Abstract
Background
Myocardial infarction (MI) is the main cause of heart failure (HF), and sympathetic nerve activity is associated with prognosis chronic heart failure. Renal sympathetic denervation (RDN) is noted for its powerful effect on the inhibition of sympathetic nerve activity. This study investigated the effect of RDN on heart failure in dogs after myocardial infarction.
Material/Methods
The experimental animals were randomized into 2 groups: the MI group (n=12) and the sham operation group (n=6). In the MI group we established an MI model by permanently ligating the left anterior descending branch. After 4 weeks, the MI dogs were randomly divided into 2 groups: the MI+RDN group (MI+renal sympathetic denervation, n=6) and the simple MI group (n=6). Animals in the MI+RDN group underwent both surgical and chemical renal denervation.
Results
Compared with sham operation group, left ventricular fraction shortening (LVFS) and left ventricular ejection fraction (LVEF) were significantly reduced in the simple MI group, while the reduction was partly reversed in the MI+RDN group. RDN reduced sympathetic nerve activity and release of B-type natriuretic peptide (BNP) and Angiotensin II (AngII) in the MI+ RDN group but not in the simple MI group.
Conclusions
Canine renal sympathetic denervation prevents myocardial malignant remodeling by lowering the activity of the systemic sympathetic nerve and inhibiting renin-angiotensin-aldosterone system (RASS) activation, providing a new target and method for the treatment of heart failure.
MeSH Keywords: Heart Failure, Myocardial Infarction, Sympathetic Nervous System
Background
Heart failure following acute myocardial infarction (AMI) accounts for a large proportion of the total incidence of heart failure. Even with timely revascularization, heart failure may still appear after myocardial infarction, seriously affecting human health and quality of life. At present, a variety of clinical methods have been used to treat heart failure, reducing the hospitalization rate and mortality of heart failure; however, 30% of HErEF (heart failure with reduced ejection fraction) patients show no improvement, even after receiving standardized drugs and cardiac resynchronization therapy (CRT). Therefore, new and more effective treatment methods are required to improve the patients’ conditions.
In the past, renal sympathetic denervation (RDN) has achieved initial success in the treatment of refractory hypertension [1]. When treating patients with hypertension, RDN reduces the activity of renal afferent and efferent sympathetic nerves, regulates the activity of cardiac autonomic nerves, and has a positive therapeutic effect on the improvement of cardiac functions [2–4]. It is speculated [5–9] that RDN can effectively enhance left ventricular functions, and may be a novel therapeutic strategy for heart failure. In the present study, a canine model of heart failure after myocardial infarction was established, and then renal sympathetic nerves were removed canines after 4 weeks of MI. We studied the influence of RDN on the occurrence and development of heart failure from aspects of cardiac structure and neurohumoral changes.
Material and Methods
Animals
We used 29 healthy adult beagle dogs (arbitrary sex, weight 13.0±2.0 kg, age=1–2 years) in the study. These canines were randomly divided into 2 groups: the sham operation group (n=6) and the myocardial infarction (MI) group (n=14). Thoracotomy was performed to ligate the anterior descending coronary artery for all subjects in the MI group, while the coronary artery was not ligated in the sham operation group. One subject in the MI group died within 3 days, and 1 did not show any manifestations of heart failure after 4 weeks. The remaining MI animals (n=12) were randomly divided into 2 groups: the MI+RDN group (n=6) and the simple MI group (n=6). In the MI+RDN group, laparotomy was performed to remove the renal sympathetic nerves, while the corresponding nerves were left intact in the simple MI group animals. There were no significant differences in breed, body weight, age, heart size, or sex between the sham operation, simple MI, and MI+RDN groups.
