Abstract
Purpose
Obstructive sleep apnea is common in patients with end-stage renal disease, and there is increasing evidence that clinical factors specific to end-stage renal disease contribute pathophysiologically to obstructive sleep apnea. It is not known whether circumstances specific to dialysis modality, in this case peritoneal dialysis, affect obstructive sleep apnea. Our study aimed to investigate the prevalence of obstructive sleep apnea in the peritoneal dialysis population and the relevance of dialysis-specific measures and kidney function in assessing this bidirectional relationship.
Methods
Participants with end-stage renal disease who were treated with nocturnal automated peritoneal dialysis for at least 3 months were recruited from a hospital-based dialysis center. Laboratory measures of dialysis adequacy, peritoneal membrane transporter status, and residual renal function were gathered by chart review. Patients participated in a home sleep apnea test using a level III sleep apnea monitor.
Results
Of fifteen participants recruited, 33% had obstructive sleep apnea diagnosed by apnea-hypopnea index ≥ 5 events per hour of sleep. Renal creatinine clearance based upon 24-h urine collection was negatively correlated with apnea-hypopnea index (ρ = – 0.63, p = 0.012). There were no significant associations between anthropometric measures, intra-abdominal dwell volume, or peritoneal membrane transporter status and obstructive sleep apnea measures.
Conclusions
The prevalence of obstructive sleep apnea and sleep disturbances is high in participants receiving peritoneal dialysis. Elevated apnea-hypopnea index is associated with lower residual renal function, whereas dialysis-specific measures such as intra-abdominal dwell volume and peritoneal membrane transporter status do not correlate with severity of obstructive sleep apnea.
Keywords: Dialysis, Obstructive sleep apnea, Sleep-disordered breathing, Peritoneal dialysis
Introduction
Obstructive sleep apnea (OSA) is a condition defined by repeated episodes of airway obstruction, apnea, and hypoxia during sleep [1]. OSA prevalence in the general population is 9–24% [2]. In chronic kidney disease (CKD), the prevalence increases with each stage of CKD, such that over 40% of patients with end-stage renal disease (ESRD) have OSA [3–6]. This striking increase in OSA prevalence may be due to shared comorbidities with ESRD, such as diabetes mellitus, hypertension, obesity, and cardiovascular disease. Recent research, however, suggests that there are unique conditions in ESRD that contribute to OSA [4, 7, 8].
Several studies have investigated the role of fluid shifts in the pathogenesis of sleep apnea in ESRD. As extracellular fluid accumulates with advancing stages of CKD, there is a positive correlation between leg fluid volume displacement, supine neck circumference, and apnea-hypopnea time, which may be mediated by an increase in pharyngeal resistance and a reduction in the upper airway cross-sectional area [7, 9, 10].
Based upon these observations, it is biologically plausible that OSA severity in ESRD is affected by dialysis-specific characteristics. In patients treated with peritoneal dialysis (PD), the presence of intra-abdominal fluid might be expected to reduce functional residual capacity, in turn leading to hypoventilation and airway collapse [11]. On the other hand, nocturnal automated PD, a dialysis modality in which extracellular volume removal occurs primarily at night, would be expected to alleviate pharyngeal and pulmonary edema. Finally, residual renal function would protect against extracellular fluid overload and thus mitigate OSA severity. These factors have not, however, been investigated to our knowledge.
The purpose of this pilot study was to determine OSA prevalence in a small cohort of PD patients and to investigate whether fluid composition measures, PD-specific parameters, and residual renal function affect OSA severity. We hypothesized that OSA prevalence among PD patients would be high and that the PD prescription, peritoneal membrane transport characteristics, and residual renal function would be associated with OSA severity.
Methods
A pilot study was designed in order to recruit participants with ESRD receiving nocturnal automated PD at a hospital-based dialysis center. Participants with a history of treated OSA and/or systolic congestive heart failure with an ejection fraction < 45% were excluded from the study. Those with a permanent pacemaker or extremity amputation were excluded from body composition measurements. All study participants were stable on PD for at least 3 months prior to study enrollment. Medical records were reviewed for medical history including cause of ESRD, medications, social habits, and laboratory data. Consent was obtained and signed by all participants in accordance with the ethical standards established by the Declaration of Helsinki. The institution’s ethical review board approved the study protocol.
