Abstract
Purpose
Integrating patient-reported outcomes (PROs) into clinical practice is an increasingly promising strategy for improving patients’ symptoms, communication, and clinical outcomes. The objective of the current study was to assess the feasibility, acceptability, and perceived effectiveness of a mobile health intervention that was designed to collect PROs and activity data as a measure of health status.
Patients and Methods
This work was a pilot intervention with 10 patients with gynecologic cancers who received palliative chemotherapy. The HOPE (Helping Our Patients Excel) study used wearable accelerometers to assess physical activity and the Beiwe research platform to collect PROs, stratify patient responses by risk, provide tailored symptom management, and notify patients and clinicians of high-risk symptoms. Feasibility and acceptability were assessed through enrollment and adherence rates, and perceived effectiveness was evaluated by patients and oncologists at study completion.
Results
The approach-to-consent rate was 100%, and participants were 90% and 70% adherent to the wearable accelerometers and smartphone surveys, respectively. Participants’ mean daily step count was 3,973 (standard deviation [SD], 2,305 steps) and increased from week 1 (mean, 3,520 steps; SD, 1,937 steps) to week 3 (mean, 4,136 steps; SD, 1,578 steps). Active monitoring of participants’ heart rates, daily steps, and PROs throughout the study identified anomalies in participants’ behavior patterns that suggested poor health for two patients (20%). Patients and clinicians indicated that the intervention improved physical activity, communication, and symptom management.
Conclusion
A mobile health intervention that collects PROs and activity data as a measure of health status is feasible, acceptable, and was perceived to be effective in improving symptom management in patients with advanced gynecologic cancers. A larger, multisite, randomized clinical trial to assess the efficacy of the HOPE intervention on patients’ symptoms, health-related quality of life, clinical outcomes, and health care use is warranted.
INTRODUCTION
The goal of palliative chemotherapy is to reduce symptoms and extend survival. Yet oncologists’ current methods of monitoring patients’ symptoms and functional status are underdeveloped. Clinicians consistently miss more than one half of patients’ symptoms,1 even when patients are enrolled in clinical trials that mandate the collection of treatment toxicities.2-4 Similarly, oncologists’ estimates of performance status are only moderately correlated with patients’ reports or other oncologists’ assessments,5-9 and may be limited by cognitive shortcuts—for example, using chronologic age as a proxy for functional status.10 Nevertheless, patients’ performance status and symptoms remain key factors that influence clinical trial eligibility, palliative chemotherapy decision making, and hospice referrals.11-13
Integrating patient-reported outcomes (PROs) into clinical practice is an increasingly promising strategy for improving symptoms, communication, and clinical outcomes.14,15 Basch et al15,16 recently demonstrated that the use of a Web-based system to assess PROs, combined with e-mail alerts to nurses, resulted in better health-related quality of life, fewer emergency department (ED) visits and hospitalizations, and improved overall survival compared with usual care.
However, this study and others have predominantly assessed PROs during ambulatory clinic visits,17 when patients may have already recovered from their last chemotherapy. A key limitation to this approach is that it relies on patients’ retrospective recall of events, which may be biased. Moments between visits—when patients are at the highest likelihood of experiencing treatment toxicities—may contain more meaningful data. Yet few studies have performed rapid, in situ assessments of patients’ symptoms and performance status in their daily lives.18,19
Smartphones offer an accessible, low-cost method for conducting in situ measures of patients’ symptoms and activity levels using ecologic momentary assessment and passively gathered sensor data.20-22 For example, smartphone applications can be used to monitor patient symptoms and reports of treatment toxicities in naturalistic settings when patient recall may be most accurate.23 It is also feasible to infer objective measures of behavior by analyzing passively gathered sensor data, such as accelerometry, GPS, screen on/off logs, and call logs,22 as well as data that were gathered by commercially available wearables; for example, Fitbits.18,19 Of importance, 77% of adults in the United States now own a smartphone, and ownership rates are rising rapidly in lower-income and older Americans.24
In this work, we describe the development, refinement, and pilot testing of a mobile health intervention, entitled HOPE: Helping Our Patients Excel, in patients with gynecologic cancers who received palliative chemotherapy. The HOPE study was designed to assess patient symptoms using the PRO version of the Common Terminology for Adverse Events (PRO-CTCAE),25 stratify responses by risk, provide tailored feedback to patients with low-risk symptoms, and notify both patients and clinicians of the presence of high-risk symptoms.
