Abstract
Insulin therapy is necessary to regulate blood glucose levels for people with type 1 diabetes and commonly used in advanced type 2 diabetes. Although subcutaneous insulin administration via hypodermic injection or pump-mediated infusion is the standard route of insulin delivery, it may be associated with pain, needle phobia, and decreased adherence, as well as the risk of infection. Therefore, transdermal insulin delivery has been widely investigated as an attractive alternative to subcutaneous approaches for diabetes management in recent years. Transdermal systems designed to prevent insulin degradation and offer controlled, sustained release of insulin may be desirable for patients and lead to increased adherence and glycemic outcomes. A challenge for transdermal insulin delivery is the inefficient passive insulin absorption through the skin due to the large molecular weight of the protein drug. In this review, we focus on the different transdermal insulin delivery techniques and their respective advantages and limitations, including chemical enhancers-promoted, electrically enhanced, mechanical force-triggered, and microneedle-assisted methods.
Keywords: Drug delivery, Transdermal delivery, Insulin, Diabetes, Iontophoresis, Electroporation, Ultrasound, Jet injection, Microneedle
Graphical Abstract
1. Introduction
Diabetes mellitus is a group of metabolic diseases characterized by increased production of glucose by the liver and decreased clearance of glucose into muscle and fat resulting in abnormal accumulation of glucose in the blood, all driven by inadequate insulin levels [1]. Approximately 425 million adults suffer from diabetes according to 2018 reports from The International Diabetes Federation [2]. Moreover, the prevalence of diabetes is increasing and expected to rise across the world in the coming decades [3]. Diabetes is usually caused by the failure of insulin secretion by the pancreas (type 1 diabetes) or the defective responsiveness of the body to insulin (type 2 diabetes) [4, 5].
Exogenous insulin administration is essential in the management of type 1 diabetes and advanced type 2 diabetes [6, 7]. Typically, people with diabetes are instructed to self-inject insulin subcutaneously several times per day, which requires both training and intensive self-management with frequent dose adjustments by patients based on glucose monitoring by the patients [8]. The need for frequent injections may be associated with poor adherence, and also carries the risk of microbial contamination, local tissue necrosis, and nerve damage [9–11]. Alternatively, patients may use continuous subcutaneous insulin infusions, also known as insulin pump therapy, which also carries limitations including technological difficulties [12]. To address these limitations, a broad range of delivery methods have been investigated as needle-free alternatives for daily insulin therapy, including oral, pulmonary, nasal, and transdermal approaches [13–20]. However, the poor permeability of insulin across the tissue barriers hinders bioavailability, which poses a major limitation in the clinical applications of these approaches [21–23].
A transdermal delivery strategy transports insulin across the skin barrier represents a minimally invasive and attractive method for insulin delivery in contrast to painful hypodermic injections [24, 25]. It also has several advantages over oral, pulmonary, and nasal administration techniques. For example, insulin delivered via a transdermal system is able to avoid the chemical and enzymatic degradation in the digestive tract [26]. This approach can also provide a sustained release to maintain therapeutic concentrations for prolonged time [27]. Finally, the convenience of this administration may increase patient adherence, leading to improved glycemic control [27].
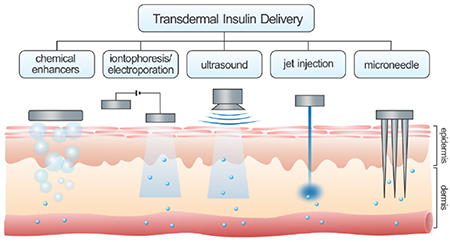
However, effective insulin delivery via the skin remains challenging due to the intrinsic, protective properties of the intact skin. Therapeutics with low molecular weight (<500 Da) can easily penetrate the skin, while the passive transport of protein drugs with higher molecular weight, such as insulin, is significantly restricted [28]. To overcome skin barriers in transdermal insulin delivery, various approaches have been explored to physically or chemically enhance the transport efficiency of the insulin molecule across the skin. This review presents the recent advances in transdermal insulin delivery systems, including chemical enhancers-promoted, electrically facilitated, mechanical force-triggered, and microneedle (MN)-assisted approaches (Fig. 1). The challenges for potential clinical applications are also discussed.
Fig. 1.
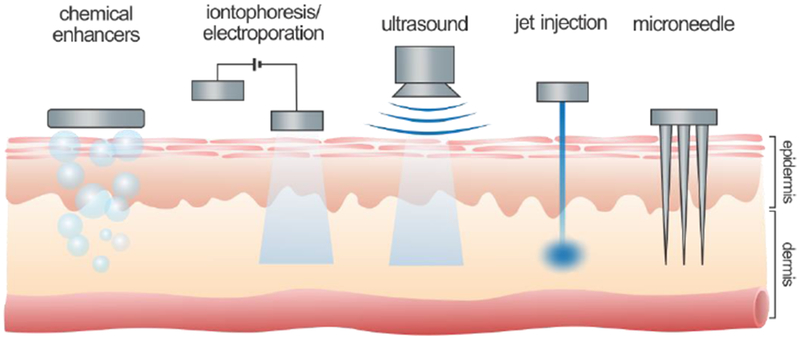
Representative transdermal strategies for insulin delivery based on diverse mechanisms, including chemical enhancers-promoted, electrically facilitated, mechanical force-triggered and microneedle-assisted delivery systems.
2. Chemical enhancers-promoted transdermal delivery
In order to improve skin permeability, chemical penetration enhancers that can disrupt the skin barrier and provide an adding driving force for transporting therapeutics have been intensively investigated [29–31]. There are various effective chemical enhancers, including conventional chemical molecules as well as membrane-permeable peptides and vehicles. Chemical enhancers can insert into the highly ordered lipid bilayer in stratum corneum to disorganize molecular packing or extract lipids to create lipid-packing defects of nanometer dimensions, thus leading higher transport efficiency of insulin [32,33].
The Gasem group has previously examined the permeation enhancement properties of 43 different chemical enhancers that have been used in insulin delivery [34]. The authors also discussed the potential criteria for further screening of enhancers. Sintov et al. reported that iodine facilitated insulin delivery across the skin as iodine could inactivate endogenous sulfhydryls, such as glutathione and gamma-glutamylcysteine, thereby reducing the formation of disulfide bonds and retaining the potency of insulin during its flux through the skin into the circulation [35]. The ability of trypsin to react with the stratum corneum was also assessed for enhanced transdermal insulin delivery, where it was shown that trypsin altered the protein structure of stratum corneum from the alpha- to the beta-form and decreased the electrical resistance of the skin, reflecting a 5.2-fold increase in insulin absorption (insulin at pH 3) [36].
Wen and coworkers identified a class of membrane-permeable peptides that can promote delivery of cargo into the systemic circulation by in vivo phage display, other than some previously characterized peptides (such as protein transduction domains (PTDs) that could only locally transport drugs [37]. The synthetic peptide, ACSSSPSKHCG (TD-1), was suggested to be capable of creating transient opening in the skin to enable penetration of insulin into hair follicles beyond a depth of 600 μm. An obvious suppression in blood glucose levels (BGLs) (~ 40% of initial values) was observed in diabetic rats, which was sustained over 11 hours (h) compared to less than 5 h with subcutaneously injected insulin. This research spurred development of an alternative series of peptide-based enhancers designed to transcutaneously deliver hydrophilic macromolecular therapeutics. For example, Chang et al. screened a number of TD-1-derived cationic cyclopeptides in Caco-2 cell monolayers-based in vitro model and assessed in vivo performance in diabetic rats [38]. The results indicated that TD-34 (ACSSKKSKHCG) with bis-substituted lysine in N-5 and N-6 sites displayed the best transdermal enhancement activity, where administration of 2.1 IU insulin with 0.5 μmol of TD-34 led to an approximately 26% reduction in BGLs of rats that was sustained for 8 h.
Nano/micro vesicles, such as liposomes and nano/microemulsions, have also been explored as chemical enhancers [39–43]. They can not only improve skin permeability but also act as the vehicles for drug solubilization and drug transport through the skin [39, 44]. A variety of nanocarriers have been demonstrated the ability to encapsulate and transdermally deliver insulin into the dermis, such as lipid-based vesicles [45–47], CaCO3 nanoparticles [48] and nanoemulsions [49, 50]. For example, King et al. reported lipid-based biphasic vesicles as skin penetration enhancers for insulin delivery [46]. These researchers incorporated insulin-encapsulated biphasic vesicles in a transdermal patch and applied the patch on the abdominal skin of diabetic mice for 48 h. The mice showed response to the patch loading 50 mg vesicle-entrapped insulin for over 51 h with a decrease in blood glucose of ~ 43%. Further analysis of the topical administration of biphasic vesicles demonstrated that the transport and absorption of insulin was primarily mediated by the lymphatic pathway in a diabetic rat model [47].
Goto and coworkers proposed an alternative formulation based on solid-in-oil (S/O) nanodispersion for protein delivery [49, 51, 52]. The prepared the S/O nanodispersions were approximately 250 nm and integrated isopropyl myristate (IPM), an oil with a penetrationenhancing effect, along with insulin and R9 peptides loaded at a molar ratio of 1:3 [49]. The R9 peptides, a type of arginine-rich peptides, was involved as PTDs to enhance the skin permeability to insulin. A synergistic effect of isopropyl myristate and PTDs to disrupt the barrier property of the skin and improve insulin penetration across the skin in vitro was demonstrated. Nose, Pissuwan and colleagues formed a different S/O formulation by introducing gold nanorods into an oil phase for transdermal delivery under irradiation of near-infrared light [50, 53]. Individually, the gold nanorods (27 nm in width and 66 nm in length) were coated with methoxy(polyethylene glycol)-thiol and formed a complex with surfactant (L-195) and insulin, followed by dispersing in the oil phase, IPM (Fig. 2) [50]. Under near-infrared light (0.4 W/cm2 for 10 min), the photothermal effect caused by gold nanorods might break the skin barrier by disrupting skin lipids or changing the size and density of the skin barrier, thus allowing insulin to permeate through the skin. Additionally, the surfactant L-195 and IPM acted as enhancers to promote transdermal transport of insulin. After treatment of nanodispersion and light irradiation, the BGLs of diabetic mice significantly decreased to approximately 58% of pre-treatment values at 4 h.
Fig. 2.
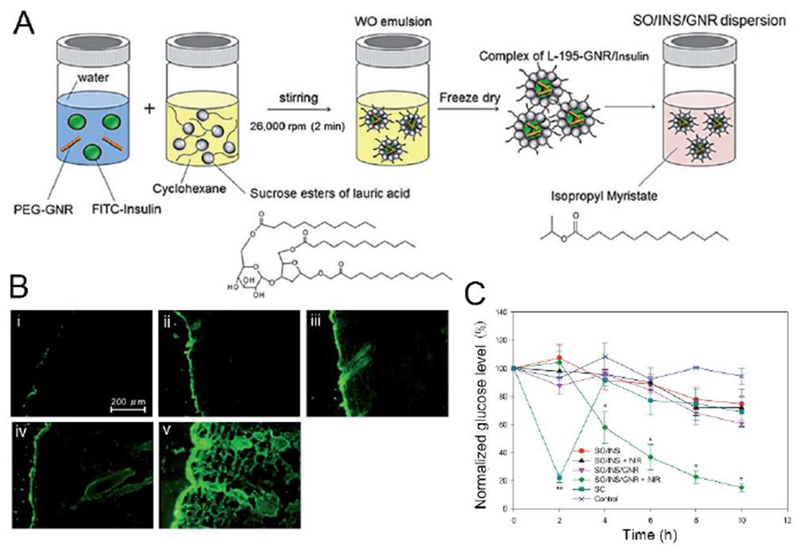
A representative solid-in-oil nanoformulation for insulin transdermal delivery. A) Scheme of the preparation of gold nanorod-insulin complexes/oil formulation (SO-INS-GNR) (SO: solid-in-oil; INS: insulin; PEG-GNR: mPEG coated gold nanorod; FITC: fluorescein isothiocyanate; WO: water in oil). B) Fluorescent images showing transdermal delivery of FITC-labeled insulin after the treatment of various insulin formulations, with or without near infrared (NIR) light irradiation: insulin in water (W-INS) treatment (i), SO-INS treatment alone (ii), SO-INS treatment with NIR irradiation (iii), SO-INS-GNR treatment alone (iv), and SO-INS-GNR treatment with NIR irradiation (v). C) Blood glucose levels of diabetic mice after administration of SO-INS and SO-INS-GNR with and without NIR light irradiation, and subcutaneous injection (SCI) of insulin (*P < 0.05, **P < 0.01). Adapted with permission from Ref [50].
Chemical penetration enhancers can disrupt the skin structure to promote permeability and improve drug solubility to provide the drug concentration-gradient driving force. Despite it, many chemical enhancers still show limited transdermal delivery efficiency of insulin. For those most effective chemical enhancers, how to prevent their diffuse out of the stratum corneum and the relevant irritation to the deeper tissue should be further addressed.
3. Electrically facilitated transdermal delivery
In addition to the chemical penetration enhancers, electrical instruments that facilitate insulin transport through the skin have also received considerable attention [54–57]. Unlike chemical penetration enhancers, these electrical instruments improve the insulin delivery efficiency through the skin by providing additional driving force via electrical interactions or introducing transient perturbation of the stratum corneum via high-voltage electrical pulse.
3.1. Iontophoresis
Iontophoresis emerged as a transdermal enhancement technique in the early 20th century. This technique uses a mild electric current for the delivery of large and/or charged molecules [54, 58]. This technology relies on a pair of electrodes that is placed on the skin to generate an electrical potential between the skin surface and the capillaries below (Fig. 3). Positively charged therapeutic molecules are driven toward the capillaries from the skin surface at the positive electrode, while negatively charged molecules transport through the skin toward the negative electrode. Extensive studies have identified that electromigration and electroosmosis are two of the predominant drive forces to affect the transport of drug ions across the skin into systemic circulation [59], and the amount of transported charge depends on the intensity of the electric field and the treatment duration [60, 61]. Nonetheless, the skin is permselective to cations under an electric current since it is negatively charged at the physiological condition [62]. Therefore, the transdermal delivery of insulin to give therapeutic levels is challenged by the negative charge of human insulin (~5800 Da) under physiological conditions [63–67].
Fig. 3.
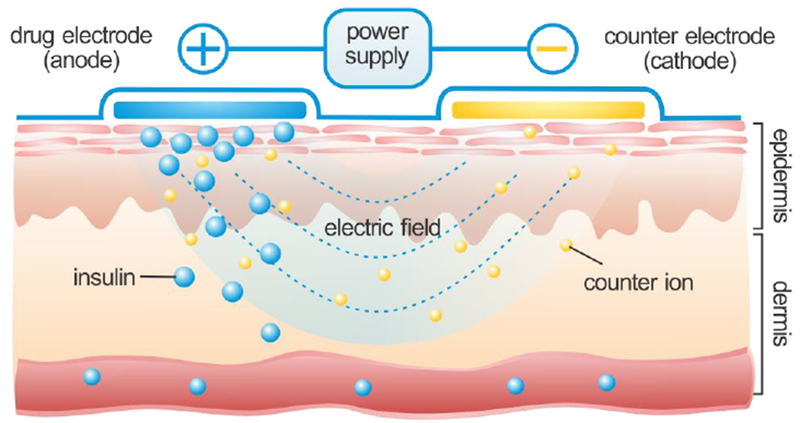
Illustration of iontophoresis-assisted insulin delivery through the skin.
Siddiqui et al. found that adjusting the aqueous solution of concentrated insulin (500 IU/ml) to a pH of 3.7 was the most effective condition for regular insulin iontophoresis [68]. Similarly, Pillai et al. revealed that anodal iontophoresis of insulin at pH 3.6 resulted in better stability and permeation of insulin [69, 70]. Kajimoto et al. also attempted to raise the transport efficiency of insulin by utilizing charged liposomes as carriers during iontophoresis [71]. The in vivo performance in a diabetic rat model showed that the transdermal iontophoresis (0.45 mA/cm2 for 1 h) of cationic liposome-encapsulated insulin through the transfollicular pathway led to a gradual decrease of 20% in BGLs at 18 h after administration which was maintained for up to 24 h. A corresponding increase in plasma insulin levels was also detected (~1.4 ng/mL 18 h post-treatment) that exceeded levels detected in rats treated with intraperitoneally injected insulin.
Pretreatment of skin such as stripping skin [72, 73], using penetration enhancers [74–77] and depilatory cream [75, 78, 79] has been reported to enhance the transport of insulin through the skin under iontophoresis. An investigation on the effect of diverse chemical enhancers including ethanol (EtOH), propylene glycol (PG), dimethylacetamide (DMA), ethyl acetate (EtOAc) and IPM demonstrated that skin permeability was severely improved with DMA, followed by EtOH and EtOAc, while IPM and PG exhibited relatively insignificant skin barrier altering potential [77]. By acting on the lipid bilayer, these chemicals led to lipid extraction (EtOH, DMA, EtOAc), changes in skin proteins (EtOH, DMA), or increased lipid fluidity (IPM), thus produced a synergistic enhancement with iontophoresis.
Furthermore, hydrogel formulations are considered to be desirable for iontophoresis, as they can act as an electroconductive base and adapt to the contours of the skin; these formulations can also be easily integrated with the iontophoresis delivery systems [80]. Kagatani et al. reported a pulsatile insulin delivery system with an electro-responsive poly(dimethylaminopropylacrylamide) (PDMAPAA) gel [81]. The insulin-loaded PDMAPAA gel was subcutaneously injected into the skin of rats as a depot. Upon stimulation with a constant current of 1.0 mA (0.36 mA/cm2), a pulsatile plasma glucose decrease was detected in the animals. Both insulin and PDMAPAA gel in this system might be positively charged at the pH of 2.3. When an electrical field is applied, the cross-linked PDMAPAA network undergoes slight expansion at the cathode side, allowing solvated insulin to quickly diffuse out the gel with the outflow of the solution, caused by the electrokinetic flow of solvated insulin with water. In another study, Pillai et al. used poloxamer 407 to prepare the insulin gel that was further integrated with chemical enhancers for the ex vivo and in vivo skin permeation analysis [82]. Application of iontophoresis, either alone or in combination with linoleic acid, resulted in a 36-40% reduction in BGLs of diabetic rats.
