Abstract
A large number of studies have attempted to use neuroimaging tools to aid in treatment prediction models for major depressive disorder (MDD). Most such studies have reported on only one dimension of function and prediction at a time. In this study, we used three different tasks across domains of function (emotion processing, reward anticipation, and cognitive control, plus resting state connectivity completed prior to start of medication to predict treatment response in 13-36 adults with MDD. For each experiment, adults with MDD were prescribed only label duloxetine (all experiments), whereas another subset were prescribed escitalopram. We used a KeyNet (both Task derived masks and Key intrinsic Network derived masks) approach to targeting brain systems in a specific match to tasks. The most robust predictors were (1) positive response to anger and (2) negative response to fear within relevant anger and fear TaskNets and Salience and Emotion KeyNet (3) cognitive control (correct rejections) within Inhibition TaskNet (negative) and Cognitive Control KeyNet (positive). Resting state analyses were most robust for Cognitive control Network (positive) and Salience and Emotion Network (negative). Results differed by whether an -fwhm or -acf (more conservative) adjustment for multiple comparisons was used. Together, these results implicate the importance of future studies with larger sample sizes, multidimensional predictive models, and the importance of using empirically derived masks for search areas.
Introduction
A rapidly growing literature implicates the use of neuroimaging markers in the prediction of response to standard treatments for those with mood and anxiety disorders (1–11). These studies have, by and large, highlighted the challenges in understanding probability of treatment response. For example, there are differences in task probes, relevance to individual patients, heterogeneity of depression, and specificity of prediction. In addition, these studies tend to have small sample sizes, variable analytic techniques, and different thresholding strategies. Within analytic techniques, one challenge in these studies is that there are four classes of studies, a priori region of interest (ROI) based, exploratory whole-brain based (WB), and prediction based upon regions identified as critical for task completion (e.g., goal-directed activity) (TaskNet) or between group (BetGroup) differences (i.e., regions implicated in psychiatric illness). As a result, a meta-analysis would fail to adequately represent the entirety of data prediction or variance in algorithms in these studies. These studies and other reviews have strongly suggested that the mechanisms in which people attain wellness are multifactorial and heterogeneous in nature (12). In a recent review, we reported on task-based fMRI studies of treatment prediction in mood disorders and highlighted the cognitive control network (CCN) and salience and emotion networks (SEN) as critical for predicting treatment response in mood disorders (3). In addition, we reviewed well-known regions and networks, such as the amygdala, insula, dorsal and dorsal anterior cingulate, but also less expected regions like occipital cortex and cerebellum that have been implicated in predicting treatment response. In light of that review, there is still a need to synthesize previous findings in order to provide a better framework for how to conceptualize brain-based prediction models of clinical outcomes, or neuroprediction. Do key region (KeyReg, BetGroup) or network (TaskNet, KeyNet) models best predict treatment response? More specifically, does a Reward network or a Salience and Emotion intrinsic network best represent nodes that are relevant for prospective prediction of treatment response? Moreover, does the task probe matter as much as the TaskNet or KeyNet?
More recently, investigations have evaluated the role of intrinsic network connectivity (e.g., KeyNet) in the prediction of treatment response in mood and related disorders (13–19). There are several advantages of the resting state-fMRI approach. Group and individual differences in effort and performance capabilities are not confounds for this approach. In addition, by looking at temporal cross-correlations of these nodes, it is possible to better address convergence across multiple nodes within a network and across and between networks (20). One of the main challenges is the need for a very large adjustment for significance, as there as hundreds of thousands of bidirectional connectivities, which is only aided slightly by the fact that in-network nodes tend to correlate moderately with each other. By using a KeyNet or ROI approach, investigators have dramatically reduced the number of comparisons, but the possibility remains that the best predictive connections (e.g., Intrinsic Networks, or CrossNetwork seed to node connections) are omitted from these models. Reducing the number of comparisons by restricting the search space could allow for a more balanced approach to type I and II error.
Three networks have been identified as particularly reliable intrinsic connectivity networks (21–23), which may be relevant to the prediction of treatment response in MDD (15), the default mode network (DMN); the salience and emotional network (SEN); and the cognitive control network (CCN). The DMN is active during rest and shows corresponding decreases in activation during cognitively demanding tasks (24–27). In contrast, the SEN is active in response to stimuli that are perceived to be relevant to current goals and involves limbic regions and ventral attention subnetworks. Finally, the CCN involves frontoparietal and dorsal attention network regions and is thought to be critical for problem-solving and executive functioning (28). Examining individual differences in connectivity within these networks during rest can provide useful information about the integrity and efficiency of these networks (23, 28). For example, prior work has suggested that attenuated connectivity within the CCN may have utility as a biomarker for individual differences in cognitive control performance and cognitive vulnerabilities for depression (e.g., (20, 29). In contrast, enhanced connectivity within the DMN often is seen in current depression and is related to elevated rumination and depression symptoms (30–34)
In this paper, we propose TaskNet and KeyNet approaches to identify treatment predictors using several small samples for illustration, balanced with our proposed whole brain uncorrected threshold strategy that could facilitate future meta-analyses (3). We aim to provide a ‘proof of principle’ framework for how data from smaller samples could be published, minimizing type I error with TaskNet and KeyNet masks using more conservative thresholds, and decreasing type II error by including WB data with effect sizes in supplemental tables and a moderately adjusted thresholded (3). We present four experiments, each using a different task that probes one of 4 subdomains of the Research Domain Criteria (RDoC), highlighting recent work suggesting specific subtypes by subdomain in MDD (35–37). 1) We use a well-published emotion face matching task (Negative Valence) (38–40), 2) a monetary incentive delay task (41–43), 3) a parametric Go/No-go task (44, 45), and 4) resting state fMRI (46, 47), all collected from an overlapping set of samples from our group.
General methods
The participants for all four experiments were recruited at the University of Michigan and surrounding area using postings in community settings and online forums. Inclusion criteria were diagnosis of Major Depressive Disorder (MDD), a Hamilton Depression Rating Score (HDRS, 17 item) greater than 13 (also completed every two weeks and at completion of the treatment) and age between 18 and 55. Those with comorbid anxiety disorders were also allowed into the study. Exclusion criteria included IQ below 75, use of psychotropic medications in the last 3 months, prior or current evidence of mania or psychosis, evidence of substance abuse (including nicotine) in the past six months, or of substance dependence in the last two years, and suicidal intent, plan, or attempt in the last six months. Participants completed informed consent in accordance with the Declaration of Helsinki.
Participants were compensated for their participation. Diagnosis was obtained with Structured Clinical Interview for DSM-IV-TR by psychologists or social workers. Imaging experiments were performed prior to initiation of treatment. Participants for all experiments were treated with duloxetine (with 1-week placebo lead-in, 30-90 mg) for 12 weeks. There were too few individuals to evaluate the effects of placebo (only for duloxetine trial) independent of the effects of medication. No individuals were excluded because of placebo response, and prior work by our group suggests that placebo responders and medication responders may show similar neural changes (12). In experiment 3, 22 additional participants were recruited and treated with escitalopram (5-10 mg and no placebo lead-in, plus the 14 who had already completed the placebo lead in study with duloxetine). If there were side effects or sleep problems, augmentation with trazadone was permitted for those in the duloxetine trial (n=2). Treatment was provided by a board-certified psychiatrist (J-K.Z. or B.J.M.). A psychologist, psychiatrist, or social worker administered the HDRS every two weeks and at completion of the treatment. Those who completed at least seven weeks and up to 12 weeks of treatment were included in the present analyses. Last measurement was carried forward in those instances where 12 weeks of treatment was not completed. Clinical and demographic information across all experiments is presented in Table 2. The dependent variable in all four experiments was percent change in HDRS score, calculated as ((HDRSpre − HDRSpost)/HDRSpre). Moreover, treatment response was defined as at least 50% reduction in HDRS score from baseline to post-treatment.
Table 2.
