Abstract
This report describes a Pd-mediated C-H radiofluorination of 8-methylquinoline derivatives with no-carrier-added Ag[18F]F. To achieve this transformation, a new method was developed for the generation of Ag[18F]F using a sep-pak cartridge. The C–H radiofluorination was then optimized and applied to a series of substituted 8-methylquinoline derivatives. Finally, this method was fully automated using a radiochemistry synthesis module.
Positron emission tomography (PET) is a functional imaging technique that is used for clinical diagnostic imaging as well as for research applications in both healthcare and the pharmaceutical industry.1,2 Fluorine-18 (18F) is one of the most commonly used PET radionuclides, mainly due to its useful half-life (110 min) and exceptional imaging properties. As such, new synthetic methods that enable the late-stage formation of a C–18F bond are of great interest to the field of radiochemistry.3
Incorporation of 18F at an sp3 carbon is one of the most widely used labelling strategies. This is typically achieved via nucleophilic displacement of an appropriate leaving group with [18F]fluoride (Figure 1a).4 Indeed, the production of 2-[18F]fluoro-2-deoxy-D-glucose ([18F]FDG) by the reaction of mannose triflate with K[18F]F is one of the most widely used labelling reactions in PET radiochemistry.5 However, this approach often necessitates complex multi-step syntheses of labelling precursors before any radiochemistry development can be undertaken. This is time consuming and not ideal for synthesizing libraries of radiotracers for screening purposes.
Fig 1.
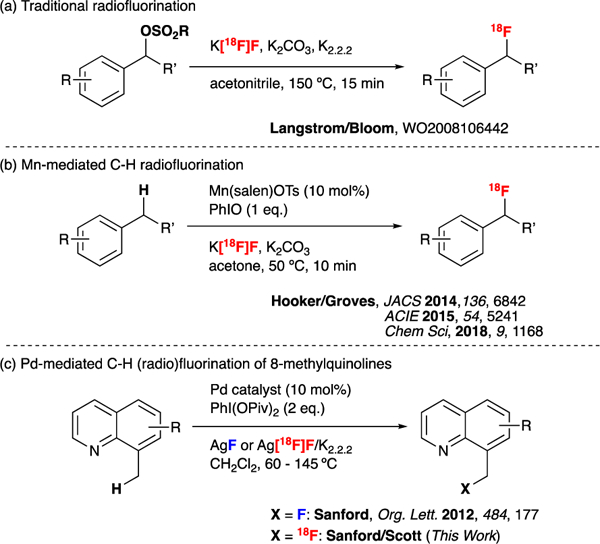
Approaches to benzylic radiofluorination
The direct conversion of carbon–hydrogen bonds to carbon–18F bonds would provide more straightforward and atom economical access to radiotracers containing C(sp3)–18F bonds.6 To date, methods for the direct conversion of C(sp3)–H bonds to C–18F bonds using high molar activity nucleophilic 18F- remain extremely limited. Recent seminal work by Hooker and Groves demonstrated proof-of-concept through Mn-mediated benzylic C-H fluorination using [18F]fluoride (Figure 1b).7 However, new, complementary methods are needed in order to realize the full potential of this approach.
In 2012, the Sanford group reported the C(sp3)–H fluorination of 8-methylquinoline derivatives using Pd-based catalysts and AgF as a fluoride source (Figure 1c).8 In this Communication we report the adaptation of this transformation for use with [18F]fluoride. As detailed below, the translation to radiofluorination required the development of a new method for the preparation of Ag[18F]F as well as significant reaction optimization. Ultimately, these studies delivered a robust and automatable process for the radiofluorination of 8-methylquinoline substrates.9
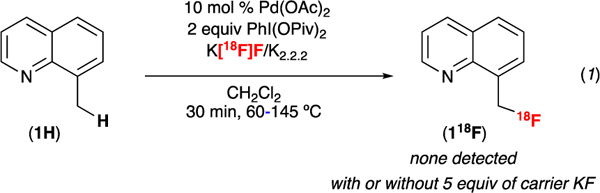
Our initial studies examined the radiofluorination of 8-methylquinoline (1H) using K[18F]F•kryptofix®2.2.2 (K[18F]F •K2.2.2), the most readily available source of 18F. These reactions were conducted using conditions otherwise analogous to those for the 19F-fluorination (10 mol % of Pd(OAc)2, 2 equiv of PhI(OPiv)2 in dichloromethane). As shown in eq. 1, none of the 18F-labeled product 118F was detected by radio-TLC or radio-HPLC under these conditions. One possible explanation for this result is the change in stoichiometry of fluoride (from 5 equiv relative to 1H in the 19F-fluorination to ≤0.0002 equiv relative to 1H in the 18F-fluorination). To test for this possibility, we next conducted the radiofluorination in the presence of 5 equiv of carrier KF. However, product 118F was still not detected under these conditions (eq. 1).
