Abstract
Cancer stem cells (CSCs) have been shown to be resistant to current anticancer therapies and the induction of oxidative stress is an important mechanism of action for many anticancer agents. However, it is still largely unknown how CSCs respond to hydrogen peroxide (H2O2)-induced oxidative stress. Here, we show that the levels of reactive oxygen species (ROS) are markedly lower in breast CSCs (BCSCs) than that in non-cancer stem cells (NCSCs). A transient exposure of breast cancer cells to sublethal doses of H2O2 resulted in a dose-dependent increase of the epithelium-specific antigen (ESA)+/CD44+/CD24−subpopulations, a known phenotype for BCSCs. Although BCSCs survived sublethal doses of H2O2 treatment, they lost the ability to form tumor spheres and failed to generate colonies as demonstrated by mammosphere-formation and clonogenic assays, respectively. Mechanistic studies revealed that H2O2 treatment led to a marked increase of senescence-associated β-galactosidase activity but only minimal apoptotic cell death in BCSCs. Furthermore, H2O2 triggers p53 activation and promotes p21 expression, indicating a role for the p53/p21 signaling pathway in oxidative stress-induced senescence in BCSCs. Taken together, these results demonstrate that the maintenance of a lower level of ROS is critical for CSCs to avoid oxidative stress and H2O2-induced BCSC loss of function is likely attributable to oxidative stress-triggered senescence induction, suggesting that ROS-generating drugs may have the therapeutic potential to eradicate drug-resistant CSCs via induction of premature senescence.
Keywords: Oxidative stress, Breast cancer, Cancer stem cells, Senescence, Hydrogen peroxide
1. Introduction
Cancer stem cells (CSCs), also known as tumor-initiating cells (TICs), are a small subpopulation of cells within a heterogeneous tumor that have the unique ability to drive tumor initiation, progression and metastasis [1, 2]. Emerging evidence has suggested that CSCs are resistant to current anticancer therapies, as demonstrated by the fact that they are enriched in residual tumors following chemotherapy and/or radiation [3–6]. Induction of oxidative stress has been shown to be a crucial mechanism of action for many anticancer agents including radiation [7–9]. However, it is largely unknown how drug-resistant CSCs respond to oxidative stress. The goal of this study was to delineate the response of breast cancer stem cells (BCSCs) to H2O2-induced oxidative stress.
Cellular senescence is a state of irreversible cell cycle arrest that can be triggered by various cell-intrinsic and extrinsic stimuli, including DNA damage, oxidative stress, and exposure to chemotherapeutic agents and ionizing radiation [10]. Although the induction of apoptotic cell death was thought to be a desirable outcome for cancer therapy, we and others have shown that senescence induction in tumor cells is a common therapeutic response to many anticancer modalities including CDK4/6 small molecule inhibitor-based targeted therapeutics and radiation treatment [10–15]. In this study, we demonstrate that H2O2-mediated oxidative stress induces premature senescence, but not apoptosis in BCSCs. We also show that H2O2-induced BCSC senescence is associated with p53 activation as well as increased p53 and p21 expression, implying a role of the p53/p21 signaling pathway in oxidative stress-induced senescence in BCSCs. These results suggest that BCSCs are susceptible to oxidative stress-induced senescence and that certain ROS-generating drugs may have the therapeutic potential to functionally disable drug-resistant CSCs via induction of premature senescence.
2. Materials and Methods
2.1. Cell lines, reagents and antibodies
The SUM series of human breast cancer cell lines including SUM159 and SUM149 cells were developed by Dr. Stephen Ethier at the Medical University of South Carolina (MUSC). We received these cell lines directly from Dr. Ethier’s laboratory at MUSC and the cells were maintained as previously described [16]. The MCF-7 human breast cancer cell line was purchased from American Type Culture Collection (ATCC) and the cells were cultured in DMEM medium containing 10% FBS, 2 mM L-glutamine and 100 microgram/mL of penicillin-streptomycin (Invitrogen). Dulbecco’s modified Eagle’s medium (DMEM), DMEM/F12 medium, recombinant human basic fibroblast growth factor (bFGF) and B27 supplement were obtained from Invitrogen (Carlsbad, CA). Recombinant human epithelial growth factor (rhEGF) was purchased from R&D systems. Heparin and hydrocortisone were purchased from Stem Cell Biotechnologies (Vancouver, BC). Rabbit anti-human phosphorylated p53 (p-p53), p53 and p21 antibodies were purchased from Cell Signaling (Danvers, MA). Mouse anti-human tubulin monoclonal antibodies were obtained from Santa Cruz Biotechnology. 2′−7′-Dichlorodihydrofluorescein diacetate (DCF-DA) and ImaGene GreenTM C12FDG lacZ gene expression kit were purchased from Invitrogen. The FITC Annexin V Apoptosis Detection kit was obtained from BD Biosciences.