Clinical and Demographic Information for Participants from Experiments 1–4.
| EFAT (Exp 1) n=13 |
MID (Exp 2) n=10 |
PGNG^ (Exp 3) n=36 |
rs-fMRI (Exp 4) n=14 |
|
|---|---|---|---|---|
| Age | 31.23 (11.26) | 28.10 (9.86) | 35.89 (11.71) | 28.93 (8.40) |
| Gender (M/F) | 5/8 | 6/5 | 14/22 | 6/8 |
| Education | 15.54 (1.66) | 16.10 (1.97) | 15.14 (2.15) | 15.57 (1.74) |
| Pre-treatment HDRS | 20.00 (3.46) | 21.00 (4.57) | 19.22 (3.46) | 19.64 (3.49) |
| Post-treatment HDRS | 6.67 (10.92) | 10.40 (14.00) | 7.43 (6.93) | 6.67 (10.92) |
| Treatment Response (HDRS % change) | 70.46 (27.82) | 64.57 (42.72) | 65.94 (25.99) | 71.36 (28.91) |
| Comorbid Anxiety | 84.6% | 53.8% | 63.6% | 76.9% |
| Medications (Escitalopram/Duloxetine) | 0/13 | 0/10 | 22/14 | 0/14 |
| Refusals/Dropouts/Data* | 3/4/4 | 3/4/7 | 7/5/1 | 3/4/3 |
Note. Values are means and standard deviations unless otherwise noted;
16 subjects were included in Langenecker et al., 2007, and 36 were included in Crane et al., 2017 (8, 48). 11 participants overlap across all experiments 1–4.
Data exclusions are because of invalid performance data or invalid imaging data, typically due to movement, and do not overlap across paradigms.
Defining TaskNets and KeyNets
We used an empirical approach of defining TaskNets and KeyNets for each probe. KeyNets were based upon the parcellation provided by the 1000 individuals recruited from the greater Boston area (49). For the sake of simplicity, we have taken this seven network parcellation, excluded visual and somatomotor networks. We have also integrated dorsal attention and executive networks based upon work of others (23, 28), to make one Cognitive Control Network (CCN), Ventral Attention (or Salience) network was integrated with the limbic network and several subcortical regions that were excluded in the prior work of Yeo and colleagues, to create a Salience and Emotion Network (49). TaskNets were created by using the Neurosynth website http://neurosynth.org , which is an online repository of imaging results in healthy individuals. Studies are organized by constructs, and we used anger and fear for Experiment 1 (Figure 1), reward anticipation for Experiment 2 (Figure 1), Errors and inhibition for Experiment 3 (Figure 2). There were no relevant TaskNets for Experiment 4 – rest only. We used a forward inference model, which looks only at what is active, and not at what is specific or unique to the particular construct. Masks thresholded based upon the aggregate set of results (p < .01) were then downloaded and used for focused analyses to reduce type I error by reducing the relevant field of view.
Figure 1.
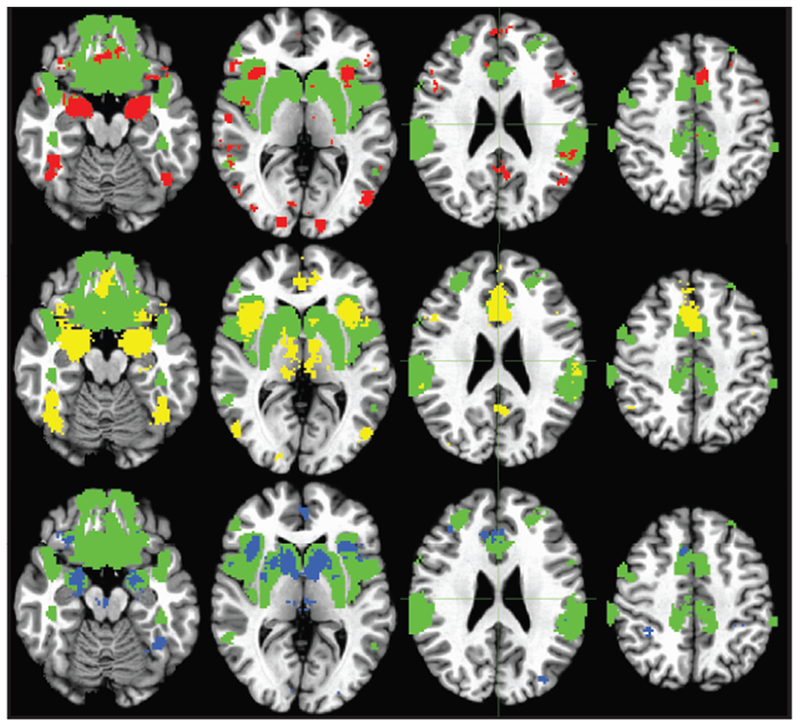
Salience and Emotion Network Intrinsic mask (green), with overlays of TaskNet for Anger (top row, red), Fear (middle row, yellow), and Reward Anticipation (bottom row, blue) derived from Neurosynth (http://neurosynth.org).
Figure 2.
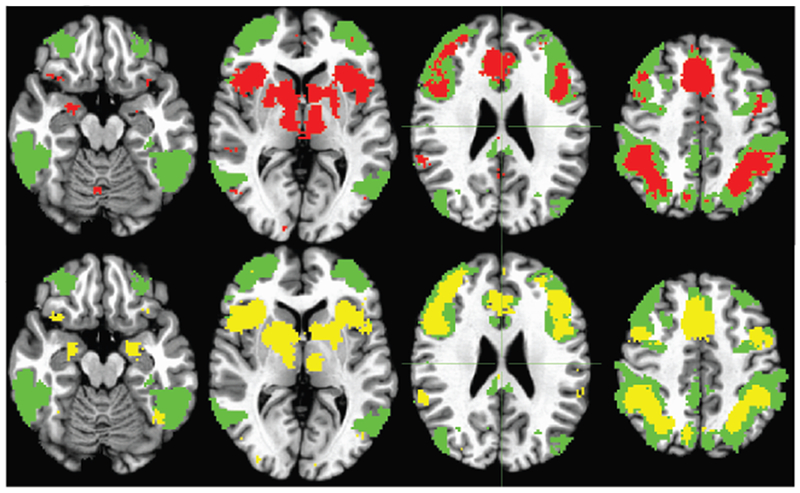
Cognitive Control Network Intrinsic mask (cyan), with overlays of TaskNet for Errors (top row, red) and Inhibition/Rejections (middle row, yellow) derived from Neurosynth masks (http://neurosynth.org).
Statistical Models and Thresholding for Neuroprediction
Each of the four experiments included models that were comprised of whole brain first level models for each individual (brain contrast or model), with change in HDRS as the predictive marker. Sex, pretreatment symptoms, movement deviation values for x, y, z translation, and age were used as covariates of no interest. To adjust for multiple comparisons, we performed clusterwise adjustment as determined with 3dClustSim where 3dFWHMx was used to estimate the spatial smoothness of the residuals with analyses using the -fwhm and -acf options separately (10,000 iterations; updated and ‘newer method’ on December 2015 and based upon subsequent commentary and papers in April and October of 2017). Moreover, and consistent with our recent synopsis of the need to balance type I and type II error, we provide partially adjusted correction values in supplemental tables that can be used for the purpose of meta-analyses (p < .005, mm3 > 80).
We add an example of the effect of masking upon adjustments for multiple comparisons. In WB analyses for Anger (EFAT), the mask consists of 510,340 voxels, and the -acf adjusted threshold would be 1473 contiguous voxels (57 for -fwhm threshold), resulting in the defacto exclusion of most subcortical regions from the models. With the neurosynth mask (TaskNet), there are 4563 voxels and the -acf adjusted threshold drops to 59 contiguous voxels (17 for -fwhm). For the SEN mask, the -acf threshold is 256 contiguous voxels (33 for -fwhm) in a mask that includes 30,532 voxels. Thus, TaskNet and KetNet approaches enable a search within a relevant network, and result in adjusted thresholds that can accommodate activation foci within smaller subcortical structures.
Acute Threat in the Negative Valence Domain with the Emotion Faces Matching Task (Experiment 1)
In the Emotion Faces Matching Task (EFMT) participants must match harsh faces (i.e., angry and fearful) to a correct same-emotion, instead of a non-matching distractor. Here, the comparison condition are shapes or happy face matching (38–40). The task has been used extensively in mood, anxiety and other disorders, and extant results have illustrated key regions and networks implicated in internalizing disorders. Regions engaged by this task include amygdala, anterior insula, and dorsal anterior cingulate. These regions tend to overlap with regions reported in our prior review as critical regions implicated in prediction of treatment response in MDD (3). These predictive studies also suggest that the Cognitive Control Network is included in important predictive regions. It is unclear if the role of the Cognitive Control Network is related to the task demands (choice between emotion in discrimination), or if it might reflect capacity for implicit emotion regulation. The KeyNet (Salience and Emotion Network, plus subgenual ACC, amygdala, and nucleus accumbens) and TaskNet models (fear, anger, from Neurosynth) are represented in Figure 11 including overlap.
Experiment 1 Methods
The sample is comprised of 13 individuals with MDD who completed treatment with duloxetine. We modeled activation in KeyNet and TaskNet regions to predict degree of change in HDRS after treatment. The demographic and clinical variables are reported in Table 2.
