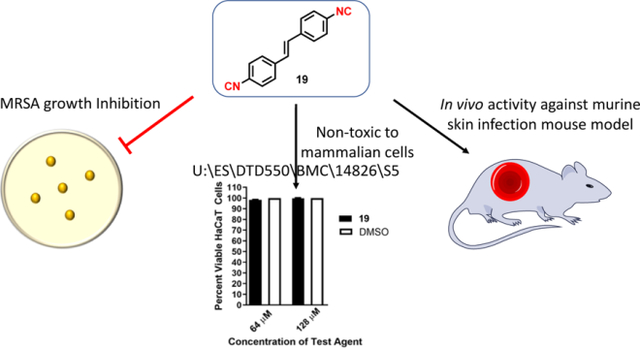
Abstract
Antibiotic resistance remains a major global public health threat that requires sustained discovery of novel antibacterial agents with unexploited scaffolds. Structure-activity relationship of the first-generation aryl isonitrile compounds we synthesized led to an initial lead molecule that informed the synthesis of a second-generation of aryl isonitriles. From this new series of 20 compounds, three analogues inhibited growth of methicillin-resistant Staphylococcus aureus (MRSA) (from 1 – 4 µM) and were safe to human keratinocytes. Compound 19, with an additional isonitrile group exhibited improved activity against MRSA compared to the first-generation lead compound. This compound emerged as a candidate worthy of further investigation and further reinforced the importance of the isonitrile functionality in the compounds’ anti-MRSA activity. In a murine skin wound model, 19 significantly reduced the burden of MRSA, similar to the antibiotic fusidic acid. In summary, 19 was identified as a new lead aryl isonitrile compound effective against MRSA.
Graphical Abstract
INTRODUCTION
Bacterial infections resistant to currently available antibiotics continue to pose a major global public health threat that requires the constant identification and development of new antibacterial agents. Though extensive research efforts and funding have recently been focused to identify new agents to treat Gram-negative bacterial infections, particularly those caused by carbapenem-resistant Enterobacteriaceae (CRE), the reality remains that Gram-positive bacteria (particularly methicillin-resistant S. aureus (MRSA)) are more prevalent sources of community-acquired and nosocomial infections, particularly in the United States of America.1–3 In its landmark report in 2013, the US Centers for Disease Control and Prevention noted that serious infections caused by MRSA (>80,000) alone outnumbered infections attributed to extended-spectrum β-lactamase producing Enterobacteriaceae (26,000), CRE (9,000), multidrug-resistant Acinetobacter (7,300), and multidrug-resistant Pseudomonas aeruginosa (6,700) combined.4 Furthermore, significantly more fatalities were attributed to MRSA infections (11,285 deaths) relative to infections caused by the aforementioned drug-resistant Gram-negative pathogens (3,240 deaths).4 Given MRSA infections continue to persist both in healthcare and community settings, and resistant isolates to key antibiotics (vancomycin and linezolid) used to treat MRSA infections have emerged,3, 5–8 new antibacterial agents are still needed.
Current antibacterial discovery programs often focus on optimizing existing antibiotic scaffolds (i.e. β-lactams, quinolones, glycopeptides, oxazolidinones) to develop new therapeutics. This approach has yielded regulatory approval for several new antibacterial agents to treat MRSA infections, including delafloxacin, dalbavancin, oritavancin, and tedizolid phosphate with improved potency relative to other antibiotics in the same drug class.9 However, bacterial resistance to these newer agents is most probably inevitable, particularly given the similarity in structure to existing antibiotics, which will further hinder clinicians’ ability to effectively treat drug-resistant bacterial infections. Identifying novel antibacterial agents with unique scaffolds or mechanisms of action is critical to circumvent the growing challenge of treating drug-resistant bacterial infections.
As part of efforts to identify compounds with unique functionalities and novel structural moieties capable of targeting multidrug-resistant bacterial infections, we recently identified aryl isonitriles as a unique class of compounds with anti-MRSA activity.10 Though extensive efforts in isolating naturally occurring isonitriles from marine sponges and cyanobacteria have been fruitful, these and other naturally occurring isonitriles with antimicrobial activity have been precluded from extensive medicinal chemistry and structure-activity-relationship (SAR) studies.11–14 This is primarily because their complex structural motifs make them very difficult to access. Therefore, the isonitrile functionality and the aryl isonitrile scaffold remain one of the least extensively studied scaffolds. Previous SAR studies on the first series of over 40 aryl isonitriles revealed the isonitrile functionality as the most essential structural component that contributed to the antibacterial activity of the compounds. The presence of a second non-isonitrile-bearing aromatic ring and an alkene bridge/linker were also shown to be important. However, substituents incorporated on this bridge did not result in improved antibacterial activity (Figure 1).
Figure 1.
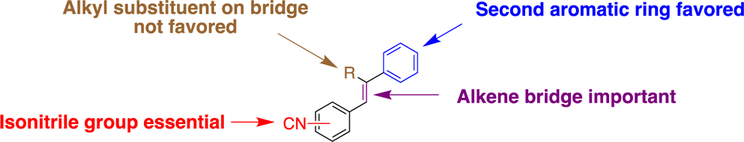
First generation SAR.
With this in mind, the current work aims to further explore the structural-anti-MRSA activity of a second generation of aryl isonitriles, characterize their spectrum of antibacterial activity, and evaluate the most promising analogue’s activity in a mouse model of MRSA infection. This exploration takes into consideration a novel group of stilbene bis-isonitriles. New stilbene aryl isonitriles with medicinally-relevant structural molecules and heterocyclic moieties, and those with a saturated linker have also been evaluated (Figure 2). From this effort, compound 19 emerged as a new lead compound with antibacterial activity in vitro and in vivo against MRSA (Figure 3).
Figure 2.
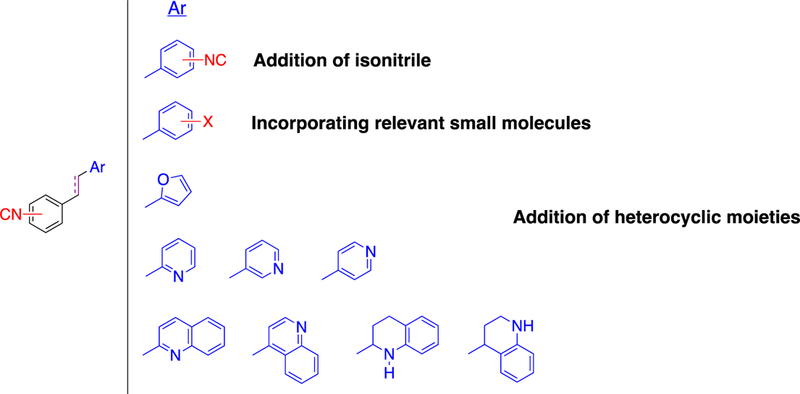
Design strategy for the second-generation aryl isonitrile compounds.
Figure 3.
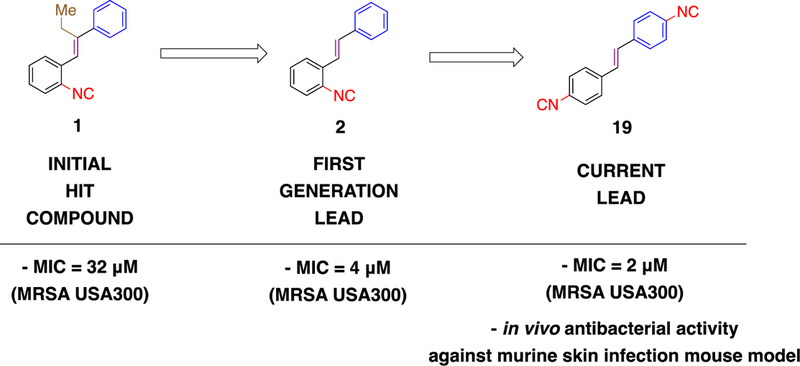
Structural evolution leading to current lead aryl isonitrile compound and minimum inhibitory concentration (MIC) values against methicillin-resistant S. aureus (MRSA).
