Abstract
De-escalation from ticagrelor to clopidogrel in acute coronary syndrome (ACS) may occur for a variety of reasons, including side effects (bleeding and non-bleeding) and costs. This study sought to assess the prevalence of de-escalation from ticagrelor to clopidogrel and the occurrence of adverse clinical outcomes following de-escalation. We conducted a systematic review of clinical trials and real-world studies in ACS patients treated with ticagrelor. Real-world data on the prevalence of de-escalation during hospitalization or at discharge, after hospital discharge, and during the whole study period were included for meta-analysis. Major adverse cardiovascular events (MACE) and bleeding events occurring after de-escalation were also assessed. A total of 12 studies were eligible for meta-analysis of the prevalence of de-escalation. De-escalation from ticagrelor to clopidogrel therapy occurred with a mean prevalence of 19.8% [95% confidence interval (CI) 11.2–28.4%]. De-escalation occurred more frequently in-hospital or at discharge than after hospital discharge (23.7% vs. 15.8%). For assessment of clinical outcomes, a total of six studies were eligible for meta-analysis. Mean rate of MACE for patients with de-escalation was 2.1% (95% CI 1.1–4.1%) and the rate of major bleeding events was 1.3% (95% CI 0.4–4.5%). In conclusion, de-escalation commonly occurs in real-world practice. Although rates of major cardiovascular and bleeding events in this analysis were generally low, the profile of patients suitable for de-escalation, the impact of de-escalation on adverse clinical outcomes and how this is affected by the timing after index ACS warrants further large-scale investigation.
Electronic supplementary material
The online version of this article (10.1007/s11239-019-01860-7) contains supplementary material, which is available to authorized users.
Keywords: Acute coronary syndrome, De-escalation, Antiplatelet therapy, Meta-analysis
Highlights
With the availability of different oral P2Y12 receptor inhibitors, antiplatelet treatment strategies can be personalized based on individual patient risk for ischemic or bleeding complications.
Recent clinical trial evidence demonstrate that an early and guided de-escalation strategy based on platelet function testing may be considered as an alternative treatment option for patients with acute coronary syndrome.
Data from real world practice shows that non-guided de-escalation is common, although the clinical implications of this approach remain unknown.
The profile of patients suitable for de-escalation, the impact of de-escalation on adverse clinical outcomes and how this is affected by the timing after index ACS warrants further large-scale investigation.
.
Introduction
Current U.S. and European guidelines recommend dual antiplatelet therapy (DAPT) with aspirin plus a P2Y12 receptor inhibitor in patients with ACS [1–3]. The use of the newer generation P2Y12 inhibitors, prasugrel and ticagrelor, is generally recommended over clopidogrel in ACS patients because of their superior efficacy, albeit at the expense of increased bleeding [4–6]. The uptake of ticagrelor is superior to that of prasugrel among these due to its broader indications and less restrictions for use [6]. However, clopidogrel still remains a commonly prescribed agent worldwide due to its lower costs, tolerability and favorable benefit–risk ratio [7].
Switching between P2Y12 receptor inhibitors frequently occurs in real-world practice and de-escalation from a more potent to a less potent agent has become part of a stage-adapted therapy [8]. In this practice, providers use more potent P2Y12 inhibitors to increase protection from ischemic events in the early phase after ACS, and later switch to clopidogrel to reduce bleeding [9]. Indeed, while the ischemic benefit of the more potent P2Y12 inhibitors over clopidogrel persists over time, their greatest benefits are seen early, when the risk of ischemic complications is highest, while most hemorrhagic events with potent platelet inhibitors arise during chronic treatment [10, 11]. However, other reasons to de-escalate in clinical practice involve bleeding or non-bleeding side-effects (e.g., dyspnea) and costs [12, 13]. Although observational data suggest that a uniform de-escalation strategy early after an ACS may increase the risk of adverse events [14], recent randomized trial data from a smaller single-center trial suggests that when this occurs 4 weeks after hospital discharge, there is a reduced risk of bleeding complications without any trade-off in efficacy [15]. Considering conflicting data, larger sample sizes are needed to better define the clinical implications associated with de-escalation, including the assets and drawbacks of guided versus unguided de-escalation strategies [9, 15]. Despite the need for further investigations in the field, the recently released 2018 ESC/EACTS Guidelines on myocardial revascularization have included a new recommendation on guided DAPT de-escalation as a strategy that may be considered as an alternative treatment option for ACS patients [16].
We conducted a systematic review and meta-analysis with the following objectives: (1) to assess the prevalence and timing of de-escalation from ticagrelor to clopidogrel in patients with ACS, and (2) to assess the rate of clinical outcomes (ischemic events and bleeding) following de-escalation from ticagrelor to clopidogrel in patients with ACS.
Materials and methods
Data sources and searches
The literature search was performed in MEDLINE (via PubMed), Embase (via Ovid), and the Cochrane Central Register of Controlled Trials (via Wiley) from inception to April 18, 2017. References were limited to those published in the English language. Conference abstracts from the American College of Cardiology, European Society of Cardiology, American Heart Association, and the European Hematology Association from 2012 to 2017 were also included in the review. The complete search strategies are provided in the Supplemental Materials. The methods recommended by the Centre for Reviews and Dissemination, University of York were used [17].
