Abstract
A novel snake venom cysteine-rich secretory protein (svCRiSP), Hellerin, was purified from C. o. helleri venom using sequential reverse phase and cation-exchange chromatography. Gel electrophoresis, N-terminal sequencing, and LC-MS/MS sequencing identified a single protein with a molecular mass of approximately 24.8 kDa and confirmed its identity as a svCRiSP. Hellerin had cytotoxic effects on human umbilical vein endothelial cells (HUVECs) in a dose-dependent manner but not in human dermal lymphatic endothelial cells (HDLECs) and human dermal blood endothelial cells (HDBECs). Hellerin produced a dramatic increase in both blood vascular permeability in vivo, and in the trans-epithelial permeability of cultured HDLEC and HDBEC cells. This is the first study that describes the effect of a svCRiSP on vascular, blood and lymphatic permeability.
Keywords: snake venom, Southern Pacific rattlesnake, snake venom cysteine-rich secretory protein (svCRiSP), vascular permeability, vasculature, lymphatic cells
1. Introduction
Snake venoms represent a highly specialized assembly of toxins that have evolved to facilitate prey capture, defense, and digestion. Although there are major differences in the specific toxins that are most prominent in the venoms of different species of snakes, nearly all snake venom toxins belong to one of 11 major families (Tasoulis and Isbister, 2017). The most dominant protein families such as metalloproteases, serine proteases, and phospholipase A2s have been characterized and their biological function is well known. Snake venom cysteine-rich secretory proteins (svCRiSPs) are ubiquitously expressed in the venom of numerous species of snakes (Sunagar et al., 2015; Tasoulis and Isbister, 2017). However, very little is known about the role svCRiSPs play in the pathophysiology of snakebite (Yamazaki et al., 2003).
svCRiSPs are non-enzymatic, single-polypeptide proteins with molecular weights between 20-30 kDa. svCRiSPs are members of a large group of structurally related vertebrate proteins, the CAP superfamily (Cysteine-rich secretory proteins, Antigen 5 and Pathogenesis-related 1 (PR-1) proteins). They share a high degree of amino acid sequence similarity and have a highly conserved domain structure including the N-terminal core domain, the PR-1 domain, and the C-terminal cysteine-rich domain (CRD) (Yamazaki and Morita, 2004; Matsunaga et al., 2009). The svCRiSPs have the highest sequence similarity with mammalian CRiSP-3 proteins, a group of secretory proteins abundant in seminal plasma, saliva, and the secretory granules of neutrophils that play possible roles in diverse aspects of reproduction, development, and immune function (Udby et al., 2002a, 2002b, 2004; Gibbs et al., 2008; Estrella et al., 2011).
It has been established that some svCRiSPs can inhibit ion channel activities (Brown et al., 1999; Yamazaki et al., 2002; Wang et al., 2005; Wang et al., 2006) and two svCRiSPs, ES-CRiSP and natrin, have been shown to affect cell-signaling pathways in vascular endothelial cells (Wang et al., 2010; Lecht et al., 2015). It is our hypothesis, based on the limited information available in the literature and our preliminary results obtained with Hellerin, a newly discovered svCRiSP isolated from the C. o. helleri venom, that svCRiSPs play an important role in the pathophysiology of snakebite by initiating an inflammatory response and a change in the physiological regulation of fluid dynamics in tissues at the site of the snakebite.
Here we describe the isolation of Hellerin from the venom of the Southern Pacific rattlesnake and its role in some important functions of vascular physiology, with special emphasis in vascular permeability.
2. Materials and Methods
2.1. Snake and venom collection
A healthy, 10 year old male (0.91 m snout-vent length) Southern Pacific rattlesnake (Crotalus oreganus helleri, Avid # 059-009-599) was obtained from California, Riverside County (USA). The snake is located and maintained at the National Natural Toxins Research Center (NNTRC), Texas A&M University-Kingsville, Kingsville, TX, USA. Venom was obtained by letting the snake bite into parafilm extended over a disposable plastic cup. Venom samples were centrifuged in a Beckman Avanti 30 at 10,000 × g for 5 min, filtered through a Millipore filtration MillexHV unit 0.45 μm (Millipore, Billerica, MA) under positive pressure, and lyophilized. Venom was kept at −90°C until use.
2.2. Protein purification
Reverse Phase Chromatography C18.
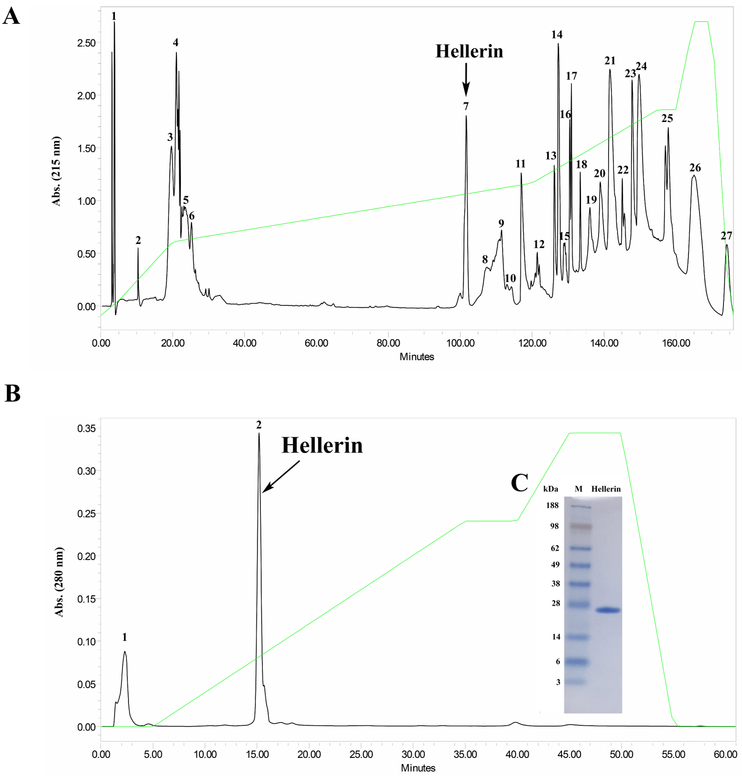
Two hundred and forty milligrams of lyophilized Crotalus oreganus helleri venom was reconstituted in 6 mL of 0.1% trifluoroacetic acid (TFA) and filtered through a 0.45 μm filter. Two hundred microliters of clear supernatant at a concentration of 40 mg/mL were fractionated by reverse phase chromatography using a Higgins Analytical PROTO 300 C18 (250 × 4.6 mm, 5 μm) column (Higgins Analytical, Inc., Mountain View, CA). Fractions were eluted using a 0.1% TFA, and 80% acetonitrile (ACN) in 0.1% TFA gradient, over 175 min with a flow rate of 1 mL/min. Protein concentrations were determined by UV spectroscopy at 280 nm. All fractions were separated by SDS-PAGE. Fraction 7 contained a protein band at molecular weight of approximately 25 kDa, which was identified as CRiSP by N-terminal sequencing.