 |
(1) |
On the basis of these preliminary results, we concluded that the counterion associated with fluoride (Ag+ in the case of the successful 19F-fluorination reactions) was likely critical for this transformation. As such, we next sought a straightforward, automatable method for accessing Ag[18F]F. Notably, Ag[18F]F has been reported in the [18F]fluorine literature,10 but its preparation typically required specialized equipment (e.g. custom cyclotron targets,10a platinum reaction vessels10e) or insoluble silver sources (Ag2O,10b,d,f silver wool10c) that are not readily adaptable to modern automated radiosynthesis modules.
We reasoned that Ag[18F]F could be prepared by the elution of [18F]fluoride from a quarternary methyl ammonium (QMA) ion exchange cartridge using an aqueous solution of a Ag+ salt (Table 1 and Supporting Information). Notably, an analogous procedure (involving elution with aqueous K2CO3) is routinely used to produce K[18F]F (Table 1, entry 1). Furthermore, we have recently reported that solutions of other eluents (e.g., copper salts, bases) are effective for eluting [18F]fluoride from QMA cartridges.11 Initial attempts to use Ag2CO3 as an eluent afforded no [18F]fluoride recovery (Table 1, entry 2), likely due to the poor water solubility of Ag2CO3. Consistent with this explanation, the use of more water-soluble AgOTf led to near quantitative recovery of Ag[18F]F (Table 1, entry 3). The use of aqueous solutions of silver salts does require an azeotropic drying step; however, attempts to elute with silver salts formulated in MeCN were unsuccessful (Table 1, entry 4). Even when using water soluble silver salts, heterogeneous mixtures were obtained following elution. This heterogeneity is likely due to the formation and co-elution of insoluble AgHCO3 due to standard QMA preconditioning with NaHCO3.‡ This issue was addressed by changing the pre-conditioning reagent. For operational simplicity, we pre-conditioned with KOTf and then used AgOTf for elution (Table 1, entry 5). §,∥
Table 1.
Elution Strategies for Generation of Ag[18F]F
 | |||
|---|---|---|---|
| Entry | Precond. Salta | QMA Eluentb (MX) |
Recovery of M[18F]F (%) |
| 1 | NaHCO3 | K2CO3 | 97 |
| 2 | NaHCO3 | Ag2CO3 | 0 |
| 3 | NaHCO3 | AgOTf | 98 |
| 4 | NaHCO3 | AgOTf | 0c |
| 5 | KOTf | AgOTf | 94 |
QMA was flushed with 10 mL of 0.5 M aq. solution;
QMA was eluted with 0.5 mL of 0.05 M aq. solution;
QMA eluent was dissolved in MeCN.
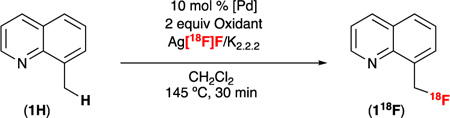
With a reliable and straightforward route to Ag[18F]F in hand, we next revisited the C–H radiofluorination of 1H. As shown in Table 2, the use of Ag[18F]F in combination with K2.2.2 afforded 118F in 3 ± 1% RCC (entry 1, n = 2). Pre-stirring PhI(OPiv)2 and Ag[18F]F in CH2Cl2 prior to the addition of Pd/substrate resulted in an enhanced RCC of 8 ± 2% (entry 2, n = 2). As such, this pre-stirring step was included in all subsequent experiments. A series of IIII oxidants was next evaluated, and PhI(OAc)2 was found to afford slightly higher RCC than PhI(OPiv)2 (11% versus 8%, entries 2 and 3). The need for a high reaction temperature (145 oC) in conjunction with CH2Cl2 (bp = 40 oC) as the reaction solvent was unexpected. However, the use of higher boiling solvents (e.g., C2H4Br2 or CH2Br2/CH2Cl2 mixtures) led to no improvement in RCC (entry 4 and Supporting Information). Changing the catalyst from Pd(OAc)2 to Pd2(dba)3 resulted in an improvement to 18 ± 2% RCC (entry 4, n = 2). Tripling the amount of precursor (to 0.042 mmol) while retaining the stoichiometry of the other reactants and reagents resulted in a further increase to 21 ± 5% (n = 7) RCC of 118F (entry 6). Notably, an even higher RCC (51 ± 10%, n = 2) was obtained upon the addition of 5 equiv of carrier AgF under otherwise analogous conditions (entry 7).¶
Table 2.
