Abstract
The draft genome sequence of a cold-adapted phosphorus-solubilizing strain Pseudomonas koreensis P2 isolated from the Sela Lake contains 6,436,246 bp with G + C content of 59.8%. The genome sequence includes 5743 protein coding genes, 68 non-protein coding genes, 1007 putative proteins, 5 rRNA genes, 64 tRNAs and two prophage regions in 40 contigs. Besides these, genes involved in phosphate solubilization, siderophore production, iron uptake, heat shock and cold shock tolerance, multidrug resistance and glycine-betaine production were also identified.
Electronic supplementary material
The online version of this article (10.1007/s13205-019-1784-7) contains supplementary material, which is available to authorized users.
Keywords: Phosphorus (P), Pseudomonas koreensis, Genome, Phosphate solubilization, PGPR
Introduction
Phosphorus (P) is a key macronutrient and very essential for the development and growth of plants. At present, P is available in a very limited amount in agricultural soil due to its low solubility and fixation (Kwak et al. 2016). Several reports indicated that Pseudomonas sp. is one of the most powerful phosphate solubilizers (Hernandez-Salmeron et al. 2016; Lafi et al. 2016). Several strains from this genus have been documented to ameliorate crop growth, nutrient mobilization and yield, in addition to the biological control of plant pathogen (Sharma et al. 2018; Solanki et al. 2014).
Pseudomonas is one of the widely distributed genera which is present in versatile environmental conditions. Pseudomonas koreensis is a non-spore forming, gram-negative, rod-shaped motile bacteria and was reported for the first time from farming soil in Korea (Kwon et al. 2003). A number of species have been documented from different environments, having excellent plant growth-promoting (PGP) attributes. The majority of these rhizobacteria exhibit multifarious PGP traits and include solubilization of phosphate, siderophore formation, 1 amino cyclopropane-1-carboxylate deaminase (ACC) and hydrogen cyanide (HCN) production etc. (Lugtenberg and Kamilova 2009). Besides these traits, rhizobacteria also acquire some environment-specific PGP attributes, for instance, heavy metal resistance, cold tolerance and antibiotic resistance. Because of these properties, they are of interest to use as inoculants for plant growth promotion (Lugtenberg and Kamilova 2009). In spite of the pervasive literature related to plant growth-promoting rhizobacteria (PGPR) and their modes of action, the molecular significance of these PGPR is still to be identified and difficult to understand, because the PGPR status is not always well defined. There are varieties of microorganisms having genes directly endure the plant beneficiary activity, for example nif, phl and pqq (Ahmad et al. 2008). There are a number of PGPR which still have to be identified. Nowadays, the next-generation sequencing technologies have numerous applications to study genomes which have been further employed to study PGPR present in several species such as Pseudomonas sp., Bacillus sp., and Klebsiella sp. (Hernandez-Salmeron et al. 2016). In the present work, draft genome sequence of Pseudomonas koreensis P2 isolated from the cold environment of the Sela Lake in the soil having inorganic phosphate-solubilizing plant growth-promoting traits with environmental tolerance was reported. The P2 strain solubilized phosphorous, synthesized siderophores, indole acetic acid (IAA) and HCN. This strain also possessed some means and skills to survive and sustain in a cold environment. The genome sequencing analysis will disclose the basics of the functioning of the organism and will provide accurate and deep understanding into evolutionary changes and future studies in Pseudomonas sp. Furthermore, the genome analysis and understanding highlight the additional factors which may contribute to the plant growth.