2.2. BCSC flow cytometry analysis and intracellular ROS detection
The ESA+/CD44+/CD24−subpopulations were identified as the CSCs in breast cancer [17]. Flow cytometry was employed to quantify ESA+/CD44+/CD24− BCSCs as previously described [16, 17]. Briefly, MCF-7 or SUM159 cells were resuspended in 2% FBS/DPBS and incubated with PE-conjugated mouse anti-human ESA (ESA), APC-conjugated mouse anti-human CD44 and BV421-conjugated mouse anti-human CD24 (both from BD Biosciences) for 20 min on ice. After washing with 2% FBS/DPBS, the frequency of ESA+/CD44+/CD24−BCSCs was analyzed using a BD LSRFortessa™ X-20 Flow Cytometer (BD Biosciences).
DCF-DA, a well-characterized ROS probe [18], was utilized to detect and measure ROS in different subpopulations of breast cancer cells using flow cytometry as previously reported [19]. Briefly, after labeling with the CSC surface markers (ESA, CD24 and CD44) as described above, the cells were incubated with DCF-DA (5 μM) at 37°C for 30 min. ROS levels in BCSC and NCSC subpopulations were measured using a BD LSRFortessaTM X-20 Flow Cytometer (BD Biosciences). Data were processed and analyzed using FlowJo software.
2.3. Mammosphere formation assay
Mammosphere formation assay (MFA) was employed to determine the sphere-forming activity of CSCs as previously described [16, 20]. Briefly, single-cell suspensions were prepared at 24 h after H2O2 exposure and cultured in 24-well ultra-low attachment plates (Corning) using serum-free DMEM/F-12 medium supplemented with 20 ng/mL basic FGF, 20 ng/mL EGF, 4 μg/mL insulin, 4 μg/mL heparin, 0.5 μg/mL hydrocortisone, 0.4% BSA and B27 nutrient supplement (Invitrogen). Culture medium was replaced in half every other day with 50% fresh medium. Mammospheres were counted and photographed after 7 days of culture.
2.4. Clonogenic assay
MCF-7 or SUM159 cells were transiently exposed to a range of sublethal doses (100 to 500 uM) of H2O2 for 2 h. Then, the cells were washed with PBS to remove H2O2 and re-fed with complete medium. At 24 h after exposure, cells were collected and cultured in 60 mm dishes at low density for 12 – 14 days to allow the formation of cell colonies. The colonies were fixed and stained with 0.5% crystal violet (Sigma) in methanol for 30 min. The number of colonies (≥ 50 cells) was scored and photographed as described previously [21].
2.5. Apoptosis analysis
After staining with CSC surface markers, apoptotic cells were labeled with FITC-conjugated Annexin V and 7-AAD using a FITC Annexin V Apoptosis Detection kit (BD Biosciences) and analyzed using a BD LSRFortessa™ X-20 Flow Cytometer as previously reported [22].
2.6. Senescence assays
In situ SA-β-gal assays were performed to determine senescent cells in bulk tumor cells using a senescence β-galactosidase staining kit (Cell Signaling) as we have reported previously [11, 21]. SA-β-gal activity in BCSCs was measured by flow cytometry using an ImaGene GreenTM C12FDG lacZ gene expression kit (Invitrogen) according to the manufacturer’s instruction and a published protocol [23] with some minor modifications. Briefly, after staining with BCSC surface markers, the cells were incubated with 25 µM chloroquine at 37°C for 30 to inhibit endogenous β-galactosidase activity. Then, the cells were incubated with 20 µM C12FDG at 37°C for 20 minutes. SA-β-gal positive senescent cells in the ESA+/CD44+/CD24− subpopulations were measured using a BD LSRFortessa™ X-20 Flow Cytometer (BD Biosciences). Data were processed and analyzed using FlowJo software.
