Abstract
Background
Acute pancreatitis is an inflammatory disease of the pancreas associated with high patient morbidity. Lycium barbarum polysaccharide (LBP), a traditional Chinese medicine with an active component extracted from the goji berry, has previously been reported to have anti-inflammatory effects. This study aimed to investigate the effects of LBP in a mouse model of cerulein-induced acute pancreatitis.
Material/Methods
Acute pancreatitis was induced by intraperitoneal injection of cerulein in C57BL/6 wild-type mice or nuclear factor erythroid-2-related factor 2 (NRF2) gene knockout mice. LBP or normal saline was administrated by gavage once daily for one week before the induction of acute pancreatitis. At 12 hours after the first intraperitoneal injection of cerulein, the mice were euthanized. Blood and pancreatic tissue were sampled for histology and for the measurement of pro-inflammatory cytokines, serum amylase, and lipase.
Results
In the untreated mouse model of cerulein-induced acute pancreatitis, amylase and lipase levels were increased, and these levels were reduced by LBP treatment when compared with vehicle treatment. In the untreated mouse model, histology of the pancreas showed edema and inflammation, which were reduced in the LBP-treated mice. In the untreated mouse model, increased levels of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) were found, which were reduced in the LBP-treated mice. NRF2 gene knockout mice with cerulein-induced acute pancreatitis showed reduced anti-inflammatory effects of LBP treatment. LBP increased the expression of NRF2 and heme oxygenase-1 (HO-1).
Conclusions
In a mouse model of cerulein-induced acute pancreatitis, LBP reduced inflammation by upregulating NRF2 and HO-1.
MeSH Keywords: Inflammation, Nuclear Respiratory Factor 1, Pancreatitis
Background
Acute pancreatitis is an inflammatory disease of the pancreas associated with high patient morbidity and may result in systemic effects that include sepsis, acute respiratory distress syndrome, and shock which are life-threatening conditions [1–3]. The pathogenesis of acute pancreatitis is complex, but inflammation and tissue necrosis occur with the release of pancreatic enzymes, including amylase and lipase [4,5]. Inflammation of the pancreas is also associated with the release of proinflammatory cytokines, and reactive oxygen species (ROS), resulting in further pancreatic inflammation and injury [6]. Previously published studies have shown that inflammation and oxidative stress have important roles in the pathogenesis of acute pancreatitis [7,8]. Inhibition of ROS has been shown to protect pancreatic cells in an animal model of the lipopolysaccharide-induced chronic pancreatitis through modulation of the signaling pathways involved in inflammation [7]. In patients with idiopathic recurrent acute pancreatitis, inhibition of oxidative stress has been proposed as a potential approach to clinical treatment [8].
Nuclear factor erythroid-2-related factor 2 (Nrf2) plays an important role in antioxidative defense mechanisms [9]. Nrf2, encoded by the NRF2 gene, belongs to the leucine zipper family and is a transcription factor that controls the expression of antioxidants. In a mouse model of alcohol-induced pancreatitis, pancreatic injury and inflammation were shown to be significantly worse when compared with wild-type mice [10]. Increased expression of NRF2 has previously been shown to protect mice with cerulein-induced acute pancreatitis by reducing tissue damage and inflammation [11,12].
Lycium barbarum polysaccharide (LBP), a traditional Chinese medicine with an active component extracted from the goji berry, has previously been reported to have anti-inflammatory and anti-oxidant effects [13–15]. LBP has been used as an anti-aging medicine in China for more than 2,000 years [13]. LBP treatment has been shown to have a protective anti-oxidant role in light-induced retinal degeneration in rats [14]. LBP has been reported to improve lipid profiles and oxygen status and reduce abdominal fat in patients with metabolic syndrome [15]. However, the effects of LBP on acute pancreatitis, including the antioxidant effects, remain unclear. Therefore, this study aimed to investigate the effects of LBP in a mouse model of cerulein-induced acute pancreatitis.