Materials and methods
PGPR strain and characterization of plant growth-promoting (PGP) attributes
P2 is a recently identified gram-negative, short-rod with yellowish white pigmentation and motile rhizobacteria isolated from the Sela lake (Latitude: 27°30′37.27″N, Longitude: 92°6′9.30″E). The full-length 16S rRNA gene sequencing was done and has been deposited to NCBI GenBank under the accession number KJ580528. The 16S rRNA sequences of different sub-species of the P. koreensis was obtained from the NCBI GenBank database and compared using MEGAX32. The 16S rRNA sequence of P2 elucidated approximately 100% sequence similarity to Pseudomonas koreensis (Fig S1). Details of the PGP properties (phosphorous solubilization, siderophore and HCN production) of P2 are shown in Table 1. Due to the advantageous characteristics of P2, it has been chosen to characterize at the molecular and genomic levels. Phosphate solubilization was quantified using three different sources of phosphate (Udaipur rock phosphate, tri-calcium phosphate and di-calcium phosphate) at a concentration of 5 g L−1 in NBRIP broth at three different temperatures (4 °C, 15 °C and 35 °C). Phosphorus was estimated in the culture supernatant by the method as described elsewhere (Fiske and Subbarow 1925). Siderophore release was estimated at three different temperatures (4 °C, 15 °C and 35 °C) on the Chrome azurol S agar plate (Sigma-Aldrich Ltd.) as described by Schwyn and Neilands (1987). Similarly, production of IAA was quantified using Salkowski reagent as described by Bric et al. (1991) and the formation of HCN was quantified using protocol mentioned by Lorck (1948) at three different temperatures (4 °C, 15 °C and 35 °C). The plant growth-promoting efficiency of P2 strain was validated in wheat, maize and chickpea plants in pot experiments (ICAR-NBAIM Annual Report 2014–2015; Kashyap et al. 2015).
Table 1.
Plant growth-promoting attributes of P2 strain at different temperature regimes
| Temperature (°C) | IAA (mg L−1) | Siderophore | HCN | P-solubilization (mg L−1) | |||
|---|---|---|---|---|---|---|---|
| Rock phosphate | Tri-calcium phosphate | Di-calcium phosphate | |||||
| 4 | – | + | + | – | − | – | |
| 15 | 14.9 | + | + | 14.00 ± 1.03 | 216.44 ± 4.27 | 667.55 ± 10.12 | |
| 35 | 14.10 | + | + | 120 ± 3.78 | 532.03 ± 5.98 | 860.40 ± 21.57 | |
Genomic DNA manipulations and draft genome sequencing
For total genomic DNA isolation, P2 was grown on LB agar (Luria Bertani) plate and a single colony was inoculated in LB broth (10 ml) and grown overnight in shaking incubation at 37 °C and 150 rpm. For draft genome sequencing of Pseudomonas koreensis strain P2, total genomic DNA was isolated according to Sharma et al. (2018) and quality of the DNA was checked on agarose gel under transilluminator. The genome sequence of P2 was determined by the Illumina Hiseq platform in paired-end module. Primary genome assembly was done using Velvet version 1.2.10. (Zerbino and Birney 2008). Bowtie version 2 (Langmead and Salzberg 2012) was employed for de novo genome validation and quality check. tRNA and rRNA were identified by employing ARAGORN version 1.2.36 (Laslett and Canback 2004) and RNAmmer version 1.2 tools (Lagesen et al. 2007), respectively. Webcutter 2.0 (https://rna.lundberg.gu.se/cutter2/) and plasmid finder version 1.3 (Carattoli et al. 2014) were used to perform plasmid contamination search and plasmid sequences determination. Prophage regions were identified by employing the PHAST server (Zhou et al. 2011). CONTIGuator version 2.7 (Galardini et al. 2011) was used to finish the draft genome with Pseudomonas koreensis as a reference genome.
Genome annotation and bioinformatic analysis
Annotation of P2 genome was done by Rapid Annotations using Subsystems Technology “(RAST, Version 2.0)” web service (Aziz et al. 2008). Ring Image Generator (BRIG) version 0.95 was used for genome comparison (Alikhan et al. 2011). The circular genomic map was constructed with BLAST+ , with standard default parameters. Pseudomonas koreensis D24 was taken as a reference genome. Next, the identified genes from P2 were functionally annotated with the help of BLASTKOALA (Kanehisa et al. 2016). This server has a modified version of the internally used KOALA algorithm for KO assignment. The result file was used further for pathway mapping and comparative pathway analysis. Phylogenetic tree construction was done using MEGAX32 version. Sequences were aligned by MUSCLE and phylogenetic tree was made by the neighbor-joining method (Tamura et al. 2013).