Study selection
A standardized review protocol was used to define the eligibility criteria for the search and screening of references using the PICO(TSS) framework, which outlines the population, interventions, comparators, outcomes, timing, setting, and study designs of interest (Table S1).
Eligibility criteria for studies on the prevalence of de-escalation included observational studies and registries on patient populations with ACS, including ST-elevation myocardial infarction (STEMI), non-ST-elevation myocardial infarction (NSTEMI), unstable angina (UA), who received treatment with ticagrelor. Outcomes included the prevalence rate of patients who switched from ticagrelor to clopidogrel, the time to switch or duration of initial ticagrelor therapy, and the reasons for de-escalation.
Eligibility criteria for studies on the clinical outcomes associated with de-escalation included clinical trials and observational studies in patients who received initial treatment with ticagrelor and subsequently switched to clopidogrel treatment. Efficacy outcomes included MI, stroke, stent thrombosis, and major adverse cardiovascular events (MACE), defined as the composite of cardiovascular death, MI, or stroke. Safety outcomes included any bleeding and major bleeding. Definitions for MACE and major bleeding reported in each study are provided in the Supplemental Materials.
Data extraction and quality assessment
Study eligibility was determined by two reviewers (R.S. and K.S.) who independently screened the abstracts and full-text. Multiple publications from the same study were mapped as primary and companion publications. A third reviewer resolved discrepancies between two primary reviewers. Additional screening information is provided in the Supplemental Materials.
Data extraction was conducted using the Digital Outcome Conversion (DOC) Data version 2.0 software platform (Doctor Evidence, LLC, Santa Monica, CA, USA) and its universal electronic extraction form, based on a standardized data configuration protocol [18].
The Cochrane Collaboration tool was used to assess the risk of bias in randomized controlled trials (RCTs) [19], and the Newcastle–Ottawa Scale (NOS) was used to assess quality of non-randomized studies [20]. A description of the methods is available in the Supplementary Material.
Statistical analysis
The prevalence and timing of de-escalation was analyzed by pooling the ticagrelor-treated cohorts to provide an overall estimate of the prevalence, or proportion, of patients switching to clopidogrel and the timing of de-escalation. When analyzing the clinical outcomes following de-escalation, a comparative analysis was preferred to make inferences regarding the choice between continuing initial ticagrelor therapy or de-escalation to clopidogrel; however, this was not feasible due to the lack of data reported for patients who remained on ticagrelor. Cohort analysis were performed instead and pooled groups that de-escalated from ticagrelor to clopidogrel therapy to determine the mean rate of outcomes, or proportion of patients experiencing the outcome, associated with de-escalation.
A random effects model using the restricted maximum likelihood (REML) method was used based on the observational study design and the heterogeneity observed between the studies [21]. The logit transformed proportions model were used for the analysis of clinical outcomes due to the probability of sparse data. The REML method was used to correct for the negative bias associated with the maximum likelihood (ML) method and is more robust to data outliers than ML estimators [22, 23]. Heterogeneity was assessed using the I2 statistic, with a value of I2 > 50% indicating significant heterogeneity. All analyses were performed using the R metaphor v2.0.0 package within the DOC Data version 2.0 software platform [24].
Results
Prevalence and timing of de-escalation
Summary of search results
The search for studies on the prevalence of de-escalation from ticagrelor to clopidogrel resulted in 1903 references. Following review, total of 12 observational studies met eligibility criteria and were included in meta-analysis [25–36]. The PRISMA flow diagram is presented in Figure S1A.
Study and group characteristics
A summary of the study characteristics is presented in Table 1, and summaries of group characteristics of the ticagrelor group across the included studies are presented in Tables S6A and S6B. Of the 12 observational studies included in the meta-analysis, seven were prospective and five were retrospective. Sample sizes for the ticagrelor group varied from 98 to 11,680 patients. Where reported, mean or median age spanned from 60 to 67.7 years of age. The proportion of females ranged from 22.5 to 36% across 11 studies reporting.
Table 1.