Material and Methods
Animals used in the study
The protocols for the animal studies were approved by the Institutional Animal Care and Use Committee of Dongguan Tungwah Affiliated Hospital of Sun Yat-sen University. Animal experiments were performed in accordance with the National Institutes of Health guidelines for the use of experimental animals. C57BL/6 mice (6–8 weeks old) and age-matched nuclear factor erythroid-2-related factor 2 (NRF2) gene knockout mice (NRF2−/− mice) were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). The mice were maintained on a 12-hour light and dark cycle and had free access to food and water.
Treatment groups and the mouse model of cerulein-induced acute pancreatitis
Lycium barbarum polysaccharide (LBP) (100 mg/kg) (Xi’an Natural Field Bio-Technique Co. Ltd., Shanxi, China) was administered by once daily gavage for one week. An equivalent volume of sterile normal saline was administrated to the vehicle-treated control group. The animals fasted for 12 h prior to induction of acute pancreatitis.
The mouse model of acute pancreatitis was created using seven intraperitoneal injections of cerulein (50 mg/kg) (Sigma-Aldrich, St. Louis, MO, USA) at one-hour intervals [16]. All mice were euthanized at 12 hours after the first intraperitoneal injection of cerulein using an intraperitoneal injection of pentobarbitone (50 mg/kg). Blood samples and pancreatic tissue samples were obtained from each mouse.
Histology of the mouse pancreatic tissue
Pancreatic tissues formalin-fixed, embedded in paraffin wax, and then cut into 5 μm sections, placed onto glass slides and stained with hematoxylin and eosin (H&E) for light microscopy. Histological examination was performed by two pathologists who were unaware of the experimental grouping or treatment of the mice. The following histological features were evaluated and graded by light microscopy: edema, acinar necrosis, inflammatory cell infiltrate, hemorrhage, fat necrosis, and perivascular inflammation. The scoring system used (0–3) was: 0, normal; 1, mild; 2, moderate; 3, severe [17].
Wet to dry (W/D) pancreatic weight ratio
The pancreas was harvested fresh from each mouse following death. The fresh pancreas was weighed immediately to obtain the wet weight. After drying in an oven at 80°C for 48 h, the dry weight of the pancreas was obtained. The W/D ratio of the pancreas was calculated for each mouse.
Measurement of amylase and lipase
Amylase kits (Zhongsheng Beikong Bio-Technology, Beijing, China) and lipase kits (Nanjing Jiancheng Corp., Nanjing, China) were used to measure the activity of amylase and lipase in the mouse blood, according to the manufacturers’ instructions.
Measurement of reactive oxygen species (ROS)
The pancreatic tissues were homogenized in tissue lysis buffer (Cell Signaling Technology Inc., Beverly, MA, USA) using a rotor-stator homogenizer. The ROS levels in pancreatic tissues were determined by measuring lipid hydroperoxide using a lipid hydroperoxide assay kit, according to the manufacturer’s instructions (Cayman Chemical, Ann Arbor, MI, USA).
Measurement of anti-oxidant enzymes
The activity of heme oxygenase-1 (HO-1) in the mouse pancreas was determined by measuring the amount of bilirubin generated in a mixture of pancreatic tissue homogenate, nicotinamide adenine dinucleotide phosphate (NADPH), glucose-6-phosphate, glucose 6-phosphate dehydrogenase (G6PD), and protohemin. The activity of superoxide dismutase (SOD) in the pancreatic tissue homogenate was determined using a specific kit (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China), according to the manufacturer’s instructions.
Enzyme-linked immunosorbent assay (ELISA)
Tumor necrosis factor (TNF)-α and interleukin (IL)-6 levels in the blood were measured using commercial ELISA kits, according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA).