Data were collected with a 3T GE scanner at the University of Michigan. The task was previously validated for use with fMRI BOLD (38, 40, 50). Angry, fearful and happy faces were selected from the Gur emotional faces set (51). There were 3 angry, 3 fearful and 3 happy blocks of trials, interspersed with shape-matching blocks. Each block lasted 20 s and consisted of 4 back-to-back 5-s trials. Shapes were used as control stimuli instead of neutral faces, because they may provide a more truly neutral baseline for comparison, particularly when using a patient sample (52). Data were analyzed using SPM8 (with despiking in AFNI and realignment in FSL) based upon prior studies in our group (48, 53). All models included movement detection and movement estimates built into first and second level models.
Experiment 1 Results
The majority of these depressed subjects responded to duloxetine treatment (Table 2). Within the TaskNet mask for Anger (Neurosynth, FDR .01, forward inference), 6 regions significantly predicted treatment response (Table 3), with clusterwise adjustment to significance using the -fwhm correction in 3dClustSim (p < .005, k > 17 for anger, k > 25 for fear). For Fear TaskNet mask (Neurosynth, FDR .01, forward inference), right inferior frontal gyrus activation was inversely correlated with eventual degree of treatment response. No clusters were significant after the -acf clusterwise adjustment for Fear TaskNet (k > 102), whereas right amygdala was significant after -acf clusterwise adjustment for anger TaskNet mask (k > 59).
Table 3.
Emotion Faces Affect Task Activation Prediction of Treatment Response in MDD
| Mask/Emotion | Region | BA | MNI | Peak Z | Cluster k | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Positive HDRS Change | |||||||
| TaskNet Mask | |||||||
| Anger | Inferior Frontal | 45 | 56 | 28 | 2 | 3.94 | 25 |
| Insula | 47 | 38 | 24 | 2 | 3.34 | 46 | |
| Gyrus Rectus | 11 | 0 | 40 | −18 | 2.79 | 22 | |
| Amygdala | 28 | 16 | 0 | −22 | 3.07 | 66^ | |
| Superior Temporal | 22 | 60 | −26 | 10 | 4.47 | 18 | |
| Inferior Occipital | 37 | 46 | −56 | −22 | 3.61 | 25 | |
| Fear | n/a | ||||||
| KeyNet Mask | |||||||
| Anger | Medial Frontal | 11 | 2 | 44 | −18 | 2.89 | 118 |
| Superior Frontal | 6 | 10 | −2 | 72 | 3.26 | 44 | |
| Subcallosal | 11 | 10 | 26 | −18 | 3.03 | 100 | |
| Insula | 48 | 32 | 12 | 10 | 3.68 | 173 | |
| Uncus | 36 | 24 | 10 | −32 | 3.21 | 90 | |
| Inferior Temporal | 20 | 44 | −12 | −28 | 3.64 | 237 | |
| Inferior Parietal | 40 | −56 | −36 | 40 | 3.50 | 55 | |
| 2 | 62 | −28 | 44 | 4.20 | 632^ | ||
| Mid-Cingulate | 23 | −12 | −30 | 36 | 4.48 | 218 | |
| Fusiform | −30 | −14 | −40 | 3.06 | 37 | ||
| Fusiform/Hippocampus | 37 | −36 | −36 | −20 | 5.52 | 183 | |
| Fear | n/a | ||||||
| Negative HDRS Change | |||||||
| TaskNet Mask | |||||||
| Anger | n/a | ||||||
| Fear | Inferior Frontal | 44 | 56 | 14 | 16 | 3.66 | 29 |
| KeyNet Mask | |||||||
| Anger | n/a | ||||||
| Fear | Inferior Frontal | 6 | 54 | 10 | 18 | 3.75 | 58 |
| Subcallosal/Subgenual | 25 | 4 | 6 | −16 | 3.36 | 229^ | |
| Fusiform | 20 | 42 | −26 | −26 | 3.10 | 35 | |
Note. TaskNet mask has an adjusted threshold of p = .005 and k > 17 contiguous voxels (-fwhm 3dClustSim, April 2017), whereas KeyNet mask uses an adjusted threshold of p = .005 and k > 33 contiguous voxels (-fwhm 3dClustSim, April 2017).
Significance after using the -acf adjustment is noted with ^.
Conversion from k to mm3 is *8.
Within the KeyNet mask (Salience and Emotion Network, plus subgenual ACC, amygdala, and nucleus accumbens, hereafter SEN), 11 anger regions (see Table 3, Figure 3) significantly predicted treatment response, with clusterwise adjustment to significance using the -fwhm correction in 3dClustSim (Dec 2015, p < .005, k > 33 for anger, k > 25 for fear, Table 3, Figure 3). For fear with SEN KeyNet mask, right inferior frontal, subcallosal, and fusiform gyrus activation was inversely correlated with eventual degree of treatment response (also Table 3). With the -acf clusterwise adjustment for fear (k > 182), subcallosal/subgenual anterior cingulate activation was significantly negatively correlated with treatment response. For anger (-acf clusterwise, k >256), right inferior parietal activation was a significant positive correlate of later treatment response.
Figure 3.
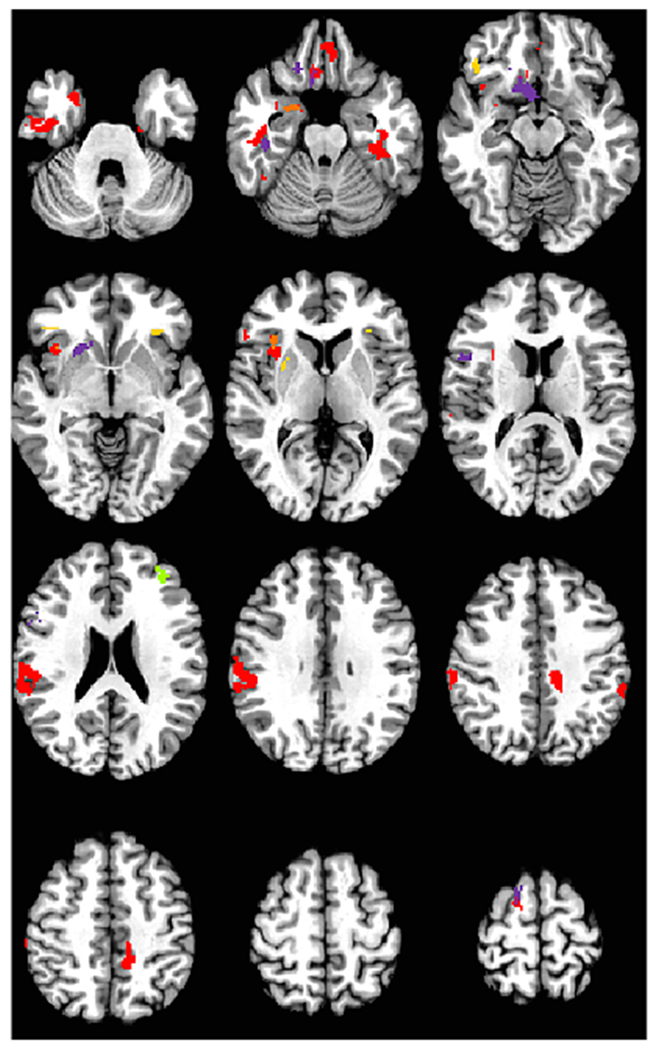
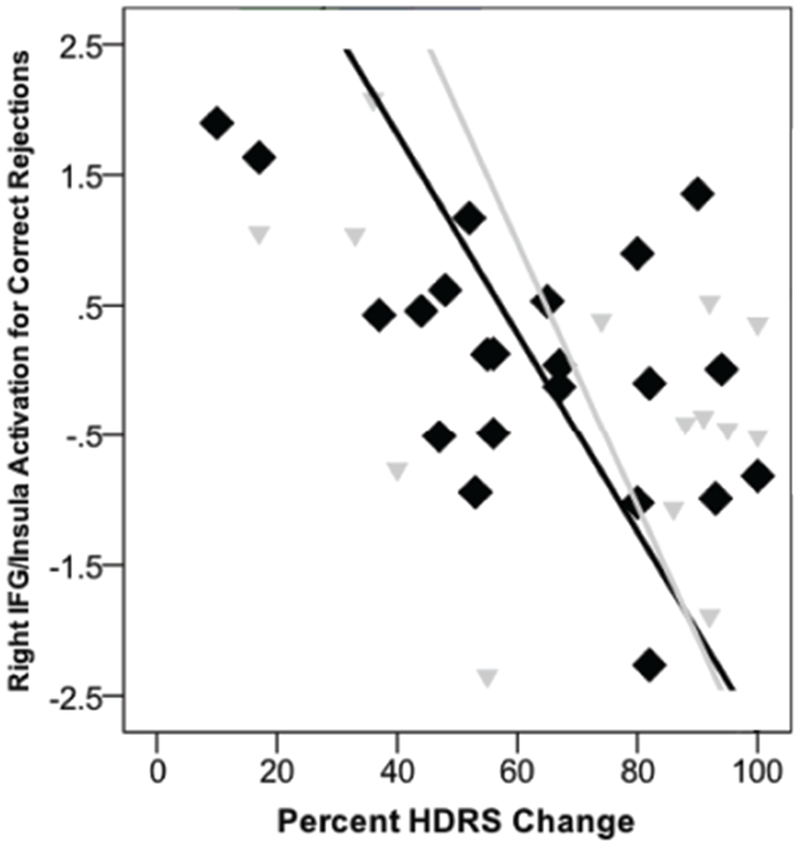
Panel A. Significant Activation Prediction Models from Experiments 1 and 3. Red = Anger positive prediction for either Anger TaskNet or SEN KeyNet. Orange is overlap of significant predictors within Anger TaskNet and SEN KeyNey for anger positive prediction. Purple is prediction for fear with Fear TaskNet or SEN KeyNet in a negative direction. Yellow is prediction of treatment response with the Inhibition TaskNet mask for PGNG Rejections in an inverse direction (see Panel B for relationship between activation in right IFG/insula and treatment response), and green is significant prediction in the positive direction for the CCN KeyNet mask for Rejections. Image is radiological format.
