Abstract
Diabetic foot ulcer infections are frequently polymicrobial in nature and exhibit increased morbidity and mortality, as well as, treatment failures. Interactions between Acinetobacter baumannii and Staphylococcus aureus were studied, which showed strain-dependent changes in growth and antibiotic susceptibility. This study examined the interactions between two clinical strains of A. baumannii (1929) and S. aureus (1928) that were recovered from skin and soft tissues of a diabetic patient. When S. aureus 1928 and A. baumannii 1929 were co-cultured together, there was no significant decrease in growth in either clinical strains, indicating that both strains can co-exist in the same site of infection. Additionally, neither strains experienced statistically significant changes in susceptibility. These findings highlight that these two pathogens can be found in the same niche of infection, which may lead to more aggressive outcome of the infection.
Keywords: Acinetobacter baumannii, Staphylococcus aureus, Diabetic foot ulcer, polymicrobial infections, commensals
1. Introduction
In recent decades, diabetes has grown to become a very serious chronic disease with an increasing global prevalence (http://www.who.int/diabetes/global-report/es/). Current data show that 25% of individuals with diabetes will develop a foot ulcer in their lifetime, and that the cost of care for a diabetic foot ulcer (DFU) is over twice that of any other chronic ulcer etiology [1, 2]. Diabetic individuals are at a higher risk of developing infections due to the effects of hyperglycemia on leukocyte function [3], although there is not enough evidence to identify the optimal method to diagnose DFU infections [4]. Relationships between clinical signs of infection and microbial load may have a role in the development of infection-related complications [5].
DFU ranging from soft tissue to bone infections- are often polymicrobial in nature, and Staphylococcus aureus is the most frequently isolated pathogen in these infections [6–9]. S. aureus belongs to the “ESKAPE” group of microorganisms and has been seen to coexist in the same habitat as Acinetobacter baumannii [10]. A. baumannii has been identified as one of the microorganisms associated with a higher incidence of major amputation [11]. While it is well-known that both pathogens can cause a variety of diseases such as pneumonia or serious blood and wound infections, there are not as many reports of S. aureus and A. baumannii together in DFU samples [8, 12]. In this study, we focus on the interaction between two clinical strains, S. aureus (1928) and A. baumannii (1929), recovered from the same site of infection from a diabetic patient, to identify changes in bacterial growth, antibiotic resistance, and morphological changes. The samples were recovered from an 80-year-old patient with a history of diabetes and hypertension presented to the Hospital de Clinicas Jose de San Martin with clinical signs of soft tissue infection associated with a chronic ulcer in her lower limb. The patient was hospitalized and taken to surgery where samples were obtained and sent for culture. In all the samples, S. aureus and A. baumannii were isolated in a polymicrobial culture.
The lack of studies on this topic leads us to explore the interactions between these two pathogens, to gain clearer insight on how one strain is affecting the other. In the present study, we address the interactions of the two strains (S. aureus 1928 and A. baumannii 1929) recovered together in the samples of the same diabetic patient.
2. Materials and methods
2.1. Bacterial strains and growth conditions
S. aureus 1928 and A. baumannii 1929 were recovered from samples of skin and soft tissue from a diabetic patient. Samples from infected soft tissue were collected in the operating room and sent immediately for processing to the microbiology laboratory. The samples were cultured on blood agar, EMB Levine agar (bioMérieux, Marcy l’Etoile, France) and thioglycolate broth and incubated at 37 °C for 48–72 hours in aerobic atmosphere. The samples were also inoculated on Brucella agar and incubated at 37 °C for 7days in anaerobic atmosphere. The isolates were identified using standard biochemical tests and by matrix assisted desorption ionization time of flight mass spectrometry (MALDI-TOF MS; Bruker Daltonics, Bremen, Germany).
Antimicrobial susceptibility of both strains was determined using an automated Phoenix system (Becton Dickinson, Argentina). The S. aureus 1928 strain was shown to be susceptible to cefoxitin (FOX), vancomycin (VAN), teicoplanin (TEC), ciprofloxacin (CIP), linezolid (LZD), clindamycin (CLI), erythromycin (ERY), rifampicin (RIF) and trimethoprim-sulfamethoxazole (SXT). The A. baumannii 1929 strain was shown to be susceptible to colistin (COL) and resistant to ampicillin/sulbactam (AMS), piperacillin/tazobactam (PTZ), 3rd and 4th generation cephalosporins, imipenem (IPM), meropenem (MEM), amikacin (AMK), gentamicin (GEN), and trimethoprimsulfamethoxazole (SXT). S. aureus LS1, strain that has been widely used in different genotypic and phenotypic studies and is methicillin-susceptible was only included for proximity assays in order to compare 1928 effect. Strains were grown in Luria-Bertani (LB) broth for 18 hours at 37 °C under shaking conditions (200 rpm).