Data characterizing peritoneal membrane transporter status, referred to as the peritoneal equilibration test (PET), were gathered from participants’ charts. Peritoneal membrane transport status is a method that classifies membrane (peritoneal) function by measuring the rate at which solutes equilibrate between the dialysate and body plasma. The standard protocol for PD patients at our center is to perform PET testing 1 month after dialysis start, and to determine both the D/P creatinine (ratio of dialysate-to-plasma creatinine concentration after a 4-h dialysate dwell) and the D/D0 glucose (ratio of dialysate glucose concentration after a 4-h dwell to initial dialysate glucose concentration), in order to characterize the peritoneal membrane transporter status as rapid (high D/P creatinine and low D/D0 glucose) or slow (low D/P creatinine and high D/D0 glucose). Rapid transporters tend to have more rapid equilibration of solute and hence less ultrafiltration, whereas slow transporters tend to have slower equilibration of solute and greater ultrafiltration.
Intraperitoneal dialysate volume was determined at the time of the sleep study. The majority of patients at our program receive a standard continuous cycling peritoneal dialysis (CCPD) prescription according to body surface area, which is adjusted every 3 months based upon patient symptoms and dialysis adequacy measurements. Adequacy measures, as defined by total Kt/V urea, dialysate Kt/V urea, and renal Kt/V urea, were collected within 1 month of the date of the sleep study, and data regarding 24-h urine volume and creatinine clearance were available by chart review within 1 month of the sleep study.
Bioelectrical Impedance Analysis (Biospace Body Composition Analyzer, Inbody 520) was used to measure body fluid content and weight on the day of the sleep study, including intracellular water (ICW), extracellular water (ECW), and total body water (TBW). Height was recorded based on patient-reported height. Neck circumference was measured in the upright position using a disposable measuring tape at the level of the hyoid bone. The site of measurement was marked, patient was asked to lie supine on an examining table, and measurement repeated within 5 min of the upright measurement. Hip circumference was measured at the largest area over the hips, while waist measurement was performed at three finger breadths above the right anterior iliac crest by the same investigator (AL). Right leg circumference was measured in the supine position at the widest calf diameter.
For the sleep study, participants were instructed on use of the Medibyte® home sleep apnea test (Braebon Medical Corporation ON, Canada), a validated Level III FDA-approved sleep-recording device that measures airflow, snoring, oxygen saturation, and body position to diagnose sleep apnea [12]. All studies were scored by the same trained polysomnography technician using recommended criteria for hypopnea with 4% desaturation and standard definition of apnea according to the AASM criteria 2012 and reviewed by the study investigators [13]. Apnea-hypopnea index (AHI) was calculated based upon the average number of apneas and hypopneas per hour of sleep.
Statistical Analyses
Analyses were conducted using IBM SPSS v.20 (IBM Statistics for Windows, Armonk, NY: IBM Corp). Descriptive statistics were used to summarize the demographic and baseline characteristics. Spearman’s correlations were performed to assess for associations between independent and dependent variables. Linear regression analyses were performed to assess for associations between independent (apnea-hypopnea index, percent time spent at SpO2 < 90%, and nadir oxygen saturation) and dependent (residual renal function, dialysis-specific measures, and body composition) variables after adjusting for covariates, when applicable. A priori covariates included CCPD dwell volume, 24-h urine creatinine clearance, and dialysate Kt/V. The association of body fluid composition with OSA was adjusted for time of day the measurement was taken.
Independent samples T tests were performed to assess if OSA groups differed on the dependent variables of interest.
Results
Ten males and five females with an average age of 46 ± 12 years were enrolled and completed the study. Participant self-identified race was Caucasian in 47% of participants, 13% identifying as Black and 33% as Latino. Main comorbidities included hypertension (80%), diabetes mellitus (47%), and asthma (20%). The main causes of ESRD identified by chart review were diabetes (20%) and hypertension (13%). Participants had been receiving peritoneal dialysis for an average of 11 months (see Table 1).