Study characteristics of included studies for prevalence and timing of de-escalation
| Study | Design | Country | Study N | Ticagrelor group (n) | Timing of de-escalation—with reasonsa |
|---|---|---|---|---|---|
| Angeras et al. [25] | Retrospective Cohort | Sweden | 1,04,012 | Ticagrelor + aspirin (n = 11,680) | After discharge—NR |
| Biscaglia et al. [26] | Prospective Cohort | Italy | 586 | Ticagrelorb (n = 586) | Varied—need for OAT, bleeding, intolerance, unwillingness, dyspnea |
| Coons et al. [27] | Retrospective Cohort | United States | 8127 | Ticagrelorb (n = 309) | In-hospital—NR |
| Dehghani et al. [28] | Prospective Cohort | Canada | 227 | Ticagrelorb (n = 227) | Varied—dyspnea, no coverage, significant bleeding |
| Dery et al. [29] | Prospective Cohort | Canada | 2179 | Ticagrelorb (n = 242/241c) | At discharge—NR |
| Gaubert et al. [30] | Prospective Cohort | France | 164 | Ticagrelorb (n = 164) | After discharge—NR |
| Green et al. [31] | Retrospective Cohort | Denmark | 7016 | Ticagrelorb (n = 3159/3066c) | After discharge—NR |
| Hamid [32] | Retrospective Cohort | United Kingdom | 98 | Ticagrelor + aspirin (n = 98) | After discharge—NR |
| Harding et al. [33] | Prospective Cohort | New Zealand | 992 | Ticagrelor + aspirin (n = 243) | Varied—NR |
| Simeone et al. [34] | Retrospective Cohort | United States | 15,788 | Ticagrelorb (n = 2323) | After discharge—NR |
| Wang et al. [35] | Prospective Cohort | China | 417 | Ticagrelor + aspirin (n = 99) | In-hospital or at discharge—NR |
| Zettler et al. [36] | Prospective Cohort | United States | 8672 | Ticagrelorb (n = 226) | After discharge—NR |
NR not reported, OAT oral anticoagulant
aReasons reported in at least 10% of those who de-escalated are listed
bStudy did not clearly specify whether all patients also received aspirin
cNumber of patients enrolled/number of patients analyzed
Meta-analysis
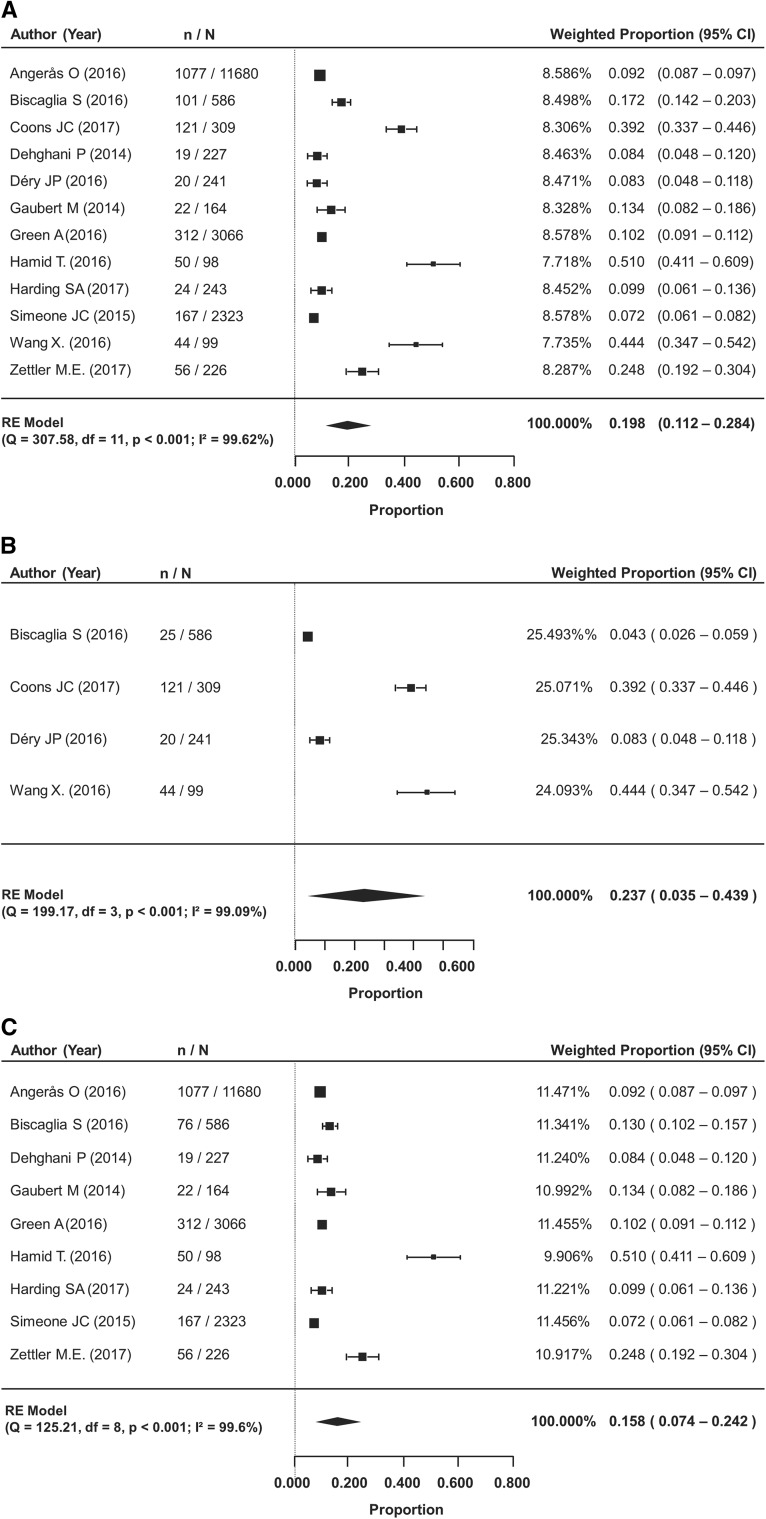
The pooled prevalence of de-escalation from ticagrelor to clopidogrel among 12 studies (n = 19,262 analyzed) was 19.8% (95% confidence interval [CI] 11.2–28.4%). The meta-analysis was also sub-grouped by the timing of de-escalation: in-hospital or at the time of discharge, or after discharge. Rates reported from baseline through 1 year after the index event were included in the post-discharge subgroup analysis. De-escalation in-hospital or at discharge was reported in four studies, and after discharge in nine studies. The timing of de-escalation in each study and the reasons for switching reported by at least 10% of the patients are provided in Table 1. The prevalence of de-escalation in-hospital or at discharge was 23.7% (95% CI 3.5–43.9%), and 15.8% (95% CI 7.4–24.2%) after hospital discharge up to 1 year follow-up (Fig. 1b and c).