2.2. Cell-free conditioned media (CFCM)
Cell-free conditioned media (CFCM) was obtained from spent media in which the bacteria had grown for 48 hours at 37 °C under shaking conditions (200 rpm). Cultures were centrifuged and the supernatant was filtered with a 0.22 μm size filter. The resulting CFCM was stored at −80°C.
2.3. Susceptibility assays and growth curve
Late stationary cultures of A. baumannii 1929 or S. aureus 1928 were grown in the presence or absence of each other and shaken at 37°C for 4 hours at 200 RPM. Following a 4-hour incubation, antibiotic susceptibility assays were performed by inoculating Mueller-Hinton (MH) agar plates. Commercial antibiotic disks were used to test the following antibiotics: AMK, ceftazidime (CAZ), GEN, IMP, MEM, CIP, ofloxacin (OFX), FOX, and VAN. Plates were incubated for 18 hours at 37°C, and zones of inhibition were measured in mm.
To test the effect of CFCM on antibiotic susceptibility, both 1929 and 1928 were grown for 18 hours, exposed to another’s CFCM, and grown for 4 hours at 37°C and 200 rpm. Disk susceptibility to AMK, CAZ, GEN, IMP, MEM, trimethoprim (TMP), and OFX were performed for A. baumannii strains; AMK, CAZ, FOX, GEN, IMP, OFX, MEM, and VAN were tested with S. aureus.
After a 4-hour incubation under the two conditions (co-culture or CFCM exposure), serial dilutions were plated and colony forming units (CFU/ml) were recorded to assess growth impairment.
2.3. Proximity assays
Following an 18-hour incubation, A. baumannii 1929, S. aureus 1928 or LS1 strains were plated onto MH, LB, CLED, and sheep blood agar in increments of 0.5 cm. Starting distance was 1 cm and final distance was 5 cm. Blood agar plates were incubated at 37 °C for 24 and 48 hours (bioMerieux, Marcy l’Etoile, France, Hardy Diagnostic). In addition, considering the effect seen with S. aureus LS1, LS1 CFCM was plated onto the top, left and bottom portion of a sheep blood agar plate. Plates were incubated for 30 minutes at 37 °C. A. baumannii 1929 was then plated on the top, bottom, and right portion of the sheep blood agar plate containing LS1 CFCM. Plates were incubated for 24 and 48 hours.
2.6. Statistical Analysis
Experiments were performed in triplicates and level of significance was set at P < 0.05 (unpaired t test or two-way ANOVA by Sidak’s comparison, accordingly). Statistical analyses were performed with the use of GraphPad Prism 7.0c software.
3. Results and Discussion
3.1. S. aureus 1928 and A. baumannii 1929 did not impair their growth
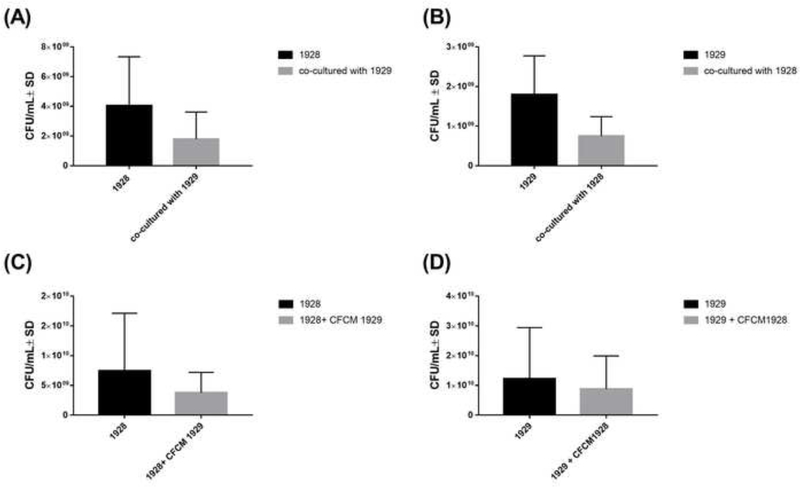
To determine the effects of the interspecies interaction on growth patterns, CFU were measured after co-culture. There was no significant decrease in growth in both S. aureus 1928 and A. baumannii 1929 when co-cultured with one another (Figure 1A and B), indicating that both strains co-exist in the same site of infection without impairing the other’s growth.