In brief, we used the Beiwe research platform,21 a digital phenotyping platform that monitors patients’ behavior passively using their own smartphones’ sensors and that can remotely deploy smartphone-based surveys, by adding a function to provide tailored responses to patients’ survey responses. We have previously defined digital phenotyping as the moment-by-moment quantification of the individual-level human phenotype in situ using data from personal digital devices, in particular, smartphones.21 We further paired this platform with two accelerometers—the Fitbit Zip and Fitbit Charge 2 (Fitbit, San Francisco, CA) —to monitor patients’ activity levels as a proxy for performance status. The goal of the current study was to assess the feasibility, acceptability, and perceived effectiveness of the HOPE intervention among patients and their clinicians.
PATIENTS AND METHODS
Study Setting, Participants, and Enrollment
We conducted a single-arm pilot study in the gynecologic oncology clinic at the Dana-Farber Cancer Institute in Boston, MA, between April 12 and June 23, 2017. Eligibility criteria included the following: diagnosis of recurrent, incurable gynecologic cancer; intent to receive chemotherapy at the Dana-Farber Cancer Institute; older than age 20 years; access to a smartphone (Android or iOS); and willingness to use the Beiwe research platform and wear two commercially available accelerometry devices for at least 30 days. Exclusion criteria included the following: participation in a clinical trial that required structured symptom or toxicity reporting; an inability to provide informed consent in English; and cognitive, visual, or physical impairments that might preclude study participation, as evaluated by the research study staff or oncology provider.
On the basis of the ORBIT model of behavioral treatment development guidelines,26 we planned to sample a diverse group of patients, including at least three patients age ≥ 65 years, three from racial/ethnic minority groups, and two who did not communicate more than weekly with text messaging as patients with less computer experience are less receptive to electronic self-reporting.27
Patients were invited by their oncology providers to enroll in the study. All oncology providers agreed to approach, recruit, and obtain consent from eligible patients with research staff and participate in debriefing interviews after study completion. Participants were specifically informed about the data that would be collected from their phone, the methods used to secure and encrypt these data, and the information that would be shared with their oncology providers. All participants provided written informed consent for all study procedures. Participants could keep the wearable accelerometers and an external battery charger that was provided after study completion; no additional financial incentives were offered. The study was approved by the institutional review boards of the Dana-Farber/Harvard Cancer Center and the Harvard TH Chan School of Public Health.
Intervention
The HOPE intervention was a mobile health intervention that consisted of both wearable accelerometers and the Beiwe research platform. Participants were asked to wear the Fitbit Zip on their waist and the Fitbit Charge 2 on their nondominant wrist during all waking hours,28 and to synchronize both devices daily. Each Fitbit was linked to the Fitabase analytics system (Small Steps Laboratories, San Diego, CA), which enabled the investigators to remotely monitor patients’ physical activity.
The Beiwe research platform was developed by the Onnela Laboratory at the Harvard TH Chan School of Public Health with funding from the National Institutes of Health.20,21 It is an open-source software program that features a study portal, a smartphone application, and data modeling and analysis tools that can be used to collect and analyze active and passive raw data from smartphones. Active data require patient participation for generation—for example, survey responses. Passive data are generated without any direct participant involvement—for example, GPS or accelerometry—and analyzed by automated data modeling methods developed by the Onnela Laboratory.29 Beiwe protects identifying data through hashing, and all data are encrypted while on the device, in transit, and stored on the server. All data are collected and stored in a Health Insurance Portability and Accountability Act–compliant manner. Once collected by the phone, raw data are encrypted and securely transmitted over Wi-Fi to an instance of Amazon S3 for storage and later retrieval for analysis.20
In the current study, we added and implemented branching logic functionality in Beiwe to enable risk stratification of participants’ responses to survey items and the delivery of tailored feedback on the basis of the severity of patient-reported symptoms using an algorithm that was developed by the study team. This also helped alleviate survey fatigue.