Western blot
Protein extracted from mouse pancreas was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride (PVDF) membrane. The membrane was incubated with primary antibodies to Nrf2 (1: 500 dilution) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), Lamin B (1: 500 dilution) (Santa Cruz Biotechnology, Dallas, TX, USA) and horseradish peroxidase (HRP)-conjugated secondary antibodies (1: 5,000 dilution) (Santa Cruz Biotechnology, Santa Cruz, CA, USA). After blocking with 5% dried skimmed milk powder, bands on the membrane were detected using enhanced chemiluminescence (ECL) (Bio-Rad Laboratories, Hercules, CA, USA) and measured by densitometric analysis using Image J software (NIH, Bethesda, MD, USA).
Statistical analysis
SPSS version 20.0 software (IBM, Armonk, NY, USA) was used for statistical calculations. Data were expressed as the mean ±SEM and analyzed with one-way analysis of variance (ANOVA) followed by Bonferroni’s t-test. Student-Newman-Keuls (SNK) method was used for comparison of the data obtained from histology of the pancreas from the mice in the different groups. A P-value <0.05 was considered to be statistically significant.
Results
Lycium barbarum polysaccharide (LBP) reduced cerulein-induced acute pancreatitis in the mouse model
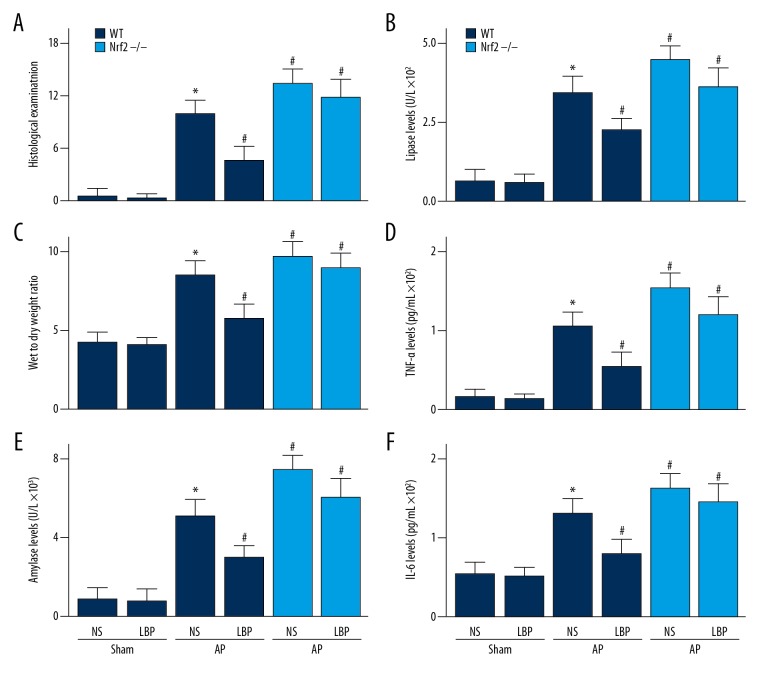
As shown in Figures 1 and 2A, in the cerulein-induced mouse model of acute pancreatitis, inflammatory cell infiltration, edema, and hemorrhage were found 12 hours after the first intraperitoneal injection of cerulein. However, the degree of the cerulein-induced pancreatic inflammation seen on histology was reduced by pre-treatment with LBP (Figures 1, 2A). The wet to dry (W/D) pancreatic weight ratio was a sensitive method for the measurement of edema in the pancreas, as in the mice in the group with cerulein-induced acute pancreatitis, there was a significant increase in the W/D ratio compared with the mice in the control group (Figure 2B), which was significantly reduced in the LBP-treated mouse model group when compared with vehicle-treated mouse model group (Figure 2B). Levels of amylase and lipase in the blood samples were significantly increased in the mouse group with cerulein-induced acute pancreatitis compared with the control group. The cerulein-induced increase in serum amylase and lipase levels were reduced in the LBP-treated mouse group compared with the vehicle-treated mouse group (Figure 2C, 2D).