Figure 1. S. aureus 1928 and A. baumannii 1929 growth patterns.
(A) CFU/ml of S. aureus 1928 mono and co-culture with A. baumannii 1929. (B) CFU/ml of A. baumannii 1929 mono and co-culture with S. aureus 1928. (C) CFU/ml of S. aureus 1928 with or without A. baumannii 1929 CFCM. (D) CFU/ml of A. baumannii 1929 with or without S. aureus 1928 CFCM. Bars and whiskers represent means ± SD of three independent experiments in duplicate. The differences between all groups of isolates were analyzed by unpaired t test.
In addition, CFCM of 1929 and 1928 were used to test potential effects of soluble molecules released during growth. In agreement with results observed in the co-culture conditions, presence of CFCM of one strain did not significantly affect the growth of another strain (Figure 1C and 1D). However, we did note a slight downward tendency in A. baumannii 1929 growth when it was exposed to S. aureus 1928 (Figure 1B and D), suggesting a dampening effect by S. aureus on A. baumannii. However, as mentioned above, these effects could not be determined with statistical strength.
3.2. Antimicrobial susceptibility of A. baumannii and S. aureus
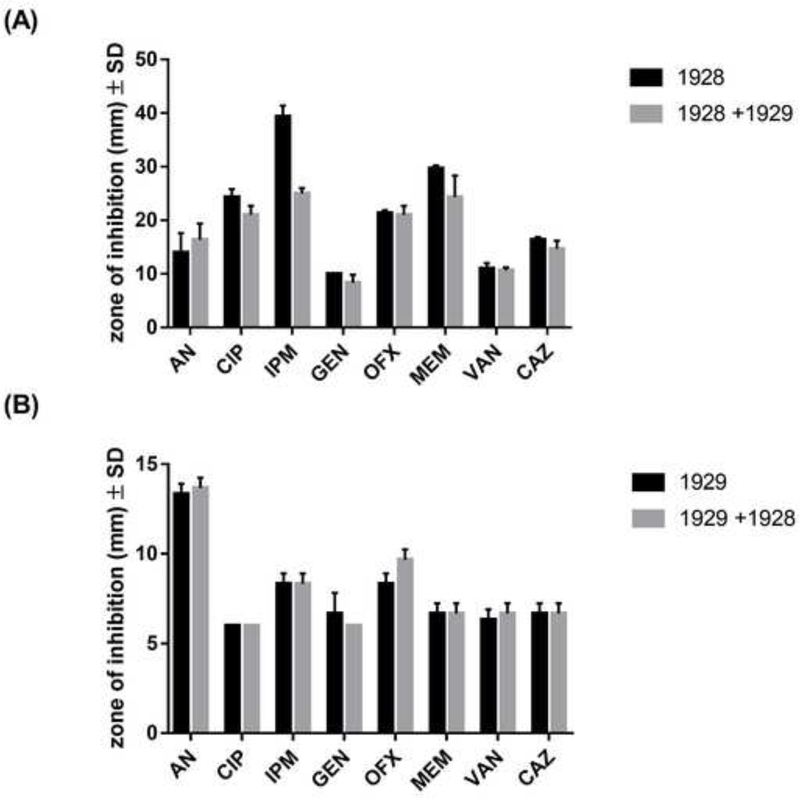
To assess the interspecies’ effects in antimicrobial susceptibility, disk diffusion tests were performed, and halos of inhibition were measured in mm (Figure 1A and 1B). When S. aureus 1928 was co-cultured with A. baumannii 1929, 1928 experienced a slight decrease in halo size (14 mm) for IMP (P > 0.05), which may have biological relevance. A 14 mm change of halo size suggests that resistance to IMP may have increased in 1928 when grown with 1929. Additionally, when 1929 and 1928 were exposed to corresponding foreign CFCM, A. baumannii 1929’s experienced a slight increase in the halo size for AMK (+1 mm). No changes in susceptibility profiles were observed in S. aureus 1928 when exposed to the CFCM of A. baumannii 1929 (Table 1).
Table 1.