Active data that were collected by Beiwe included daily assessments of patients’ quality of life and physical function using two single items from PROMIS Global-1030,31 and assessments of symptoms using two PRO-CTCAE items each day.25 PRO-CTCAE questions were paired on the basis of related symptoms—for example, nausea and vomiting, diarrhea, and dizziness—selected from a list of 10 symptoms that patients with gynecologic cancers identified as salient, including abdominal pain, nausea, vomiting, constipation, diarrhea, dizziness, peripheral neuropathy, fatigue, anxiety, and depression.32 Each of the 10 PRO-CTCAE symptoms were asked about at least once per week. PRO-CTCAE items use conditional branching for adverse events that contain multiple attributes. For example, if a participant reports a symptom, she is asked to quantify the severity and extent to which it interfered with daily activities. If she does not report a symptom, these items are skipped (Fig 1).
Fig 1.
Symptom classification and response in the HOPE (Helping Our Patients Excel) pilot intervention. PRO-CTCAE, patient-reported outcomes version of the Common Terminology for Adverse Events.
When participants reported low-risk toxicities (ie, grade 1 or 2), they received evidence-based symptom management advice within the application and a list of potential questions to ask their clinicians at the next visit.33 If participants reported high-risk symptoms (ie, grade 3 or 4), they received the following message: “Please contact your clinician immediately. Your [symptom] sounds worrisome and requires medical attention” (Fig 2). The application also included a call my clinician button that triggered the phone to call the clinic. This number transfers to the page operator after hours, so that there was 24-hour coverage using the clinic’s existing infrastructure. Passive data that were collected from Beiwe included spatial location using GPS data as a proxy for the amount of time spent away from home, accelerometer data, Wi-Fi and Bluetooth signals, screen on/off logs, and all screen touch events.20,22,29
Fig 2.
Symptom management advice displayed on participants’ smartphones. (A and B) Android smartphone screens displaying the patient-reported outcomes version of the Common Terminology for Adverse Events (PRO-CTCAE) question assessing nausea (A) and delivering tailored symptom management for a patient with low-risk symptoms (B). (C and D) iPhone smartphone screens displaying the PRO-CTCAE question assessing abdominal pain (C) and advice to contact the clinician for high-risk symptoms (D).
Monitoring of Events
Throughout the study, we monitored participants’ heart rates and mean daily step counts with the Fitbit Charge 2 using Fitabase. We hypothesized that, if the Fitbit Charge 2 registered an active heart rate but a low step count (eg, ≤ 1,000 steps in a day), this might signify poor health, whereas an undetected heart rate and step count might signify nonadherence to the devices. In either case, research staff called participants to investigate anomalies in behavioral patterns. If participants reported severe symptoms when contacted, study staff advised participants to call their clinical team and notified both the primary clinician and program nurse of their symptoms.
Active data that were collected by Beiwe were monitored daily. If participants reported high-risk symptoms and were notified to call their clinician, research staff also called the participant and notified the participant’s clinical team via e-mail. Passive data that were collected by Beiwe were not analyzed until the end of the study.
Measures
Demographics.
Basic sociodemographic information was collected from participants at enrollment (eg, age, marital status, household size, race, education, income, employment, and religion). Clinical information—for example, primary disease site, time since diagnosis, number of prior lines of chemotherapy, and Eastern Cooperative Oncology Group status—was abstracted from medical charts. We also assessed participants’ health literacy, numeracy, and comfort with technology.34-36
Feasibility.
Feasibility was defined as ≥ 60% enrollment rate among eligible patients who were approached, ≥ 70% adherence to daily smartphone surveys ≥ 4 days per week, and ≥ 80% adherence to the Fitbits ≥ 4 days per week.
Acceptability and Perceived Effectiveness.
Upon study completion, participants were asked to rate the following two questions: “Participating in this study placed a substantial burden on me”; and “I wish I had not agreed to participate in this study.” Response options included “strongly disagree,” “disagree,” “agree,” or “strongly agree.” In addition, participants were asked whether they found the study to be a positive experience and if they would recommend the study to other patients. Both patients and clinicians participated in qualitative exit interviews to elicit feedback on their experiences. Study data that were collected outside of the application and Fitbits were managed using Research Electronic Data Capture electronic data capture tools (hosted at Partners Healthcare).37 Research Electronic Data Capture is a secure, Web-based application that was designed to support data capture for research studies, providing an intuitive interface for validated data entry, audit trails for tracking data manipulation and export procedures, automated export procedures for seamless data downloads to common statistical packages, and procedures for importing data from external sources.
