Abstract
Background
Polycystic ovary syndrome (PCOS) is associated with infertility or subfertility due to impaired ovulation. Clomiphene citrate is a first-line treatment option for the induction of ovulation in women with PCOS. The study aimed to compare markers of oxidative stress or the total oxidative status (TOS), total antioxidant status (TAS), and levels of paraoxonase-1 (PON-1) before and after day 21 of the menstrual cycle in women with PCOS treated with clomiphene citrate to induce ovulation.
Material/Methods
The study included 75 women who were divided into a control group (n=25) that included healthy untreated women, untreated women with PCOS (n=24) who had spontaneous ovulation, and women with PCOS who were treated with clomiphene citrate for subfertility or infertility (n=26) (the PCOS-CC group). The study group was treated for five days with clomiphene citrate (50 mg/day). Peripheral venous blood was sampled on day 3 and day 21 of the menstrual cycle from women in all three groups, and TAS, TOS, and PON-1 levels were measured.
Results
In all three groups, TAS and PON levels were significantly reduced and TOS values were significantly increased on day 21 of the menstrual cycle. Comparison of TAS, TOS, and PON-1 levels between the three study groups on day 3 and day 21 of the menstrual cycle showed no significant difference (p=0.600, p=0.223, p=0.956, respectively).
Conclusions
This study showed that spontaneous ovulation occurs in association with an oxidative state in healthy women and women with PCOS, and women with PCOS following treatment with clomiphene citrate.
MeSH Keywords: Antioxidants, Aryldialkylphosphatase, Oxidative Stress, Polycystic Ovary Syndrome
Background
Polycystic ovary syndrome (PCOS), or Stein–Leventhal syndrome, is a syndrome that includes amenorrhea, menometrorrhagia, hirsutism, and infertility, and the ovaries contain multiple cysts and have a thickened capsule [1]. The clinical features of PCOS include hirsutism (69%), infertility (74%), amenorrhea (51%), obesity (41%), functional bleeding (29%), and irregular menstrual cycles (12%) [2]. Endocrinological studies have shown that plasma levels of luteinizing hormone (LH) and androgens in the early follicular phase are increased in women with PCOS when compared with normal women [3,4]. PCOS is also associated with metabolic disorders including obesity, insulin resistance, and diabetes mellitus [5,6]. These metabolic disorders can affect many tissues and organ systems. In women with PCOS, the prevalence of obesity is between 30–78% [7,8]. Exposure to androgens, derived from ovarian and adrenal tissue, results in low plasma adiponectin levels and adipose tissue dysfunction [9]. Clomiphene citrate induces ovulation by blocking estrogen receptors.
Oxidative stress results from a lack of antioxidant production or exposure to excess oxidants [10]. Dysfunction of adipocytes causes a local and systemic increase in the release of cytokines, resulting in oxidative stress [11,12]. Free radicals that result in oxidative stress are derived from oxygen or nitrogen and can be classified as reactive oxygen species (ROS) and reactive nitrogen species (RNS). While low and medium levels of ROS and RNS can act as a defense against infection, they sometimes serve in intracellular signaling systems. When ROS and RNS levels exceed physiological limits, they can damage DNA, intracellular lipids, and proteins. There are physiological mechanisms, repair mechanisms, and preventive mechanisms for defense against ROS [13]. Antioxidants include the enzymes superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx), non-enzymatic macromolecules (albumin and ferritin), small molecules (ascorbic acid, glutathione, and vitamin E) and paraoxonase-1 (PON-1) [14,15].
The total oxidative status (TOS) and total antioxidant status (TAS) can be measured biochemically [16]. Previously published studies have investigated the oxidative status of patients with PCOS and have demonstrated increased TOS values in women with PCOS [17]. The enzyme PON-1 is composed of 354 amino acids and is secreted from the liver and has been shown to have anti-inflammatory and anti-oxidative roles. PON-1 protects low-density lipoprotein (LDL) from oxidation [18].