Radiofluorination Optimization Studiesa
 | |||
|---|---|---|---|
| Entry | [Pd] | Oxidant | RCY (%)# |
| 1 | Pd(OAc)2 | PhI(OPiv)2 | 3±1 (n=2) |
| 2 | Pd(OAc)2 | PhI(OPiv)2 | 8±2b (n=2) |
| 3 | Pd(OAc)2 | PhI(OAc)2 | 11±2b (n=2) |
| 4 | Pd(OAc)2 | PhI(OAc)2 | 13±1b,c (n=2) |
| 5 | Pd2(dba)3 | PhI(OAc)2 | 18±2b(n=2) |
| 6 | Pd2(dba)3 | PhI(OAc)2 | 21±5b,d (n=7) |
| 7 | Pd2(dba)3 | PhI(OAc)2 | 51±10 (n = 2)b,e |
General conditions: aliquots of a prestirred stock solution containing Ag[18F]F (92.5–129.5 MBq) and oxidant (2 equiv) in CH2Cl2 (200 μL) were added to vials containing substrate (0.014 mmol) and [Pd] (10 mol %) in CH2Cl2 (550 μL);
reaction included pre-stirring step;
C2H4Br2:CH2Cl2 [2:1] used as reaction solvent;
3-fold scale up of substrate (0.042 mmol) while retaining stoichiometry of other reactants/reagents;
5 equiv. AgF added to reaction.
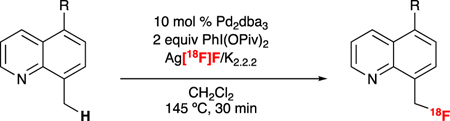
The no-carrier-added radiofluorination conditions were next applied to a series of 8-methylquinoline derivatives (2H-10H, Table 3). The method is compatible with a range of functional groups that is comparable to those used in the 19F reaction. For instance, C–H radiofluorination proceeds with 8-methylquinoline derivatives bearing electron-withdrawing (2-4) and electron-neutral (5-9) substituents. Ketone (2) and halogen (4–6) substituents on the quinoline core proved compatible, offering the potential for downstream chemistry to be conducted after radiolabeling.12 In contrast, no product was observed for a substrate bearing the electron donating methoxy substituent (10), consistent with the constraints reported for the original 19F-fluorination reaction.
Table 3.
Substrate Scope
 | ||
|---|---|---|
| Entry | R (#) | RCY (%)# |
| 1 | H (118F) | 21 ± 5 (n=7) |
| 2 | Ac (218F) | 13 ± 3 (n=5) |
| 3 | CN (318F) | 20 ± 5 (n=4) |
| 4 | F (418F) | 16 ± 2 (n=7) |
| 5 | I (518F) | 14 ± 1 (n=4) |
| 6 | Br (618F) | 12 ± 3 (n=5) |
| 7 | Cl (718F) | 11 ± 2 (n=4) |
| 8 | Me (818F) | 15 ± 2 (n=5) |
| 9 | Ph (918F) | 14 ± 2 (n=5) |
| 10 | MeO (1018F) | 0 (n=7) |
General conditions: aliquots of a prestirred stock solution containing Ag[18F]F (92.5–129.5 MBq) and PhI(OAc)2 (2 equiv) in CH2Cl2 (200 μL) were added to vials containing substrate (0.042 mmol) and Pd2(dba)3 (10 mol %) in CH2Cl2 (550 μL). Reactions were heated at 145 oC for 30 min, RCC was determined by radio-TLC.
Finally, we automated the synthesis of 118F using ~55.5 GBq of [18F]fluoride in a GE TRACERlab FXFN synthesis module (Scheme 2). The prestirring/heating of the mixture of Ag[18F]F•K2.2.2 and PhI(OAc)2 in the reactor was followed by the addition of Pd catalyst and substrate (1H). Under these automated conditions, 118F was formed in 4 ± 1% RCY,# 39 ± 15 GBq/mmol molar activity and an estimated activity yield (AY) of 1.1 ± 0.5 GBq (n = 2). We note that the automated radiochemical yield provides enough product for preclinical studies, but will require further optimization before this method can be applied in routine radiosyntheses. However, overall this operationally simple procedure demonstrates proof-of-concept that this Pd-mediated C-H radiofluorination is feasible, and that the method could ultimately be applicable to the late-stage radiofluorination of bioactive molecules.