The WB results are included in Supplemental Table 1 for purposes of meta-analyses, with the threshold of significance set at .005 and k > 15 contiguous clusters, resulting in a minimum effect size to minimize type II error (note that for WB analyses, -acf cluster size is k > 1474 for anger and k > 767 for fear). Performance for fear accuracy was not a significant predictor of treatment response (B = .34, t = 1.07, p = .31, R2 = .11). Anger accuracy was not a significant predictor of treatment response (B = −.32, t = −1.00, p = .34, R2 = .10).
Reward Anticipation in the Positive Valence Domain with the titrated Monetary Incentive Delay Task (Experiment 2)
Emerging literature suggests that reward anticipation may be a core domain of dysfunction in MDD. Although there are only a handful of imaging and performance studies using reward functioning to predict clinical outcomes in MDD (42, 54–59), most of which are ROI only, there is a strong conceptual basis for why reward anticipation might be dynamically related to probability of treatment response in MDD. Diminished reward anticipation would reduce pursuit of goals, rewards, and social interactions, thereby eliminating any positive feedback that could have been derived from these activities. The likely mechanism of behavioral activation (also part of cognitive behavioral therapy), interpersonal therapy, and exercise therapy, which are all effective therapies for depression, is increasing engagement and effectiveness of the reward and motor systems in the brain (1, 60, 61). Other work suggests that pharmacotherapy may also be helpful in increasing behavioral activity and activation. We used the Monetary Incentive Delay task (MID; Knutson et al., 2008) to probe brain activation during reward anticipation. We predicted that diminished activation during reward anticipation would predict decreased treatment response for patients MDD.
Experiment 2 Methods
The sample used for prediction with the MID task was overlapping with that investigated for Experiment 1 and the task has been described in previous work (42). Our version of the task, which we have described in previous work (42), includes an individualized titration of the task response window to give each participant about a 65% chance of being able to successfully win and avoid losing money on each trial. In the present sample, this titration resulted in adaptive performance in the HC group, but not in the MDD group, particularly MDD with low trait reward responsiveness (42). Data were collected with MID using a fixed hemodynamic response model to compare win anticipation trials relative to neutral anticipation trials and processed as above for Experiment 1.
The treatment and the demographic and clinical variables are reported in Table 2.
Experiment 2 Results
Using the TaskNet for Neurosynth for reward anticipation, activation in left rostral anterior cingulate (−2, 32, 16, Z = 3.40, p < .0003, k = 20, BA 24) was inversely correlated with treatment response in MDD after adjustment (with -fwhm (k > 20), but not after acf adjustment for multiple comparisons).
Using the SEN KeyNet mask, right inferior frontal gyrus (52, 16, −2, Z = 3.53, p = .0004, k = 34, BA 47) was negatively associated with treatment response in MDD after adjustment (for -fwhm (k > 33), but not for -acf adjustment for multiple comparisons).
The WB results are included in Supplemental Table 2 for purposes of meta-analyses, with the threshold of significance set at .005 and k > 15 contiguous clusters, resulting in a minimum effect size to reduce type II error. For performance, total amount of money won was not associated with degree of treatment response (B = −.32, t = −0.88, p = .42, R2 = .09).
Experiment 3: Cognitive Control and the Parametric Go/No-go Test
At the behavioral level, executive functioning and cognitive control tests are well-replicated predictors of treatment response in MDD (62). However, cognitive control is relatively understudied in fMRI task-based studies (63, 64). In a recently published study, we illustrated how use of complementary analytic strategies with the Parametric Go/No-go test was able to predict treatment response probability with 89% accuracy (65). The study used independent components analysis, performance, and traditional hemodynamic response function analyses in an integrated, multimodal model. In our recent review, we determined that activation within the cognitive control network, regardless of task type, was often predictive of treatment response. This was true even for emotion perception tasks (3).
Experiment 3 Methods
The individuals used in this predictive model of pharmacological treatment response have been reported in a prior study (N=36, (48)). Over 65% of these depressed subjects responded to SSRI treatment (Table 2). The Parametric Go/No-go Test is a well-validated test that includes measures of attention and inhibition errors. Data were collected with a 3T GE scanner at the University of Michigan. Data were analyzed using SPM8 based upon prior studies in our group (48, 53). We evaluated network activation during commissions (error TaskNet mask) and correct rejections (inhibition TaskNetmask).
Experiment 3 Results
Within the TaskNet mask for Errors, there were no regions for the Commissions model (Figure 3) that positively or negatively predicted treatment response (for either -fwhm (k > 27) or -acf adjustment for multiple comparisons (k>96)).
For the Inhibition Task Net mask, activation for Correct Rejections in left (−32, 30, −6, Z = 3.62, k = 43, BA 47/13) and right inferior frontal gyrus/insula (42, 30, −14, Z = 3.51, k = 43, BA 47/13, Fig3, Pan B) and right putamen (28, 6, 6, Z = 3.53, k = 29) were inversely associated with treatment response (significant with -fwhm clusterwise adjustment (k > 28), but not with -acf adjustment (k > 121).
Within the KeyNet mask (CCN from (20)), there were no regions that significantly predicted treatment response, either positive or negative, for Commissions (neither -fwhm or -acf adjustment for multiple comparisons with 3dClustSim).
For Rejections within the CCN KeyNet, activation within left middle frontal gyrus was positively correlated with treatment response (−36, 42, 24, Z = 3.39, k = 42, BA 10) significant with -fwhm adjusted (k > 29), but not with -acf adjusted threshold (k > 77)).
Activation in a number of regions for both errors and rejections was predictive of treatment response at the WB level (Supplemental Table 3). The adjusted threshold with significance of .005 and k > 15 contiguous clusters was used, in order to reduce type II error. For performance data, Percent Correct Inhibition was not a significant predictor of treatment response (B = −.33, t = −2.00, p = .06, R2 = .29).
Resting State fMRI with SEN and CCN (Experiment 4)
Recent investigations have evaluated the role of intrinsic networks as potential biomarkers of treatment response (15) given that these intrinsic networks are relevant to a variety of psychological functions. The recent review by Dichter and colleagues documented several studies that identified increased connectivity between CCN and SEN regions in association with superior response to antidepressant medication treatment, potentially representing that patients with better inhibitory control over emotional stimuli are more able to benefit from treatment with antidepressants (15). In addition, treatment-sensitive patients with MDD may have higher connectivity within the CCN (perhaps representing superior executive functioning abilities) and lower connectivity within the DMN (perhaps representing less tendency to ruminate), relative to treatment-resistant patients. Furthermore, a large study that used intrinsic networks to identify subtypes of MDD found that patients who responded to repetitive transcranial magnetic stimulation (rTMS) for MDD tended to have reduced connectivity in fronto-amygdala networks (i.e., reduced connectivity between CCN and SEN), as well as reduced connectivity in cingulate and orbitofrontal areas supporting motivation and salience evaluation (66).
Experiment 4 Methods
The sample used for prediction with the MID task was overlapping with that investigated for Experiments 1 and 2. The treatment and the demographic and clinical variables are reported in Table 1, with over 71% improvement in HDRS symptoms (Experiment 4).