Women with PCOS have difficulty with ovulation that results in subfertility and infertility. Clomiphene citrate is a nonsteroidal triphenylethylene compound that is used as the first-line treatment for induction of ovulation and is administered orally, with a half-life of approximately five days. However, despite the high ovulation rates in patients who use clomiphene citrate, pregnancy rates are low [19]. The mechanism of the low pregnancy rate in patients undergoing induction of ovulation with clomiphene citrate remains poorly understood. However, several studies have reported that this failure rate was associated with the anti-estrogenic effect of clomiphene citrate on the endometrium and cervical mucus. A preclinical study in a rat model of PCOS showed an increase in hydrogen peroxide levels and a decrease in catalase activity in the rat ovaries following clomiphene citrate administration [20]. Following melatonin treatment, hydrogen peroxide levels decreased, and catalase activity increased in the rat ovaries [20].
Therefore, this study aimed to compare markers of oxidative stress, the levels of TOS, TAS, and paraoxonase-1 (PON-1) before and after day 21 of the menstrual cycle in women with PCOS treated with clomiphene citrate to induce ovulation.
Material and Methods
Patients and study groups
The patients studied were selected from women who attended the Hitit University Faculty of Medicine, Gynecology and Obstetrics Clinic. Before the start of the study, approval was obtained from the Ethics Committee of the Hitit University Medical Faculty, and all procedures were undertaken in accordance with the Helsinki Declaration (Study Approval Number: 2017-87).
A case-control included women who were aged between 21–36 years who were evaluated between November 2017 and April 2018. The diagnosis of polycystic ovary syndrome (PCOS) was made according to the European Society of Human Reproduction and Embryology (ESHRE) and the American Society of Reproductive Medicine (ASRM) consensus PCOS criteria [21].
The study included 75 women who were divided into a control group (n=25) that included healthy untreated women, untreated women with PCOS (n=24) who had spontaneous ovulation, and women with PCOS who were treated with clomiphene citrate for subfertility or infertility (n=26) (the PCOS-CC group). The study group was treated for five days with clomiphene citrate (50 mg/day). Informed consent was obtained from the patients included in the study.
Physical examination, homeostatic model assessment (HOMA), and insulin resistance index (IRI)
Physical examination of the patients was performed. Body hair was scored and recorded using the Ferriman–Gallwey scoring system [22]. The body mass index (BMI) was calculated by measuring height and weight in the clinic. After a three-day diet that included ≥150 gm carbohydrate, the homeostatic model assessment (HOMA) and insulin resistance index (IRI) were calculated based on the fasting glucose and insulin values. The IRI calculation was performed using the formula: fasting glucose (mmol/1)×fasting insulin (μIU/mL)/22.5 [23].
Exclusion criteria
Women who had diabetes mellitus, renal disease, or cardiovascular disease, liver dysfunction, oral contraceptive use in their medical history, and anti-inflammatory or anti-oxidant use within the previous six months were excluded from the study. None of the patients had an ovarian cyst, unexplained vaginal bleeding, or pregnancy.
Clomiphene citrate treatment (the PCOS-CC group)
Following the exclusion of other causes of infertility, apart from PCOS, the PCOS-CC patients underwent five days of treatment with 50 mg/day of oral clomiphene citrate treatment (serophene, 50 mg) for ovarian stimulation. The patients were examined by transvaginal ultrasound using the Logiq P5 2015 (GE Healthcare Life Sciences, Logan, UT, USA) following treatment with clomiphene citrate.
Serum measurements of hormones and oxidation factors
Peripheral venous blood was taken on day 3 and day 21 of the menstrual cycle from all 75 women in the three study groups. Ovulation was demonstrated by measurement of the progesterone values obtained on day 21 of the menstrual cycle. Follicle stimulating hormone (FSH), luteinizing hormone (LH), thyroid stimulating hormone (TSH), and estradiol (E2) levels were measured on the 3rd day of the cycle. Approximately 2 ml of serum was used from each study participant to measure the total oxidative status (TOS) and total antioxidant status (TAS) values using an automated method as described by Erel [16,24]. Serum levels of paraoxonase-1 (PON-1) were measured using a colorimetric method [25]. On day 21 of the menstrual cycle, progesterone TAS, TOS, PON-1 and high-density lipoprotein (HDL) and low-density lipoprotein (LDL) levels were measured.