Cation-Exchange Chromatography.
Fraction 7 collected by reverse phase chromatography was purified further by cation-exchange using a Waters Protein-Pak™ SP 5PW (7.5 × 75 mm) column (Waters Corp., Milford, MA). Fractions were eluted using a 0.02 M Tris buffer, pH 7.0 and 0.02 M Tris, 0.5 M NaCl, pH 7.0 gradient, over 90 min with a flow rate of 1 mL/min. A Waters 2487 tunable detector was used to monitor the absorbance at 280 nm.
2.3. SDS-PAGE electrophoresis and N-terminal sequencing
HPLC fraction 2 contained a CRiSP, which was confirmed by SDS-PAGE and N-terminal sequencing, and named Hellerin. Purified Hellerin was applied to NuPAGE® Novex 4-12% (w/v) Bis-Tris SDS-PAGE gels (Invitrogen™, Carlsbad, CA, USA). An XCell SureLock™ system (Invitrogen™, Carlsbad, CA, USA) with MES SDS running buffer was run at 100V for 90 min using a Bio-Rad PowerPac Basic system (Bio-Rad Laboratories, CA, USA). SeeBlue® Plus2 markers (Life Technologies™, Carlsbad, CA, USA) were used as standards. The gel was stained with SimplyBlue™ SafeStain (Life Technologies™, Carlsbad, CA, USA).
For the N-terminal sequencing, purified Hellerin was transferred from an SDS-PAGE gel onto a PVDF membrane (Millipore Immobilon, Carrigtwohill, Ireland) using a Semi-Dry Transblot Cell (Bio-Rad) at 100 mA for 1.5 h. The membrane was stained with Coomassie brilliant blue R-250 for 5 min. The sample membrane was processed for N-terminal amino acid sequencing using Edman degradation method (Edman et al., 1950; Niall, 1973) on a PPSQ-33B protein sequencer (SHIMADZU, Kyoto, Japan) following the manufacturer’s instructions. The identity of the primary sequence of Hellerin compared with other proteins was evaluated using Basic Local Alignment Search Tool (BLAST - http://blast.ncbi.nlm.nih.gov/Blast.cgi).
2.4. LC-MS/MS mass spectrometry
In gel digestion.
The protein band was excised from a gel and cut into approximately 1 mm cubes and placed in an eppendorf tube. One hundred microliters of H2O was added to the tube and incubated for 15 min at room temperature. The H2O was removed and 100 μL of 50% ACN solution was added for an additional 15 min. The solution was removed and 40 μL of 100% ACN was added for 15 min, or until the gel piece turned white. The solution was removed and 40 μL of 100 mM triethylammonium bicarbonate, pH 8.0 was added for 5 min. The procedure was repeated 3 times or until the gel pieces were destained. The gel piece was dried in a vacuum for 15 min. One hundred microliters of 100 mM triethylammonium bicarbonate, pH 8.0/10 mM DTT solution was added, and the sample was incubated for 1h at 56°C. The solution was allowed to cool to room temperature and then removed. One hundred microliters of 100 mM triethylammonium bicarbonate/55 mM iodoacetamide was added and incubated for 1 h in the dark at room temperature. The gel piece was washed with 100 μL of 100 mM triethylammonium bicarbonate, pH 8.0 for 5 min. An equal volume of 100% ACN was added to the sample and incubated for 15 min. The solution was then removed. The sample was dried in vacuum for 15 min. Trypsin was added to 50 mM triethylammonium bicarbonate, pH 8.0 at a concentration of 12.5 ng/μL and 40 μL was added to the sample and incubated for 15 min at 4°C. The sample was then incubated for 37°C overnight. The supernatant was collected and the gel piece was further incubated with 100 μL of 20 mM triethylammonium bicarbonate, pH 8.0 for 10 min, followed by two times the volume of 100% ACN for 15 min. The supernatant was collected again, and the gel piece was incubated with 100 μL 0.1% TFA for 15 min, followed by two times the volume of 100% ACN. Supernatant was pooled and dried.
LC-MS/MS Analysis.
The tryptic peptides were dissolved in 5 μL of 0.25% formic acid (FA) with 3% ACN and injected into an Easy-nLC 1000 (Thermo Fisher Scientific, MA, USA). Peptides were separated on a 45 cm in-house packed column (360 μm OD × 75 μm ID) containing C18 resin (2.2 μm, 100Å) (Bischoff Chromatography, Leonberg, Germany) with a column heater (Analytical Sales and Services, Flanders, NJ, USA) set at 50°C. The mobile phase buffer consisted of 0.1% FA in ultra-pure water (buffer A) with an eluting buffer of 0.1% FA in 80% ACN (buffer B) run over a linear 60 min (method comparisons) gradient of 5%-30% buffer B at a flow rate of 250 nL/min. The Easy-nLC 1000 was coupled online with an LTQ-Orbitrap Velos Pro mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). The mass spectrometer was operated in the data-dependent mode in which a full MS scan (from m/z 3501500 with the resolution of 30,000 at m/z 400) was followed by the 10 most intense ions being subjected to collision-induced dissociation (CID) fragmentation. CID fragmentation was performed and acquired in the linear ion trap (normalized collision energy (NCE) 30%, the automatic gain control (AGC) 3e4, max injection time 100 ms, isolation window 3 m/z, and dynamic exclusion 60 s).
