Abstract
Purpose:
Implantable cardioverter-defibrillators (ICDs) improve survival of systolic heart failure (HF) patients who are at risk of sudden cardiac death (SCD). We recently showed that electrocardiographic (ECG) global electrical heterogeneity (GEH) is independently associated with SCD in the community-dwelling cohort, and developed GEH SCD risk score. The Global Electrical Heterogeneity and Clinical Outcomes (GEHCO) study is a retrospective multicenter cohort designed with two goals: (1) validate an independent association of ECG GEH with sustained ventricular tachyarrhythmias and appropriate ICD therapies, and (2) validate GEH ECG risk score for prediction of sustained ventricular tachyarrhythmias and appropriate ICD therapies in systolic HF patients with primary prevention ICD.
Methods:
All records of primary prevention ICD recipients with available data for analysis are eligible for inclusion. Records of ICD implantation in patients with inherited channelopathies and cardiomyopathies are excluded. Raw digital 12-lead pre-implant ECGs will be used to measure GEH (spatial QRS-T angle, spatial ventricular gradient magnitude, azimuth, and elevation, and sum absolute QRST integral). The primary endpoint is defined as a sustained ventricular tachyarrhythmia event with appropriate ICD therapy. All-cause death without preceding sustained ventricular tachyarrhythmia with appropriate ICD therapy will serve as a primary competing outcome. The study will draw data from the academic medical centers.
Results:
We describe the study protocol of the first multicenter retrospective cohort of primary prevention ICD patients with recorded at baseline digital 12-lead ECG.
Conclusion:
Findings from this study will inform future trials to identify patients who are most likely to benefit from primary prevention ICD.
Keywords: Sudden cardiac death, implantable cardioverter-defibrillators, electrocardiogram, vectorcardiogram, ventricular arrhythmias, risk stratification
Background:
Background and Rationale
Sudden cardiac death (SCD) remains a significant world-wide public health problem despite advances in the prevention, diagnosis, and treatment of all forms of cardiovascular disease. The incidence of SCD in the United States is approximately 111 events per 100,000 persons, accounting for approximately 356,000 deaths annually,[1] and every year SCD accounts for over 6 million deaths world-wide[2]. Patients with structural heart disease and reduced left ventricular ejection fraction (LVEF) have a significantly elevated risk of SCD, most often due to the ventricular arrhythmias ventricular tachycardia (VT) and ventricular fibrillation (VF). Implantable cardioverter-defibrillators (ICDs) have been shown to improve survival in survivors of cardiac arrest [3–6], and for primary prevention of SCD in select patients with reduced LVEF and other risk factors [7–9]. Currently accepted indications for primary prevention ICD implantation [10], however, lack both specificity and sensitivity, and many patients who are implanted with ICDs never experience a ventricular arrhythmia that requires appropriate ICD therapy, and therefore derive no benefit from ICD implantation.
A great deal of investigation has been performed evaluating patient characteristics, cardiac imaging, invasive electrophysiology studies, and various electrocardiographic and autonomic parameters to predict which patients are most likely to benefit from primary prevention ICD implantation [11], yet, results have overall been mixed, and currently, testing other than assessment of LVEF has not yet been incorporated into routine clinical practice [10]. As ICD implantation is an invasive procedure associated with risks [12] and significant costs to the medical system [13], it is critical to identify patients who are likely to benefit from primary prevention ICD implantation. A SCD risk stratification tool which can easily and inexpensively be applied to patients with reduced LVEF to further refine the risk of SCD/VT/VF, and therefore the benefit to ICD implantation, is needed.
Global Electrical Heterogeneity and Ventricular Arrhythmias:
In the 1930s, Frank Wilson introduced the foundation for the “ventricular gradient” concept [14, 15]. He conceptualized the mean electromotive forces/mean electrical axes of the heart during depolarization (QRS complex) and repolarization (T wave) as vectors with X and Y components equal to the projections of the areas of the QRS complex and T wave, respectively, along the X and Y axes in the frontal plane. He demonstrated how the sum of the QRS area and T area in a single lead would always equal zero unless there was heterogeneity involving duration of the excited state (resulting from heterogeneity of action potential duration or morphology, or heterogeneity in spatiotemporal depolarization and repolarization) along the area of myocardium represented by the ECG lead. Wilson described how calculation of a third vector, defined as the sum of the QRS area vector and the T area vector (the “ventricular gradient” vector), represented the mean electrical axis of the heart and the net effect of any local variations in the excitation and recovery process in the frontal plane.[16]
This concept was later extended into 3-dimensions (the “spatial ventricular gradient” [SVG]) by other investigators [17, 18], and subsequent theoretical and experimental evaluations confirmed Wilson’s theory that the SVG vector always points towards the area of myocardium with the shortest duration of the excited state (total recovery time) and in the direction in which non-uniformity in excitation and repolarization were maximum.[19–22] The SVG is therefore a measure of overall electrical heterogeneity in the heart. If all myocardial cells had uniform action potential morphology and duration, and there was no spatiotemporal variation in depolarization and repolarization, the SVG would equal zero. The SVG is therefore an electrophysiological measure of the degree of cardiac global electrical heterogeneity (GEH) which is present, and the direction along which it is greatest.[16] The SVG concept was later extended to the QRS-T angle (the 3-dimensional angle between the mean QRS and mean T vectors)[23], and the sum absolute QRST integral (a scalar analogue of the SVG which is represented by the sum of absolute area of the QRST complex in the orthogonal X, Y, and Z ECG leads[24], which all together comprehansively assess GEH.
Normal myocardial electrical heterogeneity results from variation in action potential duration and morphology in different parts of the heart (e.g., right ventricle vs. left ventricle, epicardium vs. endocardium, apical vs. basal, etc.)[25–27]. Although a physiological amount of electrical heterogeneity is normal, pathological amounts of electrical heterogeneity, often seen in cardiac disease states such as acute ischemia or infarction, are highly proarrhythmic [28, 29]. Assessment of GEH is therefore a physiological and logical way of improving SCD risk stratification.