RESULTS
Synthesis of new aryl isonitrile analogues
Following the identification of 1 as the initial hit aryl isonitrile and subsequent synthetic exploration resulting in 2 as the lead molecule (Figure 3), our initial synthetic efforts were focused on incorporating medicinally-relevant groups to the aryl isonitrile core. These groups included electron donating and electron withdrawing small molecules such as fluoro, chloro, trifluoromethyl, and methoxy groups.15–17 To access these novel stilbene isonitriles, Michaelis-Arbuzov reaction involving the commercially available nitrobenzyl bromide starting material (3) was used to form nitrobenzyl phosphonate (4).18 Subsequent reduction of the nitro group, followed by Hofmann isonitrile conversion of the resulting amine, led to the formation of the isonitrile phosphonate (5).19 Serving as the divergent point, the isonitrile phosphonate was then subjected to Horner-Wadsworth-Emmons (HWE)20 reaction to produce isonitrile compounds 6, 7, 8, 9, 10, 11, and 12 from the corresponding aldehydes (Scheme 1). The aforementioned synthetic procedure was also used to synthesize aryl isonitriles to explore the importance of incorporating heterocyclic moieties as the second aromatic ring. These analogues included furan (12) and quinoline (13 and 14) groups.
Scheme 1.
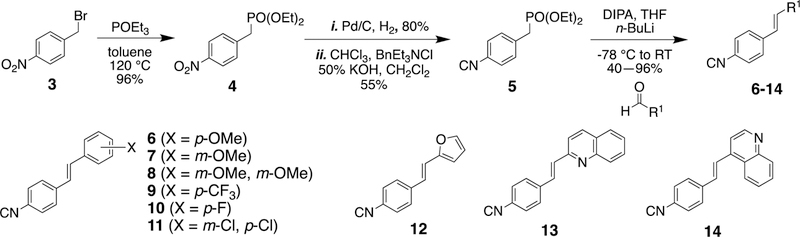
Synthesis of stilbene aryl isonitrile compounds.
As part of SAR studies on the first-generation molecules, the isonitrile functionality was identified as the integral component of the stilbene aryl isonitriles (Figure 1). Bis-isonitriles 17, 18, and 19 were therefore synthesized to explore the influence of an additional isonitrile group on the antibacterial activity of the lead molecule 2. Similarly, using 4 as the divergent point, the stilbene bis-isonitriles 17, 18, and 19 were synthesized (Scheme 2). HWE reaction involving 4 and the corresponding nitro substituted benzaldehydes was employed as the first step in the formation of dinitrostilbenes. The selective reduction of the nitro group followed by Hoffmann isonitrile synthesis led to compounds 17 and 18. Bis-isonitrile 19 was prepared by a stepwise formylation and POCl3-promoted dehydration of the corresponding diamine.
Scheme 2.
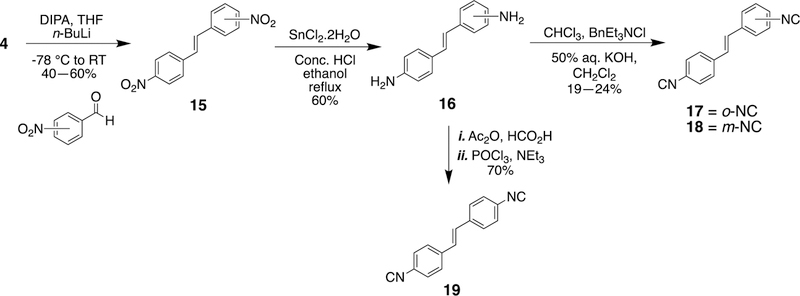
Synthesis of stilbene bis-isonitriles.
Finally, after the synthesis of the stilbene isonitriles and the bis-isonitriles, the question of the impact of an alkane bridge on the biological activity arose. This led to the synthesis of compounds 21–28 (Scheme 3). In the synthesis of aryl isonitriles with an alkane linker, HWE reaction involving 4 and various aldehydes was first employed to form the stilbene compounds. A sequence of reduction and Hofmann isonitrile synthesis afforded the corresponding aryl isonitriles in good yields.
Scheme 3.
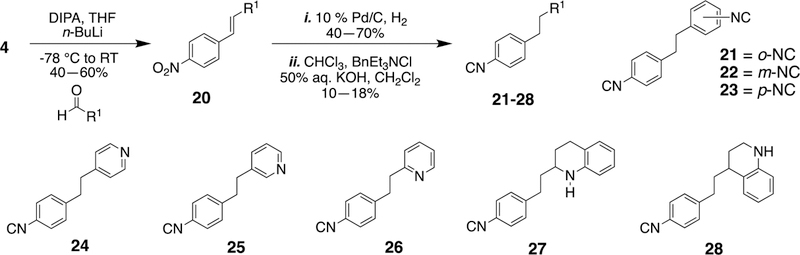
Synthesis of isonitriles with an alkane bridge.
Initial screening and structure-activity relationship of new aryl isonitrile analogues against MRSA NRS123 (USA400)
In order to investigate the antibacterial activity of the newly synthesized isonitrile analogues, the minimum inhibitory concentration (MIC) against MRSA NRS123 was determined using the broth microdilution assay (Table 1). The newly synthesized stilbene bis-isonitriles 17 (MIC = 16 µM), 18 (MIC = 4 µM), and 19 (MIC = 8 µM) all exhibited moderate to good anti-MRSA activity. The most potent analogues were compounds where the isonitrile moiety was located in the meta- (18) or para- position (19). The moderate to good activity exhibited by the stilbene bis-isonitriles further emphasized the importance of the isonitrile group for antibacterial activity against MRSA. An eight-fold reduction in activity was observed when the most potent stilbene bis-isonitrile 18 (MIC = 4 µM) was transformed to its saturated bis-isonitrile analogue 22 (MIC = 32 µM). The observance of a similar pattern for bis-isonitriles 17 (MIC =16 µM) and 19 (MIC = 8 µM) with respect to the saturated bis-isonitrile analogues 21 and 23 (MIC > 64 µM) further emphasized the importance of the alkene linker.
Table 1:
Minimum inhibitory concentration (MIC, in µM) of second-generation aryl isonitrile analogues and control antibiotics against methicillin-resistant Staphylococcus aureus (MRSA) NRS123 (USA400).
| Compound/Drug Name | MRSA NRS123 (USA 400) |
|---|---|
| MIC | |
| 6 | 32 |
| 7 | 2 |
| 8 | 64 |
| 9 | 32 |
| 10 | 64 |
| 11 | >64 |
| 12 | 32 |
| 13 | 8 |
| 14 | >64 |
| 17 | 16 |
| 18 | 4 |
| 19 | 8 |
| 21 | >64 |
| 22 | 32 |
| 23 | >64 |
| 24 | 64 |
| 25 | >64 |
| 26 | >64 |
| 27 | 16 |
| 28 | 32 |
| Linezolid | 1 |
| Vancomycin | 1 |
N.D. = Not determined
Intrigued by the importance of the non-isonitrile-bearing aromatic ring resulting from the previous SAR studies, heterocyclic moieties with established therapeutic properties and widely known in medicinal chemistry to improve the physicochemical and pharmacokinetic properties of lead molecules were investigated.21, 22 While none of these heterocyclic-containing aryl isonitriles positively improved the activity of the lead compound, the quinoline-containing compound 13 (MIC = 8 µM) was more potent than the quinoline analogue 14 (MIC > 64 µM) and furan analogue 12 (MIC = 32 µM).