Table 1.
Strategies for reducing the field of view for treatment prediction models
| KeyReg | Region of interest that is theoretically or empirically related to the research question |
| KeyNet | Intrinsic network that is theoretically or empirically related to the research question |
| TaskNet | A network or extended subset of regions that are typically engaged in a task or task contrast. |
| BetGroup | Region or set of regions that differ between groups, thought the implicate disease processes (e.g., interference, but may also implicate compensatory responses) |
Rs-fMRI data collection and analysis steps have been described in detail in prior studies (34, 67, 68). Briefly, we used a key seed approach for each of three networks, CCN, SEN, and default mode network (DMN, Figure 4). As the SEN is most heterogeneous relative to the CCN and DMN, we used two seeds for SEN (Left amygdala and left subgenual anterior cingulate). We generally use left-sided seeds (excepting right DLPFC) due to accumulating evidence that left hemisphere dysfunction is more pertinent in MDD (69). As there is no relevant TaskNet, we only use the resting state intrinsic KeyNets (Figure 4).
Figure 4.
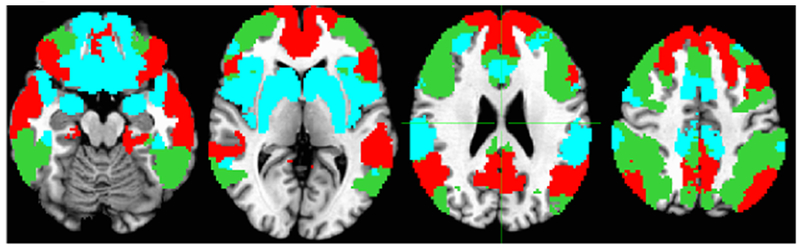
KeyNet Intrinsic masks for DMN (red), CCN (green) and SEN (blue) used in the respective rs-fMRI analyses.
Experiment 4 Results
SEN KeyNet Results
For left Amygdala, bilateral orbital frontal cortex connectivity were significant negative predictors of treatment response in MDD (Table 4, k > 25 -fwhm adjustment). For left SGAC, left middle temporal gyrus connectivity was positively correlated with treatment response, whereas left orbital frontal cortex connectivity was negatively predictive of treatment response in MDD (none were significant with -acf adjustment (k > 155) after multiple comparisons.
Table 4.
Resting State functional MRI Results in Prediction of Treatment Response in MDD
| Mask/Emotion | Region | BA | MNI | Peak Z | Cluster k | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Positive HDRS Change | |||||||
| CCN KeyNet Mask | |||||||
| Right DLPFC | Middle Frontal | 10 | −28 | 54 | 6 | 3.75 | 91 |
| Middle Frontal | 46/9 | −48 | 20 | 36 | 3.49 | 63 | |
| Precentral | 6 | −46 | −2 | 34 | 3.03 | 33 | |
| Inferior Parietal | 40 | −28 | −54 | 46 | 3.83 | 71 | |
| Inferior Parietal | 40 | 22 | −58 | 48 | 3.07 | 24 | |
| Inferior Occipital | 37 | 46 | −56 | −22 | 3.61 | 25 | |
| SEN KeyNet Mask | |||||||
| Left Amygdala | n/a | ||||||
| Left Subgenual AC | Middle Temporal | 20 | −48 | −24 | −26 | 3.83 | 40 |
| DMN KeyNet Mask | |||||||
| n/a | |||||||
| Negative HDRS Change | |||||||
| CCN KeyNet Mask | |||||||
| n/a | |||||||
| DMN KeyNet Mask | |||||||
| n/a | |||||||
| SEN KeyNet Mask | |||||||
| Left Amygdala | Inferior Frontal | 47 | −10 | 34 | −20 | 4.51 | 195^ |
| Inferior Frontal | 47 | 22 | 30 | −16 | 3.5 | 39 | |
| Left Subgenual AC | Uncus | 47 | −32 | 2 | −20 | 3.69 | 47 |
Note. KeyNet mask uses an adjusted threshold of p = .005 and k > 27 contiguous voxels (-fwhm 3dClustSim, April 2017, same threshold applies to each of three KeyNet masks, SEN, DMN, and CCN).
Significance after using the -acf adjustment is noted with ^.
Conversion from k to mm3 is *8.
CCN KeyNet Results
Connectivity of five regions within the CCN KeyNet right DLPFC was predictive of later treatment response in MDD (See Table 4), including bilateral middle frontal, inferior parietal regions (-fwhm (k > 27), but not -acf (k > 155) adjustment for multiple comparisons).
KeyNet Results
There were no regions of significant connectivity with left PCC that were significant positive or negative predictors of subsequent treatment response for MDD (for either DMN -fwhm or -acf adjustment for multiple comparisons).
Supplemental Table 4 lists predictive results with p < .005 and k > 15 for all four seeds.
General Discussion
Treatment prediction studies in MDD continue to struggle with pathways for how they can be disseminated into clinical and research advances, as results are difficult to translate to individual prediction models. The present report illustrated a way of providing supporting information for meta-analytic studies that could drive these breakthroughs. In the present experiments, emotional reactivity (to angry faces), regulation (cognitive control), and resting state networks (CCN, SEN) produced regions that were predictive of treatment response. Surprisingly, resting state connectivity of a key DMN node, the posterior cingulate, was not predictive of treatment response.
As might be expected, increased activation for angry faces (Table 2) in a number of regions included in the salience and emotion network was predictive of treatment response, highlighting that excessive neural response to threat is an important treatment target. It may also reflect the high degree of comorbidity with anxiety in this sample, which is typical for depression. Indeed, SSRIs and SNRIs are thought to modulate excessive responsivity of the salience and emotion network nodes, including amygdala, insula, fusiform and ventromedial and orbital frontal cortex, but also less studied regions like inferior parietal lobule and precuneus (3). These regions also tend to be closely associated with anxiety in response to fear, which may reflect the degree of comorbidity in this sample (70). In contrast, activation to fearful faces was inversely associated with treatment response, if in fewer areas.
Notably, activation within right inferior frontal gyrus and insula was a predictor for both anger and correct rejections, but in the opposite direction. Increased activation within this SEN node is expected for anger and is possibly reflective of excessive reactivity to threat that can be modulated by SSRIs and SNRIs. In contrast, excessive activation here when one has been successful in avoiding an error may reflect the decreased capacity for correct interaction between key CCN nodes and SEN nodes when regulation is required. Regulation was successful, yet activation in a node more reflective of errors was observed in those who are likely to be poor responders to treatment.
These results for fear as a predictor are more in line with a recent review, where increased activation in subgenual cingulate was a predictor of poorer treatment outcome (7). In most prior reviews, responses to different emotional faces are collapsed together, so we are not certain whether differential responses to fearful and angry faces in this sample is of any substantive meaning. Moreover, sad faces are studied most often in MDD, so fearful and angry faces may be understudied. The broader pattern is of excessive activation to negative stimuli in MDD relative to HC (71).
There were also regions from Cognitive Control and Salience and Emotion Networks that were predictive of treatment response using resting state analyses. Resting-state fMRI has great promise for parsing the diagnostic heterogeneity in depression (66), and may also aid in defining treatment targets, by matching treatments based on patients’ patterns of intrinsic network activity and unique symptom dimensions (66). Some excitement exists about the value of modulation of CCN circuits, and the number of foci evident in this sample suggests that there may be some reason for that excitement. The present results suggest that those with less disruption in CCN intrinsic connectivity are more likely to respond to standard pharmacological treatments (e.g., SSRI or SNRI). It is possible that those with decreased CCN connectivity might benefit from rTMS, which targets a left frontal node within the CCN. rTMS may enhance network plasticity and revert CCN functioning to normal levels. Indeed, rTMS to the dlPFC tends to enhance connectivity within the CCN (72). rTMS to the left dlPFC also has been shown to decrease DMN hyperconnectivity and to normalize CCN-DMN anticorrelations (73). Other treatment approaches may alleviate symptoms and increase wellbeing via normalization of intrinsic networks. For example, SSRIs reduced DMN hyperactivity (74); and decreased connectivity between the DMN (posterior cingulate) and CCN (inferior frontal gyrus) was associated with decreased rumination after treatment with rumination-focused CBT (68). Cognitive remediation is another pathway to improve CCN function and efficiency to facilitate and maintain wellbeing.