Fig. 1.
Prevalence of de-escalation from ticagrelor to clopidogrel. a De-escalation occurring during the entire study period (I2 = 99.62%); RE: Random Effects. b De-escalation occurring in-hospital or at discharge (I2 = 99.09%); RE: Random Effects. c De-escalation occurring after discharge (I2 = 99.60%); RE: Random Effects
To analyze the precise timing of de-escalation, three studies (14,589 patients analyzed) were meta-analyzed that followed patients over 1 year (Figure S2). The mean duration of ticagrelor therapy before de-escalation to clopidogrel or discontinuation was 115 days (95% CI 81.2–148.4).
Clinical outcomes associated with de-escalation
Summary of search results
The search for studies on the clinical outcomes associated with de-escalation from ticagrelor to clopidogrel treatment resulted in 1709 references. Following review, six studies met eligibility criteria and were included in meta-analysis [26, 32, 35, 37–39]. The PRISMA flow diagram is presented in Figure S1B.
Study and group characteristics
A summary of the study characteristics is presented in Table 2, and summaries of group characteristics of the ticagrelor group across the included studies are presented in Table S7A and 7B. Of the six studies included for meta-analysis, three were RCTs and three were observational (two prospective and one retrospective). All studies included a group taking ticagrelor followed by treatment with clopidogrel. Sample sizes for the ticagrelor followed by clopidogrel group varied from 44 to 265 patients. Where reported, mean or median age spanned from 62.1 to 72 years of age. The proportion of females ranged from 31.8% to 56% across 4 studies reporting.
Table 2.
Study characteristics of included studies for clinical outcomes associated with de-escalation
| Study | Design | Country | Study N | Ticagrelor group (n) | Timing of de-escalation—with reasonsa | Follow-up duration |
|---|---|---|---|---|---|---|
| Biscaglia et al. [26] | Prospective Cohort | Italy | 586 | Ticagrelor followed by clopidogrel (n = 101) | Varied—need for OAT, bleeding, intolerance, unwillingness, dyspnea | 12 months |
| Hamid [32] | Retrospective Cohort | United Kingdom | 98 | Ticagrelor + aspirin followed by clopidogrel + aspirin (n = 50) | 3 months—NR | 12 months |
| Wang et al. [35] | Prospective Cohort | China | 417 | Ticagrelor followed by clopidogrel (subgroup) (n = 44) | In-hospital or at discharge—NR | 6 months |
| Motovska et al. [37] | RCT | Czech Republic | 1230 | Ticagrelor followed by clopidogrel (pooled with or without bolus) (n = 265) | Varied—economic | 12 months |
| Pourdjabbar et al. [38] | RCT | Canada | 60 | Ticagrelor followed by clopidogrel (n = 60/57b) | Randomization—triple therapy, bleeding risk, cost, needing CABG, compliance concerns | 30 days |
| Xu et al. [39] | RCT | China | 114 | Ticagrelor + aspirin followed by clopidogrel + aspirin (n = 57) | 1 week—study protocol | Periprocedural |
CABG coronary artery bypass graft, NR not reported, OAT oral anticoagulant, RCT randomized controlled trial
aReasons reported in at least 10% of those who de-escalated are listed