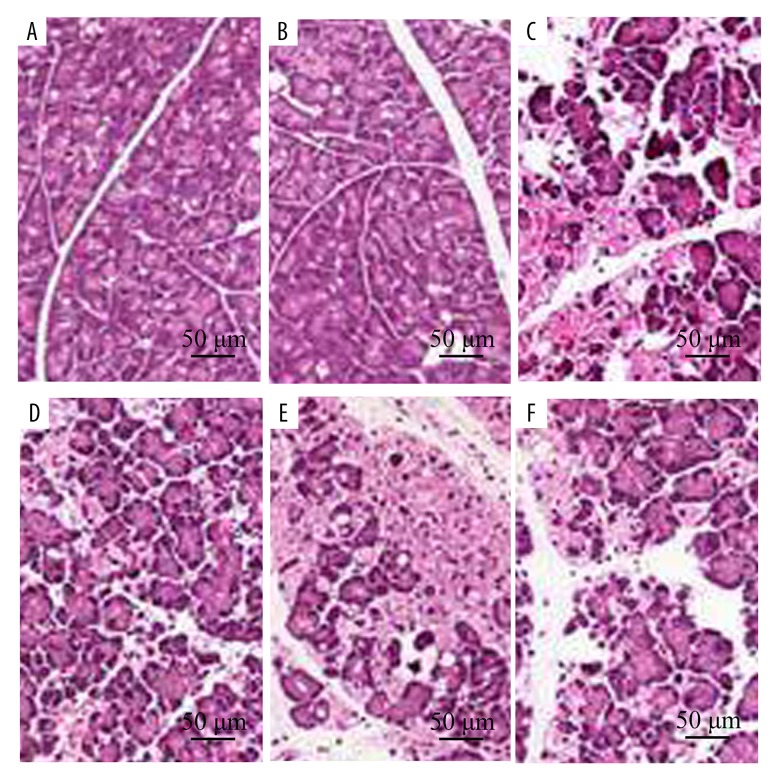
Figure 1.
Effect of Lycium barbarum polysaccharide (LBP) on cerulein-induced acute pancreatitis in wild-type (WT) or nuclear factor erythroid-2-related factor 2 (NRF2) gene knockout mice (NRF2−/−). Representative histological sections of the pancreas were stained with hematoxylin and eosin (H&E). Original magnification, ×100. (A) Wild-type (WT) sham mice and mice treated with normal saline (NS). (B) WT sham mice and mice treated LBP. (C) WT mice with acute pancreatitis treated with NS. (D) WT mice with acute pancreatitis treated with LBP. (E) NRF2−/− mice with acute pancreatitis treated with NS. (F) NRF2−/− mice with acute pancreatitis treated with LBP.
Figure 2.
Effect of Lycium barbarum polysaccharide (LBP) on cerulein-induced acute pancreatitis in wild-type (WT) or nuclear factor erythroid-2-related factor 2 (NRF2) gene knockout mice (NRF2−/−). (A) Histological scores of the mouse pancreas tissue. (B) Wet to dry weight ratio of the pancreas; serum levels of (C) amylase, (D) lipase, (E) tumor necrosis factor (TNF)-α, and (F) interleukin (IL)-6. Values shown are the mean ± SEM. * P<0.05, when compared with the sham WT mice treated with NS group; # P<0.05, when compared with WT mice in the acute pancreatitis group treated with NS. NS – normal saline.