2.7. Western blot analysis
Western blot analyses were performed as previously described [16]. Briefly, protein samples were extracted using cell lysis buffer (Cell Signaling) supplemented with a cocktail of proteinase inhibitors (Sigma). The protein concentrations were quantified using a Bio-Rad Dc protein assay kit (Bio-Rad Laboratories, Hercules, CA). Fifty microgram protein samples were resolved on 4 – 20% Mini-Protean TGX gels (Bio-Rad) and transferred onto 0.2 μM PVDF membrane (Millipore). Blots were blocked with 5% non-fat milk for one hour at room temperature and then incubated with primary antibodies at 4°C overnight. After extensive washing with TBS-T, blots were incubated with appropriate HRP-conjugated secondary antibody for 1.5 h at room temperature. Protein bands were detected using an ECL Plus Western Blotting Detection System (GE Healthcare Life Science).
2.8. Statistical analysis
Comparisons between groups were carried out using Student’s t-test. Differences were considered statistically significant at p < 0.05. The error bars indicate SEM. All analyses were carried out with the GraphPad Prism program (GraphPad Software, Inc. San Diego, CA).
3. Results
3.1. BCSCs display lower levels of ROS compared with NCSCs
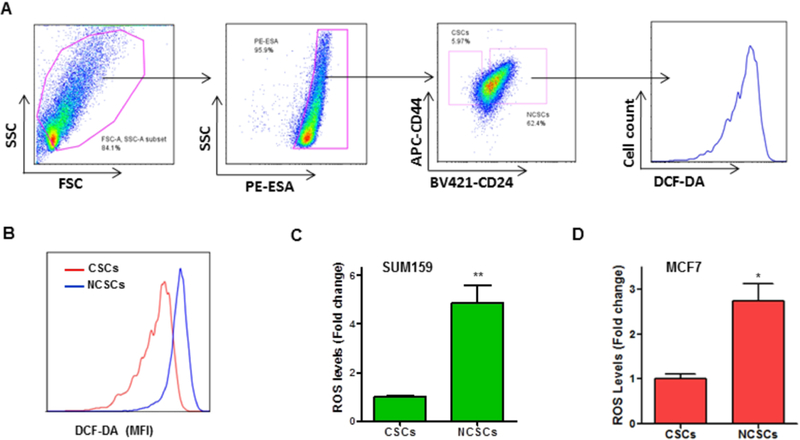
Our previous studies have demonstrated that hematopoietic stem cells (HSCs) are susceptible to ionizing radiation-induced oxidative stress [19, 24]. It also has been shown that HSCs with lower levels of ROS exhibit greater self-renewal potential and hematopoiesis-reconstituting capacity than cells with high levels of ROS [25]. Moreover, there is evidence that oxidative stress is harmful to HSCs, impairing HSC self-renewal potential and its long-term blood cellrepopulating capacity [26–28]. However, it remains to be determined how CSCs respond to oxidative stress. To gain insight into the redox status in BCSCs, we utilized a well-characterized ROS probe, DCF-DA, along with flow cytometry to quantify ROS levels in ESA+/CD24−/CD44+ BCSCs (Fig. 1A). Our data showed that ROS levels in NCSCs are approximately 5-fold higher than that in BCSCs from SUM159 cells (Fig. 1B & C). Similarly, the levels of ROS in BCSCs are markedly lower than that in NCSCs from MCF7 cells (Fig. 1D).
Fig. 1. ROS levels are markedly lower in BCSCs than that in NCSCs.
DCF-DA staining and flow cytometry assays were performed to measure ROS levels in BCSCs and NCSCs. (A) Representative flow cytometry graphs are presented, showing the gating strategy for detecting ROS levels in BCSCs and NCSCs. (B) ROS levels are presented as mean fluorescence intensity (MFI) of DCF-DA staining. Overlaid flow cytometry graphs indicate that ROS levels are significantly lower in BCSCs than that in NCSCs. (C, D) ROS levels are approximately 3- to 5-fold lower in BCSCs as compared with NCSCs. Data are presented as mean ± SEM of three independent experiments. * p < 0.05, ** p < 0.01 vs. CSCs.