Average ± Standard deviation in halo size of 1928, and 1929 when cultivated in LB and when exposed to the CFCM of one another. The experiment were performed in triplicates.
| Antibiotics | 1928 | 1929 | 1928+ 1929 CFCM | 1929+ 1928 CFCM |
|---|---|---|---|---|
| AMK | 15 ± 1.15 | 12 ± 1.15 | 13 ± 3.61 | 14 ± 1.00 |
| CAZ | 19 ± 1.00 | 7 ± 0.00 | 16 ± 0.58 | 6 ± 0.58 |
| FOX | 26 ± 0.58 | - | 22 ± 1.15 | |
| GEN | 8 ± 1.15 | 7 ± 0.58 | 8 ± 1.15 | 7 ± 0.58 |
| IMP | - | 8 ± 1.00 | - | 10 ± 0.58 |
| OFX | 20 ± 1.15 | 8 ± 0.58 | 20 ± 0.58 | 9 ± 0.58 |
| MEM | - | 7 ± 0.58 | - | 7 ± 0.58 |
| VAN | 10 ± 2.89 | - | 11 ± 1.00 | - |
Collectively, these results are in agreement with the growth results, where we observed that both strains can co-exist without imposing significant competitive or collaborative effects. Previous studies have shown that species-specific interactions can yield different outcomes depending the species involved [13–15]. For example, a previous study with two pathogens commonly found in the same site of infection, showed that released products from Pseudomonas aeruginosa could, in a strain dependent manner, potentiate antibiotic effects on S. aureus or offer protection from antibiotic killing. Another study showed that co-colonization of A. baumannii with carbapenem-resistant Enterobacteriaceae increased mortality rates, suggesting a cooperative effect [16]. Cohen et al. 2016, found that S. aureus alfa-toxin potentiates gram-negative pathogen’s proliferation and spread [17]. These observations demonstrate that exoproducts can impose effects on bacterial pathogens in a variable and opposing manners depending the species and strains [14]. In our study, we observed that both 1929 and 1928 strains can behave as commensals, without imposing a detrimental effect on the other.
3.3. Effect of soluble diffusible molecules on polymicrobial behavior
To determine if either A. baumannii or S. aureus secrete soluble diffusible proteins or molecules, both A. baumannii and S. aureus were plated at increasing distances and morphological changes were observed. The following strain-specific interactions were studied with 1) S. aureus 1928 and A. baumannii 1929, and 2) A. baumannii 1929 and S. aureus LS1.
The strains S. aureus LS1 was included in the present study, since it is methicillin-susceptible, like S. aureus 1928, and previous results from our lab (data not shown) demonstrate that S. aureus LS1 imposes an effect in A. baumannii strains [18]. For these reasons, we decided to further explore any potential effects that LS1 could cause in A. baumannii 1929.
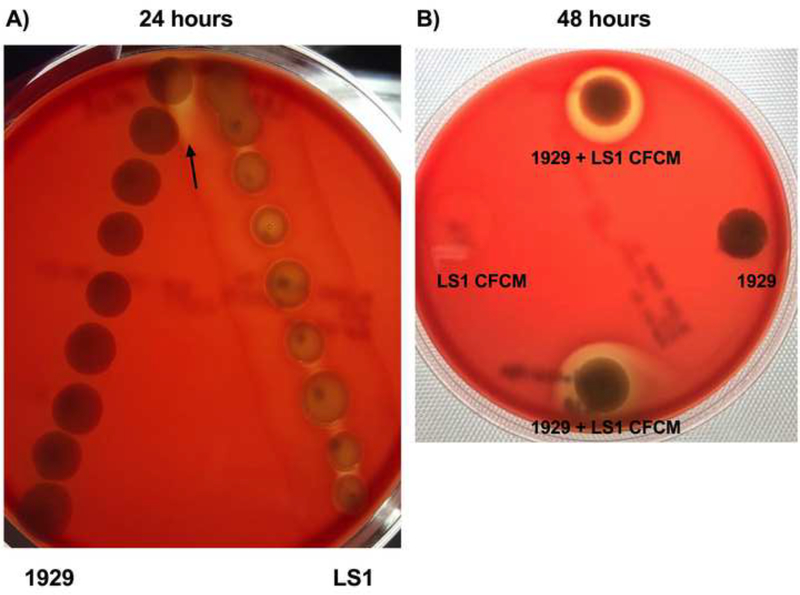
When S. aureus 1928 and A. baumannii 1929 were used, no significant morphological changes or effects were observed when plated onto LB, MH, blood agar and CLED plates. However, notable changes in hemolysis occurred when A. baumannii 1929 were plated within proximity to S. aureus LS1 strain. At decreasing distances, 1929 demonstrated an increase in hemolytic activity (Fig. 3A). We hypothesized that the change in hemolysis exhibited by A. baumannii 1929 may have been caused by soluble protein(s) secreted by the strain LS1. To test this, the LS1 CFCM was plated onto sheep blood agar and A. baumannii 1929 was plated directly on top and on separate plates. When 1929 was exposed to LS1 CFCM, hemolytic activity was observed; this change in hemolysis was increased when incubated for a prolonged period. Hemolytic activity was not observed when 1929 was grown in the absence of LS1 CFCM (Fig. 3B). To verify that the change in hemolysis was a direct result of the interaction between 1929 with the CFCM of LS1, LS1 CFCM was plated without the presence of 1929. In the absence of 1929, no hemolysis was observed, indicating that the change in hemolysis was triggered by the interaction between the A. baumannii strains and the exoproducts secreted by LS1.