Data Processing.
The raw LC-MS/MS data were searched directly against all of the Crotalus proteins available in Uniprot (downloaded July, 2018) with a 1% false discovery rate (FDR) cutoff at the protein, peptide, and modification levels. The first peptide precursor mass tolerance was set at 10 ppm, and MS/MS tolerance was set at 0.6 Da. Search criteria included a static carbamidomethylation of cysteines and variable modifications of (1) oxidation on methionine residues, (2) acetylation at the N-terminus of proteins
2.5. Multiple alignment
N-terminal amino acid sequences and sequences generated by MS were compared to the sequences in the GenBank database using BLASTX programs. Multiple alignments of the complete amino acid sequences were performed with a ClustalW program. A phylogenetic tree was generated from a multiple alignment of Hellerin within a MegAlign program using the neighbor-joining method, Lasergene 12 software (DNASTAR, Inc. Madison, WI). The bootstrap test was done using 1000 replications. The sequence of CRiSP-3 was used as an outgroup.
2.6. Vascular permeability in vivo
Quantification of in vivo vascular permeability was performed using the modified Miles assay as previously described (Fukuhara et al., 2005). 100 μL of 1% Evans blue dye in PBS was injected into the tail veins of three groups of BALB/C mice (n=3, 18-21 g body weight). Immediately following the injections, 100 μL of saline, vascular endothelial growth factor A (VEGF-A, 50 nM), or Hellerin (70 nM) was injected subcutaneously into the dorsal region. After 30 min, the mice were sacrificed by cervical dislocation and the subcutaneous injection sites were photographed, excised, and separately collected in 2 mL tubes. The skin samples were weighed and 1 mL of formamide was added to each tube containing the samples. Then, the tubes were incubated a 55°C in a water bath for 48 h to extract Evans Blue from the skins. After incubation, the tubes were centrifuged for 40 min at 12,000 rpm and the absorbance of the supernatant containing Formamide/Evans was measured at 620 nm using formamide as a blank. The ng Evans Blue extravasated per mg tissue was calculated using a standard curve for Evans Blue. Saline was used as a negative control. VEGF-A, a well-documented stimulator of vascular permeability, was used as a positive control. The animal studies were performed in accordance with the institutional guidelines. The experimental protocol had received prior approval from the Institutional Animal Care and Use Committee according to protocols ratified by the National Natural Toxins Research Center, Texas A&M University-Kingsville, Texas, USA (Viper Resource Center at Texas A&M University-Kingsville, IACUC #: 2012-12-18A-A5).
2.7. Cytotoxicity on human endothelial cells (HUVECs, HDLECs, HDBECs)
Human umbilical vein endothelial cells (HUVECs), human dermal lymphatic endothelial cells (HDLECs) and human dermal blood endothelial cells (HDBECs) at 1×105 cells/well were incubated at 37°C in 5% CO2 for 24 h, then the cells were treated with Hellerin at various concentrations (0.3 μM – 10 μM) for 24 h. Cells were incubated with 3-[4,5-dimethylthiazol-2-yl] 2,5-diphenltetrazolium bromide (MTT; 5 mg/mL) for 4 h at 37°C. MTT was aspirated and 100 μL of dimethyl sulfoxide (DMSO) was added. The absorbance of cell lysate at 570 nm was measured using a Beckman Coulter™ model AD 340 reader (Lucena et al., 2014). Cells treated only with sterile PBS were used as a negative control whereas cells treated with 1% Triton X-100 used as a positive control for toxicity comparison. The percentage of cell viability was calculated relative to the negative control, which was defined as 100%. The 50% cytotoxic concentration (CC50) of sample is defined as the protein concentration, which reduced 50% of cell viability. The percentage of cell viability was plotted against Hellerin concentrations, and the CC50 was determined.
2.8. In vitro permeability assay of HDBECs and HDLECs
HDLECs or HDBECs were seeded on 24 well plate compatible trans-well inserts (BD falcon) at ~50% density and allowed to grow to confluence. At 72 h after visual confluence was obtained, cells were treated with Hellerin (675 nM) for 60 min. FITC-BSA (10 mg/mL) was added to the upper chamber and FITC-BSA concentration in the lower chamber was measured 30 min later. The fold change in FITC-BSA signal was calculated as the ratio of FITC-BSA in the lower well of Hellerin-treated samples vs. untreated control (Cromer et al., 2014).
2.9. In vitro analysis of F-actin polymerization of HDLECs
HDLECs were grown on gelatin coated 4 well culture slides until confluent. The cells were then treated with 675 nM Hellerin for 60 min prior to fixation in 2% paraformaldehyde. The cells were washed in PBS 3 times (5 min each) and permeabilized in 0.05% Triton-X 100 in PBS for 30 min. The cells were then blocked in 5% normal goat serum in PBS for 1 h followed by staining with Phalloidin-Texas Red (100 nM, 1 h) and then washed 3 times in PBS (5 min each). The slides were then cover slipped using Pro-Long Gold with DAPI. The slides were then imaged on an Olympus widefield microscope at 20X magnification using identical settings. The resulting images were exported to ImageJ for quantification. The F-actin intensity was measured in the nuclear region of 10 cells per image and averaged.
3. Results and Discussion
Although CRISP proteins have long been recognized as ubiquitous components of many snake venoms, no clear explanation has been provided for their contribution to the biology of venoms (Yamazaki et al., 2003; Heyborne and Mackessy, 2010). In this study, we isolated and identified a new svCRiSP from the venom of the Southern Pacific rattlesnake, named Hellerin, and tested its affect on in vitro cytotoxicity, in vitro HUVEC, HDBEC, HDLEC permeability and in vivo blood vascular permeability. Hellerin was purified using sequential reverse phase and cationic exchange chromatography (Fig. 1A & 1B). However, the total protein yield was very low at approximately 0.25% yield of the total crude venom mass. According to the proteomic study (Sunagar et al., 2014), the abundance of CRiSPs varied from extremely low to large amounts (0-15%) in C. o. helleri populations from the different geographical regions. Gel electrophoresis under non-reducing conditions revealed a single protein band with a molecular mass of approximately 25 kDa (Fig. 1C). N-terminal sequencing of Hellerin and Protein BLAST analysis confirmed its identity as a svCRiSP. Its N-terminal sequence of the 12 initial residues was SVDFDSESPRKP, displaying 100% sequence homology with other crotaline CRiSPs. The protein band of Hellerin was also excised and digested with trypsin and the trypsin peptides were analyzed by mass spectrometry. The sequence for each peptide was determined by LS-MS/MS sequencing. Twelve peptides were identified from the digested protein (Table 1); several partially overlapping and redundant peptides were also obtained (Supplementary Table 1).