To mitigate the potential electrochemical damage caused during iontophoresis, such as burns and skin irritation [83], alternating current iontophoresis was investigated over conventional direct current iontophoresis; this approach showed reduced side effects [84]. Feasibility studies showed that alternating current iontophoresis-activated transdermal insulin delivery systems were associated with an average delivery of 57% of the initial insulin amount (2.85 mg in 500 μL sample) [85].
Unlike chemical penetration enhancers, iontophoresis does not disrupt the skin structure that may affect its barrier ability. Nonetheless, the low-level current of such technique limits the transport efficiency of insulin through the stratum corneum. Although increasing current intensity can lead to a higher delivery rate, the potential risk of skin irritation and pain also limits the maximum current intensity.
3.2. Electroporation
Electroporation has evolved as another attractive technique for electrically assisted transdermal drug delivery [55, 86, 87]. Different from the continuous application (hours) of low-level current in iontophoresis, the procedure of electroporation involves using short, high-voltage pulses to induce transient perturbation in the stratum corneum by creating micro-pathways across its lipid bilayers [88]. The stratum corneum forms the major barrier and contributes the major portion of the electric resistance in the skin (5-25 kΩ/cm2) [88]. In electroporation, the application of high voltage pulses that are higher than the breakdown potential of the stratum corneum (75-100 V) and thus results in the formation of temporary pores in the lipid bilayers of the stratum corneum which facilitate the transport of the drugs across skin [89, 90].
Several studies have been investigated to validate the enhanced transdermal delivery of insulin by electroporation. Mohammad and colleagues examined the effect of different electroporation parameters (number of pulses, insulin concentrations, and field strengths) and chemical enhancers (castor oil, iodine, and oleic acid) on transdermal insulin delivery in rabbits [91]. In another work, Rastogi et al. explored electroporation of polymeric nanoparticles encapsulated insulin, demonstrating a 4-fold enhancement in insulin deposition in rat skin compared to electroporation using free insulin, as well as an extended therapeutic effect from 24 h to 36 h [92]. Sen et al. reported an alternative transdermal enhancement method by mixing the anionic lipids with target molecules that were associated with enhancing the electroporative transport of negatively charged permeants up to 10 kDa in size [93]. The resultant enhancing effect was attributed to the increased number and size, as well as prolonged lifetime, of the electropores created during the electroporation in the presence of the lipid dispersion. Specifically, the anionic lipids were shown to have a positive effect in dropping the skin resistance and retard the recovery of resistance after the cessation of pulse application, thereby expanding the potential of lipid-enhanced electroporation for delivering large biomolecules. More recently, these researchers examined the impact of another anionic lipid, 1,2-dimyristoyl-3-phosphatidylserine (DMPS), on the transdermal transport of insulin using porcine epidermis model and observed a 20-fold enhancement of insulin with dispersion in DMPS electroporation for 10 min (100-105 V, 1 ms pulse width at 1 Hz) compared to that without DMPS [94]. Sen and coworkers also demonstrated a synergistic effect of coupling DMPS and anodal iontophoresis (electroosmosis) with electroporation on the transport of insulin both ex vivo and in vivo, where the combination treatment of DMPS (in 0.2 % sodium dodecyl sulfate) and electroosmosis resulted in a ~10-fold increase in plasma insulin level in a Sprague-Dawley rat model [95, 96]. This in vivo synergistic treatment of electroporation and iontophoresis was also validated by Sugibayashi and colleagues using human insulin[97]. Interestingly, this group suggested that insulin had different aggregation properties under different pH conditions in their study: insulin at pH 10 had a higher ratio of nonassociation formulation, whereas most of the insulin associated into hexamers at pH 7. Therefore, a much higher plasma level of insulin was detected with pH at 10 than at 7.
Considering the clinical application of electroporation, Wong et al. developed a painless electroporation technique using a microelectrode array block to mitigate the potential painful sensation induced by electrodes on the human skin while maintaining the delivery efficacy of insulin (Fig. 4) [98]. Studies in diabetic mice indicated a 100-fold increase in transdermal insulin delivery passive diffusion by electroporation using the electrodes array (150 V, 120 pulses at 0.2 ms, 1 Hz) compared to passive diffusion. The associated human studies suggested that the microelectrodes array design provided a feasible electroporation condition that was both painless and harmless to humans. Of note, the combination of mild hyperthermia (40 °C for 20 min) with electroporation resulted in an even higher delivery efficiency in mice, which is 23 7-fold more than the control values by passive diffusion. The delivery efficiency is consistent with pharmacodynamic studies of insulin: a significant hypoglycemic effect was observed immediately after the electroporation actuation and heating treatment that continued up to 10 h. For translation purpose, Ching et al. developed a compact, low-cost and programmable electroporation device that could easily adjust high-voltage (2-300 V) electrical waveforms including both pulsed and pulsed-biphasic forms for precise regulation of the magnitude and waveform of electroporation [99]. They also evaluated the potency of this device in vitro for transdermal delivery of medications including insulin.
Fig. 4.
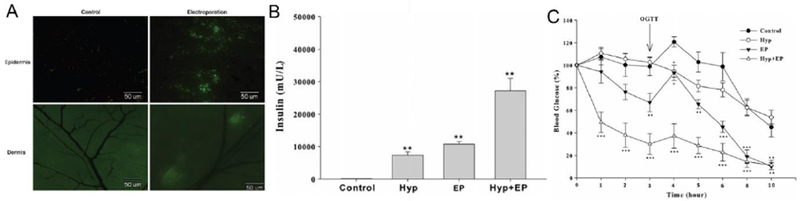
Transdermal insulin delivery based on electroporation technique. A) Fluorescence image of skin treated with electroporation of FITC-insulin. B) Transport amount of insulin in mouse skin. Control: insulin occlusion only; Hyp: hyperthermia at 40°C for 5 min before and during the 15-min insulin occlusion; EP: electroporation. C) Blood glucose levels of mice after treatment. EP (▼) (150 V, 120 pulses at 0.2 ms, 1 Hz); Hyp + EP (△) (40±0.5°C 5 min before EP and during 15-min post-pulsing); Hyp alone (○); control group (●) (insulin application only). Oral glucose tolerance test (OGTT) was performed 3 h-post EP. *P < 0.05, **P < 0.01, ***P < 0.001. Adapted with permission from Ref [98].
Since the involvement of high-intensity electrical field, electroporation leads to an enhanced delivery efficiency for insulin compared to iontophoresis. However, the high-voltage pulses inevitably affect the deeper tissues, causing potential pain and muscle stimulation. Further studies on device design to minimize the side effect should be performed to facilitate clinical translation.
4. Mechanical force-triggered insulin delivery
Besides electrical field, mechanical force is another alternative to produce transient channels on the surface of the skin for transdermal drug delivery [100]. Ultrasound and jet injection are two representative mechanical force-triggered methods for insulin delivery. Ultrasound can enhance the permeability of drugs across the skin by hyperthermia or cavitation effect. Jet injection applies the high-speed liquid to disrupt the surface of the skin to dispense insulin solution within the skin tissue.
4.1. Ultrasound
Ultrasound, a longitudinal sound wave with a frequency above 20 kHz, has long been used for biomedical purposes since the beginning of the 20th century for imaging as well as to ablate tissue, shatter kidney stones, and to facilitate transdermal drug delivery [101–106]. The mechanical force produced by ultrasound has been shown to enhance skin permeability to therapeutics compounds via ultrasound-induced hyperthermia or cavitation [107, 108]. The application of ultrasound for drug delivery through the skin is generally termed as sonophoresis, and the range of frequencies used varies between 20 kHz and 16 MHz [56, 109]. Early on, high-frequency sonophoresis (HFS) (≥ 700 kHz) was mostly investigated for transdermal drug delivery, with typical skin penetration enhancements between 1 and 10 folds comparing to passive diffusion [110, 111]. In the early to mid-1990s, scientists developed a better understanding of the cavitational effects for sonophoresis [112] and found low-frequency sonophoresis (LFS) (20-100 kHz) was more effective than HFS in enhancing skin permeability [113, 114]. Tachibana and coworkers used ultrasound with a frequency of 48 kHz or 105 kHz to yield greater transport of insulin through the skin than passive diffusion that resulted in significant decrease of BGLs in mice and rabbits [113, 115]. Mitragotri et al. also showed effective LFS-mediated transdermal transport of proteins, including insulin, interferon-γ, and erythropoietin (Fig. 5) [114]. Their experiments in a diabetic rat model showed sufficient insulin delivery by LFS, with a reduction of the BGLs from ~400 to ~200 mg/dL in 30 min. A subsequent investigation demonstrated that LFS at 20 kHz was up to three orders of magnitude more effective than HFS at 1 MHz for enhancing skin permeability [116]. Since then, LFS-mediated transdermal insulin delivery has been extensively investigated [117, 118]. Studies conducted by Boucaud et al. in rats and newborn pigs illustrated that the use LFS (20Hz) could facilitate rapid, reproducible, and reversible transdermal delivery of insulin [119, 120]. They also demonstrated that the amount of insulin transported through the skin of rats was significantly associated with the energy dose and length of an ultrasound pulse, consistent with the cavitation-related mechanism [121].
Fig. 5.
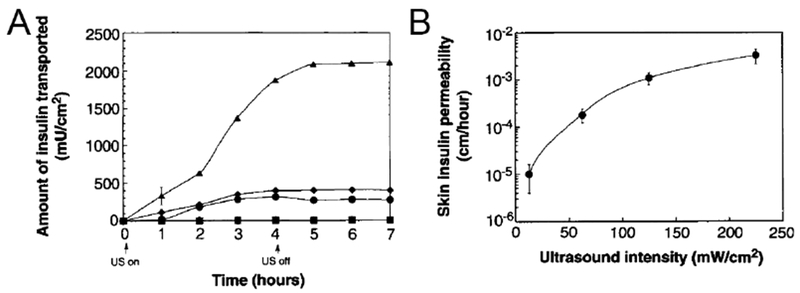
Ultrasound-mediated transdermal insulin delivery. A) In vitro transport profile of insulin across human skin in the presence of ultrasound (20 kHz, 100-ms pulses applied every second) at 12.5 (■), 62.5 (◆), 125 (●), and 225 mW/cm2 (▲) (n = 3 or 4). B) In vitro transdermal insulin permeability with different ultrasound intensity (20 kHz, 100-ms pulses applied every second) (n = 3 or 4). Adapted with permission from Ref [114].
Aside from using commercial, large, and heavy ultrasound equipment to deliver insulin across the skin, Smith et al. developed a portable and energy efficient transducer array for LFS-aided transdermal insulin delivery [122–128]. Specifically, this lightweight (<38 g) and compact (56×56×8 mm3) transducer array consisted of individual cymbal-shaped transducers, which were arranged into a multi-element array design (3×3). It also had an adjustable resonance frequency between 1 and 100 kHz, depending on the geometry. The efficacy of the portable transducer was evaluated by comparing the LFS-based insulin delivery route with subcutaneous injection route in rats [129]. The transducer was placed on top of an insulin reservoir (4mL of 50 U/mL) that adhered to the skin of rats. Ultrasound irradiation treatment at 20 kHz with an intensity of 100 mW/cm2 for 60 min resulted in a reduction of BGLs by 262 ± 40 mg/dL within 90 min; little change or less decrease (−190 ± 96 mg/dL) was observed in BGLs of rats administered with subcutaneously injected insulin (0.15, 0.2 and 0.25 U/kg). In another study, researchers assessed the effect of the cymbal transducer on Yorkshire pigs (45-64 kg) (Fig. 6) [128]. The group treated with the insulin and LFS for 60 min (20 kHz, 100 mW/cm2, 20% duty cycle) showed a decrease in BGLs of 72±5 mg/dL at 60 min post-treatment and a decrease of 91± 23 mg/dL at 90 min, indicating the feasibility of the cymbal transducer for clinical applications. In an attempt to improve the accuracy of delivered insulin doses, this team further designed a closed-loop system that allowed for on-demand delivery of insulin by coupling ultrasound-assisted insulin delivery with glucose sensing via a feedback controller [130]. The in vivo experiments were performed on 200-pound pigs, to which two ultrasound arrays were applied: one for insulin delivery (3×3, 30 kHz, 100 mW/cm2) and the other for glucose sensing (2×2, 20 kHz, 100 mW/cm2). BGLs were assessed every 20 min for 2 h and the level of automatically delivered insulin was determined according to the BGLs by a proportional feedback controller. The results suggested the feasibility of using the combined cymbal ultrasound array system for noninvasive glucose sensing and insulin delivery.
Fig. 6.
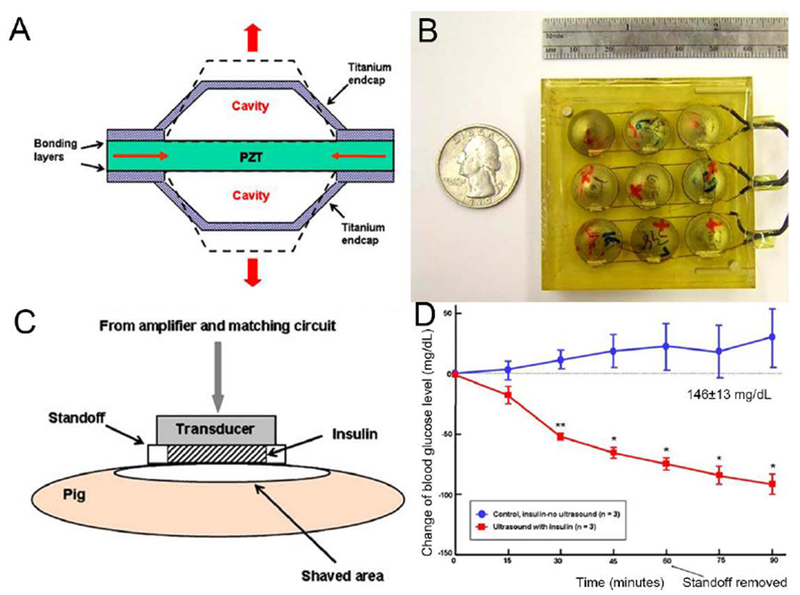
Transdermal insulin triggered by a cymbal transducer. A) Schematic of the cymbal transducer and the motion of the cymbal disk. B) Photo of a 3×3 cymbal transducers array. C) Schematic of a cymbal transducers array on pig skin for transdermal insulin delivery. D) The blood glucose levels of pig treated with pure insulin or ultrasound-mediated transdermal insulin delivery (n = 3, *P < 0.05, **P < 0.01). Adapted with permission from Ref [128].
Aside from stimulating insulin delivery from aqueous solution, Di et al. applied focused ultrasound (FUS) with an injectable nano-network for long-term regulation of BGLs [131]. The injectable polymeric nano-network was cross-linked by oppositely charged nanoparticles, which were prepared by coating chitosan (positively-charged) and alginate (negatively-charged) on insulin-loaded poly(lactic-co-glycolic acid) (PLGA) nanoparticles. The in vivo study in diabetic mice showed that after injection of the 3D nano-network, insulin was effectively released upon FUS-administration for 30 s (950 kHz, 4.31 W) with a corresponding decrease of BGLs in 10 min that reached the normoglycemic range (<200 mg/dL) in 1 h. Moreover, similar changes in BGLs were also detected under the same ultrasound treatment condition 4-day and 7-day post the injection of the nano-network, suggesting that the capability of this strategy for a long-term and pulsatile regulation of BGLs. In another study, the researchers employed chitosan microgels as the carriers to achieve the ultrasound-mediated insulin delivery [132].
More recently, Castellanos and coworkers investigated the effects of ultrasound on evoking secretory responses in pancreatic β-cells [133]. After exposure to unfocused ultrasound for 5 min at a peak intensity of 1 W/cm2 and frequencies of 400 kHz or 600 kHz, a marked release of insulin was observed from β-cells (approximately 150 ng/106 cells). Moreover, application of ultrasound at frequencies of 800 kHz resulted in 24 ng/106 cells releasing insulin, while retaining the cell viability. In their further study, researchers sought to elucidate the mechanism by which ultrasound stimulated secretion and described the role of calcium in the process [134]. These findings implicate the potential of ultrasound to augment insulin release from pancreatic cells for diabetes treatment.
Ultrasound-mediated transdermal drug delivery has been extensively investigated in in vivo animal studies and demonstrated clinical potential in small macromolecule delivery including insulin. However, HFS may lead to damage to deep skin tissue, and LFS often needs a corresponding medium or scaffold, such as hydrogel, nano-network, and cells which is invasively injected into skin tissue. Moreover, the requirement of sophisticated devices also limits the usage for people with diabetes.
4.2. Jet injection
Jet injection is another needle-free administration for transdermal insulin delivery. Instead of solid syringes, the jet injector applies a high-speed narrow stream containing insulin to create a tiny hole for insulin transport through the skin [135–137]. Jet injection has been associated with high delivery efficiency exceeding 90%, similar to hypodermic injection [135, 138, 139]. Moreover, insulin administration by jet injectors leads to a faster on-set of plasma insulin [140, 141]. Wit et al. demonstrated rapid correction of marked hyperglycemia using jet injection in overweight and obese patients with diabetes [142]. In addition, because jet injection dispenses insulin over a larger area of skin tissue than conventional injection, the pharmacokinetics of this route of insulin administration are more similar to endogenous insulin secretion by pancreas [143]. Guo et al. compared postprandial glucose control obtained using a jet injector and an insulin pen and found that the improved insulin absorption by the jet injector is beneficial for postprandial blood glucose regulation [144].