Figure 3. Hemolytic activity of A. baumannii 1929 trigger by S. aureus LS1.
(A)Proximity assay of A. baumannii 1929 and S. aureus LS1 at 24 hr. A. baumannii 1929 plated on the left and S. aureus LS1 on the right at 24hrs. Starting distance is 1cm and increases distance in increments of 0.5cm. (B) Modified proximity assay of A. baumannii 1929 and S. aureus LS1 CFCM at 48 hr. A. baumannii 1929 (top, right, bottom) and LS1 CFCM (top, left, bottom). These photos are representative of three independent experiments.
There are some evidences in the literature that showed that gene expression, affecting virulence traits and metabolic responses, can occur with other species present in the same environment [15, 19]. Changes in gene expression can in part explain the hemolytic activity observed in 1929 strain. Further analysis, to discern the molecular basis of this phenomenon is needed.
4. Conclusions
S. aureus and A. baumannii are both pathogens that comprise a portion of the “ESPAKE” group and are responsible for various bacterial infections that are especially difficult to treat. DFU have a high prevalence among diabetic patients. These infections are often polymicrobial in nature and have an increased level of morbidity that can lead to amputations if not treated properly [20]. Typically, infections are caused by P. aeruginosa, S. aureus, Escherichia coli, among others [21]. However, diabetic foot infections can be caused by Acinetobacter [22, 23].
Interestingly in our case, A. baumannii and S. aureus were recovered from soft tissues samples from diabetic patient, showing that these two pathogens can be causative agents of DFU and can coexist in the site. This was the reason that drove us to study the interaction between S. aureus 1928 and A. baumannii 1929. We found that both strains do not affect each other, either beneficially or detrimentally, showing a state of commensalism between them.
When 1929 was exposed with the well-study LS1 strain, hemolysis was observed. This suggested that A. baumannii 1929 can respond to soluble proteins secreted S. aureus strain LS1 and agreed with other studies showing strain specific effects and responses. While the presence of a single isolate in an infected sample might not contribute to a worse outcome, a polymicrobial infected sample might significantly contribute to the spread, synergistic interaction, antibiotic resistance and delayed in the outcome of a wound, soft tissue or bone infection in a diabetic patient [8]. More studies understanding the interactions, as well as co-existence, of bacterial pathogens involved in DFU infections are necessary to discern cooperative or commensal behavior of not common species. The aforementioned interaction needs to be taken into account before the establish the treatment, since an increase in infection severity may occur when more than one species is present.
Figure 2. Antibiotic susceptibility profiles of S. aureus 1928 and A. baumannii 1929 in mono and co-culture condition.
Zone of inhibition represents the average ± Standard deviation in the halo sizes. (A) S. aureus 1928 mono and co-culture with A. baumannii 1929. (B) A. baumannii 1929 mono and co-culture with S. aureus 1928. Bars and whiskers represent means ± SD of three independent experiments in duplicate. The differences between mono and co-culture were analyzed by two-way ANOVA by Sidak’s comparison test.
Acknowledgments
The authors’ work was supported by NIH 1SC3GM125556-01 to MSR. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. JF has a SOAR-ELEVAR Scholar Fellowship from Latina/o Graduate Students from the U.S. Department of Education. This study was in part supported by DAAD PPP-USA; 2016 (Proj. 57208042) from LT and MSR.