Establishment of MI model
The animals did not consume food or water for 12 h before the operation. Venous access was established after routine disinfection. Pentobarbital sodium (20 mg/kg, Na-pentobarbital) was injected intramuscularly to anesthetize the animals, and compound anesthetic analgesic solution (300 mg suxamethonium chloride injection+0.1 mg fentanyl citrate injection+250 mL 0.9% sodium chloride injection) was slowly injected intravenously during the operation. Ketamine hydrochloride (25mg/kg) was injected intravascularly to maintain intraoperative muscle relaxation. The animals were placed on a non-invasive ventilator after tracheal intubation to assist breathing (oxygen flow rate at 4–6 L/min). The LEAD-7000 multi-channel electrophysiological recorder was connected to the subjects for dynamic monitoring of heart rate, blood pressure, and oxygen saturation. In the MI group, a thoracotomy was performed at the fifth intercostal space, and 1.0 cm of the first diagonal branch artery was ligated that was stemming from the main anterior descending branch of the left coronary artery. The specific method used was based on a previous study [10]. The area of the heart surface feeding into the anterior descending artery became purple and white, the heart beat weakened, and the T wave of the electrocardiogram changed, indicating that the MI model had been successfully established (as shown in Figure 1). The same invasive operation was also performed for the sham operation group, but the coronary artery remained unligated. Afterwards, the thoracic chest was closed by suturing the muscles and skin layer by layer. Postoperative intramuscular injection of cefazolin sodium was administered at 400 000 U/day for 3 consecutive days to prevent infection.
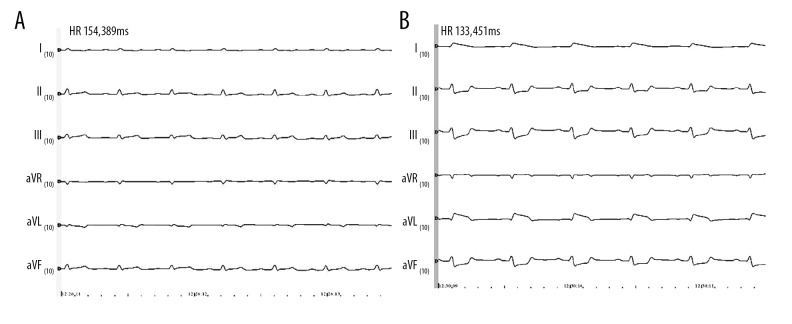
Figure 1.
Electrocardiogram before and after myocardial infarction. A is an electrocardiogram before ligating the coronary artery, and B is an electrocardiogram 30 minutes after ligating the artery. Compared with before ligation, the ST segment was significantly elevated 30 minutes after the artery was ligated. It was confirmed that the myocardial infarction model was successfully constructed.
Renal sympathetic denervation for MI canine model
After 4 weeks of MI, the renal sympathetic denervation was performed on MI canines. After routine disinfection, anesthesia, and tracheal intubation (as described above), the ventilator was connected to the canine to assist ventilation. Examination of LEAD electrophysiological recordings was also performed. Laparotomy was performed by first making a vertical incision in the middle of the abdomen. Bilateral kidneys and the renal hilum were exposed, followed by the isolation of the renal artery. Visible nerves at the surface of the renal artery were cut off mechanically for canines in the RDN+MI group, and sterile gauze soaked with 20% phenol solution was applied to the surface of the renal artery for about 10–20 min to damage the remaining sympathetic nerves around the renal artery [11]. The renal sympathetic nerves remain intact in the simple MI group. Heart rate variability was tested before and during RDN. One end of the PowerLab Bio-Signal Processor (AD Instruments Shanghai Trading Co., China) was connected to the dog’s limbs, and the other to a computer. One channel was selected to record the electrocardiogram (ECG) signal. After 1 min of pre-acquisition, the signals were collected for 5 min. The low-frequency band (LF; 0.04–0.15Hz) represented the activity of sympathetic nerves, and the high frequency band (HF; 0.15–0.4Hz) represented vagal activity. The abdominal cavity was closed through layer-by-layer suturing. The same dose of antibiotics described above was administered to prevent postoperative infection.
Echocardiography
Color Doppler sonography (Vivid E9, GE) was performed before MI (baseline level), and at 1, 2, and 4 weeks after MI, and at 1, 2, and 4 weeks after RDN (5, 6, and 8 weeks after MI) for echocardiography. Left ventricular end-diastolic diameter (LVEDD) and left ventricular end-systolic diameter (LVSD) were measured. The Teichholz correction formula was used to calculate LVEF and LVFS. Cardiac ultrasound was performed by the same person, with no knowledge of the grouping and operation of the target animals.
Measurement of blood B-type natriuretic peptide and Angiotensin II
Blood was collected before MI (baseline level) and at 1 and 4 weeks after MI, and at 4 weeks after RDN. BNP and AngII were measured using enzyme-linked immunosorbent assay (ELISA, Wuhan Huamei Company) according to the manufacturer’s instructions.
Activity of sympathetic nerves
Dynamic electrocardiogram was performed before MI (baseline level), 1 day after MI, 4 weeks after MI, and 4 weeks after RDN. The continuous R-R interval was used to perform the classical spectral estimation of the FFT, and the energy distribution in the HF and LF was quantitatively described [11].
