Abstract
Background
Schistosomiasis remains a public health problem in Central Kenya despite concerted control efforts. Access to improved water and sanitation has been emphasized as important control measures. Few studies have assessed the association between access to improved water sources and sanitation facilities with Schistosoma mansoni infection in different environmental settings. This study assessed the association between S. mansoni infection and household access to improved water sources and sanitation facilities in Mwea, Kirinyaga County, Kenya.
Methods
A cross sectional study was conducted between the months of August and October 2017. A total of 905 household heads from seven villages were interviewed and their stool samples screened for S. mansoni using the Kato Katz technique. Comparisons of demographic factors by S. mansoni infection were tested for significance using the chi-square test (χ2) or the Fisher exact test for categorical variables. Variables associated with S. mansoni infection were analyzed using univariable analysis and the strength of the association measured as odds ratio (OR) using mixed effects logistic regression at 95% CI, with values considered significant at p < 0.05.
Results
The overall prevalence of S. mansoni was, 23.1% (95% CI: 20.5–26.0%), with majority of the infections being of light intensity. Rurumi village had the highest prevalence at 33.3%, with Kirogo village having the least prevalence at 7.0%. Majority (84.1%) of the households lacked access to improved water sources but had access to improved sanitation facilities (75%). Households with access to piped water had the lowest S. mansoni infections. However, there was no significant association between S. mansoni infections with either the main source of water in the household (Odds Ratio (OR) =0.782 (95% CI: 0.497–1.229) p = 0.285 or sanitation facilities (OR = 1.018 (95% CI: 0.705–1.469) p = 0.926.
Conclusion
Our study suggests that S. mansoni is still a public health problem among all age groups in Mwea irrigation scheme, Kirinyaga County, Central Kenya. Majority of the households lacks access to improved water sources but have access to improved sanitation facilities. This study recommends initiatives to ensure adequate provision of improved water sources, and the inclusion of the adult community in preventive chemotherapy programs.
Electronic supplementary material
The online version of this article (10.1186/s12879-019-4105-1) contains supplementary material, which is available to authorized users.
Keywords: Schistosoma mansoni, Water, Sanitation, Improved, Unimproved, Kenya
Background
Schistosomiasis is a parasitic disease caused by a trematode worm of the genius Schistosoma [1]. There are two types of schistosomiasis, intestinal and urinary. Intestinal schistosomiasis is caused by Schistosoma mansoni and Schistosoma japonicum where parasite eggs are released in faeces while urinary schistosomiasis is caused by Schistosoma haematobium, and parasite eggs are released in the urine [1]. Schistosoma mansoni, transmitted by Biomphalaria snails and Schistosoma haematobium, transmitted by Bulinus snails are the most prevalent Schistosoma species [2]. According to the Global burden of disease report of 2013, more than 290 million people worldwide are estimated to be infected with schistosomiasis, about 600–780 million are at risk of infection, with morbidity due to these infections resulting to an estimated 2.8 million disability adjusted life years (DALYs) [2]. Schistosomiasis is endemic in more than 78 countries, with more than 90% of the infections occurring in sub- Saharan Africa [3].
In Kenya, approximately six million people have schistosomiasis and an additional fifteen million are at risk of infection [4]. Two species are predominant in Kenya, Schistosoma haematobium and, Schistosoma mansoni [5]. Recent findings reported prevalence of 2.1% for Schistosoma mansoni, and 14.8% for Schistosoma haematobium among school going children [6]. The distribution of Schistosomiasis in Kenya is such that Schistosoma haematobium is found mainly around the coast regions, some parts of Lake Victoria and Kano plains in Western Kenya [7], while Schistosoma mansoni occurs mainly in the Western parts of the country [8], and some parts of Central Kenya [9].
A school based schistosomiasis and soil transmitted helminths control programme was initiated in the year 2004 through collaboration between Kenya Medical Research Institute (KEMRI) and Japan International Corporation Agency (JICA). The programme entailed mass drug administration (MDA) of preventive chemotherapy to all school age children in Mwea, Kirinyaga County [10]. The preventive chemotherapy included a single dose of 40 mg/kg of Praziquantel administered using the tablet dose pole to determine the number of tablets to be taken by a child, and Albendazole as a single dose of 400 mg [11]. The programme was implemented until the year 2008, after which MDA was taken over by the Kenya National School based deworming programme.
Schistosomiasis contributes significantly to lower social economic conditions in areas where it is endemic and causes a great deal of disability thus reducing the work performance among the infected individuals. However, mortality associated with these infections is low [12]. Schistosomiasis infections have been shown to increase the susceptibility to or severity of co-infecting pathogens [1], and as a result the disease has been targeted for control and eventual elimination by the World Health Organization (WHO) [13].
Like many other endemic countries, the control of schistosomiasis is through mass drug administration (MDA) using the drug of choice Praziquantel [13]. Although chemotherapy is cost-effective [14] and reduces schistosome infections in human hosts [15], it has a limitation in that it does not kill immature worms [16] and has low impact on transmission [17]. This intervention is often delivered through school based deworming programme (SBDP) and offers many benefits to the treated children [18].
Recently, there has been global advocacy geared towards schistosomiasis transmission interruption, with a call in the year 2012 by the World Health Assembly (WHA) resolution 65.21, on countries to intensify control and initiate elimination campaigns [19]. Water, sanitation and hygiene education (WASH) have been emphasized as a component of an integrated control and elimination strategy in the WHA resolution on the bases that they should reduce schistosomiasis transmission by reducing human water contact [20]. WASH has been acknowledged in WHO prevention and control guidelines, which advices the inclusion of the same in helminth control programs [21].
The provision of access to safe drinking water, hygiene and sanitation, which has also been classified as the “forgotten foundations of health” [22, 23], though essential in the control of schistosomiasis, is inadequate in large parts of low and middle-income countries where schistosomiasis is endemic [1, 24]. WASH interventions have the potential to reduce the environmental exposure to infected schistosome eggs and larvae and thus reduce transmission of the disease ensuring a long term improvement in people’s wellbeing [25, 26].
Even though the important role of WASH has been recognized and advocated for in the World Health Assembly (WHA) resolutions on schistosomiasis [27], WASH has not been incorporated in disease specific control programs. For this to happen, there is need for data on the levels of community access to clean water, sanitation and hygiene and how each aspect of WASH associates with schistosomiasis infection in different endemic settings. This will help us understand which specific WASH intervention is most effective in reducing exposure to infection in what environmental setting. Several studies have reported on the effects of WASH on NTDs especially STHs [28–33], however, little evidence exists to inform policy decisions about the importance of including WASH as part of schistosomiasis control. Therefore, the aim of this study was to describe the association between S. mansoni infection and household access to improved water sources and sanitation facilities in Kirinyaga County, central Kenya.
Methods
Study area
The present study was conducted in two different Sub Counties of Kirinyaga County. The Sub Counties included Mwea East and West. Kirinyaga County lies between 1158 M and 5380 M above sea level in the South and at the Peak of Mount Kenya respectively, with a mean annual rainfall ranging between 1200 and 1600 mm per year. Covering an area of 1478.1 km2, the County is located about 100kms north east of Nairobi. Figures from the 2009 census indicate that the County had an estimated total population of 528,054 persons, with an annual growth rate of 1.5%. The population was projected to be 593,379 in the year 2017 [34]. There were an estimated 154, 220 households in the county. The two Sub Counties are home to the giant Mwea irrigation scheme where several water canals crisscross the area supplying irrigation water to the farms and villages respectively. The main socio-economic activities include rice and horticultural farming. Generally, Mwea East and West Sub Counties are endemic for S. mansoni [9, 35, 36]. However, data on community access to improved water and sanitation is lacking.