Despite the advantages mentioned above, several concerns limit the current use of jet injection technology. Although liquid jet injection technology is a needle-free route, the large volume of high-pressure spray may also lead to adverse reactions including bruising, bleeding, and pain [135, 145–147]. Studies have reported that jet injectors actually cause no less pain than hypodermic needles [145, 148]. To minimize the adverse reactions, Mitragotri and coworkers designed a microjet injection device that only injects solution volumes within the nanoliter range[149]. Using pulsed microjets, they reported that insulin was injected into the skin without deep penetration in a rat model, which may potentially reduce pain and bleeding. Besides, the sustained and controlled release of insulin realized by integration with biodegradable particles may also improve the application of jet injection. When Mitragotri and coworkers assessed the capability of jet injectors to deliver polymeric nanoparticles through the skin, they found the nanoparticles did not penetrate the skin as deeply but could release cargoes for prolonged periods [150]. Several jet injectors are commercially available though they have not been widely adopted. In the future, the cost, size, and performance of jet injectors may be optimized to facilitate routine usage.
5. Microneedle-assisted transdermal delivery
Recently, the emergence of microneedle (MN) techniques have provided an alternative method for transdermal protein delivery [151–156]. The micro-scaled needles are able to painlessly disrupt the stratum corneum and reach the epidermal and dermal layer for drug release [157, 158]. The micro-channels caused by MN exist temporarily for drug transport but quickly recover after removal of MN to prevent long-term damage to the skin tissue [159, 160]. Based on the material of the MN and the mechanism of drug delivery, the MN device is classified into different types (Fig. 7). Generally, solid MNs are designed to pierce the skin to improve the drug transport; hollow MNs are used for injection of a fluid drug formulation through the opening on the skin caused by needles, and dissolving or degradable MNs are made from polymers with encapsulated drugs. Below, we also describe recently-developed bioresponsive MNs that can respond to physiological glucose levels for on-demand delivery of insulin.
Fig. 7.
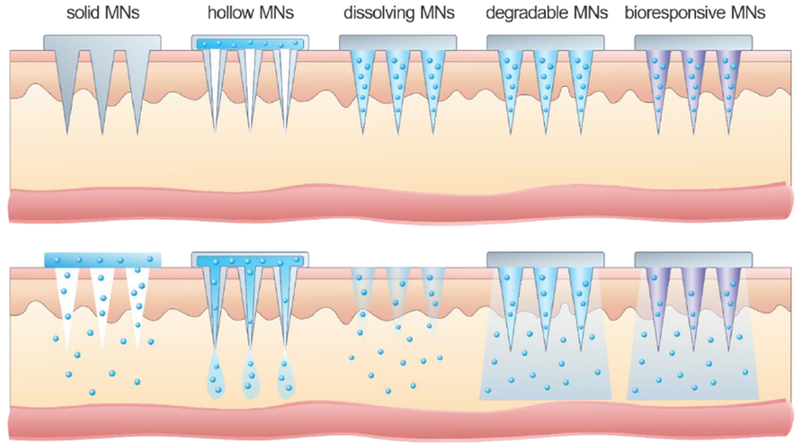
Schematic illustration of delivery mechanisms through different types of MNs.
5.1. Solid microneedles
The early generation of MN-aided insulin delivery was often based on skin perforations from solid MNs, which is also termed as “poke with patch” [151]. In this approach, the MNs pierce the skin to create microchannels where insulin can be transported during the subsequent administration of a patch or topical formulation. A plethora of studies have been reported demonstrating effective delivery of insulin through the skin during the past decades.
For example, Prausnitz and coworkers demonstrated the hypoglycemic effect of insulin in diabetic rats using MNs [161]. An array of 105 microneedles was prepared by laser-cut from stainless steel sheet and inserted into the skin of diabetic rats, after which insulin solution was administered in contact of the skin for 4 h. These solid metal MNs displayed increased transdermal delivery of insulin and reduction of BGLs in vivo as much as 80%.
Zhou et al. evaluated the potential of using a commercially available MN roller for the transdermal delivery of insulin in a rat model, testing three different lengths (250, 500, and 1000 μm) of the stainless steel MNs [162]. Rapid reduction in BGLs was observed in 1 h after application of the MN rollers, while a diminished effect with the recovery of the skin holes that were created by MNs. MN rollers with a length of 500 μm or shorter were demonstrated to be safe and able to enhance the transdermal delivery of insulin in vivo. It was also reported that the reduction of BGLs could be extended by adjusting the treating area of MNs [163].
Furthermore, a combination of microneedle and iontophoresis was studied to allow a larger range of delivered drugs for transdermal delivery [164]. Chen et al. presented a 700-fold higher absorbance rate of insulin from nanovesicles driven by iontophoresis through the microchannels induced by MNs than that by passive diffusion [165]. The positively charged nanovesicles showed significant permeation ability with the assistance of MNs and iontophoresis and reduced the BGLs of diabetic rats by 33.3% and 28.3% of the initial levels at 4 and 6 h, respectively.
To control and maximize the delivered insulin dose, solid MNs were recently modified by coating payloads directly onto the surface of MNs. Al-Qallaf el al. studied the insulin concentration profiles in blood using drug-coated microneedles with different shapes and dimensions in theoretical models [166]. The simulating results indicated a maximum insulin concentration was achieved by rocket-shaped MNs.
5.2. Hollow microneedles
Hollow MNs are designed to facilitate drugs delivery into the skin through the interior of needles (Fig. 5). Prausnitz and coworkers injected insulin into the diabetic rat skin through hollow glass MNs by microinfusion, resulting in a steady drop of up to 70% of preinfusion BGLs over a 5-h period [167]. They also designed and fabricated hollow metal MN arrays for transdermal insulin delivery [168]. The mechanical study showed that these MNs were strong enough to pierce the entire skin without breaking. In addition, silicon hollow MNs have also been explored for insulin delivery [169, 170].
Nordquist, Roxhed, and coworkers developed a “controlled release”-designed MN patch integrated with an active dispensing functionality capable of controlled release of insulin in the microliter range at low flow-rates [171, 172]. The electrically controlled dispenser consisted of a heater layer, an expandable composite, and a liquid reservoir. When current passed through the heater, the composite was heated up and then acted as the liquid reservoir, which could consequently eject the insulin solution through the hollow silicone MNs. In a diabetic rat model, this system was associated with a 5-times higher plasma insulin concentration compared to passive difiusion with a significant decrease in BGLs.
The delivery efficacy of hollow MNs has also been investigated in human studies. Gupta et al. first examined the transdermal delivery of insulin by hollow metal MNs on two type 1 diabetic adults [173]. An insulin pump was connected to the MNs and then applied on the abdominal skin to control the insulin infusion rate. Results demonstrated rapid insulin absorption and decline in BGLs with the insertion of MN in depth of 1 mm within the skin. Further clinical trials have also been conducting to evaluate the safety and efficiency of hollow MNs for insulin delivery in humans [174].
5.3. Dissolving microneedles
In addition to insoluble metal and silicon MNs, recent studies on biocompatible polymeric MNs have attracted significant attention, such as dissolving MNs [175]. Dissolving MNs are prepared by the soluble polymers to encapsulate the drug in the matrix, and upon inserted into the skin, can fully dissolve to release the drug. The therapeutic duration is dependent on the dissolution rate of the polymer material, which can be adjusted from minutes to hours to meet the goals of treatment [176]. Furthermore, the use of the biocompatible polymers could avoid any production of sharp biohazardous waste. [177, 178].
To date, various dissolving MNs made of sugar glass polymers have been reported, such as maltose [179–182], trehalose [183–186] and [187, 188]. Sugar glass MNs typically dissolve quickly in human skin after insertion [189, 190]. However, the fabrication of these MNs requires a high temperature that over 100 °C to induce rubber to glass transitions of sugar glasses, which may damage the bioactivity of bio molecules including insulin [191]. New fabrication techniques have been developed to address the thermal challenges of melting fabrication process. For example, Martin et al. used a low-temperature processing method to fabricate dissolving MNs [187].
Another strategy could be using other polymers with high solubility to form MNs, such as hyaluronic acid (HA) [192–196], carboxymethylcellulose (CMC) [183, 197], chitosan [198, 199], alginate [200, 201], polyvinylpyrrolidone (PVP) [202–205], and polyvinyl alcohol (PVA) [206–208]. The associated fabrication process can avoid high-temperatures, thereby enhancing the storage ability of drug-containing MNs. Liu et al. fabricated HA MNs via micromolding technology and characterized their application in the transdermal delivery of insulin [209]. More than 90% of the loaded insulin retained bioactivity, even after one month-storage at different temperatures (−40, 4, 20 and 40 °C). Moreover, the HA MNs exhibited higher resistance to deformation against humidity than sugar glass MNs. In vivo studies in diabetic rats demonstrated a dose-dependent hypoglycemic effect after administration with insulin-loaded HA MNs. Furthermore, the transient microchannel caused by the insertion of MNs disappeared within 24 h.
Chen and coworkers developed a dissolving MN patch composed of starch and gelatin for transdermal insulin delivery [210]. Here, gelation was blended with starch to produce tough and strong composited MNs that was suitable for skin penetration due to its film-forming ability. In vitro and in vivo penetration tests verified the sufficient mechanical strength of MNs to be inserted into porcine or rat skin with a depth of approximately 200 μm. The mild solvent casting process for MN fabrication preserved the activity of encapsulated insulin which was able to induce a significant decline in BGLs of diabetic rats upon insertion. Furthermore, the relative availability and bioactivity of insulin were still higher than 90% after one-month storage at 25 or 37°C, suggesting that these dissolving starch/gelatin MNs could be a promising device for delivery of biomolecules.
Although many formulations of dissolving MNs have been shown to successfully delivery insulin and reduce BGLs in vivo, incomplete insertion of polymeric MNs due to skin elasticity limits their delivery efficiency and also causes wastage of valuable medication [211]. To this end, researchers designed fully insertable MNs with a supporting structure that provided an extended length for counteracting skin compressive deformation during administration [212]. In this study, insulin was first loaded on tips of 600 μm-high MNs made of poly-γ-glutamic acid, while the second layer of PVA/PVP was filled in the MN molds to form the 600 μm-high supporting structures (Fig. 8). When inserted into the skin, both the MNs and supporting layer dissolved within 4 min to fully release the drug load. A comparable hypoglycemic effect was detected in diabetic mice treated the same dose of insulin (0.2 IU) via MN patches versus subcutaneous injection, indicating the feasibility and accuracy of using this proposed MN design for insulin delivery.
Fig. 8.
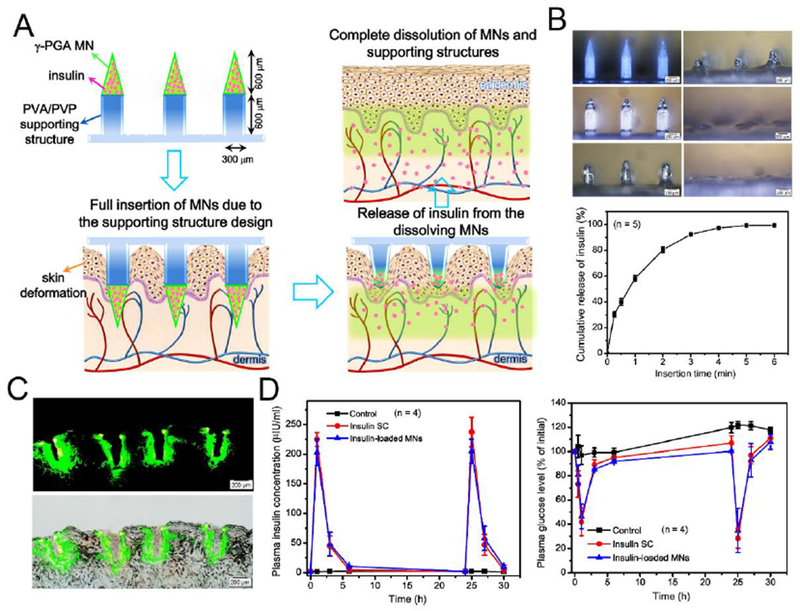
Transdermal delivery of insulin using a fully insertable MN system. A) Schematic of MNs composed of poly-γ-glutamic acid (γ-PGA) MNs and PVA/PVP supporting structures. B) In vitro release profile of insulin from MNs (n = 5). C) Fluorescence images of skin puncture sites. D) Plasma insulin concentrations (left) and plasma glucose levels (right) of diabetic rats treated with unloaded (control) and insulin-loaded MNs and subcutaneous injection of insulin (insulin SC) (n = 4). Adapted with permission from Ref [212].
Based on a similar mechanism, Liu and coworkers developed multilayered dissolving MN patches composed of stiff silk fibroin MN tips supported on flexible PVA pedestals [213]. The tips had a robust mechanical strength and were able to penetrate the skin and rapidly dissolve to release encapsulated insulin. In vivo tests in an obese mouse model indicated rapid insulin absorption through the skin into the systemic circulation, where maximum serum insulin concentrations were reached 2 h-post MN administration. The MN patch could also be stored at room temperature for 20 days, with >99.4% of insulin remained in the MNs. In this MN design, the multilayered fabrication process may condense therapeutics to reduce wastage, as well as satisfy different mechanical performance requirements for tips and pedestals. Following this rationale, two-layered MNs made from diverse materials with different ratios have been assessed for optimized insulin delivery efficiency [214, 215]. Lee et al. analyzed the penetration ability of MNs consisting of several ratios of PVP with two molecular weights (PVP10/PVP360) and found the ratio of 1:3 was the best for in vivo insulin delivery [214]. The backing layer prepared from PVP360/CMC was selected for better flexibility for skin fitting other than pure PVP360 supporting. To further strengthen the mechanical property of MNs, Liu et al. fabricated composite MNs integrating PVP matrix with insulin-loaded CaCO3 microparticles [216]. The prepared MNs exhibited enhanced stiffness and slower solubility compared to pure PVP MNs.
Traditionally, dissolving MNs are mostly fabricated using micro-size molds through a stepwise casting method. Kim et al. developed an alternative fabrication technique by applying droplet-bom air blowing (DAB) to directly shape the polymer droplets to solidified the MNs, thereby providing benign (4-25 °C) and fast (≤10 min) fabrication conditions without drug loss [218]. In this method, biopolymer droplets were first dispensed on the flat surface for MN base fabrication, and a second layer of drug-containing droplets was additionally dispensed for MN tip fabrication. Thereafter, an upper plate was used to elongate the droplets by drawing. Throughout this process, the MNs were solidified using air blowing, where the size of the MNs and the amount of loaded insulin could be tailored by the pressure and time of droplet dispenser. A decrease in BGLs in diabetic mice and quantitative bioavailability (96.6 ± 2.4%) data confirmed the efficacy of insulin delivery associated with this fabrication method. This technology may also provide multiple options with regards to the materials for fabrication of dissolving MNs, including HA, CMC, and PVP, while the layered structure enabled minimized wastage of therapeutics. To further overcome incomplete drug delivery, Yang et al. recently reported an electrospun pillar array-assisted MN delivery which allowed rapid implantation of MNs into the skin [217]. The pillar array was first coated with an electrospun fibrous PLGA sheet, after which dissolving HA droplets were dispensed on each pillar to shape MNs via DAB (Fig. 9). The resulting MNs on the electrospun pillar array was quickly separated from the porous fibrous substrate within 10 seconds once completely inserted into the porcine skin due to the tensile breakage of the fibrous sheet during the compression. The hypoglycemic effect of insulin-loaded MN device was demonstrated in vivo using a healthy mouse model.
Fig. 9.
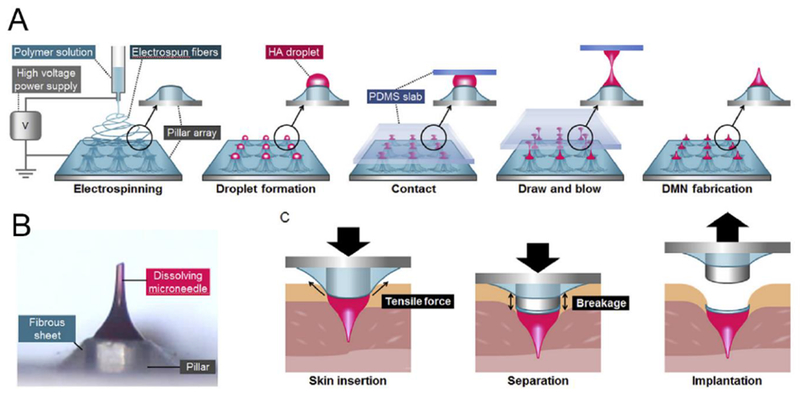
Transdermal MN based on droplet-born air blowing (DAB) technique. A) Schematic of MN fabrication. B) Rapid implantation of MN in the skin including insertion, separation, implantation steps. Adapted with permission from Ref [217].
Besides employing multilayered MNs to enhance delivery efficiency, Garland et al. investigated the incorporation of polymeric MNs with iontophoresis to improve the bioavailability of insulin and showed that a synergistic enhancement of insulin release was achieved when ITP was applied in combination of the soluble poly(methyl vinyl ether-co-maleic acid) (PMVE/MA) MN arrays [219]. Furthermore, their results revealed that the incorporation of electric current could facilitate the permeation of protein within the entire MN patch rather than the MN alone, thus increasing the delivery efficiency.
