Abstract
Background
Osteoclast precursor cells are constitutively differentiated into mature osteoclasts on bone tissues. We previously reported that the continuous stimulation of RAW264.7 precursor cells with compressive force induces the formation of multinucleated giant cells via receptor activator of nuclear factor κB (RANK)-RANK ligand (RANKL) signaling. Here, we examined the bone resorptive function of multinucleated osteoclasts induced by continuous compressive force.
Material/Methods
Cells were continuously stimulated with 0.3, 0.6, and 1.1 g/cm2 compressive force created by increasing the amount of the culture solution in the presence of RANKL. Actin ring organization was evaluated by fluorescence microscopy. mRNA expression of genes encoding osteoclastic bone resorption-related enzymes was examined by quantitative real-time reverse transcription-polymerase chain reaction. Mineral resorption was evaluated using calcium phosphate-coated plates.
Results
Multinucleated osteoclast-like cells with actin rings were observed for all three magnitudes of compressive force, and the area of actin rings increased as a function of the applied force. Carbonic anhydrase II expression as well as calcium elution from the calcium phosphate plate was markedly higher after stimulation with 0.6 and 1.1 g/cm2 force than 0.3 g/cm2. Matrix metalloproteinase-9 expression decreased and cathepsin K expression increased slightly by the continuous application of compressive force.
Conclusions
Our study demonstrated that multinucleated osteoclast-like cells induced by the stimulation of RAW264.7 cells with continuous compressive force exhibit high dissolution of the inorganic phase of bone by upregulating carbonic anhydrase II expression and actin ring formation. These findings improve our understanding of the role of mechanical load in bone remodeling.
MeSH Keywords: Bone Resorption, Carbonic Anhydrase II, Cathepsin K, Matrix Metalloproteinase 9, Osteoclasts, RANK Ligand
Background
During osteoclast differentiation, mononuclear osteoclast precursor cells in the monocyte/macrophage lineage fuse with each other in the presence of osteoclast differentiation factors, including receptor activator of nuclear factor κB (RANK) ligand (RANKL) and macrophage colony-stimulating factor (M-CSF) derived from marrow stromal cells and osteoblasts [1,2]. Morphologically, mature osteoclasts can become multinucleated giant cells and function in bone resorption; these mature osteoclasts decompose hydroxyapatite and extracellular matrix proteins in the inorganic and organic phase of bone [1,3].
Mechanical load derived from activities of daily living, regular exercise, medical devices for bone fracture healing, or orthodontic tooth movement is an important factor in the bone remodeling process [4–7]. Stimulation with a certain amount of compressive force, tension strain, or fluid shear stress induces osteoblastogenesis via the upregulation of Runx2 and Osterix, which are essential transcription factors for differentiation of mature osteoblasts with osteogenic function, including collagen synthesis and alkaline phosphatase activity, from precursor cells [8–12]. Osteoclastic bone resorption is also affected by mechanical stimulation. Several in vitro studies using osteoclast precursor cells have indicated the impacts of transient or intermittent mechanical stimuli on osteoclastogenesis. Cyclic tension force suppresses the differentiation into mature osteoclasts via the downregulation of cell fusion factors, including dendritic cell-specific transmembrane protein (DCSTAMP) and osteoclast-stimulatory transmembrane protein (OCSTAMP) [13,14]. The short-term application (up to 24 h) of compressive force generated by the superposition of a cover glass on osteoclast precursor cells pre-incubated with culture solution containing RANKL facilitates osteoclastogenesis and resorptive function in multinucleated osteoclast-like cells [15]. The effects of mechanical stimulation on osteoclastic bone resorption differ depending on the type and duration of mechanical loading; however, relatively little is known about osteoclastogenesis in vitro as compared to osteoblastogenesis owing to difficulties in applying mechanical stimuli to monocyte/macrophage lineages.
The constitutive differentiation of osteoclast precursor cells into mature osteoclasts occurs on bone tissue [1]; therefore, osteoclast precursor cells as well as mature osteoclasts and osteoblasts are continuously exposed to mechanical stimuli. In orthodontic treatments, continuous mechanical force induces alveolar bone remodeling within the physiological range during orthodontic tooth movement [6]. Recently, we investigated the effects of compressive force on osteoclastogenesis in vitro using the RAW264.7 mouse monocyte/macrophage lineage; cells were continuously exposed to compressive force created by increasing the amount of the culture solution (over 4 days) for RANKL-induced osteoclast differentiation [16]. Continuous stimulation with compressive force induced the fusion of cells by the upregulation of the two cell fusion factors described above via RANK-RANKL signaling. As a result, multinucleated cells positive for tartrate-resistant acid phosphatase (TRAP), a marker of mature osteoclasts, increased depending on the magnitude of the compressive force that was exerted on the cells [16]. To the best of our knowledge, this is the first report of the continuous effects of the direct stimulation of osteoclast precursor cells by compressive force on osteoclastogenesis. In the present study, we hypothesized that resorptive function might be enhanced in osteoclast-like multinucleated cells induced by continuous compressive force; therefore, we examined the expression of bone resorption-related enzymes as well as actin ring organization, which are typical characteristics of mature osteoclasts that efficiently degrade bone matrix. Moreover, we also conducted a pit assay using calcium phosphate-coated plate to determine bone resorption activity in cells.