RESULTS
We identified 18 potentially eligible patients during the enrollment period. Of these, eight were deemed inappropriate by their treating oncologist for the following reasons: “bad timing for the patient” (n = 4), “too distressed” (n = 3), and “ineligible as a result of language barriers” (n = 1). Among the 10 patients who were approached to participate, all provided consent and enrolled in the study.
As displayed in Table 1, mean age was 60 years (standard deviation [SD], 11), eight participants were married, and four identified as black or other, nonwhite race. Most had high rates of health literacy and numeracy, and 80% reported feeling somewhat or very comfortable using smartphone applications. Participants reported a mean of three symptoms (SD, 2) at baseline, including fatigue (n = 8), neuropathy (n = 5), abdominal pain (n = 4), and constipation (n = 4). Mean physician-assessed Eastern Cooperative Oncology Group performance status was 1 (SD, 0.66).
Table 1.
Participant Characteristics

Study participants were 70% adherent to smartphone surveys and 90% adherent to wearable accelerometers. Seven participants answered daily smartphone surveys ≥ 4 days per week throughout the study. Among the remaining participants, one did not answer any questions, one had an older version of the Android operating system (4.3, 2012) that was incompatible with Beiwe, and one answered only three surveys in the final week. Nine participants wore both Fitbits daily throughout the study period, whereas one did not wear either. Participants’ mean daily step count over the study measured with the Fitbit Charge 2 was 3,973, (SD, 2,305 steps) and increased from week 1 (mean, 3,520 steps; SD, 1,937 steps) to week 3 (mean, 4,136 steps; SD, 1,578 steps). When participants wore both devices, the Fitbit Charge 2 recorded more steps (mean difference, 445 steps; SD, 1,296 steps).
We monitored participants’ heart rates and mean daily steps throughout the study to identify anomalies in participants’ behavior patterns that might signify poor health. Two participants registered an active heart rate, but ≤ 1,000 steps in a day. When contacted, one participant reported that she planned to go to the ED for severe nausea and vomiting. Research staff contacted her clinical team and her symptoms were addressed over the phone without an ED visit. As shown in Figure 3, her mean daily steps increased to baseline the next day and she resumed normal activities. The second participant’s step count dropped after she was hospitalized for a medical procedure.
Fig 3.
Change in step and circadian behavior patterns in the setting of grade 3 nausea and vomiting. (A) Normal days. On most of the days that were recorded during the study, this participant’s step count followed a consistent circadian behavior pattern—minimal activity during daytime hours, followed by a long walk each evening. (B) Day with severe symptoms. On a day that the patient experienced severe nausea and vomiting, her circadian behavior pattern was disrupted. Her step count dropped to nearly zero during the day, and she did not take her usual evening walk. After observing this change in behavior and receiving the patient’s self-report of severe symptoms on the smartphone application, study staff contacted the patient and her clinical team instructed her on how to manage her symptoms at home. Red arrows denote time periods during which there was a sharp reduction in the patient’s steps compared with a normal day as a result of her nausea and vomiting.
Active monitoring of survey data also identified patients with high-risk symptoms. During the study period, two participants reported grade 3 symptoms that triggered an in-application alert to contact their medical teams. The first participant reported that she had completed the survey while waiting to see a physician, and her symptoms were addressed during the visit. The second participant with severe nausea and vomiting, described above, was first identified by a decrease in her step count.
Among the eight participants who used the Beiwe application, all received symptom management advice via the app (mean number of symptoms, 10; SD, 9) during the 30-day study. The Beiwe application also collected passive data from nine participants. This enabled us to estimate each participant’s time spent away from home, mean daily steps, mobility, and sleep relative to clinically meaningful events (Fig 4), which provided a quantitative measure of participants’ lived experience after chemotherapy or before an urgent clinical encounter.
Fig 4.