5.4. Degradable microneedles
The release dynamics of payloads from dissolving MNs is closely associated with the dissolution rate of polymers, which is usually fast. For protein drugs that require continuous delivery to maintain a constant therapeutic dose, MNs with a longer degradation period are preferred as the delivery device [177]. Polymers with a higher molecular weight and crosslinking density have been shown to provide extended release of insulin as well as mechanical properties of MNs [175, 220]. Drug is gradually released from biodegradable MNs through passive diffusion during the degradation process, while swelling of MNs may also accelerating drug diffusion. For instance, calcium ion cross-linked alginate/maltose composite MNs were explored for insulin delivery, where maltose was included to improve the mechanical strength of the MN [221]. The resultant biodegradable MNs exhibited a mechanical strength around 0.41 N/needle and rapidly swelled in 5 min with eventual dissolution in 40 min. The insulin-loaded MNs revealed a sustained decline in BGLs of diabetic rats and maintained the pharmacological activity of insulin with a longer period compared to the group subcutaneously injected the same dose of insulin. Yang and coworkers designed a swellable MN patch that could self-adhered in the skin for prolonged release of insulin (Fig. 10) [222]. The double-layered MN patch consisted of swellable PS-PAA MN tips that could swell by absorbing body fluids after insertion into skin and a non-swellable PS layer. The in vitro insulin released from the MNs presented a more sustained manner over 12 h without burst release. In contrast, >90% of the insulin was release from the coated MNs. Consistent with the release behavior, this swellable MN platform provided an extended hypoglycemic effect in normal mice that lasted up to 8 h. The Jin group developed a phase-transition MN patch from PVA for transdermal insulin delivery [223]. The microcrystalline crosslinking allowed the MN to swell, but not dissolve, upon insertion into the skin, thus leading to a sustained insulin release from the patch. In vivo studies in a diabetic pig model demonstrated transdermal bioavailability that exceeded 20%. Meanwhile, the glycated hemoglobin of pigs treated with the patches continuously for 2 months was lower than those treated with the insulin pen, indicating the insulin patches provided a better blood glucose regulation capability. In another recent study, Di et al. integrated a stretchable MN patch for tensile strain-triggered transdermal delivery of insulin [224]. The elastomer patch embedded insulin-loaded microgel depots inside deformed under mechanical stretch to promote the release of payloads, which further diffused into the cross-linked HA microneedle for transdermal delivery. In vivo studies demonstrated effective reduction of BGLs in diabetic mice administered with this stretchable device.
Fig. 10.
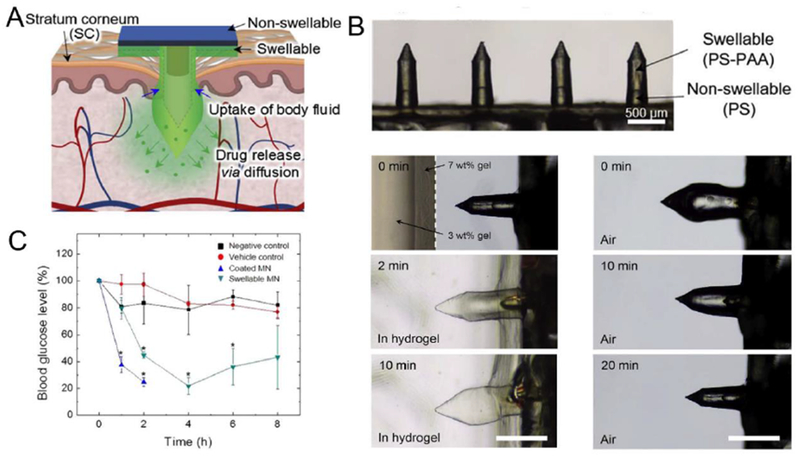
Swellable MN for transdermal insulin delivery. A) Schematic of a water-swellable MN for mechanical interlocking with skin and drug release through passive diffusion. B) Swelling/deswelling behavior of the MN in gel and air respectively. Scale bar: 500 μm. C) Blood glucose levels of normal mice treated with (▼) 10 U insulin-loaded swellable MN patches (swellable MN), (▲) 10U insulin-coated MN patches (coated MN), and (●) non-insulin-loaded swellable MN (vehicle control) (n = 3). Normal untreated mice (■) were used as a negative control (*P < 0.01). Adapted with permission from Ref [222].
Lately, bioceramics have attracted increasing interest in the field of transdermal drug delivery due to their attractive biocompatibility and strong mechanical properties. For example, the flexible porous structure of bioceramics and the electrostatic interaction between the ceramic surface and the biotherapeutics suggest promise for bioceramics to transporting biomolecules. Yu et al. prepared organic-inorganic bioceramic composite microneedles MNs made from gelatin and hydroxyapatite for extended transdermal insulin delivery [225]. Hydroxyapatite, which has a similar chemical composition as human hard tissues, is considered as a biodegradable ceramic that has been widely used in the biomedical application. The MNs composed of cross-linked gelatin and incorporated with hydroxyapatite provided sufficient mechanical strength to penetrate human skin and exhibited an effective hypoglycemic effect and extended plasma insulin release compared with that of subcutaneous injection in diabetic rats. The researchers also characterized calcium sulfate and gelatin composite MNs, which also presented similar behaviors in transdermal delivery of insulin [226].
5.5. Bioresponsive microneedles
Recently, extensive efforts have been devoted to achieving glucose-responsive smart insulin delivery [15, 17, 18, 227–230]. Bioresponsive MNs that can respond to the physiological signals have been spotlighted as a promising approach for glucose-regulated insulin delivery [231–233]. This platform generally integrates glucose-responsive components with polymeric MN matrix.
In 2015, Yu et al. reported a glucose-responsive MN patch composed of cross-linked HA matrix and hypoxia-responsive vesicles (GRVs) as a “smart insulin patch”, representing a painless and self-regulated modality (Fig. 11) [234]. The GRVs were self-assembled by the hypoxia-sensitive hyaluronic acid derivative (HS-HA), which contained a hypoxia-sensitive group, 2-nitroimidazole (NI). Under reductive conditions, the hydrophobic NI on HS-HA is reduced to hydrophilic 2-aminoimidazole, inducing the disassembly of the nanovesicles. The GRVs encapsulating insulin and glucose oxidase (GOx) were then deposited in the MNs to sense the elevated blood glucose level in the dermis. GOx, an enzyme that can convert glucose to gluconic acid, has been widely applied as a glucose-sensing element [227, 235].
Fig. 11.
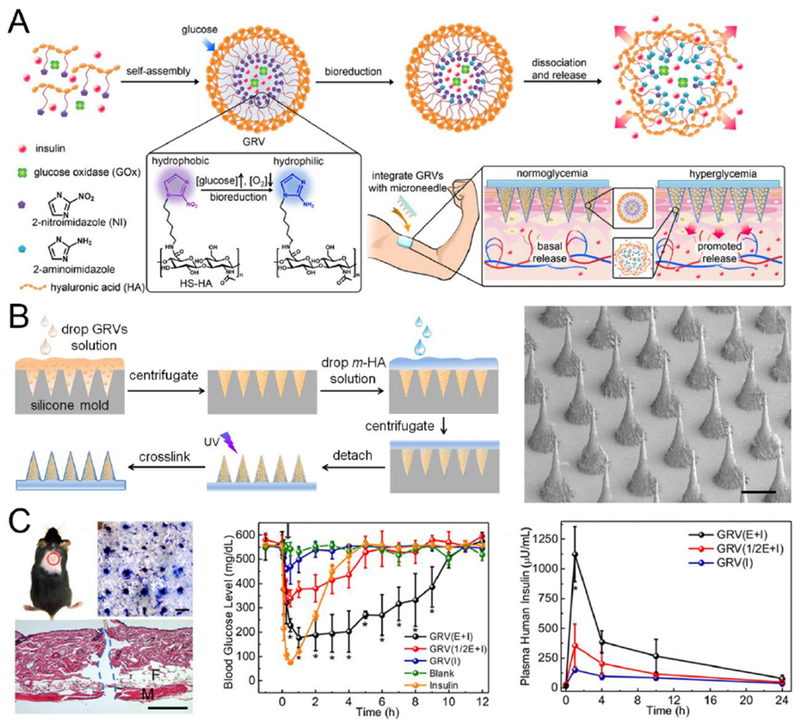
A representative transcutaneous MN patch loaded with glucose-responsive nanoformulations. A) Schematic showing the formation and mechanism of GRV-containing MN-array patch for in vivo fast insulin delivery triggered by a high blood glucose level. B) Fabrication process (left) and SEM image (right) of the MN patch. C) In vivo administration of MN-array patch in STZ-induced type 1 diabetic mice: Photograph showing penetration of mouse skin by MNs Scale bar: 500 μm (Left); BGLs (middle) and plasma insulin levels (right) of diabetic mice after treatment with blank MNs containing only cross-linked HA, MNs loaded with human recombinant insulin, MNs loaded with GRV(E + I), MNs loaded with GRV(1/2E + I), or MNs loaded with GRV(I). *P < 0.05 for administration with GRV(E + I)-loaded MNs compared with GRV(1/2E + I)-loaded MNs or GRV(I)-loaded MNs. Adapted with permission from Ref [234].
During the GOx-catalyzed oxidation of glucose, oxygen in the body fluid was consumed, leading a localized hypoxic environment [236]. The enzyme-induced hypoxic microenvironment further actuated the dissociation of GRVs as a result of bioreduction of HS-HA and led to subsequent insulin release. The hypoxia-responsive GRVs were able to rapidly release insulin in the presence of glucose and quickly reduced the BGLs of type 1 diabetic mice to around 200 mg/dL with 0.5 h with maintenance in a normal range for up to 4 h. Moreover, the administration of an additional patch was able to prolong the treatment period, while avoiding the risk of hypoglycemia.
In addition to an enzymatically-generated hypoxic or the acid environment, the generation of H2O2 during the reaction can also act as a trigger to promote insulin release from MNs [237]. Hu et al. reported bioresponsive MNs incorporated with polymeric vesicles (PVs) [238]. The PVs were prepared by self-assembly of a block copolymer composed of polyethylene glycol (PEG) and phenylboronic ester (PBE)-conjugated polyserine and further loaded into MN patch. In the presence of high glucose levels, the enzymatically-produced H2O2 oxidized the PBE pendant, leading to the disassociation of PVs and delivery of insulin through polymeric MN matrix. In this system, the release profiles responding to glucose could be modulated by adjusting the amount of GOx. The integration of H2O2-responsive PVs with MNs displayed the capability of self-regulating BGLs in a type 1 diabetic mouse model. In another example, Tong et al. synthesized glucose- and H2O2-responsive PVs by engineering phenylboronic acid (glucose-sensitive) and 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzyl acrylate (H2O2-sensitive) functional groups in the triblock copolymer [239]. The resultant PVs-loaded MNs presented an effective hypoglycemic effect in a diabetic rat model compared to that of subcutaneous injection or only insulin-loaded MNs. Xu et al. built H2O2-responsive mesoporous silica nanoparticles (MSNs), which were then loaded with insulin and entrapped into MNs for transdermal delivery [240]. The porous MSNs acted as the reservoir of insulin together with GOx for H2O2 generation in hyperglycemic states. Here, the MSNs were modified with 4-(imidazoyl carbamate)phenylboronic acid pinacol ester to form host-guest complexation with α-cyclodextrin, thereby keep insulin within the MSNs. Upon exposure to H2O2, the phenylboronic ester on the surface of the MSNs was oxidized, resulting in the destruction of the host-guest complexation and subsequent release of the preloaded insulin. Recently, mesoporous bioactive glasses was employed as the insulin carrier and integrated with MN device for glucose-responsive insulin delivery [241]. In this design, the porous bioactive glasses loaded with insulin and two enzymes (GOx/CAT) were capped with pH-sensitive ZnO quantum dots as “gatekeepers”. These ZnO quantum dots dissolved in the enzyme-mediated acid environment under hyperglycemic conditions, opening the pores on the bioactive glasses and release the encapsulated insulin.
However, the undesirable byproduct H2O2 produced during the enzymatic oxidation of glucose could reduce the activity of GOx thus hamper the response rate. The generation of H2O2 may also lead to free radical-induced damage to skin tissue during the long-term usage. Therefore, to further enhance the glucose-responsive capacity, Yu et al. designed a hypoxia and H2O2 dual-sensitive system based on polymersome-incorporated MNs for optimized insulin delivery [242]. An amphiphilic diblock copolymer consisting of PEG and polyserine were utilized to prepare the dual-sensitive polymersomes (d-GRPs), on which hypoxia-sensitive NI group was modified via H2O2-responsive thioether moiety. Rapid oxygen consumption and H2O2 generation by enzymatic reactions under high glucose levels contributed to the increased water-solubility of the copolymer, triggering the dissociation and release of the insulin from the d-GRPs. In vivo results in diabetic mice showed that this patch effectively regulated BGLs for 10 h after administration with minimal skin inflammation. In another design, Wang et al. prepared core-shell structured MNs directly from H2O2-degradable polymeric gel (Fig. 12) [243]. The core of MNs consisted of PVA network cross-linked by a H2O2-cleavable linker (TSPBA), with insulin chemically anchored on PVA via a H2O2-sensitive linkage. GOx was encapsulated into the acrylated nanogel (GOx-NG) to form a large size for covalent immobilization on PVA, restricting the leakage of GOx while maintaining the ease of insulin release. Under hyperglycemic conditions, H2O2 was locally generated by GOx, resulting in oxidation and hydrolyzation of both the PVA crosslinkers and insulin conjugates, facilitating the rapid release of free insulin from the MNs. Researchers demonstrated that this H2O2-responsive insulin patch presented rapid glucose-responsiveness and, with a consecutive administration of MNs, was able to control BGLs for 40-h without severe hypoglycemia. Of note, the MNs were further coated with a thin-layer of nanogel embedding H2O2-scavenging enzymes (catalase), thus facilitate elimination of H2O2 to mitigate its injury toward normal tissues by oxidative stress. In vivo performance of this core-shell gelated MN patch effectively improved inflammation in skin tissue treated with coated MNs compared to non-coated MNs in vivo. More recently, the same research group developed an H2O2 and pH cascade-triggered insulin delivery system based on sheath-structured MNs [244]. Insulin was entrapped by H2O2-sensitive and positively charged diblock copolymers to form nano-size complex micelles. Upon incubation in hyperglycemic conditions, this highly positively charged polymer can be oxidized by H2O2 and subsequently hydrolyzed to be weakly positive-charged materials. The reduction in pH during the oxidation of glucose also reduced the density of negative charges on insulin to weaken the interaction between insulin and polymers, further promoting its release. The trigger mechanism based on both pH and H2O2 ensured that insulin was only released in both the oxidative and acidic environment created by oxidation of glucose in the presence of GOx. By embedding catalase-nanogels in the sheath covering the insulin complex micelles-loaded MN core, this patch could regulate the glucose levels in diabetic mice within the normal range with effective mitigation of H2O2.
Fig. 12.
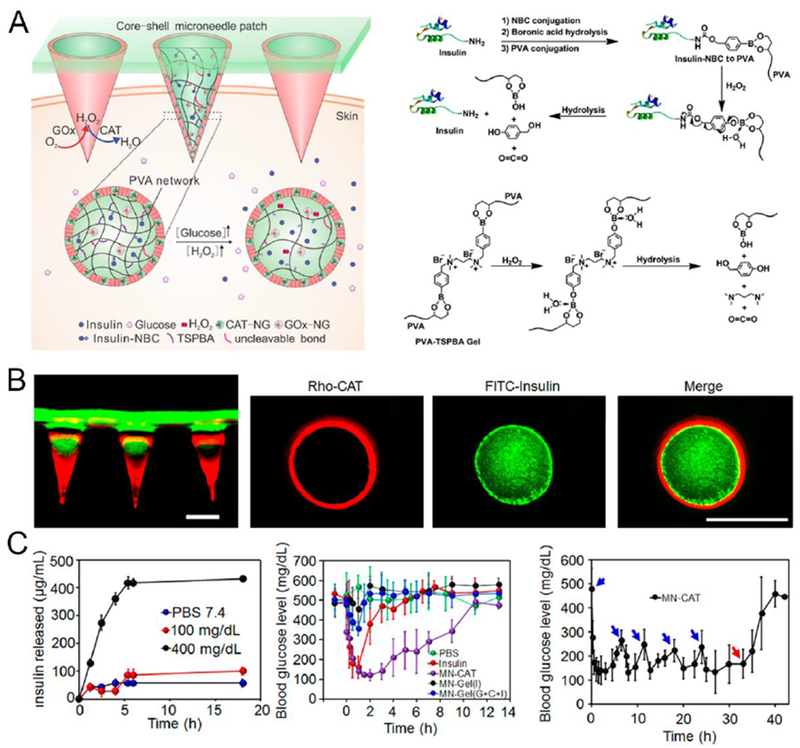
Glucose-responsive matrix-loaded MN for controllable insulin delivery. A) Schematic of the glucose-responsive insulin delivery system using H2O2 responsive PVA-TSPBA gel. B) Fluorescence images of rhodamine B-labeled insulin-loaded MN arrays with FITC-labeled HA base (left) and a cross-section of MN containing rhodamine B-labeled CAT shell and FITC-labeled insulin core (right). Scale bar: 300 μm. D) In vitro release profile of insulin from gels in PBS 7.4 in he presence of GOx (0.2 mg/mL). n = 3 (left); Blood glucose levels of diabetic mice treated with different kinds of MN array patches, n = 5 (middle); Blood glucose levels of diabetic mice treated with multiple MN array patches. The administration of MN-CAT is indicated by blue arrows. MNs were removed as indicated by red arrows, n = 5 (right). Adapted with permission from Ref [243].