Statistical analysis
Measurement data were described by mean ± standard deviation. The overall trend analysis of each time-point index was analyzed by repeated-measures data analysis. P<0.05 was considered statistically significant.
Results
Established canine model of heart failure following myocardial infarction
As shown in Table 1, LVESD and LVEDD were gradually increased, while LVFS and LVEF decreased in the MI group; however, a significant difference was observed only 4 weeks after MI between the MI and sham operation groups (P<0.05). At 4 weeks after MI, 12 of the 14 animals showed manifestations of heart failure, such as drowsiness, decreased activity, fluid retention, and shortness of breath in the MI group (of the 2 other MI animals, 1 died within 3 days and the other showed no manifestations of heart failure 4 weeks later). Echocardiography showed LVEF <50%, suggesting that the heart failure model following myocardial infarction was established successfully.
Table 1.
Changes of cardiac ultrasound after MI.
| LVESD (cm) | LVEDD (cm) | LVFS (%) | LVEF (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Sham operation | MI | Sham operation | MI | Sham operation | MI | Sham operation | MI | |
| Base | 2.63±0.17 | 2.68±0.14 | 3.53±0.25 | 3.57±0.20 | 24.97±1.77 | 25.19±1.53 | 60.32±3.77 | 59.93±4.23 |
| 1w | 2.65±0.16 | 2.89±0.12 | 3.56±0.20 | 3.73±0.27 | 25.15±1.58 | 23.37±2.13 | 59.77±3.74 | 55.25±3.43 |
| 2w | 2.62±0.18 | 3.16±0.15 | 3.54±0.22 | 4.00±0.26 | 25.20±1.52 | 21.84±2.15 | 59.34±3.97 | 52.20±2.72 |
| 4w | 2.59±0.21 | 3.43±0.10* | 3.56±0.21 | 4.17±0.22* | 24.93±1.84 | 17.85±2.04* | 59.51±3.85 | 47.13±2.63* |
P<0.05 statistical difference compared with sham operation group.
Validation of canine sympathetic denervation
Changes in the low-frequency band were obtained 4 weeks after MI. Compared with the simple MI group, the low-frequency band normalization value (LFnorm) of the 5-min heart rate variability was significantly lower in the MI+RDN group (P<0.05), suggesting that the activity of sympathetic nerves was weakened, and the renal sympathetic denervation was successful (Figure 2).
Figure 2.
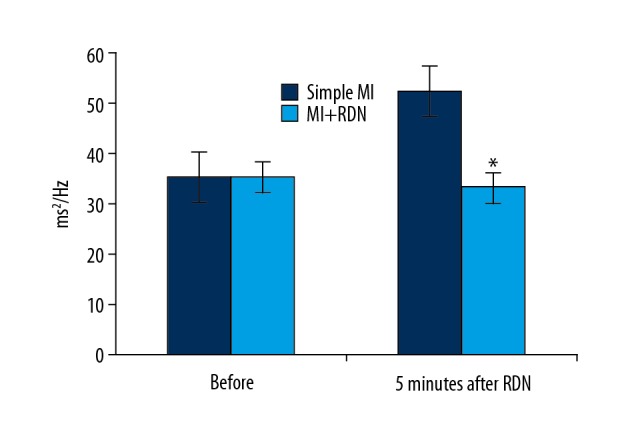
Changes of LF normalized values. * P<0.05 significant difference compared with simple MI.
The effect of RDN on heart failure
RDN alters left ventricular structure and function
As shown in Table 2, for the simple MI group, LVESD and LVEDD increased gradually with time, yet LVFS and LVEF gradually decreased (P<0.05); however, the values were only significantly different between at 4 weeks and 8 weeks (P<0.05). The changing trend of LVESD, LVEDD, LVFS, and LVEF of the MI+RDN group were similar to that of the simple MI group. Within the MI+RDN group, statistical differences in LVESD and LVEDD were noticed between the values at 8 weeks and 4 weeks (P<0.05). Compared with the simple MI group, LVESD and LVEDD values were significantly lower in the MI+RDN group after 8 weeks, while LVFS and LVEF values were significantly higher after 8 weeks (P<0.05).
Table 2.