Statistical analysis
Statistical analysis was performed using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA). Data were presented as the mean ± standard deviation (SD) and the median values. According to distribution assumptions for continuous variables, categorical data were presented using numbers and percentages. Normally distributed data were analyzed using the Kolmogorov-Smirnov, and Shapiro–Wilk tests. Homogeneity of the variables was analyzed using the Levene test. Mauchly’s sphericity test for repeated measures of analysis of variance (ANOVA) and comparison between groups of the continuous variables was performed using ANOVA or the Kruskal-Wallis variance analysis test, according to the normal distribution of the data. Bonferroni pairwise comparisons were used. A paired t-test was performed as parametric distributions were enhanced in the comparison of the mean of two dependent samples. Repeat ANOVA was performed to compare the mean TAS, TOS, and PON-1 values in the three study groups. A P-value <0.05 was considered to be statistically significant.
Results
There were 25 patients in the control group, 24 patients in the polycystic ovary syndrome (PCOS) group, and 26 patients in the group with PCOS who were treated with clomiphene citrate for subfertility or infertility (the PCOS-CC group). The average age of the patients was 27.4±3.4 years (range, 21–32 years) in the control group, 27.5±3.4 years (21–36 years) in the PCOS group, and 27.7±3.7 years (22–36 years) in the PCOS-CC group. No significant differences were found between the groups in terms of their average age (p=0.965). From the three patient groups, 44% of the control group (n=11), 33.3% of the PCOS group, and 65.4% (n=17) of the PCOS-CC group did not have children (Table 1).
Table 1.
Fertility status of the three study groups, the control group, the untreated group with polycystic ovary syndrome (PCOS), and the PCOS group treated with clomiphene citrate (PCOS-CC).
| Number of children | Total | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||||
| Diagnosis | Control group | n | 11 | 6 | 7 | 1 | 25 |
| % | 44.0% | 24.0% | 28.0% | 4.0% | 100.0% | ||
| PCOS group | n | 8 | 12 | 4 | 0 | 24 | |
| % | 33.3% | 50.0% | 16.7% | 0.0% | 100.0% | ||
| PCOS-CC group | n | 17 | 7 | 2 | 0 | 26 | |
| % | 65.4% | 26.9% | 7.7% | 0.0% | 100.0% | ||
| Total | n | 36 | 25 | 13 | 1 | 75 | |
| % | 48.0% | 33.3% | 17.3% | 1.3% | 100.0% | ||
Comparison of the biochemical values between the groups showed that levels of luteinizing hormone (LH) were significantly different between the control, the PCO, and the PCO-CC groups on the 3rd day of the cycle, and were significantly lower in the control group. No significant differences were found between the PCOS and PCOS-CC groups (p<0.001, p=0.835). The homeostatic model assessment (HOMA) index, the body mass index (BMI), and the Ferriman-Gallwey scores were increased in the PCOS and PCO-CC groups, but these indices and scores were not significantly different between the PCOS and PCOS-CC groups (Table 2).
Table 2.
Comparison of biochemical values in the three study groups, the control group, the untreated group with polycystic ovary syndrome (PCOS), and the PCOS group treated with clomiphene citrate (PCOS-CC).