3.2. H2O2 treatment has no significant effect on apoptotic cell death in BCSCs
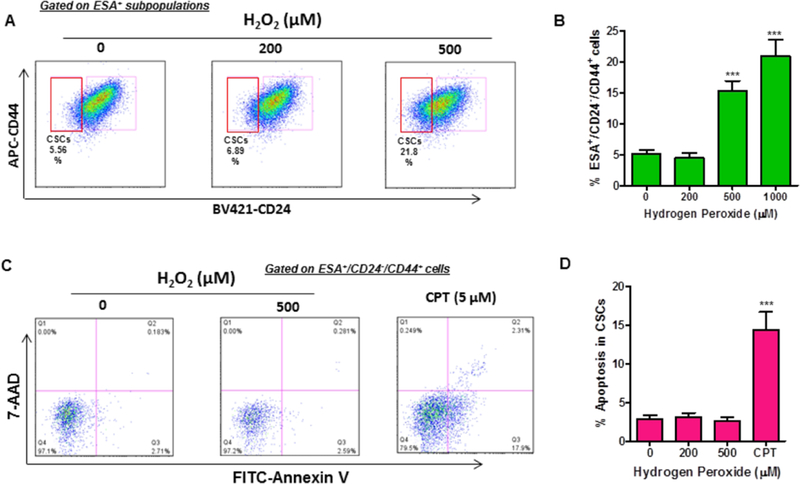
H2O2 has been widely used to induce oxidative stress in various types of cells [29, 30]. To examine how BCSCs respond to oxidative stress, we investigated the effects of H2O2 on BCSC survival and apoptotic cell death. Surprisingly, we found that sublethal doses of H2O2 treatment increases the frequency of ESA+/CD24−/CD44+ subpopulations in a dose-dependent manner (Fig. 2A & B). Furthermore, our subsequent studies revealed that a transient treatment with H2O2 failed to induce apoptotic cell death in BCSCs (Fig. 2C & D). In contrast, camptothecin (CPT) treatment led to a substantial increase of apoptosis in ESA+/CD24−/CD44+ BCSCs (Fig. 2C & D). These results suggest that sublethal dose H2O2-mediated oxidative stress has no significant effect on apoptosis induction in BCSCs.
Fig. 2. H2O2 treatment increases the number of ESA+/CD24−/CD44+ cells.
Flow cytometry was employed to analyze the number of ESA+/CD24−/CD44+ BCSCs at 24 h after H2O2 treatment. (A) Representative flow cytometry graphs are presented, showing that H2O2 treatment increases the number of ESA+/CD24−/CD44+ cells. (B) Quantification of flow cytometry data demonstrates that H2O2 treatment increases the percentage of BCSCs in a dose-dependent manner. (C) A representative flow cytometry analysis of apoptosis in the ESA+/CD24−/CD44+ subpopulations. (D) Flow cytometry analysis revealed that H2O2 treatment has no significant effect on apoptotic cell death in BCSCs. Data are presented as mean ± SEM of three independent experiments. *** p< 0.001 vs. PBS control.
3.3. Oxidative stress impairs the mammosphere-forming potential of BCSCs
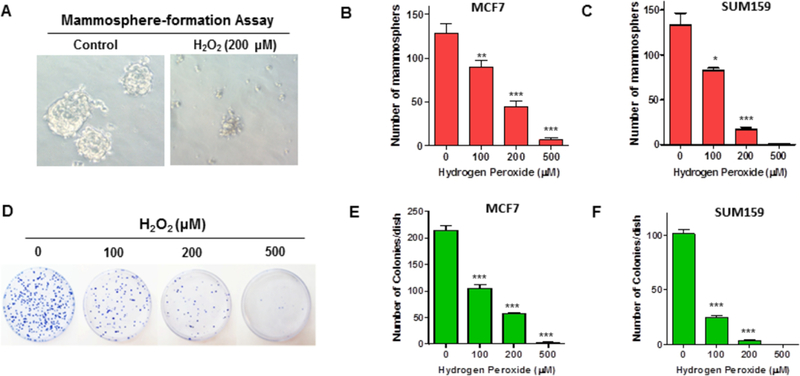
The mammosphere formation assay (MFA) is a very useful technique to measure the sphere-forming ability of CSCs and the sphere-initiating cells (SICs) have been considered as the surrogate of CSCs in culture [16, 20]. To examine the effects of H2O2-induced oxidative stress on CSC function, we took advantage of the MFA to measure the sphere-forming ability of MCF-7 and SUM159 cells after a transient exposure to H2O2 (Fig. 3A). The results showed that H2O2 treatment causes a dose-dependent decrease in the number of mammospheres generated from both MCF-7 and SUM159 cells (Fig. 3B & C).