Changes of cardiac ultrasound after RDN.
| LVESD (cm) | LVEDD (cm) | LVFS (%) | LVEF (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Simple MI | MI+RDN | Simple MI | MI+RDN | Simple MI | MI+RDN | Simple MI | MI+RDN | |
| 4w | 3.44±0.11 | 3.45±0.10 | 4.16±0.25 | 4.17±0.22 | 17.85±2.04 | 17.85±2.04 | 47.21±2.24 | 47.21±2.24 |
| 5w | 3.68±0.19 | 3.60±0.16 | 4.35±0.27 | 4.31±0.28 | 15.75±1.58 | 16.47±1.53 | 45.25±3.43 | 45.87±3.53 |
| 6w | 3.86±0.20 | 3.73±0.18 | 4.47±0.23 | 4.42±0.24 | 13.64±1.32 | 15.84±1.15 | 42.20±2.72 | 45.14±3.15 |
| 8w | 4.32±0.22* | 3.82±0.18*# | 4.86±0.26* | 4.50±0.29*# | 11.63±1.11* | 14.75±1.04# | 38.13±2.63* | 44.61±2.24# |
P<0.05 statistical difference compared with 4w;
P<0.05 statistical difference compared with simple MI.
Blood BNP and Angiotensin II levels
After 4 weeks, BNP and AngII were significantly higher than the baseline levels in the simple MI group (P<0.05). Similarly, BNP and AngII were higher than the basal level in the MI+RDN group at 4 weeks (P<0.05). There was a statistically significant difference between the MI+RDN group and simple MI group in BNP and AngII at 8 weeks (P<0.05) (Table 3).
Table 3.
Results of BNP and Angiotensin II in blood.
| BNP (pg/ml) | Ang II (pg/mL) | |||
|---|---|---|---|---|
| Simple MI | MI+RDN | Simple MI | MI+RDN | |
| Base | 52.34±12.3 | 51.84±13.03 | 156.00±21.46 | 148.00±24.72 |
| 1w | 61.64±12.58 | 60.43±12.32 | 187.00±26.36 | 175.00±29.47 |
| 4w | 83.57±15.12* | 81.35±14.13* | 262.00±30.83* | 257.00±36.04* |
| 8w | 95.64±11.46* | 57.58±11.48# | 322.00±46.04* | 168.00±27.17# |
P<0.05 statistical difference compared with base time;
P<0.05 statistical difference compared with simple MI.
RDN lowers heart rate variability after MI
The FFT-based classical spectrum estimation was performed for consecutive R-R intervals using the results of 24-h dynamic electrocardiograms before MI and at 1 day, 4 weeks, and 8 weeks after the MI operation. In the simple MI group, LF gradually increased, yet HF gradually decreased. Compared to baseline, a significant difference began to appear at 4 weeks in the simple MI group. Similarly, in the MI+RDN group, LF level was higher than the base level, while HF was lower than the base level at 4 weeks. Compared with the simple MI group, the LF level was significantly lower, but the HF was significantly increased at 8 weeks in the MI+RDN group, and the difference was statistically significant (P<0.05) (Table 4).
Table 4.
Standardized values of frequency domain at different time.
| LF (LFnorm, ms2/Hz) | HF (HFnorm, ms2/Hz) | LF/HF | ||||
|---|---|---|---|---|---|---|
| Simple MI | MI+RDN | Simple MI | MI+RDN | Simple MI | MI+RDN | |
| Base | 34.67±4.36 | 34.33±2.67 | 64.61±5.10 | 63.27±5.87 | 0.54±0.11 | 0.55±0.08 |
| 1d | 53.35±1.94 | 51.13±2.13 | 46.65±1.94 | 48.87±2.14 | 1.15±0.09 | 1.05±0.07 |
| 4w | 60.51±1.56# | 57.89±3.08# | 38.68±1.42# | 40.75±1.44# | 1.57±0.08# | 1.42±0.04# |
| 8w | 64.01±2.75# | 23.94±1.84* | 35.19±2.18# | 76.06±1.84* | 1.83±0.19# | 0.31±0.03* |
P<0.05 statistical difference compared with simple MI;
P<0.05 statistical difference compared with base line.