Results and discussion
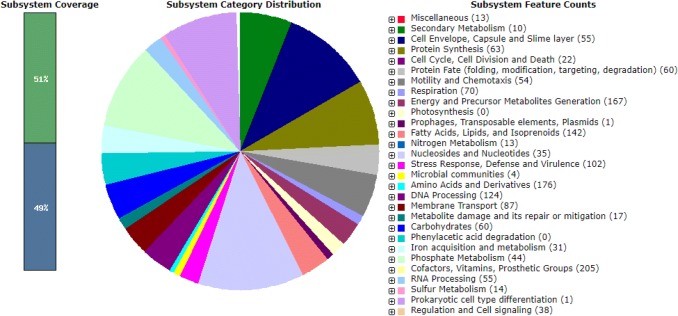
The draft genome sequencing of the strain P2 generated a total of 77.43 million reads with a total sequence data of 7820.5 Mb. High-quality reads (> 95%) with an average read length of 101 bp were considered for downstream analysis. Primary genome assembly generated 40 contigs with 59.8% GC content and a scaffold N50 value of 438,962 bp. Similar reports of genome assembly of P. koreensis strains from soybean and rice rhizosphere have been published earlier (Lozano et al. 2019; Lin et al. 2016). In this study, genome validation and quality control using Bowtie software revealed 29 million reads mapped concordantly and 6 million reads mapped discordantly with an overall alignment rate of 97.82%. Two prophage regions were present in the genome while plasmid sequences were missing. Both the prophage regions were intact and complete with 36.9 kb and 43.2 kb. Denovo genome identification and draft genome preparation were done using P. koreensis genome sequence as a reference (Table 2). Draft genome annotations and functional characterization by RAST allowed for the identification of 5743 protein coding genes, 68 non-protein coding genes, 1007 putative proteins, 5 rRNA genes and 64 tRNAs (Fig. 1; Table 2). RAST is a fully automated web-based tool and has been extensively employed in genome sequence annotation of several microbes including P.koreensis and P. moraviensis (Lin et al. 2016; Lujan et al. 2017; Srivastava et al. 2019). The intra-species sequence similarity was checked by calculating ANI value using reference of P. koreensis, P. agarici, P. moraviensis, and P. rhizosphaerae (Table 3). The ANI between the query genome and the reference genome was calculated as the mean identity of all the BLASTN matches. The ANI was calculated based on the BLAST algorithm, ANIb. The ANIb score between different subspecies of Pseudomonas is between 77 and 87%. The ANI score with references to P. koreensis was ~ 86.53. The result show more closeness to the P. koreensis.
Table 2.
Comparison of genome assembly statics of different species of Pseudomonas koreensis
| Genome | P2 | D26 | 57B-090624 | BS3658 | CI12 | CRS05-R5 | IMBL1 | LB-090714 | P19E3 |
|---|---|---|---|---|---|---|---|---|---|
| Size | 6,436,246 | 6,301,761 | 5,983,798 | 6,123,913 | 6,622,028 | 5,991,225 | 6,100,607 | 6,070,527 | 7,498,194 |
| GC content | 59.8 | 59.6 | 60 | 60.5 | 59.2 | 60.6 | 59.9 | 59.9 | 59.2 |
| N50 | 438,962 | NA | 234,574 | NA | 608,098 | NA | 324,256 | 299,940 | 6,444,290 |
| L50 | 5 | 1 | 8 | 1 | 4 | 1 | 7 | 7 | 1 |
| Number of contigs (PEGs) | 40 | 1 | 38 | 1 | 16 | 1 | 36 | 47 | 5 |
| Number of subsystems | 239 | 240 | 242 | 238 | 241 | 234 | 240 | 239 | 236 |
| Number of coding sequences | 5743 | 5638 | 5292 | 5471 | 5798 | 5345 | 5392 | 5332 | 6605 |
| Number of RNAs | 69 | 84 | 67 | 92 | 85 | 92 | 86 | 74 | 90 |
Names are written in abbreviated form. P2, D26, 57B-090624, BS3658, CI12, CRSo5-R5, IMBL1, LB-090714, P19E3 represent the subspecies of Pseudomonas koreensis
Fig. 1.
RAST annotation of Pseudomonas koreensis P2: The subsystem category distribution shows the percentage distribution of the genes in different pathways, which is labeled with different colours and the number of genes in one feature is mentioned in the bracket
Table 3.