Outside of synthetic insulin carriers, Ye et al. described an innovative transdermal insulin delivery strategy incorporating insulin-secreted pancreatic β-cells with MN array patch for diabetes treatment [245]. Transplantation of insulin-secreting cells has been intensively investigated in type 1 diabetes treatment. However, this method may be hampered by the host immune response and issues with biocompatibility of cell grafts. In this approach, the insulin secretion from exogenous β-cells are not implanted and instead are modulated by BGLs through MNs containing glucose-signal amplifiers (GSAs). The GSAs were glucose-sensitive polymeric nanovesicles entrapping GOx, α-amylase (AM) and glucoamylase (GA). GOx was selected to induce the dissociation of nanovesicles in hyperglycemic conditions. The released AM hydrolyzed α-amylose embedded in the MNs to give disaccharides and trisaccharides, which were further converted to glucose by GA. The “amplified” local-concentrated glucose effectively diffused into the externally positioned β-cell capsules, where it promoting secretion and diffusion of insulin through MNs into the skin. This design showed extended therapeutic efficacy compared to the MNs without GSAs, where one such MN patch was shown to rapidly reduce BGLs of type 1 diabetic mice and maintain the reduction in glucose levels for over 6 hours.
As microneedles do not only penetrate stratum corneum to increase skin permeability, but also directly carry insulin into the dermis layer, the microneedle-based technique demonstrated a relatively higher transport efficiency compared to other strategies. Due to the convenient, easy, and painless administration, it is considered to be suitable for people with diabetes to utilize by themselves at home. With the glucose-responsive moieties, it further shows great promising for continuous effective regulation of blood glucose. However, the potential breakage, irritation, and infection should be thoroughly investigated before clinical usage.
6. Conclusion and outlook
In this review, we have surveyed the technological advances in the development of transdermal insulin delivery systems for blood glucose regulation. Compared to passive transport through the skin, the use of chemical enhancers, electrical instruments, and microneedle devices have exhibited great potential to enhance the permeation of insulin by disrupting the skin barrier (Table 1). Unlike the traditional hypodermic injection, the transdermal insulin delivery demonstrates a more patient-friendly and minimally invasive method for daily diabetes management (Table 2). In addition, researchers also took the advantages of laser and another microdermabrasion device to facilitate insulin transport through the skin [246–248]. Besides, transdermal strategies such as power jet, heat or magnetophoresis-assisted administration routes could also be investigated for needless delivery of insulin [137,249].
Table 1.
Representative transdermal strategies for insulin delivery
| Strategies | Approaches | Advantages | Limitations |
|---|---|---|---|
| Chemical Enhancers | Disrupt the skin structure to increase permeability and improve drug solubility to provide the drug concentration-gradient driving force | • Increased skin permeability and insulin absorption • Improved patient satisfaction over injection |
• Limited transdermal delivery efficiency of macromolecule drug-insulin • Potential skin irradiation • Lack of robust controlled drug dosage |
| Iontophoresis/Electroporation | Alter permeation of cell walls | • Enhanced insulin penetration over passive transport • Improved patient satisfaction over injection • Allowing strict control of delivery period |
• Iontophoresis could be time-consuming to administer • Potential cell damage or rupture after membrane discharge by electroporation • Lack of robust controlled drug dosage |
| Ultrasound | Generate hyperthermia or cavitation effect to increase permeability of cell membrane | • Enhanced insulin penetration over passive transport • Allowing strict control of delivery period • Reduced immunization reaction • Improved patient satisfaction over injection |
• Requirement of sophisticated devices • Potential physical damage to skin tissue • Lack of robust controlled drug dosage |
| Jet injection | Deliver solution of insulin into skin powered by high-pressure gas | • Improved insulin absorption over injection as jet injection dispenses insulin over a larger area of skin tissue • Reduced immunization reaction |
• Potential bruising, bleeding, and pain due to high-pressure spray |
| Microneedle | Insert into skin to enhance skin permeability or directly deliver drug by micro-scaled needles | • Relative higher transport efficiency than other strategies by directly carrying insulin into the dermis layer • Home friendly administration method • Improved patient satisfaction over injection • Controllable drug release rates |
• Potential breakage of needle • Toxicity concern ofneedle materials • Potential skin irradiation or/and infection |
Table 2.
Representative clinical trials of transdermal insulin delivery
| Formulation | Study | Subjects | Primary Outcome Measures | Status | Phase | Related publication |
|---|---|---|---|---|---|---|
| Microporation | Transdermal Basal Insulin Patch Study in Type 1 Diabetes • (NCT00519623) |
• T1D > 10 years • Age 18-65 years • BMI 18.5-32 kg/m2 • HbA1c ≤9.0% • C-peptide negative |
PK and PD of the PassPort(R) Transdermal Insulin Delivery System in Type 1 Diabetes Patients | Completed | Phase 1/2 | [250] |
| A stable anhydrous insulin solution | Transdermally Delivered Human Insulin Product • (NCT03544996) |
• 1 male, brittle T1D patient with poor insulin saisitivity requiring more than 3,000 IUs on insulin a day • Age 60-61 |
Measurement of down modulation of serum glucose not otherwise attributable to injected insulin | Completed | ||
| Insulin by Jet-injection for Hyperglycemia in Diabetes • (NCT01947556) |
• T1D or T2D • Age 18-75 years • BMI 25-40 kg/m2 • HbA1c6.5-10% |
The time in minutes until plasma glucose concentration has dropped with ≥ 10mmol/L | Completed | [142] | ||
| Jet injection | Reliability of Insulin by Jet Injection (NCT02272296) |
30 participants • Age 18-50 years • BMI 18-32 kg/m2 • Blood pressure <160/90 mmHg |
The variability in time until maximal glucose lowering effect to maintain normoglycemia, after insulin injection; Time to maximal exogenous glucose infusion rate (GIR, in ml/min/kg) required to maintain euglycaemia. | Completed | Phase 4 | [251] |
| Pharmacology of Insulin Injected With Jet-Injection (NCT00983775) |
48 participants • T1D >1 year • Age 18-50 years • BMI 18-28 kg/m2 • HbA1c 6.5-90% • Blood pressure <160/90 mmHg |
Maximal glucose infusion rate: 0-8 hours after insulin injection | Completed | [252] | ||
| Pharmacokinetic and Pharmacodynamic Profile of Insulin Lispro UsingNeedle-Free Jet Injection Technology (NCT02443714) |
18 participants • Age 18-40 years • Height 170±10cm. • Weight ±10%kg. • Liver and renal function should be normal • Health subjects with no chronic diseases or medications. |
Early insulin exposure: AUC 0\of insulin from 0 to 30min | Completed | Phase 4 | [141] | |
| Pharmacology of Insulin Injected With Jet-injection in Diabetes (NCT01438632) |
24 participants • Duration of diabetes >1 year • Age 18-70 years • BMI 18-32 kg/m2 • HbA1c6.0-9.0% • Insulin use at least once daily or with subcutaneous pump • Blood pressure <160/90 mmHg |
AUC from time 0 to 2 h after insulin injection and meal ingestion | Completed | Phase 4 | [140] | |
| A Pilot Study to Assess the Safety, PK and PD of Insulin Injected Via Micron Jet or Conventional Needle (MicronJet) (NCT00602914) |
23 males Healthy: • Age 18-40 years • BMI <30 kg/m2 |
Blood samples for PK and PD | Completed | Early Phase 1 | [174] | |
| Pharmacokinetics/Dynamics of Basal (Continuous) Insulin Infusion Administered Either Intradermally or Subcutaneously • (NCT01061216) |
T2D: • Age 30-70 years • BMI <35 kg/m2 • HA1c 6.5-10% • 20 T1D males • Age 18-55 years • BMI ≤32 kg/m2 |
Insulin measurements will be used to compute PK model parameters | Completed | Phase 1/2 | ||
| Microneedle | Multi-day (3) In-patient Evaluation of Intradermal versus Subcutaneous Basal and Bolus Insulin Infusion (NCT01557907) |
23 participants • T1D for at least 1 year • Ages 18-55 years • BMI <32 kg/m2 • HbA1c ≤8.0% |
tmax of insulin delivered intradermally as compared to subcutaneously after a meal bolus. | Completed | Phase 1/2 | |
| Study | 20 participants • T1D > 1 year • Age≥ 18 years |
Aggregate mean difference in tmax | Completed | Phase 1/2 | ||
| Transdermal Basal Insulin Patch Study in Type 1 Diabetes (NCT00519623) |
30 males • T1D 1-15 year • Ages 18-55 years • BMI <32 kg/m2 • HbA1c≤9.0% |
AUC of the blood glucose profile after the meal | Completed | Phase 2 | [253] | |
| Transdermally Delivered Human Insulin Product (NCT03544996) |
16 participants • T1D >2 year • Age 8-18 years • BMI ≤85% for age • HbA1c≤8.5% |
Average tmax | Completed | Phase 2/3 | [254] |
T1D: type 1 diabetes; T2D: type 2 diabetes; BMI: body mass index; HbA1c: hemoglobin A1e; PK: pharmacokinetic; PD: pharmacodynamic; AUC: area under curve; tmax: time to peak insulin concentration
Data obtained from https://clinicaltrials.gov/.
Despite great successes in transdermal insulin delivery, there are several limitations associated with long-term use, delivery efficiency, and reliability that warrant further research. Further experiments should be performed to evaluate the short- and long-term side effects associated with chemical enhancers and different microneedle materials. The potential risk of irradiation and inflammation must also be assessed in clinical studies. In addition, correct dosage relies on an understanding of how to guarantee consistent insulin delivery in different patients or in different skin sites within the same patient. To this end, pharmacokinetics should also be characterized to determine the transport efficiency. More extensive investigation on transdermal insulin delivery with a closed-loop control is required to prevent the risk of hypoglycemia, which represents a severe complication of any insulin therapy method. For approaches that rely on electrical instruments, a reusable and low-cost handheld device would be beneficial for self-administration at home. Finally, innovative technologies to improve the stability, enhance bioavailability, and maintain bioactivity of insulin are critical to enable the ultimate development of effective, low-cost, and convenient transdermal insulin delivery systems.
Acknowledgement
This work was supported by the grants from the National Science Foundation (Grant No. 1708620), American Diabetes Association (ADA) (Grant No. 1-15-ACE-21), the JDRF (Grant No. 2-SRA-2016-269-A-N) to Z.G. ARK is supported by funding from the National Institute of Diabetes and Digestive and Kidney Disease of the National Institutes of Health under Award Number F30DK113728.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Collaboration ERF, Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies, The Lancet, 375 (2010) 2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].International Diabetes Federation, http://www.idf.org/diabetesatlas.
- [3].Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE, Global estimates of diabetes prevalence for 2013 and projections for 2035, Diabetes Res. Clin. Pract, 103 (2014) 137–149. [DOI] [PubMed] [Google Scholar]
- [4].Atkinson MA, Eisenbarth GS, Type 1 diabetes: new perspectives on disease pathogenesis and treatment, The Lancet, 358 (2001) 221–229. [DOI] [PubMed] [Google Scholar]
- [5].Stumvoll M, Goldstein BJ, van Haeften TW, Type 2 diabetes: principles of pathogenesis and therapy, The Lancet, 365 (2005) 1333–1346. [DOI] [PubMed] [Google Scholar]
- [6].Owens DR, Zinman B, Bolli GB, Insulins today and beyond, The Lancet, 358 (2001) 739–746. [DOI] [PubMed] [Google Scholar]
- [7].Zaykov AN, Mayer JP, DiMarchi RD, Pursuit of a perfect insulin, Nature Reviews Drug Discovery, 15 (2016) 425. [DOI] [PubMed] [Google Scholar]
- [8].Misso ML, Egberts KJ, Page M, O’connor D, Shaw J, Cochrane review: Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus, Evid. Based Child Health, 5 (2010) 1726–1867. [DOI] [PubMed] [Google Scholar]
- [9].Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M, Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study, Diabetes Res. Clin. Pract, 28 (1995) 103–117. [DOI] [PubMed] [Google Scholar]
- [10].Morris AD, Boyle DI, McMahon AD, Greene SA, MacDonald TM, Newton RW, Collaboration DM, Adherence to insulin treatment, glycaemic control, and ketoacidosis in insulin-dependent diabetes mellitus, The Lancet, 350 (1997) 1505–1510. [DOI] [PubMed] [Google Scholar]
- [11].Pickup J, Keen H, Continuous subcutaneous insulin infusion at 25 years: evidence base for the expanding use of insulin pump therapy in type 1 diabetes, Diabetes Care, 25 (2002) 593–598. [DOI] [PubMed] [Google Scholar]
- [12].Weissberg-Benchell J, Antisdel-Lomaglio J, Seshadri R, Insulin pump therapy: a meta-analysis, Diabetes Care, 26 (2003) 1079–1087. [DOI] [PubMed] [Google Scholar]
- [13].Owens DR, New horizons-alternative routes for insulin therapy, Nat. Rev. Drug Discov, 1 (2002) 529. [DOI] [PubMed] [Google Scholar]
- [14].Owens DR, Zinman B, Bolli G, Alternative routes of insulin delivery, Diabet. Med, 20 (2003) 886–898. [DOI] [PubMed] [Google Scholar]
- [15].Ravaine V, Ancla C, Catargi B, Chemically controlled closed-loop insulin delivery, J. Control. Release, 132 (2008) 2–11. [DOI] [PubMed] [Google Scholar]
- [16].Iyer H, Khedkar A, Verma M, Oral insulin–a review of current status, Diabetes Obes. Metab, 12 (2010) 179–185. [DOI] [PubMed] [Google Scholar]
- [17].Hovorka R, Closed-loop insulin delivery: from bench to clinical pract ice, Nat. Rev. Endocrinol, 7 (2011) 385. [DOI] [PubMed] [Google Scholar]
- [18].Mo R, Jiang T, Di J, Tai W, Gu Z, Emerging micro-and nanotechnology based synthetic approaches for insulin delivery, Chem. Soc. Rev, 43 (2014) 3595–3629. [DOI] [PubMed] [Google Scholar]
- [19].Veiseh O, Tang BC, Whitehead KA, Anderson DG, Langer R, Managing diabetes with nanomedicine: challenges and opportunities, Nat. Rev. Drug Discov., 14 (2015) 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bakh NA, Cortinas AB, Weiss MA, Langer RS, Anderson DG, Gu Z, Dutta S, Strano MS, Glucose-responsive insulin by molecular and physical design, Nat. Chem, 9 (2017) 937. [DOI] [PubMed] [Google Scholar]
- [21].Khafagy E-S, Morishita M, Onuki Y, Takayama K, Current challenges in non-invasive insulin delivery systems: a comparative review, Adv. Drug Del. Rev, 59 (2007) 1521–1546. [DOI] [PubMed] [Google Scholar]
- [22].Mitragotri S, Burke PA, Langer R, Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies, Nat. Rev. Drug discov., 13 (2014) 655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yang R, Wei T, Goldberg H, Wang W, Cullion K, Kohane DS, Getting Drugs Across Biological Barriers, Adv. Mater, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Prausnitz MR, Mitragotri S, Langer R, Current status and future potential of transdermal drug delivery, Nat. Rev. Drug discov, 3 (2004) 115. [DOI] [PubMed] [Google Scholar]
- [25].Prausnitz MR, Langer R, Transdermal drug delivery, Nat. Biotechnol, 26 (2008) 1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Giudice EL, Campbell JD, Needle-free vaccine delivery, Adv. Drug Del. Rev, 58 (2006) 68–89. [DOI] [PubMed] [Google Scholar]
- [27].Godin B, Touitou E, Transdermal skin delivery: predictions for humans from in vivo, ex vivo and animal models, Adv. Drug Del. Rev, 59 (2007) 1152–1161. [DOI] [PubMed] [Google Scholar]