Discussion
Heart failure is a clinical syndrome for which treatments need to address several issues: target the mechanism of myocardial remodeling, delay and prevent the development of myocardial remodeling, and reduce the hospitalization and mortality rates of heart failure. The renal sympathetic nervous system plays an important role in the development of heart failure following myocardial infarction. BNP is a prognostic factor in patients with heart failure. However, patients with heart failure mainly die due to malignant arrhythmias (ventricular tachycardia or ventricular fibrillation). Previous studies reported that sympathetic activity is similar to that of AngII, and sympathetic activity can directly increase sodium retention [12]. In HF patients with excessive sympathetic activation, β-blocker dose (BBD) might be necessary to avoid cardiovascular events [13]. Renal desympathetic nerve stimulation can improve cardiac function in patients with heart failure by attenuating adverse changes in sympathetic innervation [14]. The activation of renal sympathetic efferent nerves can cause the release of renin, and water and sodium retention; the activation of the corresponding afferent nerves during heart failure can reflexively cause increased sympathetic activity and hence promote the progression of heart failure. Transcatheter renal sympathetic denervation can selectively block the renal sympathetic nerve, which theoretically may serve as a new therapy for heart failure.
Renal sympathetic denervation affecting myocardial remodeling After MI
Clinical studies report that AMI patients show significantly increased resting muscle sympathetic nerve activity in the early stage (2–4 days) after MI, which is significantly higher than that of healthy people, and may last up to 3 months after MI [15,16]. Animal studies have confirmed that the sympathetic nerve activity increases sharply within 1 h after MI [17], and continues to increase up to 2 months after MI [18]. Cao et al. [19] found that, in 53 heart transplantation patients, the sympathetic nerves to the ventricular myocardium were not evenly distributed after myocardial injury, which was characterized by excessive localization of blood vessels and localized areas around the infarct and local denervation of myocardial necrosis. The sympathetic nerve density was significantly higher than that of patients without tachyarrhythmia, suggesting that abnormal cardiac sympathetic regeneration after myocardial infarction or other myocardial injury is closely related to HF prognosis, suggesting that excessive activation of sympathetic nerves aggravates myocardial damage and promotes myocardial remodeling. In addition, the sympathetic nervous system continues to be activated, inducing cardiomyocyte hypertrophy [20], as well as interstitial hyperplasia and remodeling, finally leading to cardiac dysfunction [3,21].
The present study revealed that LVEDD increased gradually after myocardial infarction. The difference was statistically significant starting at 4 weeks after MI. However, LVESD in the MI+RDN group was significantly lower than that in the simple MI group. Moreover, the sympathetic activity of the simple MI group was significantly higher than that of the MI+RDN group, indicating that the sympathetic nerve was activated after myocardial infarction, which promoted structural remodeling of the heart. However, RDN treatment was able to reverse this process. RDN improved ventricular remodeling by attenuating sympathetic activation.
Effects of renal sympathetic denervation on the neuroendocrine system after MI
Studies have confirmed that RAAS is activated after MI, and the blocking of neuroendocrine AngII receptor can reduce oxidative stress and inflammatory response [22], inhibit myocardial fibrosis, and suppress cardiac hypertrophy and enlargement [23]. Meanwhile, activation of the RAAS leads to sodium retention, while BNP is produced by cardiomyocytes and is mainly induced by myocardial (left ventricular) traction stimulation, and helps to retain sodium, and these are associated with the progression and prognosis of the condition [24].
In this experiment, the amount of AngII and BNP increased gradually in the simple MI group, and there was a statistically significant difference starting at 4 weeks after MI. Compared with the simple MI group, the levels of AngII and BNP in the blood were significantly lower in the MI+RDN group at 8 weeks. However, compared with the condition before myocardial infarction, there was still mild ventricular enlargement 8 weeks after MI in the MI+RDN group. Therefore, the levels of AngII and BNP were slightly higher than those before myocardial infarction, but the difference was not statistically significant. However, some studies [9,25] have shown that in the myocardial ischemia reperfusion model, BNP in the RDN group established by radiofrequency ablation was significantly higher than in the control group, suggesting that renal sympathetic nerve removal inhibits activation of the RASS system and inhibits endopeptidase. The degradation of BNP by endopeptidases is attenuated and thus increases BNP. In our experiment, LVEDD was significantly reduced, while there was no significant change in previous experiments [9,25]. The release of BNP was closely related to the traction of the left ventricle. Therefore, the results of this experiment are not consistent with other experiments and can be explained as follows.