With the knowledge of the medicinal importance of F, -OCH3, Cl and CF3 and their value in the enhancement of the pharmacokinetic and physiochemical activity of several therapeutic small molecule candidates, installing these species on the aryl isonitrile compounds was investigated.15–17 Of the methoxy substituted derivatives, the meta-substituted methoxy 7 (MIC = 2 µM) was the most potent analogue, but adding the second methoxy group (8) diminished the compound’s anti-MRSA activity (MIC = 64 µM). Interestingly, none of the analogues with electron-withdrawing fluoro, chloro, and trifluoromethyl groups exhibited a major improvement in anti-MRSA activity. This reveals that having the right electronic environment around the second aromatic ring is important.
Evaluation of most potent analogues against additional strains of S. aureus
Based upon the initial screening results, the four most potent analogues from this series (7, 13, 18, and 19) were further evaluated against additional multidrug-resistant S. aureus clinical isolates (Table 2). The S. aureus clinical isolates included strains resistant to linezolid (NRS119) and vancomycin (VRS10, VRS11a), two antibiotics frequently prescribed to treat MRSA infections. Against methicillin-sensitive S. aureus, the compounds inhibited growth from 2 – 16 µM. When evaluated against MRSA NRS384 (USA300), all four aryl isonitrile compounds inhibited growth at concentrations ranging from 1 – 4 µM. Methoxy analog 7 (MIC = 1 µM) and bis-isonitrile 19 (MIC = 2 µM) were more potent than first generation lead 2 (MIC = 4 µM) against MRSA NRS384. However, quinoline isonitrile 13 (MIC = 4 µM) and bis-isonitrile 18 (MIC = 4 µM) exhibited similar potency to 2. Against MRSA NRS119, 7 and 19 were the most potent analogues (MIC = 1 µM), followed by 13 (MIC = 4 µM), and 18 (MIC = 4 µM). Once again, the second-generation analogues 7 and 19 were more potent than the first-generation lead 2 (MIC = 4 µM). All four compounds were more potent than linezolid (MIC = 32 µM) against MRSA NRS119. The MIC results obtained against MRSA NRS384 (USA300) and MRSA NRS119 (linezolid-resistant S. aureus) demonstrate that incorporating a second isonitrile functionality (as in compound 19) improved the antibacterial activity of the first-generation lead molecule 2.
Table 2:
Minimum inhibitory concentration (MIC, in µM) of the four most potent aryl isonitrile compounds and control antibiotics against methicillin-sensitive (MSSA), methicillin-resistant (MRSA), linezolid-resistant (LRSA), and vancomycin-resistant S. aureus (VRSA).
| Compound Name | S. aureus NRS107 (MSSA) | MRSA NRS119 (LRSA) | MRSA NRS384 (USA300) | MRSA NRS385 (USA500) | VRS10 (VRSA) | VRS11a (VRSA) |
|---|---|---|---|---|---|---|
| 7 | 2 | 1 | 1 | 4 | 2 | 4 |
| 13 | N.D.1 | 4 | 4 | N.D. | 32 | N.D. |
| 18 | 16 | 4 | 4 | 16 | 8 | 16 |
| 19 | 4 | 1 | 2 | 8 | 8 | 8 |
| Linezolid | 2 | 32 | 2 | 4 | 2 | 2 |
| Vancomycin | 1 | 1 | 1 | 1 | >64 | >64 |
N.D. = Not determined
Against two S. aureus strains (VRS10 and VRS11a) resistant to vancomycin (MIC > 64 µM), compounds 7 and 19 retained their antibacterial activity (MIC ranged from 2 – 8 µM). Interestingly, 13 (MIC = 32 µM) exhibited very poor antibacterial activity against VRS10. Thus, this compound was eliminated from further biological evaluation.
Investigation of the antibacterial spectrum of activity of the aryl isonitrile compounds
To examine their spectrum of antibacterial activity, compounds 7, 18, and 19 were examined against clinical isolates from the ESKAPE pathogens (Enterococcus faecium (E), Klebsiella pneumoniae (K), Acinetobacter baumannii (A), Pseudomonas aeruginosa (P), and Enterobacter species (E)) (Table 3). Collectively, these six pathogens are a significant source of hospital-acquired bacterial infections and exhibit resistance to most clinically available antibiotics.23, 24 All four compounds were significantly less potent or inactive (MIC ≥ 64 µM) against vancomycin-resistant E. faecium (VRE), K. pneumoniae, A. baumannii, P. aeruginosa, and E. cloacae, similar to the antibiotics erythromycin, linezolid, and vancomycin. The compounds were also evaluated against two additional clinically-relevant Gram-positive bacterial pathogens, Staphylococcus epidermidis and Streptococcus pneumoniae. All three compounds exhibited potent activity against methicillin-resistant S. epidermidis (MIC = 1 µM). Against, penicillin-resistant S. pneumoniae, 7 was the most potent compound (MIC = 4 µM) followed by 19 (MIC = 8 µM). Compound 18 was the least potent analogue against S. pneumoniae (MIC = 16 µM).
Table 3:
Minimum inhibitory concentration (MIC, in µM) of the three most potent aryl isonitrile compounds and control antibiotics against clinically-relevant Gram-positive and Gram-negative bacterial pathogens.
| Bacterial Strain | |||||||
|---|---|---|---|---|---|---|---|
| Test Agent | S. epidermidis NRS101 | S. pneumoniae ATCC 700677 | E. faecium ATCC700221 | A. baumannii BAA-1605 | E. cloacae BAA-1134 | K. pneumoniae ATCC BAA-1705 | Pseudomonas aeruginosa ATCC 15442 |
| 7 | 1 | 4 | >64 | >64 | >64 | >64 | >64 |
| 18 | 1 | 16 | >64 | >64 | >64 | >64 | >64 |
| 19 | 1 | 8 | >64 | >64 | >64 | >64 | >64 |
| Erythromycin | N.D.1 | N.D. | N.D. | 16 | >64 | >64 | >64 |
| Linezolid | 2 | 2 | 0.50 | >64 | >64 | >64 | >64 |
| Vancomycin | 4 | 1 | >64 | 32 | >64 | >64 | >64 |
N.D. = Not determined
Effect of the outer membrane and efflux pump on antibacterial activity against Gram-negative bacterial pathogens
We hypothesized that the lack of antibacterial activity against Gram-negative bacteria observed for the aryl isonitrile compounds was due either to the presence of the outer membrane (OM) or due to expulsion via efflux pumps, two common resistance mechanisms utilized by Gram-negative bacterial pathogens.25 The OM has been known to prevent numerous antibiotics (including erythromycin) from gaining entry into the bacterial cell at sufficient concentrations to bind to/inhibit the molecular target. To examine if the OM was impeding the antibacterial activity of the aryl isonitrile compounds, compound 19 and control antibiotics were incubated with the same Gram-negative bacterial species presented in Table 2 in the presence of a subinhibitory concentration of the membrane-permeabilizing agent colistin. As presented in Table 4, a noticeable improvement in the antibacterial activity of compound 19 was observed against A. baumannii (MIC = 16 µM) and E. cloacae (MIC = 4 µM) indicating the OM was having a deleterious effect on the antibacterial activity of this compound. Interestingly, no improvement in antibacterial activity for 19, in the presence of colistin, was observed against K. pneumoniae and P. aeruginosa, (similar to linezolid) suggesting the presence of the outer membrane was not solely responsible for the lack of activity observed in these particular pathogens. The antibacterial activity of the antibiotic erythromycin, in the presence of colistin, improved against A. baumannii (MIC = 4 µM), E. cloacae (MIC = 1 µM), K. pneumoniae (MIC = 16 µM), and P. aeruginosa (MIC = 4 µM). Erythromycin is thought to be capable of diffusing across the OM but at a very slow rate.26 Thus permeabilizing the OM is expected to enhance penetration of the antibiotic into bacterial cells.