- [28].Bos JD, Meinardi MM, The 500 Dalton rule for the skin penetration of chemical compounds and drugs, Exp. Dermatol, 9 (2000) 165–169. [DOI] [PubMed] [Google Scholar]
- [29].Walker RB, Smith EW, The role of percutaneous penetration enhancers, Adv. Drug Del. Rev, 18 (1996) 295–301. [Google Scholar]
- [30].Finnin BC, Morgan TM, Transdermal penetration enhancers: applications, limitations, and potential, J. Pharm. Sci, 88 (1999) 955–958. [DOI] [PubMed] [Google Scholar]
- [31].Williams AC, Barry BW, Penetration enhancers, Adv. Drug Del. Rev, 56 (2004) 603–618. [DOI] [PubMed] [Google Scholar]
- [32].Karande P, Jain A, Ergun K, Kispersky V, Mitragotri S, Design principles of chemical penetration enhancers for transdermal drug delivery, Proc. Natl. Acad. Sci. U. S. A, 102 (2005) 4688–4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lane ME, Skin penetration enhancers, Int. J. Pharm, 447 (2013) 12–21. [DOI] [PubMed] [Google Scholar]
- [34].Yerramsetty KM, Rachakonda VK, Neely BJ, Madihally SV, Gasem KA, Effect of different enhancers on the transdermal permeation of insulin analog, Int. J. Pharm, 398 (2010) 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sintov AC, Wormser U, Topical iodine facilitates transdermal delivery of insulin, J. Control. Release, 118 (2007) 185–188. [DOI] [PubMed] [Google Scholar]
- [36].Li Y.-z, Quan Y.-s., Zang L, Jin M.-n., Kamiyama F, Katsumi H, Yamamoto A, Tsutsumi S, Transdermal delivery of insulin using trypsin as a biochemical enhancer, Biol. Pharm. Bull, 31 (2008) 1574–1579. [DOI] [PubMed] [Google Scholar]
- [37].Chen Y, Shen Y, Guo X, Zhang C, Yang W, Ma M, Liu S, Zhang M, Wen LP, Transdermal protein delivery by a coadministered peptide identified via phage display, Nat. Biotechnol, 24 (2006) 455–460. [DOI] [PubMed] [Google Scholar]
- [38].Chang M, Li X, Sun Y, Cheng F, Wang Q, Xie X, Zhao W, Tian X, Effect of cationic cyclopeptides on transdermal and transmembrane delivery of insulin, Mol. Pharm, 10 (2013) 951–957. [DOI] [PubMed] [Google Scholar]
- [39].Kogan A, Garti N, Microemulsions as transdermal drug delivery vehicles, Adv. Colloid Interface Sci, 123 (2006) 369–385. [DOI] [PubMed] [Google Scholar]
- [40].Touitou E, Godin B, Vesicular carriers for enhanced delivery through the skin, in: Touitou E, Barry BW (Eds.), Enhancement in Drug Delivery, CRC Press, Florida, 2006, pp. 255–278. [Google Scholar]
- [41].Pegoraro C, MacNeil S, Battaglia G, Transdermal drug delivery: from micro to nano, Nanoscale, 4 (2012) 1881–1894. [DOI] [PubMed] [Google Scholar]
- [42].Yang Y, Bugno J, Hong S, Nanoscale polymeric penetration enhancers in topical drug delivery, Polym. Chem, 4 (2013) 2651–2657. [Google Scholar]
- [43].Nastiti CM, Ponto T, Abd E, Grice JE, Benson HA, Roberts MS, Topical nano and microemulsions for skin delivery, Pharmaceutics, 9 (2017) 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Prow TW, Grice JE, Lin LL, Faye R, Butler M, Becker W, Wurm EM, Yoong C, Robertson TA, Soyer HP, Nanoparticles and microparticles for skin drug delivery, Adv. Drug Del. Rev, 63 (2011) 470–491. [DOI] [PubMed] [Google Scholar]
- [45].Guo J, Ping Q, Zhang L, Transdermal delivery of insulin in mice by using lecithin vesicles as a carrier, Drug Deliv, 7 (2000) 113–116. [DOI] [PubMed] [Google Scholar]
- [46].King MJ, Badea I, Solomon J, Kumar P, Gaspar KJ, Foldvari M, Transdermal delivery of insulin from a novel biphasic lipid system in diabetic rats, Diabetes Technol. Ther, 4 (2002) 479–488. [DOI] [PubMed] [Google Scholar]
- [47].King MJ, Michel D, Foldvari M, Evidence for lymphatic transport of insulin by topically applied biphasic vesicles, J. Pharm. Pharmacol, 55 (2003) 1339–1344. [DOI] [PubMed] [Google Scholar]
- [48].Higaki M, Kameyama M, Udagawa M, Ueno Y, Yamaguchi Y, Igarashi R, Ishihara T, Mizushima Y, Transdermal delivery of CaCO3-nanoparticles containing insulin, Diabetes Technol. Ther, 8 (2006) 369–374. [DOI] [PubMed] [Google Scholar]
- [49].Tahara Y, Honda S, Kamiya N, Goto M, Transdermal delivery of insulin using a solid-in-oil nanodispersion enhanced by arginine-rich peptides, MedChemComm, 3 (2012) 1496. [Google Scholar]
- [50].Nose K, Pissuwan D, Goto M, Katayama Y, Niidome T, Gold nanorods in an oil-base formulation for transdermal treatment of type 1 diabetes in mice, Nanoscale, 4 (2012) 3776–3780. [DOI] [PubMed] [Google Scholar]
- [51].Tahara Y, Honda S, Kamiya N, Piao H, Hirata A, Hayakawa E, Fujii T, Goto M, A solid-in-oil nanodispersion for transcutaneous protein delivery, J. Control. Release, 131 (2008) 14–18. [DOI] [PubMed] [Google Scholar]
- [52].Tahara Y, Namatsu K, Kamiya N, Hagimori M, Kamiya S, Arakawa M, Goto M, Transcutaneous immunization by a solid-in-oil nanodispersion, Chem. Commun, 46 (2010) 9200–9202. [DOI] [PubMed] [Google Scholar]
- [53].Pissuwan D, Nose K, Kurihara R, Kaneko K, Tahara Y, Kamiya N, Goto M, Katayama Y, Niidome T, A solid-in-oil dispersion of gold nanorods can enhance transdermal protein delivery and skin vaccination, Small, 7 (2011) 215–220. [DOI] [PubMed] [Google Scholar]
- [54].Kalia YN, Naik A, Garrison J, Guy RH, Iontophoretic drug delivery, Adv. Drug Del. Rev, 56 (2004) 619–658. [DOI] [PubMed] [Google Scholar]
- [55].Denet A-R, Vanbever R, Préat V, Skin electroporation for transdermal and topical delivery, Adv. Drug Del. Rev, 56 (2004) 659–674. [DOI] [PubMed] [Google Scholar]
- [56].Azagury A, Khoury L, Enden G, Kost J, Ultrasound mediated transdermal drug delivery, Adv Drug Deliv Rev, 72 (2014) 127–143. [DOI] [PubMed] [Google Scholar]
- [57].Vadlapatla R, Wong EY, Gayakwad SG, Electronic drug delivery systems: An overview, J. Drug Deliv. Sci. Technol, 41 (2017) 359–366. [Google Scholar]
- [58].Banga AK, Chien YW, Iontophoretic delivery of drugs: fundamentals, developments and biomedical applications, J. Control. Release, 7 (1988) 1–24. [Google Scholar]
- [59].Pikal MJ, The role of electroosmotic flow in transdermal iontophoresis, Adv. Drug Del. Rev, 46 (2001) 281–305. [DOI] [PubMed] [Google Scholar]
- [60].Lau DT-W, Sharkey JW, Petryk L, Mancuso FA, Yu Z, Francis L, Effect of current magnitude and drug concentration on lontophoretic delivery of octreotide acetate (Sandostatin®) in the rabbit, Pharm. Res, 11 (1994) 1742–1746. [DOI] [PubMed] [Google Scholar]
- [61].Sieg A, Jeanneret F, Fathi M, Hochstrasser D, Rudaz S, Veuthey J-L, Guy RH, Delgado-Charro MB, Extraction of amino acids by reverse iontophoresis in vivo, Eur. J. Pharm. Biopharm, 72 (2009) 226–231. [DOI] [PubMed] [Google Scholar]
- [62].Naik A, Kalia YN, Guy RH, Transdermal drug delivery: overcoming the skin’s barrier function, Pharm. Sci. Technolo. Today, 3 (2000) 318–326. [DOI] [PubMed] [Google Scholar]
- [63].Sage BH Jr., Insulin Iontophoresis, in: Sanders R LM.Hendren W (Eds.), Protein Delivery-Physical Systems, Plenum Publishing Corp, New York, NY, 1997, pp. 319–341. [Google Scholar]
- [64].Stephen R, Petelenz T, Jacobsen S, Potential novel methods for insulin administration: I. Iontophoresis, Biomed. Biochim. Acta, 43 (1984) 553–558. [PubMed] [Google Scholar]
- [65].Chien Y, Siddiqui O, Sun Y, Shi W, Liu J, Transdermal iontophoretic delivery of therapeutic peptides/proteins I: insulin, Ann. N. Y. Acad. Sci, 507 (1987) 32–51. [DOI] [PubMed] [Google Scholar]
- [66].Jue-Chen L, Ying S, Siddiqui O, Wei-min S, John L, Blood glucose control in diabetic rats by transdermal iontophoretic delivery of insulin, Int. J. Pharm, 44 (1988) 197–204. [Google Scholar]
- [67].Banga AK, Chien YW, Characterization of in vitro transdermal iontophoretic delivery of insulin, Drug Dev. Ind. Pharm, 19 (1993) 2069–2087. [Google Scholar]
- [68].Siddiqui O, Sun Y, Liu JC, Chien YW, Facilitated transdermal transport of insulin, J. Pharm. Sci, 76 (1987) 341–345. [DOI] [PubMed] [Google Scholar]
- [69].Pillai O, Kumar N, Dey CS, Borkute S, Nagalingam S, Panchagnula R, Transdermal iontophoresis of insulin. Part 1: A study on the issues associated with the use of platinum electrodes on rat skin, J. Pharm. Pharmacol, 55 (2003) 1505–1513. [DOI] [PubMed] [Google Scholar]
- [70].Panchagnula R, Bindra P, Kumar N, Shanker Dey C, Pillai O, Stability of insulin under iontophoretic conditions, Pharmazie, 61 (2006) 1014–1018. [PubMed] [Google Scholar]
- [71].Kajimoto K, Yamamoto M, Watanabe M, Kigasawa K, Kanamura K, Harashima H, Kogure K, Noninvasive and persistent transfollicular drug delivery system using a combination of liposomes and iontophoresis, Int. J. Pharm, 403 (2011) 57–65. [DOI] [PubMed] [Google Scholar]
- [72].Kari B, Control of blood glucose levels in alloxan-diabetic rabbits by iontophoresis of insulin, Diabetes, 35 (1986) 217–221. [DOI] [PubMed] [Google Scholar]
- [73].Langkjær L, Brange J, Grodsky GM, Guy RH, Iontophoresis of monomeric insulin analogues in vitro: effects of insulin charge and skin pretreatment, J. Control. Release, 51 (1998) 47–56. [DOI] [PubMed] [Google Scholar]
- [74].Choi EH, Lee SH, Ahn SK, Hwang SM, The pretreatment effect of chemical skin penetration enhancers in transdermal drug delivery using iontophoresis, Skin Pharmacol. Physiol, 12 (1999) 326–335. [DOI] [PubMed] [Google Scholar]
- [75].Rastogi SK, Singh J, Transepidermal transport enhancement of insulin by lipid extraction and iontophoresis, Pharm. Res, 19 (2002) 427–433. [DOI] [PubMed] [Google Scholar]
- [76].Pillai O, Panchagnula R, Transdermal iontophoresis of insulin, J. Control. Release, 88 (2003) 287–296. [DOI] [PubMed] [Google Scholar]
- [77].Pillai O, Nair V, Panchagnula R, Transdermal iontophoresis of insulin: IV. Influence of chemical enhancers, Int. J. Pharm, 269 (2004) 109–120. [DOI] [PubMed] [Google Scholar]
- [78].Zakzewski CA, Wasilewski J, Cawley P, Ford W, Transdermal delivery of regular insulin to chronic diabetic rats: effect of skin preparation and electrical enhancement, J. Control. Release, 50 (1998) 267–272. [DOI] [PubMed] [Google Scholar]
- [79].Kanikkannan N, Singh J, Ramarao P, Transdermal iontophoretic delivery of bovine insulin and monomeric human insulin analogue, J. Control. Release, 59 (1999) 99–105. [DOI] [PubMed] [Google Scholar]
- [80].Murdan S, Electro-responsive drug delivery from hydrogels, J. Control. Release, 92 (2003) 1–17. [DOI] [PubMed] [Google Scholar]
- [81].Kagatani S, Shinoda T, Konno Y, Fukui M, Ohmura T, Osada Y, Electroresponsive pulsatile depot delivery of insulin from poly(dimethylaminopropylacrylamide) gel in rats, J. Pharm. Sci, 86 (1997) 1273–1277. [DOI] [PubMed] [Google Scholar]
- [82].Pillai O, Transdermal delivery of insulin from poloxamer gel: ex vivo and in vivo skin permeation studies in rat using iontophoresis and chemical enhancers, J. Control. Release, 89 (2003) 127–140. [DOI] [PubMed] [Google Scholar]
- [83].Inada H, Ghanem A-H, Higuchi WI, Studies on the effects of applied voltage and duration on human epidermal membrane alteration/recovery and the resultant effects upon iontophoresis, Pharm. Res, 11 (1994) 687–697. [DOI] [PubMed] [Google Scholar]
- [84].Yan G, Peck KD, Zhu H, Higuchi WI, Li SK, Effects of electrophoresis and electroosmosis during alternating current iontophoresis across human epidermal membrane, J. Pharm. Sci, 94 (2005) 547–558. [DOI] [PubMed] [Google Scholar]
- [85].Lvovich VF, Matthews E, Riga AT, Kaza L, AC electrokinetic platform for iontophoretic transdermal drug delivery, J. Control. Release, 145 (2010) 134–140. [DOI] [PubMed] [Google Scholar]
- [86].Escobar-Chávez JJ, Bonilla-Martínez D, Villegas-González MA, Revilla-Vázquez AL, Electroporation as an efficient physical enhancer for skin drug delivery, J. Clin. Pharmacol, 49 (2009) 1262–1283. [DOI] [PubMed] [Google Scholar]
- [87].Yarmush ML, Golberg A, Serša G, Kotnik T, Miklavčič D, Electroporation-based technologies for medicine: principles, applications, and challenges, Annu. Rev. Biomed. Eng., 16 (2014). [DOI] [PubMed] [Google Scholar]
- [88].DeNuzzio JD, Berner B, Electrochemical and iontophoretic studies of human skin, J. Control. Release, 11 (1990) 105–112. [Google Scholar]
- [89].Prausnitz MR, Bose VG, Langer R, Weaver JC, Electroporation of mammalian skin: a mechanism to enhance transdermal drug delivery, Proc. Natl. Acad. Sci. U. S. A, 90 (1993) 10504–10508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Edwards DA, Prausnitz MR, Langer R, Weaver JC, Analysis of enhanced transdermal transport by skin electroporation, J. Control. Release, 34 (1995) 211–221. [Google Scholar]
- [91].Mohammad EA, Elshemey WM, Elsayed AA, Abd-Elghany AA, Electroporation parameters for successful transdermal delivery of insulin, Am. J. Ther, 23 (2016) e1560–e1567. [DOI] [PubMed] [Google Scholar]
- [92].Rastogi R, Anand S, Koul V, Electroporat ion of polymeric nanoparticles: an alternative technique for transdermal delivery of insulin, Drug Dev. Ind. Pharm, 36 (2010) 1303–1311. [DOI] [PubMed] [Google Scholar]
- [93].Sen A, Zhao Y, Zhang L, Hui SW, Enhanced transdermal transport by electroporation using anionic lipids, J. Control. Release, 82 (2002) 399–405. [DOI] [PubMed] [Google Scholar]
- [94].Sen A, Daly ME, Hui SW, Transdermal insulin delivery using lipid enhanced electroporation, Biochim. Biophys. Acta-Biomembranes, 1564 (2002) 5–8. [DOI] [PubMed] [Google Scholar]
- [95].Murthy SN, Zhao YL, Marlan K, Hui SW, Kazim AL, Sen A, Lipid and electroosmosis enhanced transdermal delivery of insulin by electroporation, J. Pharm. Sci, 95 (2006) 2041–2050. [DOI] [PubMed] [Google Scholar]
- [96].Murthy SN, Zhao Y-L, Hui S-W, Sen A, Synergistic effect of anionic lipid enhancer and electroosmosis for transcutaneous delivery of insulin, Int. J. Pharm, 326 (2006) 1–6. [DOI] [PubMed] [Google Scholar]
- [97].Tokumoto S, Higo N, Sugibayashi K, Effect of electroporation and pH on the iontophoretic transdermal delivery of human insulin, Int. J. Pharm, 326 (2006) 13–19. [DOI] [PubMed] [Google Scholar]
- [98].Wong TW, Chen TY, Huang CC, Tsai JC, Hui SW, Painless skin electroporation as a novel way for insulin delivery, Diabetes Technol. Ther, 13 (2011) 929–935. [DOI] [PubMed] [Google Scholar]
- [99].Ching CT-S, Sun T-P, Huang W-T, Huang S-H, Hsiao C-S, Chang K-M, A circuit design of a low-cost, portable and programmable electroporation device for biomedical applications, Sensors Actuators B: Chem, 166-167 (2012) 292–300. [Google Scholar]
- [100].Zhang Y, Yu J, Bomba HN, Zhu Y, Gu Z, Mechanical force-triggered drug delivery, Chem. Rev, 116 (2016) 12536–12563. [DOI] [PubMed] [Google Scholar]
- [101].Suslick KS, Price GJ, Applications of ultrasound to materials chemistry, Annu. Rev. Mater. Sci, 29 (1999) 295–326. [Google Scholar]
- [102].Mitragotri S, Kost J, Low-frequency sonophoresis: a review, Adv. Drug Del. Rev, 56 (2004) 589–601. [DOI] [PubMed] [Google Scholar]
- [103].Ferrara K, Pollard R, Borden M, Ultrasound microbubble contrast agents: fundamentals and application to gene and drug delivery, Annu. Rev. Biomed. Eng, 9 (2007). [DOI] [PubMed] [Google Scholar]
- [104].Wells PN, Liang H-D, Medical ultrasound: imaging of soft tissue strain and elasticity, J. R. Soc. Interface, 8 (2011) 1521–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Jolesz FA, MRI-guided focused ultrasound surgery, Annu. Rev. Med, 60 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Coussios CC, Roy RA, Applications of acoustics and cavitation to noninvasive therapy and drug delivery, Annu. Rev. Fluid Mech, 40 (2008) 395–420. [Google Scholar]
- [107].MIYAZAKI S, YOKOUCHI C, TAKADA M, External control of drug release: controlled release of insulin from a hydrophilic polymer implant by ultrasound irradiation in diabetic rats, J. Pharm. Pharmacol, 40 (1988) 716–717. [DOI] [PubMed] [Google Scholar]