Our group previously evaluated vectorcardiographic GEH parameters based on the concept of Wilson’s ventricular gradient (SAI QRST, QRS-T angle, and SVG) in >20,000 participants in the Atherosclerosis Risk In Communities (ARIC) study and Cardiovascular Health Study (CHS) to develop novel SCD risk scores for use in the general population[30]. We demonstrated an independent association between GEH parameters and SCD in the general population after adjustment for multiple known SCD risk factors, standard ECG parameters, and time updated incident cardiovascular events. GEH parameters were also more specific for SCD than for non-sudden cardiac death and non-cardiac death, and large changes in GEH ECG parameters over time were also associated with increased risk of SCD. Of critical importance was that the associations between GEH parameters and SCD were independent of LVEF, underscoring how they represent the electrophysiological substrate associated with ventricular arrhythmias rather than simply being markers of more advanced structural heart disease/heart failure. We then developed SCD risk scores using simple demographics and clinical characteristics (age, gender, coronary heart disease, stroke, diabetes, and hypertension) and the 5 GEH ECG parameters obtained from a standard 12-lead ECG. Our final risk score was associated with a C-statistic of 0.790 for prediction of SCD, and was specific for an elevated risk of SCD over other modes of death[30].
However, GEH parameters have been less well studied in patients with structural heart disease and/or in patients with ICDs. In a sub-analysis of the second Multicenter Automated Defibrillator Implantation Trial (MADIT-II), elevations in SAI QRST were associated with increased rates of SCD or ICD therapies for VT or VF[31], while in the PROSE-ICD study, lower SAI QRST was associated with a significantly increased risk of appropriate ICD therapies[32]. QRST angle has similarly been associated with appropriate ICD therapies for VT or VF in patients with infarct-related cardiomyopathy [33]. As of yet there are no studies evaluating the SVG in patients with structural heart disease.
Objectives
To address listed above knowledge gaps, we have designed a retrospective multicenter cohort study with two goals: 1) validate an independent association between GEH ECG parameters and sustained ventricular arrhythmias and appropriate ICD therapies in patients with structural heart disease and primary prevention ICDs, and 2) validate (and re-calibrate, if needed) GEH ECG risk score for use in patients who are candidates for primary prevention ICD implantation to accurately assess the risk of subsequent sustained ventricular arrhythmias and appropriate ICD therapies (as a marker of ICD utility).
Study design
Retrospective multicenter cohort study.
Methods:
Study protocol is reported using SPIRIT [34] guidelines; checklist is provided as a Supplement A. This is the protocol version #1, of June 8th, 2017.
Study Settings:
The current study is a retrospective, multicenter cohort study that will draw patients from the academic medical centers in the United States: Oregon Health & Science University (OHSU) in Portland, OR, Veteran Administration (VA) Portland Healthcare System in Portland, OR, Beth Israel Deaconess Medical Center (BIDMC) in Boston, MA, Cleveland Clinic in Cleveland, Ohio, University of Colorado in Aurora, CO, Cedars-Sinai Medical Center in Los Angeles, CA, Massachusetts General Hospital, Boston, MA, and Stanford University in Palo Alto, CA.
Eligibility criteria
Retrospective patients’ records are eligible for inclusion in the study if at the time of device implantation patients were 18–89 years of age, had chronic systolic HF due to infarct-related cardiomyopathy or non-ischemic cardiomyopathy, and underwent an ICD (including single chamber, dual chamber, and cardiac resynchronization defibrillator [CRT-D]) implantation for primary prevention of SCD based on guidelines-based clinical indications [10]. In order to obtain a relatively homogenous population reflecting the population for which the majority of primary prevention ICDs are implanted, records of patient with relatively uncommon indications for ICD implantation such as inherited channelopathies (eg. Long QT Syndrone and Brugada Syndrome), inherited cardiomyopathies (eg. hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy), and congenital heart disease will be excluded from the study. Patients’ records with absent digital ECG, and missing outcomes data will also be excluded.
Outcomes:
Primary outcome:
The primary outcome of this study is defined as a sustained ventricular tachyarrhythmia event with appropriate ICD therapy (either anti-tachycardia pacing (ATP) or shock). All ICD therapies will be adjudicated by local investigators blinded to the results of ECG analyses. Event adjudication form is shown in Table 1.
Table 1.
ICD Event adjudication form

|
Primary competing outcome:
All-cause death without preceding sustained ventricular tachyarrhythmia with appropriate ICD therapy will serve as a primary competing outcome. The death event data will be obtained from the local medical records and the Social Security Death Index. Cause of death will be collected if available in the medical record.
Secondary outcomes:
Two secondary outcomes will be adjudicated:
ICD therapies for monomorphic ventricular tachycardia (MMVT). MMVT is defined as a tachycardia of ventricular origin with identical or nearly identical far field electrograms (EGM), and a stable cycle length [CL] (beat-to-beat CL differences< 20 ms).
Polymorphic ventricular tachycardia (PVT) or ventricular fibrillation (VF). PVT is defined as a tachycardia of ventricular origin with beat to beat variation in far-field EGM morphology, and unstable CL (beat-to-beat CL differences ≥ 20 ms), if average CL ≥200 ms. VF is defined as a sustained ventricular tachyarrhythmia with unstable cycle length and EGM morphology, and average CL <200 ms).
Adjudication of clinical outcomes
Adjudication of the outcomes will be performed at each participating medical center, by the study investigators. At each participating institution, three-member end-points adjudication committee is comprised by physicians (cardiologists, electrophysiologists, fellows-in-training). Two adjudication committee members review all available clinical data of the event, including EGMs. Event adjudication form is shown in Table 1. In case of disagreement between two adjudicators, the 3rd investigator determines an event classification.
Retrospective timeline
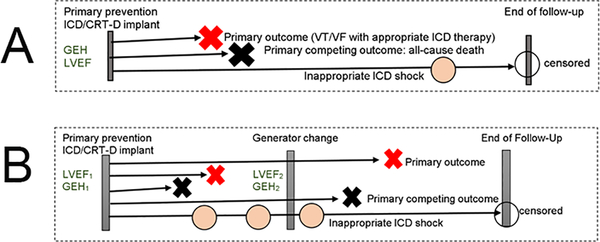
Retrospective follow-up starts at the time of baseline pre-implant ECG recording, prior to initial ICD implantation. Retrospective follow-up continues until primary outcome, or primary competing outcome (all-cause death), or end of the study period, or loss to follow-up, whichever comes first. Patients lost to follow-up will be censored at the last available follow-up visit. Retrospective follow-up timeline is shown in Figure 1. Schematic presentation of the study is shown in Figure 2.