There were also some null results with resting state connectivity within the DMN. Notably, there were not any results within DMN connectivity patterns that met the adjusted criteria for significance. The small sample size may implicate a type II error in prediction. For reward, there were results in rostral cingulate (positive) and ventral frontal areas (inverse), aligning with TaskNet and KeyNet approaches. This approach illustrates how focused network analysis approaches might reveal activation that would not survive whole brain adjustments for significance. It is possible that reward is a newer area of inquiry in the context of treatment outcome in depression, and that more studies will be emerging in due time. The notable absence of whole brain predictive models of treatment response in the published literature may also indicate a potential ‘file drawer” effect.
There are a number of limitations to consider for this study. First, the small sample size for experiments 1, 2, and 4 could have, and most likely did lead to some type II errors. The small sample size also increases the likelihood of type I error. In addition, the order of administration for the study probes was fixed. This order could have led to some fatigue in participants (1.5 hours of scanning), or order effects that obscured or confounded some of the results. In addition, the study recruited unmedicated participants could result in recruitment of more mildly ill individuals. Indeed, about 70% change in clinical outcome (and > 75% responders if defined by traditional standard of greater than 50% reduction in symptoms) is well above what would be expected in a clinical trial, so these results may not reflect those with MDD in the general community. Importantly, we cannot infer that the achievement of treatment response in this study was secondary to pharmacotherapy, as it was an open-label, one arm study. The effects could as easily represent placebo effects or a natural return to wellness (12). The placebo lead in was used only in the duloxetine sample, and there were insufficient number of completers to dissociate placebo and medication effects. Finally, the use of TaskNet and KeyNet approaches here has limitations. While they may reduce the amount of adjustment needed for multiple comparisons, there is an assumption of network unilateral function embedded within this approach. For an emotion task, the salience and emotion network may be relevant (as it was for anger and to a lesser extent, fear), but it is also the case that activation in primary visual networks or cognitive control network might be just as relevant, or even more so (75). Future studies with more well-powered samples can better address these alternative network models, or even interactions between networks.
Moreover, we did not have a large enough sample to ask the questions posed in the introduction about the value of KeyNet and TaskNet approaches relative to KeyReg and BetGroup approaches. KeyReg approaches would have potentially “missed” relevant activations in cortical regions. For example, in the TaskNet anger regression, there were 4 of 6 regions identified that were in cortical regions and not in insula or amygdala, and 6 of 7 regions in KeyNet mask were outside these ROIs. For connectivity, the results are more mixed, with about half of the regions that might be considered as significant ROIs vs not.
In summary, the present study highlights that excessive responsivity to anger and diminished reactivity to regulation challenge and connectivity for cognitive control network are most relevant in the prediction of treatment response. Prediction models with integrated, multimodal imaging techniques might provide unique, additive variance in treatment prediction. This is a likely byproduct of the heterogeneous nature of depression and the need for more personalized identification for the causes and systems involved in MDD. Multidimensional approaches can aid in addressing heterogeneity in the disease and relevance to different treatments.
Supplementary Material
Figure 5.
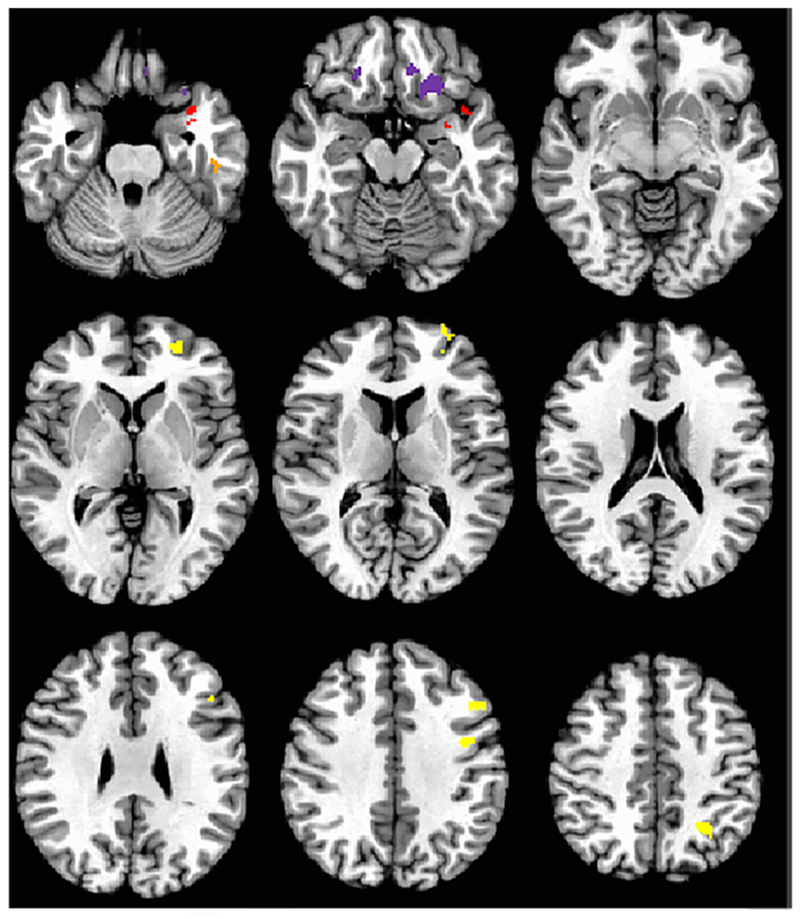
Regions of resting state networks with significant seed to node connectivity that is predictive of treatment response in MDD (Experiment 4). Left Amygdala connectivity for SEN KeyNet is in purple, with negative prediction. Red illustrates positive prediction with left subgenual cingulate seed and uncus, and orange is negative connectivity prediction with left subgenual cingulate. Yellow displays the regions of positive connectivity with right DLPFC that are predictive of treatment response within the CCN KeyNet. Image is in radiological format.
Acknowledgments:
This work was supported by MH074459 (SAL) and a NARSAD Young Investigator Award (SAL). We would like to thank the following individuals for assistance in data collection: the University of Michigan fMRI laboratory, the University of Michigan CTSA (MICHR UL1TR000433), Annie L. Weldon, Michelle T. Kassel, Kortni K. Meyers, Laura B. Gabriel, and Virginia Weinberg, RN.
Footnotes
These masks are intrinsic resting state networks derived from Yeo and colleagues (2011), including an adaptation by our group to condense the maps into three distinct networks based upon work of Menon and colleagues (2009). We also add subcortical regions to the SEN mask, as these were excluded in the work of Yeo and colleagues).