Study population and selection criteria
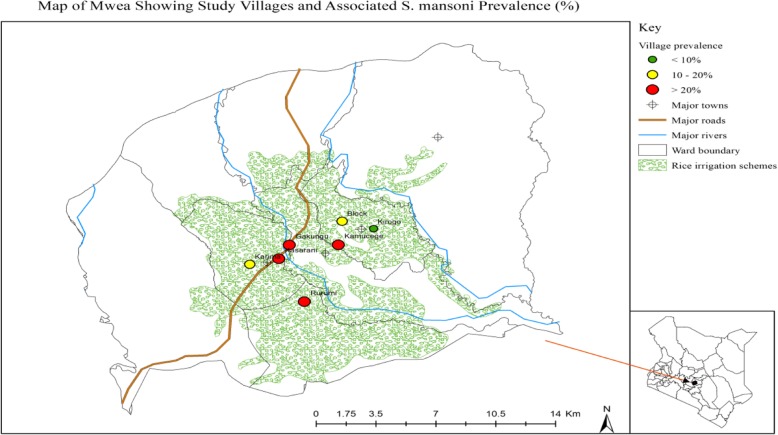
In Mwea West Sub County, there were two locations both of which were located within the irrigation scheme (Thiba and Mutithi); while in Mwea East there were a total of five locations. These included Gathigiriri, Tebere, Murinduko, Nyangati and Kangai. Tebere and Gathigiriri locations were located within the Mwea irrigation scheme. Tebere and Gathigiriri locations in Mwea East and Thiba and Mutithi in Mwea West were included in the study since they were located within the irrigation scheme. Tebere and Thiba locations from Mwea East and West respectively were then sampled randomly and included in the study. Tebere location had a total of six villages which included Kamucege, Gathigiriri, Block, Kiarukungu, Mahigaini and Kirogo, while Thiba had eight villages including Mbui Njeru, Maendeleo, Gakungu, Rurumi, Thiba, Karima, Kiratina and Kasarani. Four and three villages from Thiba and Tebere locations respectively were selected and included in the study (Fig. 1). The study villages are shown in Fig. 2.
Fig. 1.
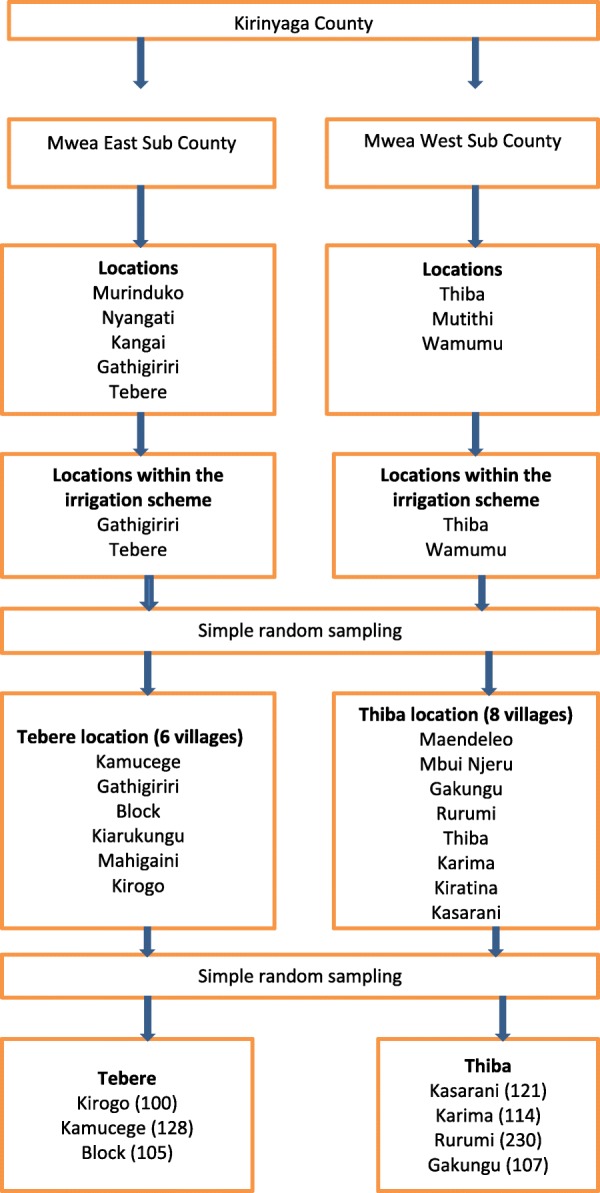
Flow diagram showing selection of study villages
Fig. 2.
Map of Mwea, showing the study villages and associated S. mansoni prevalence
Study design and sample size determination
This was a cross sectional study carried out between the months of August and October 2017. The study used prevalence of 26% for Schistosoma mansoni derived from a recent study in the area [35], level of significance and an error margin of 0.05, and standard deviation at 95% CI (1.96) to calculate the sample size [37]. A sample size of 432 per sub County was calculated, and multiplied by two since the study was covering two sub Counties to give a minimum sample size of 864 which was then adjusted to 905 to cater for refusals and incomplete data.
Lists of all households per each village were obtained from the village Community Health Workers (CHW). Using probability proportional to size sampling and the total number of households per village, each village was allocated a minimum number of households to be sampled from the total study sample size. In each village, the first household to be sampled was identified by the study team and systematic random sampling used to locate every third household during the survey. In each household sampled, the head or a representative of the sampled household was interviewed. The study team held meetings with the leadership at the County and Sub County level, the local administration and village members in all the seven study villages prior to the start of data collection to explain to them the purpose of the study and seek their consent. Each participating household head gave individual written consent before the survey.
Questionnaire administration
A structured questionnaire developed in English and translated to the local language (kikuyu) was administered to the household heads in selected households. The questionnaire included sections on social demographic indicators such as age, sex, marital status, religion, education, occupation, and the assessment of water and sanitation. The section on water and sanitation was developed based on the definitions by the joint monitoring programme (JMP), of the World Health Organization (WHO) and the United Nations Children Fund (UNICEF), for water and sanitation [38]. Improved sanitation facilities was assessed in households that had flush toilet, a piped sewer system, a septic tank, flush/ pour to a pit latrine, ventilated improved pit latrine (VIP), pit latrine with slab and a composting toilet. Unimproved toilet facilities were assessed in households that had flush pour to elsewhere, pit latrine without slab, bucket, hanging toilet/ latrine or no facility at all. Improved water source were assessed in households which had access to piped water into dwelling, piped water to yard/ plot, public tap or stand pipe, tube well or borehole, protected dug well, protected spring, bottled water and rain water, while Unimproved water source included unprotected spring, unprotected dug well, cart with small tank, tanker-truck and surface water. Surface water included rivers, dams, lakes, ponds, streams, canals and irrigation channels. Additional information in this section included time taken to the water source and whether the household head was satisfied with the source. Presence of sanitation facility and water source in the household, whether there were faeces on the toilet floor, and whether or not the toilet was in use was observed by physically visiting the facility. Information on toilet sharing was also collected (Additional file 1).
Parasitological screening for S. mansoni
Household heads or their adult representatives were asked to provide a stool sample. A clean screw capped, well labeled plastic container with scoop was provided with instructions on how to collect a stool sample. Those who were not able to provide a stool sample were visited the next day until they got the sample. The samples were collected the same day or the following morning and taken to Kimbimbi Sub County hospital laboratories for analysis. Samples that could not be processed the same day were stored at 4 °C. In the laboratory, Kato Katz techniques was used in sample processing where each sample was prepared in duplicate smears of 41.7 mg [39]. A random sample of 10% of all the positive and negative slides read each day were randomly reexamined by a third experienced laboratory technologists to ensure quality work.
Data analysis
Household data collected was entered and stored in an excel spreadsheet and counter checked for accuracy. Parasitology data was entered in the laboratory parasitology result forms and then entered in the excel spreadsheet. Data was analyzed using STATA version 14.0 (Stata Corporation, College Station, TX, USA). Presence of S. mansoni egg across the duplicate slides indicated infection while the arithmetic mean of eggs per gram (epg) of faeces across the duplicate slides expressed the intensity of the infection. The infection intensity was categorized according to the WHO classifications for S. mansoni (0) negative, (1–99) light, (100–399) moderate and (> 400) heavy [40]. The prevalence of S. mansoni was calculated by age group, gender and village of residence. Comparisons of demographic factors by S. mansoni infection were tested for significance using the chi-square test (χ2) or the Fisher exact test for categorical variables. Factors associated with S. mansoni infection were analyzed using univariable analysis and the strength of the association measured as odds ratio (OR) using mixed effects logistic regression at 95% CI, with values considered significant at p < 0.05.
Ethical considerations
Prior to the implementation of the study, ethical approvals were sought from the Scientific and Ethical Review Unit (SERU) of the Kenya Medical Research Institute (KEMRI), number (KEMRI/SERU/ESACIPAC/007/3326) and Meru University of Science and Technology Institutional Research Ethics Review Committee (MIRERC), number (MIRERC/001/2017), and the Health Management Team of Kirinyaga County. All the household heads participating in the study signed a written informed consent which had been translated into the local dialect. Those who could not read or write were asked to have an independent person as a witness who ensured that the study was clearly explained to them and guided them to give a thumb print on the consent form.