Figure 1.
A) Reverse phase C18 chromatography of C. o. helleri venom. Two hundred microliters of C. o. helleri venom (40 mg/mL) was injected in a Higgins Analytical, Inc. Proto 300 C18 5 μm HPLC column (250 × 4.6 mm) using Waters™ 1525 binary HPLC pumps. The fractions were separated with 80% in 0.1% The separation required 175 minutes with a flow rate of 1 mL/min. The absorbance was measured at 215 nm by a Waters™ 2487 Dual absorbance detector. B) Cation-exchange chromatography of the CRiSP-containing fraction collected by reverse phase chromatography was injected in cation-exchange using a Waters Protein-Pak™ SP 5PW column (7.5 × 75 mm). Protein detection was at 280 nm by a Waters™ 2487 Dual absorbance detector. The fractions were separated with a 0.02 M Tris buffer, pH 7.0, containing 0.5 M NaCl. Data acquisition was accomplished by Waters™ Breeze software. The arrow points to the purified CRiSP, named Hellerin, which was verified by N-terminal sequencing. C) SDS-PAGE analysis of Hellerin from C18 HPLC column. Hellerin (20 μg) was run on NuPAGE® Novex 4-12% (w/v) Bis-Tris SDS-PAGE under non-reducing conditions at 100 V for 90 min. The gel was stained with Simply Blue Safe Stain for 1 h and distained overnight with Milli-Q water. Lane 1: SeeBlue Plus2 Markers (Invitrogen™); Lane 2: Hellerin (25 kDa).
Table 1.
Peptide fragments from the digestion of Hellerin with trypsin and identified by LC-MS/MS sequencing.
| Number | Positions | Theo. MH+ [Da] | Sequenced fragment |
|---|---|---|---|
| 1 | 30-36 | 856.48869 | (R)KPEIQNK(I) |
| 2 | 37-45 | 1126.63675 | (K)IVDLHNFLR(R) |
| 3 | 46-70 | 2868.34034 | (R)RSVNPTASNMLKMEWYPEAAANAER(W) |
| 4 | 75-85 | 1343.61207 | (R)CIESHSPRDSR(V) |
| 5 | 86-104 | 2163.11882 | (R)VLEGIKCGENIYMSPVPIK(W) |
| 6 | 105-117 | 1620.79175 | (K)WTEIIHAWHGENK(D) |
| 7 | 121-142 | 2448.23466 | (K)YGIGADPPNAVIGHYTQVVWYK(S) |
| 8 | 143-157 | 1690.76759 | (K)SYRIGCAAAYCPSSK(Y) |
| 9 | 158-175 | 2202.99871 | (K)YSYFYVCQYCPAGNIIGK(T) |
| 10 | 176-204 | 3186.30535 | (K)TATPYKSGPPCGDCPSACDNGLCTNPCTK(E) |
| 11 | 205-223 | 2272.03326 | (K)EDKYTNCKSLVQQAGCQDK(Q) |
| 12 | 224-240 | 2128.92826 | (K)QMQSDCPAICFCQNKII(−) |
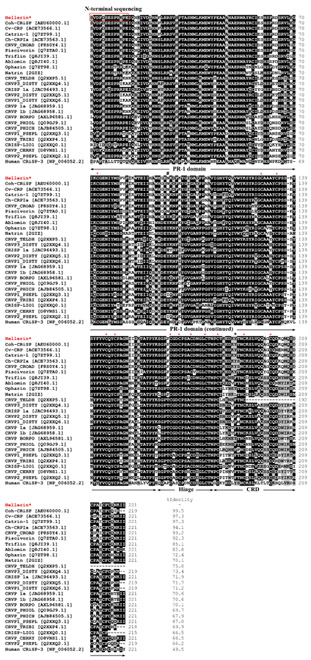
Comparison of the primary structure of Hellerin obtained by N-terminal sequencing together with sequence analysis by mass spectrometry with those of similar proteins from snake venoms in a database revealed that 16 highly conserved cysteine residues were observed among CRiSPs, and 10 of these cysteines are located in the carboxyl-terminal end (Fig. 2). NCBI protein BLAST analysis showed that Hellerin was homologous to the cDNA-derived amino acid sequences of CRiSP from C. o. helleri venom gland with 99.5% identity and shared a high similarity (80%-97%) to other viperid CRiSPs, 70%-72% to elapid CRiSPs, and 66%-75% to colubrid CRiSPs. In addition, Hellerin showed 48.5% identity with the human protein, CRiSP-3.
Figure 2.
Multiple alignments of the amino acid sequence of Hellerin with other homologous venom proteins. The alignment was generated with the ClustalW multiple sequence alignment program with manual adjustment and displayed with shaded boxes. The numbers in parenthesis are the NCBI accession numbers. All cysteine residues (an asterisk above the sequences) are conserved. Two residues numbered as 97 and 189 of CRiSPs from the North American species and the Asian species are marked by the “#” and the “+”, respectively. Coh-CRiSP (AEU60000.1) was cDNA derived amino acid sequences from a C. o. helleri venom gland cDNA library; Cv-CRP from Crotalus viridis; Catrin-1 from C. atrox; Ch-CRPIa from Crotalus horridus; CRVP_CROAD from Crotalus adamanteus; Piscivorin from Agkistrodon piscivorus piscivorus; Da-CRPa from Deinagkistrodon acutus; Triflin from Trimeresurus flavoridis; Ablomin from Agkistrodon blomhoffi; Opharin from Ophiophagus hannah; Natrin from Naja atra; CRVP 1a and CRVP 1b from Boiga irregularis; CRVP_TELDH from Telescopus dhara; CRVP_TRIBI, CRVP1_DISTY and CRVP2_DISTY from Dispholidus typus; CRVP3_DISTY from Trimorphodon biscutatus; CRISP-LEI1 from Leioheterodon madagascariensis; CRVP_PHIOL from Philodryas olfersii; CRVP_PHICH from Philodryas chamissonis; CRVP BORPO from Borikenophis portoricensis; CRISP 1a from Hypsiglena sp.; CRISP-LIO1 from Erythrolamprus poecilogyrus; CRVP1_PSEPL and CRVP2_PSEPL from Pseudoferania polylepis; CRVP_CERRY from Cerberus rynchops; and Human CRiSP-3 from Homo sapiens.
