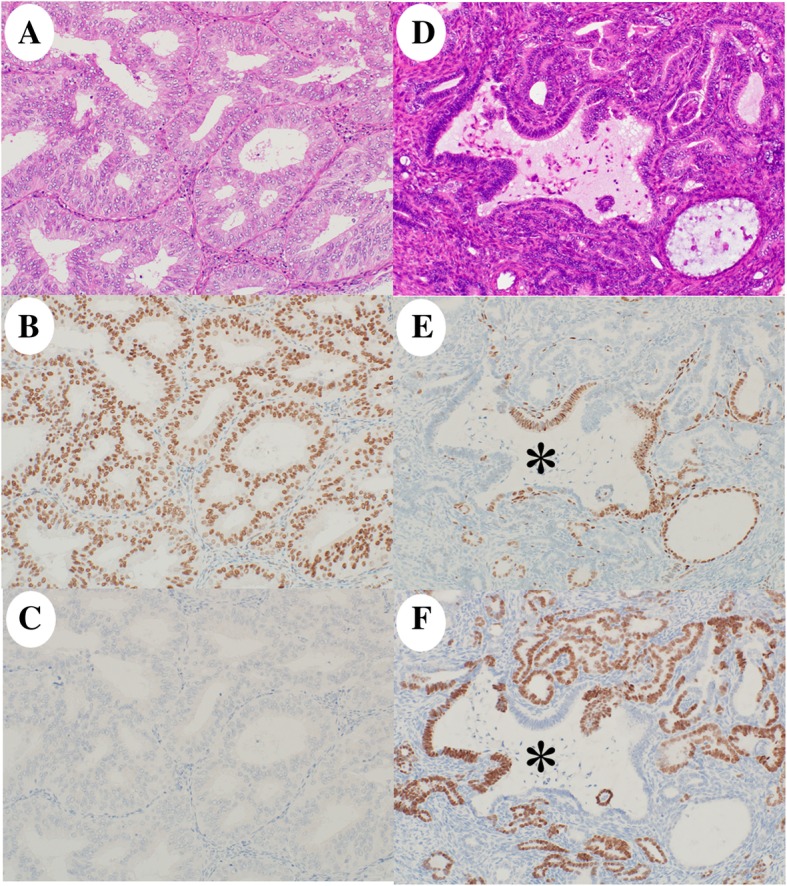
Histology and immunohistochemistry of the initially diagnosed tumor. The tumor had (a, 20×) a low-grade endometrioid carcinoma component and (d, 20×) a mesonephric-like adenocarcinoma component. Immunohistochemically, the endometrioid carcinoma was diffuse positive for (b, 20×) estrogen receptor (ER) and negative for (c, 20×) thyroid transcription factor 1 (TTF-1). The mesonephric-like adenocarcinoma showed a transition pattern with a mixture of cells positive for (e, 20×) ER- (a Müllerian duct marker) and (f, 20×) TTF-1- (a Wolffian duct marker) within the same glands (asterisk)
Based on these findings, a coexistence of endometrioid carcinoma G1 and MLA in the uterine body was diagnosed at the biopsy. After MPA therapy, tumor composed of only the component of MLA was found as recurrence.
Discussion and conclusions
Endometrial MLA occurs in patients of all ages; the results of some studies show that approximately 40 cases with MLA were been reported in women of ages between 31 and 91 years [2, 3, 6]. Furthermore, it can mimic various other endometrial cancers. In women of reproductive age, low-grade endometrial carcinoma, represented by endometrioid carcinoma G1/2, is treated with MPA to preserve fertility [8]. The MLA in our patient was coincidentally treated with MPA therapy because of misdiagnostic consideration of endometrioid carcinoma G1; to our best knowledge, there have been no previous reports with MLA which was treated with MPA. This therapy produced a good response for the endometrioid carcinoma, resulting in a complete disappearance. However, MPA therapy did not suppress the recurrence of MLA. The discrepancy in the effect of therapy between the endometrioid carcinoma G1 and MLA is considered to be attributed to overexpression of hormone receptors (ER and PgR) in the former component and weak expression of them in the latter component. Poor MPA efficacy is usually associated with weak or no expression of ER and PgR [8, 9]; hence, MPA therapy appears to be ineffective against MLA. Therefore, MLA needs to be distinguished from endometrioid carcinoma in terms of selection of appropriate treatments.