Results
Demographic information of the study participants
Overall, data was collected from a total of 905 household heads who gave a written informed consent, 70.5% of whom were female. The age of the respondents ranged from 17 to 95 years. Respondents within the age bracket of 36 and 45 years were the majority 30.3%, followed by those in the age bracket of 26 and 35 years at 28.8%. Majority of the respondents were Christians (95.0%), married (83.5%) and farmers (63.6%). Those who had no formal education were 7.6%, most of who hailed from Karima village as shown in Table 1.
Table 1.
Household demographic factors aggregated by villages
| Characteristic | Karima N (%) |
Rurumi N (%) |
Kasarani N (%) |
Kirogo N (%) |
Kamucege N (%) |
Block N (%) |
Gakungu N (%) |
Total |
|---|---|---|---|---|---|---|---|---|
| Sex | ||||||||
| Male | 34 (29.8) | 106 (46.1) | 22 (18.1) | 25 (25.0) | 33 (25.8) | 28 (26.7) | 19 (17.8) | 267 (29.5) |
| Female | 80 (70.2) | 124 (53.9) | 99 (81.9) | 75 (75.0) | 95 (74.2) | 77 (73.3) | 88 (82.2) | 638 (70.5) |
| Age (years) | ||||||||
| ≤ 25 | 15 (13.2) | 21 (9.1) | 9 (7.4) | 6 (6.0) | 12 (9.4) | 11 (10.5) | 15 (14.0) | 89 (9.8) |
| 26–35 | 22 (19.3) | 69 (30.0) | 37 (30.6) | 25 (25.0) | 30 (23.4) | 37 (35.2) | 41 (38.8) | 261 (28.8) |
| 36–45 | 27 (23.7) | 72 (31.3) | 39 (32.2) | 29 (29.0) | 37 (28.9) | 34 (32.4) | 33 (30.9) | 271 (30.0) |
| 46–55 | 21 (18.4) | 30 (13.0) | 30 (24.8) | 24 (24.0) | 23 (18.0) | 8 (7.6) | 14 (13.1) | 150 (16.6) |
| > 55 | 29 (25.4) | 38 (16.6) | 6 (5.0) | 16 (16.0) | 26 (20.3) | 15 (14.3) | 4 (3.7) | 134 (14.8) |
| Marital status | ||||||||
| Married | 97 (85.1) | 179 (77.8) | 105 (86.8) | 89 (89.0) | 109 (85.2) | 83 (79.0) | 94 (87.9) | 756 (83.5) |
| Single/ Window(wer)/ Divorced | 17 (14.9) | 51 (22.2) | 16 (13.2) | 11 (11.0) | 19 (14.8) | 22 (21.0) | 13 (12.1) | 149 (16.5) |
| Religion | ||||||||
| Christian | 106 (93) | 220 (95.7) | 116 (95.8) | 95 (95.0) | 117 (91.4) | 100 (95.2) | 106 (99.1) | 860 (95.0) |
| Others | 8 (7.0) | 10 (4.3) | 5 (4.2) | 5 (5.0) | 11 (8.6) | 5 (4.8) | 1 (0.9) | 45 (5.0) |
| Education | ||||||||
| No formal education | 20 (17.5) | 12 (5.2) | 5 (4.1) | 7 (7.0) | 16 (12.5) | 10 (9.5) | 2 (1.9) | 72 (7.6) |
| Primary (Incomplete) | 31 (27.2) | 72 (31.3) | 38 (31.4) | 26 (26.0) | 42 (32.8) | 37 (35.2) | 35 (32.7) | 280 (20.9) |
| Primary (Complete) | 41 (36.0) | 107 (46.5) | 45 (37.2) | 48 (48.0) | 47 (36.8) | 38 (36.2) | 41 (38.3) | 370 (40.9) |
| Secondary Incomplete) | 20 (17.5) | 31 (13.5) | 26 (21.5) | 16 (16.0) | 20 (15.6) | 14 (13.3) | 23 (21.5) | 152 (16.8) |
| Secondary and post-secondary | 2 (1.8) | 8 (3.5) | 7 (5.8) | 3 (3.0) | 3 (2.3) | 6 (5.8) | 6 (5.6) | 31 (13.8) |
| Occupation | ||||||||
| Business | 8 (7.0) | 24 (10.4) | 26 (21.5) | 13 (13.0) | 15 (11.7) | 17 (16.2) | 20 (18.7) | 123 (13.6) |
| Casual labourers | 9 (7.9) | 47 (20.4) | 18 (14.9) | 10 (10.0) | 19 (14.8) | 20 (19.0) | 23 (21.5) | 146 (16.1) |
| Others | 8 (7.0) | 13 (5.7) | 18 (14.9) | 1 (1.0) | 4 (3.2) | 6 (5.8) | 11 (10.3) | 61 (6.7) |
| Farmer | 89 (78.1) | 146 (63.5) | 59 (48.7) | 76 (76.0) | 90 (70.3) | 62 (59) | 53 (49.5) | 575 (63.6) |
N = 905 Karima N = 114, Rurumi N = 230, Kasarani N = 121, Kirogo N = 100, Kamucege N = 128, Block N = 105, Gakungu =107
Household access to improved water and sanitation facilities
Majority of the households (84.1%) had access to unimproved water sources mainly from surface water sources such as canals, rivers and streams. Other sources of water included piped water to plot (5.7%), borehole (5.6%) and rainwater collection (4.0%). Kasarani village had the highest households with access to piped water (19.0%), while Kirogo village had the highest access to borehole water at 49.0%. Majority of the households had improved sanitation facilities (74.9%), where the most common type was the VIP/simple latrine with concrete floor slab. Pit latrine without floor slab was the most common unimproved type at (21.9%). Rurumi village had the highest percentage (4.7%) of households without a sanitation facility (Table 2).
Table 2.
Household access to water sources and sanitation facilities aggregated by villages (N = 905)
| Characteristic | Karima N (%) |
Rurumi N (%) |
Kasarani N (%) |
Kirogo N (%) |
Kamucege N (%) |
Block N (%) |
Gakungu N (%) |
Total |
|---|---|---|---|---|---|---|---|---|
| Source of water | ||||||||
| Piped water | 0 (0) | 7 (3.0) | 19 (15.7) | 9 (9.0) | 0 (0) | 0 (0) | 13 (12.1) | 48 (5.3) |
| Borehole | 0 (0) | 0 (0) | 0 (0) | 49 (49.0) | 0 (0) | 0 (0) | 0(0) | 49 (5.4) |
| Protected dug well | 0 (0) | 0(0) | 0 (0) | 0 (0) | 0 (0) | 10(9.5) | 6 (4.7) | 6 (0.7) |
| Rainwater collection | 2 (1.8) | 21 (9.1) | 2 (1.7) | 1 (1.0) | 0 (0) | 2 (1.9) | 4 (3.7) | 32 (3.5) |
| Other sources | 0 (0) | 0 (0) | 5 (4.1) | 0 (0) | 0 (0) | 0 (0) | 0(0) | 5 (0.6) |
| Surface water/ unprotected dug well | 112(98.2) | 202 (87.2) | 95 (78.5) | 41 (41.0) | 128 (100) | 103 (98.1) | 84 (78.5) | 765 (84.5) |
| Time taken to fetch water | ||||||||
| On premises | 0 (0) | 59 (25.7) | 93 (76.9) | 49 (49.0) | 19 (14.8) | 23 (21.9) | 54 (50.5) | 297 (32.8) |
| Less than thirty minutes | 111 (97.4) | 147 (63.9) | 28 (23.1) | 51 (51.0) | 109 (85.2) | 82 (78.1) | 50 (46.7) | 578 (63.9) |
| More than thirty minutes | 3 (2.6) | 24 (10.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (2.8) | 30 (3.3) |
| Sanitation facility | ||||||||
| VIP/Simple latrine with floor slab | 98 (86.0) | 149 (64.8) | 104 (85.9) | 91 (91.0) | 87 (68.0) | 60 (57.1) | 89 (83.2) | 678 (74.8) |
| Pit latrine without floor slab | 16 (14) | 62 (27.0) | 12 (9.9) | 7 (7.0) | 38 (29.7) | 45 (42.9) | 18 (16.8) | 198 (21.9) |
| Other | 0 (0) | 8 (3.5) | 5 (4.2) | 2 (2.0) | 0 (0) | 0 (0) | 0 (0) | 15 (1.7 |
| No facility | 0 (0) | 11 (4.7) | 0 (0) | 0 (0) | 3 (2.3) | 0 (0) | 0 (0) | 14 (1.6) |
| Reported sanitation access | ||||||||
| Shared | 25 (78.1) | 45 (20.5) | 30 (24.8) | 25 (25.0) | 33 (25.8) | 18 (17.1) | 18 (16.8) | 194 (21.7) |
| Not shared | 89 (21.9) | 174 (79.5) | 91 (75.2) | 75 (75.0) | 95 (74.2) | 87 (82.9) | 89 (83.2) | 700 (78.3) |
| Toilet in use | ||||||||
| Yes | 114 (100) | 226 (98.3) | 120 (99.2) | 100 (100) | 0 (0) | 103 (98.1) | 107 (100) | 898 (99.2) |