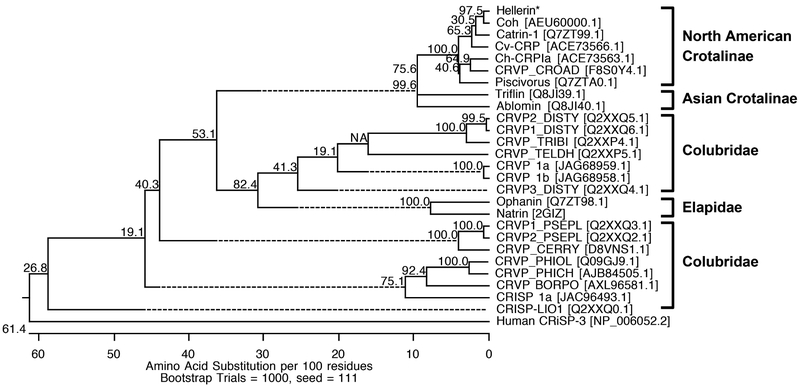
The phylogenetic relationships of the complete amino acid sequences of svCRiSPs are shown in Fig 3; human CRiSP-3 was defined as an outgroup. As expected, Hellerin was grouped with other North American crotaline CRiSPs such as Cv-CRP from Crotalus viridis (ACE73566.1), Catrin-1 from Crotalus atrox (Q7ZT99.1), and Ch-CRPIa from Crotalus horridus (ACE73563.1), CRVP_CROAD from Crotalus adamanteus (F8S0Y4.1), and Piscivorin from Agkistrodon piscivorus piscivorus (Q7ZTA0.1). Triflin from the Japanese Habu (Trimeresurus flavoviridis) and ablomin from the Japanese Mamushi (Agkistrodon blomhoffi) were clustered in the Asian Crotalinae branch. Interestingly, the primary amino acid sequence comparison between two different crotaline-clades CRiSPs revealed two residues difference at the positions 97 within the PR-1 domain and 189 within the CRD domain, which are marked by the “#” and “+”, respectively in Fig. 2, N97/Y189 in the North American species and Y97/F189 in the Asian species. The region around residues 184-189 in the CRD domain may contribute to the functional site of the svCRiSP proteins (Yamazaki et al., 2003). In addition, structural divergence studies of CRiSPs revealed that the PR-1 domain is a major contributing factor for the ligand-target recognition and the CRD domain is for the binding selectivity, which may have a role for the biological activity (Matsunaga et al., 2009). However, additional information on functional residues should be further investigated.
Figure 3.
Phylogenetic tree analysis of Hellerin based on its N-terminal amino acid sequence and MS. Hellerin is marked with an asterisk (*). The tree was constructed using the neighbor-joining methods with a bootstrap for 1000 replications. The number at the branches represents the bootstrap probability. Human-CRiSP-3 was used as an outgroup.
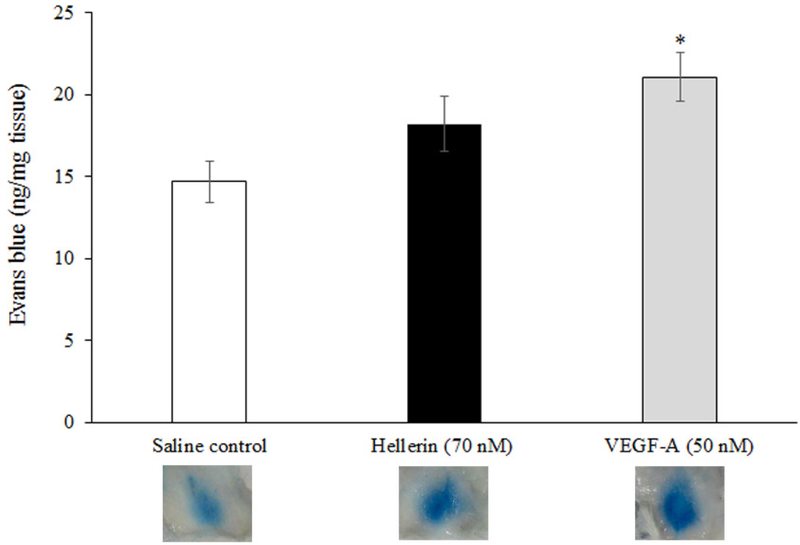
As a preliminary test of our hypothesis that svCRiSPs affect interstitial fluid dynamics, we examined the activity of Hellerin in an in vivo model of vascular permeability. As shown in Fig. 4, subcutaneous injection of Hellerin produced a rapid increase (24%) in the trans-capillary leakage of an intravascular dye (Evans blue) compared to the saline control. Treatment with the VEGF-A significantly increased vascular permeability by 43%. We next examined the effect of Hellerin on endothelial cells barrier function.
Figure 4.
Quantification of an in vivo permeability assay was performed using the modified Miles assay. Mice were injected intravenously with 100 μL of Evans blue dye, followed immediately by a subcutaneous injection of saline, Hellerin (172 ng, 70 nM), or VEGF-A (143 ng, 50 nM). The results are presented as nanograms of weight of extravasated Evans blue dye per milligram of weight of tissue sample. * P<0.05, compared with saline control.