Table 1.
Participant demographics with medians and standard deviations (SD)
| Participant demographics | Median (IQR) |
|---|---|
| Age | 51 (15) |
| Body mass index (kg/m2) | 27 (7.7) |
| Renal creatinine clearance (CrCl) (ml/min) | 4.25 (3.85) |
| Race/ethnicity | # Participants |
| White | 7 |
| Hispanic | 5 |
| Black/African American | 2 |
| Asian | 1 |
| Comorbidities | % Participants |
| Hypertension | 80 |
| Diabetes | 47 |
| Asthma | 20 |
| Causes of ESRD | # of participants |
| Diabetes | 3 |
| Hypertension | 2 |
| Unknown | 2 |
| Polycystic kidney disease | 1 |
| Focal segmental glomerulonephritis | 1 |
| Anti-neutrophil cytoplasmic antibody vasculitis | 1 |
| Renal amyloidosis | 1 |
| IgA nephropathy | 1 |
| Obstructive uropathy | 1 |
| Lupus nephritis | 1 |
Approximately 25% of the sample population had a BMI > 30 kg/m2, and median BMI was 27 kg/m2, interquartile ratio (IQR) 7.7. A significant increase was found between upright (M = 39.5 ± 5.6 cm) as compared with supine (M = 41.4 ± 5.8 cm) neck circumference measurements (p < 0.001). Median right leg circumference averaged 36.3 (IQR 4.4) cm, and median waist/hip ratio was 0.98 (IQR 0.09) (see Table 2). Right leg circumference, waist-to-hip ratio, and change in neck circumference were higher in the OSA group compared to the non-OSA group; however, this difference did not reach statistical significance.
Table 2.
Anthropometries and subjective sleep questionnaire seores
| Anthropometric data | Median (IQR) | ||
|---|---|---|---|
| OSA sample | Non-OSA samplea | Total sample | |
| Neck difference (upright vs. supine) (cm) | 2.25 (1.45) | 1.4 (1.1) | 1.4 (1.1) |
| Right leg circumference (cm) | 38.0 (4.8) | 36.1 (3.9) | 36.3 (4.4) |
| Waist:hip ratio (cm) | 1.1 + 0.06 | 0.99 + 0.11 | 1 (0.09) |
| Total body fluid (l) | 88.8 (18) | 87 (30.1) | 88.8 (23.8) |
| Extracellular water (l) | 37 (7.7) | 33.6 (12.6) | 34.6 (10.8) |
| Intracellular water (l) | 52.7 (11.3) | 53.9 + 18.2 | 53.1 (14.1) |
Of note, there was no statistically significant difference between the OSA and Non-OSA samples on any of the anthropometric data listed above
BMI was not correlated with AHI, nadir oxygen saturation, or percent time spent at SpO2 < 90% in our sample population (ρ = 0.018, p = 0.95; ρ = – 0.29, p = 0.29; ρ = 0.36, p = 0.19, respectively).
Thirty-three percent of participants had OSA as defined by an AHI greater than 5 events/h—three were severe, one moderate, and one mild. Table 3 shows median breathing parameters during sleep for reference. The median AHI was two, with six participants spending over 20% of total sleep time in SpO2 < 90%, indicating a profound hypoxemic burden.
Table 3.
Breathing parameters during sleep
| Breathing parameter | Median (IQR) N= 15 |
|---|---|
| Apnea-hypopnea index (events/h) | 2.2 (13.3) |
| Obstructive apnea index (events/h) | 0.55 (2.9) |
| Central apnea index (events/h) | 0 (0.4) |
| Mixed apnea index (events/h) | 0 (0) |
| Nadir oxygen saturation | 80 (11.8) |
| Time spent below 90% oxygen saturation | 10.6 (28.8) |
Renal creatinine clearance based upon 24-h urine collection and adjusted for body surface area (BSA) was negatively correlated with AHI (ρ = – 0.63, p = 0.012), but not correlated with oxygenation parameters (see Fig. 1). Dialysate Kt/V urea, a measure of urea clearance in the dialysate effluent, was moderately positively correlated with AHI (ρ = 0.53, p = 0.04). However, total Kt/V urea, renal Kt/V urea, CCPD dwell volume, and 24-h urine volume were not significantly correlated with respiratory parameters during sleep. PET results (D/P creatinine and D/D0 glucose) did not correlate with respiratory parameters during sleep.