bNumber of patients enrolled/number of patients analyzed
Meta-analysis
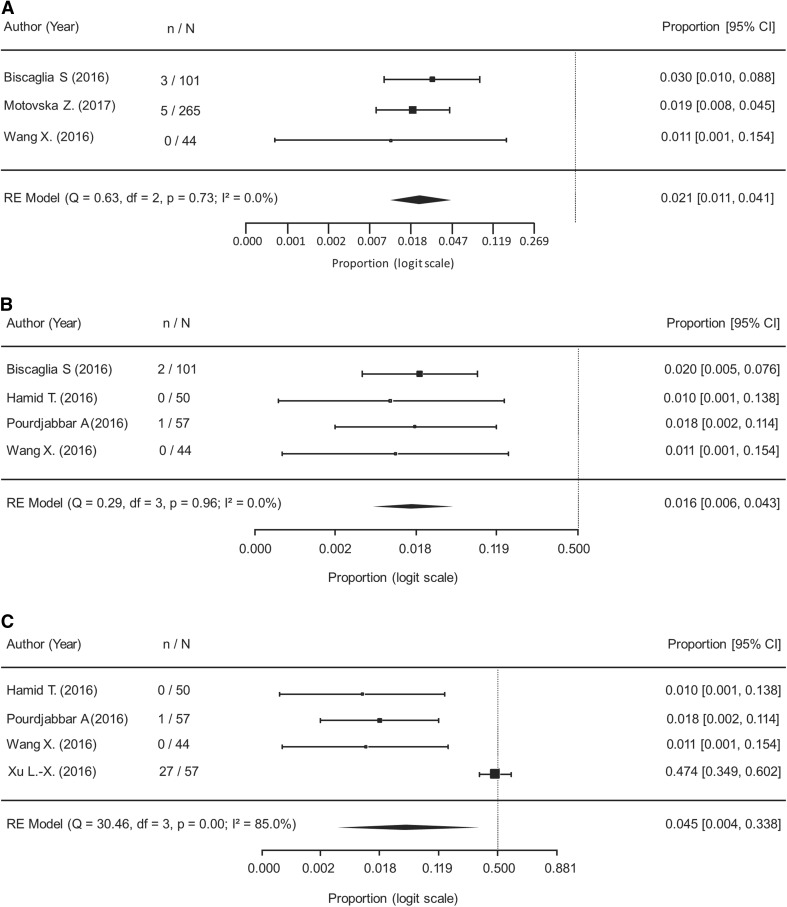
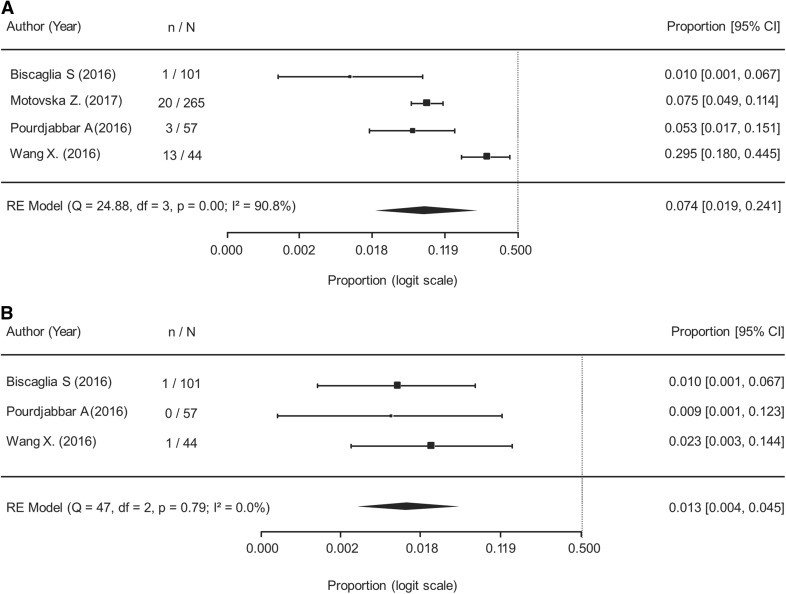
When analyzing the safety and efficacy of de-escalation (574 patients analyzed), results of the meta-analysis showed the rate of MACE was 2.1% (95% CI 1.1–4.1%) during a mean follow-up duration of 10 months and with no observed heterogeneity (Fig. 2a). The rate of cardiovascular mortality was 1.6% (95% CI 0.6–4.3%) with no observed heterogeneity (Fig. 2b). The rate of MI was 4.5% (95% CI 0.4–33.8%) with significant heterogeneity observed (Fig. 2c). There were zero cases of stroke reported in 252 patients [26, 32, 35, 38] and one case of stent thrombosis reported in 202 patients who had available data following de-escalation from ticagrelor to clopidogrel [26, 35, 38]. The rate of any bleeding event was 7.4% (95% CI 1.9–24.1%) during a mean follow-up of 7.8 months and 1.3% (95% CI 0.4–4.5%) for major bleeding during a mean follow-up of 6.3 months (Fig. 3a and b, respectively).
Fig. 2.
Cardiovascular outcomes following de-escalation from ticagrelor to clopidogrel. a Major Adverse Cardiovascular Events (I2 = 0.00%); RE: Random Effects. b Cardiovascular Mortality (I2 = 0.00%); RE: Random Effects. c Myocardial Infarction (I2 = 85.0%); RE: Random Effects
Fig. 3.
Bleeding events following de-escalation from ticagrelor to clopidogrel. a Any Bleeding (I2 = 90.8%); RE: Random Effects. b Major Bleeding (I2 = 0.00%); RE: Random Effects
Discussion
To the best of our knowledge, this is the first systematic and dedicated meta-analysis assessing the prevalence, timing, and clinical outcomes of de-escalation from ticagrelor to clopidogrel therapy. In the absence of large observational studies or randomized clinical trials assessing this modality of de-escalation, the current study aimed to pool the relevant studies to offer insights into treatment patterns in the real-world and the clinical implications associated with such practice.
Our analysis showed that it is not infrequent for ACS patients to de-escalate to clopidogrel therapy following initial treatment with ticagrelor (pooled prevalence rate of 19.8%). We observed a higher prevalence rate of de-escalation occurring in-hospital or at discharge than after hospital discharge (23.7% vs. 15.8%). The rates of de-escalation in-hospital or at discharge were more variable across the studies, compared to studies reporting de-escalation after discharge; however, both results showed significant heterogeneity among the studies. Due to the fast offset action of ticagrelor, de-escalation to clopidogrel by standard loading dose regimens is recommended, regardless of the timing (acute vs chronic) of de-escalation except for patients with bleeding complications in whom de-escalation with a maintenance dose regimen may be considered [2, 12, 40]. The time to switch or duration of DAPT with ticagrelor, individual patient characteristics, and the specific reasons for de-escalation are underreported in the literature or not often documented in registries.