Material and Methods
Materials
The RAW264.7 cells obtained from Dainippon Pharmaceutical (Osaka, Japan) and maintained in our laboratory [16] were used as osteoclast precursor cells in this study. Penicillin/streptomycin, bovine serum albumin, and Triton X-100 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine serum (FBS) was purchased from HyClone Laboratories (Logan, UT, USA). Soluble RANKL, α-minimal essential medium (α-MEM), phenol red-free α-MEM/F-12, phosphate-buffered saline (PBS), paraformaldehyde, sucrose, and sodium chloride were purchased from Wako Pure Chemical (Osaka, Japan). NucleoSpin RNA, the RNA PCR Kit (PrimeScript), and SYBR Premix Ex Taq solution were purchased from Takara Bio (Otsu, Japan). Alexa Fluor 488-phalloidin and 49,6-diamidino-2-phenylindole (DAPI) were obtained from Thermo Fisher Scientific (Rockford, IL, USA). The TRAP staining kit was purchased from Cosmo Bio (Sapporo, Japan). The mouse anti-carbonic anhydrase II (sc-48351), mouse anti-matrix metalloproteinase-9 (sc-393859), mouse anti-cathepsin K (sc-48353), and mouse anti-β-tubulin (sc-5274) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Biotin-conjugated goat anti-mouse antibodies were obtained from Abcam (Cambridge, UK). The western ECL substrate kit was obtained from Bio-Rad Laboratories (Hercules, CA, USA).
Osteoclast cultures and continuous stimulation with compressive force
Cells were stimulated with compressive force, as described previously [16]. Briefly, cells were seeded on a 96-well plate at a density of 1×105/cm2 in α-MEM containing 10% FBS and 1% (v/v) antibiotic and maintained overnight at 37°C in a humidified atmosphere of 95% air and 5% CO2. After discarding the medium, 0, 100, or 250 μL of α-MEM containing only antibiotics in addition to 100 μL of differentiation medium (α-MEM supplemented with 50 ng/mL soluble RANKL, 10% FBS, and 1% antibiotics) were added to each well. When the total volume of medium in the well was 100, 200, or 350 μL, cells that settled on the bottom of the culture plate were exposed to approximately 0.3, 0.6, or 1.1 g/cm2 compressive force, respectively, for the same total amounts of RANKL and FBS (Figure 1A). A previous study reported that RANKL is indispensable for differentiation of RAW264.7 cells into TRAP-positive osteoclast-like cells, whereas M-CSF is not required for osteoclastogenesis in in vitro study using this cell line [17]. The medium was replaced every other day. TRAP staining was conducted as previously described [16,18,19]; then, the formation of osteoclast-like cells was observed by light microscopy.
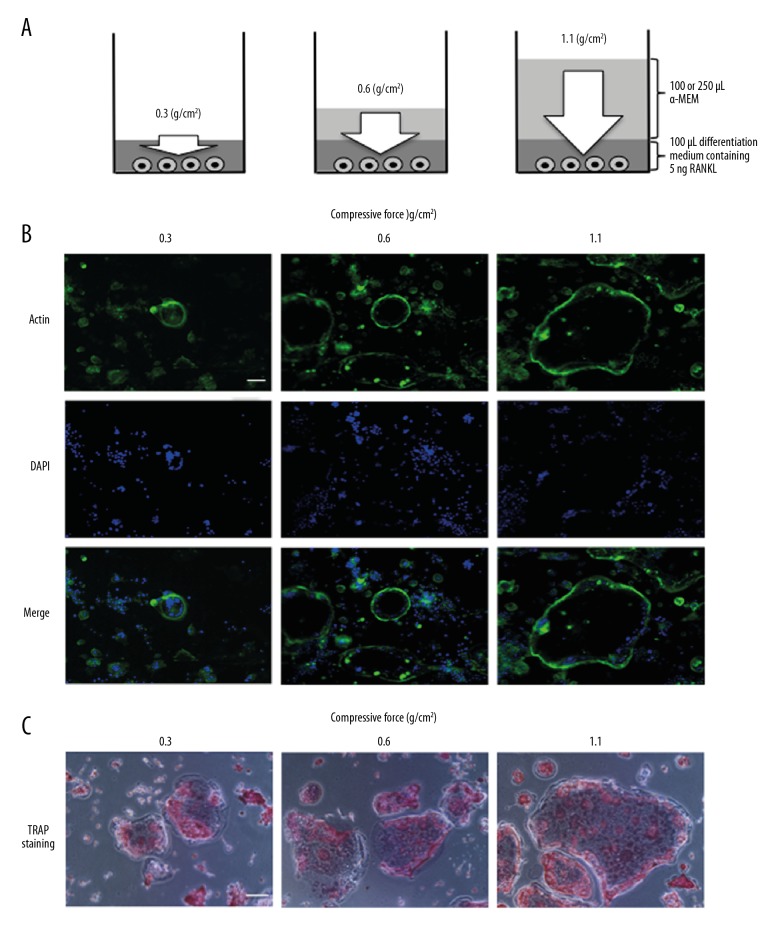
Figure 1.