Figure 1.
Retrospective Follow-Up timeline
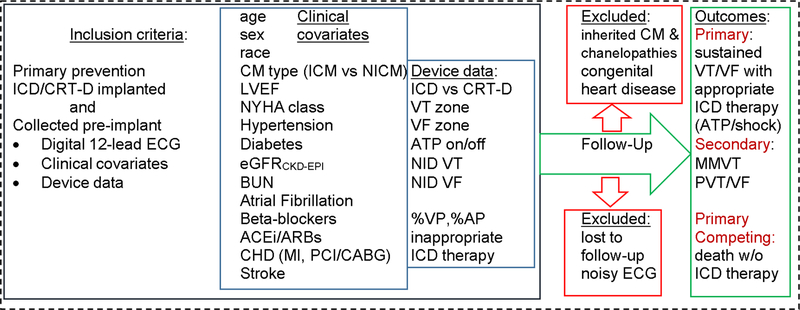
Figure 2.
Schematic presentation of the study
Sample size
In our previous work GEH variables demonstrated hazard ratio of at least 1.15 (per 1 standard deviation (SD) of GEH parameter) [30] for association with SCD. We are planning a study with a retrospective accrual interval of 8 years, and additional retrospective follow-up after the accrual interval of mean 4.5 years in our retrospective cohort. For this statistical power calculation, the rate of appropriate ICD therapies in our retrospective cohort is estimated 7.1% per year. The rate of all-cause death without preceding ICD therapy in this population was previously reported (5% per year)[35]. If the true hazard ratio per 1SD increase of GEH variable is 1.15, we will need to study 4,024 subjects to be able to reject the null hypothesis that the control (at the reference value of GEH variable) and the experimental (per 1 SD increase of GEH variable) survival curves are equal with probability (power) 0.8. The Type I error probability associated with this test is 0.05. The required sample size decreases as a function of increasing hazard ratio (Table 2).
Table 2.
Sample size estimation
| Hazard Ratio | Sample size, n |
|---|---|
| 1.15 | 4,024 |
| 1.2 | 2,400 |
| 1.25 | 1,628 |
| 1.3 | 1,202 |
| 1.35 | 924 |
| 1.4 | 748 |
| 1.5 | 528 |
Data collection methods and definition of covariates
Data will be abstracted from eligible patients’ records by local investigators. Information on patient demographics, medial history, and device programming will be collected at the time of initial ICD implantation (Figure 2). A digital 12-lead ECG from the time of initial ICD implantation will be collected and processed as noted below.
Patients’ clinical characteristics (listed in Figure 2) at the date of pre-implant clinical evaluation will be abstracted from the medical record. Glomerular filtration rate (eGFRCKD-EPI) is estimated using the Chronic Kidney Disease Epidemiology Collaboration equation [36].
Definitions of ICD/CRT-D device programming and device-saved data.
ICDs were programmed at the discretion of the attending implanting electrophysiologist. Characteristics of baseline device programming (number of intervals to detect [NID] or time to detect, the number of detection zones, heart rate for each detection zone, and anti-tachycardia pacing [ATP] programming) data are collected at the time of device implant and/or at time of appropriate ICD therapy (ATP or shock). To minimize bias due to the change of programming during follow-up, date of generator replacement and new programming data are collected. If the patient experienced inappropriate ICD shock(s), characteristics of programming changes are collected for appropriate adjustment of analyses. In patients receiving CRT-D implants, the location of the LV lead is categorized as apical, mid, or basal, and anterior, anterolateral, posterior, or posterolateral.
Data regarding inappropriate ICD shocks during follow-up will be collected as follows: date, the reason for inappropriate shock, and changes in ICD programming after inappropriate shock. Percentage of ventricular pacing (%VP), the percentage of LV pacing (%LVP) if CRT-D, and percentage of atrial pacing (%AP) will be collected at the time of device interrogation within 3 months of the primary outcome, or at the time of censoring. Patients lost to follow-up will be censored at the last available follow-up visit.
Data management
Electronic data capture will be completed using the REDCap web application. The database was constructed by the OHSU study team and provided to all participating centers. REDCap forms are provided in a Supplement B. Data dictionary codebook is provided in a Supplement C. To ensure full de-identification of the data, a blank copy of OHSU’s fully functional REDCap database was used to share between the study centers. The individual centers upload this copy to their own institution’s REDCap database. Within the original database copy, any field that contained protected health information (PHI) was indexed within the software. The research staff at each center will then select the option to redact all marked PHI fields thus allowing for secure data export. Patient-specific dates (including device implantation date, date of death, etc.) were not indexed within the system as PHI. This information will be kept confidential to the collaborators via the “date shift” option within REDCap software. At the time of export, each investigator will select this option, and all dates will be randomly shifted to an unknown date within 0 and 364 days. This protects patient-specific information while preserving the temporal relationship between our covariates. Both of these functions allow for collecting uniformly standard clinical covariates while maintaining patient confidentiality between institutions.