Long-term increase in cardiac load after MI leads to left ventricular hypertrophy, interstitial hyperplasia, enlargement of the left ventricle (stimulating BNP production), and a decrease in cardiac ejection fraction, eventually causing heart failure. The sympathetic nervous system activates RASS and aggravates the pathogenesis and process of heart failure. In particular, angiotensin can contract blood vessels, promote cardiac remodeling, and aggravate heart failure. However, RDN can reduce norepinephrine (NE) and AngII levels, inhibit myocardial fibrosis and pro-fibrotic factors, and delay left ventricular remodeling [9,25].
RDN can inhibit structural remodeling of the heart caused by myocardial infarction and reduce the degree of left ventricular muscle traction. RDN suppresses the activation of the sympathetic nervous system and RAAS, which reverses cardiac remodeling caused by activation of the sympathetic nervous system and RAAS after MI, and thus prevent and treat heart failure following myocardial infarction.
Study limitations
The study period was short, making it difficult to assess whether the difference would decrease or keep increasing between the denervation group and the simple MI group over time. In this study, cardiac ultrasound and BNP, which are clinical manifestations, were used to assess cardiac function and cardiac structural changes. There was no further evaluation of hematoxylin-eosin staining (HE)/tyrosine hydroxylase (TH) staining, and the conclusions obtained from the experiment may not be accurately evaluated. This experiment was not grouped according to sex, so we were unable to evaluate whether HF or RDN have different effects on males vs. females. In addition, there may be other limitations not mentioned.
Conclusions
Canine renal sympathetic denervation prevents myocardial malignant remodeling by lowering the activity of the systemic sympathetic nerve and inhibiting RASS activation, providing a new target and method for the treatment of heart failure.
Footnotes
Source of support: This work was supported by grants from the National Natural Science Foundation of China (No. 81660071 and 81560064)
Conflict of interest
None.
References
- 1.Bunte MC, Oliveira EID, Shishehbor MH. Endovascular treatment of resistant and uncontrolled hypertension therapies on the horizon. JACC Cardiovasc Interv. 2012;6(1):1–9. doi: 10.1016/j.jcin.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Stella A, Zanchetti A. Functional role of renal afferents. Physiol Rev. 1991;71(3):659–82. doi: 10.1152/physrev.1991.71.3.659. [DOI] [PubMed] [Google Scholar]
- 3.Guo Z, Zhao Q, Deng H, et al. Renal sympathetic denervation attenuates the ventricular substrate and electrophysiological remodeling in dogs with pacing-induced heart failure. Int J Cardiol. 2014;175(1):185–86. doi: 10.1016/j.ijcard.2014.04.189. [DOI] [PubMed] [Google Scholar]
- 4.Hu J, Yan Y, Zhou Q, et al. Effects of renal denervation on the development of post-myocardial infarction heart failure and cardiac autonomic nervous system in rats. Int J Cardiol. 2014;172(3):e414–16. doi: 10.1016/j.ijcard.2013.12.254. [DOI] [PubMed] [Google Scholar]
- 5.Polhemus DJ, Gao J, Scarborough AL, et al. Radiofrequency renal denervation protects the ischemic heart via inhibition of grk2 and increased nitric oxide signaling. Circ Res. 2016;119:470–80. doi: 10.1161/CIRCRESAHA.115.308278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polhemus DJ, Trivedi RK, Gao J, et al. Renal sympathetic denervation protects the failing heart via inhibition of neprilysin activity in the kidney. J Am Coll Cardiol. 2017;70:2139–53. doi: 10.1016/j.jacc.2017.08.056. [DOI] [PubMed] [Google Scholar]
- 7.Chang S-N, Chang S-H, Yu C-C, et al. Renal denervation decreases susceptibility to arrhythmogenic cardiac alternans and ventricular arrhythmia in a rat model of post-myocardial infarction heart failure. JACC Basic Transl Sci. 2017;2(2):184–93. doi: 10.1016/j.jacbts.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liao S-Y, Zhen Z, Liu Y, et al. Improvement of myocardial function following catheter-based renal denervation in heart failure. JACC Basic Transl Sci. 2017;2(3):270–81. doi: 10.1016/j.jacbts.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharp TE, Polhemus DJ, Li Z, et al. Renal denervation prevents heart failure progression via inhibition of the renin-angiotensin system. J Am Coll Cardiol. 2018;72:2609–21. doi: 10.1016/j.jacc.2018.08.2186. [DOI] [PubMed] [Google Scholar]