LBP reduced cerulein-induced pro-inflammatory cytokines and production of reactive oxygen species (ROS)
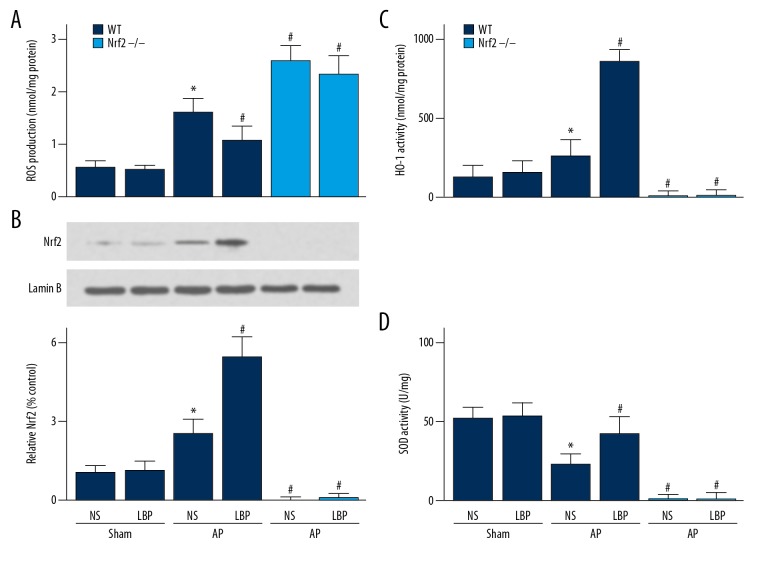
The serum levels of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) were significantly increased in the mouse group with cerulein-induced acute pancreatitis compared with the control group (Figure 2E, 2F). The upregulation of TNF-α and IL-6 levels were reduced by LBP treatment (Figure 2E, 2F). In the mouse group with cerulein-induced acute pancreatitis, there was an increase in the production of reactive oxygen species (ROS) compared with the control group, which was reduced by pre-treatment with LBP (Figure 3A).
Figure 3.
Effect of Lycium barbarum polysaccharide (LBP) on cerulein-induced acute pancreatitis (AP) in wild-type (WT) or nuclear factor erythroid-2-related factor 2 (NRF2) gene knockout mice (NRF2−/−). (A) Production of reactive oxygen species (ROS). (B) Nuclear Nrf2 expression. (C) Heme oxygenase (HO-1), and (D) superoxide dismutase (SOD) expression in the pancreas. Values shown are the mean ±SEM. * P<0.05, when compared with WT sham mice treated with NS. # P<0.05, when compared with the WT mice with AP treated with NS. NS – normal saline.
LBP treatment increased the expression of nuclear factor erythroid-2-related factor 2 (Nrf2) and antioxidant enzymes activities in the cerulein-induced mouse model of acute pancreatitis
The expression levels of Nrf2 in the cell nucleus was increased in LBP-treated mice compared with vehicle-treated group (Figure 3B). Also, the expression of heme oxygenase-1 (HO-1) and superoxide dismutase (SOD) were increased in LBP-treated mice compared with the vehicle-treated group (Figure 3C, 3D). To investigate whether the effects of LBP on cerulein-induced acute pancreatitis was Nrf2-dependent, NRF2 gene knockout mice were studied. The results showed that the protective effect of LBP on cerulein-induced acute pancreatitis was reduced in NRF2 gene knockout mice (NRF2−/−) (Figures 1–3).
Discussion
Nuclear factor erythroid-2-related factor 2 (Nrf2) has previously been shown to have a protective effect in animal models of acute pancreatitis [11,18]. Lycium barbarum polysaccharide (LBP) has previously been shown to induce the expression of Nrf2 in an insulin-resistant animal model fed a high-fat diet [19]. The findings of the present study showed that LBP increased the expression of Nrf2 in a mouse model of cerulein-induced acute pancreatitis, and reduced pancreatic edema, inflammation, hemorrhage, amylase levels, and lipase levels. However, the effects of LBP on the cerulein-induced mouse model of acute pancreatitis were reduced in NRF2 gene knockout mice. The findings of the present study indicated that the effects of LBP pre-treatment on cerulein-induced acute pancreatitis were Nrf2-dependent.
Under physiological conditions, Nrf2 is located in the cytoplasm and is specifically inhibited by Kelch-like ECH-associated protein 1 (Keap1). Under conditions of stress, Nrf2 accumulates in the cell nucleus and escapes from Keap1-mediated degradation. After translocating into the nucleus, Nrf2 regulates the transcription of antioxidative genes by binding to the antioxidant response element. Previously published studies have shown that flavonoids reduce inflammation in an animal model of acute pancreatitis by activation of Nrf2-mediated pathways [20]. Increased expression of Nrf2 was found in melatonin-treated mice with acute pancreatitis [21]. The findings of the present study were consistent with the findings from previously reported studies and showed that LBP increased the expression of Nrf2 in the mouse model of cerulein-induced acute pancreatitis. LBP treatment was also associated with an increase in levels of antioxidant enzymes and reduced pancreatic inflammation and tissue damage by the activation of Nrf2.