Table 4:
Minimum inhibitory concentration (MIC, in µM) of 19 and control antibiotics against Gram-negative bacterial pathogens in the presence of a subinhibitory concentration of colistin (COL).
| Test Agent | Bacterial Strain | |||||||
|---|---|---|---|---|---|---|---|---|
| A. baumannii BAA-1605 | E. cloacae BAA-1134 | K. pneumoniae ATCC BAA-1705 | P. aeruginosa ATCC 15442 | E. coli BW25113 | E. coli JW25113 (ΔtolC) | |||
| (−COL1) | (+COL) | (−COL) | (+COL) | |||||
| 19 | 16 | 4 | >64 | >64 | >64 | >64 | >64 | >64 |
| Erythromycin | 4 | 1 | 16 | 4 | 64 | 1 | 2 | 0.50 |
| Linezolid | >64 | 16 | >64 | >64 | >64 | >64 | 16 | 16 |
No colistin added to the media
We next examined if the presence of efflux pumps may be responsible for the lack of antibacterial activity observed for the aryl isonitrile compounds against Gram-negative bacteria (Table 4). These pathogens express different efflux pumps that confer resistance to numerous antibiotics including the AdeIJK efflux pump in A. baumannii, AcrAB-TolC efflux pump (present in E. coli E. cloacae, and K. pneumoniae) and its homologue MexAB-OprM (expressed by P. aeruginosa).26, 27 We evaluated the antibacterial activity of 19 against an Escherichia coli strain (JW25113) deficient in the AcrAB-TolC efflux pump responsible for excluding many xenobiotics from accumulating inside E. coli cells.25 No discernible improvement in antibacterial activity of 19 was observed against the mutant E. coli strain relative to the wild-type strain (BW25113). In contrast, there was noticeable improvement in the antibacterial activity of erythromycin (MIC = 2 µM) and linezolid (MIC = 16 µM), two substrates of the AcrAB-TolC efflux pump.26, 28 This suggests that the presence of efflux pumps alone on the surface of the OM on Gram-negative bacteria may not be responsible for conferring resistance to the aryl isonitrile compounds. We next investigated if the combination of the outer membrane and efflux pumps may impede the antibacterial activity of the aryl isonitrile compounds. The compound and control antibiotics were incubated with a subinhibitory concentration of colistin against E. coli BW25113 and E. coli JW25113. No improvement in the antibacterial activity of 19 (MIC > 64 µM) was observed in the presence of colistin against E. coli JW25113. Similarly, no improvement in the MIC of linezolid was observed against E. coli JW25113 in the absence and presence of colistin, indicating the lack of antibacterial activity of linezolid observed against E. coli is due primarily to efflux. The MIC of erythromycin against E. coli JW25113, in contrast, improved one-fold in the presence of colistin indicating both the OM and efflux pumps interfere with the effect of this antibiotic against Gram-negative bacteria, in agreement with previous reports.26, 29 Due to the lack of antibacterial activity observed against VRE and Gram-negative bacterial pathogens, we moved to evaluate the aryl isonitrile compounds antibacterial activity in vivo against MRSA.
In silico pharmacokinetic evaluation of compound 19
MRSA is a source of infection for both superficial skin lesions and complicated systemic infections. To determine a suitable animal model of MRSA infection to evaluate the aryl isonitrile compounds, the pharmacokinetic profile of compound 19 and linezolid were simulated utilizing a dose of 600 mg (as is commonly administered for linezolid in adult human patients). As shown in Table 5, the results indicate that compound 19 would not be suitable for oral administration for the treatment of systemic MRSA infections as it is not expected to attain a concentration in plasma/blood sufficient to inhibit bacterial growth. The maximum plasma concentration (Cmax) predicted for compound 19 is 0.17 µg/mL (0.73 µM), whereas the MIC against MRSA ranges from 1 to 8 µM. Intravenous administration of compound 19 is predicted to result in a slow rate of clearance (12.58 mL/min-kg) correlating with a long half-life (22.90 hours) which could alleviate the need for multiple daily dosing. The high values obtained for the volume of distribution at steady-state for 19 (24.94 L/kg compared to 1.11 L/kg for linezolid) indicate this compound is expected to distribute extensively into tissues. This may be due to the high degree of lipophilicity (cLogP = 3.88) present with the compound. These pharmacokinetic simulations suggest that though intravenous administration of 19 may be possible for treatment of systemic MRSA infections, the extensive distribution of the compound into tissues would necessitate a higher dose be administered/continuous infusion of compound (to ensure the concentration remained above the MIC to inhibit MRSA growth). The values obtained for linezolid via the in silico pharmacokinetic simulation overall were in close proximity to experimental values obtained by Stalker, et al. from healthy human subjects administered a single 625 mg dose of linezolid either orally or intravenously.30 However, the simulation underestimated the Cmax for linezolid and overestimated the half-life and volume of distribution compared to the experimental values. Based upon the in silico pharmacokinetic simulation, 19 appears most suitable for evaluation topically to treat localized/uncomplicated MRSA skin infections.
Table 5:
In silico pharmacokinetic analysis for compound 19 and linezolid (simulated at 600 mg).
| Oral | Intravenous | |||||
|---|---|---|---|---|---|---|
| 19 (Simulated) | Linezolid (Simulated) | Linezolid (Experimental)1 | 19 (Simulated) | Linezolid (Simulated) | Linezolid (Experimental) | |
| Cmax2 (µg/mL) | 0.17 | 5.33 | 12.7 | - | - | 13.4 |
| tmax3 (hours) | 3 | 2.75 | 1.33 | - | - | 0.5 |
| Fraction absorbed (FAlast) | 0.80 | 0.92 | 1.03 | - | - | - |
| CL4 (mL/min-kg) | - | - | - | 12.58 | 1.09 | 1.749 |
| t1/25 (hours) | - | - | - | 22.90 | 12.31 | 4.40 |
| MRT6 (hours) | - | - | - | 12.82 | 17.04 | - |
| Vd7 (L/kg) | - | - | - | 24.94 | 1.11 | 0.5810 |
| Vss8 (L/kg) | - | - | - | 9.68 | 1.12 | - |
(adapted from Stalker DJ et al. J Antimicrob Chemother 2003, 51: 1239–46 (Table 2, 625 mg dose))
Cmax = maximum concentration of drug in plasma/blood
tmax = time required to reach Cmax
CL = rate of clearance
t1/2 = half-life
MRT = mean residence time
Vd = volume of distribution
Vss = volume of distribution at steady-state
Clearance for linezolid (experimental) obtained by dividing the mean clearance value by the mean weight of patients
Volume of distribution for linezolid (experimental) obtained by dividing the mean Vd by the mean weight of patients
The active aryl isonitrile compounds are safe to mammalian keratinocytes
S. aureus is a leading source of skin and soft tissue infections, both uncomplicated and invasive, globally.31–34 As such, based upon the in silico pharmacokinetic data, we decided to investigate the antibacterial activity of the second-generation aryl isonitriles as topical antibacterial agents in a MRSA murine skin infection mouse model. Before exposing mice to the compounds, we evaluated the safety profile of 7, 18, and 19 against keratinocytes (HaCaT). All three compounds were safe up to the maximum tested concentration of 128 µM (more than 90% of HaCaT cells remained viable after 24 hours of exposure to the compounds) (Figure 5).
Figure 5. Toxicity analysis of aryl isonitrile compounds against human keratinocytes (HaCaT).