Electrocardiogram Analysis and Calculation of GEH ECG Parameters:
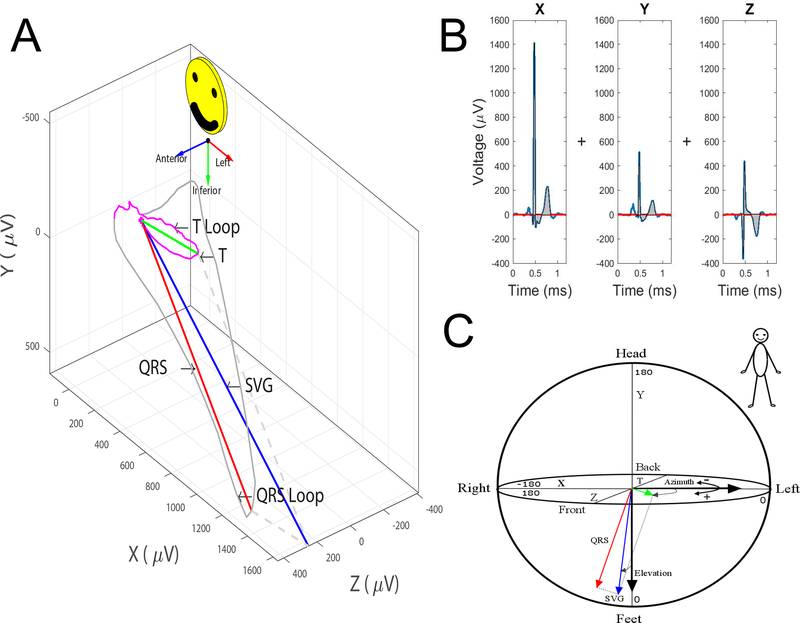
De-identified, unprocessed, digital 12-lead ECGs will be analyzed in the Tereshchenko laboratory at OHSU by investigators blinded to study outcomes. Due to the inclusion of multiple study sites with variable ECG recording equipment, ECGs were recorded on equipment used for routine clinical use at each study site. ECG processing and analysis will be performed using previously developed customized Matlab software (MathWorks, Inc, Natick, MA) that allows semi-automated calculation of GEH ECG parameters from a standard, 10 second, 12-lead ECG as previously described[30]. Briefly, 12-lead ECGs are converted to orthogonal ECGs using Kors transformation[37], as Kors transformation results in VCG signal that is closer to original orthogonal Frank VCG[38]. The median beat is then calculated and used for subsequent analyses and calculation of GEH ECG parameters (Figure 3). Spatial QRS-T angle is calculated as the angle between the peak QRS vector and the peak T vector in 3-dimensional space (Figure 3A). The SVG is a vector defined as the vector sum of the QRS area vector and the T area vector. As a vector, SVG has a magnitude which is the length of the vector, elevation (the angle of the SVG vector in the frontal/XY plane), and azimuth (the angle of the SVG vector projected in the transverse/XZ plane) (Figure 3C). SAI QRST is calculated as the sum of the absolute areas under the entire QRST complex in the X, Y, and Z leads (Figure 3B). All standard ECG parameters (including QT interval, and QRS duration, etc.) will be measured by GE 12SL algorithm on 12-lead ECG.
Figure 3:
Measurement of global electrical heterogeneity parameters: A. Spatial QRS-T angle is the angle between the QRS vector and the T vector in 3-dimensional space. Spatial ventricular gradient (SVG) is a vector defined as the vector sum of the mean QRS and the mean T vectors. SVG has a magnitude which is the length of the vector, elevation (the angle of the SVG vector projected in the XY plane), and azimuth (the angle of the SVG vector projected in the XZ plane). B. Sum Absolute QRST Intergral (SAI QRST) is the sum of the absolute area under the entire QRST complex in the X, Y, and Z leads. C. Circular coordinates system for measurement of azimuth and elevation.
All available ECGs will be analyzed, and only ECGs with extreme baseline noise/artifact that precludes waveform recognition and analysis will be excluded. There are no exclusion criteria based on cardiac rhythm, presence or absence of ventricular pacing, or intraventricular conduction abnormalities.
Statistical Data Analyses: Survival analysis
All variables will be checked for consistency and outliers will be examined. Frequencies and distributions will be examined on all variables. Transformations to normality will be performed as needed. SVG azimuth and elevation will be square root-transformed to normalize their distribution, as it was previously shown that square root transformation is a preferable transformation for these circular variables [30]. To simplify comparison of the strength of association, each predictor change will be expressed per 1 standard deviation (SD) of the predictor.
Survival analysis will be performed to evaluate associations between individual GEH ECG parameters and primary outcomes. To account for competing risks of VT/VF (surrogate of SCD) vs. death, we will construct Fine and Gray’s competing risks models[39]. GEH ECG parameters will be treated as continuous variables. GEH variables will be treated as continuous variables. Multiple models will be constructed to explore the associations between GEH variables and outcomes:
Model 1 will be adjusted for basic demographic characteristics of age, gender, race, and study center. Model 2 will be additionally adjusted for known predictors of VT/VF and mortality including LVEF, NYHA functional class, type of cardiomyopathy, hypertension, diabetes, prior stroke, atrial fibrillation, renal function (as assessed by CKD-EPI eGFR [36]), and use of cardiovascular medications (beta-blockers, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers). Model 3 will include all covariates from model 2 and also ICD characteristics (CRT vs. single/dual chamber, manufacturer, and model), and VT/VF detection parameters (number of tachycardia zones, detection rates for each zone, intervals for detection or time to detection based on manufacturer, and programmed therapy for each tachycardia detection zone), and percentage ventricular pacing. Model 4, in addition, will include time-updated generator change, as well as time-updated inappropriate ICD shocks (updated on the date of event) and percentage of ventricular pacing. Also, associations between GEH parameters and outcomes will be also evaluated using adjusted competing risk models incorporating quadratic splines with 4 knots.
Sensitivity Analyses:
To test the robustness of our findings, we will conduct several sensitivity analyses. To eliminate the effect of unmeasured improvement in VT/VF detection and treatment in each new generation of devices, we will repeat all analyses, considering only one device life (censored at the time of generation replacement). To exclude unmeasured differences in HF management at different time period, we will exclude from analysis records of patients who received device implanted before 2010 and will repeat analysis for modern patient population (device implanted in 2010 and later).
Subgroups analyses
As prior data have suggested that ventricular pacing is an effect modifier of the association between SAI QRST and clinical outcomes[31, 40, 32, 24], models 1–3 will be stratified by the type of ICD (single/dual chamber vs. CRT). In the CRT subgroup, LV lead position and percent LV/biventricular pacing will be added as covariates in model 3.
Test for interaction will be performed for the main clinical subgroups (by cohort, device type (ICD and CRT-D), CM type (ischemic and non-ischemic CM), sex and race), to determine significant interactions between GEH ECG parameters and clinical characteristics.