| No | 0 (0) | 4 (1.7) | 1 (0.8) | 0 (0) | 0 (0) | 2 (1.9) | 0 (0) | 7 (0.8) |
| Visible faeces on the edges | ||||||||
| Yes | 17 (14.9) | 52 (27.4) | 10 (8.3) | 21 (21.0) | 37 (28.9) | 31 (29.5) | 20 (18.7) | 188 (21.0) |
| No | 97 (85.1) | 167 (72.6) | 111 (91.7) | 79 (79.0) | 91 (71.1) | 74 (70.5) | 87 (81.3) | 706 (79.0) |
| Household has child (ren) under 3 years | ||||||||
| Yes | 17 (14.9) | 74 (32.2) | 37 (30.6) | 28 (28.0) | 26 (20.3) | 19 (18.1) | 47 (43.9) | 248 (27.4) |
| No | 97 (85.1) | 156 (67.8) | 84 (69.4) | 72 (72.0) | 102(79.7) | 86 (81.9) | 60 (56.1) | 657 (72.6) |
| Disposal of feaces for under 3 years | ||||||||
| Child used toilet/ latrine | 1 (6.0) | 15 (20.3) | 12 (32.4) | 8 (28.6) | 13 (50) | 13 (68.4) | 16 (30.1) | 78(31.4) |
| Other | 16 (94.0) | 55 (74.3) | 24 (64.9) | 17 (60.7) | 12 (46.2) | 0 (0) | 30 (63.8) | 154 (62.1) |
| Put/ rinsed in toilet/ latrine | 0 (0) | 4 (5.4) | 1 (0.7) | 3 (10.7) | 1 (3.8) | 6 (31.6) | 1 (6.1) | 16 (6.5) |
N = 905 Karima N = 114, Rurumi N = 230, Kasarani N = 121, Kirogo N = 100, Kamucege N = 128, Block N = 105, Gakungu =107
Prevalence and intensity of S. mansoni infections
The overall prevalence of S. mansoni was 23.1% (95% CI: 20.5–26.0%). The highest prevalence was reported in Rurumi village (31.3%), followed by Kasarani, Gakungu and Kamucege villages (28.1, 25.2 and 23%) respectively. Kirogo village had the least infection. Compared to Gakungu, residents of Kirogo village had about 78% lower odds of having S. mansoni infection (OR) 0.223(95% CI 0.092–0.540), p < 0.001). The burdens of S. mansoni infections were not significantly different in all the other villages. The number of S. mansoni eggs observed in the specimens ranged from 12 to 8148 eggs per gram (epg) of faeces. Majority of the S. mansoni infections (75%) were of light intensity (Table 3).
Table 3.
Prevalence and Intensity of Schistosoma mansoni infections by villages
| Karima N (%) |
Rurumi N (%) |
Kasarani N (%) |
Kirogo N (%) |
Kamucege N (%) |
Block N (%) |
Gakungu N (%) |
Total | |
|---|---|---|---|---|---|---|---|---|
| S. mansoni | ||||||||
| Positive | 21 | 72 | 34 | 7 | 29 | 19 | 27 | 209 |
| Negative | 93 | 158 | 87 | 93 | 99 | 86 | 80 | 696 |
| Prevalence | 18.4 | 31.3 | 28.1 | 7 | 22.7 | 18.1 | 25.2 | 23.1 |
| OR (95% CI) | 0.669(0.351–1.274) | 1.350(0.805–2.266) | 1.158(0.642–2.088) | 0.223(0.092–0.540) | 0.868(0.476–1.584) | 0.655(0.338–1.268) | REF | |
| P-value | 0.220 | 0.255 | 0.626 | < 0.001 | 0.644 | 0.207 | ||
| Intensity | ||||||||
| Light (1–99 epg) | 20 | 46 | 19 | 6 | 24 | 17 | 24 | 156 |
| Moderate (100–399 epg) | 1 | 19 | 13 | 1 | 4 | 1 | 2 | 41 |
| Heavy (≥400 epg) | 0 | 7 | 2 | 0 | 1 | 1 | 1 | 12 |
N = 905 Karima N = 114, Rurumi N = 230, Kasarani N = 121, Kirogo N = 100, Kamucege N = 128, Block N = 105, Gakungu =107
Association between S. mansoni infection and demographic variables
Table 4 summarizes the results for the association between S. mansoni infection and household demographic variables. Generally, male participants had the highest infection than female participants, though not statistically significant (26 and 22% respectively, p = 0.237). Households whose heads were farmers had the highest infection (24%) as compared to those whose occupation was business (19.8%) although not statistically significant p = 0.600. There were no statistical significant associations between S. mansoni infections and participant’s other social demographic attributes including education and marital status.
Table 4.
Association between S. mansoni infection and demographic variables
| Variables | S. mansoni | OR (95% CI) | P-value | |
|---|---|---|---|---|
| Positive | Negative | |||
| Sex | ||||
| Female | 141(22.1) | 497(77.9) | REF | |
| Male | 68(25.5) | 199(74.5) | 1.204(0.863–1.680) | 0.273 |
| Total | 209 | 696 | ||
| Age (years) | ||||
| > 55 | 36(26.1) | 102(73.9) | REF | |
| 46–55 | 36(24.2) | 113(75.8) | 0.903(0.529–1.540) | 0.707 |
| 36–45 | 53(19.3) | 221(80.7) | 0.679(0.419–1.102) | 0.116 |
| 26–35 | 69(25.7) | 199(74.3) | 0.982(0.615–1.569) | 0.941 |
| ≤ 25 | 15(19.7) | 61(80.3) | 0.697(0.353–1.376) | 0.297 |
| Total | 209 | 696 | ||
| Marital status | ||||
| Single/Widow(er)/Divorced | 66(22.5) | 227(77.5) | REF | |
| Married | 143(23.4) | 469(76.6) | 1.049(0.752–1.462) | 0.779 |
| Total | 209 | 696 | ||
| Religion | ||||
| Others | 6(13.6) | 38(86.4) | REF | |
| Christian | 203(23.6) | 658(76.4) | 1.954(0.814–4.688) | 0.127 |
| Total | 209 | 696 | ||
| Education | ||||
| Secondary & Post-secondary | 8(25.8) | 23(74.2) | REF | |
| Secondary (Incomplete) | 28(18.7) | 122(81.3) | 0.660(0.267–1.628) | 0.365 |
| Primary (Complete) | 82(22.0) | 291(78.0) | 0.810(0.349–1.878) | 0.623 |
| Primary (Incomplete) | 78(27.9) | 202(72.1) | 1.110(0.476–2.587) | 0.809 |
| No formal education | 13(18.3) | 58(81.7) | 0.644(0.236–1.759) | 0.389 |
| Total | 209 | 696 | ||
| Occupation | ||||
| Farmer | 50(8.7) | 522(91.3) | REF | |
| Business person | 64(52.0) | 59(48.0) | 1.248(0.845–1.843) | 0.265 |
| Casual labourer | 68(43.3) | 89(56.7) | 0.879(0.616–1.255) | 0.477 |
| Others | 27(50.9) | 26(49.1) | 1.195(0.680–2.098) | 0.535 |
| Total | 209 | 696 | ||
| Village | ||||
| Gakungu | 27(25.2) | 80(74.8) | REF | |
| Karima | 21(18.4) | 93(81.6) | 0.669(0.351–1.274) | 0.220 |
| Rurumi | 72(31.3) | 158(68.7) | 1.350(0.805–2.266) | 0.255 |
| Kasarani | 34(28.1) | 87(71.9) | 1.158(0.642–2.088) | 0.626 |
| Kirogo | 7(7.0) | 93(93.0) | 0.223(0.092–0.540) | < 0.001 |
| Kamucege | 29(22.7) | 99(77.3) | 0.868(0.476–1.584) | 0.644 |
| Block | 19(18.1) | 86(81.9) | 0.655(0.338–1.268) | 0.207 |
| Total | 209 | 696 | ||
N = 905
Infection with S. mansoni was highest among participants of more than 55 years of age (26.1%), followed by the age group 26 to 35 years (25.7%). The lowest prevalence of S. mansoni infections was recorded among study participants of age group 36–45 years (19.3%). In the age group ≤25 years and > 55 years, male participants had higher prevalence compared to female participants. In the 26 to 35 years’ age group men had lower prevalence of S. mansoni infections compared to women (23 and 32% respectively) (Fig. 3).