Fig. 1.
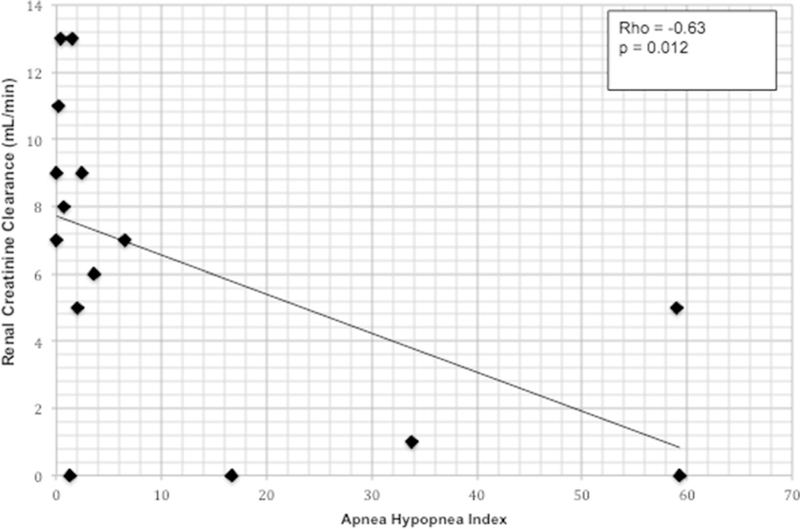
Relationship between apnea-hypopnea index and residual renal function as measured by creatinine clearance
Bioelectrical Impedance Analysis revealed that intracellular water, extracellular water, and total body water were higher in the OSA group but the difference did not reach statistical significance. There were no significant correlation of these measures with AHI, percent time spent at SpO2 < 90%, or with nadir oxygen saturation, even when adjusted for renal creatinine clearance, dialysate Kt/V, and time of day of measurement.
Discussion
This pilot study demonstrated a high prevalence of OSA in patients with ESRD receiving PD, similar to previous studies. The most significant finding was a negative correlation between OSA severity, as measured by AHI, and residual renal function, as measured by 24-h urine creatinine clearance. Contrary to our hypothesis, PD-specific measures, including peritoneal membrane transporter status and intraabdominal dwell volume, and body composition measures, did not correlate with severity of OSA.
One of the most interesting findings is the association between residual renal function and OSA. Given the crosssectional nature of this study, it is difficult to interpret the directionality of the association. One might hypothesize that repetitive insults caused by intermittent hypoxemia lead to ischemic kidney injury and loss of residual renal function; conversely, decreased residual renal function could contribute to altered nocturnal respiratory mechanics and volume overload, thus driving the apneas and hypopneas that define OSA [1, 3]. Other potential mechanisms connecting renal function to OSA relate to upper airway patency, which may be compromised in the setting of uremic myopathy [14, 15]. As more studies emerge linking decreased residual renal function to increased cardiovascular risk in ESRD and increased residual renal function to prolonged survival, this association with OSA is intriguing given that OSA has similarly been found to predict increased cardiovascular morbidity and mortality [16–18].