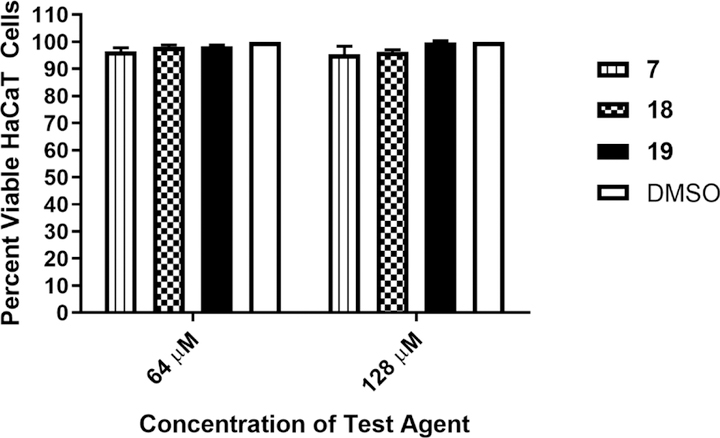
Percent viable mammalian cells (measured as average absorbance ratio (test agent relative to DMSO)) for cytotoxicity analysis of compounds 7, 18, and 19 (tested in triplicate) at 64 and 128 µM against HaCaT cells over a 24 hour period using the MTS 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay. Dimethyl sulfoxide (DMSO) was used as a negative control to determine a baseline measurement for the cytotoxic impact of each compound. The absorbance values represent an average of a minimum of three samples analyzed for each compound. Error bars represent standard deviation values. A one-way ANOVA, with post hoc Dunnet’s multiple comparisons test, determined statistical difference between the values obtained for each compound and DMSO (P < 0.05).
Compound 19 significantly reduces the burden of MRSA in a skin infection mouse model
After confirming the aryl isonitrile compounds are safe to keratinocytes, we moved to investigate the antibacterial activity of the newly synthesized analogues in a mouse model of MRSA skin infection. Given the safety profile against keratinocytes was identical for all three aryl isonitrile compounds, 19 was selected for this experiment. Given 18 and 19 are structurally similar, the more potent compound in vitro against MRSA USA300 was selected for evaluation. After the formation of an abscess at the site of infection, mice were treated twice daily for five days. As the skin wounds were uncomplicated and localized, treatment was administered topically directly onto the surface of the abscesses. Mice were euthanized 12 hours after the last dose was administered and the abscesses were harvested to enumerate MRSA CFU. As presented in Figure 6, 19 (79.02% reduction) was as effective as the control antibiotic fusidic acid (77.78% reduction) in reducing the burden of MRSA in the wounds of infected mice after five days of treatment. Importantly, no excess inflammation or toxicity was observed in wounds after exposure to the compounds or fusidic acid.
Figure 6. Reduction of MRSA USA300 in infected lesions of mice.
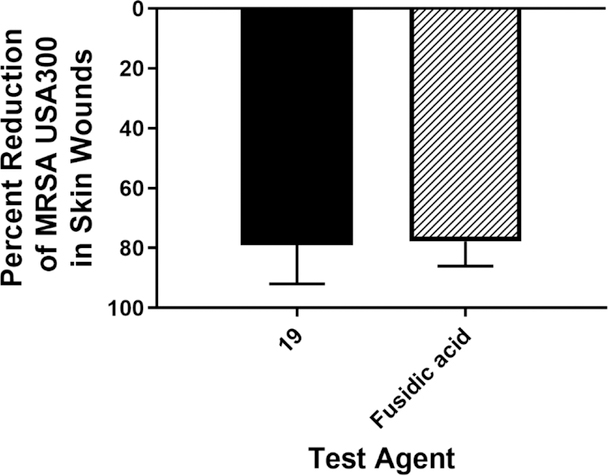
Average percent reduction of MRSA CFU/mL in murine skin lesions after treatment with 19 or fusidic acid. A one-way ANOVA with post-hoc Dunnet’s multiple comparisons found no statistical difference between mice treated with fusidic acid and mice treated with compound 19.
DISCUSSION
Antibiotics have played a critical role in resolving bacterial infections throughout the world since their initial discovery. However, bacteria have proven to be shrewd microorganisms capable of acquiring resistance to multiple antibiotics utilizing an array of clever mechanisms (e.g. through modification of the drug target, degradation of the antibiotic, and efflux of the antibiotic). As such there is a continuous need to identify and develop novel antibacterial agents capable of treating drug-resistant bacterial infections. MRSA remains a significant cause of superficial and invasive antibiotic-resistant infections globally. Though several antibacterial agents effective against MRSA are currently in clinical trials, the remarkable ability of this organism to develop resistance to different antibiotics necessitates new antibacterial agents, particularly from unexploited scaffolds, be identified and developed.
We previously synthesized and evaluated over 40 aryl isonitrile compounds for antibacterial activity against MRSA.10 The first-generation compounds inhibited MRSA growth at concentrations ranging from 2 – 64 µM10 but none of the compounds possessed a suitable physicochemical profile to evaluate their effectiveness in an animal model of MRSA infection. The present study aimed to expand the library of aryl isonitrile compounds to better understand these limitations and further characterize the compounds’ structure-antibacterial activity relationship. To this end, 20 second-generation aryl isonitrile compounds consisting of stilbene bis-isonitriles, stilbene aryl isonitriles with medicinally-relevant molecules and heterocyclic moieties, and those with a saturated linker were synthesized and evaluated for antibacterial activity.
The newly synthesized analogues were initially evaluated against a clinical isolate of MRSA USA400. From the initial screening, inclusion of an additional isonitrile moiety, the stilbene core, and the non-isonitrile-bearing aromatic ring were all found to be key for anti-MRSA activity. Four compounds (7, 13, 18, and 19) inhibited growth of MRSA at a concentration ranging from 2 – 8 µM, similar to the lead compound (2) from the first-generation aryl isonitriles. Bis-isonitrile 19 was more potent than the first-generation lead 2 against MRSA NRS384 (USA300) and MRSA NRS119 (LRSA) which further confirmed the importance of the isonitrile functionality.
Importantly, three compounds retained potent antibacterial activity against MRSA isolates exhibiting resistance to vancomycin and linezolid, antibiotics frequently used as agents of last resort for treatment of MRSA infections.35,36 Though not prevalent clinically, the identification of S. aureus strains exhibiting resistance to vancomycin and linezolid, two key therapeutic options for treatment of MRSA infections, is a noteworthy problem. The lack of cross-resistance observed between the aryl isonitrile compounds and vancomycin and linezolid is important therapeutically as it provides a potential alternative source of treatment against linezolid-resistant and vancomycin-resistant S. aureus infections.
Previously, the first-generation aryl isonitrile analogues were only evaluated against clinical isolates of S. aureus. We were curious to investigate the spectrum of antibacterial activity of the second-generation aryl isonitrile compounds. Thus, three of the most potent analogues against MRSA (7, 18, and 19) were evaluated against a panel of clinically-relevant pathogens, including members of the ESKAPE group, S. epidermidis, and S. pneumoniae. The compounds exhibited potent antibacterial activity against the Gram-positive pathogens S. epidermidis and S. pneumoniae. Interestingly, the compounds were inactive against vancomycin-resistant E. faecium. The lack of antibacterial activity against VRE (a Gram-positive pathogen similar to MRSA) is not entirely surprising given certain antibiotics that are active against S. aureus (such as ampicillin and vancomycin) are inactive against vancomycin-resistant E. faecium.37 The aryl isonitrile compounds also lacked antibacterial activity against five Gram-negative bacterial pathogens tested (A. baumannii, K. pneumoniae, E. cloacae, E. coli, and P. aeruginosa). The lack of antibacterial activity against Gram-negative pathogens is not surprising given most small molecules are unable to permeate the complex outer membrane (OM) present in Gram-negative bacteria. Those that are capable of diffusing across the OM may be susceptible to expulsion (decreasing the intracellular concentration of compound/drug) by a number of different efflux pumps expressed by Gram-negative bacteria, including the AcrAB-TolC pump present in E. coli.25 Thus we evaluated the antibacterial activity of the aryl isonitrile compounds after permeabilizing the OM (using a subinhibitory concentration of colistin) and against an E. coli strain deficient in the AcrAB-TolC efflux pump. Interestingly, there was improvement in the MIC for compound 19 observed in the presence of colistin against A. baumannii and E. cloacae but not against E. coli, K. pneumoniae, and P. aeruginosa. Against a mutant E. coli strain deficient in AcrAB-TolC, no improvement in the MIC of 19 was observed indicating that this compound may not be a substrate for efflux. This suggests that though the outer membrane may contribute to the lack of antibacterial activity for the aryl isonitrile compounds observed against specific Gram-negative bacterial pathogens, additional resistance mechanism(s) may play a role as well. Alternatively, the compounds may have a weaker affinity for the molecular target in Gram-negative bacteria as opposed to against MRSA, S. epidermidis, and S. pneumoniae. Though the molecular target of the aryl isonitrile compounds is currently unknown, this question is being intensely investigated using both genetic and phenotypic approaches. Identification of the molecular target may provide key insight into the difference in potency observed for the aryl isonitrile compounds against S. aureus relative to other bacterial pathogens.