Fig. 3.
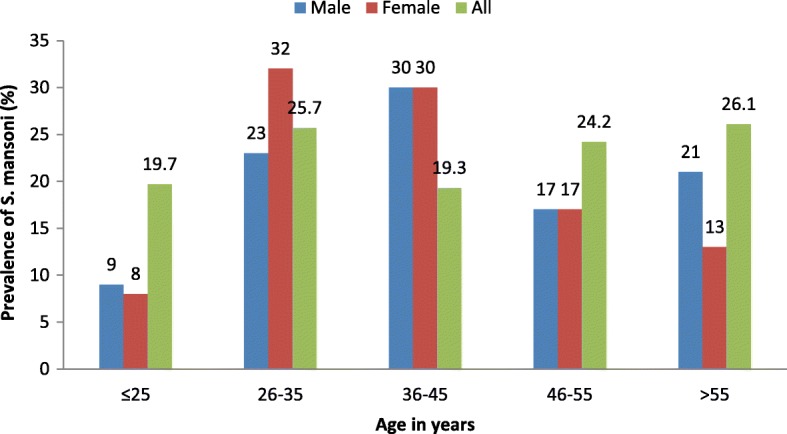
Distribution of S. mansoni infections by age and gender
Association between S. mansoni infections, water and sanitation variables
A total of 138 (15.2%) households had access to improved water sources. Out of these households, 27 (19.6%) household heads were infected with S. mansoni. Among the various sources of water, participants from households utilizing piped water had the lowest proportion of infections with S. mansoni (13%). Prevalence of S. mansoni infection were 14, 24, 31 and 50% among the participants who reported that their main source of drinking water was borehole, unprotected dug well/surface water, rainwater collection and protected dug well respectively. Nonetheless, there was no significant association between the main sources of water in households and infection with S. mansoni, (OR = 0.782 (95% CI: 0.497–1.229) p = 0.285. Being satisfied with the water supply was a significant predictor of S. mansoni infection (p < 0.004). Those who reported that they were satisfied with the water supply had 38% reduction in odds of being found to be infected with S. mansoni when assessed against their counterparts whose opinion was on the contrary (OR 0.623 (95% CI 0.452–0.859). Other attributes of water consumed in the study households that were assessed in the study, including treating of water before use, time taken to fetch water and main person who fetches water for the household, did not show significant association with the infection.
A total of 678 (74.9%) of the study households had access to an improved sanitation facility. Out of these, a total of 148 (21.8%) household heads were infected with S.mansoni. Households with unimproved sanitation facilities were 227 (25.1%), out of which 61 (26.9%) household heads were infected with S. mansoni. However, there was no significant association between the type of sanitation facility (improved / unimproved) and S. mansoni infection (OR = 1.018 (95% CI: 0.705–1.469 p = 0.926(Table 5).
Table 5.
– Association between S. mansoni infections, water and sanitation variables
| Variables | S. mansoni | OR (95% CI) | P-value | |
|---|---|---|---|---|
| Positive | Negative | |||
| Source of water in the household | ||||
| Unprotected dug well/Surface water | 180(23.5) | 585(76.5) | REF | |
| Piped | 6(12.5) | 42(87.5) | 0.464(0.194–1.110) | 0.078 |
| Borehole | 7(14.3) | 42(85.7) | 0.542(0.239–1.227) | 0.136 |
| Protected dug well | 3(50.0) | 3(50.0) | 3.250(0.650–16.243) | 0.149 |
| Rainwater collection | 10(31.3) | 22(68.8) | 1.477(0.687–3.178) | 0.315 |
| Other | 3(60.0) | 2(40.0) | 4.875(0.808–29.403) | 0.056 |
| Total | 209 | 696 | ||
| Time taken to fetch water from the source | ||||
| More than 30 min | 16(26.2) | 45(73.8) | REF | |
| On premises | 64(22.4) | 222(77.6) | 1.112(0.463–2.672) | 0.812 |
| Less than 30 min | 129(23.1) | 429(76.9) | 1.160(0.494–2.725) | 0.733 |
| Total | 209 | 696 | ||
| Satisfied with the water supply (source) | ||||
| Not satisfied | 136(26.7) | 374(73.3) | REF | |
| Satisfied | 73(18.5) | 322 (81.5) | 0.623 (0.452–0.859) | 0.004 |
| Total | 209 | 696 | ||
| Drinking water classification | ||||
| Un improved | 182(23.7) | 585(76.3) | REF | |
| Improved | 27(19.6) | 111(80.4) | 0.782(0.497–1.229) | 0.285 |
| Total | 209 | 696 | ||
| Toilet/Sanitation facility type | ||||
| No facility | 1(7.1) | 13(92.9) | REF | |
| VIP/simple pit latrine with floor/slab | 148(21.8) | 530(78.2) | 1.755(0.210–14.693) | 0.507 |
| Pit latrine without floor/slab | 46(23.2) | 152(76.8) | 1.852(0.217–15.786) | 0.999 |
| Other | 14(93.3) | 1(6.7) | 2.400(0.264–21.787) | 0.661 |
| Total | 209 | 696 | ||
| Toilet shared with other households | ||||
| Not shared | 154(22.2) | 540(77.8) | REF | |
| Shared | 54(27.4) | 143(72.6) | 1.149(0.791–1.668) | 0.466 |
| Total | 208 | 683 | ||
| Toilet in use | ||||
| No | 6(66.7) | 3(33.3) | REF | |
| Yes | 202 (22.9) | 680(77.1) | 0.400(0.089–1.802) | 0.207 |
| Total | 208 | 683 | ||
| Presence of faeces on the floor | ||||
| No | 163(23.2) | 541(76.8) | REF | |
| Yes | 45(24.1) | 142(75.9) | 1.069(0.730–1.564) | 0.732 |
| Total | 208 | 683 | ||
| Household has child(ren) under 3 years | ||||
| No | 161(22.4) | 558(77.6) | REF | |
| Yes | 48(25.4) | 138(74.6) | 1.195(0.820–1.742) | 0.353 |
| Total | 209 | 696 | ||
| Disposal of faeces for children < 3 years | ||||
| Put/rinsed into toilet or latrine | 42(25.0) | 124(75.0) | REF | |
| Child used toilet/latrine | 3(33.3) | 6(66.7) | 1.500(0.359–6.270) | 0.695 |
| Other | 3(27.3) | 8(72.7) | 1.125(0.285–4.441) | 0.999 |
| Total | 48 | 138 | ||
| Sanitation facility type | ||||
| Un-improved | 61(26.9) | 166(73.1) | REF | |
| Improved | 148(21.8) | 530(78.2) | 1.018(0.705–1.469) | 0.926 |
| Total | 209 | 696 | ||
N = 905 + Disposal of faeces for children < 3 years, n = 185
Discussion
The control intervention for most NTDs including Schistosomiasis is largely focused on preventive chemotherapy which is implemented through school based programs. However, there has been growing interest to include the adult communities and WASH interventions in the preventive chemotherapy programs. The current study has demonstrated that S. mansoni is a public health problem among the population living in Mwea, Kirinyaga County. The S. mansoni prevalence recorded in this study 23.1% (95% CI: 20.5–26.0%) places the study area under the WHO classification of moderate- risk communities [41]. Majority of the infections reported in this study were of light intensity which is consistent with findings from Western Kenya [42] and also supports previous findings that most individuals in endemic areas excrete low number of eggs [43].
In areas of moderate schistosomiasis risk, WHO recommends preventive chemotherapy as a strategy for morbidity control that will help lessen the occurrence and severity of consequences of infection for risk groups including irrigation workers, fishermen and women of child bearing age [44]. This result therefore adds to the growing concern that the adult community needs to be included in schistosomiasis control programmes since they may provide an avenue for continued re- infection of the school going children who are usually targeted for treatment in the school-based deworming programmes. Similar observations have been made in previous studies [6, 45, 46].
In this study, male participants were more at risk of infection with S. mansoni than female participants which is consistent with previous findings [47, 48]. This could be explained by the fact that, in most communities, male are more exposed to frequent water contact partly due to their economic activities like farming. This has been observed in other studies [49–52]. Participants who were advanced in age (> 55 years) were the most affected with S. mansoni at 26.1%. Schistosoma mansoni is a chronic infection which means that a person can live with the infections for a long time without seeking treatment. Similar findings have been reported elsewhere [9].