When assessing clinical outcomes after de-escalation, our analysis showed generally low rates across both ischemic outcomes and bleeding events, with no heterogeneity observed among studies for MACE and major bleeding. The observed aggregate event rates found in our review were comparable to those seen in clinical trials of de-escalation. Our results showed a rate of 2% for MACE (defined as CV mortality, MI, or stroke), 2% for CV mortality, and 1% for major bleeding. The TROPICAL-ACS study reported similar rates (3% and 1% and 1%, respectively) in patients with guided de-escalation from prasugrel to clopidogrel [9]. In the TOPIC study, CV death occurred in 0.3% and major bleeding in 0.3% of patients who were randomly assigned to downgrade from prasugrel/ticagrelor to clopidogrel [15]. Contrastingly, a different result is seen in observational data. In the SCOPE registry, a multicenter prospective non-randomized study that evaluated the incidence and short-term outcomes of switching oral P2Y12 inhibitors in ACS patients undergoing PCI, de-escalation was associated with an incidence of 20.4% for MACE and 3.8% for bleeding events [14]. In addition to the high-risk profile that patients from registries have compared with those from randomized clinical trials, these findings may be attributed to the fact that many patients switched therapy early after the index event, a time-frame in which patients are more vulnerable to thrombotic events and during which they could have benefited from more potent antiplatelet therapies.
There are several limitations regarding the findings of this study. The analysis was not performed using individual patient level data, thereby preventing adjustment of outcome data following de-escalation based on individual risk profile. Furthermore, studies did not report baseline risk variables for patients who de-escalated therapy. Duration of follow-up for outcomes, as well as the definitions for MACE and major bleeding outcomes, varied across studies. The prevalence analysis was conducted on data from observational studies, which have inherent source of bias, but do provide a more accurate assessment of prescribing behavior in the real-world setting. However, detailed information such as the timepoint of switch and patient outcomes following hospital discharge are not well reported in observational or registry data, thereby preventing landmark analyses for this review. Analyses of clinical efficacy and safety outcomes used a combination of data sourced from observational studies and RCTs. This poses challenges for determining the causal impact of de-escalation, but the inclusion of observational data may increase the generalizability of the results to real patient populations. As well, due to the limited data reported for patients remaining on ticagrelor therapy, a comparison with patients who de-escalated therapy could not be performed.
Based on the limitations and the considerable heterogeneity observed in some of our analyses, this study should be considered to be of exploratory nature. Dedicated and prospective studies are needed to provide evidence-based and practical recommendations on the optimal strategy to de-escalate DAPT therapy. These will inform on patient indicators that may benefit (or derive harm) from de-escalation, and whether the timing of de-escalation has an impact on clinical outcomes. Furthermore, further and large-scale randomized trials would allow an evaluation of de-escalation versus continuation of initial therapy. To this extent, a number of studies evaluating the use of genetic testing to guide antiplatelet treatment decisions making are currently ongoing and may add to the evidence of de-escalation guided by platelet function testing [9, 41, 42], and since the time of this review, there is also more observational data addressing the subject of pre-mature discontinuation of antiplatelet therapy [43]. Finally, defining the cost-effectiveness of de-escalation is warranted to better define its role in real-world practice. The role of platelet function and genetic testing guiding decision making on the choice of antiplatelet therapy to be used in patients undergoing PCI, has been recently revised in an updated international expert consensus document [44].
Conclusions
Following index ACS treatment with ticagrelor, it is not uncommon for patients to de-escalate to clopidogrel. The analysis showed that rates of cardiovascular outcomes were generally low following de-escalation. However, further large-scale investigations are needed to appropriately examine the clinical implications of de-escalation on the risk of recurrent ischemic events and bleeding risk, as well as the appropriate timing to de-escalate patients in whom switching occurs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Roshan Shah, Masoud Pourrahmat, and Toby Sayre, of Doctor Evidence, for medical writing and analytical assistance; Angelica Stamegna for general publication and research support.
Funding
This analysis was funded by Sanofi.
Compliance with ethical standards
Conflict of interest