| Diagnosis | N | Mean ±SD | P-value | Post-hoc p-value | ||
|---|---|---|---|---|---|---|
| TSH (IU/L) | Control | 25 | 2.46±0.60 | 2.44 (1.22–3.67) | 0.247a | – |
| PCOS | 24 | 2.75±0.69 | 2.68 (1.36–3.99) | |||
| PCOS-CC | 26 | 2.72±0.70 | 2.76 (1.25–3.98) | |||
| FSH (IU/L) | Control | 25 | 4.94±1.74 | 4.21 (2.54–8.74) | 0.720b | – |
| PCOS | 24 | 4.72±1.82 | 4.23 (2.14–9.34) | |||
| PCOS-CC | 26 | 4.73±1.96 | 3.90 (2.61–8.85) | |||
| LH (IU/L) | Control (1) | 25 | 3.97±1.76 | 3.55 (1.87–8.58) | <0.001*b | 1–2: 0.001* |
| PCOS (2) | 24 | 6.30±1.98 | 6.06 (3.74–11.41) | 1–3: <0.001* | ||
| PCOS-CC (3) | 26 | 7.15±2.18 | 6.44 (3.99–11.32) | 2–3: 0.835 | ||
| E2 (pg/ml) | Control | 25 | 101.32±25.04 | 100.00 (44,25–136.66) | 0.808b | – |
| PCOS | 24 | 98.95±20.80 | 100.23 (39.76–132.22) | |||
| PCOS-CC | 26 | 97.26±19.13 | 100.27 (56.87–121.25) | |||
| HDL (mg/dl) | Control | 25 | 55.72±5.39 | 55.56 (43.64–69.00) | 0.627a | – |
| PCOS | 24 | 57.19±6.37 | 56.96 (44.00–6.00) | |||
| PCOS-CC | 26 | 56.86±5.04 | 56.77 (49.69–66.87) | |||
| LDL (mg/dl) | Control | 25 | 111.21±13.00 | 111.21 (85.58–130.00) | <0.001*b | 1–2: 0.002* |
| PCOS | 24 | 125.02±9.77 | 125.34 (96.25–141.25) | 1–3: <0.001* | ||
| PCOS-CC | 26 | 127.04±9.24 | 128.52 (100.33–141.00) | 2–3: 1.000 | ||
| HOMA | Control | 25 | 2.05±0.25 | 2.02 (1.34–2.45) | <0.001*b | 1–2: 0.001* |
| PCOS | 24 | 2.36±0.29 | 2.36 (1.54–2.87) | 1–3: 0.001* | ||
| PCOS-CC | 26 | 2.40±0.46 | 2.43 (1.45–3.50) | 2–3: 1.000 | ||
| BMI (kg/m2) | Control | 25 | 21.62±1.10 | 21.25 (20.00–24.96) | <0.001*b | 1–2: <0.001* |
| PCOS | 24 | 24.43±2.43 | 24.28 (19.98–29.25) | 1–3: 0.001* | ||
| PCOS-CC | 26 | 24.29±3.17 | 24.39 (18.65–29.58) | 2–3: 1.000 | ||
| FER-GAL | Control | 25 | 2,.9±1.05 | 2.20 (2.00–5.00) | <0.001*b | 1–2: <0.001* |
| PCOS | 24 | 8.77±2.60 | 8.87 (4.40–12.25) | 1–3: <0.001* | ||
| PCOS-CC | 26 | 10.15±2.43 | 10.00 (5.00–14.00) | 2–3: 0.725 |
ANOVA, analysis of variance;
Kruskall-Wallis test;
TSH – thyroid stimulating hormone; FSH – follicular stimulating hormone; LH – luteinizing hormone; E2 – estradiol; BMI – body mass index; FERGAL – Ferriman-Gallwey score.
The mean scores on day 3 and day 21 of the menstrual cycle were compared between the groups. The total antioxidant status (TAS), and levels of paraoxonase-1 (PON-1) values were found to decrease significantly on the 21st day of the menstrual cycle in all groups, and TOS values were found to increase on day 21 of the menstrual cycle (p<0.001) (Table 3). Study participants in the PCOS group had hyperandrogenism and polycystic ovary morphology. Patients in the PCOS-CC group had irregular menstruation and polycystic ovarian morphology. When the TAS, TOS, and PON values of these groups were compared for the TAS cycle between day 3 to day 21 (p=0.192, 0.414), the TOS cycle between day 3 to day 21 (p=0.605, 0.593), and the PON cycle between day 3 to day 21 (p=0. 592, 0.396), there were no significant differences.
Table 3.