Passive biometric data reveals a quantitative estimate of participants’ lived experiences. Using smartphone and accelerometry data, a patient’s behavioral patterns, including their daily step count and time spent away from home, can be linked to clinical events, such as chemotherapy, urgent clinical encounters, and emergency department visits. For example, this patient took few steps and spent most of her time at home on the day of an urgent clinical encounter and for three days before an emergency department visit.
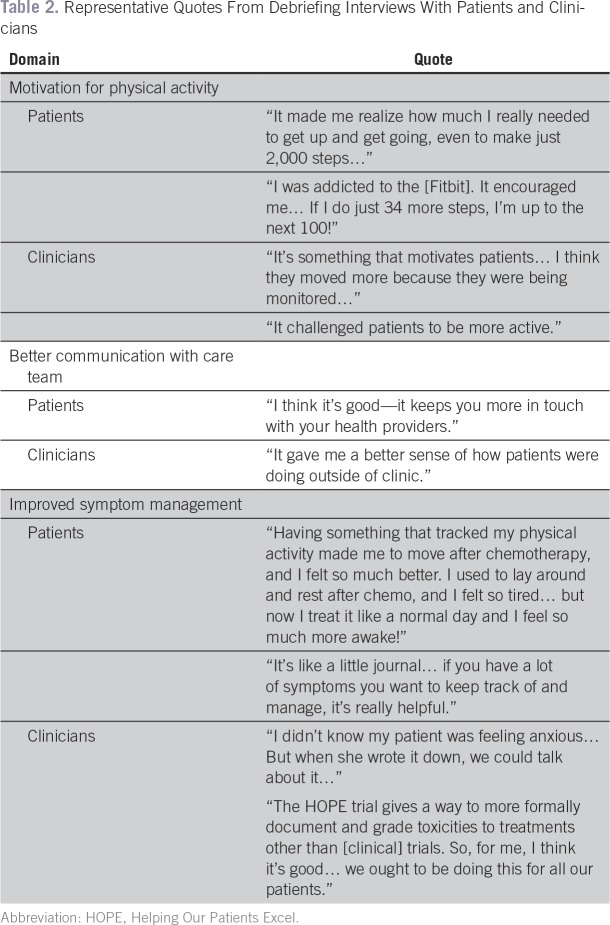
Upon study completion, nine participants disagreed or strongly disagreed with the statement, “Participating in this study placed a substantial burden on me.” Only one participant agreed with the statement, “I wish I had not agreed to participate in this study,” citing her regret about her nonadherence to the study. Nine participants reported that they “would recommend the application to a friend going through treatment.” Both participants and clinicians indicated that participating in the intervention increased participants’ physical activity, communication with the care team, and symptom management (Table 2).
Table 2.
Representative Quotes From Debriefing Interviews With Patients and Clinicians
DISCUSSION
We developed and pilot tested an intervention to assess the symptoms of patients with cancer in situ, address low-risk toxicities via a smartphone application, and alert patients and clinicians when patients report high-risk symptoms that require additional medical attention. In the current study, we demonstrated that the HOPE intervention is feasible, acceptable, and perceived to be effective by patients with advanced gynecologic cancers and their oncologists.
HOPE is innovative in several important ways. First, HOPE collects PROs using patients’ own smartphones within the context of their daily lives when they are at their greatest risk of experiencing treatment toxicities, which enables patients and clinicians to identify and manage symptoms as they occur. This also allows for more accurate ascertainment of PROs as it minimizes recall bias. Second, the HOPE intervention empowers patients to manage symptoms at home using evidence-based treatment algorithms, and notifies clinicians if patients report high-risk symptoms. Third, it allows for active the monitoring of passive biometric data (eg, mean daily steps and changes in circadian behavior patterns), which enabled us to detect unreported, yet clinically significant events, prevent at least one ED visit, and estimate the proportion of time spent at home versus out of the house for all participants, independent of their adherence with study activities.