Statistical analysis of longitudinal changes in GEH ECG parameters
We will also evaluate the association of changes in GEH ECG parameters (before initial ICD implant vs. before 1st ICD generation change) with appropriate ICD therapies for VT/VF after 1st ICD generator replacement.
Generator replacement represents an opportunity to re-evaluate risks and benefits of ICD therapy. At least a quarter of primary prevention ICD recipients improves LVEF above 35% by the time of generator change [41, 42] so that they no longer meet “primary prevention” ICD implantation guidelines. Generator replacement also carries risks, including an increased risk of infection [43–45]. Previous studies have demonstrated that LVEF remains associated with VT/VF after generator replacement [42]. However, it is unknown whether time-updated electrophysiological substrate (GEH) remains associated with VT/VF after generator replacement.
We hypothesize that time-updated GEH ECG parameters and longitudinal changes in GEH ECG parameters are associated with sustained VT/VF treated with appropriate ICD therapies after adjustment for time-updated LVEF, time-updated device programming, time-updated inappropriate ICD shocks, and time-updated generator replacement characteristics. The subgroup of patients who underwent device generator replacement and who have available data (digital ECG, LVEF, new device data), which are necessary for time-updated analyses, will be included in this analysis (Figure 1B).
Mixed models: Does GEH ECH change over time?
First, mixed effects multilevel models (adjusted for age, sex, and race with participants nested within device type (ICD or CRT-D) nested within cohort) will be constructed to determine whether GEH parameters change over time.
Time-updated survival analysis
To investigate whether longitudinal changes in GEH parameters are independently associated with primary outcome, two analyses will be performed.
First, the interaction with time will be assessed in time-updated Cox models to test the assumption of proportionality for the hazard of time-updated variables over time. Time-updated Cox models will evaluate whether an increase or decrease in GEH parameters over time (before initial ICD implant vs. before 1st ICD generation change) is associated with a proportional or non-proportional increase or decrease in the risk of sustained VT/VF over time. Models will be adjusted by time-updated generator-replaced device characteristics (type, programming, %VP/%LVP), time updated inappropriate ICD shocks, and time-updated clinical characteristics (LVEF, NYHA class, eGFRCKD-EPI, and medications use (beta-blockers and ACEi/ARBs).
In addition, to determine if large increases or decreases in GEH parameters between initial ICD implant and first generator replacement are associated with the primary outcome, the relative change in each GEH parameter between the first device implant and the first generator replacement, will be calculated. Categorical variables will be then constructed to categorize the change in each GEH parameter. For all GEH parameters except QRS-T angle, a change of < ±25% of the baseline value will be considered the reference value. Because larger changes in QRS-T angle were observed in our previous analyses of GEH ECG parameters[30], a change of < ±50% of the baseline value of QRS-T angle is defined as the reference value for analyzing changes in QRS-T angle. For SAI QRST, SVG magnitude, SVG elevation, and SVG azimuth, categories of decrease of ≥33%, decrease of 25–32%, increase of 25–32%, increase of 33–49%, and an increase of ≥50% will be compared to the reference category. Categories of decrease of ≥300%, decrease of 50–299%, increase of 50–200%, increase of 201–299%, and an increase of ≥300% will be compared to the reference category for the QRS-T angle.
To answer the question as to whether these temporal changes in GEH parameter values are independently associated with the primary outcome, we will construct Cox proportional hazards regression models and competing risks models. Minimally adjusted model 1 will be adjusted for demographic characteristics (age, gender, race, and study center). Model 2 additionally will be adjusted for known predictors of VT/VF (LVEF, type of cardiomyopathy, hypertension, diabetes), device type and characteristics and the baseline value of each GEH parameter obtained before the first device implant.
GEH Sudden Cardiac Death Risk Score validation:
Our previously developed sudden cardiac death risk scores [30] will be used to estimate the 5-year risk of appropriate ICD therapies for VT/VF (a surrogate for SCD) at the time of initial primary prevention ICD implantation. Receiver operating curves will be used to determine the ability of these existing SCD risk scores to discriminate between patients with and without appropriate ICD therapies during follow-up. We will assess calibration of these existing risk scores in the primary prevention ICD population by comparing how closely the predicted rates of appropriate ICD therapies agreed numerically with observed VT/VF outcomes. Stratification capacity of the risk scores in this population will be assessed using a risk stratification table and defining categories of risk of appropriate ICD therapy after ICD implant as low (<1%), moderate (1–30%), or high (>30%). Classification accuracy and reclassification improvement rates (the extent to which participants with VT/VF events are appropriately or inappropriately assigned/reassigned to high-, intermediate- and low-risk categories with the addition of GEH ECG parameters) will be calculated.
We will then recalibrate the existing risk scores for the primary prevention ICD population, if needed. If necessary, we will re-construct 2 risk scores using Fine and Gray’s competing risk model to test the incremental value of adding GEH parameters as continuous variables to clinical characteristics (age, gender, race, coronary heart disease, stroke, diabetes, and hypertension). Backwards selection will be performed with a cut-off p-value of 0.10 to arrive at the final risk score covariates and their relative strengths of association based on the relative size of effect estimates. A cumulative incidence function (CIF) will be used to assign 5-year VT/VF risk to each participant based on their individual risk scores, as following:
where t is time since initial primary prevention ICD implantation, x represents covariates included in each risk model, and beta represents the coefficient estimates from the risk scores models. The final risk prediction model will be made available through a free online calculator. The risk score’s discrimination capacity, calibration, goodness of fit (using the Akaike Information Criterion [AIC]), and stratification capacity, and reclassification improvement rates will be calculated.
Ethics approval, confidentiality and study registration
The multicenter study has been approved by the OHSU Institutional Review Board (IRB). In addition, each participating center obtains local IRB approval prior to participating. To simplify IRB approval at the study sites, the OHSU IRB offered to the study sites to sign an agreement with OHSU IRB, stating that OHSU IRB provide multi-center IRB oversight (optional). The study is registered at the URL: http://www.clinicaltrials.gov. Unique identifier: NCT03210883.