Scheme 2.
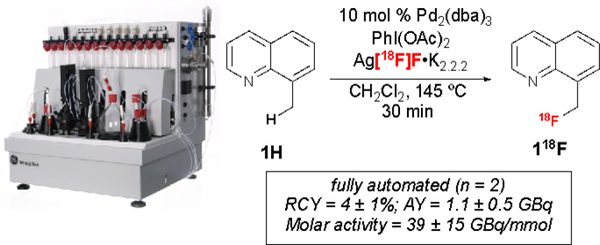
Automated Synthesis of 118F#
In conclusion, this paper reports a new method for generating no-carrier-added Ag[18F]F by using soluble silver salts to elute [18F]fluoride from a QMA cartridge. This Ag[18F]F was then applied to the Pd-catalyzed C–H radiofluorination of 8-methylquinoline derivatives. The chemistry was optimized for and applied to the radiolabeling of a series of 8-methylquinoline derivatives, providing moderate RCCs and high molar activity. Ongoing work is focused on leveraging now readily accessible Ag[18F]F to achieve a variety of other radiofluorination reactions.13
Supplementary Material
Acknowledgments
We acknowledge US DOE/NIBIB (DE-SC0012484 to PJHS) and NIH (R01EB021155 to MSS and PJHS) for financial support.
Footnotes
Conflicts of interest
There are no conflicts to declare.
Electronic Supplementary Information (ESI) available: Experimental procedures, optimization details, radio-HPLC/TLC traces and spectral data. See DOI: 10.1039/x0xx00000x
Waters QMA cartridges are shipped with the Cl- counter ion. However, this can compete with [18F]F- in downstream reactions, lowering reaction yields. Chlorinated products can also be difficult to separate from the desired fluorinated PET drugs.
This combination eluted a homogenous mixture that did not block synthesis module lines, and it provided excellent recovery of Ag[18F]F following azeotropic drying and reconstitution into the reaction solvent.
We first demonstrated that Ag[18F]F was a viable source of reactive [18F]fluoride in our established Cu-mediated radiofluorination chemistry (see Supporting Information).
Using more soluble K[18F]F in conjunction with exogenous Ag(I) salts (e.g. 3 equiv of AgOTf) also gave product, but yields were lower than those obtained using Ag[18F]F (n.c.a: 14% RCC; + 5 equiv carrier KF: 26% RCC).
Radiochemical yields (RCY) are non-isolated and were calculated by % integrated area of the 18F product versus 18F- in a radio-TLC trace.
references
- 1.For a general overview of PET, see: Ametamey SM, Honer M and Schubiger PA, Chem. Rev, 2008, 108, 1501. [DOI] [PubMed] [Google Scholar]
- 2.For an introduction to the use of PET in drug development, see: Elsinga PH, Waarde AV, Paans AMJ and Dierckx RAJO, Trends on the Role of PET in Drug Development. World Scientific, Singapore, 2012. [Google Scholar]
- 3.For reviews covering recent advances in 18F-radiochemistry, see: (a) Miller PW, Long NJ, Vilar R and Gee AD, Angew. Chem. Int. Ed, 2008, 47, 8998; [DOI] [PubMed] [Google Scholar]; (b) Cai L, Lu S and Pike VW, Eur. J. Org. Chem, 2008, 2853; [Google Scholar]; (c) Tredwell M and Gouverneur V, Angew. Chem. Int. Ed, 2012, 51, 11426; [DOI] [PubMed] [Google Scholar]; (d) Brooks AF, Topczewski JJ, Ichiishi N, Sanford MS and Scott PJH, Chem. Sci, 2014, 5, 4545; [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Jacobson O, Kiesewetter DO and Chen X, Bioconjugate Chem, 2015, 26, 1; [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Preshlock S, Tredwell M and Gouverneur V, Chem. Rev, 2016, 116, 719; [DOI] [PubMed] [Google Scholar]; (g) Deng X, Rong J, Wang L, Vasdev N, Zhang L, Josephson L and Liang S, Angew. Chem. Int. Ed, DOI: 10.1002/anie.201805501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.For a recent example, see: Langstrom B and Blom E, Synthesis of [18F]Fluoromethyl benzene using Benzyl pentafluorobenzenesulfonate. WO 2008/106442 A1, 4 September 2008.