The antioxidant effects of Nrf2 depend on its downstream antioxidant enzymes. For example, superoxide dismutase (SOD) catalyzes the dismutation of oxygen and hydrogen peroxide and has an important role in defense from oxidative damage. Reduced activity of SOD has been previously reported in an animal model of acute pancreatitis [18]. The findings of the present study are supported by previously published studies, and in the cerulein-induced mouse model of acute pancreatitis, the reduction of SOD activity was reversed by treatment with LBP.
Heme oxygenase-1 (HO-1) protects cells and tissues during inflammation and oxidative stress and catalyzes heme to carbon monoxide and biliverdin [22]. previously published studies have shown that carbon monoxide and biliverdin generated by HO-1 had a protective role in animal models of acute pancreatitis and has been shown to be upregulated [23]. Increased expression of HO-1 has previously been shown to reduce the degree of inflammation in an animal model of acute pancreatitis and inhibition of HO-1 increased the severity of acute pancreatitis [24]. Also, a preclinical animal study reported that external drainage of the biliary tract reduced the severity of acute pancreatitis by upregulation of HO-1 [25]. Overexpression of HO-1 by an adenoviral vector has been reported to reduce the severity of acute pancreatitis in an animal model [26]. Clinically, upregulation of HO-1 in peripheral blood mononuclear cells of patients with acute pancreatitis has been shown to be associated with clinical improvement [27]. Consistent with these previously published results, the results of the present study showed that the expression of HO-1 was increased in the LBP-treated mouse model of acute pancreatitis, resulting in increased defense from oxidative stress and tissue damage.
The results of this study, combined with previous studies [11,18] indicate that Nrf2 has a role in reducing inflammation and its effects in a mouse model of cerulein-induced acute pancreatitis. In the present study, the activation of Nrf2 was enhanced in LBP-treated mice. Because it was previously unclear whether the effect of LBP in acute pancreatitis was Nrf2-dependent, an NRF2 gene knockout mouse model was used. The findings showed that NRF2 gene knockout reduced the protective effect of LBP on acute pancreatitis. This finding indicated that the protective effect of LBP on cerulein-induced acute pancreatitis was at least partly Nrf2-dependent.
This study had several limitations. The mice were pre-treated with LBP before the mouse model of cerulein-induced acute pancreatitis was created. Clinically, acute pancreatitis is not a preventable disease, and the effects of a compound used before the onset of the disease are not representative of the clinical situation. However, the condition of post-endoscopic retrograde cholangiopancreatography (ERCP)-induced pancreatitis is a form of preventable acute pancreatitis that might be amenable to future studies on the effects of preventive therapy [28–30].
Conclusions
In a mouse model of cerulein-induced acute pancreatitis, Lycium barbarum polysaccharide (LBP) reduced inflammation by upregulating the nuclear factor erythroid-2-related factor 2 (NRF2) gene and heme oxygenase-1 (HO-1). Findings from this preliminary study in a mouse model of acute pancreatitis require future controlled studies to investigate the clinical effects of LBP in acute pancreatitis.
Acknowledgments
The authors thank Dong Tan and Xian Chen for their assistance in histological analysis of the mouse pancreatic tissue.
Footnotes
Ethics approval
This study was approved by the Institutional Animal Care and Use Committee of Dongguan Tungwah Affiliated Hospital of Sun Yat-sen University.
Source of support: This study was supported by the Dongguan Science and Technology Foundation of China (No. 2012105102028)
Conflict of interest
None.