- [108].Polat BE, Hart D, Langer R, Blankschtein D, Ultrasound-mediated transdermal drug delivery: mechanisms, scope, and emerging trends, J. Control. Release, 152 (2011) 330–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Mitragotri S, Sonophoresis: a 50-year journey, Drug Discov. Today, 9 (2004) 735–736. [DOI] [PubMed] [Google Scholar]
- [110].Tyle P, Agrawala P, Drug delivery by phonophoresis, Pharm. Res, 6 (1989) 355–361. [DOI] [PubMed] [Google Scholar]
- [111].Byl NN, The use of ultrasound as an enhancer for transcutaneous drug delivery: phonophoresis, Phys. Ther, 75 (1995) 539–553. [DOI] [PubMed] [Google Scholar]
- [112].Gaertner W, Frequency dependence of ultrasonic cavitation, J. Acoust. Soc. Am, 26 (1954) 977–980. [Google Scholar]
- [113].Tachibana K, Tachibana S, Transdermal delivery of insulin by ultrasonic vibration, J. Pharm. Pharmacol, 43 (1991) 270–271. [DOI] [PubMed] [Google Scholar]
- [114].Mitragotri S, Blankschtein D, Langer R, Ultrasound-mediated transdermal protein delivery, Science, 269 (1995) 850–853. [DOI] [PubMed] [Google Scholar]
- [115].Tachibana K, Transdermal delivery of insulin to alloxan-diabetic rabbits by ultrasound exposure, Pharm. Res, 9 (1992) 952–954. [DOI] [PubMed] [Google Scholar]
- [116].Mitragotri S, Blankschtein D, Langer R, Transdermal drug delivery using low-frequency sonophoresis, Pharm. Res, 13 (1996) 411–420. [DOI] [PubMed] [Google Scholar]
- [117].Al-Bataineh OM, Lweesy K, Fraiwan L, Noninvasive transdermal insulin delivery using piston-shaped PZT transducers: in vivo rabbits evaluation, JJMIE, 6 (2012). [Google Scholar]
- [118].Feiszthuber H, Bhatnagar S, Gyongy M, Coussios CC, Cavitation-enhanced delivery of insulin in agar and porcine models of human skin, Phys. Med. Biol, 60 (2015) 2421–2434. [DOI] [PubMed] [Google Scholar]
- [119].Boucaud A, Tessier L, Machet L, Vaillant L, Patat F, Transdermal delivery of insulin using low frequency ultrasound, in: Ultrasonics Symposium, IEEE, 2000, pp. 1453–1456. [Google Scholar]
- [120].Boucaud A, Machet L, Garrigue M, Vaillant L, Patat F, A practical use of low frequency ultrasound for rapid and reproducible transdermal delivery of insulin, in: Ultrasonics Symposium, IEEE, 2001, pp. 1327–1330. [Google Scholar]
- [121].Boucaud A, Garrigue MA, Machet L, L.c. Vaillant, F. Patat, Effect of sonication parameters on transdermal delivery of insulin to hairless rats, J. Control. Release, 81 (2002) 113–119. [DOI] [PubMed] [Google Scholar]
- [122].Smith NB, Lee S, Maione E, Roy RB, McElligott S, Shung KK, Ultrasound-mediated transdermal transport of insulin in vitro through human skin using novel transducer designs, Ultrasound Med. Biol, 29 (2003) 311–317. [DOI] [PubMed] [Google Scholar]
- [123].Smith NB, Lee S, Shung KK, Ultrasound-mediated transdermal in vivo transport of insulin with low-profile cymbal arrays, Ultrasound Med. Biol, 29 (2003) 1205–1210. [DOI] [PubMed] [Google Scholar]
- [124].Lee S, Newnham RE, Smith NB, Short ultrasound exposure times for noninvasive insulin delivery in rats using the lightweight cymbal array, IEEE Trans. Ultrason. Ferroelectr. Freq. Control, 51 (2004) 176–180. [PubMed] [Google Scholar]
- [125].Lee S, Snyder B, Newnham RE, Barrie Smith N, Noninvasive ultrasonic transdermal insulin delivery in rabbits using the light-weight cymbal array, Diabetes Technol. Ther, 6 (2004) 808–815. [DOI] [PubMed] [Google Scholar]
- [126].Lee S, Smith N, Markley D, Snyder B, Uzgur A, Pishko M, Newnham R, Composite transducer arrays for the treatment of diabetes, Int. J. Appl. Ceram. Tec, 2 (2005) 308–316. [Google Scholar]
- [127].Luis J, Park EJ, Meyer RJ Jr, Smith NB, Rectangular cymbal arrays for improved ultrasonic transdermal insulin delivery, J. Acoust. Soc. Am, 122 (2007) 2022–2030. [DOI] [PubMed] [Google Scholar]
- [128].Park EJ, Werner J, Smith NB, Ultrasound mediated transdermal insulin delivery in pigs using a lightweight transducer, Pharm. Res, 24 (2007) 1396–1401. [DOI] [PubMed] [Google Scholar]
- [129].Park E, Dodds J, Smith N, Dose comparison of ultrasonic transdermal insulin delivery to subcutaneous insulin injection, International journal of nanomedicine, 3 (2008) 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Park EJ, Werner J, Jaiswal D, Smith NB, Closed-loop controlled noninvasive ultrasonic glucose sensing and insulin delivery, in: AIP Conf. Proc., AIP, 2010, pp. 157–160. [Google Scholar]
- [131].Di J, Price J, Gu X, Jiang X, Jing Y, Gu Z, Ultrasound-triggered regulation of blood glucose levels using injectable nano-network, Adv. Healthc. Mater, 3 (2014) 811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Di J, Yu J, Wang Q, Yao S, Suo D, Ye Y, Pless M, Zhu Y, Jing Y, Gu Z, Ultrasound-triggered noninvasive regulation of blood glucose levels using microgels integrated with insulin nanocapsules, Nano Research, 10 (2017) 1393–1402. [Google Scholar]
- [133].Suarez Castellanos I, Jeremic A, Cohen J, Zderic V, Ultrasound Stimulation of Insulin Release from Pancreatic Beta Cells as a Potential Novel Treatment for Type 2 Diabetes, Ultrasound Med. Biol, 43 (2017) 1210–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Suarez Castellanos I, Singh T, Balteanu B, Bhowmick DC, Jeremic A, Zderic V, Calcium-dependent ultrasound stimulation of secretory events from pancreatic beta cells, J. Ther. Ultrasound, 5 (2017) 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Sonoki K, Yoshinari M, Iwase M, Tashiro K, Iino K, Wakisaka M, Fujishima M, Regurgitation of blood into insulin cartridges in the pen-like injectors, Diabetes Care, 24 (2001) 603–604. [DOI] [PubMed] [Google Scholar]
- [136].Baxter J, Mitragotri S, Needle-free liquid jet injections: mechanisms and applications, Expert Rev. Med. Devices, 3 (2006) 565–574. [DOI] [PubMed] [Google Scholar]
- [137].Mitragotri S, Immunization without needles, Nat. Rev. Immunol, 5 (2005) 905. [DOI] [PubMed] [Google Scholar]
- [138].Weller C, Linder M, Jet injection of insulin vs the syringe-and-needle method, JAMA, 195 (1966) 844–847. [DOI] [PubMed] [Google Scholar]
- [139].Katoulis E, Drosinos E, Dimitriadis G, Hadjidakis D, Mavrokefalos P, Raptis S, Efficacy of a new needleless insulin delivery system monitoring of blood glucose fluctuations and free insulin levels, Int. J. Artif. Organs, 12 (1989) 333–338. [PubMed] [Google Scholar]
- [140].Engwerda EE, Tack CJ, De Galan BE, Needle-free jet injection of rapid-acting insulin improves early postprandial glucose control in patients with diabetes, Diabetes Care, 36 (2013) 3436–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Hu J, Shi H, Zhao C, Li X, Wang Y, Cheng Q, Goswami R, Zhen Q, Mei M, Song Y, Lispro administered by the QS-M Needle-Free Jet Injector generates an earlier insulin exposure, Expert Opin. Drug Deliv, 13 (2016) 1203–1207. [DOI] [PubMed] [Google Scholar]
- [142].de Wit H, Engwerda E, Tack C, de Galan B, Insulin administered by needle-free jet injection corrects marked hyperglycaemia faster in overweight or obese patients with diabetes, Diabetes Obes. Metab, 17 (2015) 1093–1099. [DOI] [PubMed] [Google Scholar]
- [143].Malone J, Lowitt S, Grove N, Shah S, Comparison of insulin levels after injection by jet stream and disposable insulin syringe, Diabetes Care, 9 (1986) 637–640. [DOI] [PubMed] [Google Scholar]
- [144].Guo L, Xiao X, Sun X, Qi C, Comparison of jet injector and insulin pen in controlling plasma glucose and insulin concentrations in type 2 diabetic patients, Medicine, 96 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Shahani S, Shahani L, Use of insulin in diabetes: A century of treatment, Hong Kong Med. J, 21 (2015) 553–559. [DOI] [PubMed] [Google Scholar]
- [146].Houtzagers C, Visser AP, Berntzen P, Heine R, Veen E, The Medi-Jector II: Efficacy and Acceptability in Insulin-dependent Diabetic Patients With and Without Needle Phobia, Diabet. Med, 5 (1988) 135–138. [DOI] [PubMed] [Google Scholar]
- [147].Pharm DLB. D, F. Pass, Delivery of insulin by jet injection: recent observations, Diabetes Technol. Ther, 3 (2001) 225–232. [DOI] [PubMed] [Google Scholar]
- [148].Schneider U, Birnbacher R, Schober E, Painfulness of needle and jet injection in children with diabetes mellitus, Eur. J. Pediatr, 153 (1994) 409–410. [DOI] [PubMed] [Google Scholar]
- [149].Arora A, Hakim I, Baxter J, Rathnasingham R, Srinivasan R, Fletcher DA, Mitragotri S, Needle-free delivery of macromolecules across the skin by nanoliter-volume pulsed microjets, Proc. Natl. Acad. Sci. U. S. A, 104 (2007) 4255–4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Römgens AM, Rem-Bronneberg D, Kassies R, Hijlkema M, Bader DL, Oomens CW, van Bruggen MP, Penetration and delivery characteristics of repetitive microjet injection into the skin, J. Control. Release, 234 (2016) 98–103. [DOI] [PubMed] [Google Scholar]
- [151].Prausnitz MR, Microneedles for transdermal drug delivery, Adv. Drug Del. Rev, 56 (2004) 581–587. [DOI] [PubMed] [Google Scholar]
- [152].Kim Y-C, Park J-H, Prausnitz MR, Microneedles for drug and vaccine delivery, Adv. Drug Del. Rev, 64 (2012) 1547–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Bhatnagar S, Dave K, Venuganti VVK, Microneedles in the clinic, J. Control. Release, 260 (2017) 164–182. [DOI] [PubMed] [Google Scholar]
- [154].Jin X, Zhu DD, Chen BZ, Ashfaq M, Guo XD, Insulin delivery systems combined with microneedle technology, Adv. Drug Del. Rev, (2018). [DOI] [PubMed] [Google Scholar]
- [155].Rzhevskiy AS, Singh TRR, Donnelly RF, Anissimov YG, Microneedles as the technique of drug delivery enhancement in diverse organs and tissues, J. Control. Release, (2017). [DOI] [PubMed] [Google Scholar]
- [156].Donnelly RF, Larrañeta E, Microarray patches: potentially useful delivery systems for long-acting nanosuspensions, Drug Discov. Today, (2017). [DOI] [PubMed] [Google Scholar]
- [157].Henry S, McAllister DV, Allen MG, Prausnitz MR, Microfabricated microneedles: a novel approach to transdermal drug delivery, J. Pharm. Sci, 87 (1998) 922–925. [DOI] [PubMed] [Google Scholar]
- [158].Larrañeta E, McCrudden MTC, Courtenay AJ, Donnelly RF, Microneedles: A New Frontier in Nanomedicine Delivery, Pharm. Res, 33 (2016) 1055–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Narayan RJ, Transdermal Delivery of Insulin via Microneedles, J. Biomed. Nanotechnol, 10 (2014) 2244–2260. [DOI] [PubMed] [Google Scholar]
- [160].Prausnitz MR, Engineering microneedle patches for vaccination and drug delivery to skin, Annu. Rev. Chem. Biomol. Eng, 8 (2017) 177–200. [DOI] [PubMed] [Google Scholar]
- [161].Martanto W, Davis SP, Holiday NR, Wang J, Gill HS, Prausnitz MR, Transdermal delivery of insulin using microneedles in vivo, Pharm. Res, 21 (2004) 947–952. [DOI] [PubMed] [Google Scholar]
- [162].Zhou CP, Liu YL, Wang HL, Zhang PX, Zhang JL, Transdermal delivery of insulin using microneedle rollers in vivo, Int. J. Pharm, 392 (2010) 127–133. [DOI] [PubMed] [Google Scholar]
- [163].Wu Y, Gao Y, Qin G, Zhang S, Qiu Y, Li F, Xu B, Sustained release of insulin through skin by intradermal microdelivery system, Biomed. Microdevices, 12 (2010) 665–671. [DOI] [PubMed] [Google Scholar]
- [164].Wu X-M, Todo H, Sugibayashi K, Enhancement of skin permeation of high molecular compounds by a combination of microneedle pretreatment and iontophoresis, J. Control. Release, 118 (2007) 189–195. [DOI] [PubMed] [Google Scholar]
- [165].Chen H, Zhu H, Zheng J, Mou D, Wan J, Zhang J, Shi T, Zhao Y, Xu H, Yang X, Iontophoresis-driven penetration of nanovesicles through microneedle-induced skin microchannels for enhancing transdermal delivery of insulin, J. Control. Release, 139 (2009) 63–72. [DOI] [PubMed] [Google Scholar]
- [166].Al-Qallaf B, Das DB, Davidson A, Transdermal drug delivery by coated microneedles:geometry effects on drug concentration in blood, Asia-Pac. J. Chem. Eng, 4 (2009) 845–857. [Google Scholar]
- [167].McAllister DV, Wang PM, Davis SP, Park JH, Canatella PJ, Allen MG, Prausnitz MR, Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies, Proc. Natl. Acad. Sci. U. S. A, 100 (2003) 13755–13760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Davis SP, Martanto W, Allen MG, Prausnitz MR, Hollow metal microneedles for insulin delivery to diabetic rats, IEEE Trans. Biomed. Eng, 52 (2005) 909–915. [DOI] [PubMed] [Google Scholar]
- [169].Gardeniers HJ, Luttge R, Berenschot EJ, De Boer MJ, Yeshurun SY, Hefetz M, van’t Oever R, van den Berg A, Silicon micromachined hollow microneedles for transdermal liquid transport, J. Microelectromech. Syst, 12 (2003) 855–862. [Google Scholar]
- [170].Resnik D, Možek M, Pečar B, Janež A, Urbančič V, Iliescu C, Vrtačnik D, In Vivo Experimental Study of Noninvasive Insulin Microinjection through Hollow Si Microneedle Array, Micromachines, 9 (2018) 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Nordquist L, Roxhed N, Griss P, Stemme G, Novel microneedle patches for active insulin delivery are efficient in maintaining glycaemic control: an initial comparison with subcutaneous administration, Pharm. Res, 24 (2007) 1381–1388. [DOI] [PubMed] [Google Scholar]
- [172].Roxhed N, Samel B, Nordquist L, Griss P, Stemme G, Painless drug delivery through microneedle-based transdermal patches featuring active infusion, IEEE Trans. Biomed. Eng, 55 (2008) 1063–1071. [DOI] [PubMed] [Google Scholar]
- [173].Gupta J, Felner EI, Prausnitz MR, Minimally invasive insulin delivery in subjects with type 1 diabetes using hollow microneedles, Diabetes Technol. Ther, 11 (2009) 329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Kochba E, Levin Y, Raz I, Cahn A, Improved Insulin Pharmacokinetics Using a Novel Microneedle Device for Intradermal Delivery in Patients with Type 2 Diabetes, Diabetes Technol. Ther, 18 (2016) 525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Ye Y, Yu J, Wen D, Kahkoska AR, Gu Z, Polymeric microneedles for transdermal protein delivery, Adv. Drug Del. Rev, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Quinn HL, Larrañeta E, Donnelly RF, Dissolving microneedles: safety considerations and future perspectives, Ther. Deliv, 7 (2016) 283–285. [DOI] [PubMed] [Google Scholar]
- [177].Hong X, Wei L, Wu F, Wu Z, Chen L, Liu Z, Yuan W, Dissolving and biodegradable microneedle technologies for transdermal sustained delivery of drug and vaccine, Drug Des. Devel. Ther, 7 (2013) 945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].An M, Liu H, Dissolving Microneedle Arrays for Transdermal Delivery of Amphiphilic Vaccines, Small, 13 (2017). [DOI] [PubMed] [Google Scholar]
- [179].Li G, Badkar A, Nema S, Kolli CS, Banga AK, In vitro transdermal delivery of therapeutic antibodies using maltose microneedles, Int. J. Pharm, 368 (2009) 109–115. [DOI] [PubMed] [Google Scholar]
- [180].Lee K, Lee CY, Jung H, Dissolving microneedles for transdermal drug administration prepared by stepwise controlled drawing of maltose, Biomaterials, 32 (2011) 3134–3140. [DOI] [PubMed] [Google Scholar]
- [181].Kalluri H, Banga AK, Formation and closure of microchannels in skin following microporation, Pharm. Res, 28 (2011) 82–94. [DOI] [PubMed] [Google Scholar]
- [182].Li G, Badkar A, Kalluri H, Banga AK, Microchannels created by sugar and metal microneedles: characterization by microscopy, macromolecular flux and other techniques, J. Pharm. Sci, 99 (2010) 1931–1941. [DOI] [PubMed] [Google Scholar]
- [183].Lee JW, Choi SO, Felner EI, Prausnitz MR, Dissolving microneedle patch for transdermal delivery of human growth hormone, Small, 7 (2011) 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Quan F-S, Kim Y-C, Vunnava A, Yoo D-G, Song J-M, Prausnitz MR, Compans RW, Kang S-M, Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization, J. Virol, 84 (2010) 7760–7769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Kommareddy S, Baudner BC, Oh S, Kwon S.-y., Singh M, O’hagan DT, Dissolvable microneedle patches for the delivery of cell-culture-derived influenza vaccine antigens, J. Pharm. Sci, 101 (2012) 1021–1027. [DOI] [PubMed] [Google Scholar]