Schema of compressive force generated by increasing the volume of culture medium and effect of compressive force on actin ring organization and TRAP staining. White arrow indicates compressive force (A). Cells were continuously stimulated with 0.3, 0.6, or 1.1 g/cm2 compressive force in the presence of 5 ng of RANKL for 4 days. Actin labeled with fluorescently tagged phalloidin (green) and nuclei labeled with DAPI (blue) were observed with a fluorescence microscope (B). The cells were stimulated with 0.3, 0.6, or 1.1 g/cm2 compressive force for 4 days, and then stained with TRAP and observed by light microscopy (C). Scale bar=100 μm.
Actin ring observation
Cells were seeded onto glass coverslips placed at the bottom of culture plates, and then were stimulated with compressive force generated by the aforementioned procedure for 4 days. Cells were fixed and permeabilized by the same method as in the previous study [18] using 4% (v/v) paraformaldehyde/2% sucrose and 0.1% Triton X-100, respectively. Actin filaments and nuclei of cells were labeled with two fluorescent dyes, 66 nM Alexa Fluor 488-phalloidin for actin and DAPI for nucleus, after blocking nonspecific binding of the fluorescent dyes by 1% bovine serum albumin. Pictures of actin filaments and nuclei were separately obtained through fluorescence microscope BZ 9000 (Keyence, Osaka, Japan) and merged on a conventional personal computer.
Quantitative real-time reverse transcription (RT)-polymerase chain reaction (PCR)
Total RNA including mRNA was eluted from RAW264.7 cells harvested after stimulating with compressive force for 3 or 4 days using NucleoSpin RNA. cDNA synthesis was carried out with 1 μg RNA in 20 μL of a solution containing random primers, dNTP mixture, and reverse transcriptase, which were bundled with PrimeScript; 2 μL aliquots of the cDNA solution were subjected to quantitative real-time RT-PCR. Twenty-five microliters of the reaction solution contained 1×RPR buffer, 1.5 mM dNTP mixture, 1×SYBR green I, 15 mM NgCl2, 0.25 unit of Ex Taq polymerase (these components were bundled with SYBR Premix Ex Taq solution), and 20 μM forward and reverse primers. Primers were designed against mouse sequences as follows: carbonic anhydrase II (forward: CATTACTGTCAGCAGCGAGCA, reverse: GACGCCAGTTGTCCACCATC), matrix metalloproteinase-9 (forward: GCCCTGGAACTCACACGACA, reverse: TTGGAAACTCACACGCCAGAAG), cathepsin K (forward: CAGCAGA ACGGAGGCATTGA, reverse: CCTTTGCCGTGGCGTTATAC), glyceraldehyde 3-phosphate dehydrogenase (GAPDH; forward: AAATGGTGAAGGTCGGTGTG, reverse: TGAAGGGGTCG TTGATGG) [18,19]. The reaction conditions such as temperature for denaturation, annealing, and extension were the same as those for the previous study [18,19]. Results were analyzed using Smart Cycler software (Cepheid, Sunnyvale, CA, USA). The specificity of the PCR products was verified by a melting curve analysis. The expression levels of carbonic anhydrase II, matrix metalloproteinase-9, and cathepsin K were normalized to those of GAPDH.