Discussion
The proposed study will be the first to systematically evaluate the association between GEH ECG parameters and appropriate ICD therapies for ventricular arrhythmias in a large population of HF patients with primary prevention ICDs with the goal of improving the ability to identify patients who are most likely (or least likely) to benefit from ICD implantation. Although randomized controlled trials such as MADIT-II[7], the Multicenter UnSustained Tachycardia Trial (MUSTT)[46], and the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT)[9] have demonstrated a significant survival advantage associated with primary prevention ICD implantation, the benefit in all patients is not uniform. Secondary analyses of these studies have demonstrated that certain patient characteristics, such as severe renal dysfunction, are associated with a lack of benefit to primary prevention ICD implantation due to increased non-arrhythmic competing risks of death, and that despite being very heavily emphasized in the ICD guidelines, LVEF is not a strong predictor of who will or will not benefit from ICD implantation [47–50]. Attempts at refining SCD risk have yielded mixed results, and current guidelines do not recommend testing beyond assessment of LVEF (and electrophysiology studies in select cases) when selecting patients for primary prevention ICD implantation. This is problematic, as LVEF is neither sensitive nor specific for SCD, and in total, more patients with relatively preserved LVEF die suddenly than do patients with significantly reduced LVEF [51, 52]. Factors beyond LVEF, and ideally characteristics which are specific for arrhythmic risk needed to refine our understanding of SCD risk.
GEH is an attractive method for SCD risk stratification because it requires only collection of a standard 12-lead ECG which is widely available, non-invasive, inexpensive, and easily interpretable. Unlike many other risk stratification tools, variability in GEH has also been mechanistically linked to increase susceptibility for ventricular arrhythmias [29, 28]. Although current assessment of GEH ECG parameters requires special software, the algorithms which are used for GEH calculation could easily be automated and included in standard ECG machines to allow simple, wide-spread calculation of these parameters without requiring specialized software or expert interpretation of results. Digitally acquired and stored ECGs are rapidly becoming the standard of care, and automated ECG processing would also potentially allow automated, high-throughput screening of entire populations/institutional ECG databases to identify individuals who would benefit from additional follow-up, testing, and/or treatment to reduce their risk of ventricular arrhythmias or SCD.
The National Cardiovascular Data Registry (NCDR) also maintains a very large prospective registry of patients undergoing ICD implantation. Although the NCDR registry has a significantly larger total study population due to its use throughout the entire United States, our study has advantages over the NCDR registry. Unlike the NCDR, our study has the benefit of highly detailed patient level data, digital ECGs for calculation of GEH parameters, and detailed data on ICD programming and the timing of ventricular arrhythmias and ICD therapies during long-term follow-up. Importantly, outcomes in our study are adjudicated at the study centers by investigators, blinded to GEH ECG parameters. Our study could not be performed using the NCDR as it stands today.
Study data management solutions
A major logistical issue is collecting data from multiple sites while maintaining patient privacy between institutions. All study sites are provided software tools to remove all patient identifiers from digital ECGs before they are transmitted for analysis. In order to maintain patient confidentiality and avoid needing to share identifiable information, the study will utilize REDCap for data collection. Each study site will have a local REDCap database maintained on their secure institutional network where patient information will be entered. Before data is transferred via REDCap to OHSU for further analysis, all patient identifiers will be redacted, and all dates shifted to an unknown value that is only consistent within each subject’s record.
Strengths and Limitations
One significant strength of the proposed study is that it will include a very large cohort of patients from multiple institutions, which should make the results widely generalizable. The large number of study centers also affords us a large study population which will allow the study to be adequately powered (see methods section above) to detect (or refute) associations between GEH ECG parameters and ICD therapies, and to validate GEH SCD risk score, or derive a novel risk score for appropriate ICD therapies after primary prevention ICD implantation. One significant limitation is that the study is retrospective and observational. Unidentified confounders could therefore influence the results. If the results of this retrospective study are positive, however, they will provide data to support the use of GEH ECG parameters in a future prospective study. Additionally, as patients will be included who have had ICD implanted over a wide time period, changes in medical therapy, ICD technology, and ICD programming may also influence the results. This limitation will be addressed by two pre-defined sensitivity analyses, as described above. In general, however, guidelines for primary prevention ICD implantation have not changed significantly over the last decade.
Conclusion
Primary prevention ICDs improve survival in patients with structural heart disease and significantly reduced LV systolic function, but further study is needed to optimally identify those patients who are most (and least) likely to benefit from ICD implantation. This study will be the first to retrospectively investigate the associations between GEH ECG parameters and appropriate ICD therapies for ventricular arrhythmias in a large multicenter population of patients with primary prevention ICDs. The study will validate SCD risk score in patients with structural heart disease and LV systolic dysfunction and will inform future prospective randomized controlled trials.
Supplementary Material
Funding:
This study is funded through the AHA Grant-In-Aid #17GRNT33670428. AHA has no any role in study design, collection, management, analysis, and interpretation of data, and reporting the study results. This publication was made possible with support from the Oregon Clinical and Translational Research Institute (OCTRI), grant number UL1 RR024140 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Abbreviations:
- GEH
global electrical heterogeneity
- ICD
implantable cardioverter-defibrillator
- SCD
sudden cardiac death
- ECG
electrocardiogram
- LVEF
left ventricular ejection fraction
- VT
ventricular tachycardia
- VF
ventricular fibrillation
- SVG
spatial ventricular gradient
- AHA
American Heart Association
Footnotes
Trial registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT03210883
Conflict of Interests:
The Johns Hopkins University owns the patent US8880159 B2 “Methods for determining risk of ventricular arrhythmia” (Inventor LGT; 100% assigned to the Johns Hopkins University; not licensed).