References
- 1.Petrov MS, Shanbhag S, Chakraborty M, et al. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. 2010;139(3):813–20. doi: 10.1053/j.gastro.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Tao W, Li PS, Shen Z, et al. Effects of omega-3 fatty acid nutrition on mortality in septic patients: A meta-analysis of randomized controlled trials. BMC Anesthesiol. 2016;16(1):39. doi: 10.1186/s12871-016-0200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tao W, Yang L-Q, Gao J, Shao J. Neuromuscular blocking agents for adult patients with acute respiratory distress syndrome: A meta-analysis of randomized controlled trials. J Trauma Acute Care Surgm. 2018;85(6):1102–9. doi: 10.1097/TA.0000000000002057. [DOI] [PubMed] [Google Scholar]
- 4.Escobar J, Pereda J, Arduini A, et al. Cross-talk between oxidative stress and pro-inflammatory cytokines in acute pancreatitis: A key role for protein phosphatases. Curr Pharm Des. 2009;15(26):3027–42. doi: 10.2174/138161209789058075. [DOI] [PubMed] [Google Scholar]
- 5.Fisic E, Poropat G, Bilic-Zulle L, et al. The role of IL-6, 8, and 10, sTNFr, CRP, and pancreatic elastase in the prediction of systemic complications in patients with acute pancreatitis. Gastroenterol Res Pract. 2013;2013 doi: 10.1155/2013/282645. 282645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forsmark CE, Vege SS, Wilcox CM. Acute pancreatitis. N Engl J Med. 2016;375(20):1972–81. doi: 10.1056/NEJMra1505202. [DOI] [PubMed] [Google Scholar]
- 7.Choudhury S, Ghosh S, Gupta P, et al. Inflammation-induced ROS generation causes pancreatic cell death through modulation of Nrf2/NF-kappaB and SAPK/JNK pathway. Free Radic Res. 2015;49(11):1371–83. doi: 10.3109/10715762.2015.1075016. [DOI] [PubMed] [Google Scholar]
- 8.Bopanna S, Nayak B, Prakash S, et al. Increased oxidative stress and deficient antioxidant levels may be involved in the pathogenesis of idiopathic recurrent acute pancreatitis. Pancreatology. 2017;17(4):529–533. doi: 10.1016/j.pan.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10(11):549–57. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Sun J, Fu J, Zhong Y, et al. NRF2 mitigates acute alcohol-induced hepatic and pancreatic injury in mice. Food Chem Toxicol. 2018;121:495–503. doi: 10.1016/j.fct.2018.09.042. [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Zhu Q, Zhang M, et al. Isoliquiritigenin ameliorates acute pancreatitis in mice via inhibition of oxidative stress and modulation of the Nrf2/HO-1 pathway. Oxid Med Cell Longev. 2018;2018 doi: 10.1155/2018/7161592. 7161592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong Z, Shang H, Chen YQ, et al. Sulforaphane protects pancreatic acinar cell injury by modulating Nrf2-mediated oxidative stress and NLRP3 inflammatory pathway. Oxid Med Cell Longev. 2016;2016 doi: 10.1155/2016/7864150. 7864150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao Y, Wei Y, Wang Y, et al. Lycium barbarum: A traditional Chinese herb and a promising anti-aging agent. Aging Dis. 2017;8(6):778–91. doi: 10.14336/AD.2017.0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang JS, Lee YJ, Wilkie DA, Lin CT. The neuroprotective and antioxidative effects of submicron and blended Lycium barbarum in experimental retinal degeneration in rats. J Vet Med Sci. 2018;80(7):1108–15. doi: 10.1292/jvms.17-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Souza Zanchet MZ, Nardi GM, de Oliveira Souza Bratti L, et al. Lycium barbarum reduces abdominal fat and improves lipid profile and antioxidant status in patients with metabolic syndrome. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/9763210. 9763210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Q, Wei Y, Pandol SJ, et al. STING signaling promotes inflammation in experimental acute pancreatitis. Gastroenterology. 2018;154(6):1822–35.e1822. doi: 10.1053/j.gastro.2018.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kusske AM, Rongione AJ, Ashley SW, et al. Interleukin-10 prevents death in lethal necrotizing pancreatitis in mice. Surgery. 1996;120(2):284–88. doi: 10.1016/s0039-6060(96)80299-6. [DOI] [PubMed] [Google Scholar]