Use of passive data collection from participants’ smartphones is particularly important because there is considerable evidence to suggest that simply giving people wearable devices or access to mobile health applications alone does not lead to sustained engagement.38 A survey of more than 6,000 individuals demonstrated that one third of those who purchase a wearable device stop using it within 6 months.38 Moreover, mobile health interventions have alarmingly high rates of attrition. For example, in 2015, Apple funded a prospective observational study of a mobile health application that was designed to help individuals track their asthma symptoms, receive medication reminders, and share their data with their doctors. Although 40,653 users downloaded the application, only 7,593 ultimately enrolled in the study, and 6 months later, only 175 users were still using the application.39 Of importance, this application required participants to enter active data about their health, and the authors recommended the use of passive smartphone data for future research studies. In our next study, we will observe 100 participants over a 6-month period to examine the sustainability of our intervention while also developing an automated algorithm designed to predict clinically significant events on the basis of passive data that were collected from participants’ smartphones.
We encountered several challenges in this pilot. First, one of our participants did not engage in any of the study requirements. Despite this, we gleaned valuable data from the passive data that were collected from her smartphone—for example, time spent outside of the house on the basis of GPS data and mean steps on the basis of the accelerometer, which could be used to infer performance status. Second, one participant was unable to transmit data as she had a smartphone with an older version of the Android operating system that was incompatible with the Beiwe system. Third, although one of the participants received an in-application notification to contact her clinician, she did not. In response, we have changed the alerts to read, “Contact your clinician immediately. Your [symptoms] require medical attention,” and will assess whether participants respond to this in future studies. Finally, although we collected passive smartphone data in situ, we were unable to analyze and act upon these results in real time in this small sample.
Our study had some limitations. Ours was a pilot study that was conducted at a single site, and the study duration was short. Although we enrolled a racially diverse sample of patients who were older, most were well educated and had high health literacy scores. We will next assess this intervention in more diverse populations over a longer duration at several National Cancer Institute Community Oncology Research Program sites. Finally, this pilot study did not have a control arm, and whereas patients and oncologists rated HOPE favorably, this pilot does not establish efficacy. A randomized controlled trial of the HOPE intervention is needed.
In conclusion, the HOPE intervention is a feasible and acceptable mobile health intervention that collects PROs in situ to improve both symptom management and the detection of high-risk clinical events, with perceived effectiveness for increasing physical activity, communication with the care team, and symptom detection in patients with advanced gynecologic cancers and by their oncologists. This successful pilot trial provides support for conducting a larger, multisite, randomized clinical trial to assess the efficacy of the HOPE intervention on patients’ symptoms, health-related quality of life, clinical outcomes, and health care use.
ACKNOWLEDGMENT
We acknowledge the study participants for their contributions to this project, and Elizabeth Schrier for assistance with the formatting of Figure 3.
Footnotes
Supported by National Cancer Institute (NCI) Grant No. K07-CA166210, a National Palliative Care Research Center Pilot/Exploratory Project Support Grant, and a Dana-Farber Cancer Institute Department of Medical Oncology Grant. P.S., K.C., and J.-P.O. were supported by National Institute of Mental Health Grant No. 1DP2-MH103909-01. N.L.K. was supported by NCI Grant No. K24-CA181510.
The funding organizations had no role in the preparation, review, or approval of the manuscript.
Clinical Trial Information: NCT03022032.