Reference List
- 1.Dichter GS, Felder JN, Smoski MJ. The effects of Brief Behavioral Activation Therapy for Depression on cognitive control in affective contexts: An fMRI investigation. J Affect Disord. 2010;126:236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gong Q, Wu Q, Scarpazza C, Lui S, Jia Z, Marquand A, Huang X, McGuire P, Mechelli A. Prognostic prediction of therapeutic response in depression using high-field MR imaging. Neuroimage. 2011;55:1497–1503. [DOI] [PubMed] [Google Scholar]
- 3.Langenecker SA, Crane NA, Jenkins LM, Phan KL, Klumpp H. Pathways to Neuroprediction: Opportunities and Challenges to Prediction of Treatment Response in Depression. Current Behavioral Neuroscience Reports. 2018. [PMC free article] [PubMed] [Google Scholar]
- 4.Aizenstein HJ, Khalaf A, Walker SE, Andreescu C. Magnetic resonance imaging predictors of treatment response in late-life depression. J Geriatr Psychiatry Neurol. 2014;27:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borairi S, Dougherty DD. The Use of Neuroimaging to Predict Treatment Response for Neurosurgical Interventions for Treatment-Refractory Major Depression and Obsessive-Compulsive Disorder. Harvard Review of Psychiatry. 2011;19:155–161. [DOI] [PubMed] [Google Scholar]
- 6.Dunlop BW, Binder EB, Cubells JF, Goodman MM, Kelley ME, Kinkead B, Kutner M, Nemeroff CB, Newport DJ, Owens MJ, Pace TW, Ritchie JC, Rivera VA, Westen D, Craighead WE, Mayberg HS. Predictors of remission in depression to individual and combined treatments (PReDlCT): study protocol for a randomized controlled trial. Trials. 2012;13:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu CH, Steiner H, Costafreda SG. Predictive neural biomarkers of clinical response in depression: A meta-analysis of functional and structural neuroimaging studies of pharmacological and psychological therapies. Neurobiology of Disease. 2013;52:75–83. [DOI] [PubMed] [Google Scholar]
- 8.Langenecker SA, Kennedy SE, Guidotti LM, Briceno EM, Own LS, Hooven T, Young EA, Akil H, Noll DC, Zubieta JK. Frontal and limbic activation during inhibitory control predicts treatment response in major depressive disorder. Biol Psychiatry. 2007;62:1272–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roy M, Harvey PO, Berlim MT, Mamdani F, Beaulieu MM, Turecki G, Lepage M. Medial prefrontal cortex activity during memory encoding of pictures and its relation to symptomatic improvement after citalopram treatment in patients with major depression. J Psychiatry Neurosci. 2010;35:152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wessa M, Lois G. Brain Functional Effects of Psychopharmacological Treatment in Major Depression: a Focus on Neural Circuitry of Affective Processing. Current neuropharmacology. 2015;13:466–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phillips ML. Identifying Predictors, Moderators, and Mediators of Antidepressant Response in Major Depressive Disorder: Neuroimaging Approaches. American Journal of Psychiatry. 2016;172:124–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pecina M, Bohnert AS, Sikora M, Avery ET, Langenecker SA, Mickey BJ, Zubieta J-K. Placebo-Activated Neural Systems are Linked to Antidepressant Responses: Neurochemistry of Placebo Effects in Major Depression. JAMA psychiatry. 2015;72:1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klumpp H, Keutmann MK, Fitzgerald DA, Shankman SA, Phan KL. Resting state amygdala-prefrontal connectivity predicts symptom change after cognitive behavioral therapy in generalized social anxiety disorder. Biol Mood Anxiety Disord. 2014;4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salomons TV, Dunlop K, Kennedy SH, Flint A, Geraci J, Giacobbe P, Downar J. Resting-State Cortico-Thalamic-Striatal Connectivity Predicts Response to Dorsomedial Prefrontal rTMS in Major Depressive Disorder. Neuropsychopharmacology. 2013;39:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dichter GS, Gibbs D, Smoski MJ. A systematic review of relations between resting-state functional-MRI and treatment response in major depressive disorder. Journal of Affective Disorders. 2015;172:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitfield-Gabrieli S, Ghosh SS, Nieto-Castanon A, Saygin Z, Doehrmann O, Chai XJ, Reynolds GO, Hofmann SG, Pollack MH, Gabrieli JDE. Brain connectomics predict response to treatment in social anxiety disorder. Molecular Psychiatry. 2015;21:680. [DOI] [PubMed] [Google Scholar]
- 17.van Waarde JA, Scholte HS, van Oudheusden LJB, Verwey B, Denys D, van Wingen GA. A functional MRI marker may predict the outcome of electroconvulsive therapy in severe and treatment-resistant depression. Molecular Psychiatry. 2014;20:609. [DOI] [PubMed] [Google Scholar]
- 18.Ge R, Blumberger DM, Downar J, Daskalakis ZJ, Dipinto AA, Tham JCW, Lam R, Vila-Rodriguez F. Abnormal functional connectivity within resting-state networks is related to rTMS-based therapy effects of treatment resistant depression: A pilot study. Journal of Affective Disorders;218:75–81. [DOI] [PubMed] [Google Scholar]
- 19.Dunlop BW, Rajendra JK, Craighead WE, Kelley ME, McGrath C, Choi KS, Kinhead B, Nemeroff CB, Mayberg HS Functional Connectivity of the Subcallosal Cingulate Cortex And Differential Outcomes to Treatment With Cognitive-Behavioral Therapy or Antidepressant Medication for Major Depressive Disorder. American Journal of Psychiatry. 2017;174:533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stange JP, Bessette KL, Jenkins LM, Burkhouse KL, Peters AT, Feldhaus C, Crane NA, Jacobs RH, Ajilore O, Watkins ER, Langenecker SA. Attenuated Intrinsic Connectivity within Cognitive Control Network Among Individuals with Remitted Depression: Temporal Stability and Association with Negative Cognitive Styles. Human Brain Mapping. 2017;38:2939–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. [DOI] [PubMed] [Google Scholar]
- 22.Menon V, Boyett-Anderson JM, Schatzberg AF, Reiss AL. Relating semantic and episodic memory systems. Cognitive Brain Research. 2002;13:261–265. [DOI] [PubMed] [Google Scholar]
- 23.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. The maturing architecture of the brain’s default network. Proc Natl Acad Sci U S A. 2008;105:4028–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marsland AL, Kuan DCH, Sheu LK, Krajina K, Kraynak TE, Manuck SB, Gianaros PJ. Systemic inflammation and resting state connectivity of the default mode network. Brain, Behavior, and Immunity. 2017;62:162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Posner J, Cha J, Wang Z, Talati A, Warner V, Gerber A, Peterson BS, Weissman M. Increased default mode network connectivity in individuals at high familial risk for depression. Neuropsychopharmacology. 2016;41:1759–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens MC, Pearlson GD, Calhoun VD. Changes in the interaction of resting-state neural networks from adolescence to adulthood. Hum Brain Mapp. 2009;30:2356–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menon V Large-scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Sciences. 2011;15:483–506. [DOI] [PubMed] [Google Scholar]
- 29.Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive, Affective, & Behavioral Neuroscience. 2012;12:241–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berman M, Evan Nee D, Casement M, Sook Kim H, Deldin P, Kross E, Gonzalez R, Emre D, Gotlib I, Hamilton P, Joormann J, Waugh C, Jonides J. Neural and Behavioral Effects of Interference Resolution in Depression and Rumination. Cognitive, Affective, & Behavioral Neuroscience. 2010;11:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Disner SG, Beevers CG, Haigh EA, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12:467–477. [DOI] [PubMed] [Google Scholar]
- 32.Rimes KA, Watkins E. The effects of self-focused rumination on global negative self-judgements in depression. Behaviour research and therapy. 2005;43:1673–1681. [DOI] [PubMed] [Google Scholar]
- 33.Watkins E, Brown RG. Rumination and executive function in depression: an experimental study. Journal of Neurology Neurosurgery and Psychiatry. 2002;72:400–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobs RH, Jenkins lM, Gabriel LB, Barba A, Ryan KA, Weisenbach SL, Verges A, Baker AM, Peters AT, Crane NA, Gotlib IH, Zubieta J-K, Langenecker SA, Welsh RC. Increased Coupling of Intrinsic Networks in Remitted Depressed Youth Predicts Rumination and Cognitive Control. Plos ONe. 2014;9:e104366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. [DOI] [PubMed] [Google Scholar]
- 36.Sanislow CA, Pine dS, Quinn KJ, Kozak MJ, Garvey MA, Heinssen RK, Wang PSE, Cuthbert BN. Developing Constructs for Psychopathology Research: Research Domain Criteria. Journal of Abnormal Psychology. 2010;119:631–639. [DOI] [PubMed] [Google Scholar]
- 37.Webb CA, Dillon DG, Pechtel P, Goer FK, Murray L, Huys QJ, Fava M, McGrath PJ, Weissman M, Parsey R, Kurian BT, Adams P, Weyandt S, Trombello JM, Grannemann B, Cooper CM, Deldin P, Tenke C, Trivedi M, Bruder G, Pizzagalli DA. Neural Correlates of Three Promising Endophenotypes of Depression: Evidence from the EMBARC Study. Neuropsychopharmacology. 2016;41:454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. [DOI] [PubMed] [Google Scholar]
- 39.Lohoff FW, Hodge R, Narasimhan S, Nall A, Ferroro TN, Mickey BJ, Heitzeg MM, Langenecker SA, Zubieta J-K, Bogdan R, Nikolova YS, Drabant E, Hariri AR, Bevilacqua L, Goldman D, Doyle GA . Functional genetic variants in the vesicular monoamine transporter 1 (VMAT1) modulate emotion processing. Molecular Psychiatry. 2014;19:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. BiolPsychiatry. 2006;59:424–429. [DOI] [PubMed] [Google Scholar]