The aryl isonitrile compounds possessed good in vitro activity against drug-resistant S. aureus isolates and were safe to mammalian cells (non-toxic to human keratinocytes at 128 µM, more than 30-fold higher than the concentration where they inhibited MRSA growth in vitro). However, an in silico pharmacokinetic evaluation of 19 revealed this compound may not be effective in treatment of systemic MRSA infections (as the maximum predicted concentration of the compound in plasma was lower than the MIC of the compound against MRSA). As a means to address this problem while still retaining antibacterial activity, the alkene core of the current lead 19 is undergoing further structural diversification with molecular entities we hypothesize will help improve the physicochemical profile of the lead compound.
Based upon the in vitro antibacterial activity assay results and in silico pharmacokinetic simulation, we proceeded to evaluate the effectiveness of 19 as a topical antibacterial to treat MRSA skin infection. MRSA remains a major source of skin and soft tissue infections (SSTIs) throughout the world.31–34, 38, 39 Treatment of SSTIs can be challenging given the emergence of resistance to several antibiotics frequently used in the clinic. One example is the antibiotic fusidic acid, which is prescribed for use topically in Europe for the treatment of SSTIs. Extensive use of fusidic acid has resulted in the emergence of resistant isolates40 that necessitates new therapeutic agents to treat uncomplicated, localized S. aureus SSTIs. In the United States of America, MRSA USA300 is the predominant strain linked to community-acquired SSTIs.41 As such, we moved to evaluate the efficacy of compound 19 administered topically in a MRSA USA300 skin wound mouse model. Compound 19 significantly reduced the burden of MRSA USA300 in infected abscesses by over 70% (after only five days of treatment) similar to fusidicacid.42
CONCLUSIONS
In conclusion, the present study identified four new aryl isonitrile compounds bearing potent antibacterial activity against MRSA in vitro. The analogues were inactive against important Gram-negative bacterial pathogens, and their activity appeared to be negatively impacted by the presence of the outer membrane. The newly synthesized analogues were safe to keratinocytes at concentrations up to 128 µM and significantly reduced the burden of MRSA in infected wounds in a mouse model of MRSA skin infection. However, in silico pharmacokinetic simulation revealed compound 19 would not effectively permeate across the GI tract and may extensively distribute into tissues thus precluding its evaluation in a systemic animal model of MRSA infection. Addressing these limitations and deducing the molecular target are necessary questions to resolve to further develop aryl isonitrile compounds as a novel class of antibacterial agents to treat drug-resistant S. aureus infections.
EXPERIMENTAL SECTION
Biological Methods
Bacterial strains and reagents
Relevant information pertaining to all bacterial isolates used in this study are presented in Supplementary Table 1. Clinical isolates of S. aureus and S. epidermidis were obtained through the Network of Antimicrobial Resistance in Staphylococcus aureus (NARSA) program. Isolates of S. pneumoniae, E. faecium, A. baumannii, K. pneumoniae, E. cloacae, and P. aeruginosa were obtained from the American Type Culture Collection (ATCC). E. coli strains BW25113 and JW25113 were obtained from The Coli Genetic Stock Center (CGSC), Yale University. Antibiotics were purchased commercially and dissolved in DMSO (for linezolid), ethanol (for erythromycin), or sterile deionized water (for colistin and vancomycin). Stock 10 mM solutions were prepared for all antibiotics. Brain heart infusion broth (BHI), Tryptic soy broth (TSB), Tryptic soy agar (TSA), phosphate-buffered saline (PBS), Dulbeco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and 96-well plates were all purchased from commercial vendors.
Evaluation of antibacterial activity of compounds and control antibiotics
The minimum inhibitory concentration of the aryl isonitrile analogues and control antibiotics was determined using the broth microdilution assay following the Clinical and Laboratory Standards Institute guidelines, with the following modifications.43 Bacteria were cultured either in TSB or BHI (for E. faecium) and exposed to either compounds or control antibiotics, in triplicate, in 96-well plates. For permeabilization of the outer membrane, Gram-negative bacterial pathogens were exposed to a subinhibitory concentration of colistin equivalent to either ¼ × MIC (for A. baumannii, E. cloacae, E. coli, and P. aeruginosa) or ½ × MIC (for K. pneumoniae). Plates were incubated aerobically at 37 °C for 18 – 24 hours before the MIC values were recorded. S. pneumoniae was incubated with compounds at 37 °C + 5% CO2. The MICs reported represent the lowest concentration of each compound/drug necessary to inhibit visual bacterial growth.
In silico pharmacokinetic evaluation of 19
Compound 19’s pharmacokinetic profile was examined in silico, using chemPK version 2.0 (Cyprotex, Inc.) simulating a dose of 600 mg administered both orally and intravenously.
Toxicity assessment of aryl isonitrile analogues against human keratinocytes
Compounds 7, 18, and 19 were assayed (at concentrations of 16, 32, 64, and 128 µM) against a human keratinocyte (HaCaT) cell line (AddexBio, San Diego, CA, USA) to determine the potential toxic effect to mammalian skin cells in vitro, as previously described.44–48 In brief, cells were cultured in DMEM supplemented with 10% FBS at 37 °C with CO2 (5%). Control cells received DMSO alone at a concentration equal to that in drug-treated cell samples. The cells were incubated with the compounds (in triplicate) in a 96-well plate at 37 °C with CO2 (5%) for 24 hours. The assay reagent MTS 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) (Promega, Madison, WI, USA) was subsequently added and the plate was incubated for four hours. Absorbance readings (at OD490) were taken using a kinetic microplate reader (Molecular Devices, Sunnyvale, CA, USA). The quantity of viable cells after treatment with each compound was expressed as a percentage of the viability of DMSO-treated control cells (average of triplicate wells ± standard deviation). The toxicity data was analyzed via a one-way ANOVA, with post hoc Dunnet’s multiple comparisons test (P < 0.05), utilizing GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA).
Evaluation of 19 in a murine model of MRSA skin infection
The mice study was conducted under the guidelines of the Purdue University Animal Care and Use Committee (PACUC) and carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The method used for this study was similar to one described in previous reports, with slight modifications.44, 49–53 Three groups (n = 5) of eight-week old female Balb/c mice (obtained from Envigo, Indianapolis, IN, USA) received an intradermal injection (40 μL) containing 1.32 × 109 CFU/mL MRSA USA300. Following formation of an abscess at the injection site (~48 hours post-infection), topical treatment was initiated subsequently with each group of mice receiving a 2% suspension (formulated in petroleum jelly) of fusidic acid or 19. One group of mice received the vehicle alone (negative control). Each group of mice was housed separately in a ventilated cage with appropriate bedding, food, and water. Mice were checked at least four times daily during infection and treatment to ensure no adverse reactions were observed. Mice were treated twice daily for five days, before they were humanely euthanized via CO2 asphyxiation 12 hours after the last dose was administered. Wounds were aseptically extracted and subsequently homogenized in PBS (2 mL). The homogenized tissue was then serially diluted in PBS before plating onto mannitol salt agar plates. Plates were incubated for at least 16 hours at 37 °C before viable CFU were counted and MRSA reduction in the skin wound (relative to the negative control) post-treatment was determined for each group.