ANI analysis of Pseudomonas koreensis P2
| Organism name | Regression_value | ANIb value |
|---|---|---|
| Pseudomonas_koreensis | 0.98867 | 86.53 |
| Pseudomonas_moraviensis | 0.98771 | 86.55 |
| Pseudomonas_agarici | 0.9259 | 79.81 |
| Pseudomonas_rhizosphaerae | 0.91148 | 77.72 |
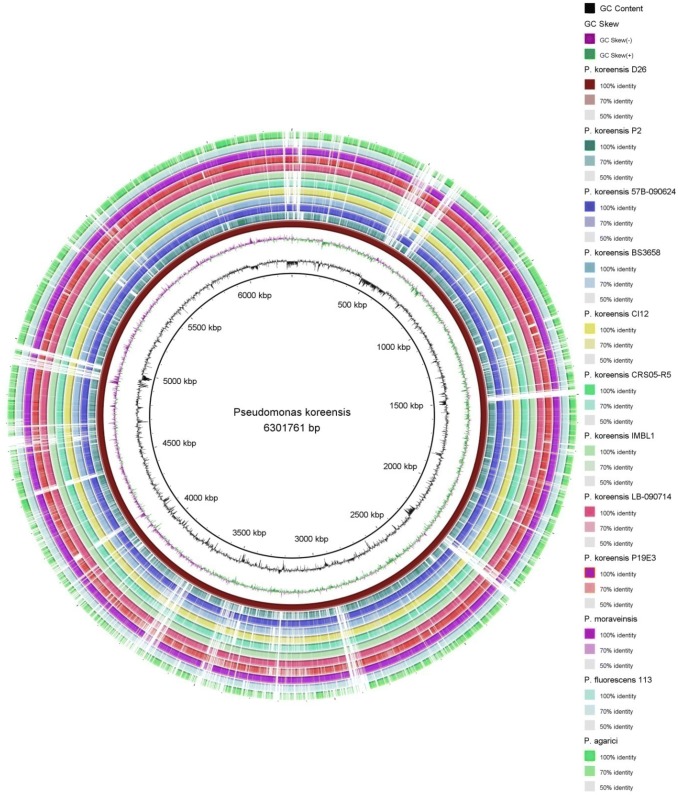
Comparative analysis of the draft genome of different sub-species Pseudomonas koreensis (P2, D26, 57B-090624, BS3658, CI12, CRSo5-R5, IMBL1, LB-090714, P19E3) using RAST (Table 2) identified around 5000–6600 coding sequences where P19E3 was identified with the highest number of coding sequences and 57B-090624 was identified with lowest coding sequences. Further, comparative study also indicated that the draft genome of different P. koreensis strains differed in the number of sub-system (234–242) and GC content (59.2–60.6%) (Table 2). Highest number of RNA (92) was identified with BS3658 and CRS05-R5, while lowest number was in 57B-090624. Further comparison of P. koreensis genome along with the P. agarici, P. moraviensis and P. fluorescens 113 using BRIG analysis (Fig. 2) indicated gap in between the sequences which represent the dissimilarity between the genome of different species of Pseudomonas. Even, there are some differences in between the P. koreensis subspecies because they have been isolated from the different geographical locations. These differences have led to the adaptation of the organism to particular climatic condition. Besides this, analysis revealed that P2 has significantly higher gene abundance of phosphorous metabolism, siderophore and HCN production and many more PGPR traits (Table S3) which allow this bacterium to be considered as a potential genomic resource for developing efficient biological fertilizer for agricultural usage.
Fig. 2.
