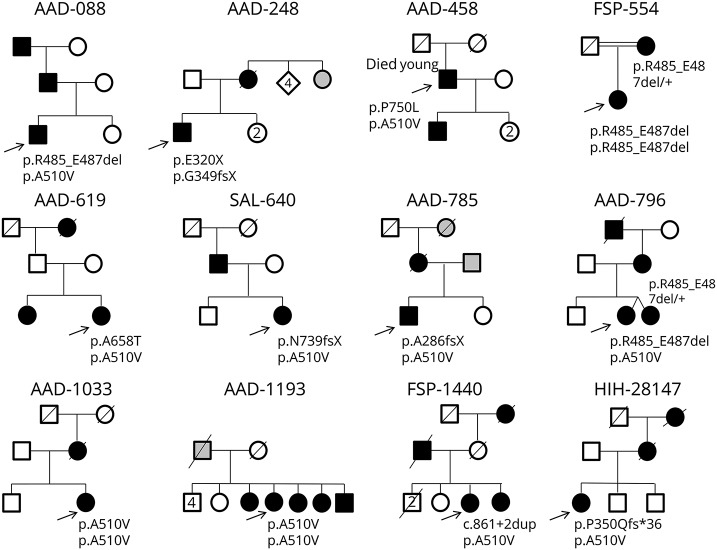
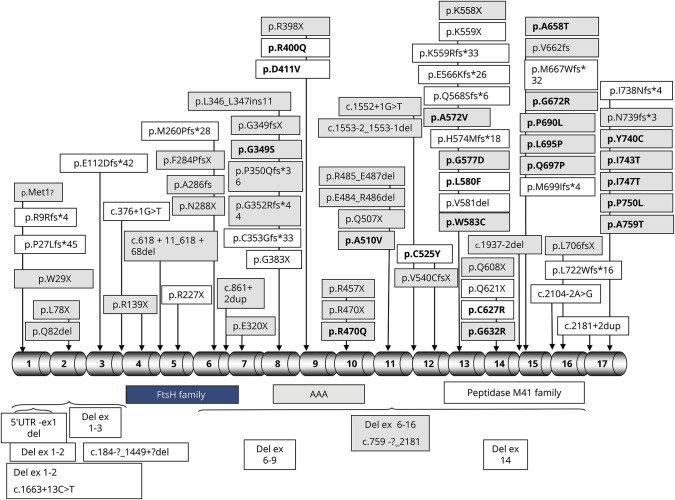
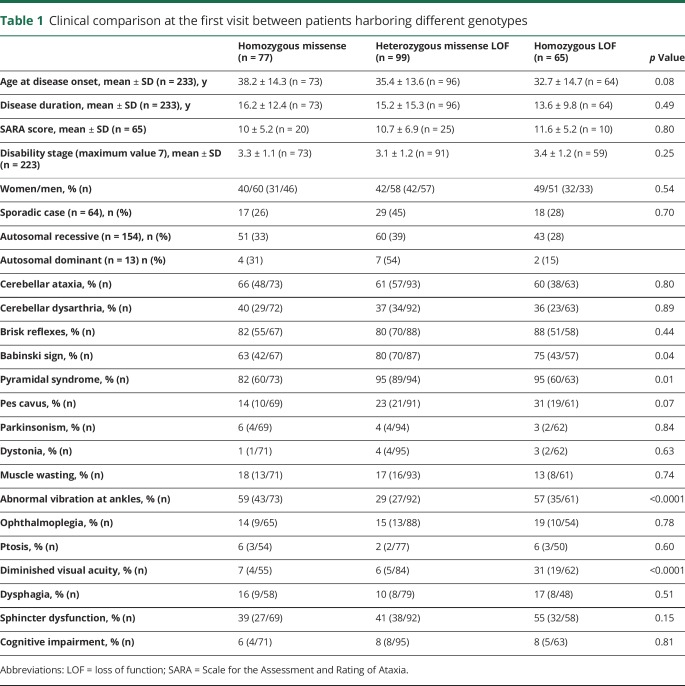
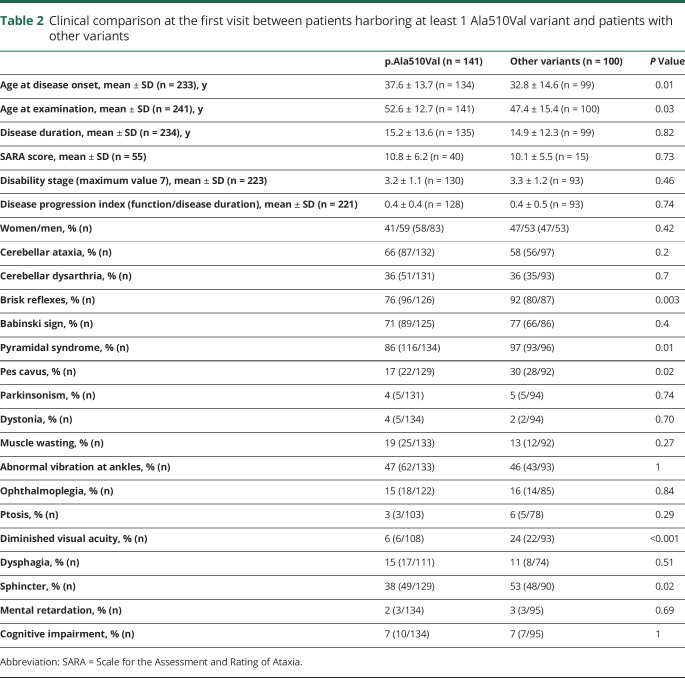
Giulia Coarelli
Giulia Coarelli, MD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Rebecca Schule
Rebecca Schule, MD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Bart PC van de Warrenburg
Bart PC van de Warrenburg, MD, PhD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Peter De Jonghe
Peter De Jonghe, MD, PhD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Claire Ewenczyk
Claire Ewenczyk, MD, PhD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Andrea Martinuzzi
Andrea Martinuzzi, MD, PhD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Matthis Synofzik
Matthis Synofzik, MD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Elisa G Hamer
Elisa G Hamer, MD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Jonathan Baets
Jonathan Baets, MD, PhD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Mathieu Anheim
Mathieu Anheim, MD, PhD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Ludger Schöls
Ludger Schöls, MD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Tine Deconinck
Tine Deconinck, MSci
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Pegah Masrori
Pegah Masrori, MD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Bertrand Fontaine
Bertrand Fontaine, MD, PhD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Thomas Klockgether
Thomas Klockgether, MD, PhD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Maria Grazia D'Angelo
Maria Grazia D'Angelo, MD, PhD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Marie-Lorraine Monin
Marie-Lorraine Monin, MD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Jan De Bleecker
Jan De Bleecker, MD, PhD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Isabelle Migeotte
Isabelle Migeotte, MD, PhD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Perrine Charles
Perrine Charles, MD, PhD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Maria Teresa Bassi
Maria Teresa Bassi, PhD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Thomas Klopstock
Thomas Klopstock, MD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Fanny Mochel
Fanny Mochel, MD, PhD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Elisabeth Ollagnon-Roman
Elisabeth Ollagnon-Roman, MD, PhD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Marc D'Hooghe
Marc D'Hooghe, MD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Christoph Kamm
Christoph Kamm, MD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Delia Kurzwelly
Delia Kurzwelly, MD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Melanie Papin
Melanie Papin, MS
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Claire-Sophie Davoine
Claire-Sophie Davoine, BS
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Guillaume Banneau
Guillaume Banneau, PhD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Sophie Tezenas du Montcel
Sophie Tezenas du Montcel, MD, PhD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Danielle Seilhean