References
- 1.Hurlow JJ, et al. , Diabetic foot infection: A critical complication. Int Wound J, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reiber GE, et al. , A comparison of diabetic foot ulcer patients managed in VHA and non-VHA settings. J Rehabil Res Dev, 200138(3): p. 309–17. [PubMed] [Google Scholar]
- 3.Gardner SE, Hillis SL, and Frantz RA, Clinical signs of infection in diabetic foot ulcers with high microbial load. Biol Res Nurs, 2009. 11(2): p. 119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Meara S, et al. , Systematic review of methods to diagnose infection in foot ulcers in diabetes. Diabet Med, 2006. 23(4): p. 341–7. [DOI] [PubMed] [Google Scholar]
- 5.Steed DL, et al. , Guidelines for the treatment of diabetic ulcers. Wound Repair Regen, 2006. 14(6): p. 680–92. [DOI] [PubMed] [Google Scholar]
- 6.Dunyach-Remy C, et al. , Staphylococcus aureus Toxins and Diabetic Foot Ulcers: Role in Pathogenesis and Interest in Diagnosis. Toxins (Basel), 2016. 8(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molina V, et al. , S. aureus pneumonia and sternoclavicular septic arthritis: An unusual complication. Arch Bronconeumol, 2018. 54(3): p. 168–169. [DOI] [PubMed] [Google Scholar]
- 8.Shettigar K, et al. , Virulence determinants in clinical Staphylococcus aureus from monomicrobial and polymicrobial infections of diabetic foot ulcers. J Med Microbiol, 2016. 65(12): p. 1392–1404. [DOI] [PubMed] [Google Scholar]
- 9.Rahim F, et al. , Frequency Of Common Bacteria And Their Antibiotic Sensitivity Pattern In Diabetics Presenting With Foot Ulcer. J Ayub Med Coll Abbottabad, 2016. 28(3): p. 528–533. [PubMed] [Google Scholar]
- 10.Ryu SY, Baek WK, and Kim HA, Association of biofilm production with colonization among clinical isolates of Acinetobacter baumannii. Korean J Intern Med, 2017. 32(2): p. 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardoso NA, et al. , Bacterial genus is a risk factor for major amputation in patients with diabetic foot. Rev Col Bras Cir, 2017. 44(2): p. 147–153. [DOI] [PubMed] [Google Scholar]
- 12.Neto RM, et al. , A case report of a multi-drug resistant bacterial infection in a diabetic patient treated in northeast Brazil. Diabet Foot Ankle, 2012. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardy BL, et al. , Corynebacterium pseudodiphtheriticum Exploits Staphylococcus aureus Virulence Components in a Novel Polymicrobial Defense Strategy. MBio, 2019. 10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radlinski L, et al. , Pseudomonas aeruginosa exoproducts determine antibiotic efficacy against Staphylococcus aureus. PLoS Biol, 2017. 15(11): p. e2003981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan AP, et al. , Multidrug resistant pathogens respond differently to the presence of co-pathogen, commensal, probiotic and host cells. Sci Rep, 2018. 8(1): p. 8656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchaim D, et al. , “Swimming in resistance”: Co-colonization with carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii or Pseudomonas aeruginosa. Am J Infect Control, 2012. 40(9): p. 830–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen TS, et al. , Staphylococcus aureus alpha toxin potentiates opportunistic bacterial lung infections. Sci Transl Med, 2016. 8(329): p. 329ra31. [DOI] [PubMed] [Google Scholar]
- 18.Bremell T, et al. , Outbreak of spontaneous staphylococcal arthritis and osteitis in mice. Arthritis Rheum, 1990. 33(11): p. 1739–44. [DOI] [PubMed] [Google Scholar]
- 19.Filkins LM, et al. , Coculture of Staphylococcus aureus with Pseudomonas aeruginosa Drives S. aureus towards Fermentative Metabolism and Reduced Viability in a Cystic Fibrosis Model. J Bacteriol, 2015. 197(14): p. 2252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gadepalli R, et al. , A clinico-microbiological study of diabetic foot ulcers in an Indian tertiary care hospital. Diabetes Care, 2006. 29(8): p. 1727–32. [DOI] [PubMed] [Google Scholar]
- 21.Bansal E, et al. , Spectrum of microbial flora in diabetic foot ulcers. Indian J Pathol Microbiol, 2008. 51(2): p. 204–8. [DOI] [PubMed] [Google Scholar]
- 22.Saseedharan S, et al. , Epidemiology of diabetic foot infections in a reference tertiary hospital in India. Braz J Microbiol, 2018. 49(2): p. 401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sekhar MS, et al. , Antimicrobial susceptibility pattern of aerobes in diabetic foot ulcers in a South-Indian tertiary care hospital. Foot (Edinb), 2018. 37: p. 95–100. [DOI] [PubMed] [Google Scholar]