BRIG output image of different sub-species of Pseudomonas koreensis along with Pseudomonas agarici, Pseudomonas moraviensis, Pseudomonas fluorescens 113 genome. Pseudomonas koreensis D24 was taken as reference genome. GC content and species name are labedded in the figure with different colour
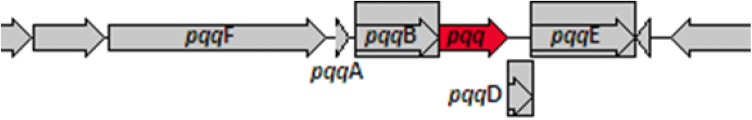
Solubilization of insoluble phosphate by bacteria is another way to enhance plant growth promotion. Gluconic acid (GA) is recognized as one of the major organic acids liable for efficient solubilization of mineral phosphates (Oteino et al. 2015). It is a well-known fact that the synthesis of GA is catalyzed by glucose dehydrogenase (GDH). P2 exhibits both UDP-dependent (UDP-GDH) and pqq-dependent glucose dehydrogenase (PQQ-GDH) and genome mining revealed the presence of pqqFABDE operon in between 156,040 and 158,264 bp in P2 genome (Fig. 3, Table S3), and still require detailed investigation to confirm their functioning in GA synthesis. However, conserved nature of phosphate regulon transcriptional regulatory proteins PhoB and sensor protein PhoR is Pseudomonads (Monds et al. 2006) strongly advocate the possibility that inorganic phosphate uptake in P2 strain is promoted by phosphate regulon transcriptional regulatory proteins PhoB and sensor protein PhoR with involvement of two other high-affinity phosphate transport systems, PstBACS and PhnE2E1. P2 also possesses sodium-dependent phosphate transporter NhaA and NhaD. Studies on in vitro mineral phosphate solubilization showed that temperature has substantial effects on TCP solubilization as P release at higher temperature (35 °C) was more than at lower temperature (15 °C and 4 °C). A significant solubilization of rock phosphate (14 and 120 mg L−1), tri-calcium (216.44 and 532.03 mg L−1) and di-calcium phosphate (667.55 and 860.40 mg L−1) by P2 at 15 °C and 35 °C temperature was observed (Table 1). Similarly, Selvakumar et al. (2011) also reported that P release by Pseudomonas lurida M2RH3 was affected by temperature and the maximum P release was observed at 30 °C as compared to 4 °C and 15 °C. They also observed that tri-calcium phosphate (TCP)-solubilizing ability of Pseudomonas sp. strain PGERs17 reduced to 42.3 mg L−1 at 4 °C from 74.1 mg L−1 at 28 °C. At 4 °C, P solubilization was absent for all the three insoluble P sources by P2 strain in the present study.
Fig. 3.
Operon structure of pqqFABDE (pqqF, pqqA, pqqB, pqqD, pqqE) in Pseudomonas koreensis P2
Siderophore-producing PGPR perform a key function in iron (Fe) nutrition of plants and act as a vital factor for improving crop health and yield (Solanki et al. 2014). P2 strain was found siderogenic at 35 °C and able to retain its siderogenic activity at a temperature of 4 °C too (Fig S2). Pseudomonas sp. has multiple systems to sense or asunder iron in its environment. There are various positive and negative regulatory factors in the Pseudomonas which are able to regulate cellular iron acquisition and storage. The genes associated with the siderophore production were identified in the P2 genome as well (Table S3). For example, the gene Fur (ferric uptake regulator) controls the transcription of PvdS, which is further needed for the transcription of several genes responsible for PVD biosynthesis and all these genes responsible for PVD biosynthesis are present in P2 genome. In addition, the P2 genome also possesses TonB- and TonR-dependent transport system along with the ABC transporters (Table S3).
IAA production by cold-tolerant bacteria like P2 strain has received little attention in the past. Recently, accumulative reports suggested that IAA production is superior in the mesophilic range relative to extremely low and chilling temperature. Selvakumar et al. (2008) recorded high levels of IAA production by Pantoea dispersa at 30 °C in contrast to 4 °C temperature. Although similar trend was observed in the present study, the IAA production in P. koreensis P2 was not much affected at 15 °C (Table 1). Similarly, cyanogenesis by PGPR is considered as another main factor to control pathogenic fungi. In the current study, P. koreensis P2 showed HCN production in all the three temperature regimes (Fig S2). In the genome analysis, association of HcnABC gene with HCN synthesis has been observed.
P2 possesses various cold shock proteins as it was identified from the cold environment. In the present study, three paralogs of CspA are determined in the P2 genome. Other cold shock proteins [CspCDG (CspC, CspD, CspG)] required for the cellular function at lower temperature were also identified (Table S2). The cold shock proteins and the heat shock proteins were identified from the subspecies of P. koreensis and some other species of Pseudomonas like P. agarici, P. moraviensis and P. fluorescens 113. In the present study, three different copies of the cspA in all the cases except P. fluorescens 113 (4 copies of cspA) were identified. The phylogenetic analysis of cspA and the GroEL subunit of heat shock protein (Hsp60) indicates a very minor differences in the data coverage (Fig S3). Furthermore, multiple sequence alignment revealed significant variation in the nucleotide as well as protein sequences of cold shock protein (Fig S4A and S4B) as compared to heat shock protein (Fig S5A and S5B). Phylogenetic analysis performed by Awasthi et al. (2019) indicated that the CspA was highly diverse in Pseudomonads (35–100%) with apparent gene transfer among different subgroups of Pseudomonas. Additionally, amino acid composition of the CspA from cold-adapted P. koreensis was different from mesophilic Pseudomonas and even a small amino acid change between CspA plays a significant role in determining cold adaptive and mesophilic species. Similarly, the findings of present study indicate that the variation of the CSP sequence may be contributing to the adaptation in a cold environment in case of P. koreensis P2, while there is not much variation in the protein as well as nucleotide sequence of heat shock protein.