- [186].Mistilis MJ, Joyce JC, Esser ES, Skountzou I, Compans RW, Bommarius AS, Prausnitz MR, Long-term stability of influenza vaccine in a dissolving microneedle patch, Drug Deliv. Transl. Res, 7 (2017) 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Martin C, Allender CJ, Brain KR, Morrissey A, Birchall JC, Low temperature fabrication of biodegradable sugar glass microneedles for transdermal drug delivery applications, J. Control. Release, 158 (2012) 93–101. [DOI] [PubMed] [Google Scholar]
- [188].Raphael AP, Crichton ML, Falconer RJ, Meliga S, Chen X, Fernando GJ, Huang H, Kendall MA, Formulations for microprojection/microneedle vaccine delivery: Structure, strength and release profiles, J. Control. Release, 225 (2016) 40–52. [DOI] [PubMed] [Google Scholar]
- [189].Ito Y, Hagiwara E, Saeki A, Sugioka N, Takada K, Feasibility of microneedles for percutaneous absorption of insulin, Eur. J. Pharm. Sci, 29 (2006) 82–88. [DOI] [PubMed] [Google Scholar]
- [190].Ito Y, Nakahigashi T, Yoshimoto N, Ueda Y, Hamasaki N, Takada K, Transdermal insulin application system with dissolving microneedles, Diabetes Technol. Ther, 14 (2012) 891–899. [DOI] [PubMed] [Google Scholar]
- [191].Miyano T, Tobinaga Y, Kanno T, Matsuzaki Y, Takeda H, Wakui M, Hanada K, Sugar micro needles as transdermic drug delivery system, Biomed. Microdevices, 7 (2005) 185–188. [DOI] [PubMed] [Google Scholar]
- [192].Hirobe S, Azukizawa H, Hanafusa T, Matsuo K, Quan Y-S, Kamiyama F, Katayama I, Okada N, Nakagawa S, Clinical study and stability assessment of a novel transcutaneous influenza vaccination using a dissolving microneedle patch, Biomaterials, 57 (2015) 50–58. [DOI] [PubMed] [Google Scholar]
- [193].Ye Y, Wang J, Hu Q, Hochu GM, Xin H, Wang C, Gu Z, Synergistic transcutaneous immunotherapy enhances antitumor immune responses through delivery of checkpoint inhibitors, ACS Nano, 10 (2016) 8956–8963. [DOI] [PubMed] [Google Scholar]
- [194].Wang C, Ye Y, Hochu GM, Sadeghifar H, Gu Z, Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PD1 antibody, Nano Lett, 16 (2016) 2334–2340. [DOI] [PubMed] [Google Scholar]
- [195].Ye Y, Wang C, Zhang X, Hu Q, Zhang Y, Liu Q, Wen D, Milligan J, Bellotti A, Huang L, A melanin-mediated cancer immunotherapy patch, Sci. Immunol, 2 (2017) eaan5692–5692. [DOI] [PubMed] [Google Scholar]
- [196].Zhang Y, Liu Q, Yu J, Yu S, Wang J, Qiang L, Gu Z, Locally Induced Adipose Tissue Browning by Microneedle Patch for Obesity Treatment, ACS Nano, 11 (2017) 9223–9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Lee JW, Park J-H, Prausnitz MR, Dissolving microneedles for transdermal drug delivery, Biomaterials, 29 (2008) 2113–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Chen M-C, Ling M-H, Lai K-Y, Pramudityo E, Chitosan microneedle patches for sustained transdermal delivery of macromolecules, Biomacromolecules, 13 (2012) 4022–4031. [DOI] [PubMed] [Google Scholar]
- [199].Chen M-C, Huang S-F, Lai K-Y, Ling M-H, Fully embeddable chitosan microneedles as a sustained release depot for intradermal vaccination, Biomaterials, 34 (2013) 3077–3086. [DOI] [PubMed] [Google Scholar]
- [200].Chen W, Tian R, Xu C, Yung BC, Wang G, Liu Y, Ni Q, Zhang F, Zhou Z, Wang J, Microneedle-array patches loaded with dual mineralized protein/peptide particles for type 2 diabetes therapy, Nature communications, 8 (2017) 1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Yu W, Jiang G, Zhang Y, Liu D, Xu B, Zhou J, Polymer microneedles fabricated from alginate and hyaluronate for transdermal delivery of insulin, Mater. Sci. Eng. C, 80 (2017) 187–196. [DOI] [PubMed] [Google Scholar]
- [202].Ke C-J, Lin Y-J, Hu Y-C, Chiang W-L, Chen K-J, Yang W-C, Liu H-L, Fu C-C, Sung H-W, Multidrug release based on microneedle arrays filled with pH-responsive PLGA hollow microspheres, Biomaterials, 33 (2012) 5156–5165. [DOI] [PubMed] [Google Scholar]
- [203].Guo L, Chen J, Qiu Y, Zhang S, Xu B, Gao Y, Enhanced transcutaneous immunization via dissolving microneedle array loaded with liposome encapsulated antigen and adjuvant, Int. J. Pharm, 447 (2013) 22–30. [DOI] [PubMed] [Google Scholar]
- [204].Chen M-C, Lin Z-W, Ling M-H, Near-infrared light-activatable microneedle system for treating superficial tumors by combination of chemotherapy and photothermal therapy, ACS Nano, 10 (2015) 93–101. [DOI] [PubMed] [Google Scholar]
- [205].Sun W, Araci Z, Inayathullah M, Manickam S, Zhang X, Bruce MA, Marinkovich MP, Lane AT, Milla C, Rajadas J, Polyvinylpyrrolidone microneedles enable delivery of intact proteins for diagnostic and therapeutic applications, Acta Biomater., 9 (2013) 7767–7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Perennes F, Marmiroli B, Matteucci M, Tormen M, Vaccari L, Di Fabrizio E, Sharp beveled tip hollow microneedle arrays fabricated by LIGA and 3D soft lithography with polyvinyl alcohol, J. Micromech. Microeng, 16 (2006) 473. [Google Scholar]
- [207].Lee I-C, He J-S, Tsai M-T, Lin K-C, Fabrication of a novel partially dissolving polymer microneedle patch for transdermal drug delivery, J. Mater. Chem. B, 3 (2015) 276–285. [DOI] [PubMed] [Google Scholar]
- [208].Zhang Y, Feng P, Yu J, Yang J, Zhao J, Wang J, Shen Q, Gu Z, ROS-responsive microneedle patch for acne vulgaris treatment, Adv. Therap, 1 (2018) 1800035. [Google Scholar]
- [209].Liu S, Jin MN, Quan YS, Kamiyama F, Katsumi H, Sakane T, Yamamoto A, The development and characteristics of novel microneedle arrays fabricated from hyaluronic acid, and their application in the transdermal delivery of insulin, J. Control. Release, 161 (2012) 933–941. [DOI] [PubMed] [Google Scholar]
- [210].Ling MH, Chen MC, Dissolving polymer microneedle patches for rapid and efficient transdermal delivery of insulin to diabetic rats, Acta Biomater, 9 (2013) 8952–8961. [DOI] [PubMed] [Google Scholar]
- [211].Leone M, Mönkäre J, Bouwstra J, Kersten G, Dissolving microneedle patches for dermal vaccination, Pharm. Res, 34 (2017) 2223–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Chen MC, Ling MH, Kusuma SJ, Poly-gamma-glutamic acid microneedles with a supporting structure design as a potential tool for transdermal delivery of insulin, Acta Biomater, 24 (2015) 106–116. [DOI] [PubMed] [Google Scholar]
- [213].Lau S, Fei J, Liu H, Chen W, Liu R, Multilayered pyramidal dissolving microneedle patches with flexible pedestals for improving effective drug delivery, J. Control. Release, 265 (2017) 113–119. [DOI] [PubMed] [Google Scholar]
- [214].Lee IC, Wu Y-C, Tsai S-W, Chen C-H, Wu M-H, Fabrication of two-layer dissolving polyvinylpyrrolidone microneedles with different molecular weights for in vivo insulin transdermal delivery, RSC Advances, 7 (2017) 5067–5075. [Google Scholar]
- [215].Lee IC, Lin WM, Shu JC, Tsai SW, Chen CH, Tsai MT, Formulation of two-layer dissolving polymeric microneedle patches for insulin transdermal delivery in diabetic mice, J. Biomed. Mater. Res. A, 105 (2017) 84–93. [DOI] [PubMed] [Google Scholar]
- [216].Liu D, Yu B, Jiang G, Yu W, Zhang Y, Xu B, Fabrication of composite microneedles integrated with insulin-loaded CaCO3 microparticles and PVP for transdermal delivery in diabetic rats, Mater. Sci. Eng. C, 90 (2018) 180–188. [DOI] [PubMed] [Google Scholar]
- [217].Yang H, Kim S, Huh I, Kim S, Lahiji SF, Kim M, Jung H, Rapid implantation of dissolving microneedles on an electrospun pillar array, Biomaterials, 64 (2015) 70–77. [DOI] [PubMed] [Google Scholar]
- [218].Kim JD, Kim M, Yang H, Lee K, Jung H, Droplet-born air blowing: Novel dissolving microneedle fabrication, J. Control. Release, 170 (2013) 430–436. [DOI] [PubMed] [Google Scholar]
- [219].Garland MJ, Caffarel-Salvador E, Migalska K, Woolfson AD, Donnelly RF, Dissolving polymeric microneedle arrays for electrically assisted transdermal drug delivery, J. Control. Release, 159 (2012) 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Park J-H, Allen MG, Prausnitz MR, Polymer microneedles for controlled-release drug delivery, Pharm. Res, 23 (2006) 1008–1019. [DOI] [PubMed] [Google Scholar]
- [221].Zhang Y, Jiang G, Yu W, Liu D, Xu B, Microneedles fabricated from alginate and maltose for transdermal delivery of insulin on diabetic rats, Mater. Sci. Eng. C, 85 (2018) 18–26. [DOI] [PubMed] [Google Scholar]
- [222].Seong KY, Seo MS, Hwang DY, O’Cearbhaill ED, Sreenan S, Karp JM, Yang SY, A self-adherent, bullet-shaped microneedle patch for controlled transdermal delivery of insulin, J. Control. Release, 265 (2017) 48–56. [DOI] [PubMed] [Google Scholar]
- [223].Yang S, Wu F, Liu J, Fan G, Welsh W, Zhu H, Jin T, Phase-Transition Microneedle Patches for Efficient and Accurate Transdermal Delivery of Insulin, Adv. Funct. Mater, 25 (2015) 4633–4641. [Google Scholar]
- [224].Di J, Yao S, Ye Y, Cui Z, Yu J, Ghosh TK, Zhu Y, Gu Z, Stretch-triggered drug delivery from wearable elastomer films containing therapeutic depots, ACS Nano, 9 (2015) 9407–9415. [DOI] [PubMed] [Google Scholar]
- [225].Yu W, Jiang G, Liu D, Li L, Tong Z, Yao J, Kong X, Transdermal delivery of insulin with bioceramic composite microneedles fabricated by gelatin and hydroxyapatite, Mater. Sci. Eng. C, 73 (2017) 425–428. [DOI] [PubMed] [Google Scholar]
- [226].Yu W, Jiang G, Liu D, Li L, Chen H, Liu Y, Huang Q, Tong Z, Yao J, Kong X, Fabrication of biodegradable composite microneedles based on calcium sulfate and gelatin for transdermal delivery of insulin, Mater. Sci. Eng. C, 71 (2017) 725–734. [DOI] [PubMed] [Google Scholar]
- [227].Wu Q, Wang L, Yu H, Wang J, Chen Z, Organization of glucose-responsive systems and their properties, Chem. Rev, 111 (2011) 7855–7875. [DOI] [PubMed] [Google Scholar]
- [228].Yu J, Zhang Y, Bomba H, Gu Z, Stimuli-responsive delivery of therapeutics for diabetes treatment, Bioeng. Transl. Med, 1 (2016) 323–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Lu Y, Aimetti AA, Langer R, Gu Z, Bioresponsive materials, Nat. Rev. Mater, 2 (2017) 16075. [Google Scholar]
- [230].Sun W, Hu Q, Ji W, Wright G, Gu Z, Leveraging physiology for precision drug delivery, Physiol. Rev, 97 (2016) 189–225. [Google Scholar]
- [231].Yu J, Zhang Y, Kahkoska AR, Gu Z, Bioresponsive transcutaneous patches, Curr. Opin. Biotechnol, 48 (2017) 28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Ma G, Wu C, Microneedle, bio-microneedle and bio-inspired microneedle: A review, J. Control. Release, 251 (2017) 11–23. [DOI] [PubMed] [Google Scholar]
- [233].Chen G, Yu J, Gu Z, Glucose-Responsive Microneedle Patches for Diabetes Treatment, J. Diabetes Sci. Technol, (2018) 1932296818778607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Yu J, Zhang Y, Ye Y, DiSanto R, Sun W, Ranson D, Ligler FS, Buse JB, Gu Z, Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery, Proc. Natl. Acad. Sci. U. S. A, 112 (2015) 8260–8265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Bankar SB, Bule MV, Singhal RS, Ananthanarayan L, Glucose oxidase-an overview, Biotechnol. Adv, 27 (2009) 489–501. [DOI] [PubMed] [Google Scholar]
- [236].Yu J, Zhang Y, Hu X, Wright G, Gu Z, Hypoxia-sensitive materials for biomedical applications, Ann. Biomed. Eng, 44 (2016) 1931–1945. [DOI] [PubMed] [Google Scholar]
- [237].Wang J, Zhang Y, Archibong E, Ligler FS, Gu Z, Leveraging H2O2 levels for biomedical applications, Adv. Biosys, 1 (2017) 1700084. [DOI] [PubMed] [Google Scholar]
- [238].Hu X, Yu J, Qian C, Lu Y, Kahkoska AR, Xie Z, Jing X, Buse JB, Gu Z, H2O2-responsive vesicles integrated with transcutaneous patches for glucose-mediated insulin delivery, ACS Nano, 11 (2017) 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Tong Z, Zhou J, Zhong J, Tang Q, Lei Z, Luo H, Ma P, Liu X, Glucose-and H2O2-responsive polymeric vesicles integrated with microneedle patches for glucose-sensitive transcutaneous delivery of insulin in diabetic rats, ACS Appl. Mater. Interfaces, 10 (2018)20014–20024. [DOI] [PubMed] [Google Scholar]
- [240].Xu B, Jiang G, Yu W, Liu D, Zhang Y, Zhou J, Sun S, Liu Y, H2O2-Responsive mesoporous silica nanoparticles integrated with microneedle patches for the glucose-monitored transdermal delivery of insulin, J. Mater. Chem. B, 5 (2017) 8200–8208. [DOI] [PubMed] [Google Scholar]
- [241].Xu B, Cao Q, Zhang Y, Yu W, Zhu J, Liu D, Jiang G, Microneedles integrated with ZnO quantum dots capped mesoporous bioactive glasses for glucose-mediated insulin delivery, CS Biomater. Sci. Eng, (2018). [DOI] [PubMed] [Google Scholar]
- [242].Yu J, Qian C, Zhang Y, Cui Z, Zhu Y, Shen Q, Ligler FS, Buse JB, Gu Z, Hypoxia and H2O2 dual-sensitive vesicles for enhanced glucose-responsive insulin delivery, Nano Lett., 17 (2017) 733–739. [DOI] [PubMed] [Google Scholar]
- [243].Wang J, Ye Y, Yu J, Kahkoska AR, Zhang X, Wang C, Sun W, Corder RD, Chen Z, Khan SA, Buse JB, Gu Z, Core-shell microneedle gel for self-regulated insulin delivery, ACS Nano, 12 (2018) 2466–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Zhang Y, Wang J, Yu J, Wen D, Kahkoska AR, Lu Y, Zhang X, Buse JB, Gu Z, Bioresponsive microneedles with a sheath structure for H2O2 and pH cascade-triggered insulin delivery, Small, (2018) 1704181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Ye Y, Yu J, Wang C, Nguyen NY, Walker GM, Buse JB, Gu Z, Microneedles integrated with pancreatic cells and synthetic glucose-signal amplifiers for smart insulin delivery, Adv. Mater, 28 (2016) 3115–3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Lee S, McAuliffe DJ, Mulholland SE, Doukas AG, Photomechanical transdermal delivery of insulin in vivo, Lasers Surg. Med, 28 (2001) 282–285. [DOI] [PubMed] [Google Scholar]
- [247].Andrews S, Lee JW, Choi S-O, Prausnitz MR, Transdermal insulin delivery using microdermabrasion, Pharm. Res, 28 (2011) 2110–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Giannos S, Skin microporation: Strategies to enhance and expand transdermal drug delivery, J. Drug Deliv. Sci. Technol, 24 (2014) 293–299. [Google Scholar]
- [249].Chen X, Current and future technological advances in transdermal gene delivery, Adv. Drug Del. Rev, (2017). [DOI] [PubMed] [Google Scholar]
- [250].Smith A, Zerkel K, Roerig P-L, Mills S, Humphries C, Durland R, Spratlin V, Transdermal delivery of insulin in patients with type 1 diabetes, in: Diabetes, Virginia, 2008, pp. A88. [Google Scholar]
- [251].Engwerda EE, Tack CJ, de Galan BE, Pharmacokinetic and pharmacodynamic variability of insulin when administered by jet injection, J. Diabetes Sci. Technol, 11 (2017) 947–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Engwerda EE, Abbink EJ, Tack CJ, De Galan BE, Improved pharmacokinetic and pharmacodynamic profile of rapid-acting insulin using needle-free jet injection technology, Diabetes Care, 34 (2011) 1804–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Pettis RJ, Hirsch L, Kapitza C, Nosek L, Hövelmann U, Kurth H-J, Sutter DE, Harvey NG, Heinemann L , Microneedle-based intradermal versus subcutaneous administration of regular human insulin or insulin lispro: pharmacokinetics and postprandial glycemic excursions in patients with type 1 diabetes, Diabetes Technol. Ther, 13 (2011) 443–450. [DOI] [PubMed] [Google Scholar]
- [254].Norman JJ, Brown MR, Raviele NA, Prausnitz MR, Felner EI, Faster pharmacokinetics and increased patient acceptance of intradermal insulin delivery using a single hollow microneedle in children and adolescents with type 1 diabetes, Pediatr. Diabetes, 14 (2013) 459–465. [DOI] [PubMed] [Google Scholar]