Western blotting
Supernatant containing intracellular protein was prepared by the same method described in previous reports [18,19]. Protein (20 μg) was resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes. After blocking of nonspecific binding using 5% skim milk solution, the membranes were incubated with primary antibodies against carbonic anhydrase II, matrix metalloproteinase-9, cathepsin K, and β-tubulin. Then, biotin-conjugated secondary antibodies were added and incubated. Membranes were labeled with streptavidin-horseradish peroxidase and visualized using ECL chemiluminescence. Western blot intensities were quantified using digital image analysis software (Quantity One; Bio-Rad Laboratories).
Mineral resorption activity and pit formation assay
RAW264.7 cells were placed on a fluoresceinated calcium phosphate-coated plate (Bone Resorption Assay Kit 48). Then, cells were continuously stimulated with compressive force generated by increasing the volume of phenol red-free α-MEM/F-12. After 4 days of culture, the amount of calcium eluted from the calcium phosphate plate into the culture medium was evaluated based on fluorescence intensity (wavelength of 485 nm for excitation and of 535 nm for emission). For the observation of the resorption pit, the plates were removed with 5% NaClO. The pit area was observed by light microscopy.
Statistical analysis
Mean differences were tested by ANOVA and Tukey’s multiple comparison tests. Results with P < 0.05 were considered statistically significant.
Results
Effect of continuous compressive force on actin ring organization
We first investigated actin ring organization in cells stimulated with continuous compressive force in the presence of RANKL for 4 days. Multinucleated osteoclast-like cells with actin rings were observed for all three magnitudes of compressive force, and the area of actin rings increased as a function of the applied force (Figure 1B). TRAP staining also revealed that the area of each osteoclast-like cell increased as a function of applied compressive force (Figure 1C).
Effects of continuous compressive force on carbonic anhydrase II, matrix metalloproteinase-9, and cathepsin K expression
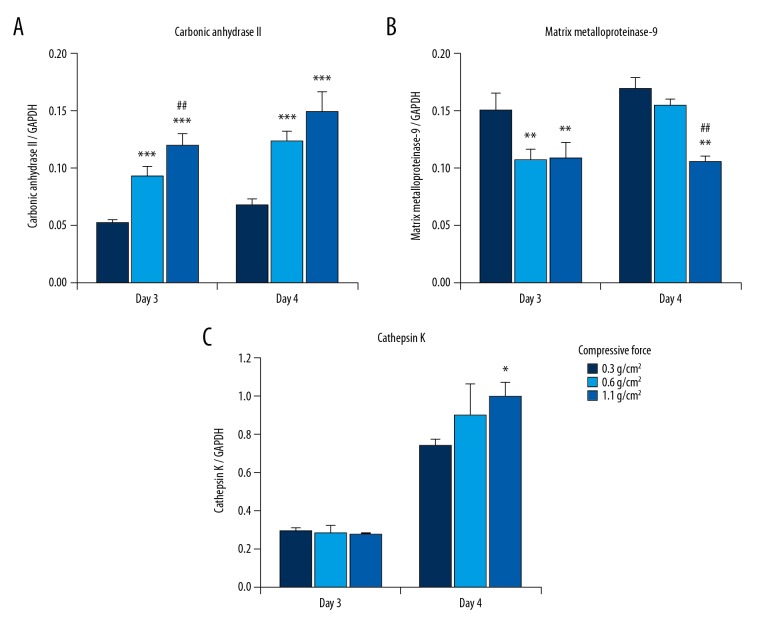
We next examined the effects of continuous compressive force on the mRNA expression of genes encoding carbonic anhydrase II, matrix metalloproteinase-9, and cathepsin K, which are osteoclastic bone resorption-related enzymes, by real-time PCR. Figure 2 shows that carbonic anhydrase II expression was significantly higher, whereas matrix metalloproteinase-9 expression was significantly lower in cells stimulated with 0.6 and/or 1.1 g/cm2 than in cells stimulated with 0.3 g/cm2 on days 3 and 4 of culture (Figure 2A, 2B). The effect of compressive force on cathepsin K expression was observed in cells only on day 4; cathepsin K expression was slightly higher in cells stimulated with 0.6 and 1.1 g/cm2 than in cells stimulated with 0.3 g/cm2, but the difference was only significant for the comparison between 0.3 g/cm2 and 1.1 g/cm2 (Figure 2C). The degree of change induced by compressive force was markedly higher for carbonic anhydrase II than for cathepsin K and matrix metalloproteinase-9; carbonic anhydrase II expression changed by 1.78–2.28-fold, cathepsin K expression changed by 0.98–1.35-fold, and matrix metalloproteinase-9 expression changed by 0.62–0.91-fold in cells stimulated with 0.6 and 1.1 g/cm2 as compared to 0.3 g/cm2. Subsequently, we examined the effects of compressive force on the protein expression of carbonic anhydrase II, matrix metalloproteinase-9, and cathepsin K by western blotting. On day 4 of culture, the protein expression of carbonic anhydrase II and cathepsin K was increased, whereas that of matrix metalloproteinase-9 was decreased, by a compressive force of 1.1 g/cm2 compared to that of 0.3 g/cm2 (Figure 3). Measurement of western blot intensities revealed that the degree of compressive force-induced change was markedly higher for carbonic anhydrase II (7.0-fold) than for cathepsin K (2.0-fold) and matrix metalloproteinase-9 (0.9-fold) in cells stimulated with 1.1 g/cm2 as compared to 0.3 g/cm2.