There is a two-component response regulator in P2, i.e., CbrA and CbrB genes which encode a sensory box histidine kinase and a response regulator, respectively. Similar orthologs have already been identified in P. aeruginosa and moreover, a paramount role in central metabolism and the knockout of orthologs with adverse effect on growth at the lower temperature have been documented (Reva et al. 2006).
Overall, the present study was able to annotate 51% of the P2 genome. Besides these, genes for phosphate solubilization, siderophore production, iron uptake, heat shock and cold shock tolerance, multi-drug resistance and glycine-betaine production were also identified. Besides these PGPR traits, we also identified many genes that are well known to be responsible for the production of antimicrobial compounds such as 4-hydroxybenzoate, phenazine and GABA (Table S3). These compounds are mainly responsible for the suppression of the pathogen and enhance the plant growth. The P2 genome sequencing confirmed the presence of 1-aminocyclopropane-1-carboxylase (ACC) deaminase (acdS) as well.
Conclusion
The current study elucidates the plant growth-promoting properties and genetic makeup of Pseudomonas koreensis P2. The draft genome sequencing and analysis of the genome support its role as a plant growth-promoting bacterium and has led to the confirmation of the presence of the several plant growth-promoting traits like phosphate solubilization, siderophore, HCN and IAA production, which can improve the growth of the associated plant. A total of 5743 protein coding genes, 68 non-protein coding genes, 4398 characterized protein and 1330 proteins with pathway annotation were identified in 40 contigs. The presence of the glucose dehydrogenase and pqq genes makes the inorganic phosphorus present in the soil bioavailable to the plant. Similarly, the presence of cold shock proteins confirms the adaptability to the cold environment. So, this genomic resource could be exploited in future for the further research and to develop a potential plant growth promoter with best suited characters for the survival under diverse environment.
Genome sequence accession number
This strain has been submitted in the NAIMCC culture collection under the accession number NAIMCC-B-01747. The whole-genome shotgun project has been deposited at NCBI GenBank under the accession number GCA_002177125.1 (BioProject ID: PRJNA328580).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thankfully acknowledge the financial assistance under CRP-Genomics Platform of Indian Council of Agricultural Research (ICAR), India. The assistance of M/S Bionivid technology Pvt. Ltd., Kasturi Nagar, Bengaluru, India is acknowledged.
Author contributions
AKS conceived the idea. AKS, PS, HC, MK and PLK are associated with the wet lab experiments and sequencing. JY, Anjney Sharma, and Anchal K. Srivastava contributed in sequencing and analysis. APB, RS and AKS analyzed the genome data. Anil K. Saxena gave critical inputs. APB and AKS wrote the paper.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Alok Kumar Srivastava, Phone: 91-547-2530080, Email: aloksrivastva@gmail.com.
Prem Lal Kashyap, Phone: 0184-2267495, Email: plkashyap@gmail.com, Email: Prem.Kashyap@icar.gov.in.