Fig. 3. H2O2 treatment impairs the mammosphere-forming potential of BCSCs.
MFA was employed to measure the mammosphere-forming capacity of BCSCs at 24 h after H2O2 treatment. (A) Representative images of a MFA showing that H2O2-treated MCF-7 cells failed to produce mammospheres in culture. (B, C) H2O2 treatment impairs the mammosphere-forming potential of BCSCs from both MCF-7 (B) and SUM159 (C) cells. (D) Representative images of a clonogenic assay showing that H2O2 inhibits the formation of colonies in MCF-7 cells. (E, F) Decreased colony-forming capacity was observed in both MCF-7 (E) and SUM159 (F) cells following H2O2 treatment. All data are presented as mean ± SEM of three independent experiments. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. PBS control.
The clonogenic assay (also known as colony formation assay) is a well-characterized in vitro experiment that measures the reproductive capacity of a single cell to generate a colony in longterm culture, thus defining a cell’s ability to replicate and form a tumor [31]. Using clonogenic assays, we demonstrated that H2O2 exposure suppresses the colony-forming capacity of both MCF-7 and SUM159 cells in a dose-dependent fashion (Fig. 3D–F). Together, these results suggest that although BCSCs can survive sublethal doses of H2O2 treatment (Fig. 2A–D), they lose the ability to produce mammospheres and to generate colonies in culture (Fig. 3A–F).
3.4. Oxidative stress induces premature senescence in BCSCs
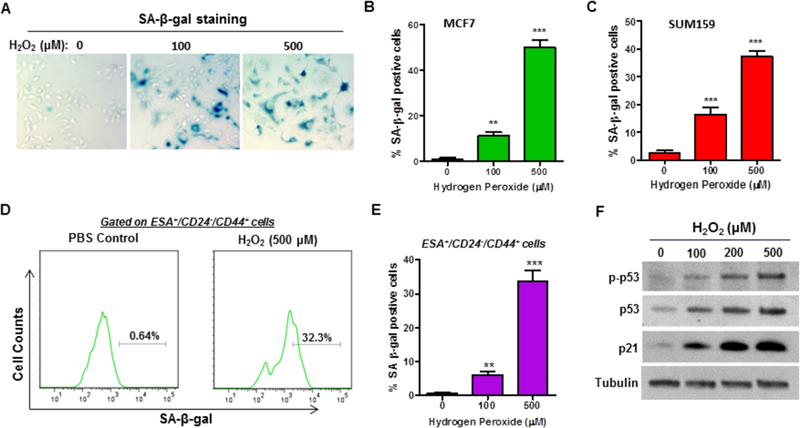
Induction of senescence is an important mechanism of tumor prevention by limiting the reproductive capacity of cells. We and others have shown that H2O2-mediated oxidative stress can readily induce premature senescence in a variety of cells [29, 32]. However, it remains to be determined if H2O2-mediated oxidative stress causes premature senescence in BCSCs. To gain insights into the role of senescence induction in H2O2-induced loss of function in BCSCs, we examined if H2O2 treatment induces senescence in breast cancer cells, and more importantly in BCSCs. SA-β-gal analyses revealed that treatment with H2O2 causes a dose-dependent increase of senescent cells in both MCF-7 and SUM159 cells (Fig. 4A–C). Our subsequent flow cytometry assays further indicated that SA-β-gal positive cells were markedly increased in the ESA/CD24/CD44 subpopulations in response to H2O2 treatment (Fig. 4D & E). These results demonstrate for the first time that H2O2-mediated oxidative stress induces premature senescence in BCSCs.