The 33% prevalence of OSA in our cohort is somewhat lower than the prevalence found in other studies of patients with ESRD [3, 5, 6]. This difference may be explained by the use of a home sleep apnea test, which may have underestimated the prevalence of OSA. Home sleep apnea monitors do not measure sleep or detect arousals, and therefore events that are associated with airflow reduction and arousals that would qualify as hypopnea on in-laboratory polysomnography testing are not detected. In addition, total sleep time is not accurately measured on home sleep monitors due to the lack of electroencephalography (EEG) recording; hence an overestimation of sleep duration may underestimate apnea-hypopnea indices and under-diagnose sleep apnea. However, the level III device used in this study has been validated against in-laboratory polysomnography with a sensitivity of 97% and a specificity of 67% for apnea and hypopnea index ≥ 5 events per hour [12]. With higher apnea-hypopnea indices, sensitivity decreases to 70% for AHI > 30 events/h but specificity rises to 100%. In our sample, the high hypoxemic burden (median time spent at saturations below 90%) in comparison to the number of apnea and hypopneas per hour of sleep may be an indication of an underestimation of sleep-disordered breathing. Other possible explanations for the lower prevalence of OSA in our cohort include the relatively younger average age of the sample and the fact that patients on PD are generally “healthier” than the average population of ESRD patients. The small sample size may have also contributed to the prevalence. On the other hand, patients with ESRD may be at risk for developing central sleep apnea related to fluid status and fluctuations in acid base status. However, central events were rare in our sample. This may be explained by the younger age of the population and the fact that patients on peritoneal dialysis in the US are generally “healthier” than those on hemodialysis for instance. In addition, though home sleep testing has excellent sensitivity and specificity for the detection of obstructive sleep apnea, its ability to accurately detect other forms of sleep-disordered breathing such as central sleep apnea remains to be determined [19, 20].
Although patients with OSA are generally known to have a certain phenotype that consists of obesity, upper airway abnormalities or both, in this population of patients with ESRD receiving peritoneal dialysis, the phenotype does not appear to be as predictive [21]. Neither body habitus nor neck circumference was associated with a diagnosis of OSA, potentially suggesting an alternative pathogenesis. Previous literature has found that body fluid composition and retention of fluid result in greater fluid-related compression of the trachea following rostral movement of fluid while in the supine position, causing increased incidence of obstructive sleep apnea during sleep [22]. In our study, however, extracellular and intracellular fluid as well as total body water were higher in the OSA group but did not reach statistical significance, even after adjusting for time of day and activity level. This finding may be related to small sample size, the confounding effect of the intra-abdominal dwell volume, or to limitations in the technology used to measure fluid status. As patients did not drain the abdomen immediately prior to bioelectrical impedance analysis measurements, intraabdominal fluid may have impacted the ability to make accurate calculations. Although bioelectrical impedance analysis is convenient, safe, and easy, measurements of fat or fluid composition are impacted by states of dehydration, illnesses, or weight change [23]. Although we attempted to control for these situations by studying patients when they were clinically stable, we were unable to eliminate other potential variables such as inter-participant variations in daytime dwell volume.
The positive association between dialysate adequacy (dialysate Kt/V urea) and severity of OSA (AHI) must be interpreted with caution. Due to the observational study design, it was impossible to ascertain whether dialysate adequacy was a predictor of OSA severity. In our center, patients with decreased residual renal function and inadequate total Kt/V urea usually have a modification in their peritoneal dialysis prescription that would include changes (e.g., increase in volume of dwells overnight) that may theoretically predispose them to OSA. The positive correlation between dialysate urea clearance and AHI is thus complicated, and we believe there is too much confounding from multiple variables to draw solid conclusions from it.
Surprisingly, data from our study do not show that dwell volume predicts OSA status or severity. This observation contrasts with preliminary data from patients with ascites showing that OSA improves after therapeutic paracentesis or data suggesting that OSA improves following parturition in pregnant women, suggesting that intra-abdominal volume plays a role in the pathogenesis of OSA, possibly via the reduction in functional residual capacity and airway collapse [23, 24].