References
- Ahmad F, Ahmad I, Khan MS. Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res. 2008;163:173–181. doi: 10.1016/j.micres.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Alikhan NF, Petty NK, Zakour NLB, Beatson SA. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics. 2011;12:402. doi: 10.1186/1471-2164-12-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi S, Sharma A, Saxena P, Yadav J, Pandiyan K, Kumar M, Singh A, Chakdar H, Bhowmik A, Kashyap PL, Srivastava AK, Saxena AK. Molecular detection and in silico characterization of cold shock protein coding gene (cspA) from cold adaptive Pseudomonas koreensis. J Plant Biochem Biotechnol. 2019 doi: 10.1007/s13562-019-00500-8. [DOI] [Google Scholar]
- Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, Meyer F. The RAST Server: rapid annotations using subsystems technology. BMC Genom. 2008;9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bric JM, Bostock RM, Silverstone SE. Rapid in situ assay for indoleacetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl Environ Microbiol. 1991;57:535–538. doi: 10.1128/aem.57.2.535-538.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carattoli A, Zankari E, Garcia-Fernandez A, Voldby Larsen M, Lund O, Villa L, Aarestrup FM, Hasman H. PlasmidFinder and pMLST: in silico detection and typing of plasmids. Antimicrob Agents Chemother. 2014;58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiske CH, Subbarow Y. The colorimetric determination of phosphorus. J Biol Chem. 1925;66:375–400. [Google Scholar]
- Galardini M, Biondi EG, Bazzicalup M, Mengoni A. CONTIGuator: a bacterial genomes finishing tool for structural insights on draft genomes. Source Code Biol Med. 2011;6:11. doi: 10.1186/1751-0473-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MR, Sambrook J. The basic polymerase chain reaction (PCR) Cold Spring Harbor Protoc. 2018 doi: 10.1101/pdb-prot095117. [DOI] [PubMed] [Google Scholar]
- Hernandez-Salmeron JE, Hernandez-Leon R, Orozco-Mosqueda MDC, Valencia-Cantero E, Moreno-Hagelsieb G, Santoyo G. Draft Genome sequence of the biocontrol and plant growth-promoting rhizobacterium Pseudomonas fluorescens strain UM270. Stand Genomic Sci. 2016;11:1. doi: 10.1186/s40793-015-0123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICAR-NBAIM Annual Report (2014–2015) ICAR-National Bureau of Agriculturally Important Microorganisms, Kushmaur, Mau, UP, India
- Kanehisa M, Sato Y, Morishima K. BlastKOALA and GhostKOALA: KEGG tools for functional characterization of genome and metagenome sequences. J Mol Biol. 2016;428:726–731. doi: 10.1016/j.jmb.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Kashyap PL, Chakdar H, Saxena P, Soni AP, Srivastava AK, Sharma AK (2015) Characterization and evaluation of plant growth promoting capabilities of phosphate solubilizing psychrotolerant bacteria from Cold Desert of Arunachal Pradesh. In: Presented at international conference on low temperature science and biotechnological advances held at NBPGR, Pusa Campus, New Delhi from 27–30th April 2015, p 112
- Kwak Y, Park G-S, Shin J-H. High quality draft genome sequence of the type strain of Pseudomonas lutea OK2T, a phosphate-solubilizing rhizospheric bacterium. Stand Genom Sci. 2016;11(1):51. doi: 10.1186/s40793-016-0173-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SW, Kim JS, Park IC, Yoon SH, Park DH, Lim CK, Go SJ. Pseudomonas koreensis sp. nov., Pseudomonas umsongensis sp. nov. and Pseudomonas jinjuensis sp. nov., novel species from farm soils in Korea. Int J Syst Evol Microbiol. 2003;53:21–27. doi: 10.1099/ijs.0.02326-0. [DOI] [PubMed] [Google Scholar]