- 5.Yu S, Biomed. Imaging Interv. J. 2006, 2, article e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.For a recent perspective on C-H fluorination, see: Neumann CN and Ritter T, Angew. Chem. Int. Ed, 2015, 54, 3216. [DOI] [PubMed] [Google Scholar]
- 7.(a) Huang X, Liu W, Ren H, Neelamegan R, Hooker JM and Groves JT, J. Am. Chem. Soc, 2014, 136, 6842. [DOI] [PubMed] [Google Scholar]; (b) Huang X, Liu W, Hooker JM and Groves JM, Angew. Chem. Int. Ed, 2015, 54, 5241; [DOI] [PubMed] [Google Scholar]; (c) Liu W, Huang X, Placzek MS, Krska SW, McQuade P, Hooker JM and Groves JT, Chem. Sci, 2018, 9, 1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMurtrey KB, Racowski JM and Sanford MS, Org. Lett, 2012, 14, 4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.For examples of PET drugs containing a quinoline core, see: (a) Vasdev N, Cao P, van Oosten EM, Wilson AA, Houle S, Hao G, Sun X, Slavine N, Alhasan M, Antich PP, Bonte FJ, and Kularni P, Med. Chem. Commun. 2012, 3, 1228. [Google Scholar]; (b) Liang SH, Holland JP, Stephenson NA, Kassenbrock A, Rotstein BH, Daignault CP, Lewis R, Collier L, Hooker JM and Vasdev N, ACS Chem. Neurosci. 2015, 6, 535. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Furumoto S, Tago T, Harada R, Kudo Y and Okamura N, Curr. Alzheimer’s Res. 2016, 14, 178. [DOI] [PubMed] [Google Scholar]
- 10.(a) Clark JC, Goulding RW and Palmer AJ, in Radiopharmaceuticals and Labelled Compounds (Copenhagen, 26–30 March 1973), IAEA, Vienna, 1973, Vol. 1, pp. 411; [Google Scholar]; (b) Gatley SJ, Hichwa RD, Shaughnessy WJ and Nickles RJ, Int. J. Appl. Rasdiat. Isot. 1981, 32, 211; [DOI] [PubMed] [Google Scholar]; (c) Yagi M, Izsawa G and Murano M, Kakuriken Kenkyu Ho Koku (Research Report of Laboratory of Nuclear Science), 1981, 14, 62; [Google Scholar]; (d) Gatley SJ, Int. J. Appl. Rasdiat. Isot. 1982, 33, 255; [Google Scholar]; (e) Shalom E, Takrouri K, Metsuyanim N, Grufi A, Katzhendler J and Srebnik M, Appl. Radiat. Isot. 2007, 65, 204; [DOI] [PubMed] [Google Scholar]; (f) Caires CC and Guccione S, Methods for Silver-Promoted Fluorination of Organic Molecules. US Patent 2010/0152502 A1, June 17, 2010.
- 11.Mossine AV, Brooks AF, Ichiishi N, Makaravage KJ, Sanford MS and Scott PJH, Sci. Rep. 2017, 7, 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.For example, halo-quinolines are well known substrates for Suzuki-Miyaura cross-coupling reactions, see: (a) Eleya N, Mahal A, Hein M, Villinger A and Langer P, Adv. Synth. Catal. 2011, 353, 2761; [Google Scholar]; (b) Arumugam A, Kaminsky W and Mallasamy D, RSC Adv, 2015, 5, 77948. [Google Scholar]
- 13.For recent fluorination reactions using AgF, see: (a) Katcher MH and Doyle AG, J. Am. Chem. Soc. 2010, 132, 17402; [DOI] [PubMed] [Google Scholar]; (b) Truong T, Klimovica K and Daugulis O, J. Am. Chem. Soc. 2013, 135, 9342; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Qin C and Davies HML, Org. Lett. 2013, 15, 6152; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Wu T, Yin G and Liu G, J. Am. Chem. Soc. 2009, 131, 16354; [DOI] [PubMed] [Google Scholar]; (e) Liu W, Huang X, Cheng M-J, Nielsen RJ, Godard WA and Groves JT, Science, 2012, 337, 1322; [DOI] [PubMed] [Google Scholar]; (f) Fier PS and Hartwig JF, J. Am. Chem. Soc. 2012, 134, 10795. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