Majority of the households in the study had inadequate access to improved sources of water; however, there was no significant association between household water sources and S. mansoni infections. The national coverage for access to improved water in Kenyan rural areas stands at 59% [53], while the global coverage as at 1990 was 76% [38]. Previous studies have observed that, most countries where schistosomiasis is endemic have inadequate access to clean water sources [1, 24]. Surface water from open water bodies such as rivers, streams and irrigation channels, was the most common source of water for majority of the study participants. This has previously been observed elsewhere by Tchuem Tchuenté et al. who noted that natural water bodies many of which are infested with snails and infective schistosome cercariae are common sources for domestic water in most schistosomiasis endemic areas [54].
Usually people become infected with schistosomiasis when they come into contact with infested water and cercariae penetrate the skin. Schistosome eggs are then excreted in human faeces or urine and when they get into water bodies, miracidia which are released from the eggs in turn infects the snails, which release cercariae which penetrates human skin [1].
Households whose heads had access to piped water had the lowest infection with S. mansoni among those with improved water sources at 13%. Infection was highest among those who utilized improved water from protected dug well at 50%. The results further show that there was no significant association between household water sources and S. mansoni infection. Previous studies have reported strong association between access to improved water sources; with significantly less infection with S. mansoni [45, 55–57]. Another study linked lack of access to clean water sources to 47% population attribute ‘able fraction (PAF) of schistosomiasis [58]. Improved water sources may not contain cercariae, but even so, its provision may not prevent all water contact with infested water. Therefore, when looking at the relationship between water and S. mansoni infection, there is need to also consider the environmental set up since areas under flood irrigation e.g. rice irrigation areas may be different from other areas.
Mwea is a rice irrigation scheme where people live in clustered villages which are surrounded by vast rice fields, and water from the rice drainage flows freely in to canals and streams which cuts across the villages, and thus even when a household has access to improved water sources, there could be high chances of them coming into contact with water from unimproved sources. Previous studies have alluded to this. Groups like irrigation workers and canal cleaners have suffered high exposure to infested water in the People’s Republic of China [59, 60]. Another study in Brazil found out that people who crossed streams were at significantly higher risk of S. mansoni infection [61].
In regards to household access to sanitation facilities, the present study did not find an association between the type of sanitation facility (Improved or unimproved) with S. mansoni infections. This is in contrast to other findings which have reported significant lower odds of S. mansoni infection among people with access to adequate sanitation [57, 62]. In order to sustain transmission, a schistosome egg must enter freshwater to infect snail which will then release cercariae which infects people who come into contact with water [1]. For sanitation to be effective in controlling schistosomiasis, it should be able to contain fecal matter and urine hence preventing the schistosome eggs from hatching and thus ensuring that there are no miracidia to infect the snails. Studies have demonstrated that schistosome reproduces exponentially within the host snail, and therefore even small numbers of eggs entering freshwater may give rise to high risk of infection to people coming into contact with the water [63]. Studies have shown that even when high sanitation coverage levels are achieved, their use may still remain low [64, 65], due to various behavioral factors [66]. Open water bodies have been shown to be particularly attractive sites for open defecation [67]. The study area being a rice irrigation scheme, where sanitation facilities are not provided for in the farms, there are possibilities of open defecation when people are working in the farms. This has been demonstrated by Chimbari et al. [68].
We acknowledge that our sample selection process could be a potential source of bias since we used the households as unit of randomization and individuals as the units of study. We however used an effect of design of two for the sample size calculation which gave us a larger sample so as to minimize this bias. Secondly, we did not factor in data on possible behavioural factors such as open defecation which could also play a role in schistosomiasis transmission. Future studies may consider incorporating this aspect. Also, the study analysed single stool sample using the Kato Katz technique which might have missed light infections because of its poor sensitivity and day to day fluctuations in egg excretion [69]. Future population studies may enhance this by collecting stool samples for at least two consecutive days.
Conclusion
In conclusion, this study reaffirms that S. mansoni is still a public health problem among communities living in Kirinyaga County. The study also shows that individuals of all age groups are infected with S. mansoni and thus supports the call for inclusion of the adult community in targeted mass drug administration. There was inadequate access to improved water sources in the study area but adequate access to improved sanitation facilities. However, there was no significant association between S. mansoni infection with either access to improved water sources or sanitation facilities. The findings of this study add to the existing knowledge on the association between specific WASH components and Schistosoma mansoni infections in areas where rice irrigation farming is practiced. The study recommends further investigations on the association of schistosomiasis infections and hygiene in similar set ups.
Additional file
Household questionnaire. (DOCX 26 kb)
Acknowledgements
The authors are sincerely grateful to all the staff of Eastern and Southern Africa Center of International Parasite Control (ESACIPA), Kirinyaga County Government, staff working in Kimbimbi sub county hospital laboratories and the residents of Karima, Rurumi, Kasarani, Gakungu, Kirogo, Kamucege and Block villages. Without them, this work would not have been possible.
Protocol ethical approval
Kenya Medical Research Institute (KEMRI), Scientific and Ethic Research Unit (SERU) Protocol Number: KEMRI/SERU/ESACIPAC/007/3326. Approval date October 31, 2016.
Abbreviations
- CHWs
Community Health Workers
- CI
Confidence interval
- DALYs
Disability Adjusted Life Years
- Epg
Eggs per gram of faeces
- ESACIPAC
Eastern & Southern Africa Center of International Parasite Control
- IQR
Interquartile range
- JICA
Japan International Corporation Agency
- JMP
Joint Monitoring Programme
- KDHS
Kenya demographic and health surveys
- KEMRI
Kenya medical research institute
- MDA
Mass drug administration
- MDG
Millennium development goals
- MIRERC
Meru Univeristy of Science and Technology Institutional Research Ethics Research Committee
- NTDs
Neglected tropical diseases
- OR
Odds ratio
- SBDP
School based deworming program
- SERU
Scientific and ethical review unit
- UNICEF
United Nations children’s fund
- VIP latrines
Ventilated improved latrines
- WASH
Water sanitation and hygiene
- WHO
World health organization
Authors’ contributions
PMG, SK, and CM were involved in the study design, data collection and manuscript writing. CK, DM, JM carried out data collection, analysis and manuscript preparation. GM and HDM critically reviewed the manuscript and result interpretation. All authors read and approved the final manuscript.
Funding
The study was funded by Kenya Medical Research Institute (KEMRI) Internal Research Grants (IRG). The funder did not have any other role in the design of the study, collection, analysis, and interpretation of data and in writing of this manuscript.
Availability of data and materials
Please contact the corresponding author for data requests.
Ethics approval and consent to participate
Prior to the implementation of the study, ethical approvals were sought from the Scientific and Ethical Review Unit (SERU) of the Kenya Medical Research Institute (KEMRI), number (KEMRI/SERU/ESACIPAC/007/3326) and Meru University of Science and Technology Institutional Research Ethics Review Committee (MIRERC), number (MIRERC/001/2017), and the Health Management Team of Kirinyaga County. All the household heads participating in the study signed a written informed consent which had been translated into the local dialect. Those who could not read or write were asked to have an independent person as a witness who ensured that the study was clearly explained to them and guided them to give a thumb print on the consent form.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Paul M. Gichuki, Email: paulmgichuki@gmail.com, Email: pgichuki@kemri.org
Stella Kepha, Email: stella.kepha@lshtm.ac.uk.
Damaris Mulewa, Email: damarismulewa@gmail.com, Email: d.mulewa@hgsf-global.org.
Janet Masaku, Email: jmasaku@kemri.org.
Celestine Kwoba, Email: abowkelec@yahoo.com.
Gabriel Mbugua, Email: gmbugua@must.ac.ke, Email: gathinguprof@gmail.com.
Humphrey D. Mazigo, Email: humphreymazigo@gmail.com
Charles Mwandawiro, Email: cmwandawiro@kemri.org.