Prior to the cellular permeability assay, Hellerin was initially tested for the cytotoxicity on three different endothelial cells including HUVECs, HDBECs, and HDLECs. Hellerin caused morphological alterations in HUVEC cells such as rounded cell shape and detachment, demonstrating the sensitivity of this cell line to Hellerin (Fig. 5A). Changes in cellular morphology have been considered as a direct indicator in assessing cytotoxicity (Al-Nazhan and Spangberg, 1990; Martin et al., 2014; Bernardes-Oliveira et al., 2016; Bradshaw et al., 2016; Gutiérrez-Praena et al., 2018). No morphological alterations were observed in HDBECs and HDLECs treated with Hellerin (data not shown). To confirm this observation, cell viability was measured by an MTT assay. Our data revealed that Hellerin dose-dependently inhibited HUVEC cell viability with a CC50 of 2.3 μM (Fig. 5B) but not to HDBECs and HDLECs (data not shown). Similar results were obtained from Lecht et al. (2015) who demonstrated that ES-CRiSP isolated from Echis carinatus sochureki was able to inhibit HUVEC cell viability and migration and was more potent than the effects on the microvascular endothelial cell isolated from human glioma (gHMVEC).
Figure 5.
Effect of Hellerin on the cell morphology (A) and cell viability (B) of HUVECs. A) Morphological changes of HUVECs after exposure to Hellerin at different concentrations for 24 h. Cell density reduction from detachment and rounded cell shape were observed by light microscopy. B) Cell viability of HUVECs treated with Hellerin was measured by an MTT assay. HUVEC cells (1×105 cells) were incubated with Hellerin at various concentrations for 24 h. The negative control consisted of cells treated with PBS buffer, pH 7.4. The results are expressed as the percentage of cell viability relative to the negative control. The results are expressed as mean ± SD (n = 3).
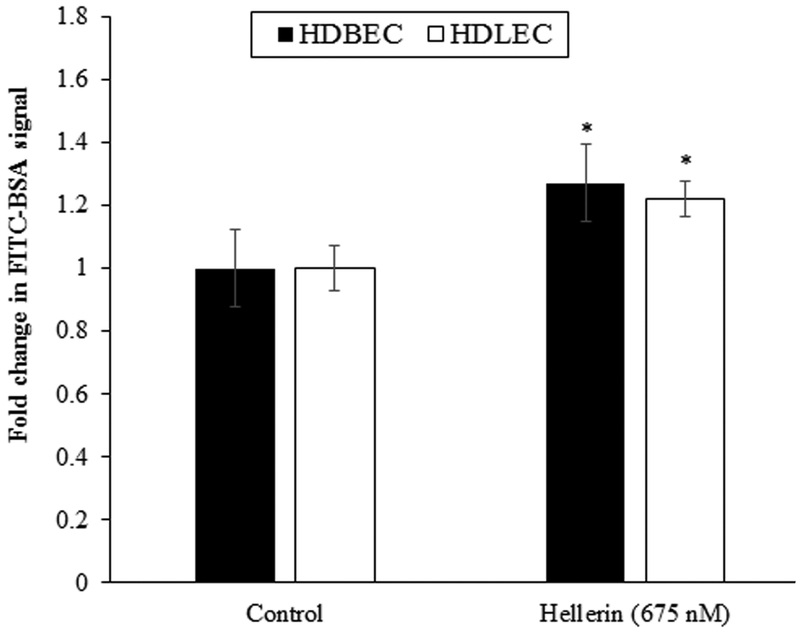
To further analyze the effect of Hellerin on blood and lymphatic permeability, we used HDBECs and HDLECs in a permeability assay. Hellerin caused 26.6% and 21.9% increase in trans-epithelial permeability after 60 min of incubation in HDBECs and HDLECs, respectively (Fig. 6). This is comparable to the effects of TNFα and LPS the authors had observed in previous studies (Cromer et. al. 2014). The actin cytoskeleton is a dynamic structure necessary for cell and tissue organization, including the maintenance of functional endothelial cell barriers. Perturbation of the actin cytoskeleton induces changes in cell morphology and various functions such as motility, cytokinesis, exocytosis, endocytosis, and increases in epithelium and endothelium permeability (Shasby et al., 1982; Bogatcheva and Verin, 2008). F-actin, one of the cytoskeletal components, is an important determinant of endothelial permeability. To explore whether the effect of Hellerin on the actin polymerization in HDLECs, we performed a fluorescence staining of F-actin, a major component of the cytoskeleton. Cells supplemented with Hellerin (675 nM) had decreased F-actin fluorescent by 20%, compared with an untreated control (Fig. 7). A similar effect was previously observed in endothelial cells treated with snake venom metalloproteinases or bacterial toxins (Ma et al., 1995; Richard et al., 1999; Wu and Huang, 2003). In addition, several studies have reported that a decrease in F-actin causes an increase in endothelial cell permeability (Phillips and Tsan, 1985; Rotrosen and Gallin, 1986; Ikeda et al., 1999; Banan et al., 2001). These results confirm that the Hellerin-induced reduction of the F-actin of HDLECs is an important part of the mechanism for the increase of endothelial monolayer permeability.
Figure 6.
Effect of Hellerin on monolayer barrier function of HDBECs and HDLECs. HDBECs and HDLECs grown on trans-well inserts, were treated with Hellerin (675 nM) for 60 min. FITC-BSA was added to the upper chamber and FITC-BSA concentration in the lower chamber was measured 30 min later. The fold change in FITC-BSA signal was calculated as the ratio of FITC-BSA in the lower well of Hellerin-treated samples vs. untreated control. Values are the mean + SE, n=6. * P<0.05, compared with untreated control.
Figure 7.
Effect of Hellerin on HDLEC F-actin levels. Cells grown on chamber slides were treated with Hellerin (675 nM) for 60 min and then stained with Phalloidin-Texas Red. The level of F-actin in relative fluorescent units was measured below the nuclear region at 20X magnification. Images presented are 40X for clarity. Values are the mean ± SE, n=4. * P<0.05, compared with untreated control.