MLA is often misdiagnosed as other malignant neoplasms, due to its unfamiliar nature. Microscopically, MLA is consistently heterogeneous in its architecture (tubular, papillary, sieve-like, ductal, solid) with cuboidal to columnar epithelium and attenuated segments [5]. Occasional small ducts or tubules can contain intraluminal eosinophilic sections. The cells tend to have frequently scant eosinophilic cytoplasm [5]. When such morphology is observed, additional studies are required in the routine clinical setting. The following panel-based immunohistochemistry results are important for differential diagnosis between endometrioid carcinoma and endometrial MLA: positive for TTF-1, GATA-3, and CD10; negative/partially positive for ER and PgR; and showing wild type p53 expression. Endometrial MLA is usually positive for TTF-1 but is more frequently ER positive and/or GATA-3 negative than cervical mesonephric carcinoma [4, 6]. The present patient also is characterized as focally containing a heterologous component of cartilaginous tissue; while cervical mesonephric carcinoma with heterologous elements such as cartilage and/or skeletal muscle were previously reported, none with endometrial MLA containing heterologous elements has been described to date [6, 10]. The present case provides evidence that even with heterologous elements it is not necessarily carcinosarcoma. We believe that carcinosarcoma could be ruled out based on the mild atypia of the cartilaginous tissue.
It remains to be clarified whether MLA represents essentially mesonephric (Wolffian) carcinoma or mesonephric carcinoma presenting like endometrioid (Müllerian) carcinoma. The present case with MLA was speculated to have a closely Müllerian lineage based on the two evidences as follows: the coexistence with endometrioid carcinoma of Müllerian duct origin and the immunohistochemical admixture/transition between ER- and TTF-1-positive cells within the same glands. In a series of patients with uterine neoplasms, McFarland et al. [2] found that MLAs predominantly involved the endometrium from which they appeared to arise, and showed subsequent invasion into the myometrium; none involved the myometrium without endometrial involvement. Moreover, 3 of their 5 patients with ovarian MLA had foci of endometriosis admixed with or adjacent to the carcinoma. Chapel et al. [11] also reported an ovarian carcinoma with combined low-grade serous and mesonephric morphologies that were indicative of a Müllerian origin. Taken together, these MLA features all suggest a Müllerian neoplasm rather than a true Wolffian/mesonephric tumor.
In conclusion, we encountered a patient with coexisting endometrial MLA and low-grade endometrioid carcinoma, which was treated using MPA therapy, resulting in recurrence of MLA alone. These clinicopathological findings support the prevailing notions that MLA is a Müllerian adenocarcinoma exhibiting mesonephric differentiation.
Acknowledgements
We thank Kouichi Kamada, Yusuke Hosonuma, and Yasuo Kamakura, Department of Pathology, Saitama Medical University International Medical Center, for their technical support. We would like to thank Editage (www.editage.jp) for English language editing.
Funding
This work was supported by Hidaka Research Projects at the Saitama Medical University (Grant numbers: 30-D-1-8) and Grants-in-Aid from the Ministry of Education, Science, Sports, and Culture of Japan (Research Project Numbers: 18 K06997).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ER
Estrogen receptor
- GATA-3
GATA binding protein-3
- MLA
Mesonephric-like adenocarcinoma
- MPA
Medroxyprogesterone acetate
- MRI
Magnetic resonance imaging
- PR
Progesterone receptor
- TTF-1
Thyroid transcription factor 1
Authors’ contributions
MY: conception, pathologic diagnosis, immunohistochemical analysis, and writing of manuscript. DS, KH: collection of clinical data. MH, KI: pathologic diagnosis and immunohistochemical analysis. EK: radiologic analysis. MY (the last author): pathologic diagnosis and revision of manuscript. All authors read and approved the final manuscript prior to submission.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for the publication of this case report.
Competing interests
The authors declare no conflicts of interest associated with this manuscript.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mitsutake Yano, Email: yano1210@oita-u.ac.jp.
Daisuke Shintani, Email: d1010goal@yahoo.co.jp.
Tomomi Katoh, Email: tomokato22@gmail.com.