Comparison of the total oxidative status (TOS), total antioxidant status (TAS), and levels of paraoxonase (PON) before and after day 21 of the menstrual cycle in women with polycystic ovary syndrome (PCOS) treated with clomiphene citrate to induce ovulation.
| Diagnosis | N | Mean ±SD | p-Value | |
|---|---|---|---|---|
| Control | TAS-3 | 25 | 1.40±0.07 | <0.001* |
| TAS-21 | 25 | 1.31±0.09 | ||
| TOS-3 | 25 | 18.99±3.20 | <0.001* | |
| TOS-21 | 25 | 21.23±3.69 | ||
| PON-3 | 25 | 138.55±17.80 | <0.001* | |
| PON-21 | 25 | 132.52±17.74 | ||
| PCOS | TAS-3 | 24 | 1.15±0.06 | <0.001* |
| TAS-21 | 24 | 1.06±0.06 | ||
| TOS-3 | 24 | 33.78±5.35 | <0.001* | |
| TOS-21 | 24 | 37.22±5.08 | ||
| PON-3 | 24 | 79.01±17.24 | <0.001* | |
| PON-21 | 24 | 73.12±15.27 | ||
| PCOS-CC | TAS-3 | 26 | 1.18±0.06 | <0.001* |
| TAS-21 | 26 | 1.08±0.078 | ||
| TOS-3 | 26 | 35.47±5.21 | <0.001* | |
| TOS-21 | 26 | 38.76±4.40 | ||
| PON-3 | 26 | 73.62±15.94 | <0.001* | |
| PON-21 | 26 | 67.24±1,26 |
Paired t-test.
PCOS – polycystic ovary syndrome (untreated); TOS – total oxidative status; TAS – total antioxidant status; PCOS-CC – patients with PCOS treated with clomiphene citrate; PON – paraoxonase; TAS-3 – TAS value on the day 3 of the menstrual cycle; TAS-21 – TAS value on day 21 of the menstrual cycle; TOS-3 – TOS value on day 3 of the menstrual cycle; TOS-21 – TOS value on day 21 of the menstrual cycle; PON-3 – PON-1 value on day 3 of the menstrual cycle; PON-21 – PON-1 value on day 21 of the menstrual cycle.
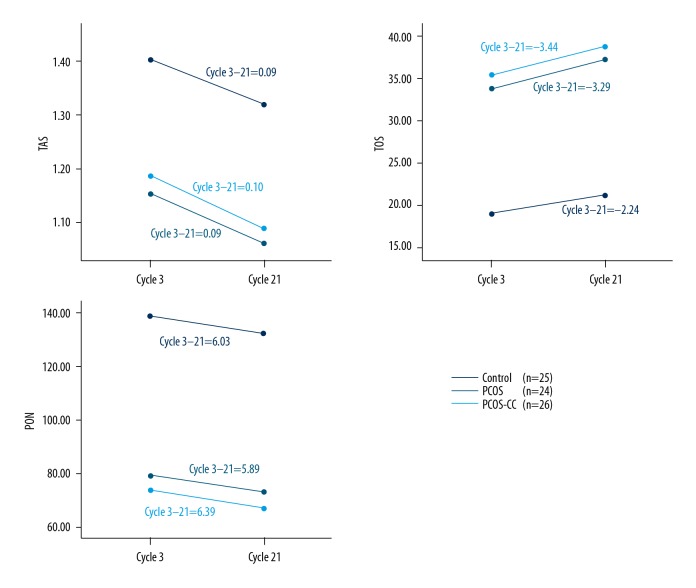
The results of the repeated analysis of variance (ANOVA) showed that the interaction on day 3 and day 21 of the menstrual cycle of the TAS, TOS, and PON measures were not significant between the groups (p=0.600, p=0.223, p=0.956 respectively), indicating that the changes in the TAS, TOS, and PON values were similar on day 3 to day 21 of the menstrual cycle in the control, PCOS, and PCOS-CC groups (Figure 1).
Figure 1.
Changes in total oxidative status (TOS), total antioxidant status (TAS), and levels of paraoxonase-1 (PON-1) from day 3 to day 21 of the menstrual cycle. Slopes of the regression lines are equal. The regression lines are parallel.