We speculate that svCRiSP-induced changes in the permeability of blood and lymphatic vessels in tissues in the proximity of the snakebite result in a localized increase in the movement of fluid out of the blood and into the interstitium. This alteration in interstitial fluid dynamics produces an increase in interstitial fluid flow that when coupled with increased lymphatic permeability, may serve to “flush” the macromolecular toxins in the venom first into the lymphatic systems and then, very rapidly, into the systemic circulation. Knowledge gained from the study of the acute effects of svCRiSPs on both blood vascular and lymphatic endothelial cell function may provide the basis for the development of classes of drugs useful in the manipulation of interstitial fluid dynamics and the treatment of diseases such as lymphedema. Further studies are underway to characterize the cellular and molecular basis for the effect of Hellerin on the function of blood and lymphatic endothelial cells.
Indeed, our data demonstrate that Hellerin, an svCRiSP recently isolated from the venom of the Southern Pacific rattlesnake (C. o. helleri) has effects on blood vascular permeability and the permeability of monolayers of blood and lymphatic endothelial cells. Moreover, this is the first observation reported that a purified svCRiSP toxin directly and acutely increases vascular permeability both in vitro and in vivo. It also shows, for the first time, that lymphatic cells are targets of svCRiSP activity.
Supplementary Material
Supplement Table 1. The trypsin digested fragments of several partially overlapping and redundant peptides for Hellerin were sequenced by mass spectrometry.
Highlights:
A novel CRiSP, Hellerin, purified from the C. o. helleri venom.
Hellerin has cytotoxic effects on HUVECs but not in HDBECs and HDLECs.
Hellerin acutely increases vascular permeability both in vitro and in vivo.
Acknowledgements.
Funding for the project was granted by the NIH/ORIP, Viper Resource Center grant# 5P40OD010960-14 (NNTRC, Texas A&M University-Kingsville, Dr. E.E. Sánchez) and NIH/AREA, NIH/NHLBI grant# 1R15HL137134-01 (Texas A&M University-Kingsville, Dr. M. Suntravat), The Texas A&M University’s Presidential Undergraduate Research Program, and the Robert A. Welch Foundation Department, grant# AC-0006 (TAMUK-Department of Chemistry). We would also like to thank, Nora Diaz DeLeon and Mark Hockmuller (NNTRC Serpentarium curator) and all the NNTRC personnel.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Nazhan S, Spangberg L, 1990. Morphological cell changes due to chemical toxicity of a dental material: an electron microscopic study on human periodontal ligament fibroblasts and L929 cells. J Endod. 16, 129–134. [DOI] [PubMed] [Google Scholar]
- Banan A, Fields JZ, Zhang Y, Keshavarzian A, 2001. iNOS upregulation mediates oxidant-induced disruption of F-actin and barrier of intestinal monolayers. Am J Physiol Gastrointest Liver Physiol. 280, G1234–4G1236. [DOI] [PubMed] [Google Scholar]
- Bernardes-Oliveira E, Gomes DL, Martelli Palomino G, Juvenal Silva Farias K, da Silva WD, Rocha HA, Gonçalves AK, Fernandes-Pedrosa MF, Crispim JC, 2016. Bothrops jararaca and Bothrops erythromelas Snake Venoms Promote Cell Cycle Arrest and Induce Apoptosis via the Mitochondrial Depolarization of Cervical Cancer Cells. Evid Based Complement Alternat Med. 2016: 1574971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogatcheva NV, Verin AD, 2008. The role of cytoskeleton in the regulation of vascular endothelial barrier function. Microvasc Res. 76, 202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw MJ, Saviola AJ, Fesler E, Mackessy SP, 2016. Evaluation of cytotoxic activities of snake venoms toward breast (MCF-7) and skin cancer (A-375) cell lines. Cytotechnology. 68, 687–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RL, Haley TL, West KA, Crabb JW, 1999. Pseudechetoxin: a peptide blocker of cyclic nucleotide-gated ion channels. Proc Natl Acad Sci U S A. 96, 754–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromer W, Zawieja SD, Tharakan B, Childs EW, Newell MK, Zawieja DC, 2014. The effects of inflammatory cytokines on lymphatic endothelial barrier function. Angiogenesis. 17, 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman P, Högfeldt E, Sillén LG, Kinell P, 1950. Method for determination of the amino acid sequence in peptides. Acta Chem Scand. 4, 283–293. [Google Scholar]
- Estrella A, Sánchez EE, Galán JA, Tao WA, Guerrero B, Navarrete LF, Rodriguez-Acosta A, 2011. Characterization of toxins from the broad-banded water snake Helicops angulatus (Linnaeus, 1758): isolation of a cysteine-rich secretory protein, Helicopsin. Arch Toxicol. 85, 305–313. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, Kangawa K, Mochizuki N, 2005. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol. 25, 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs GM, Roelants K, O'Bryan MK, 2008. The CAP superfamily: cysteine-rich secretory proteins, antigen 5, and pathogenesis-related 1 proteins--roles in reproduction, cancer, and immune defense. Endocr Rev. 29, 865–897. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Praena D, Guzmán-Guillén R, Pichardo S, Moreno FJ, Vasconcelos V, Jos Á, Cameán AM, 2018. Cytotoxic and morphological effects of microcystin-LR, cylindrospermopsin, and their combinations on the human hepatic cell line HepG2. Environ Toxicol. November 20. doi: 10.1002/tox.22679. [DOI] [PubMed] [Google Scholar]
- Heyborne W and Mackessy SP, 2010. Cysteine-rich secretory proteins in reptile venoms In: Mackessy SP (ed). Handbook of Venoms and Toxins of Reptiles. CRC Press, Boca Raton, pp. 325–336. [Google Scholar]