Dominick J. Angiolillo declares that he has received consulting fees or honoraria from Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Daiichi-Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, PhaseBio, PLx Pharma, Pfizer, Sanofi, and The Medicines Company. He has received payments for participation in review activities from CeloNova and St Jude Medical. D.J.A. and he also declares that his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, Idorsia, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, and Renal Guard Solutions. In addition, Dominick J. Angiolillo is recipient of funding from the Scott R. MacKenzie Foundation and the NIH/NCATS Clinical and Translational Science Award to the University of Florida UL1 TR000064 and NIH/NHGRI U01 HG007269, outside the submitted work. Giuseppe Patti reports speaker fees, consultancy, and/or advisory boards for Bayer, Boehringer-Ingelheim, BMS-Pfizer, Daiichi Sankyo, AstraZeneca, PIAM, Sanofi, AMGEN, Sigma-Tau, Malesci and MS. Dara Paek and Michael del Aguila report employment by Doctor Evidence, who was contracted by Sanofi to conduct the systematic review and analysis. Shalini Girotra reports employment by Sanofi. Dirk Sibbing reports grants from Roche Diagnostics, grants from Daiichi Sankyo; personal fees from Bayer AG, personal fees from Daiichi Sankyo, personal fees from Eli Lilly, personal fees from Roche Diagnostics, personal fees from MSD, personal fees from Pfizer, personal fees from AstraZeneca. All other authors report no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2016;68:1082–1115. doi: 10.1016/j.jacc.2016.03.513. [DOI] [PubMed] [Google Scholar]
- 2.Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2017;39:213–260. doi: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- 3.Capodanno D, Alfonso F, Levine GN, Valgimigli M, Angiolillo DJ. ACC/AHA versus ESC guidelines on dual antiplatelet therapy: JACC guideline comparison. J Am Coll Cardiol. 2018;72:2915–2931. doi: 10.1016/j.jacc.2018.09.057. [DOI] [PubMed] [Google Scholar]
- 4.Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 5.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 6.Franchi F, Angiolillo DJ. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol. 2015;12:30–47. doi: 10.1038/nrcardio.2014.156. [DOI] [PubMed] [Google Scholar]
- 7.Bueno H, Sinnaeve P, Annemans L, et al. Opportunities for improvement in anti-thrombotic therapy and other strategies for the management of acute coronary syndromes: insights from EPICOR, an international study of current practice patterns. Eur Heart J Acute Cardiovasc Care. 2016;5:3–12. doi: 10.1177/2048872614565912. [DOI] [PubMed] [Google Scholar]
- 8.De Luca L, Capranzano P, Patti G, Parodi G. Switching of platelet P2Y12 receptor inhibitors in patients with acute coronary syndromes undergoing percutaneous coronary intervention: review of the literature and practical considerations. Am Heart J. 2016;176:44–52. doi: 10.1016/j.ahj.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Sibbing D, Aradi D, Jacobshagen C, et al. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): a randomised, open-label, multicentre trial. Lancet. 2017;390:1747–1757. doi: 10.1016/S0140-6736(17)32155-4. [DOI] [PubMed] [Google Scholar]
- 10.Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a TRITON-TIMI 38 (TRial to assess improvement in therapeutic outcomes by optimizing platelet InhibitioN with prasugrel-thrombolysis in myocardial infarction) analysis. J Am Coll Cardiol. 2008;51:2028–2033. doi: 10.1016/j.jacc.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Becker RC, Bassand JP, Budaj A, et al. Bleeding complications with the P2Y12 receptor antagonists clopidogrel and ticagrelor in the PLATelet inhibition and patient outcomes (PLATO) trial. Eur Heart J. 2011;32(23):2933–2944. doi: 10.1093/eurheartj/ehr422. [DOI] [PubMed] [Google Scholar]
- 12.Angiolillo DJ, Rollini F, Storey RF, et al. International expert consensus on switching platelet P2YReceptor-inhibiting therapies. Circulation. 2017;136:1955–1975. doi: 10.1161/CIRCULATIONAHA.117.031164. [DOI] [PubMed] [Google Scholar]
- 13.Rollini F, Franchi F, Angiolillo DJ. Switching P2Y12-receptor inhibitors in patients with coronary artery disease. Nat Rev Cardiol. 2016;13:11–27. doi: 10.1038/nrcardio.2015.113. [DOI] [PubMed] [Google Scholar]
- 14.De Luca L, D’ascenzo F, Musumeci G, et al. Incidence and outcome of switching of oral platelet P2Y12 receptor inhibitors in patients with acute coronary syndromes undergoing percutaneous coronary intervention: the SCOPE registry. EuroIntervention. 2017;13:459–466. doi: 10.4244/EIJ-D-17-00092. [DOI] [PubMed] [Google Scholar]
- 15.Cuisset T, Deharo P, Quilici J, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38:3070–3078. doi: 10.1093/eurheartj/ehx175. [DOI] [PubMed] [Google Scholar]
- 16.Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2018 [Google Scholar]
- 17.University of York, Centre for Reviews and Dissemination . Systematic reviews: CRD’s guidance for undertaking reviews in health care. York: University of York; 2009. [Google Scholar]
- 18.Reboussin DM, Allen NB, Griswold ME, et al. Systematic review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:2176–2198. doi: 10.1016/j.jacc.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JP, Altman DG, Gøtzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells GA, Shea B, O’Connell D et al (2018) The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed 28 November 2018
- 21.Raudenbush SW. Analyzing effect sizes: Random-effects models. In: Cooper H, Hedges LV, Valentine JC, editors. The handbook of research synthesis and meta-analysis. 2. New York: Russell Sage Foundation; 2009. pp. 295–315. [Google Scholar]
- 22.Jiang J. Linear and generalized linear mixed models and their applications. New York: Springer; 2007. [Google Scholar]
- 23.Verbyla A. Modelling variance heterogeneity: residual maximum likelihood and diagnostics. J Roy Stat Soc B. 1993;55:493–508. [Google Scholar]
- 24.Viechtbauer W (2010) Conducting meta-analyses in R with the metafor package. J Stat Softw 36:1-48. http://www.jstatsoft.org/v36/i03/