Fig. 4. H2O2 treatment induces premature senescence in BCSCs.
(A) Representative images of SA-β-gal staining of senescent MCF-7 cells at 5 days after H2O2 exposure. (B, C) SA-β-gal analysis showed that H2O2 induces premature senescence in both MCF-7 (B) and SUM159 (C) cells in a dose-dependent fashion. (D) Representative flow cytometry graphs depicting that H2O2 increased SA-β-gal activity in the ESA+/CD24−/CD44+ subpopulations of MCF-7 cells at 5 days after exposure. (E) Flow cytometry analysis revealed that H2O2 treatment induces premature senescence in BCSCs in dose-dependent manner. (F) Western blot analysis indicated that H2O2 treatment-induced BCSC senescence is associated with p53 activation and increased p53 and p21 expression. ** p < 0.01, *** p < 0.001 vs. PBS control.
Our previous studies have shown that activation of the p53/p21 signaling pathway plays a pivotal role in mediating ionizing radiation-induced senescence in lung cancer cells [11]. To elucidate the mechanisms by which oxidative stress induces senescence in BCSCs, we investigated the effects of H2O2 on p53/p21 signaling. Western blot analyses revealed that H2O2 treatment led to a dose-dependent increase in the expression levels of phosphorylated p53 (p-p53), suggesting that H2O2-mediated oxidative stress may activate the p53 signaling pathway (Fig. 4F). In agreement with this suggestion, we found that both p53 and p21 expression levels were markedly increased by H2O2 treatment (Fig. 4F). Together, these results suggest that H2O2 may induce senescence in BCSCs at least in part by activation of the p53/p21 signaling pathway.
4. Discussion
Oxygen metabolism in mitochondria is a crucial process for ATP production to provide energy for cell survival and functional activity needs, but it also leads to the generation of ROS, because of electron leak during oxidative phosphorylation and reduction of O2 molecules [33]. Mitochondrial ROS include superoxide anion, hydroxyl radical, H2O2, and singlet oxygen [33]. If the accumulation of ROS exceeds the scavenging capacity of the antioxidant system, it may disturb the redox balance, resulting in oxidative stress. We and others have shown that H2O2-mediated oxidative stress could induce premature senescence in various types of cells [29, 32]. However, it was largely unknown how BCSCs respond to H2O2-mediated oxidative stress until our present study. Our flow cytometry data revealed that ROS levels are markedly lower in BCSCs than that in NCSCs. These results suggest that it may be critical for CSCs to keep ROS at a low level to avoid oxidative stress-associated toxicity. In agreement with this idea, it has been shown that increasing ROS production by L-S,R-buthionine sulfoximine (BSO) treatment could sensitize CSCs to radiation [34]. Furthermore, our studies have demonstrated that transient exposure to sublethal doses of H2O2 is sufficient to cause marked deficiency in the mammosphere-forming capacity of BCSCs. Mechanistically, we found that H2O2 treatment-induced BCSC loss of function is attributable to the induction of premature senescence.
In addition, our studies have revealed for the first time that H2O2-mediated oxidative stress increases the number of BCSCs. In agreement with our observations, previous studies showed that chemotherapy and/or radiation led to the enrichment of the CSCs in residual tumors following anticancer treatments [3–6]. The increase of the ESA+/CD24−/CD44+ subpopulations by sublethal doses of H2O2 treatment suggests that BCSCs are likely more resistant to H2O2 treatment compared to NCSCs. Another possible explanation is that H2O2-induced oxidative stress may simply reduce CD24 expression, leading to an “artificial” increase in the number of ESA+/CD24−/CD44+ cells. Nevertheless, further studies are warranted to better elucidate the precise cellular and molecular mechanisms by which low doses of H2O2 treatment increases the ESA+/CD24−/CD44+ subpopulations in breast cancer.