Figure 2.
Effect of compressive force on mRNA levels of osteoclastic bone resorption-related enzymes in RAW264.7 cells. The mRNA expression levels of carbonic anhydrase II (A), matrix metalloproteinase-9 (B), and cathepsin K (C) were determined by real-time RT-PCR on days 3 and 4 of culture. Bars indicate the means ± standard deviation of 4 independent experiments. * P<0.05, ** P<0.01, *** P<0.001 (vs. 0.3 g/cm2), ## P<0.01 (0.6 vs. 1.1 g/cm2).
Figure 3.
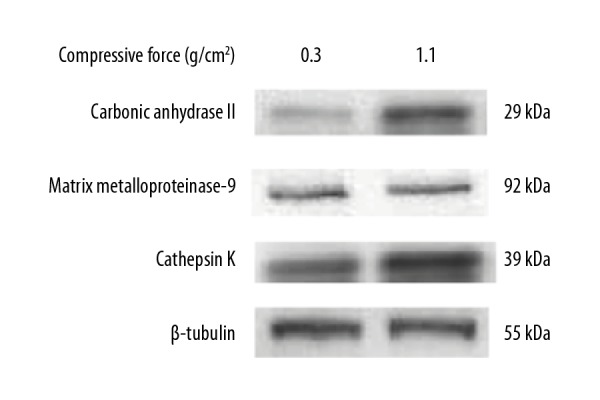
Effect of compressive force on protein levels of osteoclastic bone resorption-related enzymes in RAW264.7 cells. The protein levels of carbonic anhydrase II, matrix metalloproteinase-9, and cathepsin K were determined by western blotting on day 4 of culture. The expression of β-tubulin was used as an internal reference.
Effect of continuous compressive force on mineral resorption activity
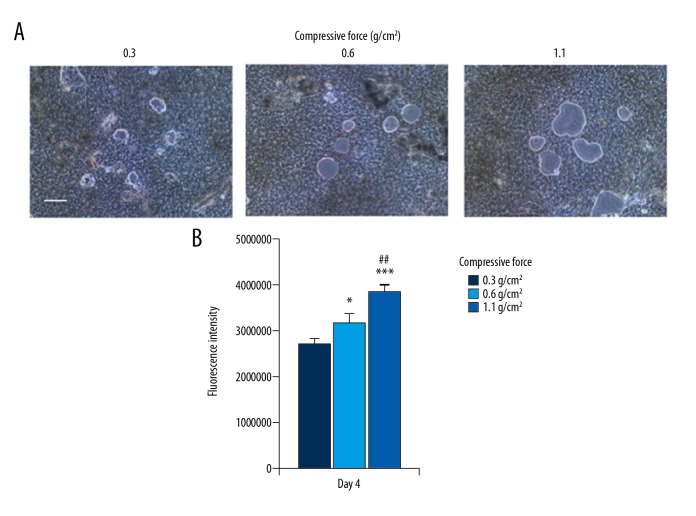
To further examine whether actin ring formation and the elevated expression of carbonic anhydrase II expression affected bone resorptive function, cells on calcium phosphate plates were stimulated with continuous compressive force. After 4 days of culture, restorative pits of various sizes were observed for all three magnitudes of compressive force (Figure 4A). Fluorescence intensity in the culture medium was significantly higher for cells stimulated with 0.6 or 1.1 g/cm2 compressive force than for cells stimulated with 0.3 g/cm2 compressive force and was significantly higher for cells stimulated with 1.1 g/cm2 compressive force than for cells stimulated with 0.6 g/cm2 compressive force (Figure 4B), suggesting that the amount of calcium eluted from the calcium phosphate plate increased as a function of the applied compressive force.
Figure 4.