- 25.Angerås O, Hasvold P, Thuresson M, Deleskog A, Öbraun O. Treatment pattern of contemporary dual antiplatelet therapies after acute coronary syndrome: a Swedish nationwide population-based cohort study. Scand Cardiovasc J. 2016;50:99–107. doi: 10.3109/14017431.2015.1119304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biscaglia S, Campo G, Pavasini R, Tebaldi M, Tumscitz C, Ferrari R. Occurrence, causes, and outcome after switching from ticagrelor to clopidogrel in a real-life scenario: data from a prospective registry. Platelets. 2016;27:484–487. doi: 10.3109/09537104.2015.1119815. [DOI] [PubMed] [Google Scholar]
- 27.Coons JC, Iasella CJ, Chanas T, et al. Comparative effectiveness and safety analysis of dual antiplatelet therapies within an integrated delivery system. Ann Pharmacother. 2017;51:649–655. doi: 10.1177/1060028017706977. [DOI] [PubMed] [Google Scholar]
- 28.Dehghani P, Chopra V, Bell A, et al. Southern saskatchewan ticagrelor registry experience. Patient Prefer Adherence. 2014;8:1427–1435. doi: 10.2147/PPA.S68423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Déry JP, Mehta SR, Fisher HN, et al. Baseline characteristics, adenosine diphosphate receptor inhibitor treatment patterns, and in-hospital outcomes of myocardial infarction patients undergoing percutaneous coronary intervention in the prospective Canadian Observational AntiPlatelet sTudy (COAPT) Am Heart J. 2016;181:26–34. doi: 10.1016/j.ahj.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Gaubert M, Laine M, Richard T, et al. Effect of ticagrelor-related dyspnea on compliance with therapy in acute coronary syndrome patients. Int J Cardiol. 2014;173:120–121. doi: 10.1016/j.ijcard.2014.02.028. [DOI] [PubMed] [Google Scholar]
- 31.Green A, Pottegård A, Broe A, et al. Initiation and persistence with dual antiplatelet therapy after acute myocardial infarction: a Danish nationwide population-based cohort study. BMJ Open. 2016;6:e010880. doi: 10.1136/bmjopen-2015-010880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamid T, Zaman M, Rose S, Malik N. Switching of ticagrelor to clopidogrel at 3 months in patients treated for acute coronary syndrome; single centre experience. Cardiovasc Pharm Open Access. 2016;5:194. doi: 10.4172/2329-6607.1000194. [DOI] [Google Scholar]
- 33.Harding SA, Holley A, Wilkins B, Fairley S, Simmonds M, Larsen PD. Contemporary antiplatelet therapy in acute coronary syndromes: are there differences in outcomes and discontinuation between clopidogrel and ticagrelor. Intern Med J. 2017;47:1298–1305. doi: 10.1111/imj.13595. [DOI] [PubMed] [Google Scholar]
- 34.Simeone JC, Molife C, Marrett E, et al. One-year post-discharge resource utilization and treatment patterns of patients with acute coronary syndrome managed with percutaneous coronary intervention and treated with ticagrelor or prasugrel. Am J Cardiovasc Drugs. 2015;15:337–350. doi: 10.1007/s40256-015-0147-y. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Xi S, Liu J, et al. Switching between ticagrelor and clopidogrel in patients who underwent percutaneous coronary intervention: insight into contemporary practice in Chinese patients. Eur Heart J Suppl. 2016;18:F19–F26. doi: 10.1093/eurheartj/suw034. [DOI] [PubMed] [Google Scholar]
- 36.Zettler ME, Peterson ED, McCoy LA, et al. Switching of adenosine diphosphate receptor inhibitor after hospital discharge among myocardial infarction patients: insights from the treatment with adenosine diphosphate receptor inhibitors: longitudinal assessment of treatment patterns and events after acute coronary syndrome (TRANSLATE-ACS) observational study. Am Heart J. 2017;183:62–68. doi: 10.1016/j.ahj.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Motovska Z, Hlinomaz O, Kala P, et al. 1-year outcomes of patients undergoing primary angioplasty for myocardial infarction treated with prasugrel versus ticagrelor. J Am Coll Cardiol. 2018;71:371–381. doi: 10.1016/j.jacc.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 38.Pourdjabbar A, Hibbert B, Chong AY, et al. A randomised study for optimising crossover from ticagrelor to clopidogrel in patients with acute coronary syndrome. The CAPITAL OPTI-CROSS study. Thromb. Haemost. 2017 doi: 10.1160/TH16-04-0340. [DOI] [PubMed] [Google Scholar]
- 39.Xu L-X, Chen K-Y, Liu T, Zheng X-T, Che J-J, Li G. Comparisons of loading doses of ticagrelor versus clopidogrel in preventing periprocedural myocardial infarction. J Am Coll Cardiol. 2016;68:C89–C90. doi: 10.1016/j.jacc.2016.07.337. [DOI] [Google Scholar]
- 40.Franchi F, Rollini F, Rivas Rios J, et al. Pharmacodynamic effects of switching from ticagrelor to clopidogrel in patients with coronary artery disease: results of the SWAP-4 study. Circulation. 2018;137:2450–2462. doi: 10.1161/CIRCULATIONAHA.118.033983. [DOI] [PubMed] [Google Scholar]
- 41.Angiolillo DJ. Dual antiplatelet therapy guided by platelet function testing. Lancet. 2017;390:1718–1720. doi: 10.1016/S0140-6736(17)32279-1. [DOI] [PubMed] [Google Scholar]
- 42.Moon JY, Franchi F, Rollini F, et al. Role of genetic testing in patients undergoing percutaneous coronary intervention. Expert Rev Clin Pharmacol. 2018;11:151–164. doi: 10.1080/17512433.2017.1353909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zanchin T, Temperli F, Karagiannis A, et al. Frequency, reasons, and impact of premature ticagrelor discontinuation in patients undergoing coronary revascularization in routine clinical practice: results from the bern percutaneous coronary intervention registry. Circ Cardiovasc Interv. 2018;11(5):e006132. doi: 10.1161/CIRCINTERVENTIONS.117.006132. [DOI] [PubMed] [Google Scholar]
- 44.Sibbing D, Aradi D, Alexopoulos D et al (2019) Updated expert consensus statement on platelet function and genetic testing for guiding P2Y12 receptor inhibitor treatment in percutaneous intervention. JACC Cardiovasc Interv (in press) [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.