Discussion
Polycystic ovary syndrome (PCOS) is the most common cause of anovulatory infertility. The arrest of folliculogenesis that occurs in PCOS is associated with an abnormal ovarian micro-environment. Clomiphene citrate induces ovulation by blocking estrogen receptors, is effective, well-tolerated, safe, and cost-effective and has been recommended as a first-line treatment for women with PCOS who plan for future pregnancy [26–28]. Clomiphene citrate use is recommended at a dose of 100 mg/day or less, but can be increased to up to 250 mg/day if required [29]. In the present study, a 5-day dose of 50 mg/day of clomiphene citrate was used. The study included women who were divided into a control group that included healthy untreated women, untreated women with PCOS who had spontaneous ovulation, and women with PCOS who were treated with clomiphene citrate for subfertility or infertility (the PCOS-CC group)
This study compared the levels of total oxidative status (TOS), total antioxidant status (TAS), and levels of paraoxonase-1 (PON-1) before and after day 21 of the menstrual cycle in women with PCOS treated with clomiphene citrate to induce ovulation. The findings showed an increased level of TOS from day 3 to day 21, and a decrease in the levels of TAS and PON-1 from day 3 to day 21 of the menstrual cycle. As all the patients in the three study groups experienced spontaneous ovulation, it is likely that this situation is physiological.
In a previously published study, plasma markers of oxidative stress were present during two-thirds of the menstrual cycle [30]. In a previous study that compared patients with premenstrual syndrome with healthy volunteers, oxidative stress markers were found to be increased on day 21 of the menstrual cycle [31]. Previous studies have also shown that high levels of oxidative stress in patients with PCOS is associated with an increased risk of cardiovascular disease [32]. These findings have resulted in studies that have investigated the effects of regulating the reduced oxidative state of PCOS. In a previously published study that investigated the effects of antioxidant supplementation in patients with PCOS, fertility rates of women were shown to improve [33]. In another study, vitamin D and omega-3 supplementation were shown to increase plasma total antioxidant levels in women with PCOS [34].
Proteolytic enzymes within the ovarian follicle during spontaneous ovulation are responsible for degrading the follicle wall [35]. The increase in gonadotropin before ovulation results in a peak increase in prostaglandin in the follicle fluid [36]. Prostaglandins and proteolytic enzymes are reported to be necessary for the success of the ovulation. Therefore, the findings of the present study support the presence of an oxidative environment in the ovary. The findings of a previously published study that included a 41 in-vitro fertilization (IVF) cycle showed a positive correlation between positive pregnancy outcomes and lipid peroxidation of the fluid in the ovarian follicle [37].
In the present study, the increased Ferriman–Gallwey scores for body hair of the PCOS and PCOS-CC groups, when compared with the control group, were associated with hyperandrogenism. Between 70–80% of women with PCOS have increased free serum testosterone levels [38]. in this study, an increased homeostatic model assessment (HOMA) index, body mass index (BMI), and low-density lipoprotein (LDL) values when compared with the control group showed that PCOS is associated with metabolic syndrome. The prevalence of metabolic syndrome in women with PCOS has previously been reported to be 45% [39].
In the present study, clomiphene citrate treatment was found to have no effects on TOS, TAS and PON-1 levels when compared with the other study groups. However, starting clomiphene citrate treatment on day 5 of the menstrual cycle, finishing treatment on day 10 of the menstrual cycle, and measuring the control levels on day 21 of the menstrual cycle indicated that the effect of clomiphene citrate on oxidative status was lost. Independent of the study grouping, comparison of the changes from day 3 to day 21 of the menstrual cycle showed no significant differences between the groups, indicating that oxidative status was a physiological state.
This study had several limitations. The number of patients included in each of the three groups was determined by power analysis. Exclusion criteria reduced the number of study participants. Also, the TAS, TOS and PON-1 levels were not examined on day 10 of menstruation, and standard laboratory diagnostic kits were used for serum analysis.
Conclusions
The findings of this study showed that spontaneous ovulation was associated with oxidation, which was independent of the use of clomiphene citrate and whether or not the woman had polycystic ovary syndrome (PCOS).