References
- 1.Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014;383:2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Study 2013 Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ezeamama AE, et al. Gaining and sustaining schistosomi-asis control: study protocol and baseline data prior to differenttreatment strategies infive African countries. BMC Infect Dis. 2016;16:229. [DOI] [PMC free article] [PubMed]
- 4.Huldah CS, Geoffrey M, Maurice O, Maurice RO, Pauline NM. Schistosoma haematobiumhotspots in South Nyanza, western Kenya: prevalence, distribution and co-endemicitywithSchistosoma mansoniand soil-transmitted helminths. Parasit Vectors. 2015;7:125. [DOI] [PMC free article] [PubMed]
- 5.GAHI . Global Atlas of Helminth Infections. 2010. [Google Scholar]
- 6.Mwandawiro CM, Nikolay B, Kihara JH, Ozier O, Mukoko DA, Mwanje MT, et al. Monitoring and evaluating the impact of national school-based deworming in Kenya: study design and baseline results. Parasit Vectors. 2013;6:198. doi: 10.1186/1756-3305-6-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hotez P, Fenwick A. Schistosomiasis in Africa: an emerging tragedy in our new global health decade. PLoS Negl Trop Dis. 2009;3(9):e485. doi: 10.1371/journal.pntd.0000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodhall DM, Wiegand RE, Wellman M, Matey E, Abudho B, Karanja DM, Mwinzi PM, Montgomery SP, Secor WE. Use of geospatial modeling to predict Schistosoma mansoni prevalence in Nyanza Province, Kenya. PLoS One. 2013;8:e71635. doi: 10.1371/journal.pone.0071635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masaku J, Mutungi F, Gichuki P, Okoyo C, Njomo D, Njenga S. High prevalence of helminths infection and associated risk factors among adults living in a rural setting, Central Kenya: a crosssectional study. Trop Med Health. 2017;45:15. doi: 10.1186/s41182-017-0055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kihara JH, Muhoho N, Njomo D, Mwobobia IK, Josyline K, et al. Drug efficacy of praziquantel and albendazole in school children in Mwea Division, Central Province, Kenya. Acta Trop. 2007;102:165–171. doi: 10.1016/j.actatropica.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 11.Montresor Antonio, Engels Dirk, Chitsulo Lester, Bundy Donald A.P., Brooker Simon, Savioli Lorenzo. Development and validation of a ‘tablet pole’ for the administration of praziquantel in sub-Saharan Africa. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2001;95(5):542–544. doi: 10.1016/S0035-9203(01)90034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–1569. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- 13.WHO . Accelerating work to overcome the global impact of neglected tropical diseases a roadmap for implementation. Geneva: World Health Organization; 2012. [Google Scholar]
- 14.Molyneux DH, Malecela MN. Neglected tropical diseases and the millennium development goals: why the “other diseases” matter: reality versus rhetoric. Parasit Vectors. 2011;4:234. doi: 10.1186/1756-3305-4-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doenhoff MJ, Hagan P, Cioli D, Southgate V, Pica-Mattoccia L. Praziquantel: its use in control of schistosomiasis in sub-Saharan Africa and current research needs. Parasitology. 2009;136:1825–1835. doi: 10.1017/S0031182009000493. [DOI] [PubMed] [Google Scholar]
- 16.Campbell SJ, Savage GB, Gray DJ, Atkinson J-AM, Soares Magalhães RJ, Nery SV, et al. Water, sanitation, and hygiene (WASH): a critical component for sustainable soil-transmitted helminth and schistosomiasis control. PLoS Negl Trop Dis. 2014;8:e2651. doi: 10.1371/journal.pntd.0002651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clements ACA, Bosqué-Oliva E, Sacko M, Landouré A, Dembélé R, Mamadou Traoré M, et al. A comparative study of the spatial distribution of schistosomiasis in Mali in 1984-1989 and 2004-2006. PLoS Negl Trop Dis. 2009;3:e431. doi: 10.1371/journal.pntd.0000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO . Helminth control in school-age children. Geneva: World Health Organization; 2011. [Google Scholar]
- 19.WHO. Accelerating work to overcome the global impact of neglected tropical diseases Da roadmap for implementation. Geneva: World Health Organization; 2012.
- 20.Grimes JE, Croll D, Harrison WE, Utzinger J, Freeman MC, Templeton MR. The roles of water, sanitation and hygiene in reducing schistosomiasis: a review. Parasit Vectors. 2015;8:156. doi: 10.1186/s13071-015-0766-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO . Helminth control in school age children: a guide for managers of control programmes. 2. Geneva: World Health Organization; 2011. [Google Scholar]
- 22.Bartram J, Cairncross S. Hygiene, sanitation, and water: forgotten foundations of health. PLoS Med. 2010;7:e1000367. doi: 10.1371/journal.pmed.1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferriman Annabel. BMJ readers choose the “sanitary revolution” as greatest medical advance since 1840. BMJ. 2007;334(7585):111.2-111. doi: 10.1136/bmj.39097.611806.DB. [DOI] [Google Scholar]
- 24.WHO, UNICEF . Progress on drinking water and sanitation - 2014 update. Geneva: World Health Organization; 2014. [Google Scholar]
- 25.Secor WE. Water-based interventions for schistosomiasis control. Pathog Glob Health. 2014;108:246–254. doi: 10.1179/2047773214Y.0000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kosinski KC, Adjei MN, Bosompem KM, Crocker JJ, Durant JL, et al. Effective control of Schistosoma haematobium infection in a Ghanaian community following installation of a water recreation area. PLoS Negl Trop Dis. 2012;6:e1709. doi: 10.1371/journal.pntd.0001709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Water sanitation and hygiene for accelerating and sustaining progress on neglected tropical diseases A global strategy 2015-2020. Geneva: World Health Organization; 2015. [DOI] [PMC free article] [PubMed]
- 28.Freeman MC, Clasen T, Brooker SJ, Akoko DO, Rheingans R. The impact of a school-based hygiene, water quality and sanitation intervention on soil-transmitted helminth reinfection: a cluster-randomized trial. Am J TropMed Hyg. 2013;89:875–883. doi: 10.4269/ajtmh.13-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freemanm MC, Chard AN, Nikolay B, Garn V, Okoyo C, Kihara J, Njenga S, Pullan R, Brooker SJ, Mwandawiro CS. Associations between school- and household-level water, sanitation and hygiene conditions and soil-transmitted helminth infection among Kenyan school children. Parasit Vectors. 2015;8:412. doi: 10.1186/s13071-015-1024-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Echazú A, Bonanno D, Juarez M, Cajal SP, Heredia V, Caropresi S, et al. Effect of poor access to water and sanitation as risk factors for soil-transmitted helminth infection: selectiveness by the infective route. PLoS Negl Trop Dis. 2015;9(9):e0004111. doi: 10.1371/journal.pntd.0004111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strunz EC, Addiss DG, Stocks ME, Ogden S, Freeman MC. Water, sanitation, hygiene, and soil-transmitted helminth infection: a systematic review and meta-analysis. PLoS Med. 2014;11(3):e1001620. doi: 10.1371/journal.pmed.1001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ziegelbauer K, Speich B, Mäusezahl D, Bos R, Keiser J, Utzinger J. Effect of sanitation on soil-transmitted helminth infection: systematic review and meta-analysis. PLoS Med. 2012;9(1):e1001162. doi: 10.1371/journal.pmed.1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell SJ, Savage GB, Gray DJ, Atkinson J-AM, Soares Magalhães RJ, et al. Water, Sanitation, and hygiene (WASH): a critical component for sustainable soil-transmitted helminth and SchistosomiasisControl. PLoS Negl Trop Dis. 2014;8(4):e2651. doi: 10.1371/journal.pntd.000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kenya National Bureau of Statistics (KNBS) 2009 Population and Housing Census. Nairobi: KNBS; 2012. [Google Scholar]
- 35.Njiru JM, Muhoho N, Simbauni JA, Kabiru E. Effects of Soil- Transmitted Helminths and Schistosoma Species on Nutritional Status of Children in Mwea Irrigation Scheme, Kenya. JALSI. 2016;5(1):1–8. doi: 10.9734/JALSI/2016/25053. [DOI] [Google Scholar]
- 36.Masaku J, Madigu N, Okoyo C, Njenga SM. Current status of Schistosoma mansoni and the factors associated with infection two years following mass drug administration programme among primary school children in Mwea irrigation scheme: a crosssectional study. BMC Public Health. 2015;15:739. doi: 10.1186/s12889-015-1991-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fisher C. A study of schistosomiassis of Stanleyville district of the Belgian Congo. Trans R Soc Trop Med Hyg. 1999;1934(28):227–306. [Google Scholar]
- 38.JMP . Progress on sanitation and drinking water- 2015 update and MDG assessment. 2015. [Google Scholar]
- 39.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 40.Montresor A. Helminth control in school-age children: a guide for managers of control programmes: World Health Organization; 2002. http://www.who.int/iris/handle/10665/42473.