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehra R Global public health problem of sudden cardiac death. Journal of electrocardiology. 2007;40(6 Suppl):S118–22. doi: 10.1016/j.jelectrocard.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 3.A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997;337(22):1576–83. doi: 10.1056/nejm199711273372202. [DOI] [PubMed] [Google Scholar]
- 4.Connolly SJ, Hallstrom AP, Cappato R, Schron EB, Kuck KH, Zipes DP et al. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. Antiarrhythmics vs Implantable Defibrillator study. Cardiac Arrest Study Hamburg. Canadian Implantable Defibrillator Study. Eur Heart J. 2000;21(24):2071–8. doi: 10.1053/euhj.2000.2476. [DOI] [PubMed] [Google Scholar]
- 5.Connolly SJ, Gent M, Roberts RS, Dorian P, Roy D, Sheldon RS et al. Canadian Implantable Defibrillator Study (CIDS). A Randomized Trial of the Implantable Cardioverter Defibrillator Against Amiodarone. 2000;101(11):1297–302. doi: 10.1161/01.cir.101.11.1297. [DOI] [PubMed] [Google Scholar]
- 6.Kuck KH, Cappato R, Siebels J, Ruppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest : the Cardiac Arrest Study Hamburg (CASH). Circulation. 2000;102(7):748–54. [DOI] [PubMed] [Google Scholar]
- 7.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS et al. Prophylactic Implantation of a Defibrillator in Patients with Myocardial Infarction and Reduced Ejection Fraction. N Engl J Med. 2002;346:877–83. [DOI] [PubMed] [Google Scholar]
- 8.Buxton A, Hafley G, Lee K, Gold M, Packer D, Lehmann M et al. Relation of ejection fraction and inducible ventricular tachycardia to mode of death in patients with coronary artery disease. Circulation. 2002;106:2466–72. [DOI] [PubMed] [Google Scholar]
- 9.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. [DOI] [PubMed] [Google Scholar]
- 10.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA 3rd, Freedman RA, Gettes LS et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51(21):e1–62. doi: 10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 11.Wellens HJJ, Schwartz PJ, Lindemans FW, Buxton AE, Goldberger JJ, Hohnloser SH et al. Risk stratification for sudden cardiac death: current status and challenges for the future(). European Heart Journal. 2014;35(25):1642–51. doi: 10.1093/eurheartj/ehu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ezzat VA, Lee V, Ahsan S, Chow AW, Segal O, Rowland E et al. A systematic review of ICD complications in randomised controlled trials versus registries: is our ‘real-world’ data an underestimation? Open Heart. 2015;2(1). doi: 10.1136/openhrt-2014-000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groeneveld PW, Matta MA, Suh JJ, Heidenreich PA, Shea JA. Costs and quality-of-life effects of implantable cardioverter-defibrillators. Am J Cardiol. 2006;98(10):1409–15. doi: 10.1016/j.amjcard.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 14.Wilson FN, Macleod AG, Barker PS, Johnston FD. The determination and the significance of the areas of the ventricular deflections of the electrocardiogram. American Heart Journal. 1934;10(1):46–61. doi: 10.1016/S0002-8703(34)90303-3. [DOI] [Google Scholar]
- 15.Wilson FN, Macleon FS, Barker PS. The T Deflection of the Electrocardiogram. Transactions of the Association of American Physicians. 1931;46:29–38. [Google Scholar]
- 16.Waks JW, Tereshchenko LG. Global electrical heterogeneity: A review of the spatial ventricular gradient. J Electrocardiol. 2016;49(6):824–30. doi: 10.1016/j.jelectrocard.2016.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burch GE, Abildskov AA, Cronvich JA. A study of the spatial vectorcardiogram of the ventricular gradient. Circulation. 1954;9(2):267–75. [DOI] [PubMed] [Google Scholar]
- 18.Simonson E, Schmitt OH, Dahl J, Fry D, Bakken EE. The theoretical and experimental bases of the frontal plane ventricular gradient and its spatial counterpart. American heart journal. 1954;47(1):122–53. doi: 10.1016/0002-8703(54)90221-5. [DOI] [PubMed] [Google Scholar]
- 19.Hurst JW. Thoughts about the ventricular gradient and its current clinical use (Part I of II). Clinical cardiology. 2005;28(4):175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurst JW. Thoughts about the ventricular gradient and its current clinical use (part II of II). Clinical cardiology. 2005;28(5):219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burger HC. A theoretical elucidation of the notion ventricular gradient. American heart journal. 1957;53(2):240–6. [DOI] [PubMed] [Google Scholar]
- 22.Gardberg M, Rosen IL. The ventricular gradient of Wilson. Annals of the New York Academy of Sciences. 1957;65(6):873–93. [DOI] [PubMed] [Google Scholar]
- 23.Oehler A, Feldman T, Henrikson CA, Tereshchenko LG. QRS-T Angle: A Review. Ann Noninvasive Electrocardiol. 2014;19(6):534–42. doi: 10.1111/anec.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tereshchenko LG, Cheng A, Fetics BJ, Butcher B, Marine JE, Spragg DD et al. A new electrocardiogram marker to identify patients at low risk for ventricular tachyarrhythmias: sum magnitude of the absolute QRST integral. Journal of electrocardiology. 2011;44(2):208–16. doi: 10.1016/j.jelectrocard.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antzelevitch C, Dumaine R. Electrical Heterogeneity in the Heart: Physiological, Pharmacological and Clinical Implications Comprehensive Physiology. John Wiley & Sons, Inc.; 2011. [Google Scholar]
- 26.Boukens BJ, Walton R, Meijborg VM, Coronel R. Transmural electrophysiological heterogeneity, the T-wave and ventricular arrhythmias. Progress in biophysics and molecular biology. 2016;122(3):202–14. doi: 10.1016/j.pbiomolbio.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Prenner SB, Shah SJ, Goldberger JJ, Sauer AJ. Repolarization Heterogeneity: Beyond the QT Interval. Journal of the American Heart Association. 2016;5(5). doi: 10.1161/jaha.116.003607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vassallo JA, Cassidy DM, Kindwall KE, Marchlinski FE, Josephson ME. Nonuniform recovery of excitability in the left ventricle. Circulation. 1988;78(6):1365–72. [DOI] [PubMed] [Google Scholar]
- 29.Kuo CS, Munakata K, Reddy CP, Surawicz B. Characteristics and possible mechanism of ventricular arrhythmia dependent on the dispersion of action potential durations. Circulation. 1983;67(6):1356–67. [DOI] [PubMed] [Google Scholar]