A growing body of evidence has suggested that CSCs play a critical role in drug resistance, metastatic progression and tumor relapse, leading to treatment failure [1, 2, 4, 35]. It has been shown that CSCs have increased DNA damage repair capacity than NCSCs [35, 36]. Oxidative stress can cause DNA damage, resulting in senescence induction. The observation that H2O2-treated BCSCs lost the ability to produce mammospheres and exhibited a significant increase in SA-β-gal staining suggests that these cells are vulnerable to oxidative stress-mediated DNA damage and senescence induction. In agreement with this idea, our studies revealed that H2O2-induced BCSC loss of function and senescence phenotype is associated with p53 activation and increased p21 expression. Given that activation of the p53/p21 signaling pathway is a well-known DNA damage response process, these results suggest that oxidative stress-triggered DNA damage response is likely involved in H2O2-induced premature senescence and loss of function in BCSCs.
In conclusion, this study provides a novel insight into BCSC’s response to H2O2-mediated oxidative stress. Even a transient exposure to sublethal doses of H2O2 can induce premature senescence in BCSCs, resulting in the loss of function for these cells to generate tumor spheres in culture. These results suggest that selective ROS-generating agents may have the therapeutic potential for targeting drug-resistant and metastatic CSCs through promoting oxidative stress-induced CSC premature senescence.
Highlights.
ROS levels are markedly lower in BCSCs than that in NCSCs
Exposure to sublethal doses of H2O2 increases the number of BCSCs
Oxidative stress causes the loss of function in BCSCs via induction of senescence
H2O2-induced BCSC senescence is associated with activation of the p53/p21 pathway
Acknowledgments
The authors thank Dr. Stephen Ethier for providing the SUM series of human breast cancer cell lines. This work was supported in part by NIH grant numbers P20GM103542 and UL1TR001450.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors declare no conflict of interest.
References
- 1.Adorno-Cruz V, Kibria G, Liu X, et al. Cancer stem cells: targeting the roots of cancer, seeds of metastasis, and sources of therapy resistance, Cancer Res 75 (2015) 924–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oskarsson T, Batlle E, Massagué J, Metastatic stem cells: sources, niches, and vital pathways, Cell Stem Cell, 14 (2014) 306–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freitas DP, Teixeira CA, Santos-Silva F, et al. Therapy-induced enrichment of putative lung cancer stem-like cells, Int. J. Cancer, 134 (2014) 1270–8. [DOI] [PubMed] [Google Scholar]
- 4.Kurtova AV, Xiao J, Mo Q, et al. Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance, Nature, 517 (2015) 209–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu H, Tran L, Park Y, et al. Reciprocal Regulation of DUSP9 and DUSP16 Expression by HIF1 Controls ERK and p38 MAP Kinase Activity and Mediates Chemotherapy-Induced Breast Cancer Stem Cell Enrichment, Cancer Res 78 (2018) 4191–4202. [DOI] [PubMed] [Google Scholar]
- 6.Creighton CJ, Li X, Landis M, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features, Proc. Natl. Acad. Sci. U S A, 106 (2009) 13820–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen BG, Bhatia SK, Buatti JM, et al. Ketogenic diets enhance oxidative stress and radio-chemo-therapy responses in lung cancerxenografts, Clin. Cancer Res 19 (2013) 3905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hou D, Liu Z, Xu X, et al. Increased oxidative stress mediates the antitumor effect of PARP inhibition in ovarian cancer, Redox Biol 17 (2018) 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov 8 (2009) 579–91. [DOI] [PubMed] [Google Scholar]
- 10.Qin S, Schulte BA, Wang GY. Role of senescence induction in cancer treatment. World J. Clin. Oncol 9 (2018) 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo H, Yount C, Lang H, et al. Activation of p53 with Nutlin-3a radiosensitizes lung cancer cells via enhancing radiation-induced premature senescence, Lung Cancer, 81 (2013) 167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo H, Yang A, Schulte BA, Wargovich MJ, Wang GY. Resveratrol Induces Premature Senescence in Lung Cancer Cells via ROS-Mediated DNA Damage, PLoS One, 8 (2013) e60065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.te Poele RH, Okorokov AL, Jardine L, Cummings J, Joel SP, DNA damage is able to induce senescence in tumor cells in vitro and in vivo, Cancer Res 62 (2002) 1876–83. [PubMed] [Google Scholar]