Mei Hamada, Email: de421042@s.okayama-u.ac.jp.
Kozue Ito, Email: ko_ito3@saitama-med.ac.jp.
Eito Kozawa, Email: 8kozawa@gmail.com.
Kosei Hasegawa, Email: koseih@gmail.com.
Masanori Yasuda, Phone: +81-42-984-6090, Email: m_yasuda@saitama-med.ac.jp.
References
- 1.Mirkovic J, Sholl LM, Garcia E, Lindeman N, MacConaill L, Hirsch M, et al. Targeted genomic profiling reveals recurrent KRAS mutations and gain of chromosome 1q in mesonephric carcinomas of the female genital tract. Mod Pathol. 2015;28:1504–1514. doi: 10.1038/modpathol.2015.103. [DOI] [PubMed] [Google Scholar]
- 2.McFarland M, Quick CM, McCluggage WG. Hormone receptor-negative, thyroid transcription factor 1-positive uterine and ovarian adenocarcinomas: report of a series of mesonephric-like adenocarcinomas. Histopathology. 2016;68:1013–1020. doi: 10.1111/his.12895. [DOI] [PubMed] [Google Scholar]
- 3.Na K, Kim HS. Clinicopathologic and molecular characteristics of mesonephric adenocarcinoma arising from the uterine body. Am J Surg Pathol. 2019;43:12–25. doi: 10.1097/PAS.0000000000000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolin DL, Costigan DC, Dong F, Nucci MR, Howitt BE. A combined morphologic and molecular approach to retrospectively identify KRAS-mutated mesonephric-like adenocarcinomas of the endometrium. Am J Surg Pathol. 2019;43:389–398. doi: 10.1097/PAS.0000000000001193. [DOI] [PubMed] [Google Scholar]
- 5.Patel V, Kipp B, Schoolmeester JK. Corded and Hyalinized mesonephric-like adenocarcinoma of the uterine Corpus: report of a case mimicking Endometrioid carcinoma. Hum Pathol. 2018; S0046-8177(18)30334-4. [DOI] [PubMed]
- 6.Pors J, Cheng A, Leo JM, Kinloch MA, Gilks B, Hoang L. A comparison of GATA3, TTF1, CD10, and Calretinin in identifying mesonephric and mesonephric-like carcinomas of the gynecologic tract. Am J Surg Pathol. 2018;42:1596–1606. doi: 10.1097/PAS.0000000000001142. [DOI] [PubMed] [Google Scholar]
- 7.Mirkovic J, McFarland M, Garcia E, Sholl LM, Lindeman N, MacConaill L, et al. Targeted genomic profiling reveals recurrent KRAS mutations in mesonephric-like adenocarcinomas of the female genital tract. Am J Surg Pathol. 2018;42:227–233. doi: 10.1097/PAS.0000000000000958. [DOI] [PubMed] [Google Scholar]
- 8.Yamazawa K, Hirai M, Fujito A, Nishi H, Terauchi F, Ishikura H, et al. Fertility-preserving treatment with progestin, and pathological criteria to predict responses, in young women with endometrial cancer. Hum Reprod. 2007;22:1953–1958. doi: 10.1093/humrep/dem088. [DOI] [PubMed] [Google Scholar]
- 9.Minaguchi T, Nakagawa S, Takazawa Y, Nei T, Horie K, Fujiwara T, et al. Combined phospho-Akt and PTEN expressions associated with post-treatment hysterectomy after conservative progestin therapy in complex atypical hyperplasia and stage Ia, G1 adenocarcinoma of the endometrium. Cancer Lett. 2007;248:112–122. doi: 10.1016/j.canlet.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Bague S, Rodriguez IM, Prat J. Malignant mesonephric tumors of the female genital tract: a clinicopathologic study of 9 cases. Am J Surg Pathol. 2004;28:601–7.11. doi: 10.1097/00000478-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Chapel DB, Joseph NM, Krausz T, Lastra RR. An ovarian adenocarcinoma with combined low-grade serous and mesonephric morphologies suggests a Mullerian origin for some mesonephric carcinomas. Int J Gynecol Pathol. 2018;37:448–459. doi: 10.1097/PGP.0000000000000444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.