- 30.Waks JW, Sitlani CM, Soliman EZ, Kabir M, Ghafoori E, Biggs ML et al. Global Electric Heterogeneity Risk Score for Prediction of Sudden Cardiac Death in the General Population: The Atherosclerosis Risk in Communities (ARIC) and Cardiovascular Health (CHS) Studies. Circulation. 2016;133(23):2222–34. doi: 10.1161/CIRCULATIONAHA.116.021306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tereshchenko LG, McNitt S, Han L, Berger RD, Zareba W. ECG Marker of Adverse Electrical Remodeling Post-Myocardial Infarction Predicts Outcomes in MADIT II Study. PloS one. 2012;7(12):e51812. doi: 10.1371/journal.pone.0051812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tereshchenko LG, Cheng A, Fetics BJ, Marine JE, Spragg DD, Sinha S et al. Ventricular Arrhythmia is predicted by Sum Absolute QRST Integral, but not by QRS width. Journal of electrocardiology. 2010;43(6):548–52. doi: 10.1016/j.jelectrocard.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borleffs CJ, Scherptong RW, Man SC, van Welsenes GH, Bax JJ, van Erven L et al. Predicting ventricular arrhythmias in patients with ischemic heart disease: clinical application of the ECG-derived QRS-T angle. Circulation Arrhythmia and electrophysiology. 2009;2(5):548–54. doi: 10.1161/circep.109.859108. [DOI] [PubMed] [Google Scholar]
- 34.Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gotzsche PC, Krleza-Jeric K et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–7. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Guallar E, Blasco-Colmenares E, Dalal D, Butcher B, Norgard S et al. Clinical and serum-based markers are associated with death within 1 year of de novo implant in primary prevention ICD recipients. Heart Rhythm. 2015;12(2):360–6. doi: 10.1016/j.hrthm.2014.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kors JA, van HG, Sittig AC, van Bemmel JH. Reconstruction of the Frank vectorcardiogram from standard electrocardiographic leads: diagnostic comparison of different methods. EurHeart J. 1990;11(12):1083–92. [DOI] [PubMed] [Google Scholar]
- 38.Cortez D, Sharma N, Devers C, Devers E, Schlegel TT. Visual transform applications for estimating the spatial QRS-T angle from the conventional 12-lead ECG: Kors is still most Frank. J Electrocardiol. 2014;47(1):12–9. doi: 10.1016/j.jelectrocard.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 40.Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA et al. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol. 2010;55(20):2212–21. doi: 10.1016/j.jacc.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Guallar E, Blasco-Colmenares E, Butcher B, Norgard S, Nauffal V et al. Changes in Follow-Up Left Ventricular Ejection Fraction Associated With Outcomes in Primary Prevention Implantable Cardioverter-Defibrillator and Cardiac Resynchronization Therapy Device Recipients. Journal of the American College of Cardiology. 2015;66(5):524–31. doi: 10.1016/j.jacc.2015.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madhavan M, Waks JW, Friedman PA, Kramer DB, Buxton AE, Noseworthy PA et al. Outcomes After Implantable Cardioverter-Defibrillator Generator Replacement for Primary Prevention of Sudden Cardiac Death. Circ Arrhythm Electrophysiol. 2016;9(3):e003283. doi: 10.1161/CIRCEP.115.003283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kramer DB, Kennedy KF, Noseworthy PA, Buxton AE, Josephson ME, Normand SL et al. Characteristics and outcomes of patients receiving new and replacement implantable cardioverter-defibrillators: results from the NCDR. Circ Cardiovasc QualOutcomes. 2013;6(4):488–97. doi:CIRCOUTCOMES.111.000054 [pii];10.1161/CIRCOUTCOMES.111.000054 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krahn AD, Lee DS, Birnie D, Healey JS, Crystal E, Dorian P et al. Predictors of short-term complications after implantable cardioverter-defibrillator replacement: results from the Ontario ICD Database. CircArrhythmElectrophysiol. 2011;4(2):136–42. doi:CIRCEP.110.959791 [pii];10.1161/CIRCEP.110.959791 [doi]. [DOI] [PubMed] [Google Scholar]
- 45.Poole JE, Gleva MJ, Mela T, Chung MK, Uslan DZ, Borge R et al. Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: results from the REPLACE registry. Circulation. 2010;122(16):1553–61. doi: 10.1161/CIRCULATIONAHA.110.976076. [DOI] [PubMed] [Google Scholar]
- 46.Buxton AE, Lee KL, DiCarlo L, Gold MR, Greer GS, Prystowsky EN et al. Electrophysiologic testing to identify patients with coronary artery disease who are at risk for sudden death. New England Journal of Medicine. 2000;342(26):1937–45. [DOI] [PubMed] [Google Scholar]
- 47.Goldenberg I, Vyas AK, Hall WJ, Moss AJ, Wang H, He H et al. Risk Stratification for Primary Implantation of a Cardioverter-Defibrillator in Patients With Ischemic Left Ventricular Dysfunction. J AM Coll Cardiol. 2007;51:288–96. [DOI] [PubMed] [Google Scholar]
- 48.Buxton AE, Lee KL, Hafley GE, Pires LA, Fisher JD, Gold MR et al. Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease. Lessons from the MUSTT study. J Am Coll Cardiol. 2007;50:1150–7. [DOI] [PubMed] [Google Scholar]
- 49.Bilchick KC, Stukenborg GJ, Kamath S, Cheng A. Prediction of mortality in clinical practice for medicare patients undergoing defibrillator implantation for primary prevention of sudden cardiac death. J Am Coll Cardiol. 2012;60(17):1647–55. doi: 10.1016/j.jacc.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Levy WC, Lee KL, Hellkamp AS, Poole JE, Mozaffarian D, Linker DT et al. Maximizing survival benefit with primary prevention implantable cardioverter-defibrillator therapy in a heart failure population. Circulation. 2009;120(10):835–42. doi: 10.1161/circulationaha.108.816884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yap YG, Duong T, Bland JM, Malik M, Torp-Pedersen C, Køber L et al. Optimising the dichotomy limit for left ventricular ejection fraction in selecting patients for defibrillator therapy after myocardial infarction. Heart. 2007;93:832–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zipes D, Camm A, Borggrefe M, Buxton A, Chaitman B, Fromer M et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death—executive summary: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). J Am Coll Cardiol. 2006;48:1064–108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.