Strengths of this study are the detailed examinations performed on patients receiving peritoneal dialysis, the objective measurement of sleep-disordered breathing, and the consistent clinical protocol followed in our peritoneal dialysis patients. Of 21 patients who were approached to participate in our study, 17 ultimately consented (two of whom withdrew), and we believe that selection bias was therefore minimal. This study needs to be interpreted in the context of several limitations. Importantly, the small sample size was meant to collect initial pilot data; larger patient cohorts are needed to establish enough power to detect specific effects. As noted previously, home sleep apnea testing may have underestimated the severity of OSA due to the lack of EEG and the inability to measure arousals or sleep duration; this fact may have impacted correlations. Additionally, prevalence of sleep apnea may have been underestimated by the level III sleep study as these devices have yet to be validated in the ESRD population. There are several confounders that were not included in this study due to limitations of studying sleep at home, including sleep duration and arousals or wake after sleep onset. Body composition measurements may have been affected by the presence of intra-abdominal (peritoneal) fluid and daytime variations in data collection, as well as sample size. Lastly, the cross-sectional nature of this study limits the ability to infer causation, and certain results (such as the dialysate Kt/V urea) must be interpreted cautiously since alterations in PD prescription prior to data collection were a source of confounding for which we could not control.
Given what is known about the high prevalence of OSA in PD patients, additional research is needed that specifically addresses OSA as a function of PD-specific parameters. Many of these parameters, such as intra-abdominal dwell volume, nocturnal urine volume, and nocturnal ultrafiltration, could be manipulated through alteration in the peritoneal dialysis and medication prescription. Others, such as PET status, may be more difficult to control. The results of this study suggest that efforts to maintain residual renal function may reduce the severity of OSA but the reverse may also be true where detection and treatment of OSA in patients with kidney disease may help preserve their kidney function.
Conclusion
The prevalence of OSA and sleep disturbances is high in participants on automated nocturnal peritoneal dialysis. Based on the measures evaluated in this study, higher AHI correlated with lower renal creatinine clearance in participants with ESRD on peritoneal dialysis. Additional research is needed to confirm these results in a larger population as well as to identify whether screening and treating OSA in this patient population impacts cardiovascular outcomes in these participants, as recent data show an increased risk for sudden death in patients on incident hemodialysis [25].
Acknowledgements
We would like to acknowledge all participants who contributed their data to this research.
Funding GB is funded by NIH R01HL130702 and R01HD078515.
Footnotes
Compliance with Ethical Standards
Conflict of interest GB has received research equipment donation from Phillips Respironics.
References
- 1.Adeseun GA, Rosas SE (2010) The impact of obstructive sleep apnea on chronic kidney disease. Curr Hypertens Rep 12(5):378–383. 10.1007/s11906-010-0135-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM (2009) Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ 108(5):246–249 [PMC free article] [PubMed] [Google Scholar]
- 3.Jhamb M, Unruh ML (2012) Volume overload as a mechanism for obstructive sleep apnea in CKD? Nephrol Dial Transplant 27(4):1291–1293. 10.1093/ndt/gfs024 [DOI] [PubMed] [Google Scholar]
- 4.Abuyassin B, Sharma K, Ayas NT, Laher I (2015) Obstructive sleep apnea and kidney disease: a potential bidirectional relationship? J Clin Sleep Med 11(8):915–924. 10.5664/jcsm.4946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuhlmann U, Becker HF, Birkhahn M, Peter JH, von Wichert P, Schutterle S et al. (2000) Sleep-apnea in patients with end-stage renal disease and objective results. Clin Nephrol 53(6):460–466 [PubMed] [Google Scholar]
- 6.Lyons OD, Bradley TD, Chan CT (2015) Hypervolemia and sleep apnea in kidney disease. Semin Nephrol 35(4):373–382. 10.1016/j.semnephrol.2015.06.008 [DOI] [PubMed] [Google Scholar]
- 7.Stepanski E, Faber M, Zorick F, Basner R, Roth T (1995) Sleep disorders in patients on continuous ambulatory peritoneal dialysis. J Am Soc Nephrol 6(2):192–197 [DOI] [PubMed] [Google Scholar]