Supplementary Material
Figure 4. Time-kill analysis of aryl isonitrile compounds 7, 18, 19, and linezolid (all at 4 × MIC) against methicillin-resistant Staphylococcus aureus (MRSA NRS123) over a 24-hour incubation period at 37°C.
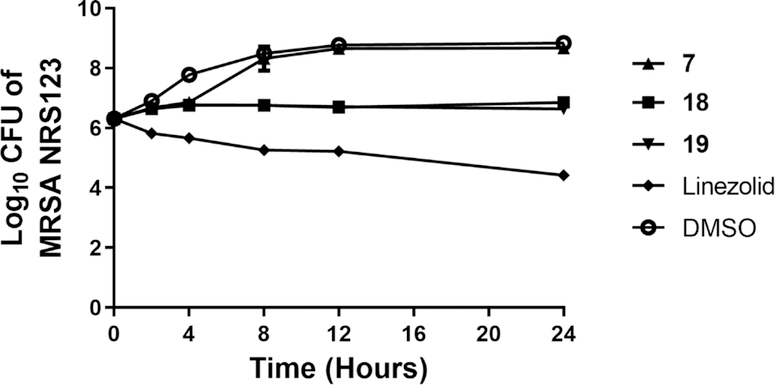
DMSO served as a negative control. The error bars represent standard deviation values obtained from triplicate samples used for each compound/antibiotic studied.
Acknowledgements:
The authors would like to thank the Network of Antimicrobial Resistance in Staphylococcus aureus (NARSA) program supported under NIAID/NIH Contract # HHSN272200700055C for providing the MRSA and VRSA strains used in this study. MD thanks financial support from NIH (R35 GM128570) and unrestricted grants from Eli Lilly and Amgen. The NIH P30 CA023168 is acknowledged for supporting shared NMR resources to Purdue Center for Cancer Research. The authors would like to thank Dr. Waleed Younis (South Valley University, Qena, Egypt) and Nader S. Abutaleb (Purdue University) for assistance with biological experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Blaskovich MAT; Hansford KA; Butler MS; Jia Z; Mark AE; Cooper MA Developments in Glycopeptide Antibiotics. ACS Infect Dis 2018, 4, 715–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sievert DM; Ricks P; Edwards JR; Schneider A; Patel J; Srinivasan A; Kallen A; Limbago B; Fridkin S; National Healthcare Safety Network, T.; Participating, N. F. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 2013, 34, 1–14. [DOI] [PubMed] [Google Scholar]
- 3.Mohammad H; Thangamani S; Seleem MN Antimicrobial peptides and peptidomimetics - potent therapeutic allies for staphylococcal infections. Curr Pharm Des 2015, 21, 2073–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC). Antibiotic Resistance Threats in the United States, 2013; 2013; pp 1–114.
- 5.de Dios Caballero J; Pastor MD; Vindel A; Maiz L; Yague G; Salvador C; Cobo M; Morosini MI; del Campo R; Canton R; Group GS Emergence of cfr-Mediated Linezolid Resistance in a Methicillin-Resistant Staphylococcus aureus Epidemic Clone Isolated from Patients with Cystic Fibrosis. Antimicrob Agents Chemother 2015, 60, 1878–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu B; Kelesidis T; Tsiodras S; Hindler J; Humphries RM The emerging problem of linezolid-resistant Staphylococcus. J Antimicrob Chemother 2013, 68, 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith TL; Pearson ML; Wilcox KR; Cruz C; Lancaster MV; Robinson-Dunn B; Tenover FC; Zervos MJ; Band JD; White E; Jarvis WR Emergence of vancomycin resistance in Staphylococcus aureus. Glycopeptide-Intermediate Staphylococcus aureus Working Group. N Engl J Med 1999, 340, 493–501. [DOI] [PubMed] [Google Scholar]
- 8.Srinivasan A; Dick JD; Perl TM Vancomycin resistance in staphylococci. Clin Microbiol Rev 2002, 15, 430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan A; Wilson B; Gould IM Current and future treatment options for community-associated MRSA infection. Expert Opin Pharmacother 2018, 19, 457–470. [DOI] [PubMed] [Google Scholar]
- 10.Davis DC; Mohammad H; Kyei-Baffour K; Younis W; Creemer CN; Seleem MN; Dai MJ Discovery and characterization of aryl isonitriles as a new class of compounds versus methicillin- and vancomycin-resistant Staphylococcus aureus. European Journal of Medicinal Chemistry 2015, 101, 384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown DG; Lister T; May-Dracka TL New natural products as new leads for antibacterial drug discovery. Bioorg Med Chem Lett 2014, 24, 413–8. [DOI] [PubMed] [Google Scholar]
- 12.Mo S; Krunic A; Chlipala G; Orjala J Antimicrobial ambiguine isonitriles from the cyanobacterium Fischerella ambigua. J Nat Prod 2009, 72, 894–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwarz O, Brun R, Bats JW, Schmalz H Synthesis and biological evaluation of new antimalarial isonitriles related to marine diterpenoids. Tetrahedron Letters 2002, 1009–1013.
- 14.Wright AD; Wang H; Gurrath M; Konig GM; Kocak G; Neumann G; Loria P; Foley M; Tilley L Inhibition of heme detoxification processes underlies the antimalarial activity of terpene isonitrile compounds from marine sponges. J Med Chem 2001, 44, 873–85. [DOI] [PubMed] [Google Scholar]
- 15.Kirk KL Fluorine in medicinal chemistry: Recent therapeutic applications of fluorinated small molecules. Journal of Fluorine Chemistry 2006, 127, 1013–1029. [Google Scholar]
- 16.Wilcken R; Zimmermann MO; Lange A; Joerger AC; Boeckler FM Principles and Applications of Halogen Bonding in Medicinal Chemistry and Chemical Biology. Journal of Medicinal Chemistry 2013, 56, 1363–1388. [DOI] [PubMed] [Google Scholar]
- 17.Yale HL The Trifluoromethyl Group in Medical Chemistry. Journal of Medicinal and Pharmaceutical Chemistry 1959, 1, 121–133. [DOI] [PubMed] [Google Scholar]
- 18.Arbusow BA Michaelis-Arbusow- und Perkow-Reaktionen. In Pure and Applied Chemistry, 1964; Vol. 9, p 307. [Google Scholar]
- 19.Weber WP; Gokel GW An improved procedure for the Hofmann carbylamine synthesis of isonitriles. Tetrahedron Letters 1972, 13, 1637–1640. [Google Scholar]
- 20.Zhang B; Studer A 2-Trifluoromethylated Indoles via Radical Trifluoromethylation of Isonitriles. Organic Letters 2014, 16, 1216–1219. [DOI] [PubMed] [Google Scholar]
- 21.Banerjee R; Hks K; Banerjee M Medicinal significance of furan derivatives: A Review 2018.