AUTHOR CONTRIBUTIONS
Conception and design: Alexi A. Wright, Stephanie Schonholz, Nancy L. Keating, Jukka-Pekka Onnela
Financial support: Alexi A. Wright, Jukka-Pekka Onnela
Administrative support: Alexi A. Wright, Kenzie Carlson, Jukka-Pekka Onnela
Provision of study materials or patients: Alexi A. Wright
Collection and assembly of data: Alexi A. Wright, Nikita Raman, Kenzie Carlson, Jukka-Pekka Onnela
Data analysis and interpretation: Alexi A. Wright, Nikita Raman, Patrick Staples, Stephanie Schonholz, Angel Cronin, Nancy L. Keating, Jukka-Pekka Onnela
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Alexi A. Wright
No relationship to disclose
Nikita Raman
No relationship to disclose
Patrick Staples
No relationship to disclose
Stephanie Schonholz
No relationship to disclose
Angel Cronin
No relationship to disclose
Kenzie Carlson
Stock and Other Ownership Interests: Accuray
Nancy L. Keating
No relationship to disclose
Jukka-Pekka Onnela
No relationship to disclose
REFERENCES
- 1.Pakhomov SV, Jacobsen SJ, Chute CG, et al. Agreement between patient-reported symptoms and their documentation in the medical record. Am J Manag Care. 2008;14:530–539. [PMC free article] [PubMed] [Google Scholar]
- 2.Fromme EK, Eilers KM, Mori M, et al. How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the Quality-of-Life Questionnaire C30. J Clin Oncol. 2004;22:3485–3490. doi: 10.1200/JCO.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 3.Di Maio M, Gallo C, Leighl NB, et al. Symptomatic toxicities experienced during anticancer treatment: Agreement between patient and physician reporting in three randomized trials. J Clin Oncol. 2015;33:910–915. doi: 10.1200/JCO.2014.57.9334. [DOI] [PubMed] [Google Scholar]
- 4.Montemurro F, Mittica G, Cagnazzo C, et al. Self-evaluation of adjuvant chemotherapy-related adverse effects by patients with breast cancer. JAMA Oncol. 2016;2:445–452. doi: 10.1001/jamaoncol.2015.4720. [DOI] [PubMed] [Google Scholar]
- 5.Sørensen JB, Klee M, Palshof T, et al. Performance status assessment in cancer patients. An inter-observer variability study. Br J Cancer. 1993;67:773–775. doi: 10.1038/bjc.1993.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutchinson TA, Boyd NF, Feinstein AR, et al. Scientific problems in clinical scales, as demonstrated in the Karnofsky index of performance status. J Chronic Dis. 1979;32:661–666. doi: 10.1016/0021-9681(79)90096-1. [DOI] [PubMed] [Google Scholar]
- 7.Blagden SP, Charman SC, Sharples LD, et al. Performance status score: Do patients and their oncologists agree? Br J Cancer. 2003;89:1022–1027. doi: 10.1038/sj.bjc.6601231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dajczman E, Kasymjanova G, Kreisman H, et al. Should patient-rated performance status affect treatment decisions in advanced lung cancer? J Thorac Oncol. 2008;3:1133–1136. doi: 10.1097/JTO.0b013e318186a272. [DOI] [PubMed] [Google Scholar]
- 9.Ando M, Ando Y, Hasegawa Y, et al. Prognostic value of performance status assessed by patients themselves, nurses, and oncologists in advanced non–small-cell lung cancer. Br J Cancer. 2001;85:1634–1639. doi: 10.1054/bjoc.2001.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broderick JM, Hussey J, Kennedy MJ, et al. Patients over 65 years are assigned lower ECOG PS scores than younger patients, although objectively measured physical activity is no different. J Geriatr Oncol. 2014;5:49–56. doi: 10.1016/j.jgo.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 12.Schnipper LE, Smith TJ, Raghavan D, et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: The top five list for oncology. J Clin Oncol. 2012;30:1715–1724. doi: 10.1200/JCO.2012.42.8375. [DOI] [PubMed] [Google Scholar]
- 13.Kumar P, Wright AA, Hatfield LA, et al. Family perspectives on hospice care experiences of patients with cancer. J Clin Oncol. 2017;35:432–439. doi: 10.1200/JCO.2016.68.9257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Detmar SB, Muller MJ, Schornagel JH, et al. Health-related quality-of-life assessments and patient-physician communication: A randomized controlled trial. JAMA. 2002;288:3027–3034. doi: 10.1001/jama.288.23.3027. [DOI] [PubMed] [Google Scholar]
- 15.Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: A randomized controlled trial. J Clin Oncol. 2016;34:557–565. doi: 10.1200/JCO.2015.63.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318:197–198. doi: 10.1001/jama.2017.7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basch E, Artz D, Dulko D, et al. Patient online self-reporting of toxicity symptoms during chemotherapy. J Clin Oncol. 2005;23:3552–3561. doi: 10.1200/JCO.2005.04.275. [DOI] [PubMed] [Google Scholar]