Effect of compressive force on mineral resorption activity. Cells on the calcium phosphate-coated plate were continuously stimulated with, 0.3, 0.6, or 1.1 g/cm2 compressive force in the presence of 5 ng of RANKL for 4 days. (A) Resorption pits were observed by light microscopy. Scale bar=100 μm. (B) Calcium phosphate released from the plate was quantified by fluorescence intensity. Bars indicate the means ± standard deviation of four independent experiments. * P<0.05, *** P<0.01 (vs. 0.3 g/cm2), ## P<0.01 (0.6 vs. 1.1 g/cm2).
Discussion
Our results indicated that decomposition of the inorganic phase of bone was promoted in osteoclast-like multinucleated cells induced by continuous compressive force during RANKL-induced osteoclastogenesis. The formation of an actin ring that surrounds and isolates the microenvironment of osteoclasts is considered a hallmark of the degradative capacity of osteoclasts because this closed ring contributes to the maintenance of effector molecules, such as hydrogen chloride and proteinases [20,21]. We previously observed TRAP-positive multinucleated cells with a large area when increasing force was applied to RAW264.7 cells in the presence RANKL [16]. In the present study, actin ring organization was observed in the multinucleated giant cells formed by the continuous stimulation of RAW264.7 cells with compressive force following the same methods and conditions used in our previous study. These results suggested that the multinucleated giant cells could create a resorptive microenvironment.
The microenvironment established by the acidic conditions of the actin ring is also important for dissolving the inorganic and organic components of bone. Carbonic anhydrase II converts CO2 into H+ and HCO3−; then, vacuolar H+-ATPase transports H+ to the resorptive zone [22,23]. Increasing H+ induces the mobilization of mineralized components of bone; subsequently, the demineralized organic matrix is degraded by proteinases [21]. In the present study, carbonic anhydrase II expression was markedly elevated by compressive force. Moreover, calcium elution from the calcium phosphate plate increased after the application of compressive force for 4 days. These results suggested that continuous compressive force facilitates the dissolution of the inorganic component of bone via the upregulation of carbonic anhydrase II expression in mature osteoclasts.
Cathepsin K and matrix metalloproteinase-9 produced by mature osteoclasts efficiently cleave type I collagen, which is abundant in the organic phase of bone [21,24,25]. In the present study, matrix metalloproteinase-9 expression decreased, whereas cathepsin K expression increased slightly by the continuous application of compressive force. This variation in the effect of continuous compressive force on proteinase expression in osteoclasts might be involved in the difference between pathologic bone resorption and physiological bone remodeling. Rheumatoid arthritis and periodontitis lead to bone destruction in articular tissue or alveolar bone. Previous studies have indicated that inflammatory cytokines, which are elevated in rheumatoid arthritis and periodontitis, or lipopolysaccharides, the cell wall component of gram-negative rods (including periodontal pathogens, such as Porphyromonas gingivalis), strongly induce the expression of cathepsin K and matrix metalloproteinase-9 as well as carbonic anhydrase II [26–30]. Mitogen-activated protein kinases (MAPKs), including extracellular signal-regulated kinase (ERK), p38, and Jun-N-terminal kinase, play important roles in the protease expression of osteoclasts [31]. Two previous studies using a specific inhibitor of each kinase revealed that the effects of these kinases on the expression of matrix metalloproteinase-9 and cathepsin K varied by the type of stimulation [32,33]. Kim and Lee [32] reported that upregulation of matrix metalloproteinase-9 expression was attenuated only by ERK inhibitor in tumor necrosis factor-α-stimulated osteoclasts. In contrast, Matsumoto et al. [33] revealed that RANKL-induced cathepsin K expression was inhibited by p38 but not ERK inhibitor. The effects of mechanical stress on the expression of matrix metalloproteinase-9 and cathepsin K may also depend on MAPK signals; however, the effects of mechanical stimulation on proteinase expression in osteoclasts remain controversial. Hayakawa et al. [15] previously revealed that the expression of both cathepsin K and matrix metalloproteinase-9 in RAW264.7 cells pre-incubated with RANKL was increased by stimulation with compressive force (~0.3 g/cm2). Xu et al. [34] reported that the effects of mechanical strain on the expression of matrix metalloproteinase-9 and cathepsin K differed, with mechanical strains of 2000 and 2500 μɛ inducing matrix metalloproteinase-9 expression, while cathepsin K expression was not affected by mechanical strain of this magnitude. Mechanical stimulation from physical activity, exercise, and adaptive stress contributes to bone tissue homeostasis [4,5]. Our present results and these previous findings suggest that decomposition of the organic phase of bone might be less aggressive in physiological bone remodeling than in pathologic bone resorption. Further studies are required to clarify the differences in intracellular signal transductions that are involved in the expression of proteases in osteoclasts between inflammatory cytokines and mechanical stimuli.