- 14.Acevedo M, Vernier M, Mignacca L, et al. A CDK4/6-Dependent Epigenetic Mechanism Protects Cancer Cells from PML-induced Senescence, Cancer Res 76 (2016) 3252–64. [DOI] [PubMed] [Google Scholar]
- 15.Klein ME, Dickson MA, Antonescu C, et al. PDLIM7 and CDH18 regulate the turnover of MDM2 during CDK4/6 inhibitor therapy-induced senescence, Oncogene, 37 (2018) 5066–5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang A, Qin S, Schulte BA, Ethier SP, Tew KD, Wang GY, MYC Inhibition depletes cancer stem-like cells in triple-negative breast cancer, Cancer Res 77 (2017) 6641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells, Proc. Natl. Acad. Sci. U S A, 100 (2003) 3983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eruslanov E, Kusmartsev S. Identification of ROS using oxidized DCFDA and flow-cytometry, Methods Mol. Biol 594 (2010) 57–72. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Liu L, Pazhanisamy S, Meng A, Zhou D, Total body irradiation selectively induces persistent oxidative stress in murine hematopoietic stem cells, Free Radic. Biol. and Med 48 (2010) 348–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreso A, van Galen P, Pedley NM, et al. Self-renewal as a therapeutic target in human colorectal cancer, Nat. Med 20 (2014) 29–36. [DOI] [PubMed] [Google Scholar]
- 21.He X, Yang A, McDonald DG, Riemer EC, Vanek KN, Schulte BA and Wang GY, MiR-34a modulates ionizing radiation-induced senescence in lung cancer cells, Oncotarget, 8 (2017) 69797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao X, Luo H, Vanek KN, LaRue AC, Schulte BA, Wang GY, Catalase Inhibits Ionizing Radiation-Induced Apoptosis in Hematopoietic Stem and Progenitor Cells. Stem Cells Dev 24 (2015) 1342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O. Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo, Nat. Protoc 4 (2009) 1798–1806. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Wang Y, Pazhanisamy SK, et al. Mn(III) meso-tetrakis-(N-ethylpyridinium-2-yl) porphyrin mitigates total body irradiation-induced long-term bone marrow suppression, Free Radic. Biol. Med 51 (2011) 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jang YY, Sharkis SJ, A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche, Blood, 110 (2007) 3056–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito K, Hirao A, Arai F, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells, Nat Med 12 (2006) 446–51. [DOI] [PubMed] [Google Scholar]
- 27.Ito K, Hirao A, Arai F, Matsuoka S, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells, Nature, 431 (2004) 997–1002. [DOI] [PubMed] [Google Scholar]
- 28.Yahata T, Takanashi T, Muguruma Y, et al. Accumulation of oxidative DNA damage restricts the self-renewal capacity of human hematopoietic stem cells, Blood, 118 (2011) 2941–50. [DOI] [PubMed] [Google Scholar]
- 29.Burova E, Borodkina A, Shatrova A, Nikolsky N. Sublethal oxidative stress induces the premature senescence of human mesenchymal stem cells derived from endometrium, Oxid. Med. Cell Longev 2013 (2013) 474931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker JR, Vuppusetty C, Colley T, et al. Oxidative stress dependent microRNA-34a activation via PI3Kα reduces the expression of sirtuin-1 and sirtuin-6 in epithelial cells, Sci Rep 6 (2006) 35871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franken NA, Rodermond HM, Stap J, et al. Clonogenic assay of cells in vitro. Nat Protoc 1 (2006) 2315–9. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Meng A, Zhou D, Inhibition of phosphatidylinostol 3-kinase uncouples H2O2-induced senescent phenotype and cell cycle arrest in normal human diploid fibroblasts, Exp. Cell Res 298 (2004) 188–96. [DOI] [PubMed] [Google Scholar]
- 33.Moloney JN, Cotter TG, ROS signaling in the biology of cancer, Semin. Cell Dev. Biol 80 (2018) 50–64. [DOI] [PubMed] [Google Scholar]
- 34.Diehn M, Cho RW, Lobo NA, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells, Nature, 458 (2009) 780–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rycaj K, Tang DG. Cancer stem cells and radioresistance. Int. J. Radiat. Biol 90 (2014) 615–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response, Nature, 444 (2006) 756–60. [DOI] [PubMed] [Google Scholar]