- 22.Kaur K; Jain M; Reddy RP; Jain R Quinolines and structurally related heterocycles as antimalarials. European Journal of Medicinal Chemistry 2010, 45, 3245–3264. [DOI] [PubMed] [Google Scholar]
- 23.Pendleton JN; Gorman SP; Gilmore BF Clinical relevance of the ESKAPE pathogens. Expert Rev Anti Infect Ther 2013, 11, 297–308. [DOI] [PubMed] [Google Scholar]
- 24.Santajit S; Indrawattana N Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. Biomed Res Int 2016, 2016, 2475067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munita JM; Arias CA Mechanisms of Antibiotic Resistance. Microbiol Spectr 2016, 4. [DOI] [PMC free article] [PubMed]
- 26.Zgurskaya HI; Lopez CA; Gnanakaran S Permeability Barrier of Gram-Negative Cell Envelopes and Approaches To Bypass It. ACS Infect Dis 2015, 1, 512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Padilla E; Llobet E; Domenech-Sanchez A; Martinez-Martinez L; Bengoechea JA; Alberti S Klebsiella pneumoniae AcrAB efflux pump contributes to antimicrobial resistance and virulence. Antimicrob Agents Chemother 2010, 54, 177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schumacher A; Trittler R; Bohnert JA; Kummerer K; Pages JM; Kern WV Intracellular accumulation of linezolid in Escherichia coli, Citrobacter freundii and Enterobacter aerogenes: role of enhanced efflux pump activity and inactivation. J Antimicrob Chemother 2007, 59, 1261–4. [DOI] [PubMed] [Google Scholar]
- 29.Krishnamoorthy G; Wolloscheck D; Weeks JW; Croft C; Rybenkov VV; Zgurskaya HI Breaking the Permeability Barrier of Escherichia coli by Controlled Hyperporination of the Outer Membrane. Antimicrob Agents Chemother 2016, 60, 7372–7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stalker DJ; Jungbluth GL; Hopkins NK; Batts DH Pharmacokinetics and tolerance of single- and multiple-dose oral or intravenous linezolid, an oxazolidinone antibiotic, in healthy volunteers. J Antimicrob Chemother 2003, 51, 1239–46 [DOI] [PubMed] [Google Scholar]
- 31.European Centre for Disease Prevention and Control (ECDC). Surveillance of antimicrobial resistance in Europe 2016. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) Stockholm, 2017; pp 1–88. [Google Scholar]
- 32.Edelsberg J; Weycker D; Barron R; Li X; Wu H; Oster G; Badre S; Langeberg WJ; Weber DJ Prevalence of antibiotic resistance in US hospitals. Diagn Microbiol Infect Dis 2014, 78, 255–62. [DOI] [PubMed] [Google Scholar]
- 33.Moet GJ; Jones RN; Biedenbach DJ; Stilwell MG; Fritsche TR Contemporary causes of skin and soft tissue infections in North America, Latin America, and Europe: report from the SENTRY Antimicrobial Surveillance Program (1998–2004). Diagn Microbiol Infect Dis 2007, 57, 7–13. [DOI] [PubMed] [Google Scholar]
- 34.Russo A; Concia E; Cristini F; De Rosa FG; Esposito S; Menichetti F; Petrosillo N; Tumbarello M; Venditti M; Viale P; Viscoli C; Bassetti M Current and future trends in antibiotic therapy of acute bacterial skin and skin-structure infections. Clin Microbiol Infect 2016, 22 Suppl 2, S27–36. [DOI] [PubMed] [Google Scholar]
- 35.Gardete S; Tomasz A Mechanisms of vancomycin resistance in Staphylococcus aureus. J Clin Invest 2014, 124, 2836–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodvold KA; McConeghy KW Methicillin-resistant Staphylococcus aureus therapy: past, present, and future. Clin Infect Dis 2014, 58 Suppl 1, S20–7. [DOI] [PubMed] [Google Scholar]
- 37.Kristich CJ; Rice LB; Arias CA Enterococcal Infection-Treatment and Antibiotic Resistance. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection, Gilmore MS; Clewell DB; Ike Y; Shankar N, Eds. Boston, 2014. [PubMed] [Google Scholar]
- 38.Grundmann H; Schouls LM; Aanensen DM; Pluister GN; Tami A; Chlebowicz M; Glasner C; Sabat AJ; Weist K; Heuer O; Friedrich AW; Markers E. S. G. o. M. E.; European Staphylococcal Reference Laboratory Working, G. The dynamic changes of dominant clones of Staphylococcus aureus causing bloodstream infections in the European region: results of a second structured survey. Euro Surveill 2014, 19. [DOI] [PubMed]
- 39.Tian L; Sun Z; Zhang Z Antimicrobial resistance of pathogens causing nosocomial bloodstream infection in Hubei Province, China, from 2014 to 2016: a multicenter retrospective study. BMC Public Health 2018, 18, 1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castanheira M; Watters AA; Mendes RE; Farrell DJ; Jones RN Occurrence and molecular characterization of fusidic acid resistance mechanisms among Staphylococcus spp. from European countries (2008). J Antimicrob Chemother 2010, 65, 1353–8. [DOI] [PubMed] [Google Scholar]
- 41.Johnson JK; Khoie T; Shurland S; Kreisel K; Stine OC; Roghmann MC Skin and soft tissue infections caused by methicillin-resistant Staphylococcus aureus USA300 clone. Emerg Infect Dis 2007, 13, 1195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vingsbo Lundberg C; Frimodt-Moller N Efficacy of topical and systemic antibiotic treatment of meticillin-resistant Staphylococcus aureus in a murine superficial skin wound infection model. Int J Antimicrob Agents 2013, 42, 272–5. [DOI] [PubMed] [Google Scholar]
- 43.Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically—Ninth Edition: Approved Standard M07-A9 In Wayne, PA, 2012. [Google Scholar]
- 44.Mohammad H; Cushman M; Seleem MN Antibacterial Evaluation of Synthetic Thiazole Compounds In Vitro and In Vivo in a Methicillin-Resistant Staphylococcus aureus (MRSA) Skin Infection Mouse Model. PLoS One 2015, 10, e0142321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kotb A; Abutaleb NS; Seleem MA; Hagras M; Mohammad H; Bayoumi A; Ghiaty A; Seleem MN; Mayhoub AS Phenylthiazoles with tert-Butyl side chain: Metabolically stable with anti-biofilm activity. Eur J Med Chem 2018, 151, 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hagras M; Abutaleb NS; Ali AO; Abdel-Aleem JA; Elsebaei MM; Seleem MN; Mayhoub AS Naphthylthiazoles: Targeting Multidrug-Resistant and Intracellular Staphylococcus aureus with Biofilm Disruption Activity. ACS Infect Dis 2018. [DOI] [PubMed]
- 47.Elsebaei MM; Mohammad H; Abouf M; Abutaleb NS; Hegazy YA; Ghiaty A; Chen L; Zhang J; Malwal SR; Oldfield E; Seleem MN; Mayhoub AS Alkynyl-containing phenylthiazoles: Systemically active antibacterial agents effective against methicillin-resistant Staphylococcus aureus (MRSA). Eur J Med Chem 2018, 148, 195–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.ElAwamy M; Mohammad H; Hussien A; Abutaleb NS; Hagras M; Serya RAT; Taher AT; Abouzid KA; Seleem MN; Mayhoub AS Alkoxyphenylthiazoles with broad-spectrum activity against multidrug-resistant gram-positive bacterial pathogens. Eur J Med Chem 2018, 152, 318–328. [DOI] [PubMed] [Google Scholar]
- 49.Thangamani S; Mohammad H; Abushahba MF; Sobreira TJ; Seleem MN Repurposing auranofin for the treatment of cutaneous staphylococcal infections. Int J Antimicrob Agents 2016, 47, 195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thangamani S; Younis W; Seleem MN Repurposing celecoxib as a topical antimicrobial agent. Front Microbiol 2015, 6, 750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thangamani S; Younis W; Seleem MN Repurposing ebselen for treatment of multidrug-resistant staphylococcal infections. Sci Rep 2015, 5, 11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thangamani S; Mohammad H; Abushahba MF; Hamed MI; Sobreira TJ; Hedrick VE; Paul LN; Seleem MN Exploring simvastatin, an antihyperlipidemic drug, as a potential topical antibacterial agent. Sci Rep 2015, 5, 16407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mohamed MF; Seleem MN Efficacy of short novel antimicrobial and anti-inflammatory peptides in a mouse model of methicillin-resistant Staphylococcus aureus (MRSA) skin infection. Drug Des Devel Ther 2014, 8, 1979–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