- Lafi FF, Alam I, Geurts R, Bisseling T, Bajic VB, Hirt H, Saad MM. Draft genome sequence of the phosphate-solubilizing bacterium Pseudomonas argentinensis strain SA190 isolated from the desert plant Indigofera argentea. Genome Announc. 2016;4:e01431-16. doi: 10.1128/genomeA.01431-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagesen K, Hallin P, Rødland EA, Staerfeldt HH, Rognes T, Ussery DW. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007;35:3100–3108. doi: 10.1093/nar/gkm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laslett D, Canback B. ARAGORN, a program to detect tRNA genes and tmRNA genes in nucleotide sequences. Nucleic Acids Res. 2004;32:11–16. doi: 10.1093/nar/gkh152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Hu S, Liu R, Chen P, Ge C, Zhu B, Guo L. Genome sequence of Pseudomonas koreensis CRS05-R5, an antagonistic bacterium isolated from rice paddy field. Front Microbiol. 2016;7:1756. doi: 10.3389/fmicb.2016.01756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorck H. Production of hydrocyanic acid by bacteria. Physiol Plantarum. 1948;1:142–146. doi: 10.1111/j.1399-3054.1948.tb07118.x. [DOI] [Google Scholar]
- Lozano GL, Park HB, Bravo JI, Armstrong EA, Denu JM, Stabb EV, Broderick NA, Crawford JM, Handelsman J. Bacterial analogs of plant tetrahydropyridine alkaloids mediate microbial interactions in a rhizosphere model system. Appl Environ Microbiol. 2019 doi: 10.1128/AEM.03058-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B, Kamilova F. Plant-growth-promoting rhizobacteria. Ann Rev Microbiol. 2009;63:541–556. doi: 10.1146/annurev.micro.62.081307.162918. [DOI] [PubMed] [Google Scholar]
- Lujan KM, Eisen JA, Coil DA. Draft genome sequences of Pseudomonas moraviensis UCD-KL30, Vibrio ostreicida UCD-KL16, Colwellia sp. strain UCD-KL20, Shewanella sp. strain UCD-KL12, and Shewanella sp. strain UCD-KL21, isolated from Seagrass. Genome Announc. 2017;5(13):e00023-17. doi: 10.1128/genomeA.00023-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monds RD, Newell PD, Schwartzman JA, O'Toole GA. Conservation of the Pho regulon in Pseudomonas fluorescens Pf0-1. Appl Environ Microbiol. 2006;72(3):1910–1924. doi: 10.1128/AEM.72.3.1910-1924.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oteino N, Lally RD, Kiwanuka S, Lloyd A, Ryan D, Germaine KJ, Dowling DN. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front Microbiol. 2015;6:745. doi: 10.3389/fmicb.2015.00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reva ON, Weinel C, Weinel M, Bohm K, Stjepandic D, Hoheisel D, Jr, Tummler B. Functional genomics of stress response in Pseudomonas putida KT2440. J Bacteriol. 2006;188:4079–4092. doi: 10.1128/JB.00101-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Selvakumar G, Kundu S, Joshi P, Nazim S, Gupta AD, Mishra PK, Gupta HS. Characterization of a cold-tolerant plant growth-promoting bacterium Pantoea dispersa 1A isolated from a sub-alpine soil in the North Western Indian Himalayas. World J Microbiol Biotechnol. 2008;24:955–960. doi: 10.1007/s11274-007-9558-5. [DOI] [Google Scholar]
- Selvakumar G, Joshi P, Suyal P, Mishra PK, Joshi GK, Bisht JK, Bhatt JC, Gupta HS. Pseudomonas lurida M2RH3 (MTCC 9245), a psychrotolerant bacterium from the Uttarakhand Himalayas, solubilizes phosphate and promotes wheat seedling growth. World J Microbiol Biotechnol. 2011;27:1129–1135. doi: 10.1007/s11274-010-0559-4. [DOI] [Google Scholar]
- Sharma D, Gupta M, Gupta S, Kashyap PL, Zargar SM, Mallick SA. Antibiotic gene specific characterization and ARDRA analysis of native isolates of Pseudomonas spp. from Jammu, India. Indian Phytopathol. 2018;71(2):225–233. doi: 10.1007/s42360-018-0028-9. [DOI] [Google Scholar]
- Solanki MK, Singh RK, Srivastava S, Kumar S, Kashyap PL, Srivastava AK, Arora DK. Isolation and characterization of siderophore producing antagonistic rhizobacteria against Rhizoctonia solani. J Basic Microbiol. 2014;54(6):585–597. doi: 10.1002/jobm.201200564. [DOI] [PubMed] [Google Scholar]
- Srivastava AK, Sharma A, Srivastava R, Tiwari PK, Singh AK, Yadav J, Jamali H, Bharati AP, Srivastava AK, Kashyap PL, Chakdar H, Kumar M, Saxena AK. Draft genome sequence of halotolerant bacterium Chromohalobacter salexigens ANJ207, isolated from salt crystal deposits in pipelines. Microbiol Resour Announc. 2019;8(15):e00049-19. doi: 10.1128/MRA.00049-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbino D, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008;18(5):821–829. doi: 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Liang Y, Lynch KH, Dennis JJ, Wishart DS. PHAST: a fast phage search tool. Nucleic Acid Res. 2011;39:W347–W352. doi: 10.1093/nar/gkr485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.