Footnotes
Source of support: This study was supported by Hitit University (Grant No: TIP19001.18.001)
Conflict of interest
None.
References
- 1.Stein IF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29:181–91. [Google Scholar]
- 2.Goldzieher JW, Axelrod LR. Clinical and biochemical features of polycystic ovarian disease. Fertil Steril. 1963;14:631–53. doi: 10.1016/s0015-0282(16)35047-6. [DOI] [PubMed] [Google Scholar]
- 3.Yen SS, Vela P, Rankin J. Inappropriate secretion of follicle-stimulating hormone and luteinizing hormone in polycystic ovarian disease. J Clin Endocrinol Metab. 1970;30:435–42. doi: 10.1210/jcem-30-4-435. [DOI] [PubMed] [Google Scholar]
- 4.DeVane GW, Czekala NM, Judd HL, Yen SS. Circulating gonadotropins, estrogens, and androgens in polycystic ovarian disease. Am J Obstet Gynecol. 1975;121:496–500. doi: 10.1016/0002-9378(75)90081-2. [DOI] [PubMed] [Google Scholar]
- 5.Burghen GA, Givens JR, Kitabchi AE. Correlation of hyperandrogenism with hyperinsulinism in polycystic ovarian disease. J Clin Endocrinol Metab. 1980;50:113–16. doi: 10.1210/jcem-50-1-113. [DOI] [PubMed] [Google Scholar]
- 6.Chang RJ, Nakamura RM, Judd HL, Kaplan SA. Insulin resistance in non-obese patients with polycystic ovarian disease. J Clin Endocrinol Metab. 1983;57:356–59. doi: 10.1210/jcem-57-2-356. [DOI] [PubMed] [Google Scholar]
- 7.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–36. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 8.Kirchengast S, Peterson B, Hauser G, Knogler W. Body composition characteristics are associated with the bone density of the proximal femur end in middle- and old-aged women and men. Maturitas. 2001;39:133–45. doi: 10.1016/s0378-5122(01)00205-5. [DOI] [PubMed] [Google Scholar]
- 9.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–51. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 10.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–44. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez-Real JM, Broch M, Vendrell J, Ricart W. Insulin resistance, inflammation, and serum fatty acid composition. Diabetes Care. 2003;26:1362–68. doi: 10.2337/diacare.26.5.1362. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez F, Rote NS, Minium J, Kirwan JP. Reactive oxygen species-induced oxidative stress in the development of insulin resistance and hyperandrogenism in polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:336–40. doi: 10.1210/jc.2005-1696. [DOI] [PubMed] [Google Scholar]
- 13.Valko M, Leibfritz D, Moncola J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Matés JM, Pérez-Gómez C, Núñez de Castro I. Antioxidant enzymes and human diseases. Clin Biochem. 1999;32:595–603. doi: 10.1016/s0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 15.Cantin AM, Fells GA, Hubbard RC, Crystal RG. Antioxidant macromolecules in the epithelial lining fluid of the normal human lower respiratory tract. J Clin Invest. 1990;86:962–71. doi: 10.1172/JCI114798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–11. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Zhang R, Liu H, Bai H, et al. Oxidative stress status in Chinese women with different clinical phenotypes of polycystic ovary syndrome. Clin Endocrinol (Oxf) 2017;86:88–96. doi: 10.1111/cen.13171. [DOI] [PubMed] [Google Scholar]
- 18.Daniil G, Phedonos AAP, Holleboom AG, et al. Characterization of antioxidant/anti-inflammatory properties and apoA-I-containing subpopulations of HDL from family subjects with monogenic low HDL disorders. Clin Chim Acta. 2011;412:1213–20. doi: 10.1016/j.cca.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Mitwally FM, Casper RF. Aromatase inhibition for ovarian stimulation: Future-avenues for infertility management. Curr Opin Obstet Gynecol. 2002;14:255–63. doi: 10.1097/00001703-200206000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Tripathi A, PremKumar KV, Pandey AN, et al. Melatonin protects against clomiphene citrate-induced generation of hydrogen peroxide and morphological apoptotic changes in rat eggs. Eur J Pharmacol. 2011;667:419–24. doi: 10.1016/j.ejphar.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS) Hum Reprod. 2012;27:14–24. [Google Scholar]