- 18.Zhang M, Wu YQ, Xie L, et al. Isoliquiritigenin protects against pancreatic injury and intestinal dysfunction after severe acute pancreatitis via Nrf2 signaling. Front Pharmacol. 2018;9:936. doi: 10.3389/fphar.2018.00936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y, Li W, Li Y, et al. Dietary Lycium barbarum polysaccharide induces Nrf2/ARE pathway and ameliorates insulin resistance induced by high-fat via activation of PI3K/AKT signaling. Oxid Med Cell Longev. 2014;2014 doi: 10.1155/2014/145641. 145641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du D, Yao L, Zhang R, et al. Protective effects of flavonoids from Coreopsis tinctoria Nutt. on experimental acute pancreatitis via Nrf-2/ARE-mediated antioxidant pathways. J Ethnopharmacol. 2018;224:261–72. doi: 10.1016/j.jep.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Jung KH, Hong SW, Zheng HM, et al. Melatonin ameliorates cerulein-induced pancreatitis by the modulation of nuclear erythroid 2-related factor 2 and nuclear factor-kappaB in rats. J Pineal Res. 2010;48(3):239–50. doi: 10.1111/j.1600-079X.2010.00748.x. [DOI] [PubMed] [Google Scholar]
- 22.Tenhunen R, Marver HS, Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969;244(23):6388–94. [PubMed] [Google Scholar]
- 23.Nuhn P, Mitkus T, Ceyhan GO, et al. Heme oxygenase 1-generated carbon monoxide and biliverdin attenuate the course of experimental necrotizing pancreatitis. Pancreas. 2013;42(2):265–71. doi: 10.1097/MPA.0b013e318264cc8b. [DOI] [PubMed] [Google Scholar]
- 24.Zhang FH, Sun YH, Fan KL, et al. Protective effects of heme oxygenase-1 against severe acute pancreatitis via inhibition of tumor necrosis factor-alpha and augmentation of interleukin-10. BMC Gastroenterol. 2017;17(1):100. doi: 10.1186/s12876-017-0651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang JL, Chen Y, Song XQ, et al. Biliary tract external drainage protects against multiple organs injuries of severe acute pancreatitis rats via heme oxygenase-1 upregulation. Pancreatology. 2017;17(2):219–27. doi: 10.1016/j.pan.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Zhang F, Fei J, Zhao B, et al. Protective effect of adenoviral transfer of heme oxygenase-1 gene on rats with severe acute pancreatitis. Am J Med Sci. 2014;348(3):224–31. doi: 10.1097/MAJ.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 27.Habtezion A, Kwan R, Yang AL, et al. Heme oxygenase-1 is induced in peripheral blood mononuclear cells of patients with acute pancreatitis: A potential therapeutic target. Am J Physiol Gastrointest Liver Physiol. 2011;300(1):G12–20. doi: 10.1152/ajpgi.00231.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li GZ, Wang F, Fang J, et al. Risk factors for post-endoscopic retrograde cholangiopancreatography pancreatitis: Evidence from 1786 cases. Med Sci Monit. 2018;24:8544–52. doi: 10.12659/MSM.913314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Shen Y, Zhong Z, et al. Risk factors for post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis and the effect of octreotide combined with nonsteroidal anti-inflammatory drugs on preventing its occurrence. Med Sci Monit. 2018;24:8964–69. doi: 10.12659/MSM.911914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parekh PJ, Majithia R, Sikka SK, Baron TH. The “Scope” of post-ERCP pancreatitis. Mayo Clin Proc. 2017;92(3):434–48. doi: 10.1016/j.mayocp.2016.10.028. [DOI] [PubMed] [Google Scholar]