- 10.Nasi-Er BG, Wenhui Z, HuaXin S, et al. Vagus nerve stimulation reduces ventricular arrhythmias and increases ventricular electrical stability. Pacing Clin Electrophysiol. 2019;42(2):247–56. doi: 10.1111/pace.13585. [DOI] [PubMed] [Google Scholar]
- 11.Zhang WH, Zhou QN, Lu YM, et al. Renal denervation reduced ventricular arrhythmia after myocardial infarction by inhibiting sympathetic activity and remodeling. J Am Heart Assoc. 2018;7(20):e009938. doi: 10.1161/JAHA.118.009938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh RB, Hristova K, Fedacko J, et al. Chronic heart failure: A disease of the brain. Heart Fail Rev. :2018. doi: 10.1007/s10741-018-9747-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Joho S, Akabane T, Ushijima R, et al. Sympathetic nerve activity efferent drive and beta-blocker treatment – effect of interaction in systolic heart failure. Circ J. 2016;80(10):2149–54. doi: 10.1253/circj.CJ-16-0464. [DOI] [PubMed] [Google Scholar]
- 14.Pinkham MI, Loftus MT, Amirapu S, et al. Renal denervation in male rats with heart failure improves ventricular sympathetic nerve innervation and function. Am J Physiol. 2017;312(3):R368–79. doi: 10.1152/ajpregu.00313.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alberto M, Nicola M. Time course of sympathetic neural hyperactivity after uncomplicated acute myocardial infarction. Circulation. 2003;107(7):793–97. doi: 10.1161/01.cir.0000055543.21433.24. [DOI] [PubMed] [Google Scholar]
- 16.Floras JS, Mak S. Muscle sympathetic nerve activity in women and men following acute myocardial infarction: A meaningful difference? Eur Heart J. 2009;30(14):1692–94. doi: 10.1093/eurheartj/ehp239. [DOI] [PubMed] [Google Scholar]
- 17.Jardine DL, Charles CJ, Ashton RK, et al. Increased cardiac sympathetic nerve activity following acute myocardial infarction in a sheep model. J Physiol (Oxford) 2005;565(1):325–33. doi: 10.1113/jphysiol.2004.082198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han S, Kobayashi K, Joung B, et al. Electroanatomic remodeling of the left stellate ganglion after myocardial infarction. J Am Coll Cardiol. 2012;59(10):954–61. doi: 10.1016/j.jacc.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao JM, Fishbein MC, Han JB, et al. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation. 2000;101(16):1960–69. doi: 10.1161/01.cir.101.16.1960. [DOI] [PubMed] [Google Scholar]
- 20.Davies JE, Manisty CH, Petraco R, et al. First-in-man safety evaluation of renal denervation for chronic systolic heart failure: Primary outcome from REACH-Pilot study. Int J Cardiol. 2013;162(3):189–92. doi: 10.1016/j.ijcard.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 21.Verloop WL, Beeftink MMA, Nap A, et al. Renal denervation in heart failure with normal left ventricular ejection fraction. Rationale and design of the DIASTOLE (DenervatIon of the renAl Sympathetic nerves in hearT failure with nOrmal Lv Ejection fraction) trial. Eur J Heart Fail. 2013;15(12):1429–37. doi: 10.1093/eurjhf/hft119. [DOI] [PubMed] [Google Scholar]
- 22.Dohi Y. Candesartan reduces oxidative stress and inflammation in patients with essential hypertension. Hyperten Res. 2003;26(9):691–97. doi: 10.1291/hypres.26.691. [DOI] [PubMed] [Google Scholar]
- 23.Yamada C, Kuwahara K, Yamazaki M, et al. The renin–angiotensin system promotes arrhythmogenic substrates and lethal arrhythmias in mice with non-ischaemic cardiomyopathy. Cardiovasc Res. 2016;109(1):162–73. doi: 10.1093/cvr/cvv248. [DOI] [PubMed] [Google Scholar]
- 24.Cantinotti M, Law Y, Vittorini S, et al. The potential and limitations of plasma BNP measurement in the diagnosis, prognosis, and management of children with heart failure due to congenital cardiac disease: An update. Heart Fail Rev. 2014;19(6):727–42. doi: 10.1007/s10741-014-9422-2. [DOI] [PubMed] [Google Scholar]
- 25.Polhemus DJ, Trivedi RK, Gao J, et al. Renal sympathetic denervation protects the failing heart via inhibition of neprilysin activity in the kidney. J Am Coll Cardiol. 2017;70:2139–53. doi: 10.1016/j.jacc.2017.08.056. [DOI] [PubMed] [Google Scholar]