- 18.Ohri N, Kabarriti R, Bodner WR, et al. Continuous activity monitoring during concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2017;97:1061–1065. doi: 10.1016/j.ijrobp.2016.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett AV, Reeve BB, Basch EM, et al. Evaluation of pedometry as a patient-centered outcome in patients undergoing hematopoietic cell transplant (HCT): A comparison of pedometry and patient reports of symptoms, health, and quality of life. Qual Life Res. 2016;25:535–546. doi: 10.1007/s11136-015-1179-0. [DOI] [PubMed] [Google Scholar]
- 20.Torous J, Onnela JP, Keshavan M. New dimensions and new tools to realize the potential of RDoC: Digital phenotyping via smartphones and connected devices. Transl Psychiatry. 2017;7:e1053. doi: 10.1038/tp.2017.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torous J, Kiang MV, Lorme J, et al. New tools for new research in psychiatry: A scalable and customizable platform to empower data-driven smartphone research. JMIR Ment Health. 2016;3:e16. doi: 10.2196/mental.5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Staples P, Torous J, Barnett I, et al. A comparison of passive and active estimates of sleep in a cohort with schizophrenia. NPJ Schizophr. 2017;3:37. doi: 10.1038/s41537-017-0038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torous J, Staples P, Shanahan M, et al. Utilizing a personal smartphone custom app to assess the Patient Health Questionnaire-9 (PHQ-9) depressive symptoms in patients with major depressive disorder. JMIR Ment Health. 2015;2:e8. doi: 10.2196/mental.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pew Research Center Record shares of Americans now own smartphones, have home broadband. http://www.pewresearch.org/fact-tank/2017/01/12/evolution-of-technology/
- 25.Dueck AC, Mendoza TR, Mitchell SA, et al. Validity and reliability of the US National Cancer Institute’s patient-reported outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) JAMA Oncol. 2015;1:1051–1059. doi: 10.1001/jamaoncol.2015.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Czajkowski SM, Powell LH, Adler N, et al. From ideas to efficacy: The ORBIT model for developing behavioral treatments for chronic diseases. Health Psychol. 2015;34:971–982. doi: 10.1037/hea0000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basch EM, Thaler HT, Shi W, et al. Use of information resources by patients with cancer and their companions. Cancer. 2004;100:2476–2483. doi: 10.1002/cncr.20261. [DOI] [PubMed] [Google Scholar]
- 28.Case MA, Burwick HA, Volpp KG, et al. Accuracy of smartphone applications and wearable devices for tracking physical activity data. JAMA. 2015;313:625–626. doi: 10.1001/jama.2014.17841. [DOI] [PubMed] [Google Scholar]
- 29.Barnett I, Onnela J-P. Inferring mobility measures from GPS traces with missing data. 2016 doi: 10.1093/biostatistics/kxy059. https://arxiv.org/pdf/1606.06328.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hays RD, Bjorner JB, Revicki DA, et al. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res. 2009;18:873–880. doi: 10.1007/s11136-009-9496-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63:1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hay CM, Courtney-Brooks M, Lefkowits C, et al. Symptom management in women with recurrent ovarian cancer: Do patients and clinicians agree on what symptoms are most important? Gynecol Oncol. 2016;143:367–370. doi: 10.1016/j.ygyno.2016.08.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Cancer Institute Chemotherapy side effects series. https://www.cancer.gov/publications/patient-education/chemo-side-effects
- 34.Wallace LS, Rogers ES, Roskos SE, et al. Brief report: Screening items to identify patients with limited health literacy skills. J Gen Intern Med. 2006;21:874–877. doi: 10.1111/j.1525-1497.2006.00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stagliano V, Wallace LS. Brief health literacy screening items predict newest vital sign scores. J Am Board Fam Med. 2013;26:558–565. doi: 10.3122/jabfm.2013.05.130096. [DOI] [PubMed] [Google Scholar]
- 36.Bakke E. A model and measure of mobile communication competence. Hum Commun Res. 2010;36:348–371. [Google Scholar]
- 37.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap): A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel MS, Asch DA, Volpp KG. Wearable devices as facilitators, not drivers, of health behavior change. JAMA. 2015;313:459–460. doi: 10.1001/jama.2014.14781. [DOI] [PubMed] [Google Scholar]
- 39.Chan YY, Wang P, Rogers L, et al. The Asthma Mobile Health Study, a large-scale clinical observational study using ResearchKit. Nat Biotechnol. 2017;35:354–362. doi: 10.1038/nbt.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]