Conclusions
Our study demonstrated that the stimulation of RAW264.7 cells with continuous compressive force in the presence of RANKL induces the formation of polynuclear giant cells with actin rings and carbonic anhydrase expression. Moreover, these osteoclast-like cells have a high capacity for decalcification, suggesting that continuous compressive force contributes to the promotion of bone resorption in bone remodeling.
Footnotes
Source of support: This study was supported by Grants-in-Aid for Scientific Research (C) (grant no. 17K12022) from the Japanese Society for the Promotion of Science; Sato Fund and Uemura Fund of Nihon University School of Dentistry; and Dental Research Center, Nihon University School of Dentistry
Conflicts of interest.
None.
References
- 1.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 2.Pereira M, Petretto E, Gordon S, et al. Common signalling pathways in macrophage and osteoclast multinucleation. J Cell Sci. 2018;131 doi: 10.1242/jcs.216267. pii: jcs216267. [DOI] [PubMed] [Google Scholar]
- 3.Väänänen HK, Laitala-Leinonen T. Osteoclast lineage and function. Arch Biochem Biophys. 2008;473:132–38. doi: 10.1016/j.abb.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 4.Skerry TM. The response of bone to mechanical loading and disuse: Fundamental principles and influences on osteoblast/osteocyte homeostasis. Arch Biochem Biophys. 2008;473:117–23. doi: 10.1016/j.abb.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 5.Karlsson MK, Rosengren BE. Training and bone – from health to injury. Scand J Med Sci Sports. 2012;22:e15–23. doi: 10.1111/j.1600-0838.2012.01461.x. [DOI] [PubMed] [Google Scholar]
- 6.Qian Y, Fan Y, Liu Z, Zhang M. Numerical simulation of tooth movement in a therapy period. Clin Biomech. 2008;23(Suppl 1):S48–52. doi: 10.1016/j.clinbiomech.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Padilla F, Puts R, Vico L, et al. Stimulation of bone repair with ultrasound. Adv Exp Med Biol. 2016;880:385–427. doi: 10.1007/978-3-319-22536-4_21. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs C, Grimm S, Ziebart T, et al. Osteogenic differentiation of periodontal fibroblasts is dependent on the strength of mechanical strain. Arch Oral Biol. 2013;58:896–904. doi: 10.1016/j.archoralbio.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Mitsui N, Suzuki N, Maeno M, et al. Optimal compressive force induces bone formation via increasing bone morphogenetic proteins production and decreasing their antagonists production by Saos-2 cells. Life Sci. 2006;78:2697–706. doi: 10.1016/j.lfs.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 10.Gao J, Fu S, Zeng Z, et al. Cyclic stretch promotes osteogenesis-related gene expression in osteoblast-like cells through a cofilin-associated mechanism. Mol Med Rep. 2016;14:218–24. doi: 10.3892/mmr.2016.5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu D, Genetos DC, Shao Y, et al. Activation of extracellular-signal regulated kinase (ERK1/2) by fluid shear is Ca2+- and ATP-dependent in MC3T3-E1 osteoblasts. Bone. 2008;42:644–52. doi: 10.1016/j.bone.2007.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L, Yuan W, Wang J. Mechanisms for osteogenic differentiation of human mesenchymal stem cells induced by fluid shear stress. Biomech Model Mechanobiol. 2010;9:659–70. doi: 10.1007/s10237-010-0206-x. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki N, Yoshimura Y, Deyama Y, et al. Mechanical stress directly suppresses osteoclast differentiation in RAW264.7 cells. Int J Mol Med. 2008;21:291–96. [PubMed] [Google Scholar]
- 14.Kameyama S, Yoshimura Y, Kameyama T, et al. Short-term mechanical stress inhibits osteoclastogenesis via suppression of DC-STAMP in RAW264.7 cells. Int J Mol Med. 2013;31:292–98. doi: 10.3892/ijmm.2012.1220. [DOI] [PubMed] [Google Scholar]
- 15.Hayakawa T, Yoshimura Y, Kikuiri T, et al. Optimal compressive force accelerates osteoclastogenesis in RAW264.7 cells. Mol Med Rep. 2015;12:5879–85. doi: 10.3892/mmr.2015.4141. [DOI] [PubMed] [Google Scholar]
- 16.Matsuike R, Tanaka H, Nakai K, et al. Continuous application of compressive force induces fusion of osteoclast-like RAW264.7 cells via upregulation of RANK and downregulation of LGR4. Life Sci. 2018;201:30–36. doi: 10.1016/j.lfs.2018.03.038. [DOI] [PubMed] [Google Scholar]