- 41.World Health Organization . Preventive chemotherapy in human helminthiasis coordinated use of antihelmintic drugs in control interventions: a manual for health professionals and programme managers. Geneva: World Health Organization; 2006. [Google Scholar]
- 42.Odiere M, Rawago F, Ombok M, Secor W, Karanja D, Mwinzi P, et al. High prevalence of schistosomiasis in Mbita and its adjacent islands of Lake Victoria, western Kenya. Parasit Vectors. 2012;5:278. doi: 10.1186/1756-3305-5-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butterworth AE, Sturrock RF, Ouma JH, Mbugua GG, Fulford AJC, Kariuki HC, et al. Comparison of different chemotherapy strategies against Schistosoma mansoni in Machako's district, Kenya. Effects on human infections and morbidity. Parasitology. 1991;103:339–355. doi: 10.1017/S0031182000059850. [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization . Schistosomiasis Progress report (2001–2011) and strategic plan (2012–2020) Geneva: World Health Organization Press; 2013. [Google Scholar]
- 45.Lelo AE, Mburu DN, Magoma GN, Mungai BN, Kihara JH, Mwangi IN, et al. No apparent reduction in schistosome burden or genetic diversity following four years of school-based mass drug administration in mwea, Central Kenya, a heavy transmission area. PLoS Negl Trop Dis. 2014;8(10):e3221. doi: 10.1371/journal.pntd.0003221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson RM, Truscott JE, Pullan RL, Brooker SJ, Hollingsworth TD. How effective is school-based deworming for the community-wide control of soil-transmitted helminths? PLoS Negl Trop Dis. 2013;7:e2027. doi: 10.1371/journal.pntd.0002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Donohue RE, Mashoto KO, Mubyazi GM, Madon S, Malecela MN, Michael E. Biosocial determinants of persistent schistosomiasis among schoolchildren in Tanzania despite repeated treatment. Trop Med Infect Dis. 2017;2:61. doi: 10.3390/tropicalmed2040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Enk MJ, Lima ACL, Barros HDS, Massara CL, Coelho PMZ, Schall VT. Factors related to transmission of and infection with Schistosoma mansoni in a village in the south-eastern region of Brazil. Mem Inst Oswaldo Cruz. 2010;105:570–577. doi: 10.1590/S0074-02762010000400037. [DOI] [PubMed] [Google Scholar]
- 49.Houmsou RS, Panda SM, Elkanah SO, Garba CL, Wama BE, Amuta EU, et al. Cross-sectional study and spatial distribution of schistosomiasis among children in northeastern Nigeria. Asian Pac J Trop Biomed. 2016;6(6):477–484. doi: 10.1016/j.apjtb.2016.04.003. [DOI] [Google Scholar]
- 50.Calasans TAS, Souza GTR, Melo CM, Madi RR, Jeraldo VLS. Socioenvironmental factors associated with Schistosoma mansoni infection and intermediate hosts in an urban area of northeastern Brazil. PLoS One. 2018;13(5):e0195519. doi: 10.1371/journal.pone.0195519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dawaki S, Al-Mekhlafi HM, Ithol I, Ibrahim J, Abdulsalam AM, Ahmed A, et al. Prevalence and riskfactors of schistosomiasis among hausa communities in Kanostate, Nigeria. Rev.Inst.Med.Trop. 2016;58:11.36. doi: 10.1590/S1678-9946201658054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanya RE, Muhangi L, Nampijja M, Nannozi V, Nakawungu PK, Abayo E. Schistosoma mansoni and HIV infection in a Ugandan population with high HIV and helminth prevalence. Tropical Med Int Health. 2015;20(9):1201–1208. doi: 10.1111/tmi.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kenya National Bureau of Statistics (KNBS) and ICF Macro . Kenya Demographic and Health Survey 2014. Calverton: KNBS and ICF Macro; 2014. [Google Scholar]
- 54.Tchuenté TLA, Rollinson D, Stothard RJ, Molyneux D, et al. Moving from control to elimination of schistosomiasis in sub-Saharan Africa: time to change and adapt strategies. Infectious Diseases of Poverty. 2017;6:42. doi: 10.1186/s40249-017-0256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagi S, Chadeka EA, Sunahara T, Mutungi F, Dan Justin YK, et al. Risk factors and spatial distribution of Schistosoma mansoni infection among primary school children in Mbita District, Western Kenya. PLoS Negl Trop Dis. 2014;8(7):e2991. doi: 10.1371/journal.pntd.0002991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meurs L, Mbow M, Boon N, Van den Broeck F, Vereecken K, Dieye TN, Abatih E, Huyse T, Mboup S, Polman K. Micro-geographical heterogeneity in Schistosoma mansoni and S. haematobium infection and morbidity in a co-endemic Community in Northern Senegal. PLoS Negl Trop Dis. 2013;7:e2608. doi: 10.1371/journal.pntd.0002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grimes JET, Croll D, Harrison WE, Utzinger J, Freeman MC, Templeton MR. The relationship between water, sanitation and schistosomiasis: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2014;8:e3296. doi: 10.1371/journal.pntd.0003296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soares Magalhaes RJ, Barnett AG, Clements AC. Geographical analysis of the role of water supply and sanitation in the risk of helminth infections of children in West Africa. Proc Natl Acad Sci U S A. 2011;108:20084–20089. doi: 10.1073/pnas.1106784108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song Y, Xiao S, Wu W, Zhang S, Xie H, Xu X, et al. Preventive effect of artemether on schistosome infection. Chin Med J. 1998;111:123–127. [PubMed] [Google Scholar]
- 60.Utzinger J, Xiao SH, N’Goran EK, Bergquist R, Tanner M. The potential of artemether for the control of schistosomiasis. Int J Parasitol. 2001;31:1549–1562. doi: 10.1016/S0020-7519(01)00297-1. [DOI] [PubMed] [Google Scholar]
- 61.Massara CL, Peixoto SV, Da Silva BH, Enk MJ, Dos Santos CO, Schall V. Factors associated with schistosomiasis mansoni in a population from the municipality of Jaboticatubas, state of Minas Gerais, Brazil. Mem Inst Oswaldo Cruz. 2004;99:127–134. doi: 10.1590/S0074-02762004000900023. [DOI] [PubMed] [Google Scholar]
- 62.Freeman MC, Garn JV, Sclar GD, Boisson S, Medlicott K, Alexander KT, et al. Thomas F. Clasen: the impact of sanitation on infectious disease and nutritional status: a systematic review and meta-analysis. Int J Hyg Environ Health. 2017;220:928–949. doi: 10.1016/j.ijheh.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 63.Ayad N. A short review of the epidemiology of schistosomiasis in Africa. Egypt J Bilharz. 1974;1:9–27. [PubMed] [Google Scholar]
- 64.Barnard S, Routray P, Majorin F, Peletz R, Boisson S, Sinha A, Clasen T. Impact of Indian Total sanitation campaign on latrine coverage and use: across-sectional study in Orissa three years following programmeimplementation. PLoS One. 2013;8:e71438. doi: 10.1371/journal.pone.0071438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Montgomery MA, Desai MM, Elimelech M. Assessment of latrine use and quality and association with risk of trachoma in rural Tanzania. Trans R Soc Trop Med Hyg. 2010;104(4):283–289. doi: 10.1016/j.trstmh.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 66.Routray P, Schmidt WP, Boisson S, Clasen T, Jenkins MW. Socio-cultural and behavioural factors constraining latrine adoption in ruralcoastal Odisha: an exploratory qualitative study. BMC Public Health. 2015;15:880. doi: 10.1186/s12889-015-2206-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tayo MA, Pugh RN, Malumfashi BAK. Endemic diseases research project, XI. Water-contact activities in the schistosomiasis study area. Ann Trop Med Parasitol. 1980;74:347–354. doi: 10.1080/00034983.1980.11687351. [DOI] [PubMed] [Google Scholar]
- 68.Chimbari M, Ndlela B, Nyati Z, Thomson A, Chandiwana SK, Bolton P. Bilharzia in a small irrigation community: an assessment of water and toilet usage. Cent Afr J Med. 1992;38:451–458. [PubMed] [Google Scholar]
- 69.Booth M, Vounatsou P, N'Goran EK, Tanner M, Utzinger J. The influence of sampling effort and the performance of the Kato-Katz technique in diagnosing Schistosoma mansoni and hookworm co-infections in rural cote d'Ivoire. Parasitology. 2003;127:525–531. doi: 10.1017/S0031182003004128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Household questionnaire. (DOCX 26 kb)
Data Availability Statement
Please contact the corresponding author for data requests.