- Ikeda K, Utoguchi N, Makimoto H, Mizuguchi H, Nakagawa S, Mayumi T, 1999. Different reactions of aortic and venular endothelial cell monolayers to histamine on macromolecular permeability: role of cAMP, cytosolic Ca2+ and F-actin. Inflammation. 23, 87–97. [DOI] [PubMed] [Google Scholar]
- Lecht S, Chiaverelli RA, Gerstenhaber J, Calvete JJ, Lazarovici P, Casewell NR, Harrison R, Lelkes PI, Marcinkiewicz C, 2015. Anti-angiogenic activities of snake venom CRISP isolated from Echis carinatus sochureki. Biochim Biophys Acta. 1850, 1169–1179. [DOI] [PubMed] [Google Scholar]
- Lucena SE, Romo K, Suntravat M, Sánchez EE, 2014. Anti-angiogenic activities of two recombinant disintegrins derived from the Mohave and Prairie rattlesnakes. Toxicon. 78, 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma TY, Hollander D, Tran LT, Nguyen D, Hoa N, Bhalla D, 1995. Cytoskeletal regulation of Caco-2 intestinal monolayer paracellular permeability. J Cell Physiol. 164, 533–545. [DOI] [PubMed] [Google Scholar]
- Martin HL, Adams M, Higgins J, Bond J, Morrison EE, Bell SM, Warriner S, Nelson A, Tomlinson DC, 2014. High-content, high-throughput screening for the identification of cytotoxic compounds based on cell morphology and cell proliferation markers. PLoS One. 9, e88338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga Y, Yamazaki Y, Hyodo F, Sugiyama Y, Nozaki M, Morita T, 2009. Structural divergence of cysteine-rich secretory proteins in snake venoms. J Biochem. 145, 365–75. [DOI] [PubMed] [Google Scholar]
- Niall HD, 1973. Automated Edman degradation: the protein sequenator. Meth Enzymol. 27,942–1010. [DOI] [PubMed] [Google Scholar]
- Phillips PG, Tsan MF, 1985. Hyperoxia causes increased albumin permeability of cultured endothelial monolayers. J Appl Physiol. 64, 1196–1202. [DOI] [PubMed] [Google Scholar]
- Richard JF, Petit L, Gibert M, Marvaud JC, Bouchaud C, Popoff MR, 1999. Bacterial toxins modifying the actin cytoskeleton. Int Microbiol. 2, 185–194. [PubMed] [Google Scholar]
- Rotrosen D, Gallin JI, 1986. Histamine type I receptor occupancy increases endothelial cytosolic calcium, reduces F-actin, and promotes albumin diffusion across cultured endothelial monolayers. J Cell Biol. 103, 2379–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shasby DM, Shasby SS, Sullivan JM, Peach MJ, 1982. Role of endothelial cell cytoskeleton in control of endothelial permeability. Circ Res. 51, 657–661. [DOI] [PubMed] [Google Scholar]
- Sunagar K, Jackson TNW, Reeks T, Fry BG, 2015. Cysteine-rich secretory proteins In: Fry BG (Ed). Venomous Reptiles and Their Toxins. Oxford University Press, New York, pp. 241–246. [Google Scholar]
- Sunagar K, Undheim EA, Scheib H, Gren EC, Cochran C, Person CE, Koludarov I, Kelln W, Hayes WK, King GF, Antunes A, Fry BG, 2014. Intraspecific venom variation in the medically significant Southern Pacific Rattlesnake (Crotalus oreganus helleri): biodiscovery, clinical and evolutionary implications. J Proteomics. 99: 68–83. [DOI] [PubMed] [Google Scholar]
- Tasoulis T, Isbister GK, 2017. A Review and Database of Snake Venom Proteomes. Toxins (Basel). 9, pii: E290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udby L, Calafat J, Sørensen OE, Borregaard N, Kjeldsen L, 2002a. Identification of human cysteine-rich secretory protein 3 (CRISP-3) as a matrix protein in a subset of peroxidase-negative granules of neutrophils and in the granules of eosinophils. J Leukoc Biol. 72, 462–469. [PubMed] [Google Scholar]
- Udby L, Cowland JB, Johnsen AH, Sørensen OE, Borregaard N, Kjeldsen L, 2002b. An ELISA for SGP28/CRISP-3, a cysteine-rich secretory protein in human neutrophils, plasma, and exocrine secretions. J Immunol Methods. 263, 43–55. [DOI] [PubMed] [Google Scholar]
- Udby L, Søorensen OE, Pass J, Johnsen AH, Behrendt N, Borregaard N, Kjeldsen L, 2004. Cysteine-rich secretory protein 3 is a ligand of alpha1B-glycoprotein in human plasma. Biochemistry. 43, 12877–12886. [DOI] [PubMed] [Google Scholar]
- Wang YL, Kuo JH, Lee SC, Liu JS, Hsieh YC, Shih YT, Chen CJ, Chiu JJ, Wu WG, 2010. Cobra CRISP functions as an inflammatory modulator via a novel Zn2+- and heparan sulfate-dependent transcriptional regulation of endothelial cell adhesion molecules. J. Biol. Chem. 285, 37872–37883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Li H, Liu MN, Song H, Han HM, Wang QL, Yin CC, Zhou YC, Qi Z, Shu YY, Lin ZJ, Jiang T, 2006. Structural and functional analysis of natrin, a venom protein that targets various ion channels. Biochem Biophys Res Commun. 351, 443–448. [DOI] [PubMed] [Google Scholar]
- Wang J, Shen B, Guo M, Lou X, Duan Y, Cheng XP, Teng M, Niu L, Liu Q, Huang Q, Hao Q, 2005. Blocking effect and crystal structure of natrin toxin, a cysteine-rich secretory protein from Naja atra venom that targets the BKCa channel. Biochemistry. 44, 10145–10152. [DOI] [PubMed] [Google Scholar]
- Wu WB, Huang TF, 2003. Activation of MMP-2, cleavage of matrix proteins, and adherens junctions during a snake venom metalloproteinase-induced endothelial cell apoptosis. Exp Cell Res. 288, 143–157. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Brown RL, Morita T, 2002. Purification and cloning of toxins from elapid venoms that target cyclic nucleotide-gated ion channels. Biochemistry. 41, 11331–11337. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Hyodo F, Morita T, 2003. Wide distribution of cysteine-rich secretory proteins in snake venoms: isolation and cloning of novel snake venom cysteine-rich secretory proteins. Arch Biochem Biophys. 412, 133–141. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Morita T, 2004. Structure and function of snake venom cysteine-rich secretory proteins. Toxicon. 44, 227–231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Table 1. The trypsin digested fragments of several partially overlapping and redundant peptides for Hellerin were sequenced by mass spectrometry.