- 8.White DP (2005) Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med 172(11):1363–1370. 10.1164/rccm.200412-1631SO [DOI] [PubMed] [Google Scholar]
- 9.White LH, Motwani S, Kasai T, Yumino D, Amirthalingam V, Bradley TD (2014) Effect of rostral fluid shift on pharyngeal resistance in men with and without obstructive sleep apnea. Respir Physiol Neurobiol 192:17–22. 10.1016/j.resp.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 10.Kasai T, Motwani SS, Elias RM, Gabriel JM, Taranto Montemurro L, Yanagisawa N et al. (2014) Influence of rostral fluid shift on upper airway size and mucosal water content. J Clin Sleep Med 10(10):1069–1074. 10.5664/jcsm.4102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee YC, Hung SY, Wang HK, Lin CW, Wang HH, Chen SW et al. (2015) Sleep apnea and the risk of chronic kidney disease: a nationwide population-based cohort study. Sleep 38(2):213–221. 10.5665/sleep.4400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Driver HS, Pereira EJ, Bjerring K, Toop F, Stewart SC, Munt PW et al. (2011) Validation of the MediByte(R) type 3 portable monitor compared with polysomnography for screening of obstructive sleep apnea. Can Respir J 18(3):137–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johns MW (2000) Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the epworth sleepiness scale: failure of the MSLT as a gold standard. J Sleep Res 9(1):5–11 [DOI] [PubMed] [Google Scholar]
- 14.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK et al. (2012) Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the American academy of sleep medicine. J Clin Sleep Med 8(5):597–619. 10.5664/jcsm.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang SC, Lam B, Lai AS, Pang CB, Tso WK, Khong PL et al. (2009) Improvement in sleep apnea during nocturnal peritoneal dialysis is associated with reduced airway congestion and better uremic clearance. Clin J Am Soc Nephrol 4(2):410–418. 10.2215/cjn.03520708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL et al. (2003) Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American heart association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation 108(17):2154–2169. 10.1161/01.cir.0000095676.90936.80 [DOI] [PubMed] [Google Scholar]
- 17. Kang SH, Choi EW, Park JW, Cho KH, Do JY (2016) Clinical significance of the edema index in incident peritoneal dialysis patients. PLoS ONE 11(1):e0147070. 10.1371/journal.pone.0147070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassan K, Hassan S, Anwar S, Zaher A, Edgem R, Hassan F (2015) Predictors of left ventricular hypertrophy and their cutoffs in peritoneal dialysis patients. Int Heart J 56(2):186–191. 10.1536/ihj.14-246 [DOI] [PubMed] [Google Scholar]
- 19.Blackman A, McGregor C, Dales R, Driver HS, Dumov I, Fleming J et al. (2010) Canadian Sleep Society/Canadian thoracic society position paper on the use of portable monitoring for the diagnosis of obstructive sleep apnea/hypopnea in adults. Can Respir J. 10.1155/2010/923718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Shayeb M, Topfer LA, Stafinski T, Pawluk L, Menon D (2014) Diagnostic accuracy of level three portable sleep tests versus level 1 polysomnography for sleep-disordered breathing: a systematic review and meta-analysis. CMAJ 186(1):E25–E51. 10.1503/cmaj.130952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perl J, Bargman JM (2009) The importance of residual kidney function for patients on dialysis: a critical review. Am J Kidney Dis 53(6):1068–1081. 10.1053/j.ajkd.2009.02.012 [DOI] [PubMed] [Google Scholar]
- 22.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A (2013) Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med 188(8):996–1004. 10.1164/rccm.201303-04480C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crespo J, Cifrian J, Pinto JA, Jimenez-Gomez A, Pons-Romero F (2003) Sleep apnea obstructive syndrome: a new complication previously undescribed in cirrhotic patients with ascites. Am J Gastroenterol 98(12):2815–2816. 10.1111/j.1572-0241.2003.08754.x [DOI] [PubMed] [Google Scholar]
- 24.Edwards N, Blyton DM, Hennessy A, Sullivan CE (2005) Severity of sleep-disordered breathing improves following parturition. Sleep 28(6):737–741 [DOI] [PubMed] [Google Scholar]
- 25.Kerns ES, Kim ED, Meoni LA, Sozio SM, Jaar BG, Estrella MM et al. (2018) Obstructive sleep apnea increases sudden cardiac death in incident hemodialysis patients. Am J Nephrol (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]