Danielle Seilhean, MD, PhD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Alexis Brice
Alexis Brice, MD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Charles Duyckaerts
Charles Duyckaerts, MD, PhD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Giovanni Stevanin
Giovanni Stevanin, PhD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,
Alexandra Durr
Alexandra Durr, MD, PhD
1From Sorbonne Université (G.C., C.E., B.F., M.-L.M., F.M., M.P., C.-S.D., G.S., A.D.), Institut du Cerveau et de la Moelle épinière (ICM), AP-HP, INSERM, CNRS, University Hospital Pitié-Salpêtrière; Department of Genetics (G.C., C.E., M.-L.M., P.C., F.M., G.B., G.S., A.D.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, Paris, France; Center for Neurology and Hertie Institute for Clinical Brain Research (R.S., M.S., L.S.), University of Tübingen, German Center for Neurodegenerative Diseases; German Center for Neurodegenerative Diseases (R.S., M.S., L.S.), Tübingen; Department of Neurology (B.P.C.v.d.W., E.G.H.), Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Neurogenetics Group (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), University of Antwerp; Laboratories of Neurogenetics and Neuromuscular Pathology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Institute Born-Bunge, University of Antwerp; Department of Neurology (P.D.J., J.B., T.D., P.M., J.D.B., M.D.), Antwerp University Hospital, Belgium; Scientific Institute IRCCS “E. Medea” (A.M.), Conegliano, Italy; Department of Neurology (M.A.), Hôpital de Hautepierre, Strasbourg; Institut de Génétique et de Biologie Moléculaire et Cellulaire (M.A.), INSERM-U964/CNRS-UMR7104/Université de Strasbourg, Illkirch; Fédération de Médecine Translationnelle de Strasbourg (M.A.), Université de Strasbourg; Department of Neurology (B.F.), Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France; Department of Neurology (T. Klockgether, D.K.), University of Bonn; German Center for Neurodegenerative Diseases (T. Klockgether, D.K.), Bonn; Scientific Institute IRCCS E. Medea Neurorehabilitation Unit (M.G.D.), Bosisio Parini, Lecco, Italy; ULB Center of Human Genetics (I.M.), Brussels, Belgium; Scientific Institute IRCCS E. Medea Laboratory of Molecular Biology (M.T.B.), Bosisio Parini, Lecco, Italy; Department of Neurology With Friedrich-Baur Institute (T. Klopstock), University Hospital of the Ludwig-Maximilians-Universität München; German Center for Neurodegenerative Diseases (T. Klopstock); Munich Cluster for Systems Neurology (T. Klopstock), Germany; Department of Genetics (E.O.-R.), Croix-Rousse University Hospital, Lyon, France; Department of Neurology (C.K.), University of Rostock, Germany; Ecole Pratique des Hautes Etudes (M.P., G.S.), PSL Research University; Sorbonne Université (S.T.d.M.), INSERM, Institut Pierre Louis de Santé Publique, Medical Information Unit, Pitié-Salpêtrière Charles-Foix University Hospital, Assistance publique–Hôpitaux de Paris; and Raymond Escourolle Neuropathology Department (D.S., C.D.), Pitié-Salpêtrière University Hospital, Assistance publique–Hôpitaux de Paris, Sorbonne Université, France.
1,✉