- 41.Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH. Neural responses to monetary incentives in major depression. BiolPsychiatry. 2008;63:686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DelDonno S, Crane NA, Weldon AL, Passarotti AM, Pruitt PJ, Gabriel LB, Yau W, Meyers KK, Hsu DT, Taylor SF, Heitzeg MM, Herbener E, Shankman SA, Mickey BJ, Zubieta JK & Langenecker SA. . Affective Personality Predictors of Disrupted Reward Learning and Pursuit in Major Depressive Disorder. Psychiatry Research. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DelDonno SR Mickey BJ, Pruitt PJ, Stange JP Hsu DT Weldon AL, Zubieta J-K, Langenecker SA. Influence of Childhood Adversity, Approach Motivation Traits, and Depression in Predicting Individual Differences in Reward Anticipation. Biological Psychology. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Langenecker S, Zubieta J, Young E, Akil H, Nielson KA. A task to manipulate attentional load, set-shifting, and inhibitory control: Convergent validity and test-retest reliability of the Parametric Go/No-Go Test. Journal of Clinical and Experimental Neuropsychology. 2007;29:12. [DOI] [PubMed] [Google Scholar]
- 45.Langenecker SA, Bieliauskas LA, Rapport LJ, Zubieta JK, Wilde EA, Berent S. Face emotion perception and executive functioning deficits in depression. J Clin Exp Neuropsychol. 2005;27:320–333. [DOI] [PubMed] [Google Scholar]
- 46.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn ResonMed. 1995;34:537–541. [DOI] [PubMed] [Google Scholar]
- 47.de Kwaasteniet BP, Rive MM, Ruhe HG, Schene AH, Veltman DJ, Fellinger L, van Wingen GA, Denys D. Decreased Resting-State Connectivity between Neurocognitive Networks in Treatment Resistant Depression. Front Psychiatry. 2015;6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crane NA, Jenkins LM, Bhaumik R, Dion C, Gowins JR, Mickey BJ, Zubieta J-K, Langenecker S. Multidimensional prediction of treatment response to antidepressants with cognitive control and functional MRI. Brain. 2017;140:472–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni D, Fischl B, Liu H, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology. 2011;106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gorka SM, Burkhouse KL, Klumpp H, Kennedy AE, Afshar K, Francis J, Ajilore O, Mariouw S, Craske MG, Langenecker S, Shankman SA, Phan KL. Error-related Brain Activity as a Treatment Moderator and Index of Symptom Change during Cognitive-Behavioral Therapy or Selective Serotonin Reuptake Inhibitors. Neuropsychopharmacology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gur RC, Sara R, Hagendoorn M, Marom O, Hughett P, Macy L, Turner T, Bajcsy R, Posner A, Gur RE. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. Journal of neuroscience methods. 2002;115:137–143. [DOI] [PubMed] [Google Scholar]
- 52.Filkowski MM, Haas BW. Rethinking the Use of Neutral Faces as a Baseline in fMRI Neuroimaging Studies of Axis-I Psychiatric Disorders. J Neuroimaging. 2017;27:281–291. [DOI] [PubMed] [Google Scholar]
- 53.Crane NA, Jenkins LM, Dion C, Meyers KK, Weldon AL, Gabriel LB, Walker SJ, Hsu DT, Noll DC, Klumpp H, Phan KL, Zubieta J-K, Langenecker SA. Comorbid Anxiety Increases Cognitive Control Activation in Major Depressive Disorder. Depression and Anxiety. 2016;33:967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vrieze E, Pizzagalli DA, Demyttenaere K, Hompes T, Sienaert P, de Boer P, Schmidt M, Claes S. Reduced Reward Learning predicts outcome in Major Depressive Disorder. Biological psychiatry. 2013;73:639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Forbes EE, Olino TM, Ryan ND, Birmaher B, Axelson DA, Moyles DL, Dahl RE. Reward-related brain function as a predictor of treatment response in adolescents with major depressive disorder. Cognitive Affective & Behavioral Neuroscience. 2010;10:107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nissen C, Holz J, Blechert J, Feige B, Riemann D, Voderholzer U, Normann C. Learning as a model for neural plasticity in major depression. BiolPsychiatry. 2010;68:544–552. [DOI] [PubMed] [Google Scholar]
- 57.Burkhouse KL, Kujawa A, Kennedy AE, Shankman SA, Langenecker SA, Phan KL, Klumpp H. Neural Reactivity to Reward as a Predictor of Cognitive Behavioral Therapy Response in Anxiety and Depression. Depress Anxiety. 2016;33:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carl H, Walsh E, Eisenlohr-Moul T, Minkel J, Crowther A, Moore T, Gibbs D, Petty C, Bizzell J, Dichter GS, Smoski MJ. Sustained anterior cingulate cortex activation during reward processing predicts response to psychotherapy in major depressive disorder. Journal of Affective Disorders. 2016;203:204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walsh E, Carl H, Eisenlohr-Moul T, Minkel J, Crowther A, Moore T, Gibbs D, Petty C, Bizzel J, Smoski MJ, Dichter GS. Attenuation of Frontostriatal Connectivity During Reward Processing Predicts Response to Psychotherapy in Major Depressive Disorder. Neuropsychopharmacology. 2017;42:831–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thompson DG, Kesler SR, Sudheimer K, Mehta KM, Thompson LW, Marquett RM, Holland JM, Reiser R, Rasgon N, Schatzberg A, O’Hara RM. fMRI Activation During Executive Function Predicts Response to Cognitive Behavioral Therapy in Older, Depressed Adults. American Journal of Geriatric Psychiatry. 2015;23:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klumpp H, Fitzgerald JM, Kinney KL, Kennedy AE, Shankman SA, Langenecker SA, Phan KL. Predicting cognitive behavioral therapy response in social anxiety disorder with anterior cingulate cortex and amygdala during emotion regulation. NeuroImage Clinical. 2017;15:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dawson EL, Caveney AF, Meyers KK, Weisenbach SL, Giordani B, Avery ET, Schallmo MP, Bahadori A, Bieliauskas LA, Mordhorst MJ, Marcus S, Kerber K, Zubieta J-K, Langenecker SA. Executive Functioning at Baseline Prospectively Predicts Depression Treatment Response. The Primary Care Companion. 2017;19:e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. BiolPsychiatry. 2007;61:198–209. [DOI] [PubMed] [Google Scholar]
- 64.Siegle GJ, Thompson WK, Collier A, Berman SR, Feldmiller J, Thase ME, Friedman ES. Toward clinically useful neuroimaging in depression treatment: prognostic utility of subgenual cingulate activity for determining depression outcome in cognitive therapy across studies, scanners, and patient characteristics. Arch Gen Psychiatry. 2012;69:913–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Natania aC, Alvaro V, Masoud K, Runa B, Kelly AR, David FM, Erika FHS, Michelle TK, Anne LW, Melvin GM, Scott AL. Developing Dimensional, Pandiagnostic Inhibitory Control Constructs With Self-Report and Neuropsychological Data. Assessment. 2018:1073191118754704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, Fetcho RN, Zebley B, Oathes DJ, Etkin A, Schatzberg AF, Sudheimer K, Keller J, Mayberg HS, Gunning FM, Alexopoulos GS, Fox MD, Pascual-Leone A, Voss HU, Casey BJ, Dubin MJ, Liston C. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nature Medicine. 2016;23:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jacobs R, Barba A, Gowins J, Klumpp H, Jenkins L, Mickey B, Ajilore O, Pecina M, Sikora M, Ryan K. Decoupling of the amygdala to other salience network regions in adolescent-onset recurrent major depressive disorder. Psychological medicine. 2016:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rh Jacobs, Watkins ER Peters AT, Feldhaus C, Barba A, Carbray J, Langenecker SA: Targeting Ruminative Thinking in Adolescents at Risk for Depressive Relapse: Rumination-Focused Cognitive Behavior Therapy in a Pilot Randomized Controlled Trial with Resting State fMRI. in PLOS-One 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jenkins LM, Barba A, Campbell MM, Lamar M, Shankman SA, Leow A, Ajilore O, Langenecker SA. Shared white matter alterations across emotional disorders: A voxel-based meta-analysis of fractional anisotropy. Neuroimage: Clinical. 2016;12:1022–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.MacNamara A, Klumpp H, Kennedy AE, Langenecker SA, Phan KL. Transdiagnostic neural correlates of affective face processing in anxiety and depression. Depress Anxiety. 2017;34:621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Groenewold NA, Opmeer EM, de Jonge P, Aleman A, Costafreda S. Emotional valence modulates brain functional abnormalities in depression: Evidence from a meta-analysis of fMRI studies. Neuroscience & Behavioral Reviews. 2013;37:152–163. [DOI] [PubMed] [Google Scholar]
- 72.Fischer AS, Keller CJ, Etkin A. The Clinical Applicability of Functional Connectivity in Depression: Pathways Toward More Targeted Intervention. Biological psychiatry Cognitive neuroscience and neuroimaging. 2016;1:262–270. [DOI] [PubMed] [Google Scholar]
- 73.Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, Voss HU, Casey BJ, Etkin A, Dubin MJ. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry. 2014;76:517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Posner J, Hellerstein DJ, Gat I, Mechling A, Klahr K, Wang Z, McGrath PJ, Stewart JW, Peterson BS. Antidepressants Normalize the Default Mode Network in Patients With Dysthymia. JAMA psychiatry. 2013;70:373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stange JP, Jenkins LM, Hamlat EJ, Bessette KL, DelDonno SR, Kling LR, Passarotti AM, Phan KL, Klumpp H, Ryan KA, Langenecker SA. Disrupted engagement of networks supporting hot and cold cognition in remitted major depressive disorder. J Affect Disord. 2017;227:183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