- 17.Takayanagi H. Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka H, Tanabe N, Kawato T, et al. Nicotine affects bone resorption and suppresses the expression of cathepsin K, MMP-9 and vacuolar-type H+-ATPase d2 and actin organization in osteoclasts. Plos One. 2013;8:e59402. doi: 10.1371/journal.pone.0059402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitami S, Tanaka H, Kawato T, et al. IL-17A suppresses the expression of bone resorption-related proteinases and osteoclast differentiation via IL-17RA or IL-17RC receptors in RAW264.7 cells. Biochimie. 2010;92:398–404. doi: 10.1016/j.biochi.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Akisaka T, Yoshida H, Inoue S, Shimizu K. Organization of cytoskeletal F-actin, G-actin, and gelsolin in the adhesion structures in cultured osteoclast. J Bone Miner Res. 2001;16:1248–55. doi: 10.1359/jbmr.2001.16.7.1248. [DOI] [PubMed] [Google Scholar]
- 21.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–8. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 22.Lehenkari P, Hentunen TA, Laitala-Leinonen T, et al. Carbonic anhydrase II plays a major role in osteoclast differentiation and bone resorption by effecting the steady state intracellular pH and Ca2+ Exp Cell Res. 1998;242:128–37. doi: 10.1006/excr.1998.4071. [DOI] [PubMed] [Google Scholar]
- 23.Maxson ME, Grinstein S. The vacuolar-type H+-ATPase at a glance-more than a proton pump. J Cell Sci. 2014;127:4987–93. doi: 10.1242/jcs.158550. [DOI] [PubMed] [Google Scholar]
- 24.Andersen T, del Carmen Ovejero M, Kirkegaard T, et al. A scrutiny of matrix metalloproteinases in osteoclasts: Evidence for heterogeneity and for the presence of MMPs synthesized by other cells. Bone. 2004;35:1107–19. doi: 10.1016/j.bone.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Cawston TE, Young DA. Proteinases involved in matrix turnover during cartilage and bone breakdown. Cell Tissue Res. 2010;339:221–35. doi: 10.1007/s00441-009-0887-6. [DOI] [PubMed] [Google Scholar]
- 26.Fujisaki K, Tanabe N, Suzuki N, et al. Receptor activator of NF-kappaB ligand induces the expression of carbonic anhydrase II, Cathepsin K, and matrix metalloproteinase-9 in osteoclast precursor RAW264.7 cells. Life Sci. 2007;80:1311–18. doi: 10.1016/j.lfs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 27.Hou GQ, Guo C, Song GH, et al. Lipopolysaccharide (LPS) promotes osteoclast differentiation and activation by enhancing the MAPK pathway and COX2 expression in. Int J Mol Med. 2013;32:503–10. doi: 10.3892/ijmm.2013.1406. [DOI] [PubMed] [Google Scholar]
- 28.Sapkota M, Li L, Kim SW, Soh Y. Thymol inhibits RANKLinduced osteoclastogenesis in. Food Chem Toxicol. 2018;120:418–29. doi: 10.1016/j.fct.2018.07.032. [DOI] [PubMed] [Google Scholar]
- 29.Inoue M, Naritani M, Raju R, et al. Effect of short-term tumors necrosis factor-alpha (TNF-α) stimulation on the growth and differentiation of MC3T3-E1 osteoblast-like cells. J Hard Tissue Biol. 2018;27:213–18. [Google Scholar]
- 30.Guo C, Hou GQ, Li XD, et al. Quercetin triggers apoptosis of lipopolysaccharide (LPS)induced osteoclasts and inhibits bone resorption in. Cell Physiol Biochem. 2012;30:123–36. doi: 10.1159/000339052. [DOI] [PubMed] [Google Scholar]
- 31.Lee K, Seo I, Choi MH, et al. Roles of mitogen-activated protein kinases in osteoclast biology. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19103004. pii: E3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim KC, Lee CH. MAP kinase activation is required for the MMP-9 induction by TNF-stimulation. Arch Pharm Res. 2005;28:1257–62. doi: 10.1007/BF02978209. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto M, Kogawa M, Wada S, et al. Essential role of p38 mitogen-activated protein kinase in cathepsin K gene expression during osteoclastogenesis through association of NFATc1 and PU.1. J Biol Chem. 2004;279:45969–79. doi: 10.1074/jbc.M408795200. [DOI] [PubMed] [Google Scholar]
- 34.Xu XY, Guo C, Yan YX, et al. Differential effects of mechanical strain on osteoclastogenesis and osteoclast-related gene expression in RAW264.7 cells. Mol Med Rep. 2012;6:409–15. doi: 10.3892/mmr.2012.908. [DOI] [PubMed] [Google Scholar]