- 22.Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440–47. doi: 10.1210/jcem-21-11-1440. [DOI] [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–19. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 24.Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277–85. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 25.Eckerson HW, Wyte CM, La Du B. The human serum paraoxonase/arylesterase polymorphism. Am J Hum Genet. 1983;35:1126–38. [PMC free article] [PubMed] [Google Scholar]
- 26.Vause TDR, Cheung AP Reproductive Endocrinology and Infertility Committee. Ovulation induction in polycystic ovary syndrome. J Obstet Gynaecol Can. 2010;32(5):495–502. doi: 10.1016/S1701-2163(16)34504-2. [DOI] [PubMed] [Google Scholar]
- 27.National Institute for Health and Care Excellence (NICE) Fertility evidence update 74. London: National Institute for Health and Care Excellence; Mar, 2015. 2015. [Google Scholar]
- 28.Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565–92. doi: 10.1210/jc.2013-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Von Hofe J, Bates GW. Ovulation induction. Obstet Gynecol Clin North Am. 2015;42:27–37. doi: 10.1016/j.ogc.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Cornelli U, Belcaro G, Cesarone MR, Finco A. Analysis of oxidative stress during the menstrual cycle. Reprod Biol Endocrinol. 2013;11:74. doi: 10.1186/1477-7827-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duvan CI, Cumaoglu A, Turhan NO, et al. Oxidant/antioxidant status in premenstrual syndrome. Arch Gynecol Obstet. 2011;283:299–304. doi: 10.1007/s00404-009-1347-y. [DOI] [PubMed] [Google Scholar]
- 32.Fenkci V, Fenkci S, Yilmazer M, Serteser M. Decreased total antioxidant status and increased oxidative stress in women with polycystic ovary syndrome may contribute to the risk of cardiovascular disease. Fertil Steril. 2003;80:123–27. doi: 10.1016/s0015-0282(03)00571-5. [DOI] [PubMed] [Google Scholar]
- 33.Panti A, Shehu C, Saidu Y, et al. Oxidative stress and outcome of antioxidant supplementation in patients with polycystic ovarian syndrome (PCOS) Int J Reprod Contracept Obstet Gynecol. 2018;7:1667–72. [Google Scholar]
- 34.Jamilian M, Samimi M, Mirhosseini N, et al. The influences of vitamin D and omega-3 co-supplementation on clinical, metabolic and genetic parameters in women with polycystic ovary syndrome. J Affect Disord. 2018;238:32–38. doi: 10.1016/j.jad.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 35.Beers WH. Follicular plasminogen and plasminogen activator and the effect of plasmin on ovarian follicle wall. Cell. 1975;3:379–86. doi: 10.1016/0092-8674(75)90187-7. [DOI] [PubMed] [Google Scholar]
- 36.Lumsden MA, Kelly RW, Templeton AA, et al. Changes in the concentration of prostaglandins in preovulatory human follicles after administration of hCG. J Reprod Fertil. 1986;77:119–24. doi: 10.1530/jrf.0.0770119. [DOI] [PubMed] [Google Scholar]
- 37.Pasqualotto EB, Agarwal A, Sharma RK, et al. Effect of oxidative stress in follicular fluid on the outcome of assisted reproductive procedures. Fertil Steril. 2004;81:973–76. doi: 10.1016/j.fertnstert.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 38.Morán C, Tapia MC, Hernández E, et al. Etiological review of hirsutism in 250 patients. Arch Med Res. 1994;25:311–14. [PubMed] [Google Scholar]
- 39.Dokras A, Bochner M, Hollinrake E, et al. Screening women with polycystic ovary syndrome for metabolic syndrome. Obstet Gynecol. 2005;106:131–37. doi: 10.1097/01.AOG.0000167408.30893.6b. [DOI] [